Abstract
The genus Plumeria of the Apocynaceae family has a rich history of traditional medicines supported by empirical evidences. This review consolidates diverse biological attributes, phytochemical compositions, physical properties (melting point, shape, optical rotation, etc.), and analytical data (UV, IR, Mass spectroscopic data, elemental analysis) of various species of Plumeria. The review covered the chemistry of wide range of natural compounds like iridoids, triterpenoids, alkaloids, flavonoids, steroids, cardiac glycosides, quinones, anthocyanins, cardenolides, fatty acid esters, lignans, coumarins, etc. found in various species of the genus Plumeria. Analytical techniques including chromatography, IR, UV, and mass spectroscopy have significantly contributed to elucidating the complex chemical profiles of extracts of various species of Plumeria which are systematically presented in a tabular format. The review also defines the historical background, geographical distribution, and traditional uses of various species of the genus Plumeria. The review also includes the mechanisms of action and biotransformation of compounds, providing a deeper understanding of their therapeutic potential. The comprehensive review reveals the significance of the natural products isolated from a number of species of genus Plumeria. It is also suggestive that there is an extensive scope for further investigation to explore new therapeutic components of the genus Plumeria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Apocynaceae family is extensively spread in tropical and subtropical areas; however, it does not grow well there because of the high temperature [1]. This family’s genera, including Alstonia, Adenium, Nerium, Alyxia, Plumeria (P.), and Vinca, have a long history of use as traditional remedies supported by empirical research. Adenium was typically employed as a skin treatment to cure lice because of its poisonous milk latex. Alstonia was traditionally used for respiratory disease [2], whereas alyxia was used for postnatal care [3]. Nerium tried to treat cancer [4], and for patients with diabetes, Vinca was used [5]. According to scientific research, certain herbs have pharmacological effects, including acting as an antioxidant for adenium.
Conversely, Alstonia is an herbal antiviral. While Nerium and Vinca are poisonous herbals utilized as CNS depressants [6] and antitumors [7], respectively, Alyxia is well-known for its antifungal properties [8]. To be more specific, Plumeria is one of the Apocynaceae plants that merits exploration for its variety and potential medical applications. Numerous studies have been done on frangipani latex, which contains alkaloid and sterol components that are pharmacologically effective as purgatives, hypotensive, and antitumors [9]. Compared to the abundance of sterol compounds like lupeol, taraxerol, and betulin, which have been shown to have anti-inflammatory [10], anticancer, and hypolipidemic properties. Plumerinine, an alkaloid, has been explored as an antianaphylaxis [11] agent.
Most tiny genus Plumeria species are deciduous shrubs and lactiferous trees. Although endemic to the Caribbean, South America, Mexico, and warm tropical regions of the Pacific Islands, Plumeria (Apocynaceae) originated in Central America. Various species may be found extensively spread over the world’s warmer areas. P. rubra, P. dichotoma, and P. bicolor are ornamental plants in India [12]. Plumeria is between thirty and forty feet tall. It has a scent unlike any other plant, and the various colored species have a distinct aroma. Certain Plumeria species smell pleasant, while others have lemony, jasmine, or peach scents [11]. Long, meaty, leathery leaves grow in clusters close to the apex of the branches. Since leaves are susceptible to cold, they often fall in early winter. Plumeria acuminata, P. rubra, P. cubensis, P. alba, P. bahamiensis, P. acutifolia, P. bicolor, P. bracteata, P. lancifolia, P. jamaicensis, P. stenopetale, P. montana, P. obtuse, P. obtusifolia, P. pudica, P. stenophylla, and P. tuberculata are among the genus that includes these species. There are as many Plumeria species as stated above, but P. acuminata, P. acutifolia, P. alba, P. bicolor, P. dichotoma, P. lancifolia, P. obtuse, P. obtusifolia, P. rubra, P. multiflora, and P. serifolia are the primary species covered in this study [11, 13].
A few review articles provide comprehensive information on the phytochemicals of the genus Plumeria. With this and the emerging potential applications of Plumeria species, this review presents an overview of their distribution, structure, biological activity, and analytical studies. The information is compiled and entered in a tabular format (Table 1). Furthermore, mechanisms of action and biotransformation of compounds and the potential pharmaceutical applications of compounds isolated from Plumeria species and their prospects were also discussed.
Brief description of the Plumeria species
Numerous hybrids and varieties of the Plumeria species, which vary widely in many traits, have emerged over time. So, while one author can count over sixty species, another might only count seven or eight, claiming the others are just hybrids and varieties [12].
Each kind of Plumeria has alternate leaves that vary in size, form, and development patterns. While P. pudica has elongated, glossy, dark green leaves, P. alba has relatively thin, corrugated leaves. P. pudica has evergreen and non-deciduous leaves. In the winter, P. obtuse keeps its leaves and blossoms. Flowers on plants can be between two and four inches across and come in white, yellow, pink, or red hues. One of the effective methods for this genus’s multiplication is tissue culture, which can be done with aseptically germinated seeds or cuttings of recently extended stems. Pruning is best done on deciduous kinds during the wintertime or when cuttings are needed.
Geographical distribution
Trees and shrubs in the genus Plumeria are native to New Zealand, Mexico, Central America, the Caribbean, and South America, extending as far south as Brazil. P. bracteata originated in the Brazilian state of Bahia. P. Alba is a native of the Caribbean and Central America. It is now standard and naturalized across southern and southeast Asia. P. obtuse is native to the Greater Antilles, northern Central America, and southern Mexico. It is typically grown in tropical areas like Hawaii, Asia, and eastern Africa. P. pudica is indigenous to Colombia, Panama, and Venezuela. P. alba is cultivated extensively in tropical and subtropical climates across the globe. It is mainly used as a plant in gardens and parks, and in many parts of India, there are also temples and cemeteries [13].
Historical background
French botanist Charles Plumier first used the name Plumeria in the 17th century. However, Plumier was one of many to call the plant Plumeria. The name was first given in 1522 by a Spanish priest, Francisco de Mendoza. The flowers were given the name frangipani when first found because the natural aroma of the Plumeria blossoms made people think of perfumed gloves. Since then, species of Plumeria have been widely used for both medicinal and decorative purposes all over the world. However, the extraction, isolation, structure elucidation, and establishment of the therapeutic activity of chemical elements of plants of the Plumeria species have been the subject of extensive investigation since the early 19th century. When Peckolt reported isolating the primary iridoid glycoside plumieride from the stem bark of P. lacifolia and P. rubra, phytochemical research on the genus Plumeria began [14].
Traditional uses
Asthma is managed by an extract from the leaves [15]. According to reports, extracts of various Plumeria species contain antifungal, antibacterial, and antiviral qualities that are used topically. Decoction of leaves is used as a purgative, anthelmintic, emmenagogue, and in several other applications [16,17,18]. Infusions of the roots and bark of Plumeria are used to calm agitation, ease constipation, induce menstruation, lower fever, and cure asthma [19, 20]. Gonorrhea is treated with crushed bark applied topically to complex tumors. Scabies are cured in Fiji using a decoction prepared from scraped bark. The leaves have rubefacient, antimicrobial, antipyretic, stimulant, and swelling properties. They treat various skin conditions [21], including ulcers, leprosy, inflammatory conditions, rheumatism, pneumonia, cholera, cough, and cold. Mexicans have used floral infusions as a traditional treatment for diabetes mellitus [22, 23]. Ruiz-Teran et al. [24] state that latex and flowers are used to treat eye hygiene, vaginal bleeding, and toothaches [24]. Fruit pulp, stem bark, and latex have all been utilized as abortifacients and purgatives in eastern Asia [25,26,27,28]. Flower tops and betel leaves (Piper betel) are eaten with ague [29]. In India, intestinal parasites, diarrhea, rheumatism, loose motions, and dysentery are all treated with the bark of the Plumeria [30]. It cleans the tongue by rubbing it on it [31]. The fruit is also used as an abortifacient [32] in southern Vietnam to treat malaria [33] and in Latin America for subcutaneous mycosis [34]. Latex is effective as a lubricant in rheumatoid arthritis and is used to heal blisters and wounds directly [35, 36]. Bark paste, when applied externally, aids in the healing of wounds [37]. Women are permanently sterilized with a specific amount of plant flowers, as Kalita et al. described in 2011 [38]. P. rubra is used to treat skin abscesses, herpes rash, dysentery, syphilis, and cough and as a laxative in Guinea and Northern Namibia.
Phytochemistry
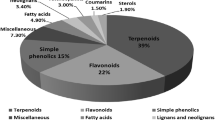
Iridoids, triterpenes, alkaloids, flavonoids, isoflavonoids, quinolizidine, and other chemical components have all been detected in the Plumeria genus. Of these, alkaloids, iridoids, and triterpenoids appear in practically every species and serve as chemotaxonomic markers for the genus. Table 1 thoroughly lists the substances discovered in Plumeria species [11].
Iridoids
Classification
As secondary metabolites, iridoids are a family of cyclic monoterpenoids found in plants and animals [39, 40]. The structural classification of iridoids is based on a glycosidic link inside the molecule [41].
Types of iridoids and their structural variations
A class of iridoid glycosides with a β-D-glucopyranosyl moiety at the C1 position is shown in Fig. 1. There are two more subtypes of iridoid glycosides: secoiridoids and carbocyclic iridoids (Figs. 1, 2) [42, 43].
The ring structure of cyclopenta[c]pyran is substituted and cis-fused in carbocyclic iridoids (Fig. 1). Substitutes of the cyclopentane moiety, such as epoxy, olefin, and hydroxy groups aid in structural variety [42]. Natural products, such as hydrogen, methyl, aldehyde, esters, and carboxylic acids, exhibit a range of modifications at the C11 position [42, 43]. Representative carbocyclic iridoids are shown to be the structure of different phytochemicals in the genus Plumeria (Figs. 3, 4). The cleaved cyclopentane ring in secoiridoids lacks a C–C link between C7 and C8 (Fig. 1). The primary precursor of iridoid alkaloids is C7 aldehyde type Iridoid, sweroside (40). Furthermore, the aldehyde moiety of 3 groups of terpenes or phenolics may be conjugated to extend this series’s structural complexity further.
Figure 1 illustrates non-glycosidic iridoids, including lactones and iridoidial iridoids. This compound exhibits regioisomeric variations involving the lactonyl carbon moiety, classified into type I and II iridoid lactones [41]. A carbonyl group is located at C3 in type I iridoid lactones, whereas in type II lactones, it is located at C1. The stereochemical difference arises from the methyl substituents at C8. Fulvolumericin is depicted as iridoid lactone type II (Fig. 2) [2, 14]. Although cis-fused ring structures have been well documented, trans-fused iridoid lactones are relatively rare [44]. Iridodials represent the aglycone of iridoid glycosides, which exist in equilibrium between the lactol and dialdehyde forms [45] (Figs. 1, 2). Therefore, the stability of the iridodial structure depends on the non-glycosidic entity.
The structures of plumieridine, α-allamcidin, β-allamcidin, and their diacetates are shown in Fig. 4. As for other iridoids, plumericin (8) (minor iridoids) [46] has appeared in Fig. 4 [47], which comprises an unusual framework based on the cis-fused ring framework. Nitrogen-containing iridoids can, moreover, be classified as iridoid-related alkaloids [45].
Iridoids in Plumeria species
List of iridoids isolated from various species of the genus Plumeria is provided in Fig. 3. From the different parts of P. rubra, fulvoplumericin (1), 15–demethyl–plumieride (2), α-allamcidin (3), β- allamcidin (4), allamcin (5), α-allamcidin diacetate (6), β-allamcidin diacetate (7), plumericin (8), isoplumericin (9), β-dihydro-plumericin (10), agoniadin (22), plumieride-p-(E)-coumarate (23), plumeridoids A (27), plumeridoids B (28), plumeridoids C (29), epiplumeridoids (30), allamandin (32), β-dihydro plumericinic acid (39), plumieride-p-(Z)-coumarate (43), p-E-coumaric acid (45) have been isolated. Plumericin (8), 8-isoplumieride (11), 13–O-caffeoyl plumieride (12), 13–deoxy plumieride (13), plumenoside (14), 1α-plumieride (15), 1α-protoplumericin A (16), protoplumericin B (17), 1α-protoplumericin B (18), 1α-protoplumericin C (19), 13–O-caffeoyl–15 demethyl plumieride (20), 15-demethyl plumieride p-E–coumarate (21), agoniadin (22), sweroside (40), 13-O-coumaroyl plumieride (44) have been reported in different parts of P. acutofolia. Isolated reported phytochemicals from P. alba are 1α-plumieride (15), agoniadin (22), plumieride-p-(E)-coumarate (23), plumieride-p-(Z)-coumarate (43). Iridoids isoplumericin (9), 1α-plumieride (15), and 3-O’-methyl plumeride (42) have been isolated from the species P. dichotoma.
Triterpenoids
Classification
Triterpenoid saponins, natural sugar conjugates of triterpenes, are common in many dicotyledonous plants, whereas they are rarely found in monocots. These compounds contain linear or branched oligosaccharides attached to hydroxyl or carboxyl groups, forming the glycone fraction. Attachment sites can be one (monodesmosides), two (bisdesmosides), or three (tridesmosides). As components of cell membranes, these plant metabolites play a central role in defense mechanisms, biological efficacy, and cell fluidity.
Types of triterpenoids and their structural variations
Classification of saponins based on aglycone skeleton structure produces six main types: Oleanane-like, Hederin-like, Lupane-like, Ursane-like, Fernane-1-like, and Fernane-2-like, determined by the chemical composition of the sapogenin arrangement (Fig. 5).
Among the triterpenoid saponins, Hederin, Oleanane, and Ursane share the same pentacyclic saponin backbone 6/6/6/6/6, which exhibits notable similarities beyond the variation in the C-23 substituent and position methyl group. In contrast, the Lupane-type saponins, Fernane-1 and Fernane-2, use a 6/6/6/6/5 pentacyclic saponin backbone, with differences mainly centered on the position of the isopropyl and carbonyl groups.
Rastogi and colleagues’ excellent comprehensive reviews of triterpenoid saponins [48, 49] covered the literature until 1978. A thorough review of Saponins’ chemical and biological importance in food and animal feed has recently been presented [50]. A new review briefly discusses advances in the structural elucidation of triterpenoid saponins [51]. Adler and Hiller have also reviewed the bisdesmosidic triterpene saponins [52], with new findings on these substances appearing in addition to those reported above [53].
Triterpenoids in Plumeria species
The triterpenoids found in various species of Plumeria and their biological and pharmacological activities are also reviewed, and their plant sources are listed in Table 1. Phytochemicals isolated from P. rubra are 6α-hydroxy-3-epi-oleanolic acid (53), ursolic acid (59), α-amyrin cinnamate (62), rubrinol (65), β-amyrin acetate (73), β-amyrin (75), cycloart-25-en-3β,24-diol (76), cycloart-22-en-3α,25-diol (77), arjunolic acid (78), lupeol acetate (79), (3α)-3,27-Dihydroxy- olean-12-ene (85), plumeric acid (104), methyl plumerate (105), taraxasteryl acetate (107). Phytochemicals extracted from P. acutofolia are ursolic acid (59), plumeriate (71), uvaol (88), lupeol (94), plumeric acid (104), methyl plumerate (105). From the species P. bicolor, phytochemicals isolated are α-amyrin cinnamate (62), 3β-acetoxy plumerian-12-ene (67), 3β-Hydroxy plumerian-12-ene (68), 3β-acetoxyurs 5,12-diene (69), 29-devinyllup-5-enol (70), β-amyrin (75), lupeol acetate (79), α-amyrin (86). From the different parts of P. obtuse, various constituents are isolated, viz. champalin A (46), champalin B (47), champalinol (48), champalinone (49), betulin (50), betulinic acid (51), α- amyrenone (52), uncarinic acid E (54), ursolic acid (59), obstusilic acid (64), (3β,20S)-dammarane-3, 20, 25-triol (72), neriucoumaric acid (81), isoneriucoumaric acid (82), alphitolic acid (83), oleanonic acid (84), α-amyrin (86), 3,27-dioxolup-12-ene (90), 3,11,27-tri-oxolup-12-ene (91), 27-hydroxy-3-oxolup-12-ene (92), 3-oxo-14-hydroxy-27-up-12-ene (93), 3β-Hydroxy-27-[(Z)-p-coumaroyl oxy]- urs-12-en-28-oic acid (95), obtusilinin (96), obtusinin (97), obtusilin (98), 3,23-dihydroxyurs-12-en-28-oic acid (99), obtusidin (100), obtusinidin (101).
Steroids
Classification
Steroid glycosides are synthetic triterpenes with a structure consisting of a six-membered tetracyclic ring and a five-membered bicyclic ring containing 27 carbon atoms.
Types of steroids and their structural variations
Steroid glycosides have two heterocyclic rings, one of which is a furan ring, and the other is a pyran ring—a common spirocarbon atom between two heterocycles (furanose and pyranose rings). Figure 6 shows an example of steroid glycosides [54]. Sterols include several major steroid groups characterized by a hydroxyl group at C3, usually in the β configuration, and a branched side chain of 8 to 10 carbon atoms at C17. They are common in all animals, especially those belonging to plant kingdoms [55].
Steroids in Plumeria species
The sterols commonly isolated from higher plants are β-stigmasterol, β-sitosterol, campesterol, and lanosterol, which are also quite common. Five sterols from the various Plumeria species have been isolated: stigmasterol (108), β-sitosterol 3-O-glucoside (109), β-sitosterol (111), campesterol (110), and stigmast-7-en-3-ol (112) (Figs. 3, 4).
Flavonoids
Classification
Flavonoids, bioactive polyphenols of relatively low molecular weight [56, 57], are pivotal in cellular photosynthesis [58, 59]. The exploration of flavonoids commenced in 1936, credited to Hungarian scientist Albert Szent-Gyorgi. He uncovered a synergistic interaction between pure vitamin C and unidentified cofactors extracted from lemon peels, initially calling them “flavonoids” before later referring to them as “vitamins P.” [60].
