Abstract
We reported a 53-year-old with late-onset mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) accompanied by aneurysm and large vessel dilations. Most studies have focused on microangiopathy causing stroke-like episodes. We report a case to describe large vessel involvement in clinical considerations, and possible mechanisms of aneurysm formation. We recommended regular angiographic examination for patients with MELAS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondrial encephalomyopathy is a rare disease affecting multiple organs and is caused by structural or functional disorders in the mitochondria, predominantly affecting the brain and muscle. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is among the most predominant subtypes of mitochondrial disorders and was first reported by Pavlakis in 1984(Pavlakis et al. 1984). MELAS is often reported to have an onset age of 30 years or earlier, and patients typically contain the m.3243A > G mutation in the MT-TL1 gene of mtDNA encoding mitochondrial tRNA. Since its discovery, at least 39 distinct mutations were found to be associated with MELAS, among which A3243G accounts for 80% of the mutations.
In mitochondrial diseases, cellular energy production is affected and concomitants such as diabetes mellitus (Wang et al. 2013), cardiomyopathy (Ping et al. 2015), gastrointestinal diseases (Fukuyama et al. 2012), and renal, pulmonary, dermatological, hematological manifestations (El-Hattab et al. 2015), and angiopathies (Takahashi et al. 2005) have been reported. In previous studies, lesions of the microvasculature were commonly observed (Takahashi et al. 2005), while large vessel involvement in patients with MELAS has been rarely reported. Several recent studies (Brunetti-Pierri et al. 2011; Finsterer and Zarrouk-Mahjoub 2016; Gabrielson et al. 2016; Tay et al. 2006) evaluating mitochondrial disorders revealed an association between MELAS with ectasia of the arteries and aneurysm formation. In this paper, a case of MELAS with carotid aneurysm and large vessel dilation is reported and its association with aneurysm formation is discussed.
Case report
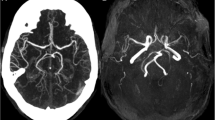
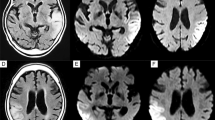
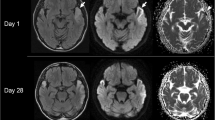
A 53-year-old man with short stature (163 cm), was admitted to our university hospital mainly complaining of visual loss in the left eye for 1 day and right eye for 12 h because of cortical blindness. He had expirenced headache and disorientation 4 days before admission. Physical examination revealed weakness of both the upper and lower limbs, auditory impairment, myoclonus of the upper limbs, hyporeflexia of the biceps and triceps reflex, areflexia of the patellar and Achilles tendon reflex, and no pathological reflex. He experienced seizures on the first day of admission. Magnetic resonance imaging (3.0 Tesla) was performed on day 4 and revealed stroke-like foci in the bilateral temporal, parietal, and occipital lobes, represented as symmetric low-intensity on T1-weighted imaging, high-intensity on T2-weighted, diffused weighted, and fluid attenuated inversion imaging. The foci were inconsistent with the vascular supply. Magnetic resonance spectroscopy revealed an elevated choline peak, decreased N-acetyl aspartate peak, and elevated, inversed lactate peak, choline/NAA ranged approximately from 1.56 to 3.32. Computerized tomographic angiography revealed an aneurysm with a diameter of approximately 0.4 mm, located in the clinoid segment of the left internal carotid artery and dilation and dissection were detected at the distal cervical segment of the bilateral internal carotid arteries. Additionally, magnetic resonance angiography showed the same results, while other cerebral vasculatures appeared to be normal. Electroencephalography revealed diffused severe encephalopathy.
Genetic examination was performed and an m.3243A > G point mutation was identified. The diagnosis of MELAS was confirmed according to the Japanese diagnostic criteria (Table 1) developed by Yatsuga et al. (Yatsuga et al. 2012). On day 12, the symptoms reached a peak and gradually improved following administration of oral coenzyme Q10 and levetiracetam therapy. Additionally, diabetes mellitus was diagnosed. For this patient, several risk factors related to aneurysm formation, including sex (females are more susceptible), smoking, alcoholism, and hypertension, were not present. Considering the associations described above, the case in this report indicates that aneurysm formation should be added to the list of vascular features in patients with MELAS, even in those with mitochondrial disorders.
