Abstract
Although a perturbed cerebral blood flow (CBF) has been reported in patients with Wernicke’s encephalopathy (WE), its clinical meaning is still elusive. A retrospective analysis of 10 patients (male, 6; mean age, 57.7 years) with WE between October 2012 and May 2018 was performed. Brain imaging was performed using fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), arterial spin labeling (ASL) perfusion-weighted imaging (PWI), and contrasted enhanced T1-weighted imaging. All patients had symmetric high signal intensity lesions in the vulnerable areas on FLAIR or DWI with focal hyperintensity on ASL-PWI (100% sensitivity). CBFlesion was variable (from 70 mL/100 g/min to 190.0 mL/100 g/min). CBFlesion/CBFwhite matter was elevated, ranging from 2.5 to 5.5. Focal hyperintensity on ASL in the vulnerable areas can be a diagnostic clue for WE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wernicke’s encephalopathy (WE) is a neurological syndrome caused by thiamine (vitamin B1) deficiency [1]. WE may present with a classical symptom triad including ophthalmoplegia, altered mental status, and ataxia. However, when symptoms are atypical, brain imaging may be helpful in the diagnosis [2]. Pathognomonic radiological findings are symmetric high signal intensity lesions in the medial thalami, the periventricular region of the third ventricle, periaqueductal gray matter, mammillary body (MB), or tectal plate [1]. In rare cases, gadolinium enhancement due to the blood-brain barrier (BBB) breakdown may be the only clue [3]. Given the BBB breakdown is closely linked to hyperperfusion, it is highly likely that patients with WE have an increased cerebral blood flow (CBF) in the vulnerable brain areas. By contrast, previous studies suggested that CBF and cerebral glucose metabolism were globally reduced in patients with WE [4]. However, those studies were performed in the subacute phase, and the exact information on focal CBF in the vulnerable structures, such as MB, does not exist. Whereas, experimental studies investigating thiamine-deficient animals showed a transient elevation in CBF, which returned to baseline after thiamine replacement [5].
Arterial spin labeling (ASL) is a method for measuring perfusion using a freely diffusible endogenous tracer [6]. It is non-invasive and does not require gadolinium-based tracers. Moreover, it is easily repeatable with high reproducibility. Therefore, we hypothesized that focal hyperintensity on ASL may have diagnostic yields in patients with the acute phase of WE.
Materials and methods
Study population
A retrospective review of medical records was performed in 63 patients with a diagnosis code of WE (International Classification of Disease, 10th Revision, code of E512) between October 2012 and May 2018. After reviewing electronic medical records, a total of 37 patients were excluded because the final diagnoses were different, such as sepsis encephalopathy or metabolic encephalopathy. Among the remaining patients, 16 were excluded either due to not undergoing ASL perfusion-weighted imaging (PWI) (n = 15) or poor quality of ASL-PWI for analysis (n = 1). A total of 10 patients were included in the final analysis.
Image protocols
All patients underwent MR imaging with a 1.5-T (Signa HDxt; GE Healthcare, Milwaukee, Wisconsin) or 3-T (Discovery 750w; GE Healthcare, Milwaukee, Wisconsin) MR imaging scanner with an 8- or 32-channel head coil. Imaging sequences included FSE T2WI, contrast-enhanced spin-echo T1WI, and ASL images. ASL images were acquired for 1.5 s of labeling followed by 1.525 s of labeling delay. Saturation pulses were used to suppress the background. The image acquisition consisted of a stack of interleaved 3D fast spin-echo spiral readouts, each of which lasted 4 ms. Each spiral arm included 512 sampling points in k-space, and a total of 8 interleaves (arms) were acquired separately. The ASL parameters were as follows: TR = 4446–4564 ms, TE = 9.4–9.9 ms, echo-train length = 1, FOV = 240 × 240 mm, matrix = 128 × 128, number of excitations = 3, number of interleaved sections = 32, section thickness = 5–6 mm, intersection gap = 0 mm, and number of sections = 32–44. The signal intensity change between the labeled image and the control image was fitted to a model from which a quantitative perfusion map of CBF was obtained.