Types of flavonoids and their structural variations
Flavonoids, characterized by a hydroxylated phenolic nature and a benzo-γ-pyrone structure, are synthesized by plants in response to bacterial contamination [61]. These compounds exist as aglycones, glycosides, and methylated derivatives [62]. Numerous primary forms of flavonoid aglycones, flavonoids without sugars, are connected in plants. The fundamental chemical structure of flavonoids may be a diphenylpropane skeleton containing 15 carbon particles in its primary center [63]. Two benzene rings (rings A and B) are primarily connected by a third heterocyclic pyrene ring containing oxygen [64]. So, this structure is additionally called C6-C3-C6, labeled A, B, and C (Fig. 7) [65, 66].
Flavonoids can be categorized into different groups based on the carbon atom of the C ring to which the B ring is attached, as well as the level of unsaturation and oxidation of the C ring. The compounds are termed isoflavones when the B ring is linked to position 3 of the C ring [67]. If the B ring connects to position 4 of the C ring, they are called neoflavonoids. Substances where the B ring attaches to position 2 of the C ring can be further subdivided into various groups according to the fundamental characteristics of the C ring. This subgroup comprises flavones, flavonols, flavanones, flavanonols, flavanols (catechins), and anthocyanins [68,69,70] (Fig. 8).
Flavonoids in Plumeria species
Eleven flavonoids (113–123) have been extracted from the Plumeria species. From P. rubra, quercetin (119), plumerubroside (120), rubranoside (121), from P. obtuse glochiflavanoside B (122), methyl p-(E)-coumarate (123), and P. acutofoila narcissin (113), kaempferol-3-rutinoside (114), ayanin (115), kaempferol (116), pillion (117), quercitrin (118).
Alkaloids
Classification
Alkaloids are heterocyclic nitrogen compounds [71]. Nitrogen is the only distinguishing feature of all alkaloids. Amino acids like tryptophan, tyrosine, and lysine are the primary metabolites from which they are derived.
Types of alkaloids and their structural variations
The primary alkaloid isolation happened in the 19th century after several alkaloid-containing drugs were presented in pharmaceuticals. The dissemination of alkaloids concurring with the essential structure of the C-N skeleton is the foremost exact and common way of classifying alkaloids (Fig. 9). The alkaloids are partitioned into the taking after fundamental bunches: pyrrolidine, pyridine, quinoline, indole, quinazoline, steroids, and diterpenoids. Depending on the structure of its representatives, each group is divided into different subgroups [72].
Alkaloids in Plumeria species
Eleven alkaloids, 124–134, have been isolated from the Plumeria species so far (Fig. 4). From the species P. rubra, plumerinine (129) and plumericidine (130) have been isolated. Six alkaloids have been identified in the species P. acutofoila viz. laurelliptine (124), phoebegrandine B (125), plumericidine (130), grandine A (132), grandine B (133), grandine C (134), vincubin (131) in P. Seriflia and two phytochemicals named uleine (126) and demethoxyaspido-spermine (127) in species P. lancifolia have been isolated [73].
Cardiac glycosides
Classification
The most significant number of druglike molecules that have been investigated in multiple studies and were found to be beneficial for the development of potential drugs has been cardioglycaemic glycosides, a type of glycosylation molecule [74,75,76,77,78]. These are chemical compounds that poison pets and treat congestive heart failure.
Types of cardiac glycosides and their structural variations
Cardiac glycosides represent a class comprising two significant groups of compounds, each with distinct aglycone structures, illustrated in Fig. 10. These glycosides are C23 or C24 steroids wherein the standard cyclopentanoperhydrophenanthrene core at C17 is replaced. Cardenolides feature a five-membered lactone group at C17, having an unsaturated γ-lactone ring (butenolide), whereas the other group, bufadienolide, was initially identified as a toxin in toad skin. The C17 substituent bears a doubly unsaturated six-membered lactone ring (α-pyrone). Plants can synthesize both cardenolides and bufadienolides. An additional subgroup, isocardenolide, exhibits the double bond of the butenolide ring positioned at either 21 or 22 instead of the conventional 20, as depicted in Fig. 10.
Cardiac glycosides in Plumeria species
P. obtuse produced the cardiac glycosides oleandrine (135) and kaneroside (136), which were isolated (Fig. 4) [79,80,81].
Quinones
Classification
The quinones are a class of organic compounds that are formally “derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C( = O)– groups with any necessary rearrangement of double bonds,” resulting in “a fully conjugated cyclic dione structure” [82,83,84].
Types of quinones and their structural variations
The prototype member of this class is 1,4-benzoquinone, also known simply as “quinone” or cyclohexadienedione. Given that this compound is one of the products of quinic acid oxidation, its name is derived from quinic acid with the suffix “one,” indicating a ketone [85]. Other notable examples include 1,2-benzoquinone (o-quinone), 1,4-naphthoquinone, and 9,10-anthraquinone [86].
Quinones in Plumeria species
From P. rubra, the quinones 2,5-dimethoxy-p-benzoquinone (137) and 2,6-dimethoxy-p-benzoquinone (138) were isolated (Fig. 4).
Nor-Terpenoids
Classification
By removing one or more carbon atoms from their carbon skeletons, nor terpenoids are a class of organic compounds that derive from terpenoids.
Types of nor-Terpenoids and their structural variations
Terpenoids derived from five-carbon isoprene units are a comprehensive and diversified class of natural chemical substances. The prefix “nor” means something has been reduced in size compared to its parent compound. This reduction usually involves the removal of methyl groups or other carbon-containing moieties from the terpenoid structure in the case of nor-terpenoids.
Nor-terpenoids in Plumeria species
From P. rubra, the nor-terpenoids P. rubrajaleelol (139) and P. rubrajaleelic acid (140) were separated (Fig. 4).
Anthocyanins
Classification
One of the most exciting groups of polyphenols is anthocyanins. These compounds, identified from their glycosylation form [86], are generally considered one of the largest groups of naturally occurring water-soluble phenolic compounds. Anthocyanins, which can produce colors ranging from red to blue, are present in many plants and natural products.
Types of anthocyanins and their structural variations
Anthocyanins derive their structure from the central framework of flavonoids [87]. Chemically, they are 2-phenylbenzopyrylium compounds composed of an aromatic ring (A) linked to a heterocyclic pyran (C), which connects to an aromatic core. A carbon-carbon bond strengthens the B aromatic ring. This ring can exhibit various hydroxylation and methoxylation patterns, with hydroxyl groups typically present at C3, C5, and C7. Additionally, the carbon atoms within the core structure can undergo glycosylation with other sugars [88] (Fig. 11).
Only the glycosylated structure is called anthocyanin, while the non-glycosylated structure is anthocyanidin and is considered an anthocyanin precursor. Anthocyanins have many structural features, from simple mongolcosides to complex structures involving multiple types of glycosylation and acylation, and to date, more than 700 have been described [88,89,90,91,92,93].
Anthocyanins in Plumeria species
Two anthocyanins, cyanidin 3-O-β-(2″-glucopyranosyl-O-β-galactopyranoside) (141) and cyanidin-3-O-β-galactopyranoside (142) were isolated from the flowers of P. rubra.
Cardenolides
Classification
Cardenolides, a group of steroid-derived compounds found naturally in plants, exist as glycosides or free genins (lactones plus steroids) [77].
Types of cardenolides and their structural variations
These compounds display diverse chemical structures but share standard features, including a 23-carbon steroid core with A/B and C/D rings in cis form (unlike sex hormones and corticosteroids) [94], an unsaturated γ-lactone (furanone) at C17, a β-hydroxyl group at C14, β-methyl groups at C10 and C13, and a glycosidic moiety at C3 [95]. Some may also bear substituents like hydroxyl, formyl, or acetyl groups at C16 (Fig. 12) [96, 97].
Recent findings indicate that the genin or aglycone segment is the pharmacologically active site or pharmacophore [77]. However, cardenolide manifests its activity when it contains at least one hydroxyl group or a sugar comprising one to five C3 monosaccharides [94]. Various sugars have been identified, including pentoses, hexoses, deoxy sugars, and others [96, 97]. While the glycoside component lacks pharmacological activity, it influences this compound family’s pharmacodynamic and pharmacokinetic properties, as discussed in [77, 96, 98]. Furthermore, the type of sugar attached impacts the potency of the compound, with monosaccharides and substances with a 3β-hydroxyl group demonstrating higher activity than disaccharides, trisaccharides, or aglycones [96, 97, 99,100,101,102].
Cardenolides in Plumeria species
P. obtuse yielded a single cardenolide named 3-epidigitoxigenin (143), which was isolated [103,104,105].
Fatty Acid Esters
From the Plumeria species, four fatty acid esters, 34-hydroxy tetratri-acontanyl ferulate, 34-O-acetyl-tetratriacontanyl ferulate, methyl n-octadecanoate, and lupeol fatty acid esters I numbered 144–147, respectively have been isolated.
Lignans
Classification
Lignans, a class of secondary metabolites resulting from the oxidative dimerization of two phenylpropanoid units, are widely distributed throughout the plant kingdom. These compounds have been discovered in the roots, rhizomes, stems, bark, leaves, seeds, and fruits of over 70 plant families [106].
Types of lignans and their structural variations
Although lignans are composed only of two phenylpropane C6–C3 units, their molecular backbone contains a sizeable structural diversity due to the various patterns of linkage for these phenylpropane units on which classification is based. The benzene ring is most lignans’ C6 partial structure of the phenylpropane unit [106].
Traditionally, there are two types of lignan: regular lignan and neolignan. Classical lignans consist of phenylpropane dimers connected through a β–β′ (8–8′) linkage, while neolignans encompass similar dimers featuring this β–β‘ bond. Classical lignans include six subgroups: dibenzyl butanes (CL1), dibenzylbutyrolactones (CL2), aryl naphthalenes (CL3), dibenzocyclooctadienes (CL4), substituted tetrahydrofurans (CL5a-c) and 2,6-diarylfurofurans (CL6) (Fig. 13), and neolignans are made up of fifteen subtypes (NL1 to NL15) (Fig. 14).
Other sorts of lignans [107,108,109,110,111,112,113,114,115], such as oligomeric lignans, hybrid lignans, and norlignans, have also been detailed, other than conventional lignans and neolignans.
Lignans in Plumeria species
From P. rubra, the lignan liriodendrin (148) was isolated.
Coumarins
Classification
Coumarins are classified into various types: simple coumarins, furanocoumarins, pyranocoumarins, pyrone-substituted coumarins.
Types of coumarins and their structural variations
Simple coumarins [116,117,118,119,120,121,122,123], which feature a hydroxylated, alkoxylated, or alkylated radical on the benzene ring; furanocoumarins, characterized by a furan ring attached to the benzene ring; pyranocoumarins, distinguished by a pyran ring connected to the benzene ring; and pyrone-substituted coumarins, wherein substitution typically occurs on the pyrone ring, often at the 3-C or 4-C position.
Coumarins in Plumeria species
Two coumarins, identified as scopoletin (149) and rel-(3 R,3'5S,4 R,4'S)-3,3',4,4' -tetrahydro-6,6'–dimethoxy[3,3'-bi-2H-benzopyran]-4,4'-diol (150), have been isolated from P. rubra and have been reported [124,125,126].
Other
P. rubra and P. acutofolia have been reported to contain six more compounds: P. rubranin, cerberic acid B, 1-(p-hydroxyphenyl) propan-1-one, 2,3 dihydroxypropyl octacosanoate, L-(+)-bornesitol and 2,4,6-trimethoxy aniline numbered (151–156).
Pharmacological activities
Because of their vast range of pharmacological properties, plants in the genus Plumeria are frequently employed in traditional medicine [11]. The extracts and substances from this genus have been shown to have substantial antioxidant, analgesic, anti-inflammatory, antidiabetic, anticancer, estrogenic/antiestrogenic, antibacterial, and antihyperglycemic activity in accumulating pharmacological research (Table 1).
Antitumor effects
By effectively stopping tumor growth, the ethanolic leaf extract of P. rubra could prolong the lives of EAC albino male Swiss mice administered at 200 and 400 mg per kg body weight over their lifetimes [127]. Likewise, methanolic leaf extract showed recovery of hemoglobin and red blood cell counts, a significant decrease in tumor volume and lipid peroxidation, and an increase in GSH, SOD, and CAT levels in mice bearing EAC tumors [128]. The ethanolic extract of the flowers showed cytotoxicity against the HepG2 cell line, inducing apoptosis at this concentration, and showing anticancer activity [129]. Compounds 1 [130], 5 [130, 131], 8 and 9 have been isolated from the bark of P. rubra. Lignan 148 [132] and quinone 137 [131] showed moderate activity against various human cancer and murine lymphocytic leukemia cell lines [130]. The six compounds isolated from plant heartwood, 8, 9 and 4-hydroxyacetophenone showed varying antibacterial and cytotoxic activity [133]. Moreover, the fraction of petroleum hydrocarbons showed high cytotoxic activity against HepG2, HCT116, and MCF-7 cell lines and moderate or lower activity levels in other fractions. Ethanolic stem bark extract has been toxic to T47D cells but not to lymphocytes with an LC50 of 273.744 gm [134]. The triterpenes 104 and 105 [11], the iridoids 22 [135], 20 [136], 2 [130], 18 and 19 [137] were found to have antitumor action. Alkaloids 130 [138] and 131 also showed antileukemic activity.
The methanol extract from leaves of P. alba has a potent antitumor activity against Dalton′s lymphoma in mice. The results indicate that a methanolic extract from P. alba has been shown to significantly extend the lives of host mice, reduce tumor size, and improve hematological parameters [139].
Antimutagenic effects
Antibacterial substances were extracted from the organic hexane and carbon tetrachloride fractions, followed by a micronucleus test to assess their antimicrobial activity. According to this hypothesis, a vital role of the antimutagenic activity detected in these isolates is that all three isolated antibacterial substances have been found to contain a hydroxyl group, particularly in the 3rd position [140]. From ethanol extract of the green leaves of P. acuminata, 59, 79, 112 [140], and 103 [11], these four isolates exhibited antimutagenic activity.
Antimicrobial effects
Antibacterial and antifungal activity
The MIC value of 125 μg/mL and the MBC/MFC value of 250 μg/mL indicated antibacterial solid activity in ethanolic stem bark extract compared to different bacteria and fungi. The various pieces of stem bark from P. rubra were characterized by multiple antibacterial activities. When the MICs of 7.81 g/mL and 3.9 g/mL were compared against some strains, a fraction of ethyl acetate showed comparable efficacy to amphotericin B and gentamicin [141]. Nevertheless, resistance to all fractions selected was found in Pseudomonas aeruginosa [142]. In comparison to ciprofloxacin and against fungal strains with MIC values of 50 g per mL compared to fluconazole, methanolic bark extract has significant activity against certain bacterial strains with MIC values of 25 g per mL [143]. The essential oils extracted from Plumeria flowers showed varying antibacterial action against the bacteria tested, indicating that gram-positive strains are more susceptible. Inhibition zone diameter ranges from 6.8 to 221.3 mm and MICs of 2.8 to 46.5 mg/mL, respectively [144]. Previous studies have investigated the antibacterial activity using a disk diffusion method. It is recommended that these results be complemented with more relevant MIC tests, as disk diffusion methods are inherently limited [145,146,147].
The agar disc diffusion method investigated methanolic extract′s in vitro antibacterial properties from P. acuminata. The crude methanol extracts MEPA inhibited the growth of both gram-positive bacteria (Micrococcus luteus, Bacillus subtilis, Staphylococcus aureus) and gram-negative bacteria (P. aeruginosa, Escherichia coli, Salmonella typhimurium). The tested gram-positive bacteria were more susceptible to the extract than the gram-negative bacteria [148].
The essential oil of the P. alba flower was evaluated against various microorganisms. The results show that gram-negative bacteria are the least susceptible to the effects of many other plant essential oils. The flower parts of P. alba were more effective in S. aureus and B. subtilis but also significantly inhibited growth at lower concentrations [149].
Rubrinol’s antimicrobial tests [150] showed its effectiveness against P. aeruginosa, P. pseudomallei, B. anthracis, and Corynebacterium pseudodiphthericum. P. acutifolia was also the source of three novel alkaloids, 132–134 [151], as well as 125 and l24 [152] and [153], respectively. From the bark and leaves, three main iridoids were isolated: plumieride [137], 16 [137], and 2 [130]. Among the phytochemicals found in P. rubra are lignan 148 from its bark [132], 137 [131] from its stem bark, 123 [11] from its leaves, 8 [39], 15 [137], 22 [135], 59 [140], 62 [154], 79 [140], 86 [155], 109 [156], 111 [157] against various pathogenic fungus and bacteria.
Iridoids such as 8, 9 [39], 27, and 28 [131], alongside 5 [130, 131], 62 [154], triterpenoid 79 [140], 80 [14], quinone 138 [131], and other compounds like 153 [131] have demonstrated fungicidal activity.
Anthelmintic activity
The methanolic stem bark extract of P. rubra showed dose-dependent anthelmintic activity, especially at 50 mg/mL, and was more effective than piperazine citrate (15 mg/mL) against Pheretima posthuma worms [158]. Similarly, against laboratory nematodes, methanol leaf extract at 25 and 50 mg/mL was as effective or more effective than piperazine citrate at 15 and 50 mg/mL [159].
Antimalarial activity
A dose-dependent antimalarial effect was observed in aqueous and chloroform extracts of Plumeria bark. The aqueous extract inhibited the growth of parasites by 74.1, 76, and 80.3% in inhibition, treatment, and prevention tests, respectively, at 200 mg/kg per day. An inhibition of 60, 62.7, and 64.1% was observed with the chloroform extracts. A comparison was made with chloroquine (5 mg/kg/day) [160]. On the other hand, the hydroalcoholic extract of dried P. rubra shoot is a potent protozoan against chloroquine-susceptible 3D7 strain and chloroquine-resistant RKL9 strain of P. falciparum with IC50 values of 2.678 μg/mL and 2.389 μg/mL, respectively [161].
