Abstract
Purpose
To investigate the cerebral macrovascular changes as well as the relationship of large vessels and cerebral blood flow (CBF) in mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) using magnetic resonance angiography (MRA) and arterial spin labeling (ASL) perfusion MR imaging (MRI).
Methods
A total of 20 patients diagnosed with MELAS (12 males, 8 females; mean age, 23.3 years) underwent conventional MRI, time-of-flight (TOF) MRA and three dimensional ASL. Follow-up scans were performed in 10 patients. The changes of cerebral arteries and branches on MRA images from both acute and recovery patients were independently evaluated by two radiologists. Lesion distribution and CBF were observed on the integrated maps of MRA and ASL.
Results
In 14 patients with clinical onsets, increased CBF was observed in all stroke-like lesions. Dilations of a single artery (four middle cerebral arteries, two posterior cerebral arteries) were found in six patients. Dilations of multiple arteries (two anterior cerebral arteries, seven middle cerebral arteries, six posterior cerebral arteries) were found in seven patients. Normal angiography was shown in one acute patient. Cortical terminal branches feeding the lesion areas were more obviously dilated than the main trunks. The dilated vessels returned to normal on follow-up scans concurrently with decreased CBF in nine patients who were resuscitated from episode attacks. Vasodilation was even seen in one preclinical patient who suffered a recurrent episode 50 days later.
Conclusion
Reversible dilation of cerebral macrovascular changes could be a new feature of MELAS and a presumed reason for fluctuant CBF. It would shed new light on the mitochondrial angiopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome is one of the most commonly diagnosed maternally inherited mitochondrial disorders. The cardinal neurological features of MELAS are stroke-like episodes, which occur in 84–99% of affected individuals. These episodes often present with seizure, severe headache, aphasia, cortical vision loss, motor weakness and dementia [1]. Although the neurological symptoms are variable, a similar acute mitochondrial encephalopathy can be found on magnetic resonance imaging (MRI). These stroke-like lesions (SLLs) predominantly affect gray matter and span arterial territories [2], dynamically progress or regress during the course of the episodes [3], and appear hyperperfused, which is a distinguishing difference from ischemia [4, 5].
As one of the etiologies of stroke in young adults [6, 7], the vascular pathogenesis of MELAS is not fully explained. Energy deficiency resulting from mitochondrial dysfunction due to genetic mutation was the initial reason [8]. Some previous studies revealed that the respiratory chain defect in the smooth muscle cell layer and endothelial cells of the microvasculature [9, 10] provided the evidence of mitochondrial angiopathy. Focal hyperemia and vasogenic edema detected in stroke-like lesions by neuroimaging further confirmed the abnormal hemodynamics and increased permeability of the small arteries [11, 12]; however, the changes of large vessels in MELAS are still unknown. Controversial results were found in several case reports. Segmental stenosis of the left carotid artery (LCA) and middle cerebral artery (MCA) was reported in an acute patient and recanalization appeared soon [13]. The reversible vascular narrowing was also found in a child with MELAS [14]. On the contrary, vasodilatation of multiple cerebral arteries was found in the early stage of stroke-like episodes [15]. Even three months prior to the clinical onset, mild vasodilatation of MCA was revealed [16]. Thus it can be seen that, besides microangiopathy, macroangiopathy may be frequently involved and play an important role in hemodynamic regulation during the stroke like episodes. Finsterer and Zarrouk-Mahjoub recently summarized the heterogeneous findings of macroangiopathy in mitochondrial disorders and pointed out macroangiopathy is a typical phenotypic manifestation of MELAS [17]. They also stated that systematic investigations for mitochondrial vasculopathy are important [18].
Therefore, in this study, we aimed to prospectively analyze the changes of cerebral arteries and branches in the acute and recovery states of MELAS using magnetic resonance angiography (MRA). Meanwhile, cerebral blood flow (CBF) was also acquired using arterial spin labeling (ASL) perfusion MRI in order to further investigate the relationship between the large vessels and CBF in stroke-like episodes.
Patients and Methods
Patients
Patients formerly diagnosed with MELAS and patients with initial onset who met the diagnostic criteria of MELAS were recruited in our study from December 2015 to July 2017. The diagnosis was based on clinical manifestation, biochemical examination, MRI, muscle biopsy and genetic testing [19]. In total, 20 patients (12 males, 8 females; mean age, 23.3 years; range, 11–49 years) were included and underwent conventional MRI, time of flight (TOF) MRA and 3D ASL perfusion MRI. Follow-up MRI scans were performed in 10 patients. This study was approved by the Institutional Review Board of our hospital and informed consent was obtained from each patient.
