Abstract
Females of two click beetle species, Cardiophorus tenebrosus and C. edwardsi (Coleoptera: Elateridae), produce methyl (3R,6E)-2,3-dihydrofarnesoate as their sex pheromone. We had serendipitously discovered that males of both species were also strongly attracted to (R)-fuscumol acetate ((E)-6,10-dimethylundeca-5,9-dien-2-yl acetate), a known longhorned beetle (Coleoptera: Cerambycidae) pheromone, due to its structural similarities to the click beetle pheromone. To further investigate the specificity of the responses of Cardiophorus males, additional analogs with different chain lengths and structural relationships compared to the natural pheromone were synthesized and tested. In field and electroantennogram bioassays, only fuscumol propionate ((E)-6,10-dimethylundeca-5,9-dien-2-yl propionate) elicited strong responses from Cardiophorus males, indicating that they were able to distinguish chain length and spatial relationships between the structural elements. In field trials, C. tenebrosus males were attracted equally to the analog and their natural pheromone, but the pheromone elicited stronger antennal responses from males. In contrast, traps baited with fuscumol propionate caught approximately 26 times as many C. edwardsi males compared to traps baited with the natural pheromone, although the analog elicited significantly smaller antennal responses from C. edwardsi males. Thus, in terms of behavioral responses, fuscumol propionate appears to be acting as a hyperactive pheromone mimic, a phenomenon which has rarely been observed in insect semiochemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Communication by means of volatile pheromones is probably the most widespread form of intraspecific communication in the Insecta, and thousands of insect pheromones have now been identified from across the insect taxa (Symonds and Elgar 2008; El-Sayed 2019). The use of pheromones as a means of communication is highly efficient, with behavioral responses and/or physiological changes often being elicited by only a few molecules of pheromone. Generally speaking, pheromone blends are also species specific, with both the efficiency and the selectivity having been tuned by natural selection over millennia (Symonds and Elgar 2008).
During the final decades of the 20th century, a substantial amount of research was conducted on analogs of the attractant pheromones used by insect species, with a focus on the sex pheromones used in attracting mates (reviewed in Renou and Guerrero 2000). These investigations had several different objectives. From a basic science viewpoint, structural modifications such as changes in the alkyl chain (elongation, shortening, saturation, and addition of alkyl branches), changes in the spatial and electronic structure by modification or replacement of polar groups (alcohols, esters, ketones, aldehydes), or isosteric replacements of hydrogen with fluorine (Pesenti and Viani 2004) were probed to see how they affected the perception of and subsequent behavioral responses to the analogs (Renou and Guerrero 2000). Similar modifications were used to test whether the clearance of pheromones from receptors by enzymatic degradation could be disrupted. From a practical viewpoint, other types of pheromone analogs were developed as a possible means of replacing inherently unstable functional groups like aldehydes by more stable formate esters or methylene groups (Renou and Guerrero 2000). Almost without exception, it was found that at best, pheromone mimics were approximately equal in activity to the natural pheromones (Briggs et al. 1986; Dawson et al. 1990; Lucas et al. 1994), or sometimes acted as synergists to the natural pheromones (Roelofs and Comeau 1971; Camps et al. 1988; Bengtsson et al. 1990; Riba et al. 1994; Grant et al. 1996). However, in most cases, the pheromone analogs varied from being much less attractive to not attractive at all compared to the natural pheromones, or they were actively inhibitory, disrupting responses to the natural pheromone when released in blends. Overall, this is perhaps not unexpected, given that pheromone receptors have evolved to be highly selective to recognize their particular ligands, which are present in minute quantities in the sea of background odors present in natural environments. To our knowledge, there are only two reported instances in which any pheromone analog has been shown to exhibit activity greater than that of the natural pheromone. In the first, Lucas et al. (1994) found that one fluorinated and one chlorinated analog of the codling moth (Cydia pomonella) pheromone component (E8,E10)-8,10-dodecadienol, among a number of halogenated analogs tested, were, respectively, 44% and 75% more attractive to codling moth males than (E8,E10)-8,10-dodecadienol itself. In the second example, with the German cockroach Blattella germanica, the three stereoisomers of (3S,11S)-3,11-dimethylnonacosan-2-one, a female-produced contact pheromone, elicited stronger behavioral responses from males at biologically relevant doses than the natural pheromone (Eliyahu et al. 2004).