Antiviral activity
Petroleum ether extract of Plumeria bark showed 35% inhibition against HIV-1 RT at 200 μg/mL, and further chromatography yielded an active fraction showing 70% inhibition at the same concentration. The IC50 value for 1, an iridoid isolated from the extract, was 45 g/mL compared to HIV-1 RT. However, there was no evident inhibitory effect from water or CHCl3 extracts. Tannins, known as RT inhibitors, have been removed from plant extracts before testing based on their activity, and they were not set out with any unique thresholds [162].
Larvicidal activity
Compared to the aqueous crude latex extract (1000, 500, 250, 125, 62.50, 31.25 ppm), silver nanoparticles (AgNPs) prepared from the latex of Plumeria were found to be highly toxic to larvae Anopheles stephensi and Aedes aegypti at different concentrations (10, 5, 2.5, 0.625, 0.3125 ppm), It has a robust larvicidal effect. Toxicity studies have not shown toxicity at LC50 or LC90 AgNP doses in the nontarget fish species Poecilia reticulata [163].
Anti-inflammatory and analgesic effects
The extract (500 mg kg−1 body weight) showed the most excellent anti-inflammatory effect. E.g., carrageenan, dextran, histamine, and serotonin were 30.51, 47.06, 34.48, and 32.50% (p < 0.001), respectively, at the end of 3 h. Administration of MEPA (500 mg kg−1 body weight) and indomethacin (10 mg kg−1 body weight) significantly reduced the granulomatous tissue formation induced by the cotton pellet method by 45.06 and 51.57%, respectively [164]. 128 (10, 25, and 50 mg/kg body weight) from a methanolic root bark extract caused a dose-dependent decrease and a similar effect to indomethacin (10 mg/kg body weight). A significant inhibitory effect on edema and granuloma tissue formation was shown by Vijayalakshmi et al. [165]. A latex-derived protease, Plumerin-R, showed a reduction in carrageenan-induced paw edema comparable to indomethacin (10 mg/kg) at various doses [166]. Furthermore, it also showed anti-irritant and anti-inflammatory effects compared to betamethasone (1 mg/kg) [167]. P. rubra methanolic leaf extract (100 and 200 mg/kg body weight) showed inhibition of carrageenan-induced paw edema in rats, with the highest inhibition at 200 mg/kg body weight and indomethacin Comparable (10 mg/kg) [159]. In different pain and inflammation models, ethanol bark extract (250 mg or 500 mg per kg) bodyweight in P. rubra is very active compared with diclofenac sodium 10 mg/kg body weight [168]. At 200 mg/kg, the triterpenoids 50 [169], 79 [140], and 62 [154] reduced the production of edema by 35.9%. However, at 100 mg/kg, no effect was seen. Methanol extract of P. acuminata leaves exhibits significant anti-inflammatory effects against dextran-, histamine-, carrageenan-, and serotonin-induced inflammation in a rat hind paw edema model. These results show that methanol extract has a substantial inhibitory effect against inflammation in the experimental animal models studied.
Antiarthritic activity
The hydroalcoholic stem bark extract of P. rubra at oral doses of 250 and 500 mg/kg body weight significantly increased body weight and spleen index over 14 days in complete Freund’s adjuvant (CFA)-induced arthritis in male albino Wistar rats, compared to a standard oral dose of piroxicam 10 mg/kg. In addition, this extract also significantly reduced the biochemical, behavioral, radiological, and hematological changes caused by CFA that showed significant antiarrhythmic activity [170].
Antialgal effects
In iridoids 22 [135], 9 [39], 16 [137], 8 [39], plumierides 23, 25, 26 [171], 27, 28 [131], 43 [171], 5 [130, 131], 44 [137], 45 [131], triterpenoid 79 [140] with other substances 153 [131], algicidal properties are demonstrated.
Anesthetic effects
Anesthetic activity was detected in the triterpenes 104 and 105 [11, 172].
Antipyretic and antinociceptive activity
In Wistar albino rats, a methanolic extract of 100, 250, and 500 mg/kg of plant leaves effectively treats brewer’s yeasty hyperthermia compared with paracetamol at 500 mg/kg body weight for 23 h. Later, the rectal temperature was lowered to 37.3 °C (100 mg/kg) at 37.2 °C. It also showed a significant antinociceptive effect in Swiss albino mice, reducing acetic acid-induced orthographic and tail motor responses comparable to aspirin [173, 174]. In addition, in albino rabbits, ethanolic extract from P. rubra leaves (200 mg/kg body weight, compared to aspirin 10 mg/kg body weight, significantly reduced the incidence of boil fever [175]. Similarly, methanol bark extract reduced the rise in body temperature in a yeast-induced fever model and showed significant dose-dependent activity compared to aspirin [176].
Furthermore, methanol bark extract effectively suppressed fever induced by rabbit typhoid vaccine and prostaglandin E (PGE1) at 100 mg/kg body weight and 200 mg/kg body weight, respectively, as well as paracetamol and aspirin, respectively [177]. In rats, a significant reduction of brewer’s yeast hyperthermia was observed with only one orally administered methanol extract at various doses between 250 and 500 mg/kg. In mouse antinociception experiments, methanolic extract of P. acuminata has also been found to have significant inhibitory effects on acetic acid-induced writhing response, hot plate responses, tail movements, and tail dipping [174].
Antioxidant activity
The methanol leaf extract of the plant increased the peroxidation inhibition of linoleic acid emulsion by 46.01, 52.83, 57.43, 61.38, and 73.14% it showed that it was at 50, 100, 200, 300, 400, and 500 μg/mL concentrations, respectively. The reduced power and scavenging capacity of DPPH, nitric oxide radicals, superoxide, and hydroxyl radicals increased dose-dependently. The IC50 values for catechin and superoxide removal were 5.27 and 72.39 μg/mL, respectively [173, 174]. Furthermore, protein fractions from P. rubra latex effectively inhibited total superoxide dismutase (SOD) and peroxidase activities at a concentration of 10 mg/mL [178]. In comparison, methanolic extracts of P. rubra flowers showed strong antioxidant activity. DPPH, iron-reducing antioxidant activity, metal chelates, hydrogen peroxide, nitric oxide, and superoxide scavenging among the tested extracts showed the highest inhibition in the test. It also exhibited significant xanthine oxidase (XO) inhibitory activity with 84.39% inhibition at 200 μg/mL [179].
A significant antioxidant and free radical scavenging activity is demonstrated in the methanol extract of P. acuminata. The results showed that at different concentrations of 50, 100, 200, 300, 400, and 500 μg mL−1, methanol extract had dose-dependent antioxidant activity and 46.01, 52.83, 57.43, 61.38, 68.27, and 73.14% inhibition, respectively on peroxidation of linoleic acid. α-tocopherol showed 81.21% inhibition at a concentration of 500 μg mL-1 simultaneously [173].
Antidiabetic and hypoglycaemic activity
Equivalent to the effects of glibenclamide 10 mg/kg orally, aqueous extract of P. rubra flowers significantly reduced blood glucose levels in STZ-induced diabetic mice at doses of 100, 200, and 400 mg/kg of body weight. After 28 days, the level decreased significantly from 380.3 mg per dL to 113.3 mg per dL at the 200 mg per kg dose. It is equivalent to reducing glibenclamide from 381.7 mg per deciliter to 100.2 mg per deciliter. An improvement in STZ-induced tubular necrosis and pancreatic injury was observed in a histopathological examination by Yadav and Undale [180]. In diabetic mice in which alloxan was induced [181], aqueous extract showed decreases in fasting glucose and glycosylated hemoglobin levels. The hydrous ethanolic stem bark extract of P. rubra (3:7) significantly lowered blood glucose levels and promoted pancreatic β-cell recovery in STZ-induced diabetic rats at doses of 250 and 500 mg/kg body weight, improving markers of pancreatic β cells. Oxidative stress compared to metformin hydrochloride (5 mg/kg body weight) [182]. Furthermore, 200 and 400 mg/kg body weight of the aqueous ethanolic extract of leaves reduced liver and kidney enzyme levels and restored antioxidant levels. It reversed histopathological damage [183] in diabetic rats, comparable to glibenclamide (120 mg/kg body weight).
Hypolipidemic activity
Flavone glycosides (250 mg/kg body weight) from the methanol fresh flower extract of P. rubra significantly lowered serum triglycerides, blood urea, and creatinine levels in rats. There was no change in serum cholesterol or glucose levels, but like glibenclamide (10 mg/kg body weight), it significantly affected AST and ALT activity [184].
Hepatoprotective activity
Methanol extract of P. rubra leaves at 400 mg/kg body weight effectively inhibited the increase in enzyme levels caused by CCl4, PCM, and antituberculosis drug intoxication in rats and mice, compared with the 200 mg/kg dose showed better results. Histopathological examination revealed reduced liver damage [185]. Similarly, administration of ethanolic extract of P. rubra pods at 200 mg/kg body weight for four days showed liver protection against CCl4-induced damage, with a decrease in the marker enzymes AST, ALT, and ALP. This dose showed similar efficacy to the standard drug silymarin (10 mg/kg body weight) [186].
Antifertility activity
Several studies have evaluated the effects of different extracts from P. rubra pods and barks on pregnancy and fertility in rats. P. rubra pods alcoholic extract administered at 200 mg/kg body weight from day 11 to 15 of gestation caused complete abortion in rats, which increased post-implantation losses and absorption index. Similarly, the ethanolic extract of P. rubra sheaths resulted in abortion rates of 13.46 to 100% in female albino rats, affected viable fetuses, and increased resorption index and post-implantation losses. In another study, methanol bark extract administered at various doses (50, 100, and 200 mg/kg body weight) over 60 days significantly reduced fertility in male rats. In comparison, a 200 mg/kg body weight dose ultimately reduces fertility. At low doses, entirely negative birth rates resulted in 58 to 64% negative birth rates [187,188,189].
Antiulcer activity
Ethanol and chloroform extracts of Plumeria leaves at 250 mg/kg p.o. in rats, ulcer index, and gastric fluid volume decreased. At the same time, SOD, CAT, and GSH levels increased, indicating potential for free radical damage. Omeprazole at 20 mg per kg showed a 91.39% protective effect against pylorus ligation compared to ulcer control. The protection was 75.05 and 63.13%, respectively, for ethanol and chloroform extracts of 250 mg per kg. Both extracts alleviated gastric glandular lesions, decreased acid secretion, and increased mucus secretion, and the ethanolic extract showed superior antiulcer activity. Soluble latex protein from P. rubra prevented ethanol-induced gastric lesions through prostaglandin involvement and antioxidant balance mediated by capsaicin-sensitive afferents and NO/cGMP/KATP pathways [190, 191].
Mechanism of action of compounds extracted from species of Plumeria
The pharmaceutical, food industry, and cosmetic sectors have made substantial use of marker components like plumeride from P. alba. [192, 193] White frangipani has been shown to have antiarthritic properties in a 2014 study. A persistent inflammatory condition that destroys cartilage and erodes bone is rheumatoid arthritis [194].
Hyperlipidemia, a risk factor for cardiovascular diseases, can potentially be mitigated by white frangipani flowers, as demonstrated by Rahman et al. [195]. Although its hypolipidemic activity was not as high as simvastatin (92%), the results were promising [195]. Additionally, white frangipani has shown hypoglycemic activities, as evaluated by an oral glucose tolerance test in mice [196].
Despite the continuous demand for novel vaccinations and anti-infectious drugs, the current scenario indicates a slowdown in identifying new active chemical entities. Natural products were the source of almost 61% of newly developed medications between 1981 and 2002; these drugs were especially effective in treating infectious disorders and cancer [197]. Thus, new natural compounds originating from plants, such as those belonging to the Plumeria genus, continue to present a prospective source of various antibacterial agents that may have novel modes of action.
Iridoids
Numerous iridoids display critical anti-inflammatory impacts by restraining basic pathways included within the inflammatory reaction. Compounds such as 8 and 9 hinder the activation of nuclear factor-kappa B (NF-κB), pivotal for the expression of pro-inflammatory cytokines and proteins like IL-1β, TNF-α, and cyclooxygenase-2 (COX-2). This inhibition decreases irritation and its related indications [198].
Iridoids like 22 [135] and plumeridoids (A, B, and C) [131] have strong antioxidant properties. They neutralize free radicals and improve the body’s antioxidant defense framework by upregulating chemicals such as catalase (CAT) and SOD. Compounds such as 1 and 5 appear to have antimicrobial action by disrupting the cell films of pathogens, leading to cell lysis and death. They interfere with microbial protein frameworks and nucleic acid synthesis, repressing the development and replication of bacteria, fungi, and viruses.
Iridoids like 32 and 39 exhibit cytotoxic effects against cancer cells. They induce apoptosis through the mitochondrial pathway, increase reactive oxygen species (ROS) production, and disrupt cell cycle progression. These actions inhibit cancer cell proliferation and tumor growth [39, 130, 136, 137]. Certain iridoids, such as 4 and its derivatives, have neuroprotective effects. They modulate neurotransmitter systems, reduce neuroinflammation, and protect neuronal cells from excitotoxicity and oxidative damage, critical factors in neurodegenerative diseases like Alzheimer’s and Parkinson’s.
Iridoids such as 23 and its derivatives manage diabetes by improving insulin sensitivity, enhancing insulin secretion, and inhibiting enzymes involved in glucose production. Some iridoids exhibit analgesic properties by interacting with opioid receptors and inhibiting pain pathways. Compounds like 6 and 7 reduce pain perception and relieve chronic pain conditions.
Triterpenoids
Many triterpenoids exhibit significant anti-inflammatory effects, including 53, 59, 75, 94, and 51. They inhibit the expression of pro-inflammatory mediators such as COX-2, nitric oxide (NO), and various cytokines (e.g., IL-1β, TNF-α), achieved by suppressing the NF-κB signaling pathway, a key regulator of the inflammatory response.
59, 86, 94, and 51 possess potent antioxidant properties. They scavenge free radicals and upregulate the expression of endogenous antioxidant enzymes like CAT, SOD, and glutathione peroxidase (GPx). This action helps reduce oxidative stress. 51, 59, and 94 induce apoptosis in cancer cells by modulating multiple pathways, including the extrinsic (death receptor) and intrinsic (mitochondrial) apoptotic pathways. They also inhibit cancer cell proliferation by arresting the cell cycle at various phases (e.g., G1, G2/M).
50, 57, and 59 exhibit hepatoprotective effects. They protect hepatocytes from damage induced by toxins, drugs, or oxidative stress. This protection is mediated through activating antioxidant defense mechanisms, inhibiting lipid peroxidation, and modulating signaling pathways involved in liver regeneration and repair. 78 and 79 have shown cardioprotective properties. They improve cardiac function and reduce myocardial damage by exerting antioxidant, anti-inflammatory, and anti-apoptotic effects. Additionally, they can improve lipid profiles and prevent the oxidation of low-density lipoprotein (LDL) and the risk of atherosclerosis by lowering cholesterol levels.
Compounds 94 and 59 exhibit broad-spectrum antimicrobial activity by disrupting microbial cell membranes, inhibiting the synthesis of vital cellular components, and interfering with microbial metabolism. 86 and 51 enhance insulin sensitivity, promote glucose uptake, and inhibit the enzymes involved in gluconeogenesis and breakdown (glycolysis), thus helping maintain blood glucose levels within normal ranges [198]. 78 also inhibit α-amylase and α-glucosidase inhibitors [199] and reduce mRNA expression levels of lipogenic genes and, as a result, inhibit lipid accumulation in HepG2 cells of obese diabetic rats [200].
Sterols
Phytosterols [55] modify prostaglandin pathways by blocking inflammatory enzymes, thus protecting platelets [201]. Compounds 108–112 reduce inflammation by inhibiting the production of COX-2 and lipoxygenase (LOX)—specifically, 109 and 111 exhibits anti-neoplastic, anti-inflammatory, and antipyretic activity in animals. A mixture of 109 and 111 improved T-lymphocyte and natural killer cell activity by testing in vitro, in animals, and in human clinical trials, targeting specific T-helper lymphocytes (Th1 and Th2 cells). Compound 108 reduces the production of NO and prostaglandins, thereby alleviating inflammation. These compounds also protect cells from oxidative damage by scavenging ROS and enhancing the activity of enzymes like CAT and SOD.
In the body, phytosterols such as 109–112 compete with cholesterol for absorption in the intestines and promote cholesterol excretion as bile acids. Saturated phytosterols are more effective than unsaturated ones in decreasing cholesterol concentrations [202, 203]. Compounds 108 and 111 inhibit the enzyme β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase involved in cholesterol synthesis [204]. Compounds 109 and 110 exhibit anticancer effects by inducing apoptosis, inhibiting cancer cell proliferation, and affecting signal transduction pathways, including phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) and mitogen-activated protein kinase (MAPK).
Compound 111 is also known for its beneficial effects on prostate health, particularly in reducing benign prostatic hyperplasia (BPH) symptoms by inhibiting 5-alpha-reductase and reducing inflammation.
Flavonoids
Flavonoids provide health benefits through modulatory effects on various metabolic and signaling enzymes beyond being direct chemical protectants [205]. They block the angiotensin-converting enzyme, which raises blood pressure, inhibit cyclooxygenase, which forms prostaglandins, and block enzymes that produce estrogen. These actions suggest that flavonoids could prevent platelet aggregation, reduce heart disease and thrombosis, and inhibit estrogen synthase, potentially decreasing the risk of estrogen-related cancers.
Hertog et al. discovered a substantial inverse relationship between consuming flavonoids and the death rate from coronary heart disease [201]. Of these, flavonol 116 is directly mutagenic and has antibacterial and anti-inflammatory properties [206]. For instance, Silva et al. [207] discovered a time-dependent biotransformation of kaempferol to quercetin by CYP in V79 cells in short-term tests such as the production of chromosomal abnormalities in eukaryotic cells. In higher plants, compound 119 is a common flavonoid that increases the mutagenicity of 116 when microsomal metabolizing systems are present. Compound 116 also suppresses the growth of rat lymphocytes, the contraction of smooth muscle, and a number of other enzymes [206].