Magnetic Resonance Imaging
The MRI examinations were performed on a 3.0 T scanner (Discovery MR750, General Electric, Boston, MA, USA) with an 8‑channal head coil. Main scan parameters were as follows: T2WI (TR/TE = 4582/96 ms), fluid-attenuated inversion recovery (FLAIR; TR/TE/TI = 8800/146/2100 ms), MRA (TR/TE = 25/3.4 ms, FA = 20°, FOV = 24 cm, 3 slabs, 128 slices), pseudocontinuous ASL (TR/TE = 5327/10.5 ms, post-labeling delay = 1.5 s, slice thickness = 4.0 mm, number of slices = 35, FOV = 24 cm). All of the MRI scans acquired in the acute phase preceded clinical treatment and two expert radiologists blinded to the clinical status of the patients analyzed the changes of cerebral arteries. Normal, vasodilation and stenosis were described by the radiologists after observing bilateral artery trunks and branches on 3D reformatted MRA images. Abnormality was confirmed only if both radiologists reached a consensus. Integrated maps of MRA and ASL derived by GE Functool package (9.4.05) were used to evaluate the distribution and CBF of the lesions.
Results
Patient Characteristics
The recruited patients presented with vomiting, headache, nausea, episodic epilepsy, hemiplegia and aphasia. Muscle biopsy revealed ragged red fibers in modified Gomori trichrome staining, ragged blue fibers in succinate dehydrogenase staining and partial absence in cytochrome C oxidase staining. Blood tests revealed a gene mutation of m.3243 A > G in 16 patients. Mutation of m.13.513 G > A was found in patient 8 diagnosed as MELAS/Leigh overlap syndrome. The whole-length mitochondrial DNA was screened in patient 5, 15 and 16 but none of the known pathogenic mutations were detected. In 12 patients in the acute state and 8 patients in the recovery state initial MRI scans were carried out when recruited. Follow-up MRI scans were performed in 10 patients, among whom 2 patients suffered recurrent episodes. The time interval between episodes appearing and MRI scans ranged from 4 days to 25 days in acute patients (Tables 1 and 2).
Magnetic Resonance Angiography
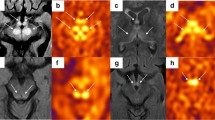
In 14 patients with clinical onset (12 acute patients at initial MRI scans, 2 recurrent patients during the follow-up), all of the stroke-like lesions showed cortical swelling, hyperintensity on T2 and FLAIR images and prominent increased CBF. Dilations of a single cerebral artery (four middle cerebral arteries, two posterior cerebral arteries) were found in six patients. Dilations of multiple cerebral arteries (two anterior cerebral arteries, seven middle cerebral arteries, six posterior cerebral arteries) were found in seven patients. Normal angiography was shown in one acute patient. Dilations of terminal branches were found more frequently and more pronounced than in the main trunks. All of the dilated vessels were ipsilateral to the lesions and corresponded to the lesion areas (Table 1). On the integrated maps of MRA and ASL, hyperperfused stroke-like lesions and dilated feeding arteries were both present. Ectatic branches of a single cerebral artery appeared in six lesions confined to a single lobe (Fig. 1). Ectatic branches of multiple cerebral arteries appeared in six extensive lesions involving more than two lobes. In patient 8 there were two acute lesions which appeared simultaneously in each parietal lobe, matching dilated branches of the left MCA and right anterior cerebral artery (ACA; Fig. 2).
