Abstract
Seeds of Securigera securidaca (Fabaceae) are used in Iranian folk medicine as an antidiabetic treatment. In this study, the antihyperglycemic activity of chloroform and methanol fractions (CF and MF) from S. securidaca seed extract was investigated and their bioactive constituents were identified. The antidiabetic effects of fractions were assessed by streptozocin-induced diabetic Naval Medical Research Institute mice. The hypoglycemic activity of MF at 100 mg/kg and CF at 400 mg/kg was comparable with glibenclamide (3 mg/kg). MF at 400 mg/kg and CF at 600 mg/kg showed equal hypoglycemic responses to 12.5 IU/kg insulin (P > 0.05). Three cardiac glycosides were isolated as active constituents responsible for the hypoglycemic activity. Securigenin-3- O -β-glucopyranosyl-(1 → 4)-β-xylopyranoside (1) was a major compound in seeds. Securigenin-3- O -inositol-(1 → 3)-β-glucopyranosyl-(1 → 4)-β-xylopyranoside (2) and securigenin-3- O -α-rhamnopyranosyl-(1 → 4)-α-glucopyranoside (3) were found as new natural products. When 1–3 were tested at 10 mg/kg there was a significant reduction of blood glucose levels in diabetic mice, comparable to that of 3 mg/kg glibenclamide (P > 0.05). The hypoglycemic effect was due to an increase in insulin secretion; the insulin levels in the diabetic mice significantly improved and were comparable with those in healthy animals (P > 0.05). Compounds responsible for the hypoglycemic properties of S. securidaca seeds were identified as cardiac glycosides and were found to act via an increase of insulin levels in a diabetic mouse model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Securigera securidaca (L.) Deg. et Dorf (Fabaceae) is a native plant of Iran [1]. Seeds of S. securidaca are traditionally used in the southern part of Iran (Fars Province) as a remedy to control diabetes. In recent years, this traditional use became known throughout the entire country and the seeds are now widely available on local markets. Use of S. securidaca does not seem to be restricted to Iran, since consumption as an antidiabetic agent has also been reported from India and Egypt [2, 3].
The antihyperglycemic activity of S. securidaca seeds has been confirmed by several in vivo studies [3–8]. Intraperitoneal and oral administration of aqueous infusions and ethanol extracts significantly reduced blood glucose levels in diabetic animals while the extracts were devoid of a hypoglycemic effect in normoglycemic animals [5]. In another study, administration of a chloroform extract in normal rats reduced fasting blood sugar and improved glucose tolerance in a dose-dependent manner, accompanied with increased food consumption, body weight, and glycogen content of the liver [6]. An aqueous extract of S. securidaca seeds decreased erythrocyte catalase activity as an antioxidant defense mechanism in diabetic animals [9]. In addition, seeds of S. securidaca reportedly also possess other pharmacological activities, such as hypolipidemic, chronotropic, gastroprotective, antinociceptive, antiepileptic and cytotoxic properties [2, 10–15].
Despite the traditional consumption of S. securidaca seeds as an antidiabetic remedy in Iran and other countries, and pharmacological studies with crude extracts, little is known about the phytochemical composition of the seeds and other plant parts of S. securidaca. Flavonoids and coumarins were reported as major constituents of aerial parts, while cardenolides and dihydrobenzofuran derivatives were found in seeds [2, 15–18]. However, the compounds responsible for the antidiabetic activity remain unknown. Here, we confirm the antidiabetic activity of seed extracts and report on the isolation and structure elucidation, and pharmacological testing of antihyperglycemic constituents in S. securidaca seeds.
Materials and methods
Chemicals
Streptozocin (STZ) from Sigma Chemical Co. (USA), glibenclamide from Pursina (Iran), and NPH insulin from Exir Co. (Iran) were purchased. Solvents used for extraction and open column chromatography were of technical grade and were purified by distillation prior to use.
Plant material
Seeds of S. securidaca were collected in September 2012 from Fars Province of Iran and were dried in shade. The plant was identified by Dr. Gh. Amin and a voucher specimen was deposited at the Herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences, Iran (6740-TEH).