Flavonoids act as potent antioxidants by neutralizing free radicals and ROS. They chelate metal ions like iron and copper, which catalyze the production of ROS. Flavonoids can induce the expression of endogenous antioxidant enzymes such as SOD, CAT, and GPx.
Flavonoids, such as those found in Plumeria species, i.e., 113-114, 116–123, exhibit antioxidant activity [208, 209]. 120–123 exhibit anti-inflammatory properties by modulating various inflammation-related signaling pathways and cytokines. 115–119 inhibit COX-1, COX-2, and LOX enzymes, reducing the production of pro-inflammatory mediators like prostaglandins and leukotrienes. 116–119 inhibit the activation of the NF-κB, which regulates the expression of inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6). Flavonoids modulate the MAPK pathway, influencing the production of inflammatory cytokines and enzymes.
Through activating caspases and regulating pro-apoptotic (like Bax) and anti-apoptotic (like Bcl-2) proteins, 115, 116, and 119 encourage apoptosis in cancer cells. 119 [210, 211] suppresses the growth of cancer cells by inducing cell cycle arrest at several phases (G0/G1, S, G2/M) [212]. 119 and 117 inhibit the formation of new blood vessels required for tumor growth by downregulating angiogenic factors like vascular endothelial growth factor (VEGF). Compound 116 [213, 214] prevents cancer cell migration and invasion by modulating matrix metalloproteinases (MMPs) and adhesion molecules [206].
113 and 114 protect the cardiovascular system through vasodilation by increasing the bioavailability of NO and modulating endothelial function. They inhibit platelet aggregation, reducing the risk of thrombosis. Flavonoids modulate lipid metabolism, reducing LDL cholesterol levels and preventing atherosclerosis.
Compound 115 exerts neuroprotective effects by scavenging free radicals and upregulating antioxidant [215, 216] defenses; flavonoids protect neurons from oxidative damage. It reduces neuroinflammation by inhibiting microglial activation and the release of pro-inflammatory cytokines. Flavonoids influence neurotransmitter systems (e.g., dopamine, serotonin), potentially improving cognitive function and mood.
Glycoside-rich fraction 116 [217] is associated with reducing glycosylated hemoglobin. In the experiment, hepatic fatty acid synthase decreased after receiving 116.
Alkaloids
Alkaloids like 125 and 129 may modulate neurotransmitter systems, affecting receptors such as acetylcholine, serotonin, dopamine, and norepinephrine, leading to changes in neural activity and potentially resulting in neuroprotective or psychoactive effects. Compounds 130, 132–134 may exert their effects by disrupting bacterial and fungal cell membranes or interfering with essential enzymatic processes, leading to cell death [151, 152].
Compounds 124, 127, and 131–134 may exhibit cytotoxicity against cancer cells by inducing apoptosis (programmed cell death) through mitochondrial pathways or disrupting cell division. It might inhibit specific enzymes in cancer cell proliferation or survival, contributing to its anticancer effects [138].
Compounds 125 and 127 may interfere with the life cycle of malaria parasites, possibly by inhibiting essential enzymes or disrupting cellular structures. Compound 131 might act on cardiovascular systems, potentially affecting heart rate and blood pressure through interactions with cardiac ion channels or neurotransmitter receptors.
Compounds 126 and 130 may act on neurons’ pain receptors or sodium channels, providing pain relief or local anesthesia. It might inhibit inflammatory pathways, reducing pain and swelling. An alkaloid was shown by Sharma et al. to have antidiabetic effects in diabetic mice by enhancing GLUT-4, glucokinase activity, and peroxisome PRAYγ [217].
Cardiac glycosides
Cardiac glycosides, such as 135 and 136 found in Plumeria species, primarily affect the heart. Cardiac glycosides cause sodium ions to build up inside heart muscle cells (myocytes) by blocking the Na+/K+-ATPase pump, which is found in the cell membrane. Usually, this pump causes a secondary inhibition of the Na+/Ca2+ exchanger by pumping potassium and calcium ions into the cell against their concentration gradients and sodium ions out of the cell. As a result, there is an increase in intracellular calcium levels (Ca2+). Elevated intracellular calcium levels increase cardiac muscle fibers’ contractility (positive inotropic effect) so the heart can contract more forcefully with each beat.
Cardiac glycosides also affect the autonomic nervous system, particularly by increasing parasympathetic (vagal) tone, which can slow heart rate (negative chronotropic effect). They may also enhance cardiac output and improve symptoms in certain heart conditions [79,80,81].
Cardiac glycoside isolated from Iranian folk medicine also mediates its hypoglycaemic activity by increasing secretion [218] While cardiac glycosides can be beneficial in medical use for conditions like heart failure, they have a narrow therapeutic window, and overdose can lead to toxicity, characterized by arrhythmias, nausea, vomiting, and potentially fatal cardiac effects.
Quinones
Quinones are highly reactive due to their ability to accept and donate electrons. They can undergo reversible redox reactions, cycling between quinone (oxidized) and hydroquinone (reduced) forms. Quinones can undergo one-electron reduction to form semiquinone radicals and then further reduction to hydroquinones. During these processes, ROS, such as hydrogen peroxide (H2O2), superoxide anion radicals (O2−), and hydroxyl radicals (OH•), can be generated as byproducts.
Notably, the compound 2,5-dimethoxy-p-benzoquinone was the inaugural benzoquinone extracted from the Plumeria genus. Its cytotoxic effects appear to be previously unreported [86].
Some quinones can inhibit enzymes by covalently modifying their active sites or essential cysteine residues, which affect various cellular processes dependent on those enzymes. Despite their potential to generate ROS, quinones can also act as antioxidants under certain conditions. Quinones can induce cytotoxicity [131] and exhibit anti-inflammatory properties and antimicrobial activity against various pathogens.
Nor-terpenoids
Nor-terpenoids such as 139 and 140 can interact with cellular signaling pathways, influencing gene expression, protein synthesis, and cell function. Depending on the specific pathways affected, this modulation can lead to diverse physiological responses.
They inhibit specific enzymes involved in biochemical pathways. For example, they may interfere with enzymes related to lipid metabolism, cell signaling, or neurotransmission, impacting cellular functions and physiological processes. Nor-terpenoids also exhibit anti-inflammatory, antioxidant, neuroprotective, antimicrobial, antifungal, antiproliferative, and anticancer properties.
Anthocyanins
Anthocyanins are potent antioxidants [88, 93, 219, 220], and some anthocyanins can chelate transition metals (e.g., iron and copper) that catalyze the production of ROS, thereby reducing their harmful effects. Anthocyanins may improve endothelial function by enhancing NO production, which helps dilate blood vessels, regulate blood pressure, and promote cardiovascular health. Anthocyanins exhibit antidiabetic activity by inhibiting α-glucosidase and pancreatic α-amylase [221]. Anthocyanins can have anticancer [219] and neuroprotective properties [88,89,90,91]. Additionally, they have been recognized as antibacterial modulators [92].
Cardenolides
Since the 1980s, cardenolide has been used to treat heart failure and blood vessel arrhythmias [77, 100]. Due to their ability to regulate the life and death of cancer cells at the molecular and cellular levels through numerous signaling pathways, research on these compounds within cancer prevention and treatment has attracted significant consideration [101, 102].
Several cardenolides in N. oleander’s leaves make up the most significant amount of oleandrin [103, 104]. It is reported in Nerium oleander, Nerium odorum, Beaumontia grandiflora, Thevetia peruviana, and P. obtuse for its high anticancer activity. Therefore, the search for biological sources where these substances could be extracted in significant quantities has increased because of several targets that may be affected by oleandrin or Cardenolides [101]. Several investigations have been carried out to clarify the mechanism of action of oleandrin on cancer cells [105].
Fatty acid esters
Fatty acid esters, particularly those with phenolic groups like ferulates (144 and 145), may exhibit antioxidant properties [211]. Some fatty acid esters, such as those containing 147, may have anti-inflammatory and anticancer properties.
146 [169] may influence lipid metabolism by affecting lipid synthesis, storage, or cell breakdown, causing metabolic disorders like obesity and dyslipidemia.
Some fatty acid esters can exhibit antimicrobial and antifungal effects. Fatty acid esters, particularly those with moisturizing or emollient properties, like 147, may benefit skin health. They can help maintain skin barrier function, improve hydration, and have wound-healing effects.
Lignans
Lignans possess antioxidant properties. Some lignans, including 148, have been found to modulate inflammatory pathways. Depending on the context, lignans can interact with estrogen receptors in the body, exerting estrogenic or anti-estrogenic effects. They may act as weak agonists or antagonists of estrogen receptors, influencing hormone balance and offering benefits in conditions influenced by estrogen levels.
Specific lignans have demonstrated antimicrobial and anticancer [132] activities. Lignans may contribute to cardiovascular health. Furthermore, bioassays of lignans have revealed critical pharmacological activities, including anticancer, anti-inflammatory, immunosuppressive, cardiovascular, antioxidant, and antiviral activities [108,109,110,111,112,113,114,115].
Coumarins
Coumarin is a phytochemical with several biological and therapeutic properties such as antibacterial, antiviral [116], antidiabetic [117], anticoagulant, [118, 119] estrogenic, skin sensitizing, vasodilators, molluscacidal, sedatives and hypnotic, analgesic, antipyretic [120, 121] and anticancer properties [122, 123]. In addition, the antioxidant, antiparasitic, antihelminthic, antiproliferative, anticonvulsant, anti-inflammatory [124], and antihypertensive [120, 121, 125, 126] activities of some coumarins also have been studied by several researchers.
Some coumarins, including scopoletin, exhibit anti-inflammatory properties by inhibiting pro-inflammatory enzymes (e.g., LOX, COX-2) and cytokines (e.g., TNF-α, IL-6). Coumarins can interact with various enzymes involved in metabolic pathways, such as those related to drug metabolism, detoxification, and cellular signaling.
Biotransformation of compounds extracted from various species of Plumeria
The bioactive compounds undergo various biotransformation processes in the body, categorized into two phases. Phase I reactions usually include hydrolysis, reduction, and oxidation. Functional groups, such as hydroxyl groups, are introduced or exposed by these events, increasing the polarity and frequently the reactivity of the molecules for phase II reactions that follow. Enzymes like cytochrome P450 oxidases (CYPs), which are essential for the oxidation of substances, aid in the oxidation process. CYPs are linked enzyme systems comprising the NADPH-containing cytochrome P450 reductase (CPR) and the heme-containing CYP [222, 223]. (Fig. 15) Hydroxylation involves the introduction of hydroxyl groups to form hydroxy derivatives. Epoxidation leads to the formation of epoxides, which can further react to form diols. Dehydrogenation converts hydroxyl groups to ketones, forming keto derivatives. Reduction reactions, in which certain iridoids undergo reduction, convert ketone or aldehyde groups to hydroxyl groups. Reduction of double bonds transforms double bonds to single bonds, and carbonyl reduction converts ketones or aldehydes to alcohols. Hydrolysis, mediated by esterases and other hydrolytic enzymes, cleaves ester bonds, forming corresponding alcohol and acid derivatives. Table 2 shows examples of oxidation reactions catalyzed by CYP [224,225,226,227].
Phase II reactions involve conjugating compounds or their phase I metabolites with endogenous substrates, increasing their water solubility and promoting excretion through urine or bile. Glucuronidation, mediated by UDP-glucuronosyltransferases (UGTs), is a significant phase II pathway for compounds. Some typical examples are shown in Table 3 [228]. Sulfation involves sulfotransferases (SULTs) catalyzing the conjugation of sulfate groups to hydroxyl or amine groups, increasing solubility. The resulting products are sulfate esters of sulfamates (Table 3). Methylation involves transferring a methyl group to hydroxyl or amino groups, mediated by methyltransferases. Acetylation is catalyzed by acetyltransferases, which transfer acetyl groups to amine or hydroxyl groups of compounds. Glycosylation involves the conjugation with glucose or other sugars, often forming glycosides. Glutathione conjugation involves the conjugation with glutathione, usually catalyzed by glutathione S-transferases (GSTs). The conjugated moieties are often identified by specific active transport mechanisms, facilitating their movement across plasma membranes and increasing their excretion rate [229]. Table 4 includes specific examples of the biotransformation of compounds in the genus Plumeria.
Glucuronides and sulfates are typically excreted via the kidneys, whereas some metabolites may undergo enterohepatic circulation and be excreted through bile. This metabolic processing is crucial for the pharmacokinetics and overall bioavailability of compounds in the body.
The liver is the primary site of biotransformation enzyme localization. The liver receives a substantial portion of blood from the splanchnic area, which contains xenobiotics that have been absorbed from the gut. As a result, most of these compounds can now be modified enzymatically by the liver. Nevertheless, biotransformation reactions can also be catalyzed by other tissues. The chemical structure, dosage, and mode of delivery are some variables that affect how much an extrahepatic organ contributes to biotransformation. Critical toxicological ramifications can result from biotransformation within extrahepatic tissues, which can have toxic effects unique to those tissues.
Phase I enzymes are primarily located in the lipoprotein membranes that connect the nucleus and mitochondria to the plasma membrane and the endoplasmic reticulum within cells. Phase I enzyme localization in membranes is important because lipophilic compounds tend to collect in lipid membranes, which can result in high concentrations of lipophilic xenobiotics at the biotransformation sites. Biotransformation processes primarily serve the function of detoxification by converting xenobiotics into more readily excreted compounds. However, metabolites more dangerous than the original substance can arise, depending on the chemical structure and the implicated enzymes. Numerous xenobiotics with minimal chemical reactivity are hazardous and carcinogenic due to a process called bioactivation. Cell death, cancer, organ failure, and other toxic symptoms can result from the interaction of hazardous metabolites. Phase II reactions and mixtures of both phases can also contribute to intoxication, even though phase I reactions are more frequently linked to the production of reactive and more hazardous metabolites [230,231,232].
Iridoids
Compounds 1–4, 21–22, 13, 15, 27–29, 32, 39, and 44 can be hydrolyzed to corresponding alcohols and then subjected to glucuronidation or sulfation. 5 undergoes oxidation to form more polar metabolites. 8–9, 11, 16–19, 30, and 40 Oxidize and then conjugate through glucuronidation or sulfation. Compound 10 can be formed through reduction of 8, further conjugated via glucuronidation. 12, 14, 20, 23, and 43 undergo conjugation via glucuronidation or sulfation. 42 undergoes further methylation, hydroxylation, and conjugation. Esterases and other hydrolytic enzymes cleave ester bonds in iridoids such as 6 and 7, forming corresponding alcohol and acid derivatives. For instance, compound 8 can undergo glucuronidation to form plumericin glucuronide, which is more water-soluble and readily excreted. Compounds like 6 and 7 can be further modified by acetylation, affecting their pharmacokinetic properties.
Triterpenoids
Functionalization through polyhydroxylation of native pentacyclic triterpenoids is one of the most promising ways to improve their pharmacological potential. These hydroxylated derivatives are widely distributed in nature, but they are usually found in trace amounts or as components of complicated mixtures. They are formed by plant P450-dependent monooxygenases [233]. Microorganisms’ ability to convert pentacyclic triterpenoids enzymatically produces hydroxylated derivatives with a high yield and regioselectivity. Not only can microbial hydroxylation happen in the A ring, but it can also happen at difficult locations on the B, D, and E rings. Moreover, though they are less common, processes including carboxylation, glycosylation, lactone production, and others might contribute to the microbial functionalization of pentacyclic triterpenoids.
59 can be hydroxylated to form 99. For instance, 75 may undergo epoxidation at the double bond. Reduction of double bonds to single bonds, such as reducing lupeol to dihydrolupeol. The reduction of betulinic acid’s carbonyl group to form 50.
Compound 59 can be conjugated to form ursolic acid glucuronide. Triterpenoids like 75 can form sulfated metabolites. For example, 94 can be glycosylated to form lupeol glucoside. GSTs usually catalyze conjugation with glutathione.
De Carvalho et al. (2006) discussed the opportunities and challenges in the industrial-scale biotransformation of monoterpenes [234]. Research on using directed biotransformation to produce novel glycyrrhetinic acid (GA), oleanolic acid (OA), and ursolic acid (UA) derivatives has demonstrated potential. More than 20 instances of these chemicals’ biotransformation utilizing bacterial and fungal cultures—mainly hydroxylation—have been reported since 2013. Less often described processes include deeper oxidation, glycosylation, esterification, acetylation, or carboxylation of triterpenoids. Only a few instances of the biocatalytic synthesis of triterpenic lactones or their derivatives with broken C–C bonds utilizing UA have been documented [235, 236].
Triterpenic acid conversion rates in fungal biotransformation processes normally vary from 2.6 to 77.5%, starting at concentrations of 0.02 g/L–1.0 g/L. The conversion rates of bacteria range from 27.5 to 70.0%, and their starting concentrations range from 0.04 g/L to 0.3 g/L. Derivatives with antioxidant, anti-inflammatory, antiviral, anticancer, antiparasitic, antibacterial, neuroprotective, and hepatoprotective qualities have been produced through the biotransformation of the pentacyclic triterpenoids oleanane and ursane.
Additional research into this multidisciplinary approach may yield powerful antibacterial medications to combat microorganisms resistant to antibiotics and novel therapeutic medicines for cancer and neurological disorders. Through the process of converting native pentacyclic triterpenoids into bioavailable chemicals and back again, industrial microbiology has the potential to establish a beneficial cycle of production for biologically active substances.
However, significant drawbacks exist with the microbial catalysts used. Fungi typically exhibit mycelial growth and produce spores and mycotoxins, while the few bacterial catalysts described are often pathogenic strains. Therefore, further studies are needed to explore the biological transformation processes of pentacyclic triterpenoids and to find new non-pathogenic bacterial strains capable of effectively synthesizing triterpene derivatives with notable biological activities.
Sterols
Compounds 108 and 110–112 undergo oxidation primarily in the liver, where CYP introduces hydroxyl groups at specific positions on the sterol nucleus or side chain. They may undergo dehydrogenation, converting hydroxyl groups to ketones, forming keto derivatives. 109 undergoes enzymatic hydrolysis to remove the glucose moiety, yielding free 111. Enzymes such as β-glucosidase hydrolyze the glucoside bond, releasing 111. The hydroxylated or ketone metabolites of 108 and 110–112 undergo conjugation reactions to increase their solubility.