While trying to identify the first pheromones for North American click beetle species (Coleoptera: Elateridae), we serendipitously discovered that a cerambycid beetle pheromone, (2R,5E)-6,10-dimethylundeca-5,9-dien-2-yl acetate (commonly known as (R)-fuscumol acetate) was an excellent mimic of the female-produced sex pheromone, methyl (3R,6E)-2,3-dihydrofarnesoate (henceforth “MDF”; structure shown in Fig. 1) of two click beetle species, Cardiophorus tenebrosus LeConte and C. edwardsi Horn (Serrano et al. 2018). The antennal responses in electroantennogram assays and behavioral responses in field trials were so strong that we initially thought that fuscumol acetate was indeed the pheromone of these two species. Having finally identified the real pheromone from volatiles extracted from female beetles, and having compared the beetles’ responses to the two enantiomers of the mimic and the natural pheromone in both electroantennogram (EAG) and behavioral bioassays, we were struck by two points. First, in EAG bioassays, only the (R)-enantiomer of the pheromone or the mimic elicited any response at all from the antennae of male beetles, suggesting that the spatial relationship between the methyl group at the polar end of each molecule and the corresponding carbonyl group was crucial. Second, the all-or-nothing response elicited by that specific arrangement, despite the long hydrophobic chains of all four molecules being identical, suggested that the functionalized end of the molecule was most important, and that variation in the length of the hydrophobic end of the molecule might still result in active analogs, as long as the crucial methyl group to carbonyl motif was still intact. Here, we report the results of testing several pheromone analogs that were designed to probe these possibilities, and specifically, the identification of a hyperactive pheromone mimic which attracted approximately 26 times more C. edwardsi males than an equivalent dose of the natural pheromone in field bioassays.
Materials and methods
Test compounds
Four compounds were synthesized for testing (Fig. 1). These included the known pheromone (R)-MDF (Figure 1.4) as a positive control and baseline for comparison. In addition, three pheromone analogs were prepared, including the terpenoid esters:
-
1.
(E)-6,10-dimethylundeca-5,9-dien-2-yl propionate (henceforth fuscumol propionate; Figure 1.3) in which the chain length of fuscumol acetate had been extended by one carbon on the ester end of the chain. This compound most closely resembles the pheromone, MDF;
-
2.
methyl citronellate (Figure 1.1), a monoterpenoid analog of MDF that shares the same three-dimensional methyl-carbonyl motif as MDF and fuscumol propionate, but lacks the terminal isoprene unit;
-
3.
citronellyl propionate (Figure 1.2), a monoterpenoid analog of fuscumol propionate which lacks the terminal isoprene group, and in which the distance between the crucial methyl and carbonyl groups is increased by two carbons.
Racemic mixtures of each of the test compounds were used in field bioassays (described below). Although it was found that C. tenebrosus and C. edwardsi males only respond to (R)-enantiomers of fuscumol acetate and MDF, the presence of the (S)-enantiomer (in racemic mixtures) did not cause a significant decrease in attraction to the (R)-enantiomer in the field (Serrano et al. 2018).
Racemic MDF was available from previous work (Ho and Millar 2001). Racemic citronellyl propionate was synthesized as follows. Propionyl chloride (4.72 ml, 55 mmol) was added dropwise to an ice-bath cooled solution of citronellol (5.6 g, 50 mmol, Aldrich Chemical, Milwaukee, WI, USA), pyridine (4.4 ml, 55 mmol), and dimethylaminopyridine catalyst (100 mg) in 100 ml methylene chloride. After the addition was complete, the ice-bath was removed and the mixture was stirred 4 h at room temp. Residual propionyl chloride was then destroyed by the addition of ethanol and stirring overnight. The resulting mixture was extracted sequentially with water, 1 M HCl, saturated NaHCO3, and brine, then concentrated to an odorous oil, which was > 95% pure by GC, and was used without further purification. 1H and 13C NMR spectral data agreed with those previously reported (Richter et al. 2014). MS (EI, 70 eV; m/z, %): 138 (37), 123 (70), 109 (35), 95 (94), 81 (100), 69 (87), 57 (74), 41 (62).
