Abstract
Ocimum, commonly known as Tulsi, is a huge genus within family Lamiaceae, comprising about 64 species of annual to perennial aromatic medicinal herbs with a long history of traditional uses. The aromatic plants of the genus Ocimum have long been used as flavouring agents, as well as diverse medicinal applications. Our comprehensive review covers the published literature through the period from 1961 to April 2019 and provides a complete survey of nearly all the studied species up to date. Additionally, all related taxonomic data, geographical distribution as well as different traditional uses are discussed here in details. The major chemical classes within the genus Ocimum include flavonoids, phenolic acids and terpenes. The bioactivities of various extracts or individual compounds, both in vitro and in vivo, include antimicrobial, cytotoxic, antinociceptive, anti-inflammatory, antihyperglycemic and antioxidant. This comprehensive review will serve as a database for future research and drug development from the genus Ocimum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural products have attracted considerable attention with their diverse pharmacological, cosmetic and food industry applications. Today, there is a growing interest towards exploring natural herbal products to solve many health care problems, via both coupling traditional knowledge with medicinal principles and obtaining the advantage of having fewer side effects. Today, many of Lamiaceous species are used as medicinal and aromatic plants such as Oregano, Mentha, Rosemary and many others. In our review, we would like to portray one genus, under the botanical name “Ocimum”, derived from the Greek meaning “to be fragrant”, and which is commonly known under the vernacular name “Tulsi” (Bhasin 2012). It is a genus of aromatic plants, belonging to the tropical tribe “Ocimeae”, comprising about 160 species indigenous to tropical regions of Asia, Africa, Central and South America, with the main center of diversity in Africa (Simon et al. 1999). Recently, a phylogenetic study has recognized 64 species (O’Leary 2016) which are highly valued for their medicinal and aromatic properties in the traditional and modern pharmacological systems. In general, plants of Ocimum are herbs, under shrubs or shrubs containing essential oils of various aromas which are valuable in pharmaceutical, perfumery and food processing industry (Singh and Chaudhuri 2018). According to published literature, the specific taxonomic description of the genus Ocimum L. is still debatable. The genus comprises three subgenera: Ocimum (classified into three sections: Ocimum, Gratissima and Hiantia), Nautochilus, and Gymnocimum (Flegkas et al. 2019).
Among the diverse bioactive compounds of the genus Ocimum, terpenoids, flavonoids and phenolic acids prevailed (Fig. 1). Moreover, biological investigations have been carried out on these compounds and demonstrated their varied bioactivities such as cytotoxic, antimicrobial, and gastroprotective ones (Tan et al. 2002; Ma et al. 2005; Sharma et al. 2012). The current review focuses on recent advances in the taxonomy, phytochemistry, synthesis, biosynthesis, bioactivity and bioavailability of about 260 different compounds as well as different fractions from diverse species of Ocimum, with a special emphasis on O. sanctum, O. basilicum and O. gratissimum, the data which have been collected over the period from 1961 till April 2019. For a comprehensive literature overview, the published phytochemical and pharmacological data have been evaluated through several search engines, such as Pubmed®, Science direct®, SciFinder, ISI®, Scopus® and Google Scholar®, along with Databases such as Metlin and DNP, using ‘Ocimum’ as the search keyword. We disregarded publications pertaining to agronomy, plant pathology, ecology and other unrelated topics (unless any phytochemical or pharmacological data were thoroughly available).
Taxonomic diversity
Morphological studies on Ocimum species showed that they reach up to 2 m or more in height with a small degree of variability in the most recorded traits including, leaf colour and shape, stem, inflorescence, flower and seed production, which further hardens the dependence on morphology for taxonomic identification. Synonyms according to http://www.theplantlist.org/ and detailed morphological features of the most well-studied species are listed in Table 1. Several aromatic compounds can be found in chemotypes of basil reflecting a great variation of available scents and flavors, where many of them contained a combination of linalool and methylchavicol and/or 1,8-cineole, reflecting the traditional sweet basil aroma (Simon et al. 1999). The cultivars showed a wide diversity in growth habit, flower, leaf and stem colors, and aromas which allowed them to be attractive ornamentals. The ‘Maenglak Thai Lemon’, ‘Osmin Purple’ and ‘Red Rubin Purple’ basils were the most attractive and best retained ornamental basils (Phippen and Simon 1998) due to their purple leaf color, which is attributed to the genetically unstable anthocyanins in purple basils, leading to an undesirable random green sectoring and reversion over the growing season (Simon et al. 1990).
In fact, interspecific hybridization and polyploidy usually occurs within the Ocimum species leading to taxonomic confusion which makes it hard to recognize the genetic relationship between many basils (Grayer et al. 1996). Moreover, taxonomy is extra complicated by the existence of chemotypes or chemical races within species that do not significantly differ in morphology (Javanmardi et al. 2002). Furthermore, chemical studies of the essential oil profiles of most species revealed some variations, that’s why those species from the same genus may have different scents, which might play a major rule as key markers in their taxonomy (Chowdhury et al. 2017).
All the aforementioned facts allow the variation of marker contents within Ocimum species, which plays a major role in the taxonomic identification. Those marker constituents in Ocimum species were produced by two different pathways: phenylpropanoids by the shikimic acid pathway and regular terpenes by the mevalonic acid pathway, and the complexity in Ocimum essential oils resulted from differences in the dominance of these species as well as their mixed pathways, morphotypes and chemotypes. Additionally, the differences in chemical composition might also be due to a number of factors including: climatic conditions, geographical locations, seasons of collection, stages of development along with the post harvest processing before oil extraction (Javanmardi et al. 2002).
Traditional uses, folkloric significance and pharmacological activities of most common species
*Ocimum sanctum Linn.
(Commonly called Sacred Tulsi, Indigenous to India, Bhasin 2012). It is known as ‘the Queen of herbs’ in India, and is one of the holiest and most cherished of many healing and healthy herbs of the orient and is renowned for its religious and spiritual sanctity. The whole plant (having a pungent bitter taste) is taken orally as a nerve tonic, adaptogenic and health promotor in cancer conditions (Singh and Chaudhuri 2018). The fresh leaves have stimulant and expectorant properties, the oral infusion is recommended for malarial fever, while the juice is applied in bronchitis, pharyngitis and chest complications (Bhasin 2012). Moreover, the essential oil extracted from leaves is rich in the treasured eugenol which is far used in food flavouring industry and synthesis of vanillin (Bhasin 2012). The therapeutic oral daily dose is 2–3 g leaf powder which is greatly distant from the oral LD50 with a value of 4505 ± 80 mg kg−1 b.wt., and also from the i.p. LD50 with a value of 3241 ± 71 mg kg−1 b.wt. signifying a wide safety range. In general, it is defined by the Ayurvedic Pharmacopeia of India (1999) as an “Elixir of life” and is recommended as an adaptogen helpful for adapting to stress (Muthu et al. 2006; Nazar et al. 2008; Pattanayak et al. 2010; Prakash and Gupta 2005; Sharkar et al. 2013).
Pharmacological activities
Several studies emphasized the immunomodulatory properties of the seed oil (fixed oil) which could modulate both humoral and cell-mediated immune responses and increase antibodies production. The seed oil (3 ml kg−1, i.p.) was daily administered for 6 days to rats in a trial to measure the humoral response and the results significantly gave a rise in anti-sheep red blood cells (SRBC) antibody titre (8.2 ± 0.6), in comparison with the effect when co-administered with diazepam as the control (1 mg kg−1, sc) with antibody titre of 8.8 ± 0.6 (Mediratta et al. 2002). The results, gained after 3 h from injecting the inflammatory stimulus, showed an inhibition of lymphokines release which allows the inflammatory cells to diverge from the site of reaction. Accordingly, besides having a direct anti-inflammatory effect, a 3 ml kg−1, i.p. dose of the oil may have a relevant effect reducing chronic inflammation by inhibiting cell-mediated immune response (Singh and Majumdar 1997).
The essential oil exhibited potent anthelmintic (Rahman et al. 2011) and antileishmanial properties (Mahajan et al. 2013).
Further studies established the strong immunity enhancing and adaptogenic properties of various extracts from the plant (Lee et al. 1985; Godhwani et al. 1988; Mahajan et al. 2013). The long-term feeding of rats with fresh leaves of tulsi (200 and 400 mg kg−1) resulted in suppressing both the sperm count and motility and also reducing the weight of testes, epididymis, seminal vesicles and ventral prostate. An extra study showed a significant increase in serum testosterone levels of rabbits fed with a small amount of fresh leaves of O. sanctum, while FSH and LH levels were significantly reduced. These results strongly support the potential use of O. sanctum as an effective male contraceptive agent (Rahman et al. 2011; Mahajan et al. 2013). Two further studies reported the anti-tuberculosis potential of crude extract of fresh leaves against respiratory disorders (Kelm et al. 2000; Vinukonda et al. 2012), while a third study evaluated the antitussive and antimalarial efficiencies of the ethanol extract, which reinforced its folkloric use as an expectorant, antitussive and against chest problems (Inbaneson et al. 2012).
The designed extract named OciBest (developped by M/s Natural Remedies Pvt. Ltd., Bangalore, India) is obtained by blending water with methanol extract of the whole plant, following the required level of active constituents from ociglycoside-I (> 0.1% w/w), rosmarinic acid (> 0.2% w/w), oleanolic acid and ursolic acid (> 2.5%). This extract proved efficacy against chronic variable stress disorders (Chandrasekaran et al. 2013).
*Ocimum basilicum L.
(Commonly known as Rehan, sweet Basil and is native to India, Bhasin 2012). Indian basils have edible leaves of which their decoction is used as a stimulant, expectorant and as a demulcent for throat congestions, besides proving efficacy against asthma, inflammation and enlarged spleen cases (Mathews et al. 1993).
Pharmacological activities
The i.p. administration of essential oil doses, higher than 0.2 ml kg−1 in mice, significantly impaired the CNS motor activity and increased the latency of convulsions and percent of animals exhibiting clonic seizures. The ED50 values of the essential oil were 0.61, 0.43 and 1.27 ml kg−1, when tested against pentylenetetrazole, picrotoxin, and strychnine-induced convulsions in mice, respectively (Zheljazkov et al. 2007a).
Both alcoholic and aqueous extracts of the whole plant of O. basilicum exhibited a stimulant cardiotonic and adrenergic effect on frog heart with significant positive ionotropic and negative chronotropic actions (P < 0.05) which were antagonized by propranolol (Bilal et al. 2012). In addition, a good hepatoprotective activity was obtained by ethanolic extract of leaves of Ocimum basilicum against liver damage induced by H2O2 (Bilal et al. 2012).
*Ocimum gratissimum L.
(Commonly known as Ram Tulsi, Indigenous to India, Bhasin 2012). The essential oil yield is 0.21% with eugenol as the major component 67% (Nakamura et al. 1999). The ethanolic extract of leaves is orally used in traditional medicine for treatment of urinary tract and GI infections (Bhasin 2012). Whereas, the essential oil is pale yellow in colour, with a high percentage of eugenol, and it is employed in flavouring food products, beverages, dental preparations, topical antiseptics and for usage in the treatment of minor wounds, boils and pimples as well as repelling mosquitoes (Bhasin 2012).
Pharmacological activities
The essential oil obtained from leaves showed significant antibacterial potency against Staphylococcus aureus at MIC of 0.75 µg ml−1 and against Shigella, Salmonella and E. coli at MIC 3–12 µg ml−1. Moderate anthelmintic (Pessoa et al. 2002), antileishmanial (Ueda-Nakamura et al. 2006) and antimalarial (Tchoumbougnang et al. 2005) activities of the oil have been stated. The methanolic extract of the plant exhibited a hypoglycemic activity (Aguiyi et al. 2000) while the alcoholic leaf extract showed an antidiarrhoeal one (Ilori et al. 1996).
*Ocimum americanum L.
(Commonly called Kali Tulsi, Indigenous to India, Bhasin 2012). A decoction of leaves is orally taken for cough, dysentery, bronchitis, immunity disturbance and as a mouth wash for reliving toothache (Chopra et al. 1958). Besides, the essential oil, with citral as the main constituent, is pale yellow in colour with a characteristic odour of lemon, which is externally used in perfumes and pharmaceutical industry.
Pharmacological activities
Many studies proved the anti-inflammatory and antioxidant potential of the essential oil of O. gratissimum (Bayala et al. 2014) as well as the mosquito repellent (Bayala et al. 2014), antimicrobial (Thaweboon and Thaweboon 2009) and larvicidal properties (Cavalcanti et al. 2004).
*Ocimum canum Sims.
(Commonly named as Dulal Tulsi, Indigenous to India). The whole plant infusion is orally administered to relieve fever, dysentry and nose haemorrage. Generally, the most common folkloric uses were directed towards alleviation of migrain and fever by the oral intake of leaves and seeds decoction (Chopra and Nayar 1956). Essential oil, light yellow in colour and rich with Linalool, is externally used in perfumes, flavour and cosmetic industry (Bhasin 2012).
