Abstract
According to the bio-isosterism theory, a series of N, N’-disubstitutedphenyl-4-ethoxylbenzene-1, 3-disulfonamides (5a-p) were designed and synthesized by two steps of reactions including chlorosulfonation and ammonolysis. The structures of all compounds have been confirmed by IR, 1H-NMR, 13C-NMR, and ESI-MS spectra. The in vitro anti-platelet aggregation activities were evaluated by Born’s test induced by adenosine diphosphate (ADP) and arachidonic acid (AA), respectively. The biological evaluation results revealed that compound 5h had the lowest IC50 value (0.32 μM) and the highest inhibition rate (40.9 %) that of three positive control agents clopidogrel (0.41 μM, 23.5 %), aspirin (0.53 μM, 28.9 %), and picotamide (0.76 μM, 32.7 %). Afterwards, compounds with higher activities were selected to further study in vitro cytotoxicity via cell counting kit-8 (CCK-8) assay. The cytotoxicity results indicated that compound 5h had simultaneously the lowest cytotoxicity, while other compounds had no significant relationship between the anti-platelet activities and cytotoxicities. Based on above in vitro anti-platelet activity data, the SAR (Structure Activity Relationship) of the target compounds was preliminarily summarized. In general, N, N’-disubstitutedphenyl-4-ethoxylbenzene-1, 3-disulfonamides have the potential of further study and very likely become safer and more effective anti-platelet agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombosis disorders are key factors responsible for morbidity and mortality all over the world (Reddy et al. 2011). Platelet aggregation is a significant reason in thrombosis, which may bring about atherosclerotic plaques that is the initiator of most thrombosis disorders (Brito et al. 2010). Therefore, anti-platelet therapy is an effective method to prevent and treat thrombus-related diseases (Mirfazli et al. 2014). Anti-platelet agents can potently hold back thrombosis through the inhibition of platelet adhesion and aggregation (Liu et al. 2017). Aspirin and clopidogrel are well-established anti-platelet agents clinically. Aspirin is cyclooxygenase-I (COX-I) inhibitor that can concurrently inhibits production of thromboxane A2 (TXA2) and prostaglandin I2 (PGI2) (Jayakumar et al. 2016; Mclewee et al. 2017). Clopidogrel is ADP receptor antagonist, which may selectively inhibit platelet aggregation by blocking ADP-mediated activation of the glycoprotein IIb/IIIa pathway (Peng et al. 2018; Metil et al. 2018). But these anti-platelet agents still have some serious limitations including the high incidence of bleeding events (aspirin and clopidogrel) (Guthrie 2011), irreversibly inhibition of platelet function (aspirin) (Mclewee et al. 2017), slow onset of action (clopidogrel) (Eskandariyan et al. 2014). On this background, we attempt to find new anti-platelet agents, which are safer and more effective are very momentous (Siwek et al. 2011).
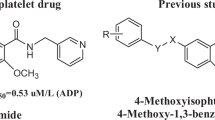
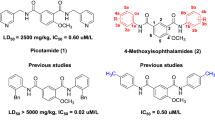
Picotamide (1, Fig. 1) is an effective platelet aggregation inhibitor with a dual inhibitory action, which can concurrently inhibit both TXA2 receptors and TXA2 synthesis (Pogliani and Milani 1996; Yip and Benavente 2011). In contrast to aspirin, it has the advantage of not interfere with PGI2 production (Celestini and Violi 2007; Wang et al. 2018). Since 2000, through the structural transformation of picotamide, our research group envisaged that replacing the two 3-pycolyl groups of picotamide with two substituted phenyl groups might open another door for anti-platelet agents. A series of 4-methoxyl-1, 3-phthalamides (2, Fig. 1) which are the structural analogs of picotamide were designed and synthesized (Liu et al. 2006, 2011, 2012). We wondrously found that among them, nearly 30% compounds exhibited higher in vitro anti-platelet activities than picotamide. It is well-known that sulfonamides have wide pharmacological activities including anti-inflammatory, anti-viral, anti-fungal and anti-neoplastic, and others (Khalid et al. 2018). Moreover, sulfonamide analogues have also shown higher inhibitory activity against platelet aggregation (Sharma et al. 2018). In view of the significance of sulfonamides in medicinal chemistry and according to the bio-isosterism theory, a series of 4-methoxy-1, 3-benzenedisulfonamides were designed and synthesized by replacing the two amide structures of picotamide with two sulfamide structures (Liu et al. 2006, 2011; Li et al. 2015).
