Abstract
Histone deacetylase (HDAC), a key regulator in controlling the acetylation status of histone, are considered to be associated with viability, migration, invasion, proliferation and apoptosis of malignant tumors. The HDAC inhibition is an effective strategy for designing compounds against malignant tumors and five compounds have been approved by FDA or NMPA for clinical therapy. In this study, we designed and synthesized a series of novel carbazole-hydroxamate analogues as HDAC inhibitors and evaluated their anti-tumor properties in vitro. Compared with vorinostat, the HDAC semi-inhibitory concentration of compounds 3f and 3g decreased 4–13 folds, compounds 8a and 8c also showed strong inhibitory HDAC activity, and compound 3g had a strong inhibitory effect on HDAC 1. The CCK8 assay showed that compounds 3g displayed good antiproliferative activity on tested tumor cells. Flow cytometric and western blot assay showed that 3g exerted anti-tumor activities by regulating the level of Ac-HH3 and activating the cleaved caspase 3. Based on these results, carbazole-hydroxamate derivative 3g might become a potential anti-tumor candidate molecule to further structural optimization research.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histone deacetylases (HDACs) and histone acetyltransferases (HATs) are crucial regulators which controlled the acetylation status of histones [1, 2]. HDACs, especially class I isozymes (1, 2, 3, and 8) are considered to be associated with the progression of tumorigenesis such as malignancies viability, migration, invasion, proliferation, as well as the induction of cell apoptosis [3,4,5,6]. The HDAC inhibition strategy have been proved to be effective for tumor therapy against malignant tumors and five compounds (vorinostat, romidepsin, belinostat, panobinostat, and chidamide) have been approved by FDA or CFDA for clinical therapy [7] (Fig. 1).
The common HDAC inhibitors, liking SAHA (vorinostat) and belinostat, fit a three-motif pharmacophoric model, comprising of a cap group, a linker and a zinc-binding group (ZBG) [8, 9]. The cap groups are crucial for identifying the surface of protein and the appropriate linkers are benefit for ZBG entering the active pocket and chelating Zn2+ [10]. Thus, exploring new cap groups and appropriate linkers might afford more stronger inhibitors.
Carbazole, an aromatic heterocyclic compound, originated from the murrayanine since 1962. Thereafter, a number of carbazole derivatives were synthesized and exerted diverse biological activities, such as anticancer, anti-inflammation, anti-Alzheimer and pro-apoptosis [11,12,13]. Following our previous design experience in HDAC inhibitors [14,15,16], substituted carbazoles were introduced as the cap groups and diverse linkers were used to connect cap groups to hydroxamate group (Fig. 2). A series of carbazole-hydroxamate derivatives were synthesized and evaluated their biological activities in this article. After biological characterizations, including HeLa nuclear extract activity, HDAC isozymes activity, pro-apoptotic activity, 3g was identified as the most potent compound. It exhibited several striking characteristics: 5.9 nM HDAC1 inhibition activity; micromolar antiproliferative activity on tested tumor cell lines equivalent to SAHA; obvious apoptosis-inducing effect. These results disclosed that 3g was a promising lead compound for further structural optimization.
Results and discussion
Chemistry
The reaction routes of all target compounds were described in Scheme 1. Starting material 1,1’-(9H-carbazole-3,6-diyl)diethanone (1) was synthesized according to the previous report [17]. Starting from compound 1, carbazole derivatives 2a-g was obtained by nucleophilic substitution. The 2a-g were coupled with NH2OH to form the target compounds 3a-g. Removal of ester group to carboxylic acid in 2a-g using NaOH in THF/H2O solution yielded compounds 4a-g. Compound 4a-g were subjected to amidation coupling reaction with methyl 4-(aminomethyl)cinnamate and methyl 4-(aminomethyl)benzoate to afford 5a-g or 7a-g, followed by coupling with NH2OH formed the target compounds 6a-g or 8a-g.
HeLa cell extract activity
HeLa cell extract activity assay was usually applied to evaluate preliminary HDAC inhibitory activity of target compounds [15]. All compounds were screened for HeLa cell extract activities at 1000 nM, and compounds 3f, 3g, 6a, 6b, 6c, 6f, 8a, 8c, 8d, 8e, 8f, 8g with inhibition rates higher than 50% were further tested to determine IC50 values (Table 1). Initially, the effects of linker length on HeLa cell extract activity was investigated. For compounds 3a-g with increasing -CH2- units in structure, the 3f and 3g with linker bearing six and seven -CH2- units showed the best inhibition potent (14 ± 0.5 nM, 5.3 ± 1.2 nM, respectively). Compared with SAHA, the IC50 values of 3f and 3g decreased 4–13 folds. Next, N-OH-benzamide or N-OH-cinnamamide were introduced to substitute for hydroxamic acid to afford 6a-g and 8a-g. The introduction of N-OH-benzamide or N-OH-cinnamamide seemed to be benefit for inhibition and most of compounds possessed good inhibition potent with IC50 values lower than 1000 nM. The best active compounds 8a and 8c respectively displayed the 23.5 nM and 66.8 nM inhibitory activities relative to 67.7 nM of SAHA.
HDAC isoforms inhibitory activity of selected compounds HDAC
Compounds 3f, 3g, 8a, and 8c with stronger inhibition potency relative to SAHA were selected to evaluated their inhibitory effects on HDAC isoform enzymes 1, 6, and 8. As shown in Table 2, these compounds were more sensitive to HDAC1 than HDAC6 and HDAC8. Compound 3g exhibited the best inhibition effects on HDAC1 with IC50 value of 5.9 nM, increasement of 10 folds than that of SAHA. Compounds 3f, 8a, and 8c showed similar inhibition against HDAC1. For HDAC6, these compounds all displayed weak inhibition activities than SAHA. For HDAC8, only compound 3f increased 2-flods inhibitory activities relative to SAHA. Taken together, the compound 3g possess potent inhibition against HDAC1 and a degree of selectivity for HDAC1 over HDAC6 and HDAC8 (27-folds and 1206-folds).
In vitro antiproliferative activity
Compounds 3f, 3g, 8a, and 8c were subsequently selected to evaluated their antiproliferative activities against HCT116, HepG2, Hela, HEL, Molt4, and U266 cell lines, with SAHA as positive control (Table 3). In terms of the inhibition influence on HCT116 and HepG2 cells, compound 3f and 3g showed higher inhibition, whereas the IC50 values of 8a and 8c exceeded 25 μM. For HeLa cells, compounds 3g and 8c showed a degree of inhibition. Against the hematologic tumor cells (HEL and Molt4), 3g and 8c exhibited much stronger antiproliferative activities than 3f and 8a. Above all, these compounds possessed different antiproliferative activities to different cancer cells.
Cell apoptosis assay
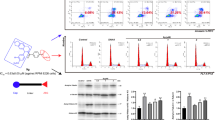
Afterward, we tested the pro-apoptosis effect of compound 3g on Molt4 cells after 24 h co-incubation. Compared with the control (3.79%) and SAHA (15.58%), compound 3g induced 24.46% and 48.50% Molt4 cell apoptosis at 0.5 and 1.5 μM. These results confirmed its notable pro-apoptosis activity on Molt4 cells (Fig. 3).