Pharmacological activities
The essential oil was evaluated against Giardia and different parasites and showed strong antigiardial and antiparasitic activities (de Almeida et al. 2007) which in turn strongly supported its anti oxidant, cytotoxic and antiproliferative activities (Manosroi et al. 2006; Selvi et al. 2015). Studies have reported the antidiabetic activity (Mahajan et al. 2013) as well as the CNS sedation (Freire et al. 2006) and antiplasmodial activities of the aqueous extract (Simonsen et al. 2001).
*Ocimum viride Willd.
(Commonly named as Van Tulsi and is native to West Africa, Bhasin 2012). A decoction of leaves is orally taken to relieve fever and cough, the fresh juice of leaves is topically applied for catarrh and as eye drops for conjunctivitis, and the poultice is externally used against rheumatism and lumbago (Bhasin 2012; CSIR 1948). The essential oil is pale yellow and viscid, with a characteristic odour of thymol and has a pungent flavor. It is extensively used in flavoring, perfumes manufacturing and in preservative purposes (Bhasin 2012).
Pharmacological activities
Studies reported the anti-inflammatory, apoptotic and cytotoxic activities of the essential oil (Sharma et al. 2010) as well as the antibacterial potential of the ethanolic extract (Pesewu et al. 2008).
*Ocimum kilimandscharicum Guerk.
(Commonly named as Kapur Tulsi, indigenous to West Africa, Bhasin 2012). The whole plant infusion has stimulant, antifungal and antibacterial properties. Essential oil is light yellow with strong odour of camphor, and it is widely used in perfumes, flavouring, pharmaceutical purposes and in various dental and oral preparations (Khosla and Bhasin 2000; Bhasin 2012). The most common traditional use is to repel insect, which was supported by studies.
Pharmacological activities
A study proved that leaves crude extract is effective via inhalation against many types of insects (Jembere et al. 1995). Another study supported the use of that extract as a stimulant with an antiproliferative activity against MCF-7 cell line (Ntezurubanza et al. 1984).
*Ocimum suave Willd.
(Commonly named as Wild Basil, indigenous to West Africa, Bhasin 2012). The oral administration of whole plant decoction is ethnomedicinally used for the treatment of cough, inflammation, abdominal pain and topically against ear and eye inflammation (Bhasin 2012). Regarding the essential oil, it is highly viscid with a light yellow colour and a balsamic woody dust odour. It is a rich source of sesquiterpene alcohols and it is widely used in flavouring of tobacco and snuff, as a body perfume and a mosquito repellent (Chopra et al. 1958).
Pharmacological activities
Tan et al. (2002) proved that the methanolic extract of leaves exhibited a gastroprotective effect.
*Ocimum carnosum.
(Commonly named as Basil Pepper, indigenous to West Africa, Bhasin 2012). The fresh leaves have been traditionally used for flavouring and its odour is useful in insect repellent purposes in Brazil (Bhasin 2012). Essential oil is light yellow, viscid with strong spicy earthy odour and is a rich source of elemicin which is highly valued for its vast pharmaceutical and flavouring properties. Elemicin is widely employed in the production of 3,4,5-trimethoxy-benzaldehyde which forms the starting material for the synthesis of trimethoprim as an important antibacterial agent. The most common traditional use of the plant was to repel insects (Bhasin 2012).
Pharmacological activities
The essential oil demonstrated a strong insect repellent activity (de Paula et al. 2003).
*Ocimum forskolei Benth.
(Commonly named as Habak and is indiginous to South Asia especially Yemen, Dekker et al. 2011). The whole plant is traditionally used as a cosmetic, antioxidant, anti-infectious and as a mosquito repellent, but the most common use is for flavouring (Waka et al. 2006; Dekker et al. 2011). Essential oil is light yellow with a high content of camphor and the oral administration of leaves extract is reported to be efficient against bacteria and dermatophytes, the results which supported its antibacterial and anti-infectious traditional uses (Al-Hajj et al. 2014; Ali et al. 2017).
Pharmacological activities
The essential oil exhibited antimicrobial, antioxidant, cytotoxic (Ali et al. 2017) and mosquito repellent activities (Dekker et al. 2011).
Out of the aforementioned collected results, the main geographical origin appears to be South Asia followed by central and west Africa, the temperate regions which may support the rule of high temperature in the production and biosynthesis of phytoconstituents. In conclusion, the main medicinal and traditional uses appeared to be concerned with immunostimulant, antimicrobial and antiinflammatory cases, as well as insect repellent purposes.
Clinical studies
Despite the long history of traditional uses of Tulsi (clinical studies usually use the term ‘Tulsi’ to refer to O. sanctum or O. gratissimum), relatively few human intervention studies have been conducted on its effectiveness against clinical conditions. The 24 human studies recognized up to date could be classified according to three main clinical domains including: metabolic disorders (15 studies), neurocognitive and mood conditions (4 studies), and immunity as well as microbial or viral infections (5 studies), all of which are extremely relevant to the growing world-wide epidemic of lifestyle-related chronic diseases (Jamshidi and Cohen 2017). Results of these studies focused on the effective adaptogenic rule of Tulsi to fight the psychological, physiological, immunological, and metabolic stress of modern living. In a further 12-week randomized trial in diabetics, 2 g of Tulsi leaf extract, whether alone or combined with neem leaf extract, produced marked reduction in diabetic symptoms with the advantage of improved efficacy as a result of the combination (Kochhar et al. 2009).
Two further clinical trials studied the effect of 10 g day−1 of an aqueous extract of fresh Tulsi leaves in acute viral infection patients as well as a study on patients with acute viral encephalitis could prove increased survival rates after 4 weeks in the Tulsi group compared to the dexamethasone control group which reported symptomatic improvement after 2 weeks (Das et al. 1983; Rajalakshmi et al. 1986). Moreover, a clinical trial has demonstrated an improvement in cognitive flexibility, short-term memory and attention in 40 healthy young adults (17–30 years) following daily treatment with 300 mg of tulsi for 4 weeks (Sampath et al. 2015). All reviewed studies reported favourable clinical effects with minimized or no side effects, except only one clinical trial which reported transient mild nausea (Satapathy et al. 2017). As the longest study was only 13 weeks, the failure to report any adverse effects does not preclude the presence of any long term side effects. However, the long traditional history of regular Tulsi use suggests that daily ingestion of Tulsi is safe especially against mode and immunity problems (Saxena et al. 2012).
Safety of Ocimum
*Ocimum sanctum
Acute toxicity studies performed on mice have used the leaves ethanol extract of O. sanctum and revealed that it did not harvest any hazardous symptoms, CNS and ANS toxicities or death when administered up to 2000 mg kg−1. Moreover, subacute treatment did not show any changes in body weight, food or water consumption and hematological and biochemical profiles when administered up to 800 mg kg−1 day−1 for 28 days (Gautam and Goel 2014).
Furthermore, the essential oil proved safety in the “in vitro” test on primary cultures of cardiomyocytes at 1200 µg mL−1, and “in vivo” when the acute oral toxicity at 2000 mg kg−1 was tested. Additionally, the essential oil of Ocimum sanctum L. var. cubensis showed no acute dermal toxicity on mice classifying this test substance as a “NON TOXIC” according to Directive No. 402 of the Organization for Economic Cooperation and Development (OECD). Consequently, the obtained results revealed that the oil could be considered safe, both topically and orally, without showing any in vitro or in vivo toxicity (LD50 of 42.5 ml kg−1 in rats). Accordingly, all the abovementioned results could prove that both the ethanol extract and essential oil of O. sanctum could be safe for human use (Chil Núñez et al. 2017).
*Ocimum basilicum
The results of the acute toxicity study performed on rats showed the LD50 of O. basilicum to be higher than 5 mg kg−1, while the subchronic study showed no adverse effects on serum parameters in both male and female rats. The hematological results showed a reduction in the hematocrit, platelets and RBC in both sexes, but no abnormalities were observed in other parameters. The overall results indicated that the hematologic system could serve as a target organ in oral toxicity of this plant (Rasekh et al. 2012).
*Ocimum gratissimum
In spite of its promising clinical relevance, its ingestion has been related with nephrotoxicity and hepatotoxicity following high doses of exposure (which is dose dependent (Onaolapo and Onaolapo 2012) and male reproductive toxicities (Njan et al. 2019). However, these toxicity studies focused on the crude extract and essential oils from the Ocimum gratissimum leaves and there have been no documented studies that investigated the probable safety of fractions from the crude leaf extract (Njan et al. 2019).
In conclusion, O. gratissimum may be useful in the treatment of certain ailments, but caution should be considered during the therapeutic applications of the plant (Ebeye et al. 2014).
In general, the majority of clinical trials showed minimized or no hazardous side effects, except only one which reported transient mild nausea So, Ocimum species can be considered safe adaptogenic herbs used to improve mode and immunity, but further standardization and wide-ranged quality control analysis are needed (Satapathy et al. 2017).
Phytoconstituents and pharmacological activities
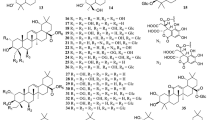
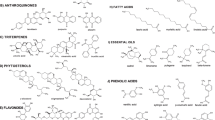
Different classes of compounds detected in different Ocimum species include: flavonoids, simple phenolics, terpenoids, coumarins, anthocyanins, essential oils, fixed oils and steroids. They are summarized in Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11, and their summarized pharmacological activities are listed in Table 2.
Flavonoids
So far, the literature survey has indicated the isolation of 58 flavonoids (from 1 to 58) from the genus Ocimum, most of them were purified from O. sanctum (Singh and Chaudhuri 2018).
A chemical study described the distribution and natural abundance of 8-oxygenated flavones on Ocimum leaf surface by the aid of APCI-MS has identified six compounds: Eupatorin 19, Cirsilineol 22, Salvigenin 34, Gardenin B 38, Apigenin 43 and Cirsimaritin 54 as majors, Luteolin-7-O-β-d-glucopyranoside 13, Luteolin-7-O-β-d-glucouronide 14, Luteolin-7-O-β-d-glucouronic acid-6″-methyl ester 15, Apigenin-7-O-glucouronide 45 and Apigenin-7-O-β-d-glucoside 46 as common ones and Galuteolin 39 and Apigenin-5-O-β-d-glucoside 40 as key markers for the genus (Grayer et al. 2002). Both Quercetin 1 and its derivative, Quercetin-3-O-rutinoside 3 which were isolated in 2002 from O. sanctum and O. kilimandscharicum leaves, respectively, showed a strong gastroprotective effect (Table 2). The latter has a strong antibacterial activity against S. epidermidis and E. faecalis (MIC = 18 µM), when compared to tetracyclin as a positive control (MIC 0.22 and 4.5 µM, respectively) (Rigano et al. 2007). The mechanism of antibacterial action of both compounds resulted mainly from the presence of an O-dihydroxy in the B ring (catechol), and additionally a 2,3 double bond in conjugation with a 4-Oxo function, as well as the presence of hydroxyl groups in positions 3, 5 and 7 in their structures (de Lira Mota et al. 2009). An analogue of Quercetin 1, is Isoquercetrin 5, with an acceptable oral daily intake of 5.4 mg kg−1 day−1, and an oral LD50 in Sprague-Dawley rats of > 25 g kg−1, showed a high degree of safety (Valentová et al. 2014). It also displayed a significant anti-asthmatic potency, in comparison with cromolyn sodium as a control, against the ovalbumin antigenic and leukotriene-induced response in guinea pigs airways at 10 mg kg−1 dose and with MIC value of 6.4 µM (Marwat et al. 2011).
Luteolin 11 (with oral LD50 of 411 mg kg−1 in rats), a flavone isolated from O. sanctum leaves (Nagaprashantha et al. 2011), exhibited a significant antiviral neuraminidase activity against neuraminidase (NA) of the H1N1 influenza virus where it gave IC50 value of 17.96 ± 2.38 µM when compared to oseltamivir as a controI with IC50 value of 0.10 ± 0.02 µM. This SAR study revealed that the presence of a hydroxyl group at both C-4′ and C-7 of flavone skeleton showed greater inhibitory activity than other flavonoids lacking hydroxyl groups at the same positions (Bang et al. 2016). Other flavonoids as Orientin 16, (with i.p. LD50 in mice of 6.5 mg kg−1) showed excellent antioxidant properties compared to dimethylsulfoxide as a control, and inhibited TBARS formation at concentrations above 0.25 µM, with % inhibition of 5.2 ± 3.13% (Uma Devi et al. 2000). It also displayed a pain relieving activity in mice with an MI of 70.3 at 10 mg kg−1 dose, and surprisingly, it was 20-fold more potent than acetylsalicylic acid (MI 90.1 ± 2.5 at 60 mg kg−1), and 3.5-fold more dynamic than indomethacin (MI 70.6 ± 2.0 at 60 mg kg−1) which gave a chance to be used as an alternative antinociceptive remedy (Da Silva et al. 2010). Salvigenin 34 displayed a strong neuroprotective potential at a concentration of 25 μM, significantly unlike chloroquine-treated cells as a standard, with verification that it acts via promoting cell servival by both inhibiting apoptosis and enhancing autophagy (Rafatian et al. 2012).