During the period, we made the contour maps of steric QSAR from CoMFA. The results indicated that the anti-platelet activity might be influenced by steric factors. In order to confirm the steric model hypotheses as above, we further synthesized 4-ethoxy-1, 3-phthalamides (3, Fig. 1) (Liu et al. 2015) and 4-ethoxy -1, 3-benzenedisulfonamides (4, Fig. 1). It was amazing for us the anti-platelet activities of above compounds are underestimated in our previous study. On the purpose of releasing the full potential of 4-ethoxy-1, 3-benzenedisulfonamides as anti-platelet agents, we carried out more detailed studies in SAR. We introduced six previous reported mono-substituted compounds (4a*–f*). The order of anti-platelet aggregation activity of the different substituents at same position was: In 2-position: 4a* (2-F) > 4b* (2-Cl) > 4c* (2-Br) > clopidogrel, aspirin, picotamide > 4d* (2-CH3). The result indicated that the derivatives bearing a strong electron-withdrawing group in the 2-position turned out to have remarkable the anti-platelet activity, which may be related to the electron-withdrawing inductive effect.
Encouraged by results of our previous studies on mono-substituted compounds (4a*–f*) and based on the above mentioned reports, fluorine, chlorine, and methyl groups are, respectively, retained at the 2-position of the phenyl rings, and fluorine, chlorine, bromine, and methyl groups are continuously introduced at the 4-position of phenyl rings, eight novel 2, 4-disubstituted compounds (5a–h) were designed and synthesized in this study. Subsequently, in order to compare the similarities and differences on anti-platelet activity between the 2, 4-disubstituted compounds (5a-h) and 3, 4-disubstituted compounds (5i–p), 2-position are replaced with 3-position and introduced same substituent groups at the 4-position of phenyl rings, eight novel 3, 4-disubstituted compounds (5i–p) were designed and synthesized.
Material and methods
General experimental instrument and reagent
All chemical reagents were purchased from Tianjin Hengshan (P.R. China), Aladdin Industrial Corporation (P.R. China) and Energy Chemical (P.R. China), and used without further purification. The reagents of cell viability were purchased from Beyotime Biotechnology (P.R. China), other biological reagents ADP was purchased from Solarbio life sciences and AA was purchased from Sigma.
The melting points were veritably measured on X-4 digital display micro melting point apparatus and were uncorrected. The IR spectra were confirmed by Fourier transfrom infrared (FTIR) 1700 infrared spectrophotometer with KBr as diluents. Nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were obtained by Bruker spectrometers (Bruker, Rheinstetten, Germany), tetramethylsilane (TMS, 0.05 % v/v) as internal standard, shift chemical are illustrated in parts per million (δppm) in deuterated solvents (DMSO-d6), coupling constant values (J) are expressed in Hertz. Signal multiplicities are reported by: s (singlet), d (doublet), t (triplet), dd (double doublet), q (quartet), m (multiplet), and brs (broad signal). Thin-layer chromatography (TLC) analysis was accomplished with Silica gel plates GF254 and observed with UV irradiation (254 nm). Electrospray ionization mass spectrometry (ESI-MS) spectra were recorded on an Agilent 6520B UPLC-Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The physicochemical parameters including Clog P value, refractivity, polarizability, molecular volume, and surface area were calculated by MarvinSketch 17.13 software.
Chemistry
The synthetic pathway of the target compounds (5a–p) was depicted in Scheme 1 and their structure were shown in Table 1. Reaction of phenetole with chlorosulfonic acid and thionyl chide to produce the intermediate 4-ethoxybenzene-1,3-disulfonyl chloride, using dichloromethane as solvent. The intermediate was reacted with the different disubstituted anilines in tetrahydrofuran (THF) or dichloromethane (CH2Cl2) to obtain the target compounds with excellent yield.
General procedure for the preparation of intermediate
4-Ethoxy-1, 3-benzenedisulfonyl chloride was synthesized according to the literature method of 4-methoxy-1, 3-benzenedisulfonyl chloride (Liu et al. 2006).