Reversible dilation of cerebral artery in MELAS episodes. Patient 13, a 20-year-old male experienced with his second attack indicated by fever, headache, vomiting, teichopsia and left hemianopia. a Axial FLAIR image demonstrates an acute stroke-like lesion in the right occipital lobe. b Increased CBF and dilated branches of PCA in the corresponding area are shown on the integrated map of ASL and MRA. c MRA shows dilations of parieto-occipital artery and calcarine artery of right PCA (redarrow). Follow-up MR images 92 days later demonstrate a resolving right occipital lesion (d), focal decreased CBF (e), and normal cerebral arteries (red arrow; f). Color bar of CBF ranges from 0 to 200 ml/100 g/min
Dilations of multiple cerebral arteries. Patient 19, a 14-year-old female presented with aphasia, dysphoria and right hemianopia for her third stroke-like episode. a An acute stroke-like lesion spanning left temporal lobe and occipital lobe appears on FLAIR images. b Remarkable increased CBF as well as dilated arteries in the lesion area. c Ectatic branches of both left MCA (parietal artery, angular artery and temporal artery) and PCA (P3, parieto-occipital artery) are observed. Meanwhile, the branches of right MCA become sparse with decreased CBF in the contralateral side. Patient 8, a 13-year-old boy suffered from headache, vomiting, blurred vision, weakness and recurrent tonic-clonic seizures. There are two lesions located in each parietal lobe asymmetrically (d and g), both of them displaying high CBF (e and h). Dilated left MCA (M2, M3, parietal artery and angular artery) and right ACA (inferior parietal artery) are also presented (red arrows; f and i). Color bar of CBF ranges from 0 to 200 ml/100 g/min
Follow-up MRI scans showed the dilated vessels returned to normal in 9 patients who experienced relief from episodes attack (Table 2; Fig. 1). Stenosis of major cerebral arteries was not found in our patients either in acute or recovery phase. Cortical branches supplying blood to the chronic hypoperfused lesion area became narrow and sparse in three patients.
One notable finding was that artery dilation could be detected in a preclinical patient. Patient 12 was recovered with no obvious central nervous symptoms when initial MRI was performed. There was no emerging lesion manifested on conventional MRI, while dilated branches of right MCA and PCA accompanied with focal increased CBF in right parietal-occipital lobes were observed, which might be suggestive of latent SLL. 50 days later, she suffered an acute stroke like episode (Fig. 3). Another patient (Patient 17) was also in his preclinical phase as the initial MRI found focal high CBF in right occipital lobe. While no observable dilation of cerebral arteries was found. He developed clinical onset after 15 days.
Cerebral artery dilation in preclinical phase. An initial MRI (upper row) from patient 12 in preclinical phase shows no acute lesion but a chronic one in left parietal lobe on the FLAIR image (a) while a slightly increased CBF in the right parietal-occipital lobe (b) and dilated parietal branches (redarrow) of right MCA can be seen (c). The patient suffered an acute episode 50 days later. The MRI in the peak of disease was a failure because of the head motion. The images acquired after peak stage (middle row) shows patchy FLAIR hyperintensity in right parietal-occipital lobe (d), as the previous high CBF indicated. Slightly high CBF (e) and dilated artery branches (red arrow) are presented (f). On follow-up MRI performed 306 days later, the lesion has resolved with cortical atrophy (g) and low CBF (h). The dilated right MCA return to normal (red arrow) in recovery state (i). Color bar of CBF ranges from 0 to 200 ml/100 g/min
Discussion
The three main hypotheses for stroke-like episodes in MELAS presented to date are mitochondrial angiopathy (impaired mitochondria in the blood vessels and inappropriate intracranial hemodynamics), mitochondrial cytopathy (mitochondrial dysfunction of neural cells) and neuronal hyperexcitability (imbalance between energy supply and demand). The hypothesis involving angiopathy is one of the most attractive ideas [20]. Mitochondrial angiopathy may be classified into either microangiopathy or macroangiopathy. Most studies assume that stroke-like lesions are associated with microangiopathy that manifested as vasculopathy of the small arteries, arterioles, capillaries, or venules [18]. Up to now, only few of the cases reported the changes of large vessels in MELAS and other mitochondrial disorders. To our knowledge, our study is the first prospective investigation to explore the changes of cerebral arteries in MELAS using MRA and perfusion MR.