Regarding the unusual flavonoids in the Lamiaceae, the rare one, Nevadensin 37, is considered to be a promising natural bioactive substance (a novel natural lead) having a wide array of bioactivities (Brahmachari 2010). It unveiled an excellent activity against the H-37Rv strain of Mycobacterium tuberculosis, on L-J medium, at MIC 0.00022 µM (compared to streptomycin as a standard at conc. 22.5 µM) (Reddy et al. 1991). Additionally, it showed a 100% cytotoxicity at a concentration of 0.00016 µM in both Dalton’s lymphoma and Ehrlich ascites tumour in another related study (Reddy et al. 1991). Concerning the significant antioxidants, Vicenin 44, is a good one which could inhibit the TBARS formation at concentrations above 0.25 µM compared to DMSO as a control which gave a % inhibition of 2.3 ± 0.26. The mechanism of action of both Vicenin and Orientin suggested a liver protection against lipid peroxidation by scavenging the radiation-induced free radicals, thus preventing their interaction with membrane lipids (Uma Devi et al. 2000). In a recent study, the same compound, Vicenin 44 exhibited potent radiosensitizer and cytotoxic activities in non-small cell lung cancer cells, only in combination with radiation and in a dose dependent manner. The bioactive concentration of 20–50 µM of this compound caused no decrease in cell survival, the one from 60 to 100 µM caused a gradual decrease while that at a 80 µM conc. resulted in a 50.08 ± 0.048% decrease (Baruah et al. 2018).
Amongst different bioactive flavonoids isolated from the genus, was Isovitexin 51, which displayed a highly significant (P < 0.001) antidiabetic potential. The mechanism of antidiabetic action was suggested to be via inhibition of both RLAR and HRAR, giving IC50 values of 0.49 ± 0.08 and 0.13 ± 0.03 µM, in comparison with quercetin with IC50 values of 0.83 ± 0.10 and 0.07 ± 0.03, respectively (Moumbock et al. 2017). On the other hand, Isothymusin 56, Luteolin 11 and Apigenin 43 with oral LD50 1.37 mg kg−1 in rats exhibited moderate anticancer activities. Anticancer molecular docking analysis illustrated their inhibitory interaction of the EGFR (one of the highly expressed proteins inducing metastasis in OSCC) and showed high gliding scores of − 9.98, − 9.51, and − 9.45 kcal mol−1 and binding energies of − 42.63, − 48.28, and − 44.95 kcal mol−1, respectively (Moumbock et al. 2017).
Biosynthesis
The biosynthetic pathway of the synthesis of salvigenin 34 via hydroxylation and methylation of apigenin 43 (the general precursor for the pathway) occurs in the peltate trichomes on basil leaf surface. The accumulating intermediates, Apigenin 7,4′-dimethylether 32, Salvigenin 34, Genkwanin 47, Ladanein 53 and Cirsimaritin 54 as well as the key compound Apigenin 43, in connection with substrate affinities of 7-O, 6-O and 4′-O-methyltransferases directs the order of the metabolite decoration with methyl and hydroxyl groups and indicates the most favourable biosynthetic way (Berim et al. 2012). Apigenin 43 is also considered as a general precursor for the production of nevadensin 37 with a step of O-demethylation at the 7 position on the ring A of Gardenin B 38 as an intermediate (Berim and Gang 2013).
Bioavailability
The highest bioavailability has been recorded for isoflavones, followed by flavanols, flavanones and flavonol glycosides, while the least one was observed with proanthocyanidins, flavanol gallates and anthocyanins. Flavonoid glycosides are deglycosylated prior to the intestinal uptake, whereas aglycones are being transported to the liver to undergo extensive metabolism producing different conjugates which in turn are proposed to be responsible for the health-promoting effects of flavonoids (Viskupicova et al. 2008; Thilakarathna and Rupasinghe 2013). For example, the bioavailability of quercetin metabolites were five times higher when given to healthy human volunteers (Hollman 2004) as Quercetin-4′-O-glucoside (Cmax: 2.1 ± 1.6 µg ml−1) instead of Quercetin-3-O-rutinoside (Cmax: 0.3 ± 0.3 µg ml−1) which indicate the great effect of the type and position of sugar moiety on rate of absorption (Thilakarathna and Rupasinghe 2013).
Lignans and neolignans
Chemical investigation of the genus Ocimum resulted in the isolation of 2 lignans and 7 neolignans from 59 to 67 (Table 2; Fig. 3). Lignans and neolignans are polyphenolic compounds rarely detected in Ocimum, but known for their leishmanicidal properties (Suzuki et al. 2009). Tulsinol A 61 and Tulsinol B 62 have very interesting structures comparable to the flavonoidal skeleton and were suggested to be synthesized from eugenol through oligomerization. Thus, they might belong to the neolignan group in a biosynthetic standpoint, and to the flavone group in a structural standpoint via having a flavan-3-ol skeleton (Suzuki et al. 2009).
Biosynthesis
Lignans share a common biosynthetic pathway, consisting of two propyl-benzene units coupled by a β,β′-bond, thus being classified under diphenolic compounds (Rodríguez-García et al. 2019).
Bioavailability
Lignans metabolized after ingestion, by intestinal bacteria, followed by transformation to mammalian lignans (enterolactones and enterodiols) prior to absorption, which considerably decreases the risk of different types of cancer, particularly of the colon, prostate and breast (Rodríguez-García et al. 2019). Among the 9 lignans and neolignans detected in various species of Ocimum, Tulsinol C showed the highly significant leishmanicidal activity with IC50 value of 20.5 µM (P < 0.001), followed by both Tulsinols A & F (P < 0.05), while the others were considered weakly active (Suzuki et al. 2009). On the other hand, Rabdosiin and shimobashiric acid were bioactive compounds having a diversity of moderate efficacies, but were considered as weakly active antiproliferative agents (Flegkas et al. 2019).
Phenolic compounds
Chemical investigation of ocimum resulted in the identification of 41 phenolic compounds over the period from 1974 to 2019 (68–107, Table 2) (Fig. 4)
Quantitative analysis of phenolic acids (PhAs) in both flower and leaf tissues gave a value of 99.62 mg g−1 DW, which may be due to dry and warm conditions of the geographical origin and which is protective for the plants against the fluctuating environmental conditions. Accordingly, the production of caffeic acid is highly dependent on climatic conditions. In the same context, the highest amounts of lithospermic acid 86 were observed in the flowers (2500 mg g−1 DW), where Syringic 68, Protocatechuic 72, Vanillic 74, p-coumaric 91 and Ferulic acids 93, in most of the accessions ranged around 10–250 mg g−1 DW and were significantly correlated with Rosmarinic acid (RA). Caffeic acid 94 concentration was the lowest in the flower and leaf extracts (< 10 mg g−1 DW) (Javanmardi et al. 2002). In general, since phenolics are stress-related compounds produced under harsh environmental conditions, it seems that in humid and semihumid conditions there is no need to produce these compounds (Javanmardi et al. 2002).
Considering different bioactivities of PhAs, Ferulaldehyde 98 exhibited a highly significant leishmanicidal activity against Leishmania major with IC50 2.27 μM, compared to amphotericin B as a standard with IC50 0.09 μM (Suzuki et al. 2009). Moreover, Chicoric acid 99, displayed a moderate but selective inhibitory activity of HIV-1 integrase at conc. 50 µM, with IC50 0.1–0.5 μM for the end-processing/strand transfer reactions and 0.1–0.2 μM for the disintegration reactions, respectively (ED50 1–2 µM and CT > 200 µM), compared to Oseltamivir as a control with IC50 = 0.10 ± 0.02 µM (Bang et al. 2016). Furthermore, Rosmarinic acid (RA) 100 (with a safe oral LD50 up to 100 mg kg−1 in rats) was investigated for neuraminidase (NA) protecting potential of cells from destruction during H1N1 viral replication and gave IC50 of 16.65 ± 0.19, EC50 of 22.6 ± 2.76 and CC50 of ~ 200 μM. Molecular docking analysis revealed that the 3,4-dihydroxyphenyl moiety of caffeic acid is essential for the activity and suggested that the exploration of potent NA inhibitors from caffeic acid derivatives is promising to deal with influenza virus and that the two units of caffeic acid moiety definitely exhibit strong NA inhibitory activity (Bang et al. 2016). SAR analysis indicated that at least one carboxylic acid moiety and a bisphenol, as caffeoyl (3,4-dihydroxycinnamoyl) or galloyl (3,4,5-trihydroxybenzoyl), are necessary for the anti-HIV activity, while removal of the carboxylic acids or replacement with either cyclic or acyclic alkyl groups resulted in a comparable in vitro HIV-IN inhibition and no antiviral activity. The antibacterial activity of hydroxybenzoic acids was found to decrease with an increasing number of hydroxyl groups in correlation with their lipophilicity, while that of hydroxycinnamic acids doesn’t depend on the substitutions of the aromatic ring with hydroxyl or methoxy groups, but is strongly dependent on the double bonds of the side chain (Sánchez-Maldonado et al. 2011).
Biosynthesis
Phenolic compounds are formed via the shikimate pathway, with shikimic acid as the main resulted metabolite. The pathway consists of seven reaction steps, beginning with an aldol-type condensation of phosphoenolpyruvic acid (PEP) from the glycolytic pathway, and d-erythrose-4-phosphate, from the pentose phosphate cycle, to produce 3-deoxy-d-arabino-heptulosonic acid 7-phosphate (DAHP). A key branch-point compound is chorismic acid which is considered to be the final product of the shikimate pathway (Santos-Sánchez et al. 2019).
Bioavailability
Benzoic acid derivatives, as gallic acid, are well absorped after being methylated or glucouronated (Shahrzad et al. 2001). Both chlorogenic and chicoric acids were cleaved by microbial esterase, hydrolyzed to caffeic acid (CA) and then reduced to dihydrocaffeic acid, which was then dehydroxylated to m-hydroxyphenylpropionic acid (Qiang 2011). On the other hand, cinnamic acid derivatives, as Ferulic acid, when supplemented to rats gave Tmax reached 30 min of the acid with a reduction of bioavailability of sulfoconjugates after esterification (Adam et al. 2002). Comparing the bioactivity of CA with RA, after oral administration to rats at 100 µM kg−1 BW, gave a 9.7-fold higher absorption efficacy in the former than that of the latter (Konishi et al. 2005).
Amongst the 41 phenolic compounds detected in Ocimum, 32 compounds gave a wide range of biological activities while the other nine were inactive. Gallic acid methyl ester was considered as the most potent and highly significant (P < 0.001) antibacterial phenolic compound (Mondal et al. 2009), while rosmarinic acid was a potent (P < 0.05) antiviral one. Both citrusin C and lithospermic acid exhibited a significant capacity (P < 0.05) to suppress NO, while Ferulaldehyde was the most potent leishmanicidal compound (P < 0.001) isolated from the genus, followed by bieugenol (P < 0.01). Considering the field of apoptosis, Syringin was highlighted as the most active and highly significant (P < 0.001) anti-apoptotic bioactive compound isolated from the genus exhibiting about 0.98 ± 0.02 and 0.90 ± 0.01 folds suppression of neuronal toxicity leading to apoptosis at concentrations of 5 and 20 μM, respectively (US et al. 2015).
Anthocyanins
Eight anthocyanins 108–115 (Fig. 5) of both the cyanidin- and peonidin-based types were purified in 1998 from crude extracts of O. basilicum leaves, where they showed attractive antisickling activity (Phippen and Simon 1998). Anthocyanins are polyphenolic compounds abundantly found in plants as well as different types of foods (Panche et al. 2016). The total extractable anthocyanins yield among commercial purple basil cultivars ranged from (6.5 ± 1.1 mg 100−1 g FW) in Purple Bush and small leafed cultivar to (18.7 ± 0.8 mg 100−1 g) in Dark Opal, Purple Ruffles and large leafed cultivars (Phippen and Simon 1998).
Biosynthesis
From a biosynthetic point of view, both flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase catalyze the hydroxylation of dihydrokaempferol to form (2R,3R)-dihydroquercetin and dihydromyricetin, respectively. Both enzymes determine the hydroxylation pattern of the B-ring of flavonoids and anthocyanins for cyanidin and delphinidin production, respectively, together with being the key enzymes determining the structures of anthocyanins and specifying their colours (Tanaka et al. 2008). After oral administration, they could be absorbed from the stomach or intestine and appear in the bloodstream in 6–20 min after consumption to reach maximum levels after 15–60 min. Anthocyanins such as cyanidin-3-glucoside could be absorbed in its intact form into the GI wall, undergo extensive first-pass metabolism and enter the systemic circulation as Phenolic acid metabolites which were found in the blood stream in much higher concentrations than their parent compounds. These metabolites could be responsible for the associated health benefits (Fang 2014; Pojer et al. 2013).