Racemic fuscumol propionate was prepared in analogous fashion, substituting racemic fuscumol (Bedoukian Research, Danville CT, USA) for citronellol. The product was purified by Kugelrohr distillation (oven temp ~ 99 °C, 0.25 mm Hg), yielding a colorless oil which was > 95% pure by GC. 1H NMR (400 MHz, CDCl3) δ 5.18–5.03 (m, 2H), 4.89 (m, 1H), 2.30 (q, J = 7.6 Hz, 2H), 2.14–1.90 (m, 6H), 1.70–1.65 (m, 3H), 1.59 (d, m, 6H), 1.55–1.43 (m, 2H), 1.21 (d, J = 6.3 Hz, 3H), 1.13 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 174.28, 135.85, 131.52, 124.42, 123.54, 70.61, 39.83, 36.16, 28.11, 26.81, 25.82, 24.05, 20.15, 17.82, 16.05, 9.38. MS (EI, 70 eV; m/z, %): 209 (2), 178 (12), 163 (7), 135 (18), 123 (9), 109 (100), 93 (16), 81 (20), 69 (59), 57 (37), 41 (31).
Racemic methyl citronellate was prepared from the corresponding carboxylic acid by esterification with methyl iodide (Sun et al. 2016). Thus, methyl iodide (1.44 g, 10.1 mmol) and K2CO3 (1.06 g, 7.64 mmol) were added to a solution of citronellic acid (0.85 g, 5 mmol; Aldrich Chemical) in dry dimethylformamide. The reaction mixture was stirred at 22 °C for 30 min, then quenched with saturated aqueous NaHCO3 (30 ml). The resulting mixture was extracted with hexane (3 × 50 ml) and the combined organic phases were washed with brine, dried over anhydrous Na2SO4, and concentrated. The crude product was purified by vacuum flash chromatography on silica gel, eluting with 10% EtOAc in hexane to yield a colorless oil (0.78 g, 84%) which was > 95% pure by GC. 1H and 13C NMR spectral data agreed with those previously reported (Whittaker and Dong 2015). MS (EI, 70 eV; m/z, %): 184 (M + , 7), 152 (39), 141 (2), 137 (5), 129 (4), 123 (4), 119 (3), 110 (62), 101 (12), 95 (67), 87 (12), 82 (32), 74 (24), 69 (100), 59 (23), 55 (40), 41 (69).
Field bioassays
Field bioassays were carried out from 9 April to 30 June 2018 at the same two sites near Jenks Lake (34° 09′ 45.8″ N 116° 54′ 08.6″ W and 34° 09′ 48.1″ N 116° 54′ 13.0″ W) in the San Bernardino National Forest in San Bernardino Co., CA, USA as the original studies (Serrano et al. 2018). Modified flight intercept traps (Serrano et al. 2018) were placed 10–15 m apart, with treatments initially assigned randomly to traps within each five-trap replicate (4 treatments and a control). One replicate of treatments was tested at each field site. Traps were checked twice weekly and beetles were live trapped so that they could be brought back to the laboratory for sex determination and coupled gas chromatography-electroantennogram (GC-EAD) analyses. The lures were replaced weekly, at which time traps were rotated one position. Lures consisted of 5 × 7.5 cm (~ 0.05 mm wall thickness) low-density polyethylene zipper seal bags (Fisher Scientific, #01-816-1A), filled with 1 ml of a 20 mg/ml solution of the test compound in isopropanol. Treatments consisted of methyl citronellate, citronellyl propionate, fuscumol propionate, and MDF (each as racemic mixtures), with isopropanol as a solvent control.
Gas chromatography–electroantennogram detection
GC–EAD analyses were conducted as previously described (Serrano et al. 2018), with the exception that the GC was fitted with a DB-5 column (30 m × 0.25 mm ID × 0.25 μm film; J&W Scientific, Folsom CA, USA). Antennae from 4 C. tenebrosus males and 3 C. edwardsi males were used, with each antennal preparation being used for 1–3 analyses.