We found dilation of cerebral arteries and branches in 13 out of 14 patients with clinical onset. Meanwhile, increased CBF was shown in acute lesions located within the vascular territory of the dilated arteries. The dilation is reversible since it returned to normal along with the regression of acute lesions. These findings indicated that macroangiopathy might play a role in the pathophysiology of the MELAS and contribute to the understanding of the disease. The pathogenesis of large vessels involvement has not been clearly elucidated. An autopsy found disrupted smooth muscle and elastic layers, decreased cytochrome-C oxidase (COX) I in the aorta from an A3243G mutation/MELAS patient who died of aortic rupture. [21]. Other macroangiopathies in MELAS including progressive stenosis [22], dissection [23], ectasia [24] and aneurysm formation [25] have been reported. Carotid artery, cerebral artery and aortic root were primarily affected. All of these findings presumed that a high mitochondrial DNA mutation load in the blood vessels leads to degenerative vascular changes. We agree with this point of view and regard the pathologic abnormality as the basis of macroangiopathy; however, this theory could not fully explain the reversible changes of cerebral arteries. Some functional mechanisms of vascular regulation might participate in the hemodynamics of stroke-like episodes. A study of vascular changes evoked by visual stimuli found significantly increased PCA diameter, which was strongest near the primary visual cortex and progressively decreased as a function of distance [26]. This indicated that neural activity interacted with neurovascular changes. Our results showed a similar upstream pattern of vasodilation being more common in the cortical branches than in the main trunks. It was deduced that cerebral artery dilation might probably be trigged by focal neuronal energy depletion or hyperexcitability due to mitochondrial dysfunction. Although the mechanism of neurovascular coupling in MELAS is still unknown, several factors such as lactic acid accumulation, [19] microvascular pressure, [27] and ion concentration [28] might be involved. Different cell types within the neurovascular unit, including astrocytes, vascular smooth muscle cells, pericytes and endothelial cells were presumed to contribute to neurovascular regulation [29]. Through vasodilation, energy substrates and nitric oxide can be quickly and efficiently delivered to the SLL area so as to preserve neural activity.
Another indicator to reflect the capacity of blood vessels dilating in response to a vasoactive stimulus is cerebral vascular reactivity (CVR), an important marker for brain vascular reserve. Increased cerebral CBF and decreased CVR were found in three interictal MELAS patients. The CBF correlated inversely with CVR in the frontal lobe [12]. One earlier case reported generalized CVR reduction in the acute phase which improved thereafter [30]. Both studies demonstrated the interactive relationship between CBF and capacity of vessel dilation. In this study, similar findings were found showing that hyperperfused lesions co-occurred with dilated feeding arteries in the acute patients, even in a preclinical case. It could also be speculated that the dilated cerebral vessels might be one of the reasons resulting in the restriction of CVR during the episode attacks, and vasodilation returned to normal followed by CVR improvement.
Large vessel dilation could occur prior to clinical onset, which was detected in our patient 12. Dilation of the right MCA and PCA were shown 2 months before the SLE emerged. The subsequent responsible foci located in the right parietal occipital lobe corresponded with the territory of the dilated vessels. A previous study detected left MCA dilatation as well as regional hyperperfusion 3 months before the occurrence of acute episodes. [16] Our findings are consistent with this and vasodilation of the cerebral artery in the preclinical stage might be an alternative imaging sign to predict the emergence of upcoming SLE. In our previous study, increased CBF proved to be an efficient predictor of episodes in MELAS [31]. Whether vasodilation of the cerebral artery combined with high CBF could serve as co-biomarker to improve predictive accuracy of latent SLE, further studies should be performed. In addition, dilations of multiple cerebral arteries were common in the acute phase provided that the SLL involved two or three lobes. These dilated vessels were found in 6 patients during 12–20 days after clinical onset, usually a peak period of the episodes. A case described that prominent dilation of the posterior and middle cerebral artery was observed in early stage of SLE, prior to the development of clinical symptoms [15]. As we know, the SLLs might gradually spread from one lobe to the adjacent lobe, which is an imaging feature suggesting disease progression [4, 11]; therefore, it was presumed that vasodilation of multiple cerebral arteries in early stage of the episodes might be an imaging sign to predict the progressive tendency.
Cerebral arteries showed normal in seven interictal patients on initial MRA. The dilated arteries returned to normal in the follow-up patients who have recovered from the SLE. In three patients, cortical branches feeding the chronic hypoperfused lesion areas became narrow and sparse. This degenerative vascular change might result from the neuronal dysfunction and necrosis; therefore, the synchronization of cerebral artery and CBF was evident during the process of MELAS. Cerebral vasodilation appeared in attack episodes concurrently with increased CBF, thereafter, both were resolved during the recovery state. Furthermore, it might be indicated that the reversible cerebral macrovascular dilation could be a presumed reason for fluctuant CBF not merely because of the microvascular pathology. It would shed new light on the mitochondrial angiopathy.
Due to the limitation of MRA, only large intracranial arteries can be observed. Although dilation of small arteries and arterioles might occur directly in response to the neural energy depletion, it could not be detected. According to our findings, it was still difficult to illuminate whether vasodilation served as a primary cause leading to the stroke episodes or a secondary phenomenon. Other macroangiopathies reported in MELAS patient, such as premature atherosclerosis, dissection and aneurysms were not present in our patients. Systematic investigation of larger patient cohorts based on high-resolution MRA and other new MRI techniques will be performed in our further study to discover more signs of large vessels impairment in MELAS.