Out of the eight detected anthocyanins, only two proved bioactivity, while the other six were considered inactive. Cyanidine-3-glucoside is one of the most potent apoptotic agents isolated from Ocimum which could, at a concentration of 10 µM tested for 48 h in comparison with Doxorubicin as a control, modulate the expression of caspase-3 and PARP in HS578T cells, inhibit tumor cell growth, induce apoptosis in vitro and suppress tumor growth in vivo as determined by western blot analysis (P < 0.001) (Chen et al. 2005).
Coumarins
Four coumarins 116–119 (Fig. 6) were isolated from the genus Ocimum, where Aesculetin 117, Aesculin 118 and coumarin 116 are the most bioactive ones (Yordi et al. 2017). Aesculin 118 displayed a significant antioxidant activity with IC50 4.7 and 1.35 μM in DPPH and superoxide anion radical assays, respectively, in comparison with BHA as the control with IC50 of 32.4 and 72.9 μM, respectively (Lee et al. 2007). Aesculetin 117 exhibited a significant cytotoxicity on the viability and apoptosis of leukemia cells as determined by MTT assay, cell cycle analyses, DNA fragmentation, and chromatin condensation. The results revealed that concentrations of 0–225 μM of the compound administered for 24 h could inhibit the U937 cells growth compared to K562 and HL-60 cells (α-tubulin was used as internal control) (Chu et al. 2001).
Biosynthesis
A cytochrome P450 reaction is involved in the biosynthesis of coumarins which originate from the phenylpropanoid pathway with an ortho-hydroxylation of hydroxycinnamic acids as a fundamental step that has received inadequate attention in literature (Bourgaud et al. 2006). After oral administration of 120 mg kg−1 of both Aesculin and Aesculetin, the mean Cmax values were 340.3 and 316.5 ng mL−1, respectively. The bioavailability of esculin was calculated to be 0.62% (Rehman et al. 2015).
Terpenoids
Terpenoids (compounds 131–238) are the major class of compounds prevailing in the genus Ocimum, which are divided into: Triterpenes 120–130 (Fig. 7), monoterpenes, diterpenes, and sesquiterpenes 131–238 (Fig. 8). Monoterpenes are generally found in oils of aromatic plants especially in the Lamiaceae which encounters most of the terpenoidal compounds occuring in plants in nature as Mentha, Rosemary and Thyme (Singh and Chaudhuri 2018).
Triterpenes
The quantification studies highlighted Ursolic acid (UA) 120 (ursane-type triterpene, with oral LD50 9.26 g kg−1 in mice), as the most prevalent compound within the genus with 0.252–0.478% w/w and 0.62–19.10 mg g−1 using HPTLC and UPLC-ESI–MS/MS, respectively (Prabhu et al. 2009). It possesses a wide range of activities, where it showed a significant antiproliferative action against MCF-7 human breast cancer cells, with the maximum effect obtained with 15 and 20 µM (about 30–40% of increase with respect to control). The mechanism of antiproliferative action was proposed to occur by inducing autophagy and apoptosis, and in vitro suppression of inflammatory responses via the PI3K/AKT and NF-KB signaling pathway (Luo et al. 2017). A further study examined the SAR of UA which revealed that the triterpenes possess two hydrogen-bond forming groups (an H-donor and a carbonyl group) at positions 3 and 28 which are responsible for the cytotoxic activity. Such activity concerns with the configuration at C-3, at which the introduction of an amino group greatly increases the potency up to 20 times than the parent UA, while selectivity greatly increases in the 28-aminoalkyl dimer compounds (Ma et al. 2005). Moreover, UA also proved an anti HIV-1 protease efficacy with IC50 value of 8 μM, and a potent leishmanicidal activity against L. major with IC50 of 4.95 µM (compared to amphotericin B as a control with IC50 0.09 μM) (Suzuki et al. 2009).
On the other hand, the six triterpenic acids: UA 120, 3-epimaslinic acid 122, Oleanolic acid 124, Alphitolic acid 125, Betulinic acid 127 and Euscaphic acid 128 showed a significant hepatoprotective potential against CCl4-induced oxidative stress, and gave a % inhibition ranged from 52.7 ± 15.7 to 56.8 ± 1.6% at MIC 0.22 μM when compared to silymarin as the positive control (Marzouk 2009). One of the bioactive compounds detected in Ocimum is Oleanolic acid 124, (LD50 1500 mg kg−1 i.p. mice) which was considered a highly significantly active cytotoxic compound which was Brine shrimp lethality-tested against a panel of seven human solid tumor cell lines: lung carcinoma, breast carcinoma, colon adenocarcinoma, renal carcinoma, prostate adenocarcinoma, pancreatic carcinoma and yellow fever mosquito larvae Aedes aegypti, and gave ED50 values of 7.11, 5.5, 7.02, 7.04, 5.8, 7.8 μM, and LC50 of 0.15 μM, respectively. Such results were compared to adriamycin as positive control which obtained ED50 values 0.04, 0.78, 0.08, 0.098, 0.075, 0.017 μM, and LC50 2.27 × 10−1, respectively) (Njoku et al. 1997).
The inclusive SAR of the previous triterpenes revealed that they possess two hydrogen-bond forming groups (an H-donor and a carbonyl group) at positions 3 and 28 which exhibited cytotoxic activity, especially the configuration at C-3 which proved a great influence on the activity (Prabhu et al. 2009).
Biosynthesis
Triterpenoids and sesquiterpenoids are biosynthesized via the mevalonate pathway, whereas monoterpenoids, diterpenoid, and tetraterpenoids are biosynthesized via the methylerythritol phosphate pathway. Generally, a cyclization of 2,3-oxidosqualene is catalyzed by oxidosqualene cyclase oxidation, then further modifications occur via glycosylation (Sawai and Saito 2011). In the western world, the individual average human consumption of triterpenes is estimated, and found to be approximately 250 mg day−1, and reached up to 400 mg day−1 in the Mediterranean countries. Both Betulinic and oleanolic acids are fairly absorped from the GIT, so derivatives have been synthesized to improve permeability to reach up to 4.9–32.7%.
Out of the 11 isolated triterpenes from the genus Ocimum, 8 compounds were reported to be bioactive while the other 3 had no reported activity. Ursolic acid was outlined as the king of bioactive triterpenes with a diversity of bioactivities. Although being a moderate hepatoprotective agent, it gave, as well as oleanolic acid, the highest significant results as cytotoxic one (P < 0.001) when compared to doxorubicin. It was also emphasized as the strongest both antiviral and antileishmanial triterpene with the least IC50 values, as previously mentioned.
Essential oils
About 107 different compounds (from 131 to 238) belonging to the essential oils were detected in the genus Ocimum. Many of them showed a wide range of bioactivities, where methyl eugenol 84 showed a significant leishmanicidal activity (IC50 = 9 µM and IC90 = 15.76 µM) (Zheljazkov et al. 2007b), while β-caryophylline oxide 208 exhibited a significant antiproliferative activity against MCF-7 cell line with IC50 10.8 µM. (Compared with doxorubicin as the control with IC50 < 0.2 μM, Singh et al. 2014).
Biosynthesis
Essential oils are biosynthesized via two complex natural biochemical pathways: isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) as the universal precursors, and they are produced by the cytosolic enzymatic MVA pathway or by the MEP pathway. In the particular plant cell part, prenyl diphosphate synthases condense isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) further to form prenyl diphosphates, which are used as substrates for geranyl diphosphate (GPP, C10) or for fernesyl diphosphate (FPP, C15) (Rehman et al. 2016).
Bioavailability
Essential oils can be absorbed from the food matrices or as pure products and cross the blood brain barrier easily due to their lipophilic character and small size. When 10% aqueous solution of α-pinene, limonene, camphor and borneol was given orally to mice, the bioavailability obtained was 61%, 66%, 54%, and 58%, respectively, and when the same compounds were topically applied to skin shaved mice, no one was absorped preferably indicating that the oral bioavailability is better than the dermal one, besides the role of inhalation which provides the most efficient bioavailability results (Kohlert et al. 2000; Pattanayak et al. 2010; Djilani and Dicko 2012).
Out of all the simple terpenoids classified under the essential oils, 29 monoterpenes out of the detected 45 compounds had no reported activities up to date, and amongst the 44 isolated sesquiterpenes, 39 compounds were considered inactive. Regarding the most bioactive ones, methyl eugenol and α-cadinene gave the best ever leishmanicidal activity with nearly equal IC50 values. Moreover, both β-caryophylline and its oxide were highly significant antiproliferative agents against MCF-7 cell line with also nearly equal IC50 values (Ntezurubanza et al. 1984). On the other hand, essential oil extracted from O. gratissimum leaves proved a highly significant inhibition of S. aureus at MIC of 0.75 µg ml−1 and against Shigella, Salmonella and E. coli at MIC 3–12 µg ml−1, compared with DMSO (100 µl) as a standard (Nakamura et al. 1999). The best ever cytotoxic results amongst the Ocimum bioactive fractions were assessed by the colorimetric SRB assay of the essential oil extracted from O. viride aerial parts, where they inhibited COLO 205 cells, with IC50 values of 0.070, 0.058 and 0.033 µg ml−1 at 24, 48, and 72 h, respectively. The results were highly significant (P < 0.001) when compared with DMSO as a control which didn’t produce any efficacy (Sharma et al. 2010).
Fixed oils (fatty acids)
About 13 fatty acids and fatty acid derivatives (239–251) have been detected up to date in the genus Ocimum (available studies concerned only with O. sanctum and O. basilicum) over the period from 2002 to 2014 (Fig. 9). In general, most of them exhibited good antihypercholesterolemic properties, as well as other diverse bioactivities. Both Oleic acid 239 and Linoleic acid 240, which were isolated from O. sanctum seed oil (Mondal et al. 2009), proved efficacy as antimicrobial agents, and when they were SAR investigated, it was found that the number and position of double bonds as well as modifying the carboxyl group in their structures could greatly influence their activity (Huang et al. 2010). On the other hand, the antiarthritic potential of the fixed oils obtained from O. sanctum was investigated, and showed a significant inhibition of turpentine oil-induced joint edema in rats at a concentration of 3.0 ml kg−1, i.p. at 1, 2, 3, 4, and 5 h compared with aspirin as control (100 mg kg−1) (Singh and Majumdar 1996).
A further related study reported that the antiulcer potential of O. sanctum seed oil could probably be due to its anti secretory effects (Bilal et al. 2012). Concerning antihypercholesterolemic potential of that oil, it significantly reduced serum cholesterol, triacylglycerol, LDL, VLDL and cholesterol in cholesterol fed rabbits, at a concentration of 0.8 g kg−1 day−1 for 4 weeks (dose 100 mg kg−1 day−1). Interestingly, two new fatty acid derivatives (cerebrosides), Ocimumoside A 244 and Ocimumoside B 245 were purified in 2007 from O. sanctum leaves extract, and were found to possess strong antistress activity (Gupta et al. 2007).
Biosynthesis
Fatty acids (FA) naturally occur in the two subcellular compartments: chloroplasts and endoplasmic reticulum (ER). Both de novo synthesis from acetyl-CoA of such FAs, as palmitic or stearic as well as desaturation of stearic acid to oleic acid, occur in plastids, whereas the further conversion of oleic acid into linoleic acid and further to linolenic acid occurs in the ER (Sidorov and Tsydendambaev 2014).
Bioavailability
A great variation in absolute bioavailability occurs after oral administration of some FAs to rats. α-Linolenic acid was rapidly absorbed (0.76 h) and distributed when given in a dose of 31 mg kg−1 body weight, while the absolute oral bioavailability was 0.7% after oral doses of 25 and 50 mg kg−1 of oleanolic acid, indicating poor absorption and extensive metabolism in vivo (Rodríguez-Alcalá et al. 2015; Cholewski et al. 2018).
Amongst the 13 detected FAs, 6 were active and the other 7 had no reported activities. In general, linolenic and palmitic acids were considered as highly significant bioactive antibacterial agents (P < 0.001) while oleic and linoleic acids were well marked antiulcerogenic ones (P < 0.05). The two cerebrosides A and B were emphasized as significant (P < 0.05) anti stress compounds among all the antistress and immunomodulatory compounds isolated from the genus.
Sterols
Five sterols and sterol derivatives 252–256 were isolated from the genus Ocimum over the period form 1961 to 2012 (Fig. 10), among them were β-sitosterol, stigmasterol and daucosterol which possess diverse bioactivities. β-Sitosterol 252, with oral LD50 more than 25 g kg−1 in mice, was synthesized from stigmasterol 254 via selective hydrogenation of the side chain Δ 22–23, and biosynthesized from both mevalonate and deoxyxylulose pathways which is regulated during membrane biogenesis. Both β-sitosterol 252 and stigmasterol 254 (1.8-fold stronger than β-sitosterol) possesses the capability to bind to chondrocyte membrane and exert a series of anti-inflammatory and anti-catabolic actions at a concentration of 45 µM via counteracting expression of the MMPs involved in cartilage degradation as well as inhibiting the pro-inflammatory mediators PGE2 via counteracting the NF-KB pathway (Gabay et al. 2010).