Image analysis
FLAIR, DWI, or T1 contrast imaging (if applicable) were analyzed for the presence of abnormalities in vulnerable areas such as the medial thalamus, periventricular area, periaqueductal gray matter, MB, tectal plate, or medial vestibular nucleus. ASL-PWI data were reviewed to correlate abnormalities in the corresponding areas. For quantitative analysis of CBF (CBFlesion), a region of interest (ROI) was manually placed at the hottest appearing part in the areas of abnormal ASL-PWI. A CBF within the region was recorded for the analysis. For normalized CBF, a reference ROI (15 mm × 15 mm × 5 mm) was placed in the frontal subcortical white matter and calculated as CBFnormalized = CBFlesion/CBFwhite matter [7].
Results
Among the 10 included patients (male, 6; mean age, 57.7 years), the majority (n = 7) had poor oral intake due to systemic conditions such as advanced cancers or gastrointestinal problems, and the remaining three were chronic alcoholics (Table 1).
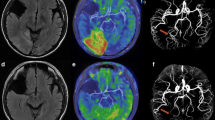
Brain imaging was performed in the acute phase (median 0 days after symptom onset, interquartile range 0–1) and exhibited symmetric high signal intensity lesions in the medial thalamus, MB, hypothalamus, fornix, tectum, inferior colliculus, or medial vestibular nuclei on FLAIR or DWI (Table 1). Five patients had abnormalities in the hypothalamus and tectum, and four patients exhibited an abnormality in the medial thalamus. Five patients underwent contrast-enhanced T1WI and showed abnormality in the area of on FLAIR or DWI. Interestingly, all lesions on FLAIR, DWI, or T1-contrast imaging were identified as hyperintensity on ASL-PWI (sensitivity 100%) (Fig. 1, Supplemental Table 1). The average measured CBF using ASL-PWI was different among the affected anatomical structures (from 70.3 mL/100 g/min in the medial thalamus to 190.0 mL/100 g/min in the hypothalamus). Indeed, CBFnormalized ranged from 2.5 (fornix) to 5.5 (tectum and medial vestibular nuclei) compared with frontal subcortical white matter (Supplemental Table 1).
Representative figure of the patients. Pathognomonic hyperintensity lesions were observed in the mammillary body and red nucleus (a and b, arrows, case 1); medial thalamus and fornix (c and d, arrows, case 2); tectal plate (e and f, arrows, case 7) and mammillary body (g and h, arrows, case 5). a, c, e, and f were fluid-attenuated inversion recovery imaging; b, d, f, and g were arterial spin labeling imaging
Discussion
This study demonstrated that focal hyperintensity on ASL-PWI is obvious in the lesions on FLAIR, DWI, or contrast-enhanced T1WI in the acute symptomatic phase of WE. Moreover, CBF in the lesions was elevated by a factor of 2.5 to 5.5 compared with that of frontal subcortical white matter.
Previous studies have reported that cortical CBF was globally decreased, accompanied by a reduction in glucose and oxygen metabolism in patients with WE [4]. However, in those studies, CBF imaging was performed using positron emission tomography, xenon computed tomography (CT), or single-photon emission tomography, which has limitations in spatial resolution. Therefore, it is not clear whether a similar CBF change is expected in the small deep structures such as the MB or medial thalamus, which are known for pathognomonic lesions in WE. Moreover, the majority of subjects in those studies were chronic alcoholics, who may have decreased metabolism and hypoperfusion in the cerebral cortex. Therefore, it remains elusive whether CBF reduction is mechanistically related to WE, or simply a reflection of low metabolism in chronic alcoholism.