Western blot assay
Western blot assay was performed to test compound 3g influence on the acetylation of histone h3 (Ac-HH3), acetylation of tubulin (Ac-Tubulin) and cleaved caspase 3. As shown in Fig. 4, compound 3g enhanced the acetylation of histone h3 at the concentration of 1.5 μM, much stronger than SAHA on the acetylation-induced capacity at the same concentration. For acetylation of tubulin, compound 3g didn’t displayed significant acetylation-induced capacity at the concentration of 1.5 μM, whereas SAHA obviously increased the acetylation of tubulin. These results indicated that compound 3g had a more significant inhibitory effect on HDAC1 than on HDAC6, which agreed with the results in the HDAC isoforms inhibitory activity. Besides, 3g induced the cleavage of caspase-3, which indicated the activation of apoptosis.
Molecular docking study
Molecular docking was performed to investigate the interactions between 3g with HDAC1 protein (PDB code: 5ICN). As shown in Fig. 5A, B, the carbazole core occupied the outer surface of HDAC1 catalytic pocket and forms key interactions with Gly97 and Cys100. The fatty chain linkers and hydroxamic acids extended to the catalytic pocket around Zn2+. The carbonyl group chelated with zinc ions. Furthermore, the hydroxyl and amino groups interacted with Cys151 and Gly149 through hydrogen bond interactions. The docking of SAHA with HDAC1 was also performed and the result was as shown in Fig. 5C, D. Similar to 3g, the O of carbonyl chelated with Zn401 and the hydroxyl and amino groups interacted with Cys151 and Gly149. These results explained why the inhibition activity of 3g was similar to SAHA.
Conclusion
In this study, a series of novel carbazole-hydroxamate analogues were designed and synthesized as HDAC inhibitors. Through screening, compound 3g displayed potent enzyme and tumor cells inhibition activities. Flow cytometry and western blot assay showed that compound 3g could efficiently induce apoptosis through caspase-3 pathway. Western blot assay also revealed that compound 3g possessed stronger histone h3 acetylation-induced capacity, consistent with its stronger HDAC1 inhibitory activity. Based on these results, carbazole-hydroxamate derivative 3g might become a potential anti-tumor candidate molecule to further structural optimization research.
Experimental
General synthesis of compounds
Reagents and solvents used were of commercially available and without further purification. Melting points were recorded by the electrothermal melting point apparatus. 1H and 13C NMR spectra were recorded on a Brucker DRX spectrometer at 400 MHz. High-resolution mass spectra (HRMS) were conducted on an Agilent 6510 Quadrupole Time-of-Flight LC/MS deliver.
ethyl 2-(3,6-diacetyl-9H-carbazol-9-yl)acetate (2a)
1,1’-(9H-carbazole-3,6-diyl)diethanone (0.76 g, 3.0 mmol, 1.0 eq) was dissolved in a mixture of DMF (5.0 mL) and K2CO3 (0.84 g, 6.0 mmol, 2.0 eq). Then the ethyl bromoacetate (0.60 g, 3.6 mmol, 1.2 eq) was added and heated to 80 °C for 4–6 h. After that, 25 mL H2O was added into the mixture. The mixture was extracted by EtOAc (×3). Later, the EtOAc layer was washed by saturated brine solution, dried with anhydrous MgSO4, filtered and reduced to yield an light yellow solid. The crude residue was further purified by flash column chromatography on a silica gel using CH2Cl2/CH3OH (100:1), yield 74%. Mp: 202–203 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.81 (d, J = 1.8 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.40 (d, J = 8.6 Hz, 2H), 5.07 (s, 2H), 4.23 (q, J = 7.1 Hz, 2H), 2.75 (s, 6H), 1.25 (t, J = 7.2 Hz, 3H).
2b-2g were synthesized in the same way as 2a.
methyl 3-(3,6-diacetyl-9H-carbazol-9-yl)propanoate (2b)
White solid. Mp:158–160 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.81 (d, J = 1.8 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.43 (d, J = 8.7 Hz, 2H), 4.43 (q, J = 7.1 Hz, 2H), 3.72 (s, 3H), 2.75 (s, 6H), 2.25 (t, J = 7.2 Hz, 2H).
methyl 4-(3,6-diacetyl-9H-carbazol-9-yl)butanoate (2c)
White solid. Mp:146–147 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.7 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.50 (d, J = 8.7 Hz, 2H), 4.47 (t, J = 7.2 Hz, 2H), 3.69 (s, 3H), 2.75 (s, 6H), 2.38 (t, J = 6.8 Hz, 2H), 2.29–2.18 (m, 2H).
ethyl 5-(3,6-diacetyl-9H-carbazol-9-yl)pentanoate (2d)
White solid. Mp: 145–146 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.8 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.47 (d, J = 8.7 Hz, 2H), 4.39 (t, J = 7.2 Hz, 2H), 4.10 (q, J = 7.2 Hz, 2H), 2.75 (s, 6H), 2.33 (t, J = 7.2 Hz, 2H), 1.95 (q, J = 7.6 Hz, 2H), 1.73 (q, J = 7.6 Hz, 2H), 1.21 (t, J = 7.2 Hz, 3H).
methyl 6-(3,6-diacetyl-9H-carbazol-9-yl)hexanoate (2e)
White solid. Mp: 140–141 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.8 Hz, 2H), 8.18 (dd, J = 8.7, 1.8 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 4.37 (t, J = 7.2 Hz, 2H), 3.64 (s, 3H), 2.75 (s, 6H), 2.29 (t, J = 7.3 Hz, 2H), 1.93 (p, J = 7.4 Hz, 2H), 1.68 (p, J = 7.4 Hz, 2H), 1.42 (tt, J = 9.7, 6.2 Hz, 2H).
ethyl 7-(3,6-diacetyl-9H-carbazol-9-yl)heptanoate (2f)
White solid. Mp:110–111 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.8 Hz, 2H), 8.18 (dd, J = 8.7, 1.7 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 4.36 (t, J = 7.2 Hz, 2H), 4.10 (q, J = 7.2 Hz, 2H), 2.75 (d, J = 2.5 Hz, 6H), 2.26 (t, J = 7.4 Hz, 2H), 1.91 (t, J = 7.2 Hz, 2H), 1.60 (d, J = 6.5 Hz, 2H), 1.39 (dd, J = 7.3, 3.5 Hz, 4H), 1.23 (t, J = 7.2 Hz, 3H).
ethyl 8-(3,6-diacetyl-9H-carbazol-9-yl)octanoate (2g)
White solid. Mp: 95–96 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.84 (d, J = 1.8 Hz, 2H), 8.28 (dd, J = 8.7, 1.7 Hz, 2H), 7.35 (d, J = 8.7 Hz, 2H), 4.32 (t, J = 7.2 Hz, 2H), 3.75 (m, 2H), 2.73 (s, 6H), 2.32 (t, J = 7.4 Hz, 2H), 1.91 (t, J = 6.6 Hz, 2H), 1.54 (t, J = 7.3 Hz, 2H), 1.40–1.22 (m, 9H).
2-(3,6-diacetyl-9 H -carbazol-9-yl)-N-hydroxyacetamide (3a)
The synthesis method was according to our previous published article [16]. Yield 49%. Light yellow solid. Mp: 202–203 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (d, J = 1.8 Hz, 2H), 7.83 (dd, J = 8.7, 1.8 Hz, 2H), 7.55 (d, J = 8.8 Hz, 2H), 5.23 (s, 2H), 2.30 (s, 6H). 13C NMR (101 MHz, DMSO) δ 170.47, 153.92, 141.53, 129.26, 124.06, 122.77, 118.53, 109.89, 44.71, 12.42. HRMS (ESI) m/z Calcd [M + H]+ 325.1183, found: 325.1184.