References
Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, Kakuma T, Koga Y; Taro Matsuoka for MELAS Study Group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. 2012;1820:619-24.
El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015;116:4-12.
Tzoulis C, Bindoff LA. Acute mitochondrial encephalopathy reflects neuronal energy failure irrespective of which genome the genetic defect affects. Brain. 2012;135(Pt 12):3627–34.
Ito H, Mori K, Harada M, Minato M, Naito E, Takeuchi M, Kuroda Y, Kagami S. Serial brain imaging analysis of stroke-like episodes in MELAS. Brain Dev. 2008;30:483-8.
Li R, Xiao HF, Lyu JH, Wang D JJ, Ma L, Lou X. Differential diagnosis of mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) and ischemic stroke using 3D pseudocontinuous arterial spin labeling. J Magn Reson Imaging. 2017;45:199-206.
Bersano A, Markus HS, Quaglini S, Arbustini E, Lanfranconi S, Micieli G, Boncoraglio GB, Taroni F, Gellera C, Baratta S, Penco S, Mosca L, Grasso M, Carrera P, Ferrari M, Cereda C, Grieco G, Corti S, Ronchi D, Bassi MT, Obici L, Parati EA, Pezzini A, De Lodovici ML, Verrengia EP, Bono G, Mazucchelli F, Zarcone D, Calloni MV, Perrone P, Bordo BM, Colombo A, Padovani A, Cavallini A, Beretta S, Ferrarese C, Motto C, Agostoni E, Molini G, Sasanelli F, Corato M, Marcheselli S, Sessa M, Comi G, Checcarelli N, Guidotti M, Uccellini D, Capitani E, Tancredi L, Arnaboldi M, Incorvaia B, Tadeo CS, Fusi L, Grampa G, Merlini G, Trobia N, Comi GP, Braga M, Vitali P, Baron P, Grond-Ginsbach C, Candelise L; Lombardia GENS Group*. Clinical pregenetic screening for stroke monogenic diseases: results from lLombardia GENS Registry. Stroke. 2016;47:1702-9.
Tatlisumak T, Putaala J, Innilä M, Enzinger C, Metso TM, Curtze S, von Sarnowski B, Amaral-Silva A, Jungehulsing GJ, Tanislav C, Thijs V, Rolfs A, Norrving B, Fazekas F, Suomalainen A, Kolodny EH. Frequency of MELAS main mutation in a phenotype-targeted young ischemic stroke patient population. J Neurol. 2016;263:257-62.
Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med. 2012;366:1132-41.
Sarnat HB, Flores-Sarnat L, Casey R, Scott P, Khan A. Endothelial ultrastructural alterations of intramuscular capillaries in infantile mitochondrial cytopathies: “mitochondrial angiopathy”. Neuropathology. 2012;32:617-27.
Lax NZ, Pienaar IS, Reeve AK, Hepplewhite PD, Jaros E, Taylor RW, Kalaria RN, Turnbull DM. Microangiopathy in the cerebellum of patients with mitochondrial DNA disease. Brain. 2012;135(Pt 6):1736-50.
Kim JH, Lim MK, Jeon TY, Rha JH, Eo H, Yoo SY, Shu CH. Diffusion and perfusion characteristics of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode) in thirteen patients. Korean J Radiol. 2011;12:15-24.
Rodan LH, Poublanc J, Fisher JA, Sobczyk O, Wong T, Hlasny E, Mikulis D, Tein I. Cerebral hyperperfusion and decreased cerebrovascular reactivity correlate with neurologic disease severity in MELAS. Mitochondrion. 2015;22:66–74.
Yoshida T, Ouchi A, Miura D, Shimoji K, Kinjo K, Sueyoshi T, Jonosono M, Rajput V. MELAS and reversible vasoconstriction of the major cerebral arteries. Intern Med. 2013;52:1389-92.
Noguchi A, Shoji Y, Matsumori M, Komatsu K, Takada G. Stroke-like episode involving a cerebral artery in a patient with MELAS. Pediatr Neurol. 2005;33:70-1.
Minobe S, Matsuda A, Mitsuhashi T, Ishikawa M, Nishimura Y, Shibata K, Ito E, Goto Y, Nakaoka T, Sakura H. Vasodilatation of multiple cerebral arteries in early stage of stroke-like episode with MELAS. J Clin Neurosci. 2015;22:407-8.
