Abstract
Herein, two classic animal behavior despair tests-the forced swim test and tail suspension test were used to evaluate the antidepressant-like activity of saringosterol from Sargassum fusiforme in mice. Saringosterol was found to significantly shorten immobility time in the forced swim test and tail suspension test at doses of 10, 20, and 30 mg/kg in mice. The measurement of locomotor activity indicated that saringosterol had no central nervous system-stimulating effects. In addition, it was found that the saringosterol significantly increased noradrenaline, serotonin (5-HT), and the metabolite 5-hydroxyindoleacetic acid in the mouse brain, suggesting that the antidepressant-like activity may be mediated through these neurotransmitters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is one of the most prevalent psychopathologies. Symptoms of depression include lowered mood and reduced pleasure and interest. According to WHO’s prediction, it is expected to become the second leading cause of disease-related disability by 2020 (Gaffrey et al. 2013; Lopez and Murray 1998). The main biochemical causes of depression are metabolic disorders of monoamine neurotransmitters that are involved in serotonin (5-HT), noradrenaline (NE), and dopamine (DA) signaling. These neurotransmitters play important roles in mediating behavioral activity induced by antidepressant drugs (Hao et al. 2013; Bergner et al. 2016). γ-Aminobutyric acid and brain-derived neurotrophic factors are believed to be associated with depressive disorders (Luo et al. 2015). The function of the hypothalamic-pituitary-adrenal axis was impaired in many depressed patients (Naert et al. 2011). The glutamate (L-Glu) neuroreceptor system displayed the mechanisms of antidepressant on the pharmacological therapeutic (Pilc et al. 2013). α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid L-Glu receptors (AMPARs) or N-methyl-d-aspartate receptors (NMDARs) are major targets of the various chronic depressive disorders (Lohmann and Kessels 2014).
Sargassum fusiforme (Harv.) Setchel, a kind of brown algae widely distributed in China, Korea and Japan has been applied as a therapeutic for thousands of years (Chen et al. 2014). Steroids are biological signaling molecules with profound chemical, clinical, and scientific significance. Steroids have diverse biological actions that are mediated through different functional groups surrounding a rigid tetracyclic core (Malyarenko et al. 2015). In recent years, steroids have displayed antidepressant effects. Han 2005 displayed that steroids could significantly reduce the immobility time in the forced swim test (FST), indicating an antidepressant effect. We previously showed that fucosterol, a sterol compound significantly reduced the immobility time during the FST and the tail suspension test (TST), indicating an antidepressant-like effect and the action of fucosterol is likely mediated by an increase in central nervous system 5-HT and NE (Zhen et al. 2015). Saringosterol belongs to sterol compound (Fig. 1). However, the antidepressant-like activity of saringosterol was extracted from Sargassum fusiforme has not been reported in mice. Herein, in the present study, we evaluate possible antidepressant-like activity of saringosterol from Sargassum fusiforme in the FST and TST in mice. And levels of the main monoamine neurotransmitters and their metabolites were also measured in the mice brain.
Materials and methods
Materials and agents
Sargassum fusiforme was collected in Donghai, Zhejiang Province (China). Saringosterol was provided by the Zhejiang Ocean University (Zhejiang, China). A positive control: fluoxetine-HCl (Sigma, Shanghai, purity > 99%) was included. Reference standards for simultaneous determination of: 5-HT, NE, DA, and 5-hydroxyindoleacetic acid (5-HIAA) were all purchased from Sigma, Shanghai, purity > 99%. Melting points were determined in open capillary tubes. Infrared (IR) spectra were recorded (in KBr) on a FT-IR1730 (Bruker, Switzerland), 1H-NMR and 13C-NMR spectra were measured on an AV-300 (Bruker, Switzerland) and all chemical shifts were given in ppm relative to tetramethylsilane. High resolution mass spectra were measured on an MALDI-TOF/TOF mass spectrometer (Bruker, Germany). The major chemicals were purchased from Aldrich Chemical Corporation (Shanghai, China). All other chemicals were of the analytical grade.