3b-3g were synthesized in the same way as 3a.
3-(3,6-diacetyl-9H-carbazol-9-yl)-N-hydroxypropanamide (3b)
White solid. Mp: 213–215 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (d, J = 1.8 Hz, 2H), 7.85 (dd, J = 8.7, 1.8 Hz, 2H), 7.63 (d, J = 8.7 Hz, 2H), 4.64 (t, J = 7.1 Hz, 2H), 2.75 (t, J = 7.0 Hz, 2H), 2.30 (s, 6H). 13C NMR (101 MHz, DMSO) δ 173.05, 153.89, 140.72, 128.99, 124.00, 122.70, 118.54, 110.02, 34.14, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 337.1194, found: 337.1197.
4-(3,6-diacetyl-9H-carbazol-9-yl)-N-hydroxybutanamide (3c)
White solid. Mp: 175–177 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 2H), 7.86 (dd, J = 8.7, 1.8 Hz, 2H), 7.62 (d, J = 8.8 Hz, 2H), 4.43 (d, J = 7.3 Hz, 2H), 2.30 (s, 6H), 2.06–1.94 (m, 4H). 13C NMR (101 MHz, DMSO) δ 168.98, 153.91, 140.95, 128.88, 124.02, 122.58, 118.65, 109.81, 42.47, 29.69, 25.05, 12.39. HRMS (ESI) m/z Calcd [M + H]+ 353.1496, found: 353.1499.
5-(3,6-diacetyl-9H-carbazol-9-yl)-N-hydroxypentanamide (3d)
White solid. Mp: 191–192 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (s, 2H), 7.88–7.82 (m, 2H), 7.61 (d, J = 8.7 Hz, 2H), 4.42 (t, J = 7.0 Hz, 2H), 2.30 (s, 6H), 2.22 (t, J = 7.4 Hz, 2H), 1.82–1.75 (m, 2H), 1.51 (t, J = 7.8 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 197.51, 174.78, 153.91, 140.98, 128.80, 123.98, 122.54, 118.61, 109.85, 42.70, 33.95, 28.61, 22.60, 12.37. HRMS (ESI) m/z Calcd [M + H]+ 367.1652, found: 367.1656.
6-(3,6-diacetyl-9H-carbazol-9-yl)-N-hydroxyhexanamide (3e)
White solid. Mp: 161–163 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.46 (s, 2H), 7.85 (dd, J = 8.7, 1.8 Hz, 2H), 7.58 (d, J = 8.7 Hz, 2H), 4.37 (t, J = 7.2 Hz, 2H), 2.30 (s, 6H), 1.84 (t, J = 7.3 Hz, 2H), 1.75 (t, J = 7.5 Hz, 2H), 1.55–1.40 (m, 2H), 1.33–1.20 (m, 2H). 13C NMR (101 MHz, DMSO) δ 178.17, 153.79, 140.96, 128.78, 123.98, 122.51, 118.55, 109.77, 43.03, 38.38, 29.12, 27.27, 26.55, 12.36. HRMS (ESI) m/z Calcd [M + H]+ 379.1663, found: 379.1668.
7-(3,6-diacetyl-9H-carbazol-9-yl)-N-hydroxyheptanamide (3f)
White solid. Mp:171–173 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.32 (s, 1H), 8.64 (s, 1H), 8.50–8.43 (m, 2H), 7.91–7.82 (m, 2H), 7.61 (dd, J = 8.9, 5.4 Hz, 2H), 4.40 (d, J = 6.7 Hz, 2H), 2.30 (s, 6H), 1.89 (t, J = 6.9 Hz, 2H), 1.77 (q, J = 7.4, 7.0 Hz, 2H), 1.43 (q, J = 6.9 Hz, 2H), 1.38–1.20 (m, 4H). 13C NMR (101 MHz, DMSO) δ 169.54, 153.91, 140.98, 128.77, 123.99, 122.53, 118.63, 109.82, 42.89, 32.64, 28.90, 28.76, 26.60, 25.44, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 395.1965, found: 395.1968.
8-(3,6-diacetyl-9H-carbazol-9-yl)-N-hydroxyoctanamide (3g)
White solid. Mp: 171–172 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.31 (s, 1H), 8.64 (s, 1H), 8.47 (d, J = 1.9 Hz, 2H), 7.86 (dd, J = 8.7, 1.8 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 4.40 (t, J = 7.0 Hz, 2H), 2.30 (s, 6H), 1.89 (t, J = 7.4 Hz, 2H), 1.82–1.71 (m, 2H), 1.42 (q, J = 7.5 Hz, 2H), 1.27 (d, J = 6.1 Hz, 4H), 1.20–1.08 (m, 2H). 13C NMR (101 MHz, DMSO) δ 169.57, 153.92, 140.99, 128.76, 123.98, 122.53, 118.62, 109.84, 42.91, 32.66, 29.05, 28.98, 28.95, 26.85, 25.51, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 409.2122, found: 409.2124.
2-(3,6-diacetyl-9H-carbazol-9-yl)acetic acid (4a)
Compound 2a (0.68 g, 2.0 mmol, 1.0eq) was dissolved in MeOH (6 mL), then the solution of NaOH (1 M, 6 mL) was added. The mixture was stirred at reflux for 4 h. Then the mixture was neutralized with 1 M HCl to PH 2-3. White precipitate obtained was filtered and vacuum drying. Yield 63%. Light yellow solid. Mp: 289–290 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.78 (d, J = 1.8 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.43 (d, J = 8.6 Hz, 2H), 5.01 (s, 2H), 2.74 (s, 6H).
4b-4g were synthesized in the same way as 4a.
3-(3,6-diacetyl-9H-carbazol-9-yl)propanoic acid (4b)
Light yellow solid. Mp:191–192 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.81 (d, J = 1.8 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.43 (d, J = 8.7 Hz, 2H), 4.43 (q, J = 7.1 Hz, 2H), 3.72 (s, 3H), 2.75 (s, 6H), 2.25 (t, J = 7.2 Hz, 2H).
4-(3,6-diacetyl-9H-carbazol-9-yl)butanoic acid (4c)
Light yellow solid. Mp:220–221 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.74 (d, J = 1.8 Hz, 2H), 8.13 (d, J = 8.7 Hz, 2H), 7.44 (d, J = 8.7 Hz, 2H), 4.42 (t, J = 6.8 Hz, 2H), 2.68 (s, 6H), 2.38 (t, J = 6.8 Hz, 2H).
5-(3,6-diacetyl-9H-carbazol-9-yl)pentanoic acid (4d)
Light yellow solid. Mp:215-216 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.8 Hz, 2H), 8.19 (dd, J = 8.7, 1.7 Hz, 2H), 7.47 (d, J = 8.7 Hz, 2H), 4.39 (t, J = 7.2 Hz, 2H), 3.90 (q, J = 7.2 Hz, 2H), 2.75 (s, 6H), 2.33 (t, J = 7.2 Hz, 2H), 1.95 (q, J = 7.6 Hz, 2H).