Flavonoids
The biotransformation of flavonoids from Plumeria species involves metabolic reactions in phases I (oxidation, reduction, hydrolysis) and II (glucuronidation, sulfation, methylation).
CYP enzymes, such as CYP1A1, CYP1A2, and CYP3A4, oxidize flavonoids, i.e., 119, 115-116, 123, to form hydroxylated metabolites. For example, 119 can be oxidized to quercetin-3′-O-glucuronide. Some flavonoids undergo reduction reactions, which are less common compared to oxidation. Esterases can hydrolyze flavonoid glycosides into their aglycone forms and sugar moieties. For instance, 118 can be hydrolyzed to 119. In 114 and 118, esterases hydrolyze it to release 116, rutinose, and 119 and rhamnose, respectively. Both phytochemicals then undergo phase I and II metabolism, as described above.
By UGTs catalysis, 119 can be conjugated to form quercetin-3-O-glucuronide. 119 is commonly glucuronidated at the 3, 4’, or 7 positions. 116 is glucuronidated, primarily at the 3 and 7 positions. 115 and 123 Likely conjugated with glucuronic acid and sulfate. With the help of SULTs, 119 can be sulfated to quercetin-3-O-sulfate—catechol-O-methyltransferases (COMTs) methylate hydroxyl groups on flavonoids. Compound 119 can be methylated to isorhamnetin (3′-O-methyl quercetin).
The gut microbiota also plays a significant role in flavonoid metabolism. Microbial enzymes can do de-glycosylation, ring cleavage, and reduction. These processes occur mainly in the liver and gastrointestinal tract, with significant contributions from the gut microbiota.
Alkaloids
124, 132–134, 127, and 129 may undergo oxidation by CYP enzymes, introducing hydroxyl groups to increase their polarity. The resulting metabolites can be conjugated with glucuronic acid or sulfate to enhance solubility for excretion.
Compound 130 may undergo dealkylation, hydroxylation, or demethylation by CYP enzymes, producing more polar metabolites. Metabolites from phase I are often conjugated with glucuronic acid, sulfate, or glutathione, facilitating renal or biliary excretion. Compound 125 might be reduced or hydrolyzed to form simpler metabolites. The metabolites can be further conjugated with polar molecules like glucuronic acid.
Compound 131 may be metabolized by hydroxylation or dealkylation. Compound 126 can undergo oxidation or reduction to form more polar metabolites. Resulting metabolites are often conjugated with glucuronic acid or sulfate, enhancing excretion through urine or bile.
Cardiac glycosides
The biotransformation of cardiac glycosides typically involves several steps within the body, often in the liver. After ingestion, cardiac glycosides are absorbed from the gastrointestinal tract into the bloodstream. They are transported via the bloodstream to various tissues, including the liver, where much of the biotransformation occurs.
The biotransformation of cardiac glycosides involves Phase I and II metabolic processes, primarily mediated by liver enzymes. The specific pathways and enzymes involved may vary depending on the chemical structure, but the general mechanisms include oxidation, reduction, hydrolysis, and conjugation.
Quinones
The biotransformation of quinones, such as 137 and 138 found in Plumeria species, involves several enzymatic processes, primarily in the liver.
CYP enzymes in the liver catalyze oxidation reactions, converting quinones into hydroquinones or other oxidized forms. This process can involve introducing hydroxyl (-OH) or different functional groups. Quinones can undergo either enzymatically or non-enzymatically reduction reactions to form hydroquinones. Some quinones may undergo hydrolysis reactions, resulting in the cleavage of chemical bonds and the formation of smaller molecules. The metabolites produced in phase I reactions are conjugated with endogenous molecules such as glucuronic acid, sulfate, or amino acids (e.g., glycine).
Biotransformation of other classes of compounds
The biotransformation of nor-terpenoids, anthocyanins, cardenolides, fatty acid esters, lignans, and coumarins found in Plumeria species involves enzymatic processes primarily in the liver. The general process of their biotransformation is same as followed by quinones (Table 4).
Result and discussion
The study delved into the Apocynaceae family, specifically focusing on the genus Plumeria, renowned for its traditional medicinal use and empirical research support [1,2,3,4,5,6,7,8]. Plumeria species have multifaceted applications addressing conditions from skin treatments to respiratory ailments and postnatal care [15,16,17,18,19,20,21,22,23,24,25].Their pharmacological effects span antioxidants [57, 65], antiviral actions [17], CNS depression, and antitumor potential [128]. Plumeria encompasses diverse deciduous shrubs and lactiferous trees, primarily found in tropical regions.
Out of 274 research articles cited, a total of 129 articles containing 156 phytochemicals, including 45 iridoids, 62 triterpenes, 11 flavonoids, 11 alkaloids, steroids, cardiac glycosides, quinones, nor-terpenoids, anthocyanins, cardenolides, fatty acid esters, lignans, coumarins, and others have been displayed in the search results (Table 1).
These compounds exhibit diverse pharmacological activities, including anti-inflammatory, anticancer, hypolipidemic, and antianaphylaxis effects [39, 120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214, 237,238,239,240,241,242]. Their structures (Fig. 4) and properties have been meticulously analyzed and organized in a tabular format (Table 1). Aerial parts, bark [130, 143], flowers [32, 129, 136, 144, 145], fruits [69], leaves [169], stem, stem bark [132, 142, 159, 160], root [165], whole plants, and heartwood were among the Plumeria species parts that were extracted, according to the literature. However, the extraction of phytochemicals with biological activity was regularly carried out using the stem bark [132, 142], root bark [165], and leaves [127, 139]. The genus Plumeria has several species with potential medical uses. This study explains the main bioactive phytoconstituents that have been separated from this genus Plumeria and how they are used in various traditional medical systems to treat different illnesses such as diarrhea, venereal disease, blennorhea, rheumatism, and leprosy [30]. Thephytoconstituents of genus Plumeria are excellent analgesics [2], antioxidants [128, 173, 179, 184], anticancer, antibacterial [150, 239, 240], and anti-hyperproliferative agents [135, 243], among other properties. Numerous phytochemical components of the terpenoid, iridoid, alkaloid, and other classes are present in the plants [9, 12]. The pharmacological activities of extracts obtained from various parts of plants, varied chemical constituents, their distribution, structure, the solvents used for extraction (primarily petroleum ether, chloroform, and methanol), physical properties, and the analytical data (mass, IR, and UV spectroscopical data) of phytoconstituents of the genus Plumeria are all summarised in this review.
In addition to these findings, the review also explores the general mechanisms of action and biotransformation [244] of the phytoconstituents found in Plumeria species. The bioactive compounds interact with various biological targets, leading to their diverse pharmacological effects. [244] For instance, triterpenoids [198] and iridoids [200] have been identified as key agents in anticancer and anti-inflammatory activities through their ability to modulate signaling pathways and inhibit specific enzymes. The biotransformation processes, including metabolic pathways and enzymatic modifications, contribute significantly to the bioavailability and therapeutic efficacy of these compounds [244]. Understanding these mechanisms provides deeper insights into the therapeutic potential and optimization of Plumeria-derived compounds in pharmaceutical applications.
While several comprehensive review articles discuss the phytochemicals of the genus Plumeria, this review aims to provide an overview of their distribution, structure, pharmacological properties, and analytical studies. Additionally, it delves into the potential pharmaceutical applications of compounds isolated from Plumeria species, shedding light on their prospects in medicine.
Conclusion
The therapeutic potential of various species of the genus Plumeria is quite broad. The essential bioactive phytoconstituents that were extracted from this genus and their functions in various traditional medical fields to treat various ailments are discussed in this review. Phytoconstituents of the genus Plumeria work as very effective analgesic, anticancer, antibacterial, anti-hyperproliferative, and antioxidant. The plants include several terpenoids, iridoids, steroids, fatty acid esters, coumarins, anthocyanins, cardenolides, lignans and alkaloids etc. as phytochemical components. The following review summarizes the phytochemical constituents, obtained from the extracts of various species of genus Plumeria, analytical data and their pharmacological properties.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- BPH:
-
Benign prostatic hyperplasia
- CAT:
-
Catalase
- CCl4 :
-
Carbon tetrachloride
- CHCl3 :
-
Chloroform
- CNS:
-
Central Nervous System
- COX-2:
-
Cyclooxygenase-2
- CPR:
-
Cytochrome P450 reductase
- CYP:
-
Cytochrome P450
- CYPs:
-
Cytochrome P450 oxidases
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- EAC:
-
Ehrlich ascites carcinoma
- GA:
-
Glycyrrhetinic acid
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- GSTs:
-
Glutathione S-transferases
- HCT:
-
Human Colorectal Carcinoma
- HepG:
-
Hepatoblastoma
- HIV-1 RT:
-
human immunodeficiency virus type I- reverse transcriptase
- HMG-CoA:
-
β-Hydroxy β-methylglutaryl-CoA
- IC50 :
-
Half maximal inhibitory concentration
- IR:
-
Infrared spectroscopy
- LC50 :
-
Lethal concentration
- LD50 :
-
Median lethal dose
- LD90 :
-
90% lethal dose
- LDL:
-
Low-density lipoprotein
- LOX:
-
Lipoxygenase
- MAPK:
-
Mitogen-activated protein kinase
- MCF:
-
Michigan Cancer Foundation
- MEPA:
-
Methanolic extract of P. acuminata
- MIC:
-
Minimal inhibitory concentration
- MBC/MFC:
-
Minimal bactericidal or fungicidal concentration
- MMPs:
-
Metalloproteinases
- NF-κB:
-
Nuclear factor-kappa B
- NK:
-
Natural killer
- NO:
-
Nitric oxide
- OA:
-
Oleanolic acid
- P.:
-
Plumeria
- PCM:
-
Paracetamol
- PI3K/Akt:
-
Phosphatidylinositol 3-kinase/protein kinase B
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- STZ:
-
Streptozotocin
- SULTs:
-
Sulfotransferases
- UA:
-
Ursolic acid
- UGTs:
-
UDP-glucuronosyltransferases
- UV:
-
Ultraviolet spectrophotometry
- VEGF:
-
Vascular endothelial growth factor
- XO:
-
Xanthine oxidase
References
El-Kashef DF, Hamed A, Khalil HE, Kamel SM. Triterpenes and sterols of family Apocynaceae (2013–1955), a review. J Pharmacol Phytochem. 2015;4:21–39. https://doi.org/10.2139/ssrn.3776357
Shang JH, Cai XH, Feng T, Zhao YL, Wang JK, Zhang LY, et al. Pharmacological evaluation of Alstonia scholaris: anti-inflammatory and analgesic effects. J Ethnopharmacol. 2010;129:174–8. https://doi.org/10.1016/j.jep.2010.02.011
Jamal JA, Ghafar ZA, Husain K. Medicinal plants used for postnatal care in Malay traditional medicine in the Peninsular Malaysia. Pharmacogn J 2011;3:15–24. https://doi.org/10.5530/pj.2011.24.4
Erdemoglu N, Küpeli E, Yeşilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J ethnopharmacol. 2003;89:123–9. https://doi.org/10.1016/S0378-8741(03)00282-4
Ghosh S, Suryawanshi SA. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. J Exp Biol. 2001;39:748–59. http://nopr.niscpr.res.in/handle/123456789/23851
Siddiqui BS, Sultana R, Begum S, Zia A, Suria A. Cardenolides from the methanolic extract of Nerium oleander leaves possessing central nervous system depressant activity in mice. J nat prod. 1997;60:540–4. https://doi.org/10.1021/np960679d
Cutts JH, Beer CT, Noble RL. Biological properties of Vincaleukoblastine, an alkaloid in Vinca rosea Linn, with reference to its antitumor action. Cancer Res. 1960;20:1023–31. https://aacrjournals.org/cancerres/article/20/7/1023/474394/Biological-Properties-of-Vincaleukoblastine-an
Rattanapan J, Sichaem J, Tip-pyang S. Chemical constituents and antioxidant activity from the stems of Alyxia reinwardtii. Rec Nat Prod. 2012;6:288 http://acgpubs.org/doc/2018080620063539-RNP_1103-545.pdf
Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer–an extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–77. https://doi.org/10.1016/S0378-8741(00)00341-X
Geetha T, Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharmacol. 2001;76:77–80. https://doi.org/10.1016/S0378-8741(01)00175-1
Sharma G, Chahar MK, Dobhal S, Sharma N, Sharma TC, Sharma MC, et al. Phytochemical constituents, traditional uses, and pharmacological properties of the genus Plumeria. Chem Biodivers 2011;8:1357–69. https://doi.org/10.1002/cbdv.201000159
Choudhary M, Kumar V, Singh S. Phytochemical and pharmacological activity of genus Plumeria: an updated review. Int J Biomed Adv Res. 2014;5:266–71. https://doi.org/10.7439/ijbar
Devprakash TR, Gurav S, Kumar GP, Mani TT. An review of phytochemical constituents and pharmacological activity of Plumeria species. Int J Curr Pharm Res 2012;4:1–6. http://www.ijcpr.org/Issues/Vol4Issue1/449.pdf
Akhtar N, Malik A. Oleanene type triterpenes from Plumeria rubra. Phytochem. 1993;32:1523–5. https://doi.org/10.1016/0031-9422(93)85171-M
Pand RR, Mehrotra BN. Compendium of Indian Medicinal Plants. Vol 2, vol. 1. CDRI. New Delhi: Lucknow and NISCAIR; 1960. 320–2 1969
Leven M, Berghe DA, Mertens F, Vlietinck A, Lammens E. Screening of higher plants for biological activities I. Antimicrobial activity. Planta Med. 1979;36:311–21. https://doi.org/10.1055/s-0028-1097277
Vanden Berghe DA, Jeven M, Mertens F, Vlietink A, Lammens E. Screening of higher plants for biological activities. II. Antiviral activity. J Nat Prod 1978;41:463–7. https://doi.org/10.1142/S0219030307000584
Sticher O. Plant mono-, di-and sesquiterpenoids with pharmacological or therapeutical activity. In New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity: Proceedings of the First International Congress on Medicinal Plant Research, Section A, held at the University of Munich, Germany, September 6–10, 1976 1977 (pp. 137–76). Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-66682-7_6
Patil GG, Mali PY, Bhadane VV. Folk remedies used against respiratory disorders in Jalgaon district, Maharashtra. Nat Product Radiance. 2008;7:354–8. https://nopr.niscpr.res.in/handle/123456789/5703
Wiart C. Medicinal plants of Southeast Asia. Kuala Lumpur: Prentice Hall. Pearson Malaysia Sdn. Bhd; 2002. p. 524–45. https://www.cabdirect.org/cabdirect/abstract/20046798394
Peckolt G. Brazilian anthelmintic plants. Rev Flora Med. 1942;9:333.
Jarald E, Joshi SB, Jain D. Diabetes and herbal medicines. Iran. J Pharmacol Ther. 2008; 97–106. https://www.sid.ir/en/vewssid/j_pdf/101020080119.pdf
Andrade-Cetto A, Heinrich M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol. 2005;99:325–48. https://doi.org/10.1016/j.jep.2005.04.019
Ruiz-Terán F, Medrano-Martínez A, Navarro-Ocaña A. Antioxidant and free radical scavenging activities of plant extracts used in traditional medicine in Mexico. Afr J Biotechnol. 2008;7:1886–93. https://doi.org/10.5897/AJB2008.000-5034
Datta Q, Datta S. PC. Bark drugs of Plumeria. Q J Crude Drug Res 1976;14:129–42. https://doi.org/10.3109/13880207609081913
Sawhney AN, Khan MR, Ndaalio G, Nkunya MHH, Wevers H. Studies on the rationale of African traditional medicine, Part III. Preliminary screening of medical plants for antifungal activity. Pak J Sci Ind Res 1978;21:193–6. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL8050176954
Quisumbing E. 1951 Medicinal plants of the Philippines. Department of Agriculture and Commerce, Philippine Islands Technical Bulletin. (16). https://www.cabdirect.org/cabdirect/abstract/20057007481
Wasuwat S. A list of Thai medicinal plants. ASRCT Research Report. 1967. https://cir.nii.ac.jp/crid/1573950399128916352
Nandkarni KM. Indian Meteria Medica, vol. 1. Bombay: Popular Prakashan; 1976. p. 993.
Basavaraju R, Raj JV, Bhiravamurthy PV. Medicinal plant resources of Puttaparthi Mandal: Taxonomic overview and need for conservation. Ethnobotanical Leafl. 2009;2009:6. https://opensiuc.lib.siu.edu/ebl/vol2009/iss11/6
Sharief MU, Kumar S, Diwakar PG, Sharma TV. Traditional phytotherapy among karens of middle Andaman. Indian J Traditional Knowl. 2005;4:429–36. http://nopr.niscpr.res.in/handle/123456789/8533
Zaheer ZA, Konale AG, Patel KA, Khan SU, Ahmed RZ. Comparative phytochemical screening of flowers of Plumeria alba and Plumeria rubra. Asian J Pharm Clin Res. 2010;3:88–9.
Nguyen-Pouplin J, Tran H, Tran H, Phan TA, Dolecek C, Farrar J, et al. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J Ethnopharmacol. 2007;109:417–27. https://doi.org/10.1016/j.jep.2006.08.011
Gaitán I, Paz AM, Zacchino SA, Tamayo G, Giménez A, Pinzón R, et al. Subcutaneous antifungal screening of Latin American plant extracts against Sporothrix schenckii and Fonsecaea pedrosoi. Pharma boil. 2011;49:907–19. https://doi.org/10.3109/13880209.2011.555916
Verma C, Bhatia S, Srivastava S. Traditional medicine of the Nicobarese. Indian J Tradit Knowl. 2010;9:779–85. http://nopr.niscpr.res.in/handle/123456789/10336
Warrier PK. Indian medicinal plants: a compendium of 500 species. Orient Blackswan. Orient Longman. 1993;4:329 https://books.google.com/books?hl=en&lr=&id=y3_vZIUVVj8C&oi=fnd&pg=PA1&ots=ntAni2H-6h&sig=XFa8NzDCQlVhR4qdGbl4yM3ByHk
Rajakumar N, Shivanna MB. Traditional herbal medicinal knowledge in Sagar taluk of Shimoga district, Karnataka, India. Indian J Nat Prod Resour. 2010;1:102–8. http://nopr.niscpr.res.in/handle/123456789/7704
Kalita JC, Chakrabarty A, Tanti B. Assessment of Antifertility activity of some traditionally used plants by different ethnic communities in three districts of Assam, India. J Herb Med Toxicol 2011;5:65–72.