Animals
Male ICR mice (20–22 g) obtained for experiments were provided by the Laboratory of Animal Research, College of Pharmacy, Zhejiang Academy of Medical Sciences. The animals were acclimatized for 1 week before being used for the experiment. Before and during the experiment the mice were housed under controlled environmental conditions of temperature (23 ± 2 °C) and a 12 h light and dark cycle and maintained on (unless otherwise stated) standard food pellets and tap water ad libitum. All animal handling procedures in the experiment were conducted in accordance with China.
Isolation and purification of saringosterol
Saringosterol was extracted from Sargassum fusiforme with root cutoff according to the procedures described in previous study (Wang et al. 2008; Chen and Liu 2012). Briefly, the air dried Sargassum fusiforme 2000 g were extracted with five-time 95% ethanol under reflux and three repeated for 2 h. All ethanol-extracts were combined, filtered and concentrated under vacuum to afford 430 g. The mixture was added and dissolved with water and extracted sequentially with n-hexane and acetic ether. Then, n-hexane fraction was subjected to a silica gel column eluted with n-hexane-acetic ether (9:1, 7:1, 5:1, 3:1,1:1) and acetic ether-MeOH(4:1, 1:1) affording ten sub-fractions (sfr): sfr.1 to sfr.10. Subfraction 8 was subjected to isocratic semi-preparation high-performance liquid chromatography (HPLC) using with n-hexane-acetic ether(3:1) afford the white solid saringosterol. The melting point and spectral data of saringosterol are given as below. Mp. 159–161 °C; IR (KBr) cm−1: 3401, 2943, 1642, 1471, 1382, 1059, 1038; 1H-NMR (CDCl3, 500 MHz):δ 0.67(3H, s), 0.82(3H), 0.92(3H), 0.94(3H), 1. 04(3H), 5.12 (dd, 1H), 5.19(1H), 5.36(1H, dd), 5.29(1H,q, J = 6.82 Hz, H-28), 5.40(1H, br d, J = 5.16 Hz, H-6); 13C-NMR (CDCl3, 100 MHz):δ 145.09(C-24), 140.69(C-5), 121.88(C-6), 115.86(C-25), 71.74(C-3), 58.32(C-17), 56.74(C-14), 50.16 (C-9), 44.03(C-13), 42.89(C-26), 41.80(C-4), 38.13(C-12), 37.83(C-10), 36.49(C-20), 35.98(C-23), 31.95(C-2), 31.93(C-8), 30.75(C-7), 30.12(C-1), 27.70(C-16), 27.58(C-22), 27.04(C-15), 22.30(C-11), 21.67(C-19), 21.20 (C-18), 19.36(C-21), 15.34(C-27), 14.68(C-28), 13.34(C-29); ESI-HRMS calcd for C29H48O2 +([M+H]+): 428.3654; found: 428.3650. The comprehensive analysis of above the data consistent with the reported the literature (Khan et al. 2011; Ayyad et al. 2003).
Drug treatment
Saringosterol was dissolved in dimethyl sulfoxide. Other drugs were dissolved in isotonic saline solution (NaCl 0.9%) Vehicle solvent served as a negative control, while fluoxetine served as a positive control. All drugs were administered by intraperitoneal (i.p.) route 30 min before in the FST, TST, or open-field (at a dose of 30 mg/kg) and the volume of administration for vehicle and drug solutions was 0.1 mL/20 g of mice.
The forced swimming test (FST)
Male ICR mice were housed in groups of six. On the test day, mice were dropped one at a time into a plexiglass cylinder (height 25 cm, diameter 10 cm) containing 10 cm of water at 22 ± 3°C. Mice were assigned into different groups (n = 8 for each group). Then, the mice were dropped individually into the pelxiglass cylinder and left in the water for 6 min. After the first 2 min of the initial vigorous struggling, the animals were immobile. The duration of immobility was recorded during the last 4 min of the 6 min test. All test swim sessions were recorded by a video camera positioned directly above the cylinder. Two competent observers, who were unaware of the treatment each mouse had received, scored the videotapes. Immobility period was regarded as the time spent by the mouse floating in the water without struggling and making only those movements necessary to keep its head above the water (Porsolt et al. 1997; Guan et al. 2013).