6-(3,6-diacetyl-9H-carbazol-9-yl)hexanoic acid (4e)
Light yellow solid. Mp:155–156 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.8 Hz, 2H), 8.18 (dd, J = 8.7, 1.8 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 4.37 (t, J = 7.2 Hz, 2H), 2.75 (s, 6H), 2.29 (t, J = 7.3 Hz, 2H), 1.93 (p, J = 7.4 Hz, 2H), 1.68 (p, J = 7.4 Hz, 2H), 1.42 (tt, J = 9.7, 6.2 Hz, 2H).
7-(3,6-diacetyl-9H-carbazol-9-yl)heptanoic acid (4f)
Light yellow solid. Mp:175–176 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.8 Hz, 2H), 8.18 (dd, J = 8.7, 1.7 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 4.36 (t, J = 7.2 Hz, 2H), 2.75 (s, 6H), 2.26 (t, J = 7.4 Hz, 2H), 1.91 (t, J = 7.2 Hz, 2H), 1.60 (d, J = 6.5 Hz, 2H), 1.39 (dd, J = 7.3, 3.5 Hz, 4H).
8-(3,6-diacetyl-9H-carbazol-9-yl)octanoic acid (4g)
Light yellow solid. Mp:155–156 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.79 (d, J = 1.8 Hz, 2H), 8.18 (dd, J = 8.7, 1.7 Hz, 2H), 7.45 (d, J = 8.7 Hz, 2H), 4.35 (t, J = 7.2 Hz, 2H), 2.75 (s, 6H), 2.30 (t, J = 7.4 Hz, 2H), 1.90 (t, J = 6.6 Hz, 2H), 1.58 (t, J = 7.3 Hz, 2H), 1.44 – 1.21 (m, 6H).
methyl 4-((2-(3,6-diacetyl-9H-carbazol-9-yl)acetamido)methyl)benzoate (5a)
Compound 4a (0.31 g, 1.0 mmol, 1.0eq) was dissolved in 20 mL DCM, TBTU (0.39 g, 1.2 mmol, 1.2eq) and DIEA (0.39 g, 3 mmol, 3eq) was added. After stiring 0.5 h, methyl 4-(aminomethyl)benzoate (0.26 g, 1.1 mmol, 1.1eq) was added and the reaction was stirred at RT for 8–10 h. Then the mixture was washed with 1 M citric acid (×3), saturate solution of NaHCO3(×3) and brine solution (×3). The organic layer was concentrated in vacuo, then purified via chromatography on silica (DCM/MeOH 80:1) to give a white solid as the target compound. Yield 67%. Mp:274–275 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.74 (d, J = 1.7 Hz, 2H), 8.17 (dd, J = 8.6, 1.7 Hz, 2H), 7.88 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.6 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 6.04 (d, J = 6.6 Hz, 1H), 5.07 (s, 2H), 4.45 (d, J = 6.1 Hz, 2H), 3.87 (s, 3H), 2.70 (s, 6H).5b-5g were synthesized in the same way as 5a.
Ethyl 4-((3-(3,6-diacetyl-9H-carbazol-9-yl)propanamido)methyl)benzoate (5b)
White solid. Mp:207–208 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.77 (d, J = 1.7 Hz, 2H), 8.13 (dd, J = 8.7, 1.7 Hz, 2H), 7.87–7.70 (m, 2H), 7.54 (d, J = 8.6 Hz, 2H), 6.91 (d, J = 8.0 Hz, 2H), 5.74 (s, 1H), 4.80 (t, J = 6.3 Hz, 2H), 4.31 (d, J = 5.9 Hz, 2H), 3.89 (s, 3H), 2.80 (q, J = 4.3, 2.5 Hz, 2H), 2.73 (s, 6H).
Methyl 4-((4-(3,6-diacetyl-9H-carbazol-9-yl)butanamido)methyl)benzoate (5c)
White solid. Mp:195–196 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.78 (d, J = 1.6 Hz, 2H), 8.13 (dd, J = 8.7, 1.7 Hz, 2H), 8.00 (d, J = 8.1 Hz, 2H), 7.49 (d, J = 8.7 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 5.69 (s, 1H), 4.56–4.47 (m, 4H), 3.91 (s, 3H), 2.73 (s, 6H), 2.30 (p, J = 6.6 Hz, 2H), 2.27–2.20 (m, 2H).
Methyl 4-((5-(3,6-diacetyl-9H-carbazol-9-yl)pentanamido)methyl)benzoate (5d)
White solid. Mp:207–208 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.7 Hz, 2H), 8.18 (dd, J = 8.6, 1.7 Hz, 2H), 7.95 (d, J = 8.2 Hz, 2H), 7.47 (d, J = 8.6 Hz, 2H), 7.28 (d, 2H), 4.45 (d, J = 5.9 Hz, 2H), 4.41 (t, J = 7.0 Hz, 2H), 3.91 (s, 3H), 2.75 (s, 6H), 2.23 (t, J = 7.2 Hz, 2H), 1.97 (t, J = 8.0 Hz, 2H), 1.82–1.74 (m, 2H).
methyl 4-((6-(3,6-diacetyl-9H-carbazol-9-yl)hexanamido)methyl)benzoate (5e)
White solid. Mp:199–200 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.79 (d, J = 1.8 Hz, 2H), 8.17 (dd, J = 8.7, 1.7 Hz, 2H), 7.98 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 8.7 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 5.71 (s, 1H), 4.46 (d, J = 6.0 Hz, 2H), 4.37 (t, J = 7.1 Hz, 2H), 3.91 (s, 3H), 2.74 (s, 6H), 2.19 (t, J = 7.3 Hz, 2H), 1.94 (p, J = 7.4 Hz, 2H), 1.73 (p, J = 7.5 Hz, 2H), 1.42 (p, J = 8.0 Hz, 2H).
methyl 4-((7-(3,6-diacetyl-9H-carbazol-9-yl)heptanamido)methyl)benzoate (5f)
White solid. Mp:194–195 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.79 (d, J = 1.7 Hz, 2H), 8.18 (dd, J = 8.7, 1.7 Hz, 2H), 7.98 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.7 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 5.72 (s, 1H), 4.47 (d, J = 5.9 Hz, 2H), 4.35 (t, J = 7.1 Hz, 2H), 3.90 (s, 3H), 2.75 (s, 6H), 2.18 (t, J = 7.4 Hz, 2H), 1.98–1.86 (m, 2H), 1.73 – 1.61 (m, 2H), 1.46–1.32 (m, 4H).
methyl 4-((8-(3,6-diacetyl-9H-carbazol-9-yl)octanamido)methyl)benzoate (5g)
White solid. Mp:180–181 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.7 Hz, 2H), 8.18 (dd, J = 8.7, 1.7 Hz, 2H), 7.99 (d, J = 8.1 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 5.73 (s, 1H), 4.48 (d, J = 5.9 Hz, 2H), 4.35 (t, J = 7.2 Hz, 2H), 3.91 (s, 3H), 2.75 (s, 6H), 2.19 (t, J = 7.5 Hz, 2H), 1.88 (d, J = 7.6 Hz, 2H), 1.69 – 1.60 (m, 2H), 1.40–1.25 (m, 6H).
6a-6g were synthesized in the same way as 3a.