El-Naggar LJ, Beal JL. Iridoids. A review. J Nat Prod 1980;43:649–707. https://doi.org/10.1021/np50012a001
Boros CA, Stermitz FR. Iridoids. An Updated Review. Part I. J Nat Prod 1990;53:1055–147. https://doi.org/10.1021/np50071a001
Inouye H, Jaenicke L, Lounasmaa M, Marner FJ, Séquin U, Somersalo P, et al. Biosynthesis of iridoids and secoiridoids. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. 1986:169-236. https://doi.org/10.1007/978-3-7091-8888-0_5
Braekman JC, Daloze D, Franzyk H, Leclercq S, PasteeIs JM, Franzyk H. Synthetic aspects of iridoid chemistry. Fortschritte der Chemie organischer Naturstoffe/progress in the chemistry of organic natural products. 2000:1-14. https://doi.org/10.1007/978-3-7091-6341-2_1
Hussain H, Green IR, Saleem M, Raza ML, Nazir M. Therapeutic potential of iridoid derivatives: patent review. Inventions. 2019;4:29 https://doi.org/10.3390/inventions4020029
Khera S, Woldemichael GM, Singh MP, Suarez E, Timmermann BN. A Novel Antibacterial Iridoid and Triterpene from Caiophora coronata. J Nat prod 2003a;66:1628–31. https://doi.org/10.1021/np030314a
Liblikas I, Santangelo EM, Sandell J, Baeckström P, Svensson M, Jacobsson U, et al. Simplified isolation procedure and interconversion of the diastereomers of nepetalactone and nepetalactol. J Nat prod. 2005;68:886–90. https://doi.org/10.1021/np049647d
Little JE, Johnstone DB. Plumericin: an antimicrobial agent from Plumeria multiflora. Arch Biochem. 1951;30:445–52.
Gousiadou C, Gotfredsen CH, Matsa M, Hadjipavlou-Litina D, Skaltsa H. Minor iridoids from Scutellaria albida ssp. albida. Inhibitory potencies on lipoxygenase, linoleic acid lipid peroxidation and antioxidant activity of iridoids from Scutellaria sp. J Enzym Inhib Med Chem 2013;28:704–10. https://doi.org/10.3109/14756366.2012.672415
Agarwal SK, Rastogi RP. Triterpenoid saponins and their genins. Phytochem. 1974;13:2623–45. https://doi.org/10.1016/0031-9422(74)80217-7
Chandel RS, Rastogi RP. Triterpenoid saponins and sapogenins: 1973–1978. Phytochem. 1980;19:1889–908. https://doi.org/10.1016/0031-9422(80)83001-9
Price KR, Johnson IT, Fenwick GR, Malinow MR. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit Rev Food Sci Nutr. 1987;26:27–135. https://doi.org/10.1080/10408398709527461
Cai P. Advances in structure elucidation of triterpenoid saponins. Yaoxue Tongbao. 1982;17:668–74.
Adler C, Hiller K. Bisdesmosidic triterpene saponins. Pharmazie. 1985;40:676. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=8578153
Hiller K, Adler C. New findings on triterpene saponins. A review. Die Pharmazie. 1982;37:619–34. https://europepmc.org/article/med/6755497
Chaieb I. Saponins as insecticides: a review. Tunis J Plant Prot. 2010;5:39–50. https://www.academia.edu/download/53840293/saponins_as_insectiside.pdf
Ju YH, Clausen LM, Alrd KF, Almada AL, Helferich WG. β-sterol, β-sitosterol glucoside and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and ovariectomized athymic mice. J Nutr. 2004;134:1145–51. https://doi.org/10.1093/jn/134.5.1145
Fernandez SP, Wasowski C, Loscalzo LM, Granger RE, Johnston GAR, Paladini AC, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharm. 2006;539:168–76. https://doi.org/10.1016/j.ejphar.2006.04.004
Heim KE, Tagliaferro AR, Bobliya DJ. Flavonoids antioxidants: Cemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002;13:572–84. https://doi.org/10.1016/S0955-2863(02)00208-5
Hollman PH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food chem Toxicol. 1999;37:937–42. https://doi.org/10.1016/S0278-6915(99)00079-4
Cushnie TT, Lamb AJ. Antimicrobial activity of flavonoids. Inter J Antimicrobial agents. 2005;26:343–56. https://doi.org/10.1016/j.ijantimicag.2005.09.002
Murray MT. Quercetin: Nature’s antihistamine. Better Nutrition 1998. NTP Technical Report (no.409) on the toxicology and carcinogenesis studies of quercetin in F344/N rats. NIH Publication No. 91-3140. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; 1991.
Dixon RA, Dey PM, Lamb CJ. Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat areas Mol Biol. 1983;55:1–36. https://doi.org/10.1002/9780470123010.ch1
Harborne JB. The flavonoids- Advances in Research Since 1980. ed 1. London: Chapman and Hall; 1988.
Middleton E. The flavonoids. Trends Pharm Sci. 1984;5:335–38.
Kuhnau J. The flavonoids. A class of semiessential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–91. https://doi.org/10.1159/000399407
Pietta PG. Flavonoids as antioxidants. J nat prod 2000;63:1035–42. https://doi.org/10.1021/np9904509
Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Bio Med. 1996;20:933–56. https://doi.org/10.1016/0891-5849(95)02227-9
Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J med food. 2005;8:281–90. https://doi.org/10.1089/jmf.2005.8.281
He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev food sci tech 2010;1:163–87. https://doi.org/10.1146/annurev.food.080708.100754
Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–35. https://doi.org/10.1016/j.foodchem.2010.12.026
Mills S, Bone K. Principles and Practice of Phytotherapy–Modern Herbal Medicine. New York: Churchill Livingstone; 2000. p. 31–34.
Abed El Aziz M, Ashour A, Madbouly H, Melad AS, El Kerikshi K. Investigations on green preparation of heavy metal saponin complexes. J Water Environ Nanotechnol. 2017;2:103–11. https://doi.org/10.22090/jwent.2017.02.005
Ahmed S. Isolation and structural elucidation of chemical constituents from fumaria indica, ferula oopoda and withania somnifer. Doctorate Thesis. International center for chemical researche, university of Karachi 1998: 10-25. http://142.54.178.187:9060/xmlui/handle/123456789/7752
Roberts MF, Wink M. Alkaloids: Biochemistry, ecology, and medicinal applications. New York, USA: Plenum Press; 1998. p. 1–7. https://books.google.com/books?hl=en&lr=&id=TMEGCAAAQBAJ&oi=fnd&pg=PA1&dq=Roberts+MF,+Michael+Wink.+Alkaloids:+Biochemistry,+ecology,+and+medicinal+applications.+Plenum+Press,+New+York,+USA,+1998:+1-7.&ots=p7w69NlRHr&sig=I4EupFtUobzMJOCAeQq6NtJ8xys
Akhtar N, Malik A, Ali SN, Kazmi SU. Proceragenin, an antibacterial cardenolide from Calotropis procera. Phytochem. 1992;31:2821–24. https://doi.org/10.1016/0031-9422(92)83639-G
Brüschweiter F, Stöckel K, Reichstein T. Supposed partial structure of Calotropis Glycosides. Glycosides and aglycones. Helvetica Chim Acta. 1969;52:2276–303.
Crout DH, Curtis RF, Hassall CH, Jones TL. The cardiac glycosides of Calotropis procera. Tetrahedron Lett. 1963;4:63–7. https://doi.org/10.1016/S0040-4039(01)90578-7
Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926–35. https://doi.org/10.1038/nrd2682
Shaker KH, Morsy N, Zinecker H, Imhoff JF, Schneider B. Secondary metabolites from Calotropis procera (Aiton). Phytochem Lett 2010;3:212–6. https://doi.org/10.1016/j.phytol.2010.07.009
Haynes GS. The pharmacological action of Digitalis, Strophanthus, and Squill on the heart. Biochemical J. 1906;1:62–87. https://doi.org/10.1042/bj0010062
Kelly RA. Cardiac glycosides and congestive heart failure. Am J Cardio. 1990;65:10–16. https://doi.org/10.1016/0002-9149(90)90245-V
Farnsworth NF. Biological and phytochemical screening of plants. J Pharma‐ Ceutical Sci. 1966;55:225–76.
IUPAC (1997), Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”). Online corrected version: (2006–) “Quinones”. https://doi.org/10.1351/goldbook.Q05015
^ Patai, Saul; Rappoport, Zvi, eds. (1988). The Quinonoid Compounds: Vol. 1 (1988). https://doi.org/10.1002/9780470772119
^ Patai, Saul; Rappoport, Zvi, eds. (1988). The Quinonoid Compounds: Vol. 2 (1988). https://doi.org/10.1002/9780470772126
Crookes W. The Chemical News and Journal of Physical Science. Griffin: Bohn and Company; 1870. https://books.google.com/books?hl=en&lr=&id=jFDJuXhWjnkC&oi=fnd&pg=PA1&dq=85.%09%5E+The+Chemical+News+and+Journal+of+Physical+Science.+Griffin,+Bohn+and+Company.+1773.&ots=g3DvwIl5Yg&sig=ueqvoydgsaFeWzRzGVm41QJZsP4
Glover BJ, Martin C. Anthocyanins. Curr Biol. 2012;22:R147–50. https://doi.org/10.1016/j.cub.2012.01.021
Pina F, Oliveira J, de Freitas V. Anthocyanins and derivatives are more than flavylium cations. Tetrahedron. 2015;71:3107–14. https://doi.org/10.1016/j.tet.2014.09.051
Wallace T, Giusti M. Anthocyanins in Health and Disease. New York, NY, USA: CRC Press; 2014. ISBN 978-1-4398-9471-2
Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, et al. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2017;8:684–93. https://doi.org/10.3945/an.116.014852
Fernandes I, Pérez-Gregorio R, Soares S, Mateus N, De Freitas V. Wine flavonoids in health and disease prevention. Molecules. 2017;22:292 https://doi.org/10.3390/molecules22020292
Gowd V, Jia Z, Chen W. Anthocyanins as promising molecules and dietary bioactive components against diabetes–A review of recent advances. Trends Food Sci Technol. 2017;68:1–3. https://doi.org/10.1016/j.tifs.2017.07.015
Ma Y, Ding S, Fei Y, Liu G, Jang H, Fang J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control. 2019;106:106712. https://doi.org/10.1016/j.foodcont.2019.106712
Krga I, Milenkovic D. Anthocyanins: From sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J Agric Food Chem. 2019;67:1771–83. https://doi.org/10.1021/acs.jafc.8b06737
Bruneton J, Villar del Fresno A, Carretero Accame ERLM. Cardenólido Bufadienólido 82. 1ra ed. España: Acribia; 1991.
Nebauer SG, Segura J, Kreis W, Arrillaga I, Clemente ES, Frieder M. Wild Crop Relatives: Genomic and Breeding Resources. Wild Crop Relatives: Genomic and Breeding Resources. 2011. https://doi.org/10.1007/978-3-642-21201-7
Wen S, Chen Y, Lu Y, Wang Y, Ding L, Jiang M. Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia. 2016;112:74–84. https://doi.org/10.1016/j.fitote.2016.04.023
El-seedi HR, Khalifa SAM, Taher EA, Farag MA, Saeed A, Gamal M, et al. SC. Pharmacol Res. 2018; https://doi.org/10.1016/j.phrs.2018.12.015
Cerella C, Dicato M, Diederich M. Mitochondrion Assembling the puzzle of anticancer mechanisms triggered by cardiac glycosides. MITOCH. 2013;13:225–34. https://doi.org/10.1016/j.mito.2012.06.003
Mestre J, Matheu MI, Díaz Y, Castillón S, Boutureira O. Chemical Access to d - Sarmentose Units Enables the Total Synthesis of Cardenolide Monoglycoside N1 from Nerium oleander. J Org Chem. 2017;82:3327–33. https://doi.org/10.1021/acs.joc.7b00210
Stenkvist B, Bengtsson E, Eklund G, Eriksson O, Holmquist J, Nordin BW-NS. Evidence of a modifying influence of heart glucosides on the development of breast. Anal Quant Cytol. 1980;2:49–54.
Diederich M, Muller F, Cerella C. Cardiac glycosides: From molecular targets to immunogenic cell death. Biochem Pharm. 2017;125:1–11. https://doi.org/10.1016/j.bcp.2016.08.017
Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, et al. Trial watch: Cardiac glycosides and cancer therapy. Oncoimmunology. 2013;2:e23082 https://doi.org/10.4161/onci.23082
Cao YL, Zhang MH, Lu YF, Li CY, Tang JS, Jiang MM. Cardenolides from the leaves of Nerium oleander. Fitoterapia. 2018;127:293–00. https://doi.org/10.1016/j.fitote.2018.03.004
Ko YS, Rugira T, Jin H, Park SW, Kim HJ. Oleandrin and its derivative odoroside a, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the stat-3 signaling pathway. Int J Mol Sci. 2018;19:3350. https://doi.org/10.3390/ijms19113350
Kumar A, De T, Mishra A, Mishra AK. Oleandrin: A cardiac glycosides with potent cytotoxicity. Pharmacogn Rev. 2013;7:131. https://doi.org/10.4103/0973-7847.120512
Pan J-Y, Chen S-L, Yang M-H, Wu J, Sinkkonen J, Zou K. An update on lignans: natural products and synthesis. Nat Prod Repo. 2009;26:1251–92. https://doi.org/10.1039/b910940d
Whiting DA. Ligans and neolignans. Nat Prod Repo. 1985;2:191–211. https://doi.org/10.1039/NP9850200191
Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life sci. 1994;55:1061–9. https://doi.org/10.1016/0024-3205(94)00641-5
Thompson LU, Seidl MM, Rickard SE, Orcheson LJ, Fong HH. Antitumorigenic effect of a mammalian lignan precursor from flaxseed. Fong, Nutr Cancer. 1996;26:159–65. https://doi.org/10.1080/01635589609514472
Kangas L, Saarinen N, Mutanen M, Ahotupa M, Hirsinummi R, Unkila M, et al. Antioxidant and antitumor effects of hydroxymatairesinol (HM-3000, HMR), a lignan isolated from the knots of spruce. Eur. J. Cancer Prev. 2002 Aug:S48-57. http://www.jstor.org/stable/45051298
Lu H, Liu GT. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992;58:311–3. https://doi.org/10.1055/s-2006-961473
Ghisalberti EL. Cardiovascular activity of naturally occurring lignans. Phytomed. 1997;4:151–66. https://doi.org/10.1016/S0944-7113(97)80063-3
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem, 1999;202:91–100. https://doi.org/10.1023/A:1007022329660
Yamauchi S, Ina T, Kirikihira T, Masuda T. Synthesis and antioxidant activity of oxygenated furofuran lignans. Biosci Biotechnol Biochem 2004;68:183–92. https://doi.org/10.1271/bbb.68.183
Charlton JL. J Nat Prod 1998;61:1447–51. https://doi.org/10.1021/np980136z
Hassan MZ, Osman H, Ali MA, Ahsan MJ. Therapeutic potential of coumarins as antiviral agents. Eur J Med Chem. 2016;123:236–55. https://doi.org/10.1016/j.ejmech.2016.07.056
Pari L, Rajarajeswari N, Saravanan S, Rathinam A. Antihyperlipidemic effect of coumarin in experimental type 2 diabetic rats. Bio Prev Nut. 2014;4:171–76. https://doi.org/10.1016/j.bionut.2014.02.003
Xu J, Ma L, Jiang D, Zhu S, Yan F, Xie Y, et al. Content evaluation of 4 furanocoumarin monomers in various citrus germplasms. Food Chem. 2015a;187:75–81. https://doi.org/10.1016/j.foodchem.2015.04.007
Xu L, Wu YL, Zhao XY, Zhang W (2015b) The study on biological and pharmacological activity of coumarins. Adv Eng Res:135–38. https://doi.org/10.2991/ap3er-15.2015.33
Yamahara J, Kobayashi G, Matsuda H, Katayama T, Fujimura H. Vascular dilatory action of Artemisia capillaris bud extracts and their active constituent. J Ethnopharm. 1989a;26:129–36. https://doi.org/10.1016/0378-8741(89)90060-3
Yamahara J, Kobayashi G, Matsuda H, Iwamoto M, Fujimura H. Vascular dilatory action of the Chinese crude drug. II. Effects of scoparone on calcium mobilization. Chem Pharm Bull. 1989b;37:485–89. https://doi.org/10.1248/cpb.37.485
Thakur A, Singla R, Jaitak V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur J Med Chem. 2015;101:476–95. https://doi.org/10.1016/j.ejmech.2015.07.010
Dandriyal J, Singla R, Kumar M, Jaitak V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur J Med Chem. 2016;119:141–68. https://doi.org/10.1016/j.ejmech.2016.03.087
Wanga YT, Yanb W, Chena QL, Huanga WY, Yanga Z, Lic X, et al. Inhibition viral RNP and anti-inflammatory activity of coumarins against influenza virus. Biomed Pharm. 2017;87:583–88. https://doi.org/10.1016/j.biopha.2016.12.117
Tandan SK, Chandra S, Tripathi HC, Lal J. Pharmacological actions of seselin, a coumarin from Seseli indicum seeds. Fitoterapia. 1990;61:360–63. https://www.cabdirect.org/cabdirect/abstract/19910302135
Kayser O, Kolodziej H. Antibacterial activity of simple coumarins: structural requirements for biological activity. Z Naturforsch. 1999;54:169–74. https://doi.org/10.1515/znc-1999-3-405
Rekha JB, Jayakar B. Anti cancer activity of ethanolic extract of leaves of Plumeria rubra (Linn). J Curr Pharma Res. 2011;1:175 https://search.proquest.com/openview/c259d15abb8196cafde57cef6b8d9a03/1?pq-origsite=gscholar&cbl=1936342
Periyasamy G, Gupta M, Mazumder UK, Gebrelibanos M, Sintayehu B. Antioxidant and antitumor activity of Plumeria acuminata in ehrlich ascites carcinoma bearing swiss albino mice. Br J Pharm Res. 2013;3:671–85. https://doi.org/10.9734/BJPR/2013/4472
Muruganantham N, Solomon S, Senthamilselvi MM. Anti-cancer activity of Plumeriarubra (flowers) against human liver cancer. Int J Pharmacogn Phytochem Res 2014;6:1007–9.