Extraction and fractionation
The dried seeds of S. securidaca (1790 g) were powdered and macerated with 80% methanol at room temperature. The extract was concentrated under reduced pressure, and remaining water was removed by freeze drying. The crude extract (323 g) was re-extracted with chloroform to afford chloroform fraction (CF; 80 g), and the residue named methanol fraction (MF; 243 g). The dried fractions were kept at 4 °C prior to testing.
Animals
Male Naval Medical Research Institute (NMRI) mice weighing between 20 and 25 g were purchased from the Pasteur Institute (Tehran, Iran). They were kept at an ambient temperature (25 ± 2 °C) in a 12-h light/dark cycle. All animals had free access to laboratory rodent diet and water. The animals were allowed to acclimatize to the laboratory environment for a week. All experimental procedures followed the principles of laboratory animal care and were carried out according to a protocol approved by the Animal Ethics Committee of the Toxicology and Pharmacology Department, Faculty of Pharmacy, Tehran University of Medical Sciences.
Induction of diabetes in mice
Diabetes mellitus type II was induced by a single intraperitoneal (i.p.) injection of 200 mg/kg STZ to overnight-fasted mice [19]. STZ was freshly dissolved in 0.05 M phosphate buffer (pH 4) prior to injection. Three days later, blood samples were obtained from the tips of mice tails, and glucose levels were determined using a glucometer (Accu Chek Sensor; Roche, Germany). Mice with fasting blood glucose of ≥250 mg/dl were considered as diabetic and were used for the studies.
Evaluation of antidiabetic activity
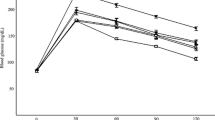
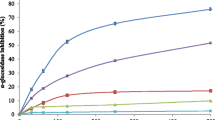
Animals were randomly divided into 13 groups, each containing 6 mice. Freshly prepared dilutions of 100–600 mg/kg of fractions, and 10 mg/kg of pure compounds were administered by i.p. injection in a fixed volume of 0.5 ml normal saline. Normal control and diabetic control groups were treated with normal saline. NPH insulin (12.5 IU/kg) and glibenclamide (3 mg/kg) were applied as positive controls [20]. Blood glucose was measured immediately before treatment (t 0), and after treatment at four time points (1, 2, 4 and 6 h). For pure compounds, heart blood was also collected after 4 h for measurement of insulin levels. Results were calculated as mean ± SD. Statistical significance was evaluated by ANOVA and Tukey posthoc tests. P < 0.05 was considered significant.
Isolation and purification of active constituents
A portion of CF (35 g) was separated by column chromatography on silicagel (7.0 × 16.0 cm), using a step gradient of petroleum ether/ethyl acetate (10:0, 9:1, etc.), followed by ethyl acetate/methanol (9:1, 5:5, etc.) and methanol (M). Eleven fractions (CF1–CF11) were obtained on the basis of thin-layer chromatography (TLC) patterns. CF9 (3 g) was submitted to column chromatography on silicagel (3 × 53 cm) using a step gradient of CHCl3/MeOH (9:1, 8:2, 7:3, 6:4, 2:8, 0:10). Ten subfractions were obtained (CF9-1 to CF9-10). Compound 1 (45.7 mg) was isolated from subfraction CF9-7 (838 mg) by chromatography on Sephadex LH-20 (2 × 83 cm) (mobile phase CHCl3/MeOH 4:6) and on an RP-18 column (1.5 × 23 cm) (mobile phase H2O/MeOH 5:5). Subfraction CF8 (504.4 mg) was separated on a silicagel column (2 × 39 cm) using a step gradient of CHCl3/MeOH (9:1, 7:3, 5:5, 3:7, 0:10) to afford subfractions CF8-1 to CF8-4. CF8-3 (114.8 mg) was further separated on a silicagel column (0.8 × 30 cm) eluted with CHCl3:MeOH (8:2) to give 4 subfractions (CF8-3-1 to CF8-3-4). Compound 2 (25.9 mg) was isolated from CF8-3-4 (28.4 mg) using a Sephadex LH-20 column (1.2 × 67 cm) and methanol as eluant.