Quantification of release rates
The release rates of MDF and fuscumol propionate were measured by performing headspace collections from the polyethylene lures loaded with 20 mg each of the two test compounds in 1 ml isopropanol. Collections (N = 10) were conducted at 31 °C in a temperature-controlled room. Volatiles were collected from the lures using two-piece cylindrical glass chambers (28 cm × 8 cm ID) with O-ring-sealed center joints and Swagelok® unions (Solon OH, USA) on either end for making connections. The lures (as described above) were hung on wire stands centered within the chambers and oriented perpendicular to the inlet and outlet. Air was pulled through the chambers at ~ 1 L/min using house vacuum, with incoming air being filtered through activated charcoal (6–14 mesh; Fisher Scientific, Pittsburgh PA, USA). Volatiles were collected on 200 mg of thermally-desorbed activated charcoal (GAC-2050 ground down to ~ 100 mesh; Charcoal House, Crawford NE, USA) held between glass wool plugs in a short piece of glass tubing (0.9 cm ID). Collections were conducted for 4 h, beginning on the day the lure was made and then sampling again on days 3, 5, and 7. After collections were completed, trapped volatiles were eluted from the charcoal with 2 ml of dichloromethane containing dodecyl acetate (0.5 mg/ml) as in internal standard.
To quantify the amount of MDF and fuscumol propionate being released from the polyethylene lures, authentic standards of racemic MDF and racemic fuscumol propionate were diluted in hexane in a range spanning 4 different concentrations (1 mg/ml, 0.2 mg/ml, 0.1 mg/ml, and 0.02 mg/ml). The dilutions were then mixed 1:1 with a solution of dodecyl acetate (1 mg/ml in hexane) as an internal standard and analyzed to prepare a calibration curve. Solutions were analyzed with an Agilent 78020A GC coupled to an Agilent 5977E MSD fitted with a DB-5MS column. The oven temperature was programmed from 50 °C for 1 min, then increased at 10 °C/min to 280 °C, with an injector temperature of 280 °C. Injections (1 μl) were made in splitless mode and the amounts of recovered pheromone were determined from the calibration curve.
Statistical analyses
All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc. 2019). Replicates for field bioassays of both Cardiophorus species were based on both spatial and temporal replicates, with temporal replicates being the total number of times the traps were checked. The mean number of male beetles captured per treatment replicate was compared among treatments by logistic regression (PROC GLIMMIX) while specifying a negative binomial distribution. When the overall ANOVA indicated significant differences among treatments, differences among means were compared using simulation-based test for multiple comparisons (ADJUST = SIMULATE option of the LSMEANS statement). The average release rates of racemic MDF and fuscumol propionate were determined for each day of the analysis and data were analyzed by repeated measures using the GLIMMIX procedure with compound, day, and the compound by day interaction as fixed effects, and replicate nested within a day was included as a random effect. Differences among means were compared using the procedure described above. The antennal responses of males of both Cardiophorus species to MDF and fuscumol propionate were analyzed separately for each species using a generalized linear model (PROC GLIMMIX) by comparing mean area counts of the response curves with the individual as a random effect, specifying a normal distribution.
Results
Field bioassays
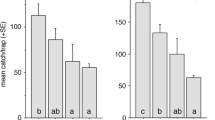
In field bioassays of the pheromone analogs with C. tenebrosus, a total of 408 males were caught between 12 April to 29 May 2018 in 26 treatment replicates. There was a significant difference among treatment means (F4,125 = 32.08; P < 0.0001), but the MDF and fuscumol propionate treatments were the only treatments that were significantly attractive compared to controls (Table 1) (P < 0.0001). The mean numbers of beetles caught in traps baited with fuscumol propionate were not statistically different than MDF (P > 0.05). For C. edwardsi, a total of 941 males were caught from 18 May to 26 June 2018 in 24 treatment replicates. Traps that contained the control and methyl citronellate treatments did not capture any male beetles and were eliminated from the statistical analysis. There was a significant difference among the mean number of beetles captured in the MDF, fuscumol proprionate, and citronellyl propionate treatments (F2,69 = 42.26; P < 0.0001). In contrast to C. tenebrosus, traps baited with fuscumol propionate attracted ~ 26 times as many C. edwardsi males as traps baited with MDF, and both MDF and fuscumol propionate were more attractive than citronellyl propionate (Table 1) (P < 0.005 and P < 0.0001, respectively).