According to scales used to describe the progression and prognosis of mitochondrial diseases, such as the Newcastle mitochondrial diseases adult scale (Schaefer et al. 2006) and Japanese mitochondrial disease rating scale (Yatsuga et al. 2012), the patient was considered a severe MELAS case. We did not treat the aneurysm because it was a mini unruptured intracranial aneurysm (mUIA) and because of the severity of the disease. Researches (Wiebers et al. 2003) from the International Study of Unruptured Intracranial Aneurysms demonstrated that the 5-year rupture rate of UIAs less than 7 mm is approximately 0–0.7%, and this value may be nearly zero for UIAs in anterior circulation. Factors increasing the risk of rupture, such as a history of hypertension, smoking, multifocal aneurysm, posterior circulation, and history of ruptured aneurysms, were all negative for the patient. Thus, surgical interventions and digital subtraction angiography (DSA) examinations were not conducted for this patient.
Discussion
Mitochondrial disease with aneurysm formation has recently been examined in several studies. The pathogenesis of vascular involvement in MELAS remains unclear. However, several hypotheses have been proposed; the most commonly accepted is an aberrant vascular tone caused by abnormalities in the vascular smooth muscle and endothelium. A study by Vattemi et al. (Vattemi et al. 2011) suggested that in MELAS patients, the vessel wall is a target of oxidative stress and that the degree of endothelial dysfunction is related to age and oxidative stress. First, regarding endothelial dysfunction, nitric oxide (NO) synthesis by endothelial nitric oxide synthase (eNOS) was decreased. Second, asymmetric dimethylarginine, an NOS inhibitor, was found to be increased in MELAS patients (El-Hattab et al. 2012b). Third, availability of the NO precursors arginine and citrulline availability was low in MELAS patients (El-Hattab et al. 2016), and thus a lower level of NO was observed in MELAS. Previous studies demonstrated that deficiencies in NO can result in impaired microvasculature, myopathy, lactic acidosis, diabetes, and, in turn stroke-like episodes (El-Hattab et al. 2012a). Interestingly, a recent study (Yang et al. 2015) revealed an association between eNOS and intracranial aneurysm (IA) risk. The study suggested that T786C polymorphisms are associated with the susceptibility to IA in the Asian population; however, G894T and 27-bp-VNTR likely have no influence on this condition. Similarly, downregulated NO may trigger cerebral aneurysm (Tamura et al. 2009). Therefore, in patients with MELAS, eNOS may be important in the formation of aneurysm.
Another mechanism explaining the association between MELAS and aneurysm formation involves interleukin (IL). During the early immunoinflammatory process of injury, cell signalling and the release of cytokines and growth factors, such as partially damage associated molecular patterns (DAMPs), are involved, with γδ T cells playing a key role. In mitochondrial DAMPs, pro-inflammatory cytokine production was induced by γδ T cells, while anti-inflammatory cytokines such as IL-10 were not affected or decreased to some extent (Raju et al. 2016). A study by Jan suggested that a general inflammatory process and its association with hyperexpressed IL-6 involve B plasma cell infiltration (plasmacytosis) (Lindeman et al. 2008). Moreover, IL-10 gene containing 1082G/A polymorphisms are significantly associated with aneurysm in the Chinese population (Wang et al. 2015). Compared to the IL-10 gene 1082G, the A allele was generally associated with a lower level of IL-10 (Bown et al. 2007), which may contribute to the formation of IA. In the microenvironment of mitochondrial dysfunction in MELAS, hyperexpressed IL-6 and stochastic regulation of IL-10 are vital for the formation of IA. Additionally, other cytokines in inflammatory processes, such as IL-1β and IL-8, which are positively regulated by the inflammatory factor NF-κB, are essential in aneurysm formation in MELAS, but the mechanisms require further investigation.