4-((2-(3,6-diacetyl-9H-carbazol-9-yl)acetamido)methyl)-N-hydroxybenzamide (6a)
White solid. Mp:226–227 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.51–8.46 (m, 2H), 7.89 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 8.6 Hz, 2H), 7.71 (d, J = 7.9 Hz, 1H), 7.57 (d, J = 8.7 Hz, 2H), 7.39 (d, J = 8.0 Hz, 1H), 7.34 (d, J = 7.9 Hz, 1H), 5.18 (s, 2H), 4.36 (dd, J = 12.4, 5.8 Hz, 2H), 2.30 (s, 6H). 13C NMR (101 MHz, DMSO) δ 167.99, 167.62, 153.93, 144.79, 141.69, 129.80, 129.20, 127.71, 127.52, 124.00, 122.83, 118.54, 109.98, 46.22, 42.54, 12.44. HRMS (ESI) m/z Calcd [M + H]+ 458.1710, found: 458.1711.
4-((3-(3,6-diacetyl-9H-carbazol-9-yl)propanamido)methyl)-N-hydroxybenzamide (6b)
White solid. Mp: 202-204 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (d, J = 2.1 Hz, 2H), 7.85 (dd, J = 8.7, 2.0 Hz, 2H), 7.66 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 8.7 Hz, 2H), 7.54 (d, J = 7.9 Hz, 1H), 7.00 (d, J = 7.9 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 4.68 (t, J = 6.4 Hz, 2H), 4.20 (d, J = 6.3 Hz, 2H), 2.70 (q, J = 7.1, 6.6 Hz, 2H), 2.31 (s, 6H). 13C NMR (101 MHz, DMSO) δ 170.57, 153.95, 153.92, 144.55, 142.76, 140.77, 129.61, 128.98, 127.40, 127.24, 123.96, 122.71, 118.56, 110.16, 110.09, 42.27, 35.38, 12.40. HRMS (ESI) m/z Calcd [M + H]+ 472.1867, found: 472.1866.
4-((4-(3,6-diacetyl-9H-carbazol-9-yl)butanamido)methyl)-N-hydroxybenzamide (6c)
White solid. Mp: 170–172 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (d, J = 1.8 Hz, 2H), 7.85 (dd, J = 8.8, 1.8 Hz, 2H), 7.69 (d, J = 7.9 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.29 (d, J = 7.9 Hz, 2H), 4.43 (t, J = 7.1 Hz, 2H), 4.28 (d, J = 5.9 Hz, 2H), 2.30 (s, 6H), 2.27–2.19 (m, 2H), 2.09–1.99 (m, 2H). 13C NMR (101 MHz, DMSO) δ 172.01, 153.92, 143.34, 140.98, 131.73, 129.82, 128.87, 127.72, 127.56, 127.38, 124.02, 122.59, 118.63, 109.81, 42.33, 32.53, 25.00, 12.40. HRMS (ESI) m/z Calcd [M + H]+ 486.2023, found: 486.2028.
4-((5-(3,6-diacetyl-9H-carbazol-9-yl)pentanamido)methyl)-N-hydroxybenzamide (6d)
White solid. Mp: 151–153 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.97 (s, 2H), 8.47 (d, J = 1.8 Hz, 2H), 7.85 (dd, J = 8.5, 1.8 Hz, 4H), 7.61 (d, J = 8.8 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 4.43 (t, J = 6.9 Hz, 2H), 4.28 (d, J = 5.9 Hz, 2H), 2.30 (s, 6H), 2.18 (t, J = 7.3 Hz, 2H), 2.08 (s, 2H), 1.78 (s, 2H), 1.60–1.54 (m, 2H). 13C NMR (101 MHz, DMSO) δ 172.49, 167.68, 153.94, 145.19, 140.98, 129.79, 128.80, 127.55, 123.99, 122.54, 118.60, 109.85, 42.70, 42.24, 35.42, 28.72, 23.35, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 500.2180, found: 500.2183.
4-((6-(3,6-diacetyl-9H-carbazol-9-yl)hexanamido)methyl)-N-hydroxybenzamide (6e)
White solid. Mp: 184–186 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (s, 2H), 7.91–7.81 (m, 3H), 7.70 (d, J = 7.9 Hz, 1H), 7.59 (d, J = 8.7 Hz, 2H), 7.31 (d, J = 7.9 Hz, 1H), 7.27 (d, J = 7.9 Hz, 1H), 4.46–4.36 (m, 2H), 4.26 (dd, J = 12.2, 5.9 Hz, 2H), 2.30 (s, 6H), 2.10 (t, J = 7.6 Hz, 2H), 1.79 (d, J = 8.9 Hz, 2H), 1.60–1.49 (m, 2H), 1.34–1.24 (m, 2H). 13C NMR (101 MHz, DMSO) δ 172.60, 167.64, 153.91, 145.40, 140.98, 129.82, 128.78, 127.61, 127.45, 123.98, 122.54, 118.63, 109.84, 42.88, 42.24, 35.60, 28.84, 26.59, 25.47, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 514.2336, found: 514.2332.
4-((7-(3,6-diacetyl-9H-carbazol-9-yl)heptanamido)methyl)-N-hydroxybenzamide (6f)
White solid. Mp: 172–174 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (s, 2H), 7.86 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.0 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H), 4.39 (t, J = 7.0 Hz, 2H), 4.26 (d, J = 5.8 Hz, 2H), 2.30 (s, 6H), 2.10 (t, J = 7.2 Hz, 2H), 1.77 (t, J = 7.2 Hz, 2H), 1.47 (t, J = 7.0 Hz, 2H), 1.36–1.23 (m, 4H). 13C NMR (101 MHz, DMSO) δ 172.65, 153.92, 143.52, 141.00, 131.68, 129.81, 128.78, 127.44, 127.37, 124.00, 122.54, 118.63, 109.83, 42.91, 42.19, 35.71, 28.96, 28.93, 26.68, 25.60, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 526.2347, found: 526.2377.
4-((8-(3,6-diacetyl-9H-carbazol-9-yl)octanamido)methyl)-N-hydroxybenzamide (6g)
White solid. Mp: 213–214 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (s, 2H), 7.93–7.81 (m, 4H), 7.60 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 7.9 Hz, 2H), 4.40 (t, J = 7.0 Hz, 2H), 4.30 (d, J = 5.9 Hz, 2H), 2.30 (s, 6H), 2.11 (t, J = 7.4 Hz, 2H), 1.82–1.71 (m, 2H), 1.48 (t, J = 7.5 Hz, 2H), 1.34–1.24 (m, 4H), 1.22–1.14 (m, 2H). 13C NMR (101 MHz, DMSO) δ 172.78, 167.65, 153.93, 145.46, 140.99, 129.80, 129.69, 128.76, 127.62, 123.98, 122.52, 118.62, 109.82, 42.92, 42.24, 35.71, 29.03, 28.94, 26.83, 25.67, 12.37. HRMS (ESI) m/z Calcd [M + H]+ 542.2649, found: 542.2656.