GC–EAD analyses
Coupled GC–EAD analyses showed that only MDF and fuscumol propionate elicited detectable responses from the antennae of male C. tenebrosus and C. edwardsi (Fig. 2). For males of both species, the antennal responses to MDF were significantly larger than the responses to fuscumol propionate (F1,15 = 5.89; P < 0.05 for C. tenebrosus; F1,12 = 15.64; P < 0.005 for C. edwardsi).
Representative coupled gas chromatography-electroantennogram detection (GC-EAD) analyses of racemic methyl citronellate (MC), citronellyl propionate (CP), fuscumol propionate (FP), and MDF using an antenna from a male Cardiophorus beetle. Top trace shows the GC chromatogram and the bottom, inverted trace shows the responses from antennae of Cardiophorus males. a GC-EAD of C. tenebrosus; b GC-EAD of C. edwardsi
Release rates
The mean amounts of racemic MDF and fuscumol propionate released by the polyethylene bags in a 4 h period were not significantly different within each of the four days that were sampled (Fig. 3; P > 0.05). However, there was a day effect (F3,36 = 3.08; P < 0.05) and the highest release rates for both MDF and fuscumol propionate were on day 3.
Discussion
Our initial study in which we identified MDF as the female-produced sex attractant pheromone of the two study species suggested that several features of the MDF structure might be crucial for activity, specifically the methyl group on the chiral carbon, the carbonyl, the spatial relationship between these two functionalities, and the terpenoid hydrocarbon chain (Serrano et al. 2018). The cerambycid beetle pheromone, fuscumol acetate, shared these structural properties with MDF, which provided a plausible explanation for the strong attraction of male Cardiophorus beetles to fuscumol acetate. The study described here further probed the roles of those structural features. Of the three pheromone analogs tested, fuscumol propionate was the most bioactive in both GC-EAD analyses and field trials, and structurally most similar to MDF, with the only difference between the two structures being the transposition of the oxygen and the methylene group flanking the carbonyl. These data, together with the good biological activity of fuscumol acetate, reinforced the importance of the structural similarities at the ester end of the structures.
However, the additional importance of the structural details at the other end of the chain was dramatically illustrated by the fact that methyl citronellate, which has exactly the same structure as MDF at the ester end of the molecule but is missing the terminal isoprene group, was completely inactive in both electrophysiological and field bioassays, for both species. In particular, the lack of EAD activity was unexpected because in our previous study, the (R)-enantiomers of fuscumol acetate and MDF elicited strong EAD responses, whereas the (S)-enantiomers, with identical structures except for the configuration of the methyl group closest to the carbonyl, elicited no discernable responses from antennae. This had suggested that the isoprenoid end of the structure was less important for structural recognition by the receptors (or possibly by pheromone binding proteins that transport pheromone molecules to receptors), but this is clearly not the case: both the isoprenoid and the ester portions of the pheromone structure are crucial for recognition, and hence any downstream behavioral response. This was further reinforced by the complete lack of activity of the third analog, citronellyl propionate, which both lacked the terminal isoprene unit, and in which the carbonyl to methyl group distance was altered. When comparing the electrophysiological results between C. tenebrosus and C. edwardsi males to fuscumol propionate and MDF, for both species, fuscumol propionate elicited significantly smaller antennal responses than MDF. However, the behavioral responses between the two species differed considerably, with fuscumol propionate attracting similar numbers of C. tenebrosus as the natural pheromone MDF, whereas fuscumol propionate attracted much higher numbers of C. edwardsi males than MDF. In fact, the relatively low numbers of C. edwardsi males caught in traps baited with MDF, in comparison to previous studies in which MDF was shown to be a good attractant for this species (Serrano et al. 2018) suggest that fuscumol propionate may have been outcompeting MDF, and pulling beetles away from the MDF-baited traps. Thus, for this species, the significantly smaller EAG responses to fuscumol propionate than to MDF were not congruent with the markedly stronger attraction to fuscumol propionate in field bioassays. In addition, this greatly enhanced level of attraction could not be due to differing release rates of the two test compounds because their measured release rates were similar (Fig. 3).