A portion of MF (25 g) was submitted to column chromatography on an RP-18 (4 × 13 cm) column, using a gradient of aqueous methanol (20–80% MeOH) followed by methanol/ethyl acetate (80:20). Eight subfractions (MF1–MF8) were achieved on the basis of TLC patterns. MF4 (100 mg) was further fractionated on an RP-18 column (2.5 × 17.5 cm) eluted with a gradient of aqueous methanol (40–70% MeOH). Five subfractions (MF4-1 to MF4-5) were obtained. Compound 3 (22.5 mg) was purified from MF4-4 (69.6 mg) using Sephadex LH-20 (1.2 × 67 cm), and methanol as the mobile phase.
Characterization of compounds
Structures were established with the aid of 1D and 2D NMR, ESIMS and EIMS data. Absolute configuration of compounds 1 and 2 were determined by electronic circular dichroism (ECD), and X-ray crystallographic analysis of 1.
Sugar analysis
Sugars were identified after acid hydrolysis [20]. Glycosides (10 mg) were heated on a steam bath in 10 ml HCl (2N) for 45 min. Solutions were cooled and extracted with diethyl ether. The aqueous phase was chromatographed on Whatman No. 1 paper with ethyl acetate:pyridine:water (12:5:4) as solvent. Glucose, galactose, rhamnose, mannose, xylose and myoinositol were used as reference compounds. The chromatogram was sprayed with p-anisidine hydrochloride/sodium hydrosulfite reagent and heated for 10 min until brown spots of sugars appeared.
X-ray crystallography
Crystal of compound 1 was measured on a Bruker Kappa Apex2 diffractometer. Apex2 software [21] was used for data collection and integration. The structure was solved by direct methods using the program SIR92 [22]. Least-squares refinement against F was carried out on all non-hydrogen atoms using the program CRYSTALS [23]. Chebychev polynomial weights [24, 25] were used to complete the refinement. Plots were produced using MERCURY [26]. Detailed experimental data are provided in Supplementary material.
Computational methods
Conformational analysis for the aglycon of 1 was performed with Schrödinger Macro Model 9.1 (Schrödinger LLC, New York, USA) employing the OPLS2005 (optimized potential for liquid simulations) force field in H2O. Conformers within a 2 kcal/mol energy window from the global minimum were selected for geometrical optimization and energy calculation applying DFT-B3LYP/6-31 G** basis set in the gas phase with the Gaussian 09 program package. Vibrational evaluation was performed at the same level to confirm minima. Excitation energy (denoted by wavelength in nm), rotatory strength dipole velocity (R vel), and dipole length (R len) were calculated in MeOH by TDDFT/CAM-B3LYP/6-31 G**, using the SCRF method with the CPCM model. ECD curves were obtained on the basis of rotator strengths with a half-band of 0.25 eV using Spec Dis v1.61.
Results
Spectral analysis
The isolated compounds were identified using different spectroscopic methods (Fig. 1).
Securidaside:Securigenin-3- O -β-glucopyranosyl-(1 → 4)-β-xylopyranoside (1); white needles (recrystallized with anhydrous methanol); UV (MeOH) λ max: 238 and 312 nm; ECD (MeOH, c 0.6 mM, 0.1 cm), [θ]223 = −5515, [θ]242 = 5706, [θ]314 = 6332; ESIMS m/z 703.5 [M + Na]+, 719.3 [M + K]+, 725.9 [M + HCOO]−; IR (KBr) ν max 3444, 2924, 2723, 1740, 1725, 1623, 1461, 1379, 1167, 756 cm−1.
Securigenin-3- O -inositol-(1 → 3)-β-glucopyranosyl-(1 → 4)-β-xylopyranoside (2); white needles (recrystallized with anhydrous methanol); UV (MeOH) λ max: 238 and 312 nm; ECD (MeOH, c 0.3 mM, 0.1 cm), [θ]223 = −2349, [θ]242 = 13944, [θ]314 = 5545.
Securigenin-3- O -α-rhamnopyranosyl-(1 → 4)-α-glucopyranoside (3); white needles (recrystallized with anhydrous methanol); UV (MeOH) λ max: 238 and 312 nm.
1H NMR and 13C NMR data of compound 1–3 were recorded in Table 1.