7a-7g were synthesized in the same way as 5a.
ethyl (E)-3-(4-((2-(3,6-diacetyl-9H-carbazol-9-yl)acetamido)methyl)phenyl)acrylate (7a)
White solid. Mp:238–240 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.66 (s, 2H), 8.10 (dd, J = 8.6, 1.6 Hz, 1H), 7.50 (d, J = 16.0 Hz, 1H), 7.38 (d, J = 8.6 Hz, 2H), 7.30 (d, J = 7.9 Hz, 2H), 7.02 (d, J = 7.9 Hz, 2H), 6.28 (d, J = 16.1 Hz, 1H), 6.02 (s, 1H), 4.99 (s, 2H), 4.34 (d, J = 6.0 Hz, 2H), 4.17 (d, J = 7.2 Hz, 2H), 2.63 (s, 6H), 1.25 (t, J = 7.1 Hz, 3H).
ethyl (E)-3-(4-((3-(3,6-diacetyl-9H-carbazol-9-yl)propanamido)methyl)phenyl)acrylate (7b)
White solid. Mp:232–233 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.77 (d, J = 1.7 Hz, 2H), 8.14 (dd, J = 8.7, 1.7 Hz, 2H), 7.62–7.48 (m, 3H), 7.28 (d, J = 8.3 Hz, 2H), 6.92 (d, J = 7.9 Hz, 2H), 6.34 (d, J = 16.0 Hz, 1H), 5.71 (s, 1H), 4.79 (t, J = 6.4 Hz, 2H), 4.27 (dd, J = 9.2, 6.4 Hz, 4H), 2.79 (t, J = 6.5 Hz, 2H), 2.72 (s, 6H), 1.34 (t, J = 7.1 Hz, 3H).
Ethyl (E)-3-(4-((4-(3,6-diacetyl-9H-carbazol-9-yl)butanamido)methyl)phenyl)acrylate (7c)
White solid. Mp:221–222 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.76 (d, J = 1.7 Hz, 2H), 8.12 (dd, J = 8.6, 1.7 Hz, 2H), 7.64 (d, J = 16.0 Hz, 1H), 7.48 (dd, J = 8.3, 2.5 Hz, 4H), 7.29 (d, 2H), 6.41 (d, J = 16.1 Hz, 1H), 5.74 (t, J = 5.9 Hz, 1H), 4.50 (t, J = 6.7 Hz, 2H), 4.45 (d, J = 5.8 Hz, 2H), 4.25 (q, J = 7.2 Hz, 2H), 2.72 (s, 6H), 2.29 (q, J = 6.7 Hz, 2H), 2.22 (t, J = 6.4 Hz, 2H), 1.33 (t, J = 7.1 Hz, 3H).
ethyl (E)-3-(4-((5-(3,6-diacetyl-9H-carbazol-9-yl)pentanamido)methyl)phenyl)acrylate (7d)
White solid. Mp:201–202 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.79 (d, J = 1.8 Hz, 2H), 8.17 (dd, J = 8.7, 1.7 Hz, 2H), 7.63 (d, J = 16.0 Hz, 1H), 7.45 (dd, J = 12.5, 8.4 Hz, 4H), 7.23 (d, J = 7.9 Hz, 2H), 6.39 (d, J = 16.0 Hz, 1H), 4.40 (t, J = 6.7 Hz, 4H), 4.26 (q, J = 7.2 Hz, 2H), 2.74 (s, 6H), 2.22 (t, J = 7.2 Hz, 2H), 1.95 (d, J = 8.2 Hz, 2H), 1.77 (t, J = 7.9 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H).
ethyl (E)-3-(4-((6-(3,6-diacetyl-9H-carbazol-9-yl)hexanamido)methyl)phenyl)acrylate (7e)
White solid. Mp:189–190 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.79 (d, J = 1.8 Hz, 2H), 8.17 (dd, J = 8.7, 1.7 Hz, 2H), 7.65 (d, J = 16.0 Hz, 1H), 7.46 (t, J = 8.8 Hz, 4H), 7.24 (d, 2H), 6.41 (d, J = 16.0 Hz, 1H), 5.65 (s, 1H), 4.42 (d, J = 5.9 Hz, 2H), 4.37 (t, J = 7.1 Hz, 2H), 4.26 (q, J = 7.1 Hz, 2H), 2.74 (s, 6H), 2.18 (t, J = 7.4 Hz, 2H), 1.93 (t, J = 7.7 Hz, 2H), 1.72 (q, J = 7.6 Hz, 2H), 1.43 (q, J = 8.3 Hz, 2H), 1.34 (t, J = 7.1 Hz, 3H).
ethyl (E)-3-(4-((7-(3,6-diacetyl-9H-carbazol-9-yl)heptanamido)methyl)phenyl)acrylate (7f)
White solid. Mp:159–160 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.7 Hz, 2H), 8.18 (dd, J = 8.7, 1.7 Hz, 2H), 7.63 (d, J = 16.0 Hz, 1H), 7.46 (dd, J = 8.4, 3.5 Hz, 4H), 7.27 (d, 2H), 6.39 (d, J = 16.0 Hz, 1H), 5.67 (s, 1H), 4.42 (d, J = 5.8 Hz, 2H), 4.35 (t, J = 7.2 Hz, 2H), 4.26 (q, J = 7.2 Hz, 2H), 2.75 (s, 6H), 2.17 (t, J = 7.4 Hz, 2H), 1.91 (t, J = 7.1 Hz, 2H), 1.64 (t, J = 7.3 Hz, 2H), 1.43–1.30 (m, 7H).
ethyl (E)-3-(4-((8-(3,6-diacetyl-9H-carbazol-9-yl)octanamido)methyl)phenyl)acrylate (7g)
White solid. Mp:150–151 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.80 (d, J = 1.7 Hz, 2H), 8.18 (dd, J = 8.6, 1.7 Hz, 2H), 7.65 (d, J = 16.0 Hz, 1H), 7.46 (dd, J = 8.4, 3.7 Hz, 4H), 7.28 (d, 2H), 6.41 (d, J = 16.0 Hz, 1H), 4.44 (d, J = 5.8 Hz, 2H), 4.35 (t, J = 7.2 Hz, 2H), 4.26 (q, J = 7.1 Hz, 2H), 2.75 (s, 6H), 2.18 (t, J = 7.5 Hz, 2H), 1.88 (d, J = 8.1 Hz, 2H), 1.62 (dd, J = 15.0, 7.7 Hz, 4H), 1.38–1.30 (m, 7H).
8a-8g were synthesized in the same way as 6a.
(E)-3-(4-((2-(3,6-diacetyl-9H-carbazol-9-yl)acetamido)methyl)phenyl)-N-hydroxyacrylamide (8a)
White solid. Mp: 222–224 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 2H), 7.84 (dd, J = 8.7, 1.8 Hz, 2H), 7.53 (dd, J = 15.6, 8.3 Hz, 4H), 7.43 (d, J = 15.8 Hz, 1H), 7.31 (d, J = 7.8 Hz, 2H), 6.47 (d, J = 15.9 Hz, 1H), 5.15 (s, 2H), 4.32 (d, J = 5.8 Hz, 2H), 2.30 (s, 6H). 13C NMR (101 MHz, DMSO) δ 167.86, 163.28, 153.94, 141.70, 141.07, 138.44, 134.02, 129.18, 128.27, 127.92, 124.00, 122.82, 119.35, 118.52, 109.97, 46.22, 42.55, 12.44. HRMS (ESI) m/z Calcd [M + H]+ 482.1940, found: 482.1945.