To our knowledge, this level of agonistic “hyperactivity” of an analog of an insect’s sex attractant pheromone is unprecedented. Among the many studies of pheromone analogs, if analogs or mimics have had appreciable activity at all, their activity typically has been less than or approximately equal in activity compared to the natural pheromone (reviewed in Renou and Guerrero 2000). A few examples of instances in which pheromone analogs were approximately as attractive as the natural pheromone have been observed in studies with lepidopteran species such as the red-banded leaf roller, Argyrotaenia velutinana (Walker) (Cardé and Roelofs 1977), the soybean pod borer, Leguminivora glycinivorella (Matsumura) (Hu et al. 2012), and the lichen moth, Miltochrosta calamina Butler (Muraki et al. 2014).
However, to our knowledge, there have been only two reports of volatile pheromone analogs being significantly more active than natural pheromones. In the first example, two pheromone analogs, (8E,10E)-10,11-difluoro-8,10-dodecadienol and (8E,10E)-11-chloro-8,10-undecadienol, were found to elicit EAG responses from antennae of male codling moths that were similar in intensity to those elicited by their natural pheromone component (8E,10E)-8,10-dodecadienol (Lucas et al. 1994). In addition, the detection thresholds of all three compounds in EAG assays were similar (between 1 and 10 ng). However, in field bioassays, traps baited with (8E,10E)-10,11-difluoro-8,10-dodecadienol and (8E,10E)-11-chloro-8,10-undecadienol attracted 44% and 75% more male moths than traps baited with equivalent doses of (8E,10E)-8,10-dodecadienol, respectively. Differing volatilities were discounted as a possible explanation for these results, suggesting that the analogs were indeed more attractive than the natural pheromone (Lucas et al. 1994).
In another study conducted with the gypsy moth, Lymantria dispar, traps baited with an oxaspiropentane compound, 4-(1-oxaspiro[2.2]pent-2-yl)butan-1-ol, caught ~ 1.7 times as many moths as traps baited with the natural pheromone (+)-disparlure (Solari et al. 2007). However, the estimated boiling points of 4-(1-oxaspiro[2.2]pent-2-yl)butan-1-ol and (+)-disparlure differ by ~ 100 °C (244 vs. 341 °C, respectively, at atmospheric pressure; Advanced Chemistry Development Labs 2019), and consequently the vapor pressures of these two compounds differ by at least an order of magnitude [estimated with EPI Suite software; (US EPA 2019)]. Consequently, the release rate of the analog in the field tests would have been substantially higher than that of (+)-disparlure, confounding any estimates of the comparative attractiveness of the two compounds.
The ~ 26-fold higher trap catch of C. edwardsi in traps baited with fuscumol propionate as compared to MDF may be the result of one or a number of different factors because the units of measurement (trap catch) are the end result of a series of neurophysiological and behavioral steps. These in turn are mediated by both the chemical properties of the test compounds (e.g. volatility) and the biochemistry of the sequence of events from the capture of an odor molecule by the insect’s antenna through to the triggering of a behavioral output resulting in upwind flight towards a lure. Differing volatilities of the attractive test substrates were ruled out because the measured release rates of fuscumol propionate and MDF were not statistically different. In terms of the biochemistry, it also seems unlikely that the rates of adhesion of the compounds to the antennal cuticle or dissolution in the antennal lymph would be markedly different given the very similar structures and consequently lipophilicities of the two molecules. However, slight differences in structure could indeed be crucial in the next steps. That is, the capture of the ligands by a pheromone binding protein for transport through the sensillar lymph and transfer to the pheromone receptor would both be expected to be sensitive to structure, and this was demonstrated indirectly by the complete lack of an EAD signal from the other two analogs tested, or of the (S)-enantiomers of MDF and fuscumol acetate (Serrano et al. 2018). Thus, the relative affinities of the transport proteins and the pheromone receptors, and the kinetics of the capture and transfer of fuscumol propionate and MDF between the transport proteins and the receptors, would likely be different. Similarly, the clearance of the pheromone from the active site of the receptor by pheromone degrading enzymes could also be different. These effects, in turn, could alter the signal transmitted to higher brain centers, and the eventual output to motor neurons resulting in attraction to the odor source. We also cannot rule out the possibility that fuscumol propionate and MDF may actually be detected by different receptors and that the receptors may be expressed differently in either species. At this point, all we can say is that the hyperattraction to fuscumol propionate demonstrated by the enhanced trap captures may be a consequence of any one of these factors, or some combination of them.