Phenetole (2.40 mL; 0.02 mol) was dropwise added to a solution of chlorosulfonic acid (9.20 mL; 0.14 mol) in anhydrous dichloromethane (15 mL) at 0 °C under mechanical stirring and the reaction mixture was stirred at 40 °C for about 5 h. Subsequently, thionyl chide (2.90 mL; 0.04 mol) was dropwise added to the reaction mixture and the reaction mixture was stirred at 50 °C for about 2 h. The reaction was monitored by TLC (PE : EA = 1:1, Rf = 0.67). After that, the mixture was poured into a mixture of water and ice with stirring. The white solid was filtered with excellent yield. The crude product was purified by recrystallization from cyclohexane five times to obtain the high purity intermediate. Yield: 65.4 %; m.p.: 99.3–100.2 °C.
General procedure for the preparation of target compounds (5a–p)
N1, N3-bis (2, 4-difluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide (5a)
The intermediate 4-ethoxy-1, 3-benzenedisulfo chloride (1.2 g, 3.7 mmol) was dropwise added to a solution of 2, 4-difluoro aniline (1.0 g, 7.5 mmol) with dichloromethane (15 mL) as solvent at room temperature under magnetic stirring. After 10 min, triethylamine (0.5 mL) as acid-binding agent was dropwise added to the reaction mixture. The mixture was stirred under reflux at 35 °C for about 8 h. After the reaction completed as monitored by TLC, the solvent was removed under reduced pressure. Under the condition of ice bath, the mixture was dissolved in cold 5% NaOH aqueous solution to obtain filtrate. The solid was obtained by adjusting the pH of filtrate with hydrochloric acid (VHCl: Vwater = 1:1) in the condition of ice salt bath. After filtrating and drying, crude product was prepared. The crude product was purified by recrystallization from ethanol with excellent yield (Scheme 1).
Other target compounds (5b–p) were prepared by the same process. The results of each compound are described as follows.
N1, N3-bis (2-fluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(4a *): Yellow solid (The crude product was recrystallized by ethanol.)
Yield: 70.1%; m.p.: 201.2–207.3 °C; IR (KBr) cm−1: 3278.67 (υNH), 2898.25 (υCH), 1417.35, 1348.66 (υSO2), 1290.12, 1258.09 (υC-O-C), 920.38, 797.62, 759.45 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.18 (1H, s, –SO2NH–), 9.85 (1H, s, –SO2NH–), 8.42 (1H, d, J = 2.5 Hz, 2-H), 7.82 (1H, dd, J = 8.9, 2.5 Hz, 6-H), 7.35 (1H, d, J = 8.9 Hz, 5-H), 7.24 (4H, m, 3A-H, 5A-H, 3B-H, 5B-H), 7.05 (2H, m, 6A-H, 6B-H), 6.92 (2H, m, 4A-H, 4B-H), 4.23 (2H, q, J = 7.0 Hz, –OCH2–), 1.29 (3H, t, J = 7.0 Hz, –OCH2CH3); 13C-NMR (101 MHz, DMSO-d6, TMS) δ (ppm): 159.31 (C, 4-C), 157.56 (C, 2a-C), 157.51 (C, 2b-C), 133.90 (C, 1a-C), 133.28 (C, 1b-C), 132.16 (C, 1-C), 129.15 (CH, 6-C), 125.11 (CH, 5a-C), 125.05 (CH, 5b-C), 123.87 (CH, 4a-C), 123.35 (CH, 4b-C), 122.66 (CH, 2-C), 120.12 (C, 3-C), 117.64 (CH, 5a-C), 117.45 (CH, 5b-C), 115.50 (CH, 3a-C), 115.34 (CH, 3b-C), 112.28 (CH, 5-C), 65.87 (CH2, –OCH2–), 14.27 (CH3, –CH3); HR-ESI-MS (m/z): 469.0700 [M + H]+.
N1, N3-bis (2-chlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(4b *): White solid (The crude product was recrystallized by ethanol.)