In patients with MELAS, because of the oxidative stress caused by mitochondrial dysfunction, deficiency in a generalized cytochrome c oxidase (COX), the main component of the respiratory chain in mitochondria, via mitochondrial protein kinase A was discovered (Srinivasan and Avadhani 2012; Srinivasan et al. 2013). When COX is deficient, electron transport is impaired, reducing ATP synthesis and increasing reactive oxidative species, leading to apoptosis. Moreover, COX deficiency may activate and promote ceramide synthase 6, resulting in the accumulation of ceramide and cytochrome c release via mitochondrial outer membrane permeabilization caused by Bcl-2 proteins, lipid composition, and accumulated ceramide (Schüll et al. 2015). Thus, the intrinsic apoptotic pathway is initiated by activation of caspase-9. In fact, COX deficiency is not restricted to ischemic regions and cerebral blood vessels, and the aorta may also be affected by COX deficiency. Furthermore, a study by Sinha et al. revealed that intrinsic mitochondrial-dependent apoptosis of cells in the aortic wall was associated with aneurysm formation (Sinha et al. 2005).
The heteroplasmy rate is approximately 10%. Furthermore, we carried out a family study, but no relatives were found to be similar to the proband. Several studies in other countries suggested maternal inheritance in a considerable number of patients with m.3243A > G mutation, while a Chinese study reported the opposite. Among patients with the m.3243A > G mutation in China, only 18.7% showed a maternal inheritance pattern. A lack of family history may be because: (1) mitochondrial DNA mutations often exhibit a threshold pattern, and the relatives we investigated may have low mutation rates that were lower than the threshold and (2) the patient may be sporadic, referring to a spontaneous mutation in the oocyte or fertilized egg.
Large vessel angiopathy in patients with MELAS may not be an occasional issue. The mechanisms involved in this process may include eNOS deficiency, cytokine disorders, and mitochondrial-dependent apoptosis. MELAS is a mitochondrial disorder with various biomolecular changes, and the mechanisms discussed above may be essential; however, other factors, such as angiotensin-converting enzyme, endoglin, apolioprotein E, and the regional matrix metalloproteinase family balance, may also be significant. The mechanisms causing aneurysm require further analysis.
Conclusion
We describe an association between mitochondrial disorders, particularly MELAS and aneurysm formation. In clinical assessments of a patient diagnosed with or suspected as having MELAS, aneurysmal formation should be considered and angiographic examinations such as computed tomography angiography, magnetic resonance angiography, or digital subtraction angiography should be conducted for patients at high risk.
Abbreviations
- MELAS:
-
mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke like episodes
- NAA:
-
N-acetyl aspartate
- NO:
-
nitric oxide
- eNOS:
-
endothelial nitric oxide synthase
- UIA:
-
unruptured intracranial aneurysm
- DSA:
-
digital subtraction angiography
- IA:
-
intracranial aneurysm
- IL:
-
interleukin
- DAMPs:
-
damage associated molecular patterns
- COX:
-
cytochrome c oxidase
References
Bown MJ, Lloyd GM, Sandford RM, Thompson JR, London NJ, Samani NJ, Sayers RD (2007) The interleukin-10-1082 'A' allele and abdominal aortic aneurysms. J Vasc Surg 46:687–693. doi:10.1016/j.jvs.2007.06.025
Brunetti-Pierri N et al (2011) Dilation of the aortic root in mitochondrial disease patients. Mol Genet Metab 103:167–170. doi:10.1016/j.ymgme.2011.02.007
El-Hattab AW, Emrick LT, Craigen WJ, Scaglia F (2012a) Citrulline and arginine utility in treating nitric oxide deficiency in mitochondrial disorders. Mol Genet Metab 107:247–252. doi:10.1016/j.ymgme.2012.06.018
El-Hattab AW, Hsu JW, Emrick LT, Wong LJ, Craigen WJ, Jahoor F, Scaglia F (2012b) Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 105:607–614. doi:10.1016/j.ymgme.2012.01.016
El-Hattab AW, Adesina AM, Jones J, Scaglia F (2015) MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab 116:4–12. doi:10.1016/j.ymgme.2015.06.004
El-Hattab AW et al (2016) Impaired nitric oxide production in children with MELAS syndrome and the effect of arginine and citrulline supplementation. Mol Genet Metab 117:407–412. doi:10.1016/j.ymgme.2016.01.010