However, the fact that fuscumol propionate elicited substantial, albeit significantly smaller responses from the antennae of males of both beetle species in EAG assays would seem to suggest that all the steps up to and including the firing of the pheromone receptors may be similar between fuscumol propionate and the natural pheromone. This, in turn, might suggest that the differences in the behavioral responses to the two compounds are the result of processes that occur after rather than before the receptors fire.
In summary, our results suggest that fuscumol propionate may be the most powerful analog or mimic ever reported for a volatile insect pheromone. This finding is even more remarkable in light of the fact that insect pheromone systems have been tuned by natural selection for millennia, to provide species-specific signals with very low detection thresholds. Thus, it was entirely unexpected and difficult to explain, how an analog could be so much more attractive than the natural pheromone. Further studies incorporating tools such as single sensillum recording might provide further insight into the mechanisms underlying the hyperactive response.
References
Advanced Chemistry Development (2019) ACD/Labs. Software V. 11.02. Toronto, ONT, Canada
Bengtsson M, Rauscher S, Arn H, Sun W-C, Prestwich GD (1990) Fluorine-substituted pheromone components affect the behavior of the grape berry moth. Experientia 46:1211–1213. https://doi.org/10.1007/BF01936940
Briggs GG, Cayley GR, Dawson GW, Griffiths DC, Macaulay EDM, Pickett JA, Pile MM, Wadhams LJ, Woodcock CM (1986) Some fluorine-containing pheromone analogs. Pestic Sci 17:441–448. https://doi.org/10.1002/ps.2780170415
Camps F, Fabriàs G, Gasol V, Guerrero A, Hernández R, Montoya R (1988) Analogs of the sex pheromone of processionary moth Thaumetopoea pityocampa: synthesis and biological activity. J Chem Ecol 14:1331–1346. https://doi.org/10.1007/BF01020138
Cardé RT, Roelofs WL (1977) Attraction of redbanded leafroller moths, Argyrotaenia velutinana, to blends of (Z)-and (E)-11-tridecenyl acetates. J Chem Ecol 3:143–149. https://doi.org/10.1007/BF00994141
Dawson GW, Mudd A, Pickett JA, Pile MM, Wadhams LJ (1990) Convenient synthesis of mosquito oviposition pheromone and a highly fluorinated analog retaining biological activity. J Chem Ecol 16:1779–1789. https://doi.org/10.1007/BF01020494
Eliyahu D, Mori K, Takikawa H, Leal WS, Schal C (2004) Behavioral activity of stereoisomers and a new component of the contact sex pheromone of female German cockroach, Blattella germanica. J Chem Ecol 30:1839–1848. https://doi.org/10.1023/B:JOEC.0000042405.05895.3a
El-Sayed AM (2019) The pherobase: database of pheromones and semiochemicals. https://www.pherobase.com/. Accessed 1 Nov 2019
Grant GG, Langevin D, Liska J, Kapitola P, Chong JM (1996) Olefin inhibitor of gypsy moth, Lymantria dispar, is a synergistic pheromone component of nun moth, L. monacha. Naturwissenschaften 83:328–830. https://doi.org/10.1007/BF01152217
Ho H-Y, Millar JG (2001) Identification and synthesis of a male-produced sex pheromone from the stink bug Chlorochroa sayi. J Chem Ecol 27:1177–1201. https://doi.org/10.1023/A:1010368013235
Hu D-H, He J, Zhou Y-W, Feng J-T, Zhang X (2012) Synthesis and field evaluation of the sex pheromone analogues to soybean pod borer Leguminivora glycinivorella. Molecules 17:12140–12150. https://doi.org/10.3390/molecules171012140
Lucas P, Renou M, Tellier F, Hammoud A, Audemard H, Descoins C (1994) Electrophysiological and field activity of halogenated analogs of (E, E)-8,10-dodecadienol, the main pheromone component, in the codling moth (Cydia pomonella L.). J Chem Ecol 20:489–503. https://doi.org/10.1007/BF02059592