Biosynthesis
The usual pathway of sterol biosynthesis proceeds along the well-established isoprenoid trail passing from the “active isoprene unit”, through isopentenyl diphosphate, to the C30 triterpenoid squalene via three major stages: acetate → isoprenoid intermediate → cyclization product → cholesterol and with the aid of acetoacetyl CoA thiolase, hydroxyl-3-methylglutaryl CoA synthase, 3-hydroxy-3-methylglutaryl CoA reductase, phosphomevalonate kinase and finally mevalonate diphosphate decarboxylase. As a result, C30 symmetric olefin is formed, followed by oxidation to form S-oxidosqualene via an NADPH-dependent mono-oxygenase reaction catalyzed by squalene epoxidase (SQE), and this substrate can be cyclized by an oxidosqualene–sterol synthase to yield the steroidal backbone structure represented in lanosterol which is further converted into cholesterol (Nes 2011).
Bioavailability
Phytosterols are generally poorly absorpable, and it was stated that less than 10% of dietary phytosterols are systematically absorbed, in contrast to about 50–60% of dietary cholesterol (Ogbe et al. 2015).
Polysaccharides
A polysaccharide fraction (constituted of 23.3% of rhamnose 257, 19.2% of xylose 258 and 10.3% of glucose 259) was detected in O. basilicum seeds (Anjaneyalu and Gowda 1979) (Fig. 11). The fraction isolated from mucilage of O. canum contained the same constituents but with different concentrations, where d-glucose, d-mannose, d-galactose, l-arabinose, d-xylose and l-rhamnose have existed in the approximate ratio 8:5:2:1:1:2 (Anjaneyalu and Tharanathan 1971). In the same context, the mucilaginous capsular polysaccharide-complex isolated from the seeds of O. gratissimum yielded an acidic xylan composed of d-xylose (48%), l-arabinose (16%), d-galactose (16%), and d-galacturonic acid (~ 20%) (Anjaneyalu et al. 1983). Polysaccharide fraction of O. basilicum exhibited a potent DPPH free radical scavenging activity with IC0.2 11.26 μM compared to α-tocopherol (IC0.2 value of 11.9 ± 0.2 μM) and BHA (IC0.2 value of 14.5 ± 2.5 μM), while the % inhibition at both concentrations (20 and 40 µg ml−1) were 60.7 ± 4.1% and 76.6 ± 3.8%, respectively (Subramanian et al. 2005). In the same context, it inhibited the lipid peroxidation at IC50 12.6 µM in a significant and a concentration-dependent manner where it showed 39.1 ± 4.5% and 80.3 ± 4.5% protection in AAPH and Fe(II)-ascorbic acid induced lipid peroxidation, respectively. The results revealed that effectiveness of the fraction in the Fe(II)-ascorbic acid system with IC50 30.18 µM was far superior to that in the AAPH system with IC50 78.6 µM (Subramanian et al. 2005). In addition, the polysaccharide fraction isolated from O. sanctum, at 100 μg ml−1, protected 30 ± 3.2% mouse splenocytes against γ-ray irradiation, the activity which was explained by the presence of reactive oxygen species scavenging and iron chelating properties (Singh and Chaudhuri 2018).
Nutraceutical value (minerals, pigments and mucilage)
Minerals in routine dietary intake play an important role in food and nutraceutical industry. In the same context, herbs of Ocimum which have been used to add distinctive flavor to food have also been used as a home remedy for various health conditions. In addition, they have been considered as rich sources of vitamins, minerals, fats, proteins, polysaccharides, fibers, pigments and mucilage. Interestingly, the elemental analysis of macro and micro contents of O. sanctum leaves using LIBS and ICAP-AES techniques revealed the presence of elements as C, H, O and N which suggest its application in maintaining electrolytic balance and obtaining organic compounds. The same study also showed the presence of vitamin A, vitamin C, β-carotene, ribiflavone, chlorophyll, insoluble oxalates, proteins (30 kcal), fats (0.5 g), carbohydrates (2.3 g) and minerals (Tripathi et al. 2015). Each 100 g of O. sanctum leaves contains vitamin C (83 μg), carotene (2.5 μg), Ca (3.15%), P (0.34%), Cr (2.9 μg), Cu (0.4 μg), Zn (0.15 μg), V (0.54 μg), Fe (2.32 μg) and Ni (0.73 μg) (Pattanayak et al. 2010). Besides, ascorbic acid (8.21 mg), riboflavin (0.06 mg) and thiamine (0.3 mg) contents which suggest its leaves intake as an edible plant to be a dietary supplement and an alternative economic source of vitamins and natural antioxidants (Bhattacharya et al. 2014). Similarly, the seed mucilage of O. sanctum (yield ~ 30%) contains hexouronic acid (27.25%), pentoses (38.9%), protein and amino acids (Hameed et al. 2016). In the same context, O. basilicum contains high amounts of such minerals where 100 g of fresh plant contains Mg (64 mg), K (295 mg) and Fe (3.17 mg). The plant is also rich in a variety of important nutrients, where a 100 g fresh plant was found to contain vitamin A (264 µg), vitamin C (18 mg), riboflavin (76 µg), vitamin K (414 µg), Ca (177 mg) and P (56 mg) (Filip 2017). A related study concerned with O. gratissimum revealed that each 100 g of powdered leaves or stems of the plant contained Ca (5.2 and 3.73 mg l, s), Mg (0.53 and 0.33 mg l, s), Fe (13.9, 6.76 mg l, s) and P (4.25 and 3.05 mg l, s). 3.33 ± 0.07% and 1.65 ± 0.02% crude protein content for leaves and stems; 8.50 ± 0.04% and 3.00 ± 0.15% crude lipid content for leaves and stems and 9.52 ± 0.01% and 19.65 ± 0.03% crude fiber content for leaves and stems.
Accordingly, the nutraceutical value of the studied Ocimum species arise from the biological importance of such elements, as Mg and K which are considered among the seven essential macrominerals improving the health of cardiovascular system and transmission of nerve impulses, as well as providing protection from a number of chronic diseases. Moreover, the higher K content in the stems also qualify the plant to be efficient for hypertensive patients, the polyphenolics play an important role as powerful antioxidants and the presence of different minerals play an important role in proper tissue functioning (Idris et al. 2011).
Chemical difference between Ocimum species
The variation in the essential oil content and chemical composition of the leaves and inflorescences of most Ocimum species were determined for two harvesting seasons. The essential oil extracted using hydro-distillation and chemical characterization was performed using GC/MS techniques, and the recovery ranged from 0.18 to 0.774% during pre-monsoon and 0.141–0.748% during post-monsoon. Out of the different analyzed species, O. gratisimum showed the highest percentage of eugenol (53–89%), followed by O. santum ‘Purple and green varieties’ while O. viride and O. gratisimum were rich in methyl isoeugenol (40.26–53.21%). Analyzing the flavonoidal profile of the nine studied species of Ocimum, substantial infraspecific differences were found. For example, O. americanum var. pilosum accumulated the flavone C-glycoside, vicenin-2, which existed in var. americanum in traces. The major flavonoids found in all the investigated species were flavonol 3-O-glucosides and 3-O-rutinosides, while many other species produced the more unusual compound, quercetin 3-O-(6-O-malonyl) glucoside, and small amounts of flavone O-glycosides. The level of flavonol glycosides was significantly reduced in glasshouse-grown plants, but the levels of flavone glycosides were unaffected. O. sanctum was characterised by the accumulation of flavone-O-glycosides as the 7-O-glucuronides of luteolin and apigenin, while Luteolin 5-O-glucoside, which was found in all nine species of Ocimum studied, and is considered to be a key character for the genus (Grayer et al. 2002).
In general, it seems that the high degree of polymorphism within the genus Ocimum evoked a large number of subspecies and different varieties with varying chemical composition, which offers a variable level of medicinal potential.
Conclusion and future perspectives
Keeping the various medical benefits together with the ancient traditional uses of many Ocimum species, several studies have been carried out towards discovery of the bioactive components (Fig. 12). Two hundred and sixty compounds were reported from the genus Ocimum over the period from 1961 to April 2019, 100 of them have been isolated in the period from 2001 to 2010 (Fig. 13). Our research results highlighted O. Sanctum, O. gratissimum and O. basilicum as the most well-studied species with the most well-known traditional uses and chemopharmcological importance (Table 2). However, other Ocimum species such as O. viride, O. americnum and O. canum are underexplored and need more chemical and biological investigations. Different classes of compounds including phenolics, flavonoids, neolignans, terpenoids, coumarins and fatty acid derivatives were discovered, with terpenes and flavonoids as the major prevailing classes of compounds which are responsible for most of the genus bioactivities (Fig. 1). Among those valuable bioactive compounds, essential oils isolated from different Ocimum species provided good sources of natural eugenol and a high commercial importance in pharmaceutical, cosmetics and food industry. In addition, they have been a new lead for prevention and treatment of hyperglycemia, atherosclerosis and serious cardiovascular disorders. Concerning flavonoids as the second prevalent class, orientin and vicenin which showed a significant radioprotection to human peripheral lymphocytes were suggested for clinical application in cancer radiotherapy and as a new choice for the treatment of UT bacterial infections, with the need for further investigation to ensure complete safety. On the other hand, the fixed oil of seeds which is rich in ω-3 fatty acids is considered as of recent interest of most researchers, due to its wide range of pharmacological properties especially in cardioprotection. The review highlighted O. sanctum as the “Queen of herbs” with a wide range of biological activities discovered by the traditional users, in particular the immunomodulatory and adaptogenic ones, which were further confirmed by pre-clinical studies and finally tagged with the clinical ones, and which could opportunely provide more assurance on the efficacy coupled-safety of Ocimum as a herbal remedy.
Nevertheless, analysis of the reviewed data has revealed some considerable gaps in studying Ocimum. Firstly, some species as O. canum, O. suave and O. forskolei, with a plenty of phytonutrients and a considerable history of folkloric uses, have been insufficiently investigated and mainly limited to insect repellent activity studies. Additionally, roots and stems of the genus Ocimum have also been chemically and biologically underinvestigated. In general, there is a need for further investigation of the chemical aspects of Ocimum to get novel molecules with new pharmacological potentials. More SAR studies are needed to illuminate the possible mechanisms of action of the isolated compounds as antitumor, antiviral, gastroprotective and anti-inflammatory agents. Additionally, new approaches such as metabolomics based on HRMS and NMR data could be applied to compare the various species of ocimum and to correlate chemical diversity to biological potential. Moreover, several aspects are also needed to work on the large scale plans to isolate sufficient amounts of the major as well as the minor chemical constituents to explore their pharmacological activities. Furthermore, synthetic analogues of bioactive metabolites of Ocimum should be developed to focus on improving their efficacy and safety.
Despite the lack of long-term clinical trials, the 24 studies published to date have suggested that Tulsi is a safe herbal intervention that may assist in normalising blood glucose, blood pressure and lipid profiles and which can fight psychological and immunological disorders. In the same context, the studies indicated that daily addition of Tulsi to the diet and/or as adjunct to drug therapy can potentially assist in prevention or reduction of various health conditions, the results which really warrants further clinical evaluation. Finally, more efforts should be directed towards the investigation of these species to translate them into existing market products which may be potentially beneficial, in addition to be less toxic than currently available synthetic compounds to enable Ocimum plants to contribute a lot towards economy and health care.