Antidiabetic activities
Antidiabetic activities of seed fractions of S. securidaca were investigated in STZ-induced diabetic mice. Hypoglycemic activities were assessed at four time points (1, 2, 4, 6 h after i.p. injection) (Table 2). The activity of MF at 100–500 mg/kg was equivalent to that of 3 mg/kg glibenclamide (P > 0.05); this effect was dose-dependent. MF at 400 and 500 mg/kg gave an antihyperglycemic response that was comparable to that of 12.5 IU/kg insulin (P = 0.23 and 0.43, respectively). CF at 400 mg/kg showed a hypoglycemic effect comparable to glibenclamide (P = 0.23). There was no significant difference in blood sugar reduction percentage of 600 mg/kg of CF and glibenclamide (P = 0.68) or insulin (P = 0.12). Interestingly, onset of action (beginning of blood sugar reduction) for CF was ≤1 h, while glibenclamide showed a similar effect after 6 h.
The antidiabetic activity of pure isolated compounds was studied at 10 mg/kg BW. Securigenin glycosides 1–3 reduced blood glucose equivalent to glibenclamide (P > 0.05) (Table 3). Blood glucose levels in groups treated with securigenin glycosides and in the group treated with glibenclamide were reduced by approximately 40% after 4 and 6 h, respectively. The onset of action of securigenin glycosides was faster than with glibenclamide. Blood glucose levels in animals treated with compounds 1–3 were significantly lower than for the diabetic control group. Insulin levels in diabetic mice treated with securigenin glycosides were elevated up to normal levels and were comparable with those in non-diabetic animals (P > 0.05). These findings were in line with previous observations with seed extracts of S. securidaca [3, 5].
Conformational analysis and X-ray crystallography
The experimental ECD spectrum of 1 showed two sequential positive cotton effects (CEs) at 314 and 242 nm, along with a negative CE at 223 nm. ECD spectra of cardenolides have been previously studied [27]. Hence, the spectra of 1 could not be compared with references. Therefore, we calculated the ECD spectrum of 1 and compared with experimental data. Detailed experimental data are provided in Supplementary Material. Comparison of experimental data showed an excellent fit with calculated data of the 3S, 8R, 9S, 10S, 13R, 14S, 17R stereoisomer (Fig. 2).
Configuration of sugars, and inter-glycosidic linkage in 1 was confirmed by single-crystal X-ray diffraction analysis of the compound (Cu/Kα radiation) (Fig. 3). Crystal data for compound (1): formula C37H61.46O17.73, M = 790.00, F(000) = 1701.156, colorless needle, size 0.030–0.050–0.270 mm3, orthorhombic, space group P 21 21 21, Z = 4, a = 6.3288(5) Å, b = 18.0687(13) Å, c = 34.032(2) Å, α = 90°, β = 90°, γ = 90°, V = 3891.7(5) Å3, D calc. = 1.348 Mg m−3. The data unambiguously confirmed the structure of 1. The crystallographic data for the aglycon structure have been deposited with the Cambridge Crystallographic Data Center (No 1034938).
Discussion
S. securidaca has a long history in folk medicine as an antidiabetic, but the nature of the active constituent(s) and molecular modes of action have not been established. We confirmed the activity of fractions obtained from seed extracts, and isolated three cardenolide glycosides as active compounds. Compounds 2 and 3 are reported here for the first time, while compound 1 (securidaside) has been previously reported from S. securidaca. We were able to show that these cardiac glycosides decrease blood glucose level due to an increase in blood insulin level, and thereby provide a rational explanation for the use of this traditional remedy.
The structure of compound 1 was confirmed as securidaside (securigenin-3- O -β-glucopyranosyl-(1 → 4)-β-xylopyranoside) by 1D and 2D NMR spectra, and by comparison with previously published data [16, 28–30]. The absolute configuration of 1 was established by ECD measurements and X-ray crystallographic analysis.