Muraki Y, Taguri T, Yamakawa R, Ando T (2014) Synthesis and field evaluation of stereoisomers and analogues of 5-methylheptadecan-7-ol, an unusual sex pheromone component of the lichen moth, Miltochrista calamina. J Chem Ecol 40:250–258. https://doi.org/10.1007/s10886-014-0405-5
Pesenti C, Viani F (2004) The influence of fluorinated molecules (semiochemicals and enzyme substrate analogues) on the insect communication system. ChemBioChem 5:590–613. https://doi.org/10.1002/cbic.200300829
Renou M, Guerrero A (2000) Insect parapheromones in olfaction research and semiochemical-based pest control strategies. Annu Rev Entomol 45:605–630. https://doi.org/10.1146/annurev.ento.45.1.605
Riba M, Eizaguirre M, Sans A, Quero C, Guerrero A (1994) Inhibition of pheromone action in Sesamia nonagrioides by haloacetate analogues. Pestic Sci 41:97–103. https://doi.org/10.1002/ps.2780410205
Richter C, Schaepe K, Glorius F, Jan Ravoo B (2014) Tailor-made N-heterocyclic carbenes for nanoparticle stabilization. Chem Commun 50:3204–3207. https://doi.org/10.1039/C4CC00654B
Roelofs WL, Comeau A (1971) Sex pheromone perception: synergists and inhibitors for the red-banded leaf roller attractant. J Insect Physiol 17:435–448. https://doi.org/10.1007/BF00987721
SAS Institute Inc. (2019) SAS/STAT 9.4 user’s guide. Cary NC
Serrano JM, Collignon RM, Zou Y, Millar JG (2018) Identification of sex pheromones and sex pheromone mimics for two North American click beetle species (Coleoptera: Elateridae) in the genus Cardiophorus Esch. J Chem Ecol 44:327–338. https://doi.org/10.1007/s10886-018-0940-6
Solari P, Crnjar R, Frongia A, Sollai G, Secci F, Spiga M, Masala C, Liscia A (2007) Oxaspiropentane derivatives as effective sex pheromone analogues in the gypsy moth: electrophysiological and behavioral evidence. Chem Senses 32:755–763. https://doi.org/10.1093/chemse/bjm043
Sun Y, Meng Z, Chen P, Zhang D, Baunach M, Hertweck C, Li A (2016) A concise total synthesis of sespenine, a structurally unusual indole terpenoid from Streptomyces. Org Chem Front 3:368–374. https://doi.org/10.1039/C5QO00416K
Symonds MRE, Elgar MA (2008) The evolution of pheromone diversity. Trends Ecol Evol 23:220–228. https://doi.org/10.1016/j.tree.2007.11.009
US EPA (2019) Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11. United States Environmental Protection Agency, Washington, DC, USA
Whittaker AM, Dong VM (2015) Nickel-catalyzed dehydrogenative cross-coupling: direct transformation of aldehydes into esters and amides. Angew Chem Int Ed 54:1312–1315. https://doi.org/10.1002/anie.201410322
Acknowledgements
We thank Stacy Hishinuma for providing access to field sites, Rebecca Schmidt-Jeffris and W. Rodney Cooper for assistance with statistics in SAS, and Sean Halloran and J. Steven McElfresh for assistance with field work. We also thank Ring Cardé, Teun Dekker, and John Hildebrand for helpful discussions concerning possible mechanisms.
Funding
This work was funded in part by financial support from the Robert van den Bosch Scholarship to JMS, a UC Riverside Committee on Research grant to JGM, and funding from Hatch project CA-R*ENT-5181-H to JGM.
Author information
Authors and Affiliations
Contributions
JMS and JGM designed the research. JMS, YZ, and JGM conducted the chemical syntheses. JMS performed the experiments, analyzed the data, and wrote the first draft of the manuscript. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing interests.
Additional information
Handling Editor: Günther Raspotnig.
Rights and permissions
About this article
Cite this article
Serrano, J.M., Zou, Y. & Millar, J.G. Identification of a hyperactive pheromone analog in field tests of pheromone mimics for two click beetle species in the genus Cardiophorus (Coleoptera: Elateridae). Chemoecology 30, 297–304 (2020). https://doi.org/10.1007/s00049-020-00319-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00319-z