(E)-3-(4-((3-(3,6-diacetyl-9H-carbazol-9-yl)propanamido)methyl)phenyl)-N-hydroxyacrylamide (8b)
White solid. Mp: 112–113 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.49 (s, 2H), 8.40 (t, J = 6.0 Hz, 1H), 7.90 – 7.81 (m, 2H), 7.61 (d, J = 8.7 Hz, 2H), 7.48 (d, J = 16.0 Hz, 1H), 7.40 (d, J = 7.8 Hz, 2H), 6.92 (d, J = 8.0 Hz, 2H), 6.42 (d, J = 16.0 Hz, 1H), 4.69 (t, J = 6.3 Hz, 2H), 4.17 (d, J = 5.9 Hz, 2H), 2.69 (t, J = 6.8 Hz, 2H), 2.31 (s, 6H). 13C NMR (101 MHz, DMSO) δ 197.57, 168.01, 153.91, 144.03, 143.76, 140.76, 129.96, 129.92, 128.97, 128.41, 127.86, 126.89, 123.94, 122.98, 122.88, 122.73, 119.14, 118.57, 110.59, 72.09, 42.27, 27.21, 12.39. HRMS (ESI) m/z Calcd [M + H]+ 498.2023, found: 498.2025.
(E)-4-(3,6-diacetyl-9H-carbazol-9-yl)-N-(4-(3-(hydroxyamino)-3-oxoprop-1-en-1-yl)benzyl)butanamide (8c)
White solid. Mp: 154–156 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 2H), 8.42 (d, J = 5.9 Hz, 1H), 7.84 (d, J = 8.6 Hz, 2H), 7.64 – 7.56 (m, 3H), 7.49 (d, J = 7.6 Hz, 1H), 7.41 (d, J = 15.7 Hz, 1H), 7.26 (d, J = 7.8 Hz, 2H), 6.52–6.42 (m, 1H), 4.43 (t, J = 7.0 Hz, 2H), 4.26 (d, J = 5.7 Hz, 2H), 2.30 (s, 6H), 2.20 (d, J = 7.2 Hz, 2H), 2.03 (t, J = 7.2 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 171.96, 163.21, 153.84, 140.98, 138.37, 133.90, 128.86, 128.44, 128.28, 127.91, 124.01, 122.58, 119.29, 118.62, 109.80, 42.48, 32.50, 24.98, 12.39. HRMS (ESI) m/z Calcd [M + H]+ 512.2180, found: 512.2187.
(E)-5-(3,6-diacetyl-9H-carbazol-9-yl)-N-(4-(3-(hydroxyamino)-3-oxoprop-1-en-1-yl)benzyl)pentanamide (8d)
White solid. Mp: 152–153 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.52–8.44 (m, 2H), 8.32 (s, 1H), 7.85 (dd, J = 8.7, 1.8 Hz, 2H), 7.61 (d, J = 8.8 Hz, 2H), 7.49–7.40 (m, 2H), 7.20 (d, J = 7.8 Hz, 2H), 6.41 (d, J = 15.8 Hz, 1H), 4.42 (d, J = 7.6 Hz, 2H), 4.22 (d, J = 5.8 Hz, 2H), 2.30 (s, 6H), 2.17 (t, J = 7.4 Hz, 2H), 1.77 (dd, J = 12.4, 6.6 Hz, 2H), 1.63–1.49 (m, 2H). 13C NMR (101 MHz, DMSO) δ 172.38, 163.36, 153.97, 141.63, 140.99, 138.63, 133.81, 128.81, 128.08, 127.91, 124.00, 122.56, 119.05, 118.64, 109.85, 42.73, 42.22, 35.50, 28.76, 23.44, 12.40. HRMS (ESI) m/z Calcd [M + H]+ 524.2336, found: 524.2359.
(E)-6-(3,6-diacetyl-9H-carbazol-9-yl)-N-(4-(3-(hydroxyamino)-3-oxoprop-1-en-1-yl)benzyl)hexanamide (8e)
White solid. Mp: 121–123 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (s, 2H), 8.28 (t, J = 5.7 Hz, 1H), 7.90 – 7.79 (m, 2H), 7.59 (d, J = 8.7 Hz, 2H), 7.52–7.45 (m, 2H), 7.41 (d, 1H), 7.23 (d, J = 7.8 Hz, 2H), 6.43 (d, J = 15.8 Hz, 1H), 4.45–4.33 (m, 2H), 4.21 (d, J = 5.8 Hz, 2H), 2.30 (s, 6H), 2.09 (t, J = 7.4 Hz, 2H), 1.77 (d, J = 7.7 Hz, 2H), 1.64–1.44 (m, 2H), 1.29 (d, J = 10.3 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 172.51, 163.25, 153.94, 141.68, 140.99, 138.47, 133.84, 128.76, 128.14, 127.90, 123.99, 122.54, 119.18, 118.62, 109.84, 42.90, 42.23, 35.61, 28.85, 26.59, 25.47, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 538.2347, found: 538.2372.
(E)-7-(3,6-diacetyl-9H-carbazol-9-yl)-N-(4-(3-(hydroxyamino)-3-oxoprop-1-en-1-yl)benzyl)heptanamide (8f)
White solid. Mp: 113–115 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (d, J = 1.8 Hz, 2H), 8.29 (d, J = 6.1 Hz, 1H), 7.85 (dd, J = 8.7, 1.8 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.48 (d, J = 7.8 Hz, 2H), 7.42 (d, J = 15.8 Hz, 1H), 7.24 (d, J = 7.9 Hz, 2H), 6.42 (d, J = 15.8 Hz, 1H), 4.39 (t, J = 7.0 Hz, 2H), 4.23 (d, J = 5.9 Hz, 2H), 2.30 (s, 6H), 2.09 (t, J = 7.3 Hz, 2H), 1.77 (s, 2H), 1.52 – 1.40 (m, 2H), 1.35–1.21 (m, 4H). 13C NMR (101 MHz, DMSO) δ 172.61, 163.31, 153.94, 141.75, 141.00, 138.53, 133.82, 128.78, 128.14, 127.90, 123.99, 122.54, 119.15, 118.62, 109.82, 60.22, 42.91, 42.23, 35.72, 28.93, 26.65, 25.60, 12.38. HRMS (ESI) m/z Calcd [M + H]+ 552.2504, found: 552.2514.
(E)-8-(3,6-diacetyl-9H-carbazol-9-yl)-N-(4-(3-(hydroxyamino)-3-oxoprop-1-en-1-yl)benzyl)octanamide (8g)
White solid. Mp: 167–168 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (d, J = 1.7 Hz, 2H), 8.31 (d, J = 6.2 Hz, 1H), 7.85 (dd, J = 8.7, 1.8 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.47 (d, J = 7.9 Hz, 2H), 7.40 (d, J = 15.3 Hz, 1H), 7.23 (d, J = 7.9 Hz, 2H), 6.47 (d, J = 15.9 Hz, 1H), 4.40 (t, J = 7.0 Hz, 2H), 4.23 (d, J = 5.9 Hz, 2H), 2.30 (s, 6H), 2.09 (t, J = 7.4 Hz, 2H), 1.76 (s, 2H), 1.47 (q, J = 7.4 Hz, 2H), 1.27 (s, 4H), 1.19 (s, 2H). 13C NMR (101 MHz, DMSO) δ 172.65, 163.27, 153.89, 141.54, 140.99, 137.72, 134.03, 128.77, 128.12, 127.79, 123.97, 122.53, 119.69, 118.62, 109.83, 42.93, 42.29, 35.72, 29.03, 26.84, 25.68, 12.36. HRMS (ESI) m/z Calcd [M + H]+ 568.2805, found: 568.2800.