Kardono LB, Tsauri S, Padmawinata K, Pezzuto JM, Kinghorn AD. Cytotoxic constituents of the bark of Plumeria rubra collected in Indonesia. J Nat Products. 1990;53:1447–55. https://doi.org/10.1021/np50072a008
Kuigoua GM, Kouam SF, Ngadjui BT, Schulz B, Green IR, Choudhary MI, et al. Minor secondary metabolic products from the stem bark of Plumeria rubra Linn. displaying antimicrobial activities. Planta Med. 2010;76:620–5. https://doi.org/10.1055/s-0029-1240611
Dickey EE. Liriodendrin, a new lignan diglucoside from the inner bark of yellow poplar (Liriodendron tulipifera L.). J Org Chem 1958;23:179–84. https://doi.org/10.1021/jo01096a007
Hamburger MO, Cordell GA, Ruangrungsi N. Traditional medicinal plants of Thailand XVII Biologically active constituents of Plumeria rubra. J ethnopharmacol. 1991;33:289–92. https://doi.org/10.1016/0378-8741(91)90091-Q
Kuswanti N, Widyarti S, Widodo W, Rifa’i M. Cytotoxicity of ethanolic extract of Plumeria rubra L. stem bark to cancer cells and lymphocytes. Res J Pharm Technol. 2018;11:5545–50. https://doi.org/10.5958/0974-360X.2018.01009.0
Coppen JJ, Cobb AL. The occurrence of iridoids in Plumeria and Allamanda. Phytochem 1983;22:125–8. https://doi.org/10.1016/S0031-9422(00)80071-0
Xia YY, Lin CZ, Lu XJ, Liu FL, Wu AZ, Zhang L, et al. New iridoids from the flowers of Plumeria rubra “Acutifolia”. Phytochem Lett. 2018;25:81–5. https://doi.org/10.1016/j.phytol.2018.02.003
Abe F, Chen RF, Yamauchi T. Minor iridoids from the roots of Plumeria acutifolia. Chem pharm bull. 1988;36:2784–9. https://doi.org/10.1248/cpb.36.2784
Ye G, Li ZX, Xia GX, Peng H, Sun ZL, Huang CG. A new iridoid alkaloid from the flowers of Plumeria rubra L. cv. acutifolia. Helv Chim Acta. 2009;92:2790–4. https://doi.org/10.1002/hlca.200900222
Radha R, Kavimani S, Ravichandran V. Antitumour activity of methanolic extract of Plumeria alba L. leaves against Dalton lymphoma ascites in mice. Inter J Health Res. 2008;1:79–85. https://doi.org/10.4314/ijhr.v1i2.47919
Guevara AP, Amor E, Russell G. Antimutagens from Plumeria acuminata ait. Mutat Res /Environ Mutagenesis Relat Subj. 1996;361:67–72. https://doi.org/10.1016/S0165-1161(96)90240-X
Rasool SN, Jaheerunnisa S, Chitta SK, Jayaveera KN. Antimicrobial activities of Plumeria acutifolia. J Med Plants Res. 2008;2:77–80.
Alhozaimy GA, Al-Sheddi ES, Ibrahim TA. Biological Activity and isolation of compounds from stem bark of Plumeria acutifolia. Phcog Mag 2017;13:S505–11. https://doi.org/10.4103/pm.pm_22_17
Sharma SK, Kumar N. Antimicrobial potential of Plumeria rubra Syn Plumeria acutifolia bark. Pharma Chem. 2012;4:1591–3. https://www.academia.edu/download/86748475/antimicrobial-potential-of-Plumeria-rubra-syn-Plumeria-acutifolia-bark.pdf
Liu Y, Wang H, Wei S, Yan Z. Chemical composition and antimicrobial activity of the essential oils extracted by microwave-assisted hydrodistillation from the flowers of two Plumeria species. Anal lett. 2012;45:2389–97. https://doi.org/10.1080/00032719.2012.689905
Muruganantham N, Solomon S, Senthamilselvi MM. Anti-oxidant and anti-inflammatory activity of Plumeria rubra (flowers). Int J Pharm Sci Rev Res. 2015;30:132–5.
Lawal U, Egwaikhide PA, Longbap DB. Preliminary phytochemical and anti-bacterial studies on the leaf extracts of plumeria rubra linn. J Nat Sci Res. 2014;4:14 https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=60fb8d0860cf20284bf7c6839768770dc511fcaa
Sulaiman SF, Yaacob SS, Tan ML, Tengku MST. Chemical components of the essential oils from three species of Malaysian Plumeria L. and their effects on the growth of selected microorganisms. J Biosci. 2008;19:1–7. https://ejournal.usm.my/tlsr/article/view/tlsr_vol19-no-2-2008_1
Gupta M, Mazumder UK, Gomathi P, Selvan VT. Antimicrobial activity of methanol extracts of Plumeria acuminata Ait. leaves and Tephrosia purpurea (Linn.). Pers roots Nat Prod Radiance. 2008;7:102–5. https://nopr.niscpr.res.in/handle/123456789/5651
Zahid Z, Khan SW, Patel KA, Konale AG, Lokre S. Antimicrobial activity of essential oil of flowers of Plumeria alba Linn. J Pharm Phytochem Sci. 2010;2:155–7.
Akhtar NMAASN, Rubrinol KSU. a new antibacterial triterpenoid from Plumeria rubra. Fitoterapia. 1994;65:162–6. http://pascalfrancis.inist.fr/vibad/index.php?action=getRecordDetail&idt=4090595
Mukhtar MR, Aziz AN, Thomas NF, Hadi AH, Litaudon M, Awang K. Grandine A, a new proaporphine alkaloid from the bark of Phoebe grandis. Molecules. 2009;14:1227–33. https://doi.org/10.3390/molecules14031227
Mukhtar MR, Martin MT, Domansky M, Pais M, Hamid A, Hadi A, et al. Phoebegrandines A and B, proaporphine-tryptamine dimers, from Phoebe grandis. Phytochem 1997;45:1543–6. https://doi.org/10.1016/S0031-9422(97)00189-1
Le Quesne PW, Larrahondo JF, Raffauf RF. Antitumor plants. X. Constituents of Nectandra rigida. J nat prod 1980;43:353–9. https://doi.org/10.1021/np50009a006
Siddiqui S, Siddiqui BS, Naeed A, Begum S. Three pentacyclic triterpenoids from the leaves of Plumeria obtuse. J nat prod 1990;53:1332–6. https://doi.org/10.1021/np50071a029
Siddiqui S, Siddiqui BS, Naeed A, Begum S. Pentacyclic triterpenoids from the leaves of Plumeria obtuse. Phytochemistry. 1992;31:4279–83. https://doi.org/10.1016/0031-9422(92)80458-Q
Njinga NS, Sule MI, Pateh UU, Hassan HS, Abdullahi ST, Ache RN. Isolation and antimicrobial activity of β-sitosterol-3-O-glucoside from Lannea kerstingii engl. & K. Krause (Anacardiacea). J Health Allied Sci NU. 2016;6:004–8. https://doi.org/10.1055/s-0040-1708607
Schmidt JL, Lien NT, Khoi NH, Adam G. Lupeol long-chain fatty acid esters and other triterpenoid constituents from Plumeria obtusifolia. Phytochem 1983;22:1032–3. https://doi.org/10.1016/0031-9422(83)85051-1
Srivastava A, Gupta AK, Rajendiran A. Phytochemical screening and in-vitro anthelmintic activity of methanolic extract from the stem bark of Plumeria rubra Linn. Int J Pharm Sci Res 2017;8:5336–41.
Sameer Rastogi SR, Harshita Rastogi HR, Vijender Singh VS. Anti-inflammatory and anthelmintic activities of methanolic extract of Plumeria rubra leaves. Ind J Nat Prod. 2009;25:15–18.
Katsayal UA, Abubakar MS, Ahmed A, Abdurahman EM. Perceptions of the Traditional Medical Practitioners of North-Western Nigeria on Malaria Treatment and the Potential Antiplasmodial Properties of Plumeria rubra Stem-Bark. Mod Adv Pharm Res. 2019;2:31–43. https://www.researchgate.net/profile/Umar-Katsayal-2/publication/276933239_Perceptions_of_the_Traditional_Medical_Practitioners_of_North-Western_Nigeria_on_Malaria_Treatment_and_the_Potential_Antiplasmodial_Properties_of_Plumeria_rubra_Stem-Bark/links/5671c69b08aecc73dc095879/Perceptions-of-the-Traditional-Medical-Practitioners-of-North-Western-Nigeria-on-Malaria-Treatment-and-the-Potential-Antiplasmodial-Properties-of-Plumeria-rubra-Stem-Bark.pdf
HN H, Mathew S, Jani DV, George LB. In-vitro evidence of effective anti-plasmodium activity by Plumeria rubra (L) Extracts. Int J Pharm Phytochem Res 2016;8:1377–84. https://www.researchgate.net/profile/Dhara-Jani/publication/306308164_In-vitro_evidence_of_effective_anti-plasmodium_activity_by_Plumeria_rubra_L_extracts/links/63b2b28ea03100368a476c9c/In-vitro-evidence-of-effective-anti-plasmodium-activity-by-Plumeria-rubra-L-extracts.pdf
Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J nat prod 1991;54:143–54. https://doi.org/10.1021/np50073a012
Patil CD, Patil SV, Borase HP, Salunke BK, Salunkhe RB. Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol Res 2012;110:1815–22. https://doi.org/10.1007/s00436-011-2704-x
Gupta M, Mazumder UK, Gomathi P, Selvan VT. Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Compl Alternative Med. 2006;6:1–6. https://doi.org/10.1186/1472-6882-6-36.
Vijayalakshmi A, Ravichandiran V, Velraj M, Hemalatha S, Sudharani G, Jayakumari S. Anti–anaphylactic and anti–inflammatory activities of a bioactive alkaloid from the root bark of Plumeria acutifolia Poir. Asian Pac J Trop Biomed. 2011;1:401–5. https://doi.org/10.1016/S2221-1691(11)60088-9
Chanda I, Sarma U, Basu SK, Lahkar M, Dutta SK. A protease isolated from the latex of Plumeria rubra linn (apocynaceae) 2: Anti-inflammatory and wound-healing activities. Trop J Pharm Res. 2011;10:755–60. https://doi.org/10.4314/tjpr.v10i6.8.
Aziz A, Saqib F, Khan IA, Ashraf MM, Ashraf MN, Raza MA. Dermatological Evaluation of Anti-Irritant and Anti-Inflammatory Effect of Plumerin-R Isolated from the Latex of Plumeria rubra Linn. Lat Am J Pharm. 2018;37:317–20. https://www.researchgate.net/profile/Fatima-Saqib/publication/322147357_Dermatological_evaluation_of_anti-_irritant_and_anti-inflammatory_effect_of_Plumerin-R_isolated_from_the_latex_of_Plumeria_rubra_Linn/links/5a7c9b10aca272341aeb73e7/Dermatological-evaluation-of-anti-irritant-and-anti-inflammatory-effect-of-Plumerin-R-isolated-from-the-latex-of-Plumeria-rubra-Linn.pdf.
Banibrata Das BD, Ferdous T, Mahmood QA, Hannan JM, Rajib Bhattacharjee RB, Das BK. Antinociceptive and anti-inflammatory activity of the bark extract of Plumeria rubra on laboratory animals. Eur J Med Plants. 2013;3:114–26. https://pesquisa.bvsalud.org/portal/resource/pt/sea-164008
Siddiqui BS, Ilyas F, Rasheed M, Begum S. Chemical constituents of leaves and stem bark of Plumeria obtuse. Phytochem 2004;65:2077–84. https://doi.org/10.1016/j.phytochem.2004.04.024
Mondal P, Das S, Mahato K, Borah S, Junejo JA, Zaman K. Evaluation of anti-arthritic potential of the hydro-alcoholic extract of the stem bark of Plumeria rubra in freund’s complete adjuvant-induced arthritis in rats. Int J Pharm Sci Res. 2016;7:3675 https://doi.org/10.13040/IJPSR.0975-8232.7(9).3675-88.
Siddiqui BS, Naeed A, Begum S, Siddiqui S. Minor iridoids from the leaves of Plumeria obtuse. Phytochem. 1994;37:769–71. https://doi.org/10.1016/S0031-9422(00)90355-8
Yadav K, Divyadeepika, Joshi J. Biological activity of phytochemicals extracted from medicinal plants of Apocynaceae family. Materials Today: Proceedings. 2024 Apr. https://doi.org/10.1016/j.matpr.2024.04.003
Gupta M, Mazumder UK, Gomath P. Evaluation of antioxidant and free radical scavenging activities of Plumeria acuminata leaves. J Biol Sci. 2007;7:1361–7.
Gupta M, Mazumder UK, Gomath P. Evaluation of antipyretic and antinociceptive activities of Plumeria acuminata leaves. J Med Sci. 2007;7:835–9.
Misra V, Uddin SM, Srivastava V, Sharma U. Antipyretic activity of the Plumeria rubra leaves extract. Int J Pharm. 2012;2:330–2.
Aziz A, Khan IA, Munawar SH, Sadr-ul-Shaheed SU. Antipyretic study of methanolic bark extract of plumeriarubra, linn. in various pyrexia induced models. Int J Res Dev Pharm Life Sci 2013;2:680–5. https://pesquisa.bvsalud.org/portal/resource/pt/sea-149352
Khan IA, Aziz A, Raza MA, Saleem M, Bashir S, Alvi A. Study pertaining to the hypothermic activity of Plumeria rubra, Linn. in prostaglandin 1 and typhoid vaccine-induced pyrexia models in rabbits. W. Indian Med. J. 2015;12. https://doi.org/10.7727/wimj.2015.172
de Freitas CD, Souza DP, Araújo ES, Cavalheiro MG, Oliveira LS, Ramos MV. Anti-oxidative and proteolytic activities and protein profile of laticifer cells of Cryptostegia grandiflora, Plumeria rubra and Euphorbia tirucalli. Braz J Plant Physiol. 2010;22:11–22. https://doi.org/10.1590/S1677-04202010000100002
Mohamed Isa SS, Ablat A, Mohamad J. The antioxidant and xanthine oxidase inhibitory activity of Plumeria rubra flowers. Molecules. 2018;23:400 https://doi.org/10.3390/molecules23020400
Yadav AV, Undale VR. Antidiabetic effect of Plumeria rubra linn. in streptozotocin induced diabetic rats. Int Int J Pharma Sci Res. 2017;8:1806.
Yadav AV, Undale VR, Bhosle AV. Antidiabetic activity of Plumeria rubra L. in normal and alloxan induced diabetic mice. Int J Basic Clin Pharm. 2016;5:884–9.
Zaman K. Evaluations of antidiabetic potential of the hydro-alcoholic extract of the stem bark of Plumeria rubra a traditionally used medicinal source in North-East India. Int. J Green Pharm. (IJGP). 2016;10. https://doi.org/10.22377/ijgp.v10i04.763
Viswanathan S, Doss DV. Ameliorative effect of Plumeria rubra leaf extract against Streptozotocin induced diabetic Rats. Malaya J Biosci (MJB). 2014;92–9. https://scholar.archive.org/work/yos2ihgjzfewziifpzexmckfcu/access/wayback/.
Merina AJ, Sivanesan D, Begum VH, Sulochana N. Antioxidant and hypolipidemic effect of Plumeria rubra L. in alloxan induced hyperglycemic rats. J Chem. 2010;7:1–5. https://doi.org/10.1155/2010/576704
Sangeetha J, Abbulu K, Sudhakar M. Study on the effect of Aganosma cymosa and Plumeria rubra methanol extract on different models of induced liver toxicity in experimental rats. J Pharma Res. 2013;3:49.
Dawada SD. Hepatoprotective activity of pod extract of Plumeria rubra against carbontetrachloride induced hepatic injury in rats (wistar). Int J Pharm Pharm Res 2015;3:218–27.
Zade V, Dabhadkar D. Antifertility effect of Alcoholic extract of Plumeria rubra on estrous cycle of female Albino rat. Int J Pharm Sci Rev Res. 2012;12:75–9. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=afd6b234ff0f4ada34a99c3594b9f9727907c9fe
Dabhadkar D, Zade V. Abortifacient activity of Plumeria rubra (Linn). Pod Extr female albino rats Indian J Exp Biol. 2012;50:702–7. https://nopr.niscpr.res.in/bitstream/123456789/14793/1/IJEB%2050%2810%29%20702-707.pdf
Gupta RS, Sharma D. A pragmatic antifertility assessment of methanolic bark extract of Plumeria rubra (L.) in male albino rats. World J Pharm Res. 2017;6:1315–17.
Misra V, Yadav G, Uddin SM, Srivastava V. Determination of antiulcer activity of Plumeria Rubra leaves extract. Int Res J Pharm 2012;3:194–7.
Alencar NM, Pinheiro RS, Figueiredo IS, Luz PB, Freitas LB, Souza TD, et al. The preventive effect on ethanol-induced gastric lesions of the medicinal plant Plumeria rubra: involvement of the latex proteins in the NO/cGMP/K ATP signaling pathway. Evidence-Based Complem. Alt. Med. 2015 Jan;2015. https://doi.org/10.1155/2015/706782
Aiyambo D. Traditional uses of selected members of the Apocynaceae family in Namibia. Minist Agric Water Forestry Windhock. 2010;115:1–2.