Yield: 72.1%; m.p.: 213.3–215.0 °C; IR (KBr) cm−1: 3277.54 (υNH), 2946.12 (υCH), 1470.26, 1328.25 (υSO2), 1289.56, 1250.37 (υC-O-C), 923.14, 792.13 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.38 (1H, s, -SO2NH-), 10.27 (1H, s, –SO2NH–), 8.35 (1H, d, J = 2.5 Hz, 2-H), 7.87 (1H, dd, J = 8.6, 2.4 Hz, 6-H), 7.36 (1H, d, J = 8.0 Hz, 5-H), 7.28 (2H, m, 3A-H, 3B-H), 7.15 (2H, m, 5A-H, 5B-H), 6.99 (4H, m, 4A-H, 4B-H, 6A-H, 6B-H), 4.21 (2H, q, J = 7.0 Hz, -OCH2-), 1.40 (3H, t, J = 6.9 Hz, –OCH2CH3); 13C-NMR (101 MHz, DMSO-d6, TMS) δ (ppm): 158.58 (C, 4-C), 131.89 (C, 1-C), 129.80 (CH, 3a-C), 129.56 (CH, 3b-C), 128.89 (CH, 6-C), 127.66 (2 × C, 1a-C, 5a-C), 127.09 (2 × C, 1b-C, 5b-C), 125.65 (2 × C, 2a-C, 4a-C), 125.20 (2 × C, 2b-C, 4b-C), 124.66 (CH, 6a-C), 124.21 (CH, 6b-C), 122.39 (CH, 2-C), 121.39 (C, 3-C), 114.35 (C, 5H-C), 65.72 (CH2, –OCH2–), 14.55 (CH3, –CH3); HR-ESI-MS (m/z): 531.0426 [M + H]+.
N1, N3-bis (2-bromophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(4c *): White solid (The crude product was recrystallized by ethanol.)
Yield: 80.3%; m.p.: 167.5–168.9 °C; IR (KBr) cm−1: 3281.06 (υNH), 2890.33 (υCH), 1417.23, 1327.02 (υSO2), 1288.24 (υC-O-C), 1163.33, 923.29, 758.14 (υph-H); 1H NMR (400-MHz, DMSO-d6, TMS), δ (ppm): 10.34 (1H, s, –SO2NH–), 10.18 (1H, s, –SO2NH), 8.53 (1H, d, J = 2.2 Hz, 2-H), 7.92 (1H, dd, J = 8.7, 2.3 Hz, 6-H), 7.55 (2H, m, 3A-H, 3B-H), 7.42 (1H, d, J = 7.6 Hz, 5-H), 7.13 (2H, m, 5A-H, 5B-H), 6.79 (4H, m, 4A-H, 4B-H, 6A-H, 6B-H), 4.25 (2H, q, J = 6.8 Hz, –OCH2–), 1.41 (3H, t, J = 7.5 Hz, –OCH2CH3); 13C-NMR (101 MHz, DMSO-d6, TMS), δ (ppm): 158.65 (C, 4-C), 137.30 (C, 1a-C), 136.69 (C, 1b-C), 132.81 (CH, 3a-C), 132.63 (CH, 3b-C), 131.90 (C, 1-C), 130.32 (CH, 6-C), 128.81 (CH, 5a-C), 128.63 (CH, 5b-C), 126.63 (CH, 4a-C), 126.52 (CH, 4b-C), 124.88 (CH, 2-C), 123.27 (CH, 6a-C), 123.06 (CH, 6b-C), 120.15 (CH, 2-C), 117.81 (C, 2a-C), 117.63 (C, 2b-C), 114.4 (CH, 5-C), 65.70 (CH2, –OCH2–), 14.62 (CH3, –CH3); HR-ESI-MS (m/z): 587.9082 [M + H]+.
N1, N3-bis (2-methylphenyl)-4-ethoxybenzene-1, 3-disulfonamide
(4d *): White solid (The crude product was recrystallized by ethanol.)