Abbreviations
- AAPH:
-
2,2′-Azobis (2-amidinopropane) dihydrochloride
- APCI-MS:
-
Atmospheric pressure chemical ionization mass spectrometry
- BW:
-
Body weight
- 13C-NMR:
-
Carbon nuclear magnetic resonance
- CC:
-
Column chromatography
- CC50 :
-
Half cytotoxic concentration
- DMBA:
-
7,12-Dimethylbenzo anthracene
- DPPH:
-
2,2-Diphenyl-1-picryl-hydrazyl-hydrate
- DW:
-
Dry weight
- ED50 :
-
Half effective dose
- EGFR:
-
Epidermal growth factor receptor
- FW:
-
Fresh weight
- Gal:
-
Galactose
- GC/MS:
-
Gas chromatography/mass spectrometry
- Glc:
-
Glucose
- GlcA:
-
Glucouronic acid
- 1H:
-
Proton nuclear magnetic resonance
- HIV:
-
Human immunodeficiency virus
- HPTLC:
-
High performance thin layer chromatography
- IC50 :
-
50% Inhibitory concentration
- IC90 :
-
90% Inhibitory concentration
- LC50 :
-
50% Lethal concentration
- ICAP-AES:
-
Inductively coupled argon plasma atomic spectroscopy
- Ip.:
-
Intraperitoneal
- LD50 :
-
50% Lethal dose
- LDL:
-
Low density lipoproteins
- LIBS:
-
Laser induced breakdown spectroscopy
- LMI%:
-
Percentage leucocyte migration inhibition
- Me:
-
Methyl
- MES:
-
Maximal electric shock
- MI:
-
Maximal inhibition
- MIC:
-
Minimum inhibitory concentration
- µM:
-
Micromolar
- NF-KB:
-
Nuclear factor Kappa B
- OMe:
-
Methoxy
- OSCC:
-
Oral squamous cell carcinoma
- Pg:
-
Pico gram
- PGE2:
-
Prostaglandin E2
- PhAs:
-
Phenolic acids
- RAP:
-
Randomly amplified polymorphic DNA
- Rha:
-
Rhamnose
- SAR:
-
Structure activity relationship
- UPLC–ESI–MS/MS:
-
Ultra performance liquid chromatography
- VLDL:
-
Very low density lipoproteins
- ZOI:
-
Zone of inhibition
- Xyl:
-
Xylose
References
Adam A, Crespy V, Levrat-Verny M-A, Leenhardt F, Leuillet M, Demigné C, Rémésy C (2002) The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J Nutr 132:1962–1968
Aguiyi J, Obi C, Gang S, Igweh A (2000) Hypoglycaemic activity of Ocimum gratissimum in rats. Fitoterapia 71:444–446
Al-Hajj NQM, Rashid H, Wang H, Thabit R, Rashed M (2014) Antioxidant, antimicrobial and Pulicaria inuloides and Ocimum froskolei: a review. Am Res Thoughts 1:973–1000
Ali A, Ali M (2012) New fatty acid derivatives from Ocimum sanctum L. leaves. Indian Drugs 49:13–18
Ali NAA, Chhetri BK, Dosoky NS, Shari K, Al-Fahad AJ, Wessjohann L, Setzer WN (2017) Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines 4(17):1–14
Alves MJ, Ferreira IC, Froufe HJ, Abreu R, Martins A, Pintado M (2013) Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J Appl Microbiol 115:346–357
Al-Zahrani SH (2012) Antibacterial activities of gallic acid and gallic acid methyl ester on methicillin-resistant Staphylococcus aureus. J Am Sci 8:7–12
Anjaneyalu YV, Gowda DC (1979) Structural studies of an acidic polysaccharide from Ocimum basilicum seeds. Carbohydr Res 75:251–256
Anjaneyalu Y, Tharanathan R (1971) Composition and preliminary fractionation of the seed mucilage of Ocimum canum. Aust J Chem 24:1501–1507
Anjaneyalu YV, Khan M-R, Tharanathan RN (1983) An acidic xylan from the capsular polysaccharide-complex of Ocimum gratissimum seeds. Carbohydr Res 116:83–88
Bang S, Ha TKQ, Lee C, Li W, Oh W-K, Shim SH (2016) Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br J Ethnopharmacol 192:398–405
Baruah TJ, Sharan R, Kma L (2018) Vicenin-2: a potential radiosensitizer of non-small cell lung cancer cells. Mol Biol Rep 45:1219–1225
Bayala B, Bassole IHN, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema J-B, Lobaccaro J-MA, Simpore J (2014) Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One 9(3):1–12
Berim A, Gang DR (2013) The roles of a flavone-6-hydroxylase and 7-O-demethylation in the flavone biosynthetic network of sweet basil. J Biol Chem 288:1795–1805
Berim A, Hyatt DC, Gang DR (2012) A set of regioselective O-methyltransferases gives rise to the complex pattern of methoxylated flavones in sweet basil. Plant Physiol 160:1052–1069
Bhasin M (2012) Ocimum—taxonomy, medicinal potentialities and economic value of essential oil. J Biosph 1:48–50
Bhattacharya A, Aggarwal A, Sharma N, Cheema J (2014) Evaluation of some anti-oxidative constituents of three species of Ocimum. Int J Life Sci 8:14–17
Bilal A, Jahan N, Ahmed A, Bilal SN, Habib S, Hajra S (2012) Phytochemical and pharmacological studies on Ocimum basilicum Linn—a review. Int J Curr Res Rev 4(23):73–83
Bourgaud F, Hehn A, Larbat R, Doerper S, Gontier E, Kellner S, Matern U (2006) Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem Rev 5:293–308
Bouzaiene NN, Chaabane F, Sassi A, Chekir-Ghedira L, Ghedira K (2016) Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci 144:80–85
Brahmachari G (2010) Nevadensin: isolation, chemistry and bioactivity. Int J Green Pharm 4:213–219
Brožič P, Kocbek P, Sova M, Kristl J, Martens S, Adamski J, Gobec S, Rižner TL (2009) Flavonoids and cinnamic acid derivatives as inhibitors of 17β-hydroxysteroid dehydrogenase type 1. Mol Cell Endocrinol 301:229–234
Carović-Stanko K, Orlić S, Politeo O, Strikić F, Kolak I, Milos M, Satovic Z (2010) Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem 119:196–201
Cavalcanti ESB, Morais SMd, Lima MAA, Santana EWP (2004) Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz 99:541–544
Chagonda LS, Makanda CD, Chalchat JC (2000) The essential oils of Ocimum canum Sims (basilic camphor) and Ocimum urticifolia Roth from Zimbabwe. Flavour Fragr J 15:23–26
Chandrasekaran C, Srikanth H, Anand M, Allan JJ, Viji MH, Amit A (2013) Evaluation of the mutagenic potential and acute oral toxicity of standardized extract of Ocimum sanctum (OciBest™). Hum Exp Toxicol 32:992–1004
Charles DJ, Simon JE, Wood KV (1990) Essential oil constituents of Ocimum micranthum Willd. J Agric Food Chem 38:120–122
Chen P-N, Chu S-C, Chiou H-L, Chiang C-L, Yang S-F, Hsieh Y-S (2005) Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr Cancer 53:232–243
Chil Núñez I, Escalona Arranz J, Berenguer Rivas C, Mendonça P, Mateo Pérez K, Dutok Sánchez C, Cortinhas L, Silva C, Carvalho M, Queiroz M (2017) Chemical composition and toxicity of Ocimum sanctum L. var. cubensis essential oil up-growing in the eastern of Cuba. Int J Pharmacogn Phytochem Res 9:1021–1028
Cholewski M, Tomczykowa M, Tomczyk M (2018) A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients 10:1662
Chopra RN, Nayar SL (1956) Glossary of Indian medicinal plants. Council of Scientific And Industrial Research, New Delhi
Chopra R, Chopra I, Handa K, Kapoor L (1958) Jatropha curcas (Euphorbiaceae). Chopra’s indigenous drugs of India, 2nd edn. UN Dhar and Sons, Calcutta, pp 587–676
Chowdhury T, Mandal A, Roy SC, De Sarker D (2017) Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J Gen Eng Biotechnol 15:275–286
Chu C-Y, Tsai Y-Y, Wang C-J, Lin W-L, Tseng T-H (2001) Induction of apoptosis by esculetin in human leukemia cells. Eur J Pharmacol 416:25–32
CSIR DK (1948) The wealth of India. Council of Scientific and Industrial Research, New Delhi
Da Silva RZ, Yunes RA, de Souza MM, Monache FD, Cechinel-Filho V (2010) Antinociceptive properties of conocarpan and orientin obtained from Piper solmsianum C. DC. var. solmsianum (Piperaceae). J Nat Med 64:402–408
Das S, Chandra A, Agarwal S, Singh N (1983) Ocimum sanctum (Tulsi) in the treatment of viral encephalitis (A preliminary clinical trial). Antiseptic 80:323–327
de Almeida I, Alviano DS, Vieira DP, Alves PB, Blank AF, Lopes AHC, Alviano CS, Maria do Socorro SR (2007) Antigiardial activity of Ocimum basilicum essential oil. Parasitol Res 101:443–452
de Lira Mota KS, Dias GEN, Pinto MEF, Luiz-Ferreira Â, Monteiro Souza-Brito AR, Hiruma-Lima CA, Barbosa-Filho JM, Batista LM (2009) Flavonoids with gastroprotective activity. Molecules 14:979–1012
de Paula JP, Gomes-Carneiro MR, Paumgartten FJ (2003) Chemical composition, toxicity and mosquito repellency of Ocimum selloi oil. J Ethnopharmacol 88:253–260
Dekker T, Ignell R, Ghebru M, Glinwood R, Hopkins R (2011) Identification of mosquito repellent odours from Ocimum forskolei. Parasite Vectors 4(183):1–7
Devi PU, Ganasoundari A, Rao B, Srinivasan K (1999) In vivo radioprotection by Ocimum flavonoids: survival of mice. Rad Res 151:74–78
Djilani A, Dicko A (2012) The therapeutic benefits of essential oils, nutrition, wellbeing and health. In: Bouayed J (ed) InTech, pp 158–177. ISBN 978-953-51-0125-3
Ebeye O, Ekundina O, Wilkie I (2014) Histological and biochemical effects of aqueous extract of Ocimum gratissimum on the liver and kidney of adult wistar rats. Afr J Cell Pathol 2:59–64
Fang J (2014) Bioavailability of anthocyanins. Drug Metab Rev 46:508–520
Farjam MH, Rustaiyan A, Ezzatzadeh E, Jassbi AR (2013) Labdane-type diterpene and two flavones from Salvia sharifii Rech. f. and Esfan. and their biological activities. Iran J Pharm Res 12(2):395–411
Fatope MO, Marwah RG, Al Hadhrami NM, Onifade AK, Williams JR (2008) Identification of the chemotypes of Ocimum forskolei and Ocimum basilicum by NMR spectroscopy. Chem Biodivers 5:2457–2463
Filip S (2017) Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int J Clin Nutr Diet 3(118):1–5
Flegkas A, Milosević Ifantis T, Barda C, Samara P, Tsitsilonis O, Skaltsa H (2019) Antiproliferative activity of (–)-rabdosiin isolated from Ocimum sanctum L. Medicines 6(37):1–10
Freire CMM, Marques MOM, Costa M (2006) Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. J Ethnopharmacol 105:161–166
Fu X, Li S, Wang M (2006) Vasorelaxant action of orientin on isolated rabbit thoracic aortic rings and its mechanism. J China Pharm Univ 37(6):539–543
Fu JT, Tang L, Li WS, Wang K, Cheng DM, Zhang ZX (2015) Fumigant toxicity and repellence activity of camphor essential oil from Cinnamonum camphora siebold against solenopsis invicta workers (Hymenoptera: Formicidae). J Ins Sci (Online) 15:129
Gabay O, Sanchez C, Salvat C, Chevy F, Breton M, Nourissat G, Wolf C, Jacques C, Berenbaum F (2010) Stigmasterol: a phytosterol with potential anti-osteoarthritic properties. Osteoarthr Cartil 18:106–116
Gautam MK, Goel RK (2014) Toxicological study of Ocimum sanctum Linn leaves: hematological, biochemical, and histopathological studies. J Toxicol 2014:1–9
Godhwani S, Godhwani J, Was D (1988) Ocimum sanctum—a preliminary study evaluating its immunoregulatory profile in albino rats. J Ethnopharmacol 24:193–198
Granger RE, Campbell EL, Johnston GA (2005) (+)-And (−)-borneol: efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem Pharmacol 69:1101–1111
Grayer RJ, Kite GC, Goldstone FJ, Bryan SE, Paton A, Putievsky E (1996) Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 43:1033–1039
Grayer RJ, Veitch NC, Kite GC, Price AM, Kokubun T (2001) Distribution of 8-oxygenated leaf-surface flavones in the genus Ocimum. Phytochem 56:559–567
Grayer RJ, Kite GC, Veitch NC, Eckert MR, Marin PD, Senanayake P, Paton AJ (2002) Leaf flavonoid glycosides as chemosystematic characters in Ocimum. Biochem Syst Ecol 30:327–342
Gupta P, Yadav DK, Siripurapu KB, Palit G, Maurya R (2007) Constituents of Ocimum sanctum with antistress activity. J Nat Prod 70:1410–1416
Gupta R, Sharma AK, Dobhal M, Sharma M, Gupta R (2011) Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J Diabetes 3:29–37
Hameed H, Aydin S, Başaran AA, Başaran N (2016) Assessment of cytotoxic properties of sinapic acid in vitro. Turk J Pharm Sci 13:225–232
He M, Min J-W, Kong W-L, He X, Li J-X, Peng B-W (2016) A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 115:74–85
Hiltunen R, Holm Y (2003) Basil: the genus Ocimum. CRC Press, Amsterdam, pp 1–146
Hollman PC (2004) Absorption, bioavailability, and metabolism of flavonoids. Pharm Biol 42:74–83
Huang CB, George B, Ebersole JL (2010) Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch Oral Biol 55:555–560