Pande M, Dubey VK, Yadav SC, Jagannadham MV. A novel serine protease cryptolepain from Cryptolepis buchanani: purification and biochemical characterization. J Agric Food Chem 2006;54:10141–50. https://doi.org/10.1021/jf062206a
Choudhary M, Kumar V, Gupta P, Singh S. Investigation of antiarthritic potential of Plumeria alba L. leaves in acute and chronic models of arthritis. BioMed Res Int 2014;2014:474616 https://doi.org/10.1155/2014/474616
Rahman H, Reddy VB, Ghosh S, Mistry SK, Pant G, Sibi G. Antioxidant, cytotoxic and hypolipidemic activities of Plumeria alba L. and Plumeria rubra L. Am J Life sci. 2014;2:11–5. https://doi.org/10.11648/j.ajls.s.20140204.13
Kadébé ZT, Metowogo K, Bakoma B, Poevi Lawson-Evi S, EkluGadegbeku K, Aklikokou K. et al. Antidiabetic activity of Plumeria Alba Linn (Apocynaceae) root extract and fractions in streptozotocin-induced diabetic rats. Trop J Pharm Res. 2016;15:87. https://doi.org/10.4314/tjpr.v15i1.12.
Anand U, Nandy S, Mundhra A, Das N, Pandey DK, Dey A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: Possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. 2020;51:100695 https://doi.org/10.1016/j.drup.2020.100695
Tan MJ, Ye JM, Turner N, Hohnen-Behrens C, Ke CQ, Tang CP, et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol 2008;15:263–73. https://doi.org/10.1016/j.chembiol.2008.01.013
Ghosh J, Sil PC. Arjunolic acid: a new multifunctional therapeutic promise of alternative medicine. Biochimie. 2013;95:1098–109. https://doi.org/10.1016/j.biochi.2013.01.016
Uemura T, Goto T, Kang MS, Mizoguchi N, Hirai S, Lee JY, et al. Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr. 2011;141:17–23. https://doi.org/10.3945/jn.110.125591
Hertog MG, Feskens EJ, Kromhout D, Hollman PC, Katan MB. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. lancet. 1993;342:1007–11. https://doi.org/10.1016/0140-6736(93)92876-U
Ling WH, Jones PJ. Dietary phytosterols: a review of metabolism, benefits and side effects. Life sci. 1995;57:195–206. https://doi.org/10.1016/0024-3205(95)00263-6
Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–56. https://doi.org/10.1002/1097-0010(20000915)80:123.0.co;2-w.
Jones PJ, Ntanios FY, Raeini-Sarjaz M, Vanstone CA. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am J Clin Nutr. 1999;69:1144–50. https://doi.org/10.1093/ajcn/69.6.1144
Kinsella JE, Frankel E, German B, Kanner J. Possible mechanisms for the protective role of antioxidants in wine and plant foods: physiological mechanisms by which flavonoids, phenolics, and other phytochemicals in wine and plant foods prevent or ameliorate some common chronic diseases are discussed. Food technol (Chic). 1993;47:85–9. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=3770646
Baxter H, Puri B, Harborne JB, Hall A, Moss GP. Phytochemical dictionary: a handbook of bioactive compounds from plants. CRC press; 1998 Dec. https://doi.org/10.4324/9780203483756
Silva ID, Rodrigues A, Gaspar J, Mala R, Laires A, Rueff J. Mutagenicity of kaempferol in V79 cells: The role of cytochromes P450. Teratogenesis Carcinogenesis Mutagenesis. 1996;16:229–41. https://doi.org/10.1002/(SICI)1520-6866(1996)16:43.0.CO;2-K.
Al-Numair KS, Chandramohan G, Veeramani C, Alsaif MA. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015;20:198–209. https://doi.org/10.1179/1351000214Y.0000000117
Abo-Salem OM. Kaempferol attenuates the development of diabetic neuropathic pain in mice: Possible anti-inflammatory and anti-oxidant mechanisms. Open Access Maced J Med Sci 2014;2:424–30. https://doi.org/10.3889/oamjms.2014.073
Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–82. https://doi.org/10.1093/ajcn/62.6.1276
Alinezhad H, Azimi R, Zare M, Ebrahimzadeh MA, Eslami S, Nabavi SF, et al. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of Hyssopus officinalis L. Var. angustifolius. Int J Food Prop. 2013;16:1169–78. https://doi.org/10.1080/10942912.2011.578319
Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin‐induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res. 2009;53:859–68. https://doi.org/10.1002/mnfr.200800310
An G, Gallegos J, Morris ME. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos. 2011;39:426–32. https://doi.org/10.1124/dmd.110.035212
Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47:2274–9. https://doi.org/10.1021/jf9811065
Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res 2005;51:117–23. https://doi.org/10.1016/j.phrs.2004.06.002
Stewart LK, Wang Z, Ribnicky D, Soileau JL, Cefalu WT, Gettys TW. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia. 2009;52:514–23. https://doi.org/10.1007/s00125-008-1252-0
Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J ethnopharmacol. 2010;127:457–62. https://doi.org/10.1016/j.jep.2009.10.013
Tofighi Z, Moradi-Afrapoli F, Ebrahimi SN, Goodarzi S, Hadjiakhoondi A, Neuburger M. et al. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J Nat Med. 2017;71:272–80. https://doi.org/10.1007/s11418-016-1060-7.
Nasri S, Roghani M, Baluchnejadmojarad T, Rabani T, Balvardi M. Vascular mechanisms of cyanidin-3-glucoside response in streptozotocin-diabetic rats. Pathophysiology. 2011;18:273–8. https://doi.org/10.1016/j.pathophys.2011.03.001
Zhu W, Jia Q, Wang Y, Zhang Y, Xia M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radic Biol Med. 2012;52:314–27. https://doi.org/10.1016/j.freeradbiomed.2011.10.483
Akkarachiyasit S, Charoenlertkul P, Yibchok-Anun S, Adisakwattana S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int J Mol Sci. 2010;11:3387–96. https://doi.org/10.3390/ijms11093387
Guengerich FP. Reactions and significance of cytochrome P-450 enzymes. JBC. 1991;266:10019–22. https://doi.org/10.1016/S0021-9258(18)99177-5
Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. https://doi.org/10.1021/tx700079z
Guengerich FP, Liebler DC, Reed DL. Enzymatic activation of chemicals to toxic metabolites. CRC Crit Rev Toxicol. 1985;14:259–307. https://doi.org/10.3109/10408448509037460
Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–50. https://doi.org/10.1021/tx0002583
Rendic S, Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997;29:413–580. https://doi.org/10.3109/03602539709037591
Brown CM, Reisfeld B, Mayeno AN. Cytochromes P450: a structure-based summary of biotransformations using representative substrates. Drug Metab Rev. 2008;40:1–00. https://doi.org/10.1080/03602530701836662
Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. https://doi.org/10.1146/annurev.pharmtox.40.1.581
Buters JT. 2.2A Phase I Metabolism. Toxicology and risk assessment: a comprehensive. 2008 Apr:49. http://ndl.ethernet.edu.et/bitstream/123456789/32168/1/Helmut%20Greim.pdf#page=71
Anders MW. Glutathione‐dependent bioactivation of haloalkanes and haloalkenes. Drug Metab Rev. 2004;36:583–94. https://doi.org/10.1081/DMR-200033451
Anders MW. Bioactivation mechanisms and hepatocellular damage. In: Arias IM, Jakoby WB, Popper H, Schachter D, Shafritz DA, (eds). The Liver: Biology and Pathology. 2nd edition. New York: Raven Press; 1988. p. 389–400.
DeBethizy JD, Hayes JR. Metabolism, a determinant of toxicity. In: Hayes AW, (ed.). Principles and Methods of Toxicology. New York: Raven Press; 1994. p. 59–100.
Ghosh S. Triterpene structural diversification by plant cytochrome P450 enzymes. Front Plant Sci 2017;8:1886.
De Carvalho CC, da Fonseca MM. Biotransformation of terpenes. Biotechnol Adv. 2006;24:134–42. https://doi.org/10.1016/j.biotechadv.2005.08.004
Fu SB, Yang JS, Cui JL, Sun DA. Biotransformation of ursolic acid by Syncephalastrum racemosum CGMCC 3.2500 and anti-HCV activity. Fitoterapia. 2013;86:123–8. https://doi.org/10.1016/j.fitote.2013.02.007
Fu S, Meng Q, Yang J, Tu J, Sun DA. Biocatalysis of ursolic acid by the fungus Gliocladium roseum CGMCC 3.3657 and resulting anti-HCV activity. RSC Adv. 2018;8:16400–5. https://doi.org/10.1039/C8RA01217B
Afifi MS, Salama OM, Gohar AA, Marzouk AM. Iridoids with antimicrobial activity from plumeria alba L. Bull Pharm Sci Assiut Univ. 2006;29:215–23. https://doi.org/10.21608/bfsa.2006.65194
Ali N, Ahmad D, Bakht J. Antimicrobial activity of different solvent extracted samples from the flowers of medicinally important Plumeria obstusa. Pak J Pharm Sci. 2015;28:195–200. https://www.academia.edu/download/97330216/Paper-26.pdf
Baghel AS, Mishra CK, Rani A, Sasmal D, Nema RK. Antibacterial activity of Plumeria rubra Linn. plant extract. J Chem Pharm Res. 2010;2:435–40. https://www.academia.edu/download/111662452/antibacterial-activity-of-plumeria-rubra-linn-plant-extract.pdf
Dey A, Das T, Mukherjee S. In vitro antibacterial activity of n-Hexane fraction of methanolic extract of Plumeria rubra L.(Apocynaceae) stem bark. J Plant Sci. 2011;6:135–42. https://doi.org/10.3923/jps.2011.135.142
Arcelino AI, Almeida AM, Rodrigues LV, Moraes MM, da Camara CA. Chemical Composition, Antioxidant, And Insecticidal Activity of Essential Oils from the Leaves and Flowers of Plumeria pudica. Chem Nat Compd. 2024;60:353–5. https://doi.org/10.1007/s10600-024-04323-5
Bouic PJ, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Alter Med Rev. 1999;4:170–7. https://lavierebelle.org/IMG/pdf/1999_plant_sterols_and_sterolins_review_of_their_immune-modulating_properties.pdf
Ulubelen A, Berkan T. Triterpenic and steroidal compounds of cnicus benedictus. Planta Med. 1977;31:375. https://doi.org/10.1055/s-0028-1097546
Dekant W. The role of biotransformation and bioactivation in toxicity. Mol. Clin. Environ. Toxicol.: Vol. 1: 2009 Jan:57-86. https://doi.org/10.1007/978-3-7643-8336-7_3
Albers‐Schönberg G, Schmid H. Über die Struktur von Plumericin, Isoplumericin, β‐Dihydroplumericin und der β‐Dihydroplumericinsäure. Helv Chim Acta. 1961;44:1447–73. https://doi.org/10.1002/hlca.19610440604
Akhtar N, Saleem M, Riaz N, Ali MS, Yaqoob A, Jabbar A. Isolation and characterization of the chemical constituents from Plumeria rubra. Phytochem lett. 2013;6:291–8. https://doi.org/10.1016/j.phytol.2013.03.007
Saleem M, Akhtar N, Riaz N, Ali MS, Jabbar A. Isolation and characterization of secondary metabolites from Plumeria obtuse. J Asian nat prod res 2011;13:1122–7. https://doi.org/10.1080/10286020.2011.618452
Ye G, Yang YL, Xia GX, Fan MS, Huang CG. Complete NMR spectral assignments of two new iridoid diastereoisomers from the flowers of Plumeria rubra L. cv. acutifolia. Mag Reso Chem. 2008;46:1195–7. https://doi.org/10.1002/mrc.2331
Schliemann W, Adam G. Enzymatic hydrolysis of plumieride plumieridine. Phytochem 1982;21:1438–9. https://doi.org/10.1016/0031-9422(82)80160-X
Krohn K, Gehle D, Dey SK, Nahar N, Mosihuzzaman M, Sultana N, et al. Prismatomerin, a new iridoid from Prismatomeris tetrandra. Structure elucidation, determination of absolute configuration, and cytotoxicity. J nat prod. 2007;70:1339–43. https://doi.org/10.1021/np070202
Van Beek TA, Lankhorst PP, Verpoorte R, Svendsen AB. Isolation of the secoiridoid-glucoside sweroside from Tabernaemontana psorocarpa. Planta Med. 1982;44:30–1. https://doi.org/10.1055/s-2007-971394
Lee JS, Kim J, Kim BY, Lee HS, Ahn JS, Chang YS. Inhibition of phospholipase Cγ1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla. J nat prod. 2000;63:753–6. https://doi.org/10.1021/np990478k
Siddiqui S, Siddiqui BS, Naeed A, Begum S. Pentacyclic triterpenoids from the leaves of Plumeria obtuse. Phytochem 1989;28:3143–7. https://doi.org/10.1016/0031-9422(89)80295-X
Siddiqui BS, Begum S. Two triterpenoids from the leaves of Plumeria obtuse. Phytochem 1999;52:1111–5. https://doi.org/10.1016/S0031-9422(99)00270-8
Hasan AM, Joshi BC, Dobhal MP, Sharma MC. A brief review on chemical constituents of some medicinally important species of the genus Plumeria. Asian J Chem. 1997;9:571 https://asianjournalofchemistry.co.in/user/journal/viewarticle.aspx?ArticleID=9_4_2
Mahato SB, Kundu AP. 13C NMR spectra of pentacyclic triterpenoid-A compilation and some salient features. Phytochem 1994;37:1517–75. https://doi.org/10.1016/S0031-9422(00)89569-2
Hassan EM, Shahat AA, Ibrahim NA, Vlietinck AJ, Apers S, Pieters L. A new monoterpene alkaloid and other constituents of Plumeria acutifolia. Planta med. 2008;74:1749–50. https://doi.org/10.1055/s-0028-1088317
Siddioui S, Siddiqui B, Naeed A, Begum S. Isolation and structure elucidation of obtusilinin, a new triterpenoid and 27-pZ-coumaroyloxyursolic acid from the leaves of Plumeria obtuse. Jour Chem Soc Pak Vol. 1991;13:115–19. https://jcsp.org.pk/issueDetail.aspx?aid=59a49afc-2eb0-452e-8f05-6b634dda1301
Siddiqui S, Siddiqui BS, Begum S, Naeed A. Pentacyclic triterpenoids from Plumeria obtuse. Phytochem 1990;29:3615–20. https://doi.org/10.1016/0031-9422(90)85287-P
De Oliveira PV, Lemos RPL, Conserva LM. Chemical constituents of Rourea doniana. Revista Brasileira de Farmacognosia Brazilian. J Pharmacogn. 2012;22:451–4. https://doi.org/10.1590/S0102
Zhao M, Liang Z, Xie Z, Yang D, Xu X. Separation and purification of 15-demethylplumieride, cerberic acid B, and kaempferol-3-rutinoside from plumeria rubra ‘acutifolia’by high-speed counter-current chromatography. Sep Sci Technol. 2015;50:2360–6. https://doi.org/10.1080/01496395.2015.1056357
Matsuda H, Morikawa T, Toguchida I, Yoshikawa M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem Pharm bull. 2002;50:788–95. https://doi.org/10.1248/cpb.50.788
Ye G, Huang C. Flavonoids of Limonium aureum. Chem nat comp 2006;42:232–4. https://doi.org/10.1007/s10600-006-0089-3
Fang SH, Rao YK, Tzeng YM. Anti-oxidant and inflammatory mediator’s growth inhibitory effects of compounds isolated from Phyllanthus urinaria. J Ethnopharmacol. 2008;116:333–40. https://doi.org/10.1016/j.jep.2007.11.040
Lim TK. 2014. Plumeria obtuse, in: Edible Medicinal and Non-Medicinal Plants. Springer Netherlands, 2014; pp. 87–93. https://doi.org/10.1007/978-94-007-7395-0_3.
Otsuka H, Hirata E, Shinzato T, Takeda Y, Glochiflavanosides AD. flavanol glucosides from the leaves of Glochidion zeylanicum (Gaertn) A. Juss. Chem Pharm Bull 2001;49:921–3. https://doi.org/10.1248/cpb.49.921
França OO, Brown RT, Santos CA. Uleine and demethoxyaspidospermine from the bark of Plumeria lancifolia. Fitoterapia. 2000;71:208–10. https://doi.org/10.1016/S0367-326X(99)00141-0
Kazmi SN, Ahmed Z, Ahmed W, Malik A. Plumerinine―A novel lupin alkaloid from Plumeria rubra. Heterocycles (Senda). 1989;29:1901–6. https://doi.org/10.3987/COM-89-5043
Dobhal MP, Hasan AM, Sharma MC, Joshi BC. Ferulic acid esters from Plumeria bicolor. Phytochem 1999;51:319–21. https://doi.org/10.1016/S0031-9422(99)00006-0
Yang G, Yin X, Li Y. Chemical constituents of Tripterygium wilfordii. Helv Chim Acta. 2000;83:3344–50. https://doi.org/10.1002/1522-2675(20001220)83:123.0.CO;2-B.
Dekant W. The role of biotransformation and bioactivation in toxicity. Molecular, Clinical and Environmental Toxicology: Volume 1: Molecular Toxicology. 2009 Jan:57-86. https://doi.org/10.1007/978-3-7643-8336-7_3
Acknowledgements
Divyadeepika acknowledge the vital financial support from UGC, New Delhi, for SRF which enabled us to dedicate time to this study.
Author contributions
Conceptualization, literature survey, data collection and manuscript writing, visualization: DD; Supervision: JJ. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Divyadeepika, Joshi, J. A review on phytochemical constituents, analytical data, and pharmacological properties of the genus Plumeria. Med Chem Res (2024). https://doi.org/10.1007/s00044-024-03303-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00044-024-03303-2