The tail suspension test (TST)
Mice were individually suspended by tail with clamp (2 cm from the tip of the end) in a box (25 × 25 × 30 cm) with the head 5 cm to the bottom. Testing was carried out in a darkened room with minimal background noise. All animals were suspended for total 6 min, and the duration of immobility was observed and measured during the final 4-min interval of the test. All test sessions were recorded by a video camera positioned directly above the box. Two competent observers blind to treatment scored the videotapes. Mice were considered to be immobile only when they hung passively and completely motionless (Steru et al. 1985).
The open-field test
The open-field test was carried out in mice according to the method of Archer with slight modifications (Archer 1973; Elliott et al. 1986). Each mouse was placed individually in the center of the open-field apparatus, and the locomotor activity was assessed. The open-field apparatus was a non-transparent plastic container (80 × 60 × 30 cm), with the underside divided into 48 units of size 10 × 10 cm, without walls. The animals were gently placed in the center of the platform and were allowed to explore their surroundings. Hand-operated counters were used to score locomotion (ambulation, numbers of crossing lines with all four paws) and rearing frequencies (number of times an animal stood on its hind legs) for 5 min. The researchers, who did not know which groups had been treated, scored the behaviors in the open field. The experiments were performed in a dark room and the apparatus was illuminated by a 60-W bulb giving a yellowish light, positioned 1 m above the center of the apparatus.
The sample preparation
The doses of saringosterol was 30 mg/kg and fluoxetine was 20 mg/kg (Wang et al. 2014) employed for testing the effect on monoamine neurotransmitter concentrations in the rats brain. Mice were randomly divided into five groups (10 mice per group were used). Normal vehicle, stress vehicle, total sterols, saringosterol, and fluoxetine were given orally daily for 7 days. On the last day, the drugs were given 1 h prior to the test. At the end of the experiment, the mice were immediately sacrificed by cervical dislocation, the brain tissue was quickly removed and rapidly frozen and stored at −80 °C until they were processed for biochemical estimations.
HPLC condition and test
The brain tissues were sonicated in 0.1 M NaH2PO4 aqueous solution including 0.85 mM OSA, 0.5 mM EDTA·Na2 and centrifuged (12,000 rpm, 30 min). Then 5-HT, NE, DA, 5-HIAA, and DHBA were assayed by HPLC-ECD (electrochemical detection). The HPLC system consisted of a microbore reverse-phase column (Shimadzu LC-10ATVP HPLC system, Shimadzu L-ECD-6A electrochemical detector, N2000 HPLC workstation software, Hypersil ODS C18 Column 4.6 × 150 mm 5 μM. The mobile phase consisted of 0.1 M NaH2PO4 aqueous solution including 0.85 mM OSA, 0.5 mM EDTA·Na2, and 11% methanol adjusted to pH 3.4 with phosphate acid and filtered through 0.45 μM pore size filter. External standard curves were used to quantify the amounts of 5-HT, 5-HIAA, NE, and DA in each sample calculated by area under curve. The volume of injection was 20 μL. The detection limit of the assay was 20 pg/g sample. The filtrate sample was used for quantification of 5-HT, NE, DA, and 5-HIAA by HPLC coupled with electro-chemical detection in brain region.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Prism 5.0, version 2.0; GraphPad Software, Inc., San Diego, CA, USA). The results are expressed as the mean ± SEM. The Student’s t-test was used to compare the differences between the two groups, with a one-way analysis of variance followed by Tukey’s multiple comparison tests. A value of P < 0.05 was considered significant.