HeLa nuclear extract and HDAC enzymatic assay
The mixed solution containing enzyme solution (HeLa nuclear extract, HDAC1, HDAC6 or HDAC8), the tested compounds solution and the fluorogenic substrate solution [Boc-Lys (acetyl)-AMC or Boc-Lys (triflouroacetyl)-AMC] were plated to enzyme-labeled plate. After incubation (30 min, 37 °C), the reaction was stopped by the trypsin and trichostatin A. Fluorescence was analyzed with EX/EM 360/460 nm by Microplate reader (Tecan).
In vitro antiproliferative activity
HCT-116, HepG2, Hela, HEL, Molt-4, and U266 cells were treated with different concentrations of compounds after seeded into 96-well plates, respectively. After co-incubation for 48 h, 20 ul CCK8 was added to each well and incubated for 4 h at 37 °C. Microplate reader (Tecan) was used to detect the absorbance at 450 nm for each well.
Flow cytometric assay
Molt4 cells were treated with 3g (0.5 μM, 1.5 μM) for 24 h. Then the cells were collected pellets for annexin V-FITC/PI staining. The stained cells were analyzed by a flow cytometer.
Western blot assay
Molt4 cancer cells were treated with 3g (0.17 μM, 0.5 μM, 1.5 μM) for 24 h, then the cells were collected pellets for protein extraction. The protein content was determined by the BCA protein determination assay. Equal amounts of the total protein were measured for Western blot, such as Ac-HH3, Ac-Tubulin and cleaved caspase 3.
Molecular docking
The crystal structures of HDAC1 (PDB code: 5ICN) were downloaded from Protein Data Bank. Molecular docking was conducted using the MOE software. The top-scored results were selected as the most favorable binding model.
References
Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. https://doi.org/10.1038/icb.2011.100.
Bajbouj K, Al-Ali A, Ramakrishnan RK, Saber-Ayad M, Hamid Q. Histone modification in NSCLC: molecular mechanisms and therapeutic targets. Int J Mol Sci. 2021;22:11701 https://doi.org/10.3390/ijms222111701.
Kunadis E, Lakiotaki E, Korkolopoulou P, Piperi C. Targeting post-translational histone modifying enzymes in glioblastoma. Pharmacol Ther. 2021;220:107721 https://doi.org/10.1016/j.pharmthera.2020.107721.
Abu-Zhayia ER, Machour FE, Ayoub N. HDAC-dependent decrease in histone crotonylation during DNA damage. J Mol Cell Biol. 2019;11:804–6. https://doi.org/10.1093/jmcb/mjz019.
Liang T, Zhou Y, Elhassan RM, Hou X, Yang X, Fang H. HDAC–Bax multiple ligands enhance Bax-dependent apoptosis in Hela cells. J Med Chem. 2020;63:12083–99. https://doi.org/10.1021/acs.jmedchem.0c01454.
Punpai S, Saenkham A, Jarintanan F, Jongrungruangchok S, Choowongkomon K, Suksamrarn S, et al. HDAC inhibitor cowanin extracted from G. fusca induces apoptosis and autophagy via inhibition of the PI3K/Akt/mTOR pathways in Jurkat cells. Biomed Pharmacother. 2022;147:112577 https://doi.org/10.1016/j.biopha.2021.112577.
Cui H, Hong Q, Wei R, Li H, Wan C, Chen X, et al. Design and synthesis of HDAC inhibitors to enhance the therapeutic effect of diffuse large B-cell lymphoma by improving metabolic stability and pharmacokinetic characteristics. Eur J Med Chem. 2022;229:114049 https://doi.org/10.1016/j.ejmech.2021.114049.
Liu M, Gao S, Liang T, Qiu X, Yang X, Fang H, et al. Discovery of novel Src homology-2 domain-containing phosphatase 2 and histone deacetylase dual inhibitors with potent antitumor efficacy and enhanced antitumor immunity. J Med Chem. 2022;65:12200–18. https://doi.org/10.1021/acs.jmedchem.2c00866.
Vaidya GN, Rana P, Venkatesh A, Chatterjee DR, Contractor D, Satpute DP, et al. Paradigm shift of “classical” HDAC inhibitors to “hybrid” HDAC inhibitors in therapeutic interventions. Eur J Med Chem. 2021;209:112844 https://doi.org/10.1016/j.ejmech.2020.112844.
Hesham HM, Lasheen DS, Abouzid KAM. Chimeric HDAC inhibitors: comprehensive review on the HDAC-based strategies developed to combat cancer. Med Res Rev. 2018;38:2058–109. https://doi.org/10.1002/med.21505.
Huang L, Feng Z-L, Wang Y-T, Lin L-G. Anticancer carbazole alkaloids and coumarins from Clausena plants: a review. Chin J Nat Med. 2017;15:881–8. https://doi.org/10.1016/S1875-5364(18)30003-7.
Głuszyńska A. Biological potential of carbazole derivatives. Eur J Med Chem. 2015;94:405–26. https://doi.org/10.1016/j.ejmech.2015.02.059.
Humphries PS, Bersot R, Kincaid J, Mabery E, McCluskie K, Park T, et al. Carbazole-containing amides and ureas: discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg Med Chem Lett. 2018;28:293–7. https://doi.org/10.1016/j.bmcl.2017.12.051.
Chen C, Chu H, Wang A, Yin H, Gao Y, Liu S, et al. Discovery of 2,5-diphenyl-1,3,4-thiadiazole derivatives as HDAC inhibitors with DNA binding affinity. Eur J Med Chem. 2022;241:114634 https://doi.org/10.1016/j.ejmech.2022.114634.
Chen C, Hou X, Wang G, Pan W, Yang X, Zhang Y, et al. Design, synthesis and biological evaluation of quinoline derivatives as HDAC class I inhibitors. Eur J Med Chem. 2017;133:11–23. https://doi.org/10.1016/j.ejmech.2017.03.064.
Chen C, Li X, Zhao H, Liu M, Du J, Zhang J, et al. Discovery of DNA-targeting HDAC inhibitors with potent antitumor efficacy in vivo that trigger antitumor immunity. J Med Chem. 2022;65:3667–83. https://doi.org/10.1021/acs.jmedchem.1c02225.
Velasco D, Castellanos S, López M, López-Calahorra F, Brillas E, Juliá L. Red organic light-emitting radical adducts of carbazole and tris(2,4,6-trichlorotriphenyl)methyl radical that exhibit high thermal stability and electrochemical amphotericity. J Org Chem. 2007;72:7523–32. https://doi.org/10.1021/jo0708846.
Funding
This work was supported by the Key Research and Development Program of Shandong Province (No. 2019JZZY011115 and 2021CXGC011101), Weihai Zhengsheng Biotechnology Foundation and Rongxiang Regenerative Medicine Foundation of Shandong University (No. 2019SDRX-05), National Natural Science Foundation of China (22101146), Natural Science Foundation of Shandong Province (ZR2020QB005), Weihai-Shandong Academy of Sciences Industry University research collaborative innovation fund (2020CXY08, 2020GC04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, L., Han, L., Zhang, L. et al. Design, synthesis, and antitumor activity evaluation of carbazole derivatives with potent HDAC inhibitory activity. Med Chem Res 32, 1677–1689 (2023). https://doi.org/10.1007/s00044-023-03084-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03084-0