The NMR data of compound 2 were very similar to those of 1. The aglycon was also securigenin, and notable differences were detected in the signals attributable to the sugar moiety. In the 1H-NMR spectrum, signals of anomeric protons were seen at 4.40 and 4.38 ppm (d, H-1′ and H-1″) and 3.27 ppm (m, H-1′″). The β configuration of the glucopyranosyl and xylopyranosyl units were confirmed from the doublet signal of its anomeric protons with J constant 7.9 and 8.0 Hz, respectively. All other proton signals appeared as multiples between 3.19 and 3.91 ppm, and the carbon signals were observed between 72.5 and 84.8 ppm. COSY and HMBC spectra revealed a securigenin-β-glucopyranosyl-(1 → 4)-β-xylopyranosyl structure as in compound 1, but with an additional sugar residue attached. The HMBC and COSY spectral analysis of third glycosyl moiety of compound 2 are displayed in Fig. 4. A COSY correlation between H-1′″ (3.27 ppm) and H-3″ (3.39 ppm), and a strong HMBC correlation of H-1′″ (3.27 ppm)/C-3″ (77.7 ppm) confirmed that the third sugar moiety was attached to C-3 of xylose (Fig. 4). The presence of 6 hydroxyl groups on carbons of a cyclitol ring was obvious from the 13C NMR chemical shifts of remaining carbons, and the residue was identified as inositol. The identity of sugars was confirmed by PC after acid hydrolysis. The ECD spectrum of 2 was identical with that of 1, and compound 2 was thus identified as securigenin-3- O -inositol-(1 → 3)-β-glucopyranosyl-(1 → 4)-β-xylopyranoside, a new natural product.
NMR data of compound 3 indicated that it was also a securigenin glycoside. A notable difference was detected in the chemical shift and coupling constant of H-3 (3.21, dd, J = 8.9, 8.0 Hz) compared to compound 1 (δ H-3 4.34, 4.32, d, J = 8.0 Hz) which is due to the connection to a different conformation of sugar molecule. Signals of anomeric protons of α-glucose and α-rhamnose units appeared at 4.88 ppm (d, J = 1.5 Hz) and 4.59 ppm (brs), respectively. The presence of a rhamnose unit was corroborated by doublet at 1.31 ppm (J = 6.5 Hz, 3H) indicative of the methyl group at C-6′. The α configuration of the glucopyranosyl unit was assumed from the doublet signal of its anomeric proton at 4.88 with J constant 1.5 Hz in the 1H NMR spectrum while the configuration of the rhamnopyranosyl unit was deduced from the upfield shift of its C-5 (δ c 68.7) in the 13C NMR spectrum [31].
The structural assignment of compound 3 was confirmed by carrying out 2D NMR techniques such as HSQC, HMBC and H–H COSY. Carbons of the sugar moiety and their attached protons were correlated by an HSQC spectrum. An HMBC correlation between the anomeric proton of glucose (H-1′, 4.88 ppm) and C-3 (76.0 ppm) confirmed the 1 → 3 connectivity of the glucose unit to the aglycone. An HMBC correlation between the anomeric proton of rhamnose (H-1″, 4.59 ppm) and C-4′ (83.4 ppm) of glucose indicated a 1 → 4 connection of the two sugar units. The HMBC spectral analysis of glycosyl moiety of compound 3 displayed correlation peaks between H-1′ and C-2′, H-2′ and C-4′, H-4′ and C-2′, C-1″, C-5″, H-6′ and C-4′ of glucose sugar and H-1″ and C-4′, C-5′, H-5″ and C-3″, and methyl unit on the H-6″ of rhamnose with C-4′ and C-5″. Thus, compound 3 was identified as securigenin-3- O -α-rhamnopyranosyl-(1 → 4)-α-glucopyranoside, a new natural product.
Numerous plant secondary metabolites reportedly possess antidiabetic properties, including cardiac glycosides [32, 33]. Cardenolide glycoside 1, securidaside, has been previously reported from S. securidaca, Coronilla hyrcana and C. varia, and showed Na+–K+ATPase inhibitory activity in a concentration range of 10−9–10−6 mol/l. The activity was between that of ouabain and digitoxin [34]. It increased the amplitude of heart contraction, and bradycardia at 0.5–1 mg/kg, and decreased the arterial pressure and respiration at 0.2–0.25 mg/kg in mice. For securidaside, an LD50 of 25 mg/kg in mice has been reported after subcutaneous injection [35].
Earlier studies showed that infusion of ouabain at 1.0 µg/kg caused a significant decrease in plasma glucose and glycerol concentrations, but did not produce any marked changes in the electrocardiogram or plasma potassium [36]. Subsequently it was shown that the hypoglycemic effect of ouabain was due to an increase of insulin secretion [37]. Enhancement of insulin levels may be a reason for the apparent lack of toxicity of cardiac glycosides in diabetic animals, since insulin has been shown to interact directly with Na+ –K+ATPase and thus protects from cardiac toxicity [38]. The d-glucose on the terminal position of glucose moiety of cardiac glycosides plays an important role in the inhibition of the digitalis receptor [39]. This could be another reason which described non-toxicity of cardiac glycosides without deoxy sugars. The increase of insulin and decrease of glucose levels by cardiac glycosides were shown in our experiment, but more studies are required to prove the efficacy and lack of toxicity of this plant on the heart muscle.