Results and discussion
Effect of saringosterol on the immobility time in the FST and TST
In the present work, we demonstrated that saringosterol, a sterol from Sargassum fusiforme was effective in producing antidepressant effects when assessed in the FST and TST in mice. This behavioral effect was accompanied by saringosterol-induced modulation of the central neurochemical. Both the FST and TST are the accepted stress models of depression, which induce a state of despair in animals and have good reliability and predictive validity in mice (Borsini et al. 1986; De Oliveira et al. 2011). In the two models, the mice are restricted and cannot escape, inducing a characteristic behavior of immobility. The immobility displayed in both of these behavioral despair models has been hypothesized to reflect behavioral despair, which in turn may reflect depressive disorders in human. There was a significant correlation between clinical potency and the potency of antidepressants in both models (Możdżeń et al. 2014; Li et al. 2013). Thus, two models are usually used to screen or evaluate the antidepressant activity (Ishola et al. 2014). The total immobility time is reduced by the majority of the antidepressants when administered acutely or subchronically to animals in the FST or TST model.
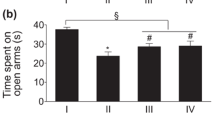
The antidepressant-like effect of saringosterol on the immobility time in these tests is presented in Table 1 and Fig. 2. Saringosterol significantly reduced the immobility time in the FST and TST at doses of 10, 20, and 30 mg/kg. Saringosterol had a similar effect to the drug fluoxetine (20 mg/kg) at a dose of 30 mg/kg, which induced the greatest reduction in the immobility time compared with the control group (P < 0.001). To better understand the antidepressant-like activity of saringosterol, we calculated the percentage decrease in immobility duration (% DID) using the formula % DID = [(Y−X)/Y] × 100, where Y is the duration of immobility (s) in the control group, and X is the duration of immobility (s) in the sterol-treated group. The duration of immobility in the FST was reduced and the % DID increased by three doses of saringosterol in the FST. The same effects were observed for three doses of total sterols in the TST (Table 1 and Fig. 3). The % DID values were as follows: FST: 32.67% (10 mg/kg) (F 4, 35 = 11.18, P < 0.05), 44.71% (20 mg/kg) (F 4, 35 = 15.30, P < 0.01), and 53.60% (30 mg/kg) (F 4, 35 = 18.34, P < 0.001) and fluoxetine (20 mg/kg) (F 4, 35 = 18.81, P < 0.001); TST: 32.06% (10 mg/kg) (F 4, 35 = 9.94, P < 0.05), 42.38% (20 mg/kg) (F 4, 35 = 13.14, P < 0.01)and 50.83% (30 mg/kg) (F 4, 35 = 17.46, P < 0.001) and fluoxetine (20 mg/kg) (F 4, 35 = 16.32, P < 0.001). These results indicate that saringosterol has a significant antidepressant activity during the FST and TST in mice. Furthermore, saringosterol exhibited the antidepressant-like activity in a dose-dependent manner.
Open-field test
In these behavioral tests, false-positive results are occasionally obtained with agents that stimulate locomotor activity (Elliott et al. 1986; Kaster et al. 2004). Therefore, the effect of saringosterol on locomotor activity was evaluated using in open-field test, saringosterol treatment showed no differences compared with control animals in 5 min at the dose range used in the present study. The administration of saringosterol in the FST did not cause any significant change in the number of crossing, rearing and grooming in open field test (Fig. 4), demonstrating that the antidepressant-like effect of saringosterol in the FST and TST was not caused by altered locomotor behavior. A variety of antidepressant drugs are known to reduce the immobility time in the FST and TST. However, drugs that affect motor function may give false-positive or -negative results in the FST and TST. Psychomotor stimulants enhance locomotor activity, which reduces the immobility time (Huang et al. 2014).
Effects of saringosterol on whole-brain monoamine neurotransmitter levels
Monoamine neurotransmitters including 5-HT, NE, and DA are believed to be involved in mental depression and play important roles in mediating behavioral effects of antidepressant drugs (Xu et al. 2015; Li et al. 2014). The monoamine hypothesis supposed that depression was a result of the depletion of 5-HT, NE, and DA in addition to activation of monoamine oxidase in the central nervous system (CNS). A metabolic disorder of monoamine neurotransmitters is believed to be the main biochemical cause of depression in the CNS. Depression can be alleviated by increasing the levels of monoamine neurotransmitters in the CNS (Machado et al. 2010; Dhanda et al. 2015; Waszkielewicz et al. 2015; Mahesh et al. 2014). The levels of monoamine neurotransmitters and their metabolites detected in mice brain are summarized in Table 2. In the present study, saringosterol did not change DA levels, but significantly increased 5-HT and NE levels at the highest doses during the FST in mice brain, similar to the positive control drug fluoxetine. In addition, saringosterol significantly increased 5-HIAA levels, indicating a reduced 5-HT metabolism. These findings suggested that the probable mechanism of action of saringosterol is thought to be related to the increase in 5-HT and NE in the CNS.