Yield: 77.8%; m.p.: 170.3–171.0 °C; IR (KBr) cm−1: 3319.63 (υNH), 2912.35 (υCH), 1498.12, 1432.11 (υSO2), 1234.53 (υC-O-C), 854.34, 752.12, 712.36 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS), δ (ppm): 9.69 (1H, s, –SO2NH–), 9.58 (1H, s, –SO2NH–), 8.47 (1H, d, J = 2.4 Hz, 2-H), 7.95 (1H, dd, J = 8.8, 2.4 Hz, 6-H), 7.30 (1H, d, J = 8.9 Hz, 5-H), 7.11 (4H, m, 3A-H, 3B-H, 5A-H, 5B-H), 6.85 (4H, m, 4A-H, 4B-H, 6A-H, 6B-H), 4.23 (2H, q, J = 7.0 Hz, –OCH2–), 3.79 (3H, s, 2a-CH3), 3.62 (3H, s, 2b-CH3), 1.36 (3H, t, J = 7.3 Hz, –OCH2CH3); 13C-NMR (101 MHz, DMSO-d6, TMS), δ (ppm): 160.15 (C, 4-C), 139.65 (C, 1a-C), 139.23 (C, 1b-C), 133.20 (C, 2a-C), 133.07 (C, 2b-C), 130.11 (C, 1-C), 129.66 (CH, 3a-C), 129.24 (CH, 3b-C), 128.35 (CH, 6-C), 127.82 (CH, 5a-C), 127.08 (CH, 5b-C), 125.34 (CH, 6a-C), 125.01 (CH, 6b-C), 123.65 (CH, 4a-C), 123.33 (CH, 4b-C), 122.54 (CH, 2-C), 119.66 (C, 3-C), 113.57 (CH, 5-C), 65.87 (CH2, –OCH2–), 15.12 (CH3, 2a–CH3), 14.96 (CH3, 2b–CH3), 14.27 (CH3, –CH3); HR-ESI-MS (m/z): 461.1203 [M + H]+.
N1, N3-bis (3-methylphenyl)-4-ethoxybenzene-1, 3-disulfonamide
(4e *): White solid (The crude product was recrystallized by ethanol.)
Yield: 80.9%; m.p.: 177.1–178.0 °C; IR (KBr) cm−1: 3302.54 (υNH), 2978.21 (υCH), 1398.22, 1342.56 (υSO2), 1184.43 (υC-O-C), 952.44, 753.52, 708.45 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS), δ (ppm): 10.58 (1H, s, –SO2NH–), 9.68 (1H, s, –SO2NH–), 9.01 (1H, d, J = 2.4 Hz, 2-H), 7.76 (1H, dd, J = 8.8, 2.4 Hz, 6-H), 7.31 (1H, d, J = 8.9 Hz, 5-H), 7.04 (2H, m, 5A-H, 5B-H), 6.84 (2H, s, 2A-H, 2B-H), 6.73 (4H, m, 4A-H, 4B-H, 6A-H, 6B-H), 4.21 (2H, q, J = 6.8 Hz, –OCH2–), 3.72 (3H, s, 3a-CH3), 3.54 (3H, s, 3b-CH3), 1.58 (3H, t, J = 7.1 Hz, –OCH2CH3); 13C-NMR (101 MHz, DMSO-d6, TMS), δ (ppm): 158.12 (C, 4-C), 142.69 (C, 3a-C), 142.01 (C, 3b-C), 138.69 (C, 1a-C), 138.14 (C, 1b-C), 131.61 (C, 1-C), 129.33 (CH, 5a-C), 129.05 (CH, 5b-C), 129.16 (CH, 6-C), 124.28 (CH, 2-C), 120.16 (C, 3-C), 119.25 (2 × CH, 2a-C, 2b-C), 118.78 (2 × CH, 4a-C, 4b-C), 117.44 (2 × CH, 6a-C, 6b-C), 116.30 (CH, 5-C), 67.43 (CH2, –OCH2–), 15.43 (CH3, 3a–CH3), 15.26 (CH3, 3b–CH3), 14.6 (CH3, -CH3); HR-ESI-MS (m/z): 461.1200 [M + H]+.
N1, N3-bis (4-methylphenyl)-4-ethoxybenzene-1, 3-disulfonamide
(4f *): White solid (The crude product was recrystallized by ethanol.)