Conclusions
Although the use of S. securidaca has a long history in folk medicine as an antidiabetic agent, the nature of its active ingredient has not been established. Our study confirmed that the seed extract and three isolated cardenolide glycosides are responsible for this activity and compounds 2 and 3 are reported here for the first time. These cardiac glycosides showed a reduction of blood sugar due to an increase of insulin level and thereby provide a rational explanation for the use of this traditional remedy.
References
Rechinger KH (1984) Papilionaceae II. In: Rechinger KH (ed) Flora Iranica 157. Akademische Druck und Verlagsanstalt, Austria
Ali AA, Mohamed MH, Kamel MS, Fouad MA, Spring O (1998) Studies on Securigera securidaca (L.) Deg. Et Dorfl. (Fabaceae) seeds, an antidiabetic Egyptian folk medicine. Pharmazie 53:710–715
Porchezhian E, Ansari SH (2001) Effect of Securigera securidaca on blood glucose levels of normal and alloxan-induced diabetic rats. Pharm Biol 39:62–64
Nagarajan S, Jain HC, Aulakh GS (1982) Indigenous plants used in the control of diabetes. In: Atal C, Kapur B (eds) Cultivation and utilization of medicinal plants. Regional Research Laboratory, India, pp 584–604
Hosseinzadeh H, Ramezani M, Danaei AR (2002) Antihyperglycaemic effect and acute toxicity of Securigera securidaca L. seed extracts in mice. Phytother Res 16:745–747
Zahedi Asl S, Marahel H, Zare B (2005) Study on the effects of chloroformic extract of Securigera securidaca on serum glucose level and liver glycogen content of mice. J Kerman Univ Med Sci 12:32–38
Ghitasi I, Nikbakht MR, Sadeghi H, Sabzali V, Sabzali S, Shahrani M (2007) The hypoglycemic effects of a hydro-alcoholic extract from Securigera securidaca seeds on induced diabetic in male rats. J Shahrekord Univ Med Sci 8:68–73
Pouramir M, Shahaboddin ME, Moghadamnia AA, Parastouei K (2011) To study the effects of Securigera securidaca (L.) seed against alloxan-induced hyperglycemia. J Med Plants Res 5:3188–3191
Roostazadeh A, Firoozrai M, Shabani M (2008) Effect of aqueous seed extract of Securigera securidaca on erythrocytes catalase activity in type 1 diabetic rats. Qom Univ Med Sci J 1:9–14
Al-Hachhim G, Maki B (1969) Effect of Securigera securidaca on electroshock seizure threshold in mice. Psychol Rep 24:551–553
Mard SA, Bahari Z, Eshaghi N, Farbood Y (2008) Antiulcerogenic effect of Securigera securidaca L. seed extract on various experimental gastric ulcer models in rats. Pak J Biol Sci 11:2619–2623
Azarmiy Y, Zakheri A, Allaf Akbari N, Fathi Azad F, Fakhrjo A, Andalib S, Maleki Dizaji N, Babaei H, Garjani A (2009) The effect of total extract of Securigera securidaca L. seed on thoracic aorta function in nigh-fat fed rats. Pharm Sci 15:83–92
Garjani A, Fathiazad F, Zakheri A, Akbari NA, Azarmie Y, Fakhrjoo A, Andalib S, Maleki Dizaji N (2009) The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol 126:525–532
Shahidi S, Pahlevani P (2013) Antinociceptive effects of an extract of Securigera securidaca and their mechanisms in mice. Neurophysiology 45:34–38
Tofighi Z, Asgharian P, Goodarzi S, Hadjiakhoondi A, Ostad SN, Yassa N (2014) Potent cytotoxic flavonoids from Iranian Securigera securidaca. Med Chem Res 23:1718–1724
Zamula VV, Maksyutina NP, Kolesnikov DG (1965) Cardenolides of Securigera securidaca II. Chem Nat Compd 1:117–119
Zatula VV, Chernobrovaya NV, Kolesnikov DG (1966) The structure of securigenin and its biosidesecuridaside. Khim Prir Soedin 2:438–439
Komissarenko AN, Kovalev VN (1987) Hydroxycoumarins and flavones of Securigera securidaca. Khim Prir Soedin 2:298–299