Ikawa M, Yoneda M, Muramatsu T, Matsunaga A, Tsujikawa T, Yamamoto T, Kosaka N, Kinoshita K, Yamamura O, Hamano T, Nakamoto Y, Kimura H. Detection of preclinically latent hyperperfusion due to stroke-like episodes by arterial spin-labeling perfusion MRI in MELAS patients. Mitochondrion. 2013;13:676-80.
Finsterer J, Zarrouk-Mahjoub S. Macroangiopathy is a typical phenotypic manifestation of MELAS. Metab Brain Dis. 2017;32:977-9.
Finsterer J, Zarrouk-Mahjoub S. Mitochondrial vasculopathy. World J Cardiol. 2016;8:333-9.
Iizuka T, Sakai F, Suzuki N, Hata T, Tsukahara S, Fukuda M, Takiyama Y. Neuronal hyperexcitability in stroke-like episodes of melas syndrome. Neurology. 2002;59:816-24.
Yoneda M, Ikawa M, Arakawa K, Kudo T, Kimura H, Fujibayashi Y, Okazawa H. In vivo functional brain imaging and a therapeutic trial of L‑arginine in MELAS patients. Biochim Biophys Acta. 2012;1820:615-8.
Tay SH, Nordli DR Jr, Bonilla E, Null E, Monaco S, Hirano M, DiMauro S. Aortic rupture in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes. Arch Neurol. 2006;63:281-3.
Iizuka T, Goto Y, Miyakawa S, Sato M, Wang Z, Suzuki K, Hamada J, Kurata A, Sakai F. Progressive carotid artery stenosis with a novel tRNA phenylalanine mitochondrial DNA mutation. J Neurol Sci. 2009;278:35-40.
Mancuso M, Montano V, Orsucci D, Peverelli L, Caputi L, Gambaro P, Siciliano G, Lamperti C. Mitochondrial vasculopathy due to the m.3243A > G mutation is not restricted to the carotid artery. Mol Genet Metab Rep. 2016;9:12-4.
Finsterer JBA. Dilative arteriopathy and leucencephalopathy as manifestations of a neurometabolic disease. Open Neurol J. 2015;26:28-31.
Zhu K, Li S, Chen H, Wang Y, Yu M, Wang H, Zhao W, Cao Y. Late onset MELAS with m.3243A > G mutation and its association with aneurysm formation. Metab Brain Dis. 2017;32:1069-72.
Bizeau A, Gilbert G, Bernier M, Huynh MT, Bocti C, Descoteaux M, Whittingstall K. Stimulus-evoked changes in cerebral vessel diameter: a study in healthy humans. J Cereb Blood Flow Metab. 2017 Jan 1 [Epub ahead of print]
Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8-17.
Muñoz MF, Puebla M, Figueroa XF. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front Cell Neurosci. 2015;9:59.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419-34.
Gropen TI, Prohovnik I, Tatemichi TK, Hirano M. Cerebral hyperemia in MELAS. Stroke. 1994;25(9):1873–6.
Li Y, Lin J, Sun C, Zhao C, Li H. Increased cerebral blood flow as a predictor of episodes in MELAS using multimodal MRI. J Magn Reson Imaging. 2017;46:915–8.
Acknowledgements
We would like to thank all patients who participated in this study. We also acknowledge the grants from the National Natural Science Foundation of China (No. 81301203, 81401035) which supported this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Li, W. Xu, C. Sun, J. Lin, J. Qu, J. Cao, H. Li and L. Yang declare that they have no competing interests.
Ethical standards
All investigations performed on humans in this study were carried out in accordance with national law and the Helsinki Declaration from 1964 (in its current revised form). This study was approved by the ethics committee of our hospital. Informed consent was obtained from each patient.
Additional information
Dr. Weixingzi Xu and Dr. Chong Sun both participated in the project design and revised the manuscript. In addition, Dr. Xu analyzed the imaging data and Dr. Sun managed the patients.
Caption Electronic Supplementary Material
62_2018_662_MOESM1_ESM.pdf
ESM-Fig. 1 MRA images (inferior-superior view) of each patient with MELAS at the initial MR scans; Fig. 2 MRA images (inferior-superior view) of ten patients with MELAS at the follow-up MR scans
Rights and permissions
About this article
Cite this article
Li, Y., Xu, W., Sun, C. et al. Reversible Dilation of Cerebral Macrovascular Changes in MELAS Episodes. Clin Neuroradiol 29, 321–329 (2019). https://doi.org/10.1007/s00062-018-0662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0662-8