Hussain AI, Anwar F, Sherazi STH, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108:986–995
Idris S, Iyaka Y, Ndamitso M, Paiko Y (2011) Nutritional composition of the leaves and stems of Ocimum gratissimum. J Emerg Trends Eng Appl Sci 2:801–805
Ilori M, Sheteolu A, Omonigbehin E, Adeneye A (1996) Antidiarrhoeal activities of Ocimum gratissimum (Lamiaceae). J Diarrhoeal Dis Res 14:283–285
Inbaneson SJ, Sundaram R, Suganthi P (2012) In vitro antiplasmodial effect of ethanolic extracts of traditional medicinal plant Ocimum species against Plasmodium falciparum. Asian Pac J Trop Med 5:103–106
Jamshidi N, Cohen MM (2017) The clinical efficacy and safety of Tulsi in humans: a systematic review of the literature. Evid Based Complement Alternat Med 2017:1–13
Javanmardi J, Khalighi A, Kashi A, Bais H, Vivanco J (2002) Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J Agric Food Chem 50:5878–5883
Jembere B, Obeng-Ofori D, Hassanali A, Nyamasyo G (1995) Products derived from the leaves of Ocimum kilimandscharicum (Labiatae) as post-harvest grain protectants against the infestation of three major stored product insect pests. Bull Entomol Res 85:361–367
Jia C, Zhang J, Yu L, Wang C, Yang Y, Rong X, Xu K, Chu M (2018) Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front Cell Infect Microbiol 8:1–13
Joseph B, Nair VM (2013) Ocimum sanctum Linn. (Holy basil): pharmacology behind its anti-cancerous effect. Int J Pharm Biol Sci 4:556–575
Joshi R (2013) Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Ind J Pharm Sci 75(4):457–477
Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP (1972) Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother 2:23–28
Karawya MS, Hashim FM, Hifnawy MS (1974) Oils of Ocimum basilicum and Ocimum rubrum grown in Egypt. J Agric Food Chem 22:520–522
Kelm M, Nair M, Strasburg G, DeWitt D (2000) Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine 7:7–13
Khosla M, Bhasin M (2000) Biosynthetic correlations of major essential oil components in Ocimum carnosum LK & Otto. Ind Perfum 44:55–60
Kochhar A, Sharma N, Sachdeva R (2009) Effect of supplementation of Tulsi (Ocimum sanctum) and Neem (Azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics. Stud Ethno-Med 3:5–9
Kohlert C, Van Rensen I, März R, Schindler G, Graefe E, Veit M (2000) Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med 66:495–505
Konishi Y, Hitomi Y, Yoshida M, Yoshioka E (2005) Pharmacokinetic study of caffeic and rosmarinic acids in rats after oral administration. J Agric Food Chem 53:4740–4746
Koriem KM, Soliman RE (2014) Chlorogenic and caftaric acids in liver toxicity and oxidative stress induced by methamphetamine. J Toxicol 2014:1–10
Kotan R, Kordali S, Cakir A (2007) Screening of antibacterial activities of twenty-one oxygenated monoterpenes. J Biosci 62:507–513
Kothari S, Bhattacharya A, Ramesh S (2004) Essential oil yield and quality of methyl eugenol rich Ocimum tenuiflorum Lf (syn. O. sanctum L.) grown in south India as influenced by method of harvest. J Chromatogr A 1054:67–72
Kulkarni KV, Adavirao BV (2018) A review on: Indian traditional shrub Tulsi (Ocimum sanctum): the unique medicinal plant. J Med Plants 6:106–110
Lee J, Scagel CF (2009) Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem 115:650–656
Lee TH, Hoover RL, Williams JD, Sperling RI, Ravalese J III, Spur BW, Robinson DR, Corey E, Lewis RA, Austen KF (1985) Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med 312:1217–1224
Lee B-C, Lee SY, Lee HJ, Sim G-S, Kim J-H, Kim J-H, Cho Y-H, Lee D-H, Pyo H-B, Choe T-B (2007) Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis. Arch Pharm Res 30:1293
Lu M, Kong Q, Xu X, Lu H, Lu Z, Yu W, Zuo B, Su J, Guo R (2014) Pectolinarigenin—a flavonoid compound from Cirsium japonicum with potential anti-proliferation activity in mcf-7 breast cancer cell. Trop J Pharm Res 13:225–228
Luo J, Hu Y-L, Wang H (2017) Ursolic acid inhibits breast cancer growth by inhibiting proliferation, inducing autophagy and apoptosis, and suppressing inflammatory responses via the PI3K/AKT and NF-κB signaling pathways in vitro. Exp Therap Med 14:3623–3631
Ma C-M, Cai S-Q, Cui J-R, Wang R-Q, Tu P-F, Hattori M, Daneshtalab M (2005) The cytotoxic activity of ursolic acid derivatives. Eur J Med Chem 40:582–589
Machado MIL, de Vasconcelos Silva MG, Matos FJA, Craveiro AA, Alencar JW (1999) Volatile constituents from leaves and inflorescence oil of Ocimum tenuiflorum L. f. (syn. O. sanctum L.) grown in Northeastern Brazil. J Ess Oil Res 11:324–326
Mahajan N, Rawal S, Verma M, Poddar M, Alok S (2013) A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum. Biomed Prev Nutr 3:185–192
Manosroi J, Dhumtanom P, Manosroi A (2006) Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett 235:114–120
Martins AP, Salgueiro LR, Vila R, Tomi F, Cañigueral S, Casanova J, da Cunha AP, Adzet T (1999) Composition of the essential oils of Ocimum canum, O. gratissimum and O. minimum. Planta Med 65:187–189
Marwat SK, Khan MS, Ghulam S, Anwar N, Mustafa G, Usman K (2011) Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae). Asian J Chem 23(9):3773–3782
Marzouk AM (2009) Hepatoprotective triterpenes from hairy root cultures of Ocimum basilicum L. Z Für Nat C 64:201–209
Mathews S, Singhal R, Kulkarni P (1993) Ocimum basilicum: a new non-conventional source of fibre. Food Chem 47:399–401
Mediratta P, Sharma K, Singh S (2002) Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J Ethnopharmacol 80:15–20
Mondal S, Mirdha BR, Mahapatra SC (2009) The science behind sacredness of tulsi (Ocimum sanctum Linn.). Ind J Physiol Pharmacol 53:291–306
Moumbock AF, Simoben CV, Wessjohann L, Sippl W, Günther S, Ntie-Kang F (2017) Computational studies and biosynthesis of natural products with promising anticancer properties. Phytoch–Nat Prod Cancer. InTech, Rijeka, pp 257–285
Mousavi L, Salleh RM, Murugaiyah V (2018) Phytochemical and bioactive compounds identification of Ocimum tenuiflorum leaves of methanol extract and its fraction with an anti-diabetic potential. Int J Food Prop 21:2390–2399
Muthu C, Ayyanar M, Raja N, Ignacimuthu S (2006) Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J Ethnobiol Ethnomed 2(43):1–10
Nagaprashantha LD, Vatsyayan R, Singhal J, Fast S, Roby R, Awasthi S, Singhal SS (2011) Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem Pharmacol 82:1100–1109
Nakamura CV, Ueda-Nakamura T, Bando E, Melo AFN, Cortez DAG, Dias Filho BP (1999) Antibacterial activity of Ocimum gratissimum L. essential oil. Mem Inst Oswaldo Cruz 94:675–678
Narwal S, Rana A, Tiwari V, Gangwani S, Sharma R (2011) Review on chemical constituents and pharmacological action of Ocimum kilimandscharicum. Indo Glob J Pharm Sci 1:287–293
Naveen P, Lingaraju H, Anitha K (2017) Simultaneous determination of rutin, isoquercetin, and quercetin flavonoids in Nelumbo nucifera by high-performance liquid chromatography method. Int J Pharm Invest 7(2):94–104
Nazar S, Ravikumar S, Prakash Williams G (2008) Ethnopharmacological survey of medicinal plants along the southwest coast of India. J Herb Spice Med Plants 14:219–239
Nes WD (2011) Biosynthesis of cholesterol and other sterols. Chem Rev 111:6423–6451
Njan AA, Olaoye SO, Afolabi SO, Ejimkonye BC, Soje A, Olorundare OE, Iwalewa EO (2019) Safety effect of fractions from methanolic leaf extract of Ocimum gratissimum on reproduction in male wistar rats. Toxicol Rep 6:496–504
Njinga N, Sule M, Pateh U, Hassan H, Abdullahi S, Ache R (2016) Isolation and antimicrobial activity of [beta]-sitosterol-3-O-glucoside from Lannea kerstingii Engl. & K. Krause (Anacardiacea). Nitte Univ J Health Sci 6(1):4–8
Njoku C, Zeng L, Asuzu I, Oberlies NH, McLaughlin JL (1997) Oleanolic acid, a bioactive component of the leaves of Ocimum gratissimum (Lamiaceae). Int J Pharmacogn 35:134–137
Nour AH, Elhussein SA, Osman NA, Nour AH (2009) Characterization and chemical composition of the fixed oil of fourteen basil (Ocimum basilicum L.) accessions grown in Sudan. Int J Chem Technol 1:52–58
Ntezurubanza L, Scheffer J, Looman A, Svendsen AB (1984) Composition of essential oil of Ocimum kilimandscharicum grown in Rwanda. Planta Med 50:385–388
Ogbe RJ, Ochalefu DO, Mafulul SG, Olaniru OB (2015) A review on dietary phytosterols: their occurrence, metabolism and health benefits. Asian J Plant Sci Res 5:10–21
O’Leary N (2016) Taxonomic revision of Ocimum (Lamiaceae) in Argentina. J Torrey Bot Soc 144:74–88
Onaolapo A, Onaolapo O (2012) Ocimum gratissimum Linn causes dose dependent hepatotoxicity in streptozotocin-induced diabetic Wistar rats. Maced J Med Sci 5:17–25
Panat NA, Amrute BK, Bhattu S, Haram SK, Sharma GK, Ghaskadbi SS (2015) Antioxidant profiling of C3 quercetin glycosides: quercitrin, quercetin 3-β-d-glucoside and quercetin 3-O-(6″-O-malonyl)-β-d-glucoside in cell free environment. Free Radic Antioxid 5:90–100
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5(e47):1–15
Paton A (1992) A synopsis of Ocimum L. (Labiatae) in Africa. Kew Bull 47:403–435
Pattanayak P, Behera P, Das D, Panda SK (2010) Ocimum sanctum Linn. A reservoir plant for therapeutic applications: an overview. Pharmacogn Rev 4(7):95–105
Pesewu GA, Cutler RR, Humber DP (2008) Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. J Ethnopharmacol 116:102–111
Pessoa L, Morais S, Bevilaqua C, Luciano J (2002) Anthelmintic activity of essential oil of Ocimum gratissimum Linn. and eugenol against Haemonchus contortus. Vetr Parasitol 109:59–63
Phippen WB, Simon JE (1998) Anthocyanins in basil (Ocimum basilicum L.). J Agric Food Chem 46:1734–1738
Pojer E, Mattivi F, Johnson D, Stockley CS (2013) The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf 12:483–508
Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101:379–385
Prabhu K, Lobo R, Shirwaikar A (2009) Ocimum gratissimum: a review of its chemical, pharmacological and ethnomedicinal properties. Open Complement Med J 1:1–15
Prakash P, Gupta N (2005) Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Ind J Physiol Pharmacol 49(2):125–131
Qiang Z (2011) Bioavailability and metabolism of botanical constituents and enhancement of intestinal barrier function by caffeic acid derivatives in Caco-2 cells. Dissertation, Iowa State University
Rafatian G, Khodagholi F, Farimani MM, Abraki SB, Gardaneh M (2012) Increase of autophagy and attenuation of apoptosis by Salvigenin promote survival of SH-SY5Y cells following treatment with H2O2. Mol Cell Biochem 371:9–22
Rahman S, Islam R, Kamruzzaman M, Alam K, Jamal A (2011) Ocimum sanctum L: a review of phytochemical and pharmacological profile. Am J Drug Disc Dev. https://doi.org/10.3923/rjmp.2011
Rahmana S, Muktaa Z, Hossainb M (2009) Isolation and characterization of β-sitosterol-d-glycoside from petroleum extract of the leaves of Ocimum sanctum L. Asian J Food Agro-Ind 2:39–43
Rajalakshmi S, Sivanandam G, Veluchamy G (1986) Role of tulsi (Ocimum sanctum Linn.) in the management of Manjal Kamalai (viral hepatitis). J Res Ayurv Siddha 9:118–123
Rasekh HR, Hosseinzadeh L, Mehri S, Kamli-Nejad M, Aslani M, Tanbakoosazan F (2012) Safety assessment of Ocimum basilicum hydroalcoholic extract in wistar rats: acute and subchronic toxicity studies. Iran J Basic Med Sci 15:645–653
Reddy G, Melkhani A, Kalyani G, Rao JV, Shirwaikar A, Kotian M, Ramani R, Aithal K, Udupa A, Bhat G (1991) Chemical and pharmacological investigations of Limnophila conferta and Limnophila heterophylla. Int J Pharmacogn 29:145–153
Rehman SU, Kim IS, Kang KS, Yoo HH (2015) HPLC determination of esculin and esculetin in rat plasma for pharmacokinetic studies. J Chromatogr Sci 53:1322–1327