Tofighi Z, Alipour F, Hadavinia H, Abdollahi M, Hadjiakhoondi A, Yassa N (2014) Effective antidiabetic and antioxidant fractions of Otostegia persica extract and their constituents. Pharma Biol 52:961–966
Yassa N, Saeidnia S, Pirouzi R, Akbaripour M, Shafiee A (2007) Three phenolic glycosides and immunological properties of Achillea millefolium from Iran, population Golestan. Daru 15:49–52
Bruker (2006) Bruker Analytical X-Ray Systems, Inc. apex 2, version 2 ed. Madison, M86-E01078
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A, Burla MC, Polidori G, Camalli M (1994) A program for automatic solution of crystal structures by direct methods optimized for powder data. J Appl Crystallogr 27:435–436
Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ (2003) CRYSTALS version 12: software for guided crystal structure analysis. J Appl Crystallogr 36:1487
Prince E (1982) Mathematical techniques in crystallography and materials science. Springer, New York, pp 12–13 (Duoc Hoc 6)
Watkin DJ (1994) The control of difficult refinements. Acta Crystallogr A 50:411–437
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, Streek JV, Wood PA (2008) Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J Appl Crystallogr 41:466–470
Ripperger HH, Lindig C, Snatzke G (1998) Circular dichroism of cardenolides. J Prak Chem-Chem ZTG 340:476–478
Bagirov RB, Komissarenko NF (1966) New cardenolides from the seeds of Coronilla hyrcana. Khim Prir Soedin 2:251–257
Hembree JA, Chang CJ, McLaughlin JL, Peck G, Cassady JM (1979) Potential antitumor agents: a cytotoxic cardenolide from Corronilla varia. J Nat Prod 42:293–298
Ankli A, Heilmann J, Heinrich M, Sticher O (2000) Cytotoxic cardenolides and antibacterial terpenoids from Crossopetalum gaumeri. Phytochemistry 54:531–537
Kasai R, Okihara M, Asakawa J, Mizutani K, Tanaka O (1979) 13C NMR study of a- and b-anomeric pairs of d-mannopyranosides and l-rhamnopyranosides. Tetrahedron 35:1427–1432
Marles RJ, Farnsworth NR (1995) Antidiabetic plants and their active constituents. Phytomedicine 2:137–189
Ross IA (2001) Medicinal plants of the world-chemical constituents. In: Traditional and modern uses. Human Press Inc, Totowa
Mraz M, Opletal L, Sovova M, Drasar P, Havel M (1992) Inhibition of Na+–K+ATPase by the glycosides from Coronilla varia. Planta Med 58:467–468
Ahmad VU, Basha A (2006) Cardenolides and pregnanes. In: Ahamad VU, Basha A (ed) Spectroscopic data of steroid glycosides. Springer, New York, p 2335
Triner L, Killian P, Nahas GG (1968) Ouabain hypoglycemia: insulin mediation. Science 162:560–561
Triner L, Papayoanou J, Killian P, Vulliemoz Y, Castany R, Nahas GG (1969) Effects of ouabain on insulin secretion in the dog. Circ Res 25:119–129
Oubaassinea R, Weckering M, Kessler L, Breidert M, Roegel JC, Eftekhari P (2012) Insulin interacts directly with Na+/K+ATPase and protects from digoxin toxicity. Toxicology 299:1–9
Aubie A, Naranjan D, Grant P, Pawan S (2001) Diabetes and cardiovascular disease etiology, treatment and outcomes. Springer, NewYork
Acknowledgements
This research was a part of PhD thesis and it was supported by Tehran University of Medical Sciences and Health Services Grants (Nos. 11981 and 13726).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tofighi, Z., Moradi-Afrapoli, F., Ebrahimi, S.N. et al. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J Nat Med 71, 272–280 (2017). https://doi.org/10.1007/s11418-016-1060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1060-7