Yield: 89.1%; m.p.: 214.5–215.3 °C; IR (KBr) cm−1: 3269.49 (υNH), 2892.05 (υCH), 1394.17, 1382.21 (υSO2), 1064.44 (υC-O-C), 824.31, 722.33, 702.58 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS), δ (ppm): 11.09 (1H, s, –SO2NH–), 10.81 (1H, s, –SO2NH–), 7.99 (1H, d, J = 2.4 Hz, 2-H), 7.72 (1H, dd, J = 8.8, 2.4 Hz, 6-H), 7.39 (1H, d, J = 8.9 Hz, 5-H), 7.05 (4H, m, 3A-H, 3B-H, 5A-H, 5B-H), 6.98 (4H, 2A-H, 2B-H, 6A-H, 6B-H), 4.25 (2H, q, J = 7.0 Hz, –OCH2–), 3.58 (3H, s, 4a-CH3), 3.76 (3H, s, 4b-CH3), 1.69 (3H, t, J = 7.3 Hz, –OCH2CH3); 13C-NMR (101 MHz, DMSO-d6, TMS), δ (ppm): 159.92 (C, 4-C), 136.75 (C, 1a-C), 136.13 (C, 1b-C), 133.78 (C, 4a-C), 133.35 (C, 4b-C), 131.60 (C, 1-C), 129.33 (2 × CH, 3a-C, 5a-C), 129.12 (2 × CH, 3b-C, 5b-C), 128.57 (CH, 6-C), 122.6 (CH, 2-C), 119.5 (C, 3-C), 116.5 (2 × CH, 2a-C, 6a-C), 116.2 (2 × CH, 2b-C, 6b-C), 112.6 (CH, 5-C), 66.8 (CH2, -OCH2), 22.8 (2 × CH3, 4a-CH3, 4b-CH3), 14.7 (CH3, -CH3); HR-ESI-MS (m/z): 461.1212 [M + H]+.
N1, N3-bis (2, 4-difluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5a): White solid (The crude product was recrystallized by ethanol.)
Yield: 37.5%; m.p.: 189.3–191.2 °C; IR (KBr) cm−1: 3126.44 (υNH), 2983.11 (υCH), 1400.87, 1336.40 (υSO2), 1287.53, 1170.76 (υC-O-C), 967.47, 847.63, 732.06 (υph-H); 1H-NMR (400MHz, DMSO-d6, TMS) δ (ppm): 10.97 (2H, s, 2 × –SO2NH–), 8.31 (1H, d, J = 2.4 Hz, H-2), 7.91 (1H, dd, J = 10.8, 2.0 Hz, H-6), 7.37 (1H, d, J = 7.2 Hz, H-5), 6.78 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 6.56 (2H, s, 3A-H, 3B-H), 4.25 (2H, q, J = 6.9 Hz, –OCH2–), 1.31 (3H, t, J = 6.8 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 161.21 (C, 4-C), 159.56 (2 × C, 2a-C, 2b-C), 154.53 (2 × C, 4a-C, 4b-C), 131.39 (C, 1-C), 129.65 (C, 1a-C), 129.12 (C, 1b-C), 128.23 (CH, 6-C), 123.74 (CH, 2-C), 121.10 (C, 3-C), 119.36 (2 × CH, 5a-C, 5b-C), 119.03 (2 × CH, 6a-C, 6b-C), 112.06 (CH, 5-C), 104.80 (2 × CH, 3a-C, 3b-C), 66.29 (CH2, –OCH2–), 14.56 (CH3, –CH3); HR-ESI-MS (m/z): 505.0512 [M + H]+.
N1, N3-bis (2-fluoro-4-chlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5b): White solid (The crude product was recrystallized by ethanol.)
Yield: 42.2%; m.p.: 176.3–178.0 °C; IR (KBr) cm−1: 3260.59 (υNH), 2932.12 (υCH), 1387.66, 1325.78 (υSO2), 1276.33, 1159.74 (υC-O-C), 1032.77, 987.32, 855.43, 759.61 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.54 (1H, s, –SO2NH–), 10.26(1H, s, –SO2NH–), 8.44 (1H, d, J = 3.4 Hz, H-2), 7.93 (1H, dd, J = 8.8, 2.7 Hz, H-6), 7.55 (2H, s, 3A-H, 3B-H), 7.23 (1H, d, J = 8.9 Hz, H-5), 6.75 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 4.43 (2H, q, J = 6.8 Hz, –OCH2–), 1.37 (3H, t, J = 6.5 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 158.21 (C, 4-C), 156.79 (C, 2a-C), 156.22 (C, 2b-C), 133.88 (C, 1a-C), 133.63 (C, 1b-C), 131.66 (C, 1-C), 128.79 (CH, 6-C), 127.53 (2 × C, 4a-C, 4b-C), 125.76 (2 × CH, 5a-C, 5b-C), 123.98 (CH, 2-C), 120.55 (C, 3-C), 119.32 (2 × CH, 6a-C, 6b-C), 117.76 (CH, 5-C), 113.24 (2 × CH, 3a-C, 3b-C), 68.87 (CH2, –OCH2–), 15.65 (CH3, –CH3); HR-ESI-MS (m/z): 538.3738 [M + H]+.