Rehman R, Hanif MA, Mushtaq Z, Al-Sadi AM (2016) Biosynthesis of essential oils in aromatic plants: a review. Food Rev Int 32:117–160
Rigano D, Formisano C, Basile A, Lavitola A, Senatore F, Rosselli S, Bruno M (2007) Antibacterial activity of flavonoids and phenylpropanoids from Marrubium globosum ssp. libanoticum. Phytother Res 21:395–397
Rodríguez-Alcalá LM, Ares I, Fontecha J, Juarez M, Castellano V, Martínez-Larrañaga MR, Anadón A, Martínez MA (2015) Oral absorption and disposition of alpha-linolenic, rumenic and vaccenic acids after administration as a naturally enriched goat dairy fat to rats. Lipids 50:659–666
Rodríguez-García C, Sánchez-Quesada C, Toledo E, Delgado-Rodríguez M, Gaforio JJ (2019) Naturally Lignan-rich foods: a dietary tool for health promotion. Molecules 24:917–926
Sacchetti G, Medici A, Maietti S, Radice M, Muzzoli M, Manfredini S, Braccioli E, Bruni R (2004) Composition and functional properties of the essential oil of Amazonian basil, Ocimum micranthum Willd., Labiatae in comparison with commercial essential oils. J Agric Food Chem 52:3486–3491
Saeidnia S, Manayi A, Gohari AR, Abdollahi M (2014) The story of beta-sitosterol—a review. Eur J Med Plants 4(5):590–597
Sampath S, Mahapatra S, Padhi M, Sharma R, Talwar A (2015) Holy basil (Ocimum sanctum Linn.) leaf extract enhances specific cognitive parameters in healthy adult volunteers: a placebo controlled study. Indian J Physiol Pharmacol 59:69–77
Sánchez-Maldonado A, Schieber A, Gänzle M (2011) Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J Appl Microbiol 111:1176–1184
Sanda K, Koba K, Nambo P, Gaset A (1998) Chemical investigation of Ocimum species growing in Togo. Flavour Fragr J 13:226–232
Santos-Sánchez NF, Salas-Coronado R, Hernández-Carlos B, Villanueva-Cañongo C (2019) Shikimic acid pathway in biosynthesis of phenolic compounds, plant physiological aspects of phenolic compounds. IntechOpen, pp 1–15
Satapathy S, Das N, Bandyopadhyay D, Mahapatra SC, Sahu DS, Meda M (2017) Effect of tulsi (Ocimum sanctum Linn.) supplementation on metabolic parameters and liver enzymes in young overweight and obese subjects. Ind J Clin Biochem 32:357–363
Sawabe A, Nesumi C, Morita M, Matsumoto S, Matsubara Y, Komemushi S (2005) Glycosides in African dietary leaves, Hibiscus sabdariffa. J Oleo Sci 54:185–191
Sawai S, Saito K (2011) Triterpenoid biosynthesis and engineering in plants. Front Plant Sci 2(25):1–8
Saxena RC, Singh R, Kumar P, Negi MPS, Saxena VS, Geetharani P, Allan JJ, Venkateshwarlu K (2012) Efficacy of an extract of Ocimum tenuiflorum (OciBest) in the management of general stress: a double-blind, placebo-controlled study. Evid-Based Compl Alternat Med 2012:1–7
Schinella G, Aquila S, Dade M, Giner R, del Carmen Recio M, Spegazzini E, de Buschiazzo P, Tournier H, Ríos JL (2008) Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya. Planta Med 74:215–220
Schühly W, Heilmann J, Calis I, Sticher O (1999) New triterpenoids with antibacterial activity from Zizyphus joazeiro. Planta Med 65:740–743
Selvi MT, Thirugnanasampandan R, Sundarammal S (2015) Antioxidant and cytotoxic activities of essential oil of Ocimum canum Sims. from India. J Saud Chem Soc 19:97–100
Senthil S, Sridevi M, Pugalendi K (2007) Protective effect of ursolic acid against myocardial ischemia induced by isoproterenol in rats. Toxicol Mech Methods 17:57–65
Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I (2001) Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr 131:1207–1210
Sharkar P, Rahman MM, Haque Masum GZ, Nayeem MA, Hossen MM, Azad AK (2013) Ethnomedicinal importance of the plants in villages in Kushtia Sador and Mirpur Upozila, Bangladesh. J Herb Spice Med Plants 19:401–417
Sharma M, Agrawal SK, Sharma P, Chadha B, Khosla M, Saxena A (2010) Cytotoxic and apoptotic activity of essential oil from Ocimum viride towards COLO 205 cells. Food Chem Toxicol 48:336–344
Sharma A, Meena A, Meena R (2012) Antimicrobial activity of plant extracts of Ocimum tenuiflorum. Int J Pharm Tech Res 4:76–180
Sharma P, Prakash O, Shukla A, Singh Rajpurohit C, Vasudev P, Luqman S, Kumar Srivastava S, Bhushan Pant A, Khan F (2016) Structure–activity relationship studies on holy basil (Ocimum sanctum L.) based flavonoid orientin and its analogue for cytotoxic activity in liver cancer cell line Hepg2. Comb Chem High Throughput Screen 19:656–666
Shoeb M, Jaspars M, MacManus SM, Celik S, Nahar L, Kong-Thoo-Lin P, Sarker SD (2007) Anti-colon cancer potential of phenolic compounds from the aerial parts of Centaurea gigantea (Asteraceae). J Nat Med 61:164
Siddiqui BS, Aslam H, Ali ST, Begum S, Khatoon N (2007) Two new triterpenoids and a steroidal glycoside from the aerial parts of Ocimum basilicum. Chem Pharm Bull 55:516–519
Sidorov R, Tsydendambaev V (2014) Biosynthesis of fatty oils in higher plants. Russ J Plant Physiol 61(1):1–18
Silva MGV, Vieira ÍG, Mendes FN, Albuquerque IL, Dos Santos RN, Silva FO, Morais SM (2008) Variation of ursolic acid content in eight Ocimum species from northeastern Brazil. Molecules 13:2482–2487
Silva-Alves KS, Ferreira-da-Silva FW, Peixoto-Neves D, Viana-Cardoso KV, Moreira-Júnior L, Oquendo MB, Oliveira-Abreu K, Albuquerque AAC, Coelho-de-Souza AN, Leal-Cardoso JH (2013) Estragole blocks neuronal excitability by direct inhibition of Na+ channels. Braz J Med Biol Res 46:1056–1063
Simon JE, Quinn J, Murray RG (1990) Basil: a source of essential oils. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, pp 484–489
Simon JE, Morales MR, Phippen WB, Vieira RF, Hao Z (1999) Basil: a source of aroma compounds and a popular culinary and ornamental herb. In: Janick J (ed) Perspectives on new crop new uses. ASHS Press, Alexandria, pp 499–505
Simonsen HT, Nordskjold JB, Smitt UW, Nyman U, Palpu P, Joshi P, Varughese G (2001) In vitro screening of Indian medicinal plants for antiplasmodial activity. J Ethnopharmacol 74:195–204
Singh D, Chaudhuri PK (2018) A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Ind Crop Prod 118:367–382
Singh S, Majumdar D (1996) Effect of fixed oil of Ocimum sanctum against experimentally induced arthritis and joint edema in laboratory animals. Int J Pharmacogn 34:218–222
Singh S, Majumdar D (1997) Evaluation of antiinflammatory activity of fatty acids of Ocimum sanctum fixed oil. Ind J Exp Biol 35:380–383
Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R (2005) Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure–activity relationship with linarin and linarin acetate. Carcinogenesis 26:845–854
Singh D, Kumar Chaudhuri P, Darokar MP (2014) New antiproliferative tricyclic sesquiterpenoid from the leaves of Ocimum sanctum. Helv Chim Acta 97:708–711
Skaltsa H, Tzakou O, Singh M (1999) Note polyphenols of Ocimum sanctum from suriname. Pharm Biol 37:92–94
Souto-Maior FN, Fonsêca DVd, Salgado PRR, Monte LdO, de Sousa DP, de Almeida RN (2017) Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm Biol 55:63–67
Subramanian M, Chintalwar GJ, Chattopadhyay S (2005) Antioxidant and radioprotective properties of an Ocimum sanctum polysaccharide. Redox Rep 10:257–264
Suzuki A, Shirota O, Mori K, Sekita S, Fuchino H, Takano A, Kuroyanagi M (2009) Leishmanicidal active constituents from Nepalese medicinal plant tulsi (Ocimum sanctum L.). Chem Pharm Bull 57:245–251
Tada H, Murakami Y, Omoto T, Shimomura K, Ishimaru K (1996) Rosmarinic acid and related phenolics in hairy root cultures of Ocimum basilicum. Phytochemistry 42:431–434
Tan PV, Nyasse B, Dimo T, Mezui C (2002) Gastric cytoprotective anti-ulcer effects of the leaf methanol extract of Ocimum suave (Lamiaceae) in rats. J Ethnopharmacol 82:69–74
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749
Tchoumbougnang F, Zollo PA, Dagne E, Mekonnen Y (2005) In vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimum on mice infected with Plasmodium berghei. Planta Med 71:20–23
Tewari D, Sah A, Pandey H, Meena H, Meena R, Ramaswamy R, Reddy RC, Deo YK, Bandari S, Bhadra Dev P (2012) A review on phytoconstituents of Ocimum (Tulsi). Int J Ayurvedic Med 3:1–9
Thaweboon S, Thaweboon B (2009) In vitro antimicrobial activity of Ocimum americanum L. essential oil against oral microorganisms. SE Asian J Trop Med 40(5):1025–1033
Thilakarathna S, Rupasinghe H (2013) Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5:3367–3387
Tripathi DK, Pathak A, Chauhan D, Dubey N, Rai A, Prasad R (2015) An efficient approach of laser induced breakdown spectroscopy (LIBS) and ICAP-AES to detect the elemental profile of Ocimum L. species. Biocatal Agric Biotechnol 4:471–479
Ueda-Nakamura T, Mendonça-Filho RR, Morgado-Díaz JA, Maza PK, Dias Filho BP, Cortez DAG, Alviano DS, Maria do Socorro SR, Lopes AHC, Alviano CS (2006) Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol Int 55:99–105
Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan K, Unnikrishnan M (2000) Radiation protection by the Ocimum flavonoids orientin and vicenin: mechanisms of action. Rad Res 154:455–460
US MR, Zin T, Abdurazak M, Ado Ahmad B (2015) Chemistry and pharmacology of syringin, a novel bioglycoside: a review. Asian J Pharm Clin Res 8:20–25
Valentová K, Vrba J, Bancířová M, Ulrichová J, Křen V (2014) Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol 68:267–282
Vani SR, Cheng S, Chuah C (2009) Comparative study of volatile compounds from genus Ocimum. Am J Appl Sci 6:523
Vieira RF, Grayer RJ, Paton AJ (2003) Chemical profiling of Ocimum americanum using external flavonoids. Phytochemistry 63:555–567
Vinukonda VP, Palakeerti SK, Nalakurthi BC, Palleti JD (2012) In silico studies of Justicia adhatoda, Ocimum sanctum plant compounds as mycobacterium tuberculosis FTSZ inhibitors. Int J Bioassays 1(8):22–25
Viskupicova J, Ondrejovic M, Sturdik E (2008) Bioavailability and metabolism of flavonoids. J Food Nutr Res 47(4):151–162
Waka M, Hopkins R, Glinwood R, Curtis C (2006) The effect of repellents Ocimum forskolei and deet on the response of Anopheles stephensi to host odours. Med Vet Entomol 20:373–376
Wang J, Fang X, Ge L, Cao F, Zhao L, Wang Z, Xiao W (2018) Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One 13:1–12
Xaasan CC, Ciilmi CX, Faarax MX, Passannanti S, Piozzi F, Paternostro M (1980) Unusual flavones from Ocimum canum. Phytochemistry 19:2229–2230
Yordi EG, Matos M, Martínez AP, Tornes A, Santana L, Molina E, Uriarte E (2017) In s∆ilico genotoxicity of coumarins: application of the phenol-explorer food database to functional food science. Food Funct 8:2958–2966
Zhao J, Li R, Pawlak A, Henklewska M, Sysak A, Wen L, Yi J-E, Obmińska-Mrukowicz B (2018) Antitumor activity of betulinic acid and betulin in canine cancer cell lines. In Vivo 32:1081–1088
Zheljazkov VD, Callahan A, Cantrell CL (2007a) Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J Agric Food Chem 56:241–245
Zheljazkov VD, Cantrell CL, Tekwani B, Khan SI (2007b) Content, composition, and bioactivity of the essential oils of three basil genotypes as a function of harvesting. J Agric Food Chem 56:380–385
Zhu J, Sanidad KZ, Sukamtoh E, Zhang G (2017) Potential roles of chemical degradation in the biological activities of curcumin. Food Funct 8:907–914
Acknowledgements
We would like to thank both Minia and Deraya Universities, Minia, Egypt, for all facilities, support and encouragement offered to us during this work.
Author information
Authors and Affiliations
Contributions
Conflict of interest
All authors declare that they have no conflict of interest.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zahran, E.M., Abdelmohsen, U.R., Khalil, H.E. et al. Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae). Phytochem Rev 19, 907–953 (2020). https://doi.org/10.1007/s11101-020-09690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-020-09690-9