Conclusion
In conclusion, the present study demonstrated that saringosterol appeared to produce significant antidepressant-like effects in the FST and TST. The probable mechanism of action of saringosterol is thought to be related to the increase in 5-HT and NE in the CNS. The mechanism involved in may be mediated by neurochemical systems. These findings suggested that saringosterol might be a potentially valuable drug for the treatment of depression.
References
Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235
Ayyad SE, Sowellim SZ, el-Hosini MS, Abo-Atia A (2003) The structural determination of a new steroidal metabolite from the brown alga Sargassum asperifolium. Z Naturforsch C 58:333–336
Bergner CL, Smolinsky AN, Hart PC, Dufour BD, Egan RJ, LaPorte JL, Kalueff AV (2016) Mouse models for studying depression-like states and antidepressant drugs. Methods Mol Biol 1438:255–269
Borsini F, Voltera G, Meli A (1986) A dose the behavioral ‘despair’ test measure ‘despair’. Physiol Behav 38:385–389
Chen Z, Liu HB (2012) Progress in research on the chemical constituents and bioactivities of sargassum. Chin J Mar Drugs 31:41–51
Chen Z, Liu J, Fu Z, Ye C, Zhang R, Song Y, Zhang Y, Li H, Ying H, Liu H (2014) 24(S)- Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J Agric Food Chem 62:6130–6137
De Oliveira KN, Costa P, Santin JR, Mazzambani L, Bürger C, Mora C, Nunes RJ, de Souza MM (2011) Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonylhydrazones. Bioorg Med Chem 19:4295–4306
Dhanda S, Sandhir R (2015) Role of dopaminergic and serotonergic neurotransmitters in behavioral alterations observed in rodent model of hepatic encephalopathy. Behav Brain Res 286:222–235
Elliott PJ, Chan J, Parker YM (1986) Behavioral effects of neurotensin in the open field: structure-activity studies. Brain Res 381:259–265
Gaffrey MS, Luby JL, Barch DM (2013) Towards the study of functional brain development in depression: Aninteractive specialization approach. Neurobiol Dis 52:38–48
Guan LP, Zhao DH, Chang Y, Sun Y, Ding XL, Jiang JF (2013) Design, synthesis and antidepressant activity evaluation2’-hydroxy-4’,6’-diisoprenyloxychalcone derivatives. Med Chem Res 22:5218–5226
Han YC (2005) Research progress of depression and the natural antidepressant drug. J Pharmaceut Practice 23:3–5
Hao CW, Lai WS, Ho CT, Sheen LY (2013) Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test. J Funct Foods 5:370–379
Huang X, Mao YS, Li C, Wang H, Ji JL (2014) Venlafaxine inhibits apoptosis of hippocampal neurons by up-regulating brain-derived neurotrophic factor in a rat depression model. Pharmazie 69:909–916
Ishola IO, Ochieng CO, Olayemi SO, Jimoh MO, Lawal SM (2014) Potential of novel phytoecdysteroids isolated from Vitex doniana in thetreatment depression: Involvement of monoaminergic systems. Pharmacol Biochem Behav 127:90–100
Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL (2004) Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett 355:21–24
Khan AM, Noreen S, Imran ZP, Choudhary MI (2011) A new compound, jolynamine, from marine brown alga Jolyna laminarioides. Nat Prod Res 25:898–904
Li YW, Langdon S, Pieschl R, Strong T, Wright RN, Rohrbach K, Lelas S, Lodge NJ (2014) Monoamine reuptake site occupancy of sibutramine: Relationship to antidepressant-like and thermogenic effects in rats. Eur J Pharmacol 737:47–56