With recent advances in non-invasive imaging, ASL-PWI enables the identification of perturbed CBF in various clinical situations. In our study, all patients exhibited dramatic hyperintensity on ASL-PWI in vulnerable areas related to WE, including the medial thalamus, hypothalamus, tectum, or MB with 100% sensitivity. There are a few hypotheses on the mechanism of focal hyperemia in WE. In the acute phase of WE, an increase in lactate production by neurons and astrocytes was identified, which leads to endothelial dysfunction and increased production of nitric oxide [8], and may result in increased vascular permeability, vasodilation, and hyperemia [9]. Therefore, hyperperfusion or hyperemia may exist in the acute phase of WE. Consistent with this, one study reported the focal hyperemia using CT perfusion in a patient with WE who presented with acute onset confusion, dysarthria, and facial palsy [10]. In addition, an animal study using thiamine deficiency reported that focal hyperemia was evident and slowly improved over several weeks after symptom development [5]. Taken together, increased CBF is mechanistically relevant in the pathogenesis of WE, especially in the acute phase. When necrosis occurs in the chronic phase, focal hyperemia may disappear. This may partly explain the previous results of decreased CBF in WE because CBF was measured in the subacute phase.
There are several limitations in this study. First, ASL-PWI scans were not performed on the same MR scanner, which could have led to high individual variations of CBF. However, we tried to normalize CBF using reference CBF from the frontal white matter. Second, the lesions on ASL-PWI were usually small as shown in the figure, and small ROIs usually have poor signal to nose ratio. However, we manually drew ROIs on the hottest area on ASL imaging, and the hyperintensity on ASL imaging suggests that those values were true rather than artifact originated from small ROI. Third, we set post labeling delay (PLD) at 1.525 s regardless of age and sex, which is relatively short for elderly patients [6]. However, relatively short PLD is associated with underestimation of CBF. Therefore, we do not think that PLD value was the main factor for elevated CBF in WE.
Conclusion
In conclusion, focal hyperintensity in the vulnerable area on ASL imaging is a mechanistically relevant finding in WE, especially in acute symptomatic phase. Identifying hyperintensity using ASL-PWI may be used as a sensitive diagnostic protocol in WE. Large-scale prospective studies are needed for validation.
References
Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A (2014) MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. BioMed Res Int. 2014:503596
Jung YC, Chanraud S, Sullivan EV (2012) Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychol Rev. 22:170–180
Shogry ME, Curnes JT (1994) Mamillary body enhancement on MR as the only sign of acute Wernicke encephalopathy. AJNR Am J Neuroradiol. 15:172–174
Matsuda K, Yamaji S, Ishii K, Sasaki M, Sakamoto S, Kitagaki H et al (1997) Regional cerebral blood flow and oxygen metabolism in a patient with Korsakoff syndrome. Ann Nucl Med. 11:33–35
Hakim AM (1986) Effect of thiamine deficiency and its reversal on cerebral blood flow in the rat. Observations on the phenomena of hyperperfusion, “no reflow,” and delayed hypoperfusion. J Cereb Blood Flow Metab. 6:79–85
Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M (2015) A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 57:1181–1202
Yoo RE, Yun TJ, Yoon BW, Lee SK, Lee ST, Kang KM et al (2017) Identification of cerebral perfusion using arterial spin labeling in patients with seizures in acute settings. PloS one. 12:e0173538
Navarro D, Zwingmann C, Hazell AS, Butterworth RF (2005) Brain lactate synthesis in thiamine deficiency: a re-evaluation using 1h-13c nuclear magnetic resonance spectroscopy. J Neurosci Res. 79:33–41
Desjardins P, Butterworth RF (2005) Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol. 31:17–25
Bhan A, Advani R, Kurz KD, Farbu E, Kurz MW (2014) Wernicke’s encephalopathy mimicking acute onset stroke diagnosed by CT perfusion. Case Rep Neurol Med. 2014:673230
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ko, SB., Kim, T.J. & Sohn, CH. Focal hyperemia in Wernicke’s encephalopathy: a preliminary arterial spin labeling MRI study. Neuroradiology 62, 105–108 (2020). https://doi.org/10.1007/s00234-019-02298-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02298-7