Finsterer J, Zarrouk-Mahjoub S (2016) Mitochondrial vasculopathy. World J Cardiol 8:333–339. doi:10.4330/wjc.v8.i5.333
Fukuyama K, Ishikawa Y, Ogino T, Inoue H, Yamaoka R, Hirose T, Nishihira T (2012) Mucosal necrosis of the small intestine in myopathy, encephalopathy, lactic acidosis, and stroke-like episodes syndrome. World J Gastroenterol 18:5986–5989. doi:10.3748/wjg.v18.i41.5986
Gabrielson M et al (2016) Altered PPARgamma Coactivator-1 alpha expression in abdominal aortic aneurysm: possible effects on mitochondrial biogenesis. J Vasc Res 53:17–26. doi:10.1159/000446653
Lindeman JH, Abdul-Hussien H, Schaapherder AF, Van Bockel JH, Von der Thusen JH, Roelen DL, Kleemann R (2008) Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond) 114:687–697. doi:10.1042/CS20070352
Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP (1984) Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 16:481–488. doi:10.1002/ana.410160409
Ping P et al (2015) Harnessing the power of integrated mitochondrial biology and physiology: a special report on the NHLBI mitochondria in heart diseases initiative. Circ Res 117:234–238. doi:10.1161/CIRCRESAHA.117.306693
Raju R et al (2016) Dermal γδ T-cells can Be activated by mitochondrial damage-associated molecular patterns. PLoS One 11:e0158993. doi:10.1371/journal.pone.0158993
Schaefer AM, Phoenix C, Elson JL, McFarland R, Chinnery PF, Turnbull DM (2006) Mitochondrial disease in adults: a scale to monitor progression and treatment. Neurology 66:1932–1934. doi:10.1212/01.wnl.0000219759.72195.41
Schüll S et al (2015) Cytochrome c oxidase deficiency accelerates mitochondrial apoptosis by activating ceramide synthase 6. Cell Death and Disease 6:e1691. doi:10.1038/cddis.2015.62
Sinha I, Sinha-Hikim AP, Hannawa KK, Henke PK, Eagleton MJ, Stanley JC, Upchurch GR Jr (2005) Mitochondrial-dependent apoptosis in experimental rodent abdominal aortic aneurysms. Surgery 138:806–811. doi:10.1016/j.surg.2005.07.011
Srinivasan S, Avadhani NG (2012) Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med 53:1252–1263. doi:10.1016/j.freeradbiomed.2012.07.021
Srinivasan S, Spear J, Chandran K, Joseph J, Kalyanaraman B, Avadhani NG (2013) Oxidative stress induced mitochondrial protein kinase a mediates cytochrome c oxidase dysfunction. PLoS One 8:e77129. doi:10.1371/journal.pone.0077129
Takahashi N et al (2005) Vascular involvement in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. The American journal of the medical sciences 329:265–266
Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M, Nagahiro S (2009) Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens 27:1284–1292. doi:10.1097/HJH.0b013e328329d1a7
Tay SH, Nordli DR Jr, Bonilla E, Null E, Monaco S, Hirano M, DiMauro S (2006) Aortic rupture in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes. Arch Neurol 63:281–283. doi:10.1001/archneur.63.2.281
Vattemi G et al (2011) Increased protein nitration in mitochondrial diseases: evidence for vessel wall involvement. Molecular & cellular proteomics : MCP 10:M110.002964. doi:10.1074/mcp.M110.002964
Wang S, Wu S, Zheng T, Yang Z, Ma X, Jia W, Xiang K (2013) Mitochondrial DNA mutations in diabetes mellitus patients in Chinese Han population. Gene 531:472–475. doi:10.1016/j.gene.2013.09.019
Wang F, Quan QQ, Zhang CL, Li YB, Jiang TB (2015) Association between polymorphisms in the interleukin-10 gene and risk of abdominal aortic aneurysm. Genet Mol Res 14:17599–17604. doi:10.4238/2015.December.21.32
Wiebers DO et al. (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet (London, England) 362:103–110
Yang C, Qi ZY, Shao C, Xing WK, Wang Z (2015) Association between three eNOS polymorphisms and intracranial aneurysms risk: a meta-analysis. Medicine (Baltimore) 94:e452. doi:10.1097/MD.0000000000000452
Yatsuga S et al (2012) MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta 1820:619–624. doi:10.1016/j.bbagen.2011.03.015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhu, K., Li, S., Chen, H. et al. Late onset MELAS with m.3243A > G mutation and its association with aneurysm formation. Metab Brain Dis 32, 1069–1072 (2017). https://doi.org/10.1007/s11011-017-9989-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-9989-0