Li J, Geng D, Xu J, Weng LJ, Liu Q, Yi LT (2013) Antidepressant-like effect of macranthol isolated from Illicium dunnianum tutch in mice. Eur J Pharmacol 707:112–119
Lohmann C, Kessels HW (2014) The developmental stages of synaptic plasticity. J Physiol 592:13–31
Lopez AD, Murray CC (1998) The global burden of disease. 1990-2020. Nat Med 4:1241–1243
Luo L, Liu XL, Mu RH, Wu YJ, Liu BB, Geng D, Liu Q, Yi LT (2015) Hippocampal BDNF signalingrestored with chronic asiaticoside treatment in depression-like mice. Brain Res Bull 114:62–69
Machado RA, Espinosa AG, Montoto AP (2010) Cholesterol concentrations and clinical response to sertraline in patients with epilepsy: preliminary results. Epilepsy Behav 19:509–512
Mahesh R, Dhar AK, Jindal A, Bhatt S (2014) Design, synthesis and evaluation of antidepressant activity of novel 2-methoxy 1, 8 naphthyridine 3-carboxamides as 5-HT3 receptor antagonists. Chem Biol Drug Des 83:583–591
Malyarenko TV, Vishchuk OSM, Ivanchina NV, Kalinovsky AI, Popov RS, Kicha AA (2015) Four new sulfatedpolarsteroidsfromthefareasternstarfishLeptasteriasochotensis: Structuresandactivities. Mar Drugs 13:4418–4435
Możdżeń E, Papp M, Gruca P, Wąsik A, Romańska I, Michaluk J, Antkiewicz-Michaluk L (2014) 1,2,3,4-Tetrahydroisoquinoline produces an antidepressant-like effect in the forced swim test and chronic mild stress model of depression in the rat: Neurochemical correlates. Eur J Pharmacol 729:107–115
Naert G, Ixart G, Maurice T, Tapia-Arancibia L, Givalois L (2011) Brain-derived neurotrophic factor and hypothalamic pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol Cell Neurosci 46:55–66
Pilc A, Wieronska JM, Skolnick P (2013) Glutamate-based antidepressants: Preclinical psychophar- macology. Biol Psychiatry 73:1125–1132
Porsolt RD, Bertin A, Jalfre M (1997) Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn 229:327–336
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370
Wang W, Li HY, Wang YY, Xia X, Yoshihto O, Okuyama T (2008) Chemical constituents from brown alga Sargassum fusiforme. Chin Trad Herbal Drugs 39:657–661
Wang JM, Cui Y, Feng WS, Zhang YY, Wang GF, Wang XX, Zhou G (2014) Involvement of the central monoaminergic system in theantidepressant-like effect of catalpol in mice. BioScience Trends 8:248–252
Waszkielewicz AM, Pytka K, Rapacz A, Wełna E, Jarzyna M, Satała G, Bojarski A, Sapa J, Żmudzki P, Filipek B, Marona H (2015) Synthesis and evaluation of antidepressant-like activity of some 4-substituted 1-(2-methoxyphenyl)piperazine derivatives. Chem Biol Drug Des 85:326–335
Xu J, Xu H, Liu Y, He H, Li G (2015) Vanillin-induced amelioration of depression-like behaviors in rats by modulating monoamine neurotransmitters in the brain. Psychiatry Res 225:509–514
Zhen XH, Quan YC, Jiang HY, Wen ZS, Qu YL, Guan LP (2015) Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur J Pharmacol 768:131–138
Acknowledgements
This work was supported by the Science and Technology Planning Project of Zhoushan City of China (No. 2015C41015) and the Medical and Health Science and Technology Planning Project of Zhoushan City of China (No. 2015G01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Jin, HG., Zhou, M., Jin, QH. et al. Antidepressant-like effects of saringosterol, a sterol from Sargassum fusiforme by performing in vivo behavioral tests. Med Chem Res 26, 909–915 (2017). https://doi.org/10.1007/s00044-017-1804-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1804-2