N1, N3-bis (2-chloro-4-fluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5c): White solid (The crude product was recrystallized by ethanol)
Yield: 44.1%; m.p.: 202.1–204.0 °C; IR (KBr) cm−1: 3126.50 (υNH), 2916.64 (υCH), 1400.80, 1336.51 (υSO2), 1287.34, 1170.82(υC-O-C), 907.55, 847.76, 732.28 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.14 (1H, s, -SO2NH-), 9.83 (1H, s, -SO2NH-), 8.34 (1H, d, J = 3.6 Hz, H-2), 7.89 (1H, dd, J = 7.2, 2.4 Hz, H-6), 7.63 (2H, s, 3A-H, 3B-H), 7.48 (1H, d, J = 12.0 Hz, H-5), 6.81 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 4.25 (2H, q, J = 6.5 Hz, –OCH2–), 1.31 (3H, t, J = 7.2 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.14 (C, 4-C), 153.79 (C, 4a-C), 153.36 (C, 4b-C), 131.22 (C, 1-C), 129.35 (CH, 6-C), 127.21 (2 × C, 2a-C, 2b-C), 124.93 (C, 1a-C), 124.29 (C, 1b-C), 122.01 (CH, 2-C), 119.87 (C, 3-C), 118.99 (CH, 6a-C), 118.56 (CH, 6b-C), 117.87 (2 × CH, 3a-C, 3b-C), 115.66 (CH, 5-C), 113.87 (2 × CH, 5a-C, 5b-C), 65.96 (CH2,–OCH2–), 14.30 (CH3, –CH3); HR-ESI-MS (m/z): 538.4125 [M + H]+.
N1, N3-bis (2, 4-dichlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5d): White solid (The crude product was recrystallized by ethanol.)
Yield: 42.2%; m.p.: 176.3–178.0 °C; IR (KBr) cm−1: 3131.84 (υNH), 2826.07 (υCH), 1400.72, 1341.16(υSO2), 1285.55, 1146.34(υC-O-C), 1066.78, 997.93, 844.78, 742.19 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.97 (1H, s, –SO2NH–), 10.78 (1H, s, –SO2NH–), 8.31 (1H, d, J = 3.6 Hz, H-2), 7.99 (1H, dd, J = 9.0, 2.5 Hz, H-6), 7.56 (2H, s, 3A-H, 3B-H), 7.37 (1H, d, J = 8.7 Hz, H-5), 6.90 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 4.29 (2H, q, J = 6.8 Hz, –OCH2–), 1.29 (3H, t, J = 6.5 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.21 (C, 4-C), 134.24 (C, 1-C), 130.39 (2 × CH, 3a-C, 3b-C), 128.56 (CH, 6-C), 126.87 (CH, 5a-C), 126.38 (CH, 5b-C), 126.06 (2 × C, 2a-C, 2b-C), 124.80 (C, 1a-C), 124.67 (C, 1b-C), 124.26 (2 × C, 4a-C, 4b-C), 122.03 (CH, 2-C), 120.23 (C, 3-C), 117.18 (2 × CH, 6a-C, 6b-C), 115.97 (CH, 5-C), 66.09 (CH2, –OCH2–), 14.33 (CH3, –CH3); HR-ESI-MS (m/z): 571.3266 [M + H]+.
N1, N3-bis (2-methyl-4-fluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5e): Red solid (The crude product was recrystallized by isopropanol.)
Yield: 54.0%; m.p.: 188.3–190.0 °C; IR (KBr) cm−1: 3133.45 (υNH), 2887.62 (υCH), 1401.59, 1337.53 (υSO2), 1284.83, 1166.48 (υC-O-C), 997.08, 834.44, 723.16 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 9.58 (2H, s, 2 × -SO2NH-), 7.99 (1H, d, J = 2.0 Hz, H-2), 7.73 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.39 (1H, d, J = 8.8 Hz, H-5), 6.92 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 6.70 (2H, s, 3A-H, 3B-H), 4.27 (2H, q, J = 6.4 Hz, –OCH2–), 2.12 (3H, s, 2a-CH3), 1.99 (3H, s, 2b-CH3), 1.33 (3H, t, J = 6.6 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.61 (C, 4-C), 159.57 (C, 4a-C), 159.13 (C, 4b-C), 135.41 (C, 1a-C), 135.21 (C, 1b-C), 131.09 (C, 1-C), 130.71 (C, 2a-C), 130.26 (C, 2b-C), 128.77 (CH, 6-C), 124.58 (CH, 2-C), 120.69 (C, 3-C), 117.67 (CH, 6a-C), 117.52 (CH, 6b-C), 115.45 (CH, 3a-C), 115.30 (CH, 3b-C), 114.33 (CH, 5-C), 112.66 (CH, 5a-C), 112.38 (CH, 5b-C), 65.94 (CH, –OCH2–), 18.01 (2×-CH3, 2a-CH3, 2b-CH3), 14.35(CH3,–CH3); HR-ESI-MS (m/z): 497.1031 [M + H]+.
N1, N3-bis (2-methyl-4-chlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5f): White solid (The crude product was recrystallized by ethanol.)
Yield: 51.5%; m.p.: 194.3–195.8 °C; IR (KBr) cm−1: 3133.95 (υNH), 2818.41 (υCH), 1400.25, 1336.66 (υSO2), 1165.30 (υC-O-C), 923.53, 872.28, 713.78 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 9.72 (1H, s, –SO2NH–), 9.57 (1H, s, –SO2NH–), 7.94 (1H, d, J = 2.2 Hz, H-2), 7.75 (1H, dd, J = 12.8, 2.4 Hz, H-6), 7.39 (1H, d, J = 8.8 Hz, H-5), 7.19 (2H, s, 3A-H, 3B-H), 6.89 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 4.25 (2H, q, J = 6.7 Hz, –OCH2–), 2.11 (3H, s, 2a-CH3), 1.97 (3H, s, 2b-CH3), 1.31 (3H, t, J = 7.0 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.15 (C, 4-C), 137.26 (C, 1a-C), 137.20 (C, 1b-C), 135.63 (CH, 3a-C), 135.03 (CH, 3b-C), 131.77 (C, 1-C), 130.33 (C, 2a-C), 130.19 (C, 2b-C), 128.74 (CH, 6-C), 126.74 (CH, 5a-C), 126.72 (CH, 5b-C), 124.89 (C, 4a-C), 124.80 (C, 4b-C), 122.64 (CH, 2-C), 119.22 (C, 3-C), 117.97 (CH, 6a-C), 117.73 (CH, 6b-C), 113.69 (CH, 5-C), 65.87 (CH2, –OCH2–), 17.76 (2 × –CH3, 2a-CH3, 2b-CH3), 14.31(CH3, –CH3); HR-ESI-MS (m/z): 530.0676 [M + H]+.
N1, N3-bis (2-methyl-4-bromophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5g): Red solid (The crude product was recrystallized by methanol.)
Yield: 41.3%; m.p.: 207.0–209.0 °C; IR (KBr) cm−1: 3249.19 (υNH), 2889.18 (υCH), 1339.13, 1281.83 (υSO2), 1170.15 (υC-O-C), 1107.85 (υC-N), 922.61, 816.70, 729.89 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 9.72 (1H, s, –SO2NH–), 9.56 (1H, s, –SO2NH–), 7.95 (1H, d, J = 2.0 Hz, H-2), 7.75 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.41 (1H, d, J = 8.4 Hz, H-5), 7.17 (4H, m, 3A-H, 3B-H, 5A-H, 5B-H), 6.79 (2H, m, 6A-H, 6B-H), 4.25 (2H, q, J = 6.8 Hz, -OCH2-), 2.11 (3H, s, 2a-CH3), 1.97 (3H, s, 2b-CH3), 1.31 (3H, t, J = 6.5 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.15 (C, 4-C), 137.51 (C, 1a-C), 137.48 (C, 1b-C), 135.71 (CH, 3a-C), 135.68 (CH, 3b-C), 132.67 (C, 2a-C), 132.55 (C, 2b-C), 131.77 (C, 1-C), 129.96 (CH, 5a-C), 129.61 (CH, 5b-C), 127.54 (CH, 6-C), 124.59 (CH, 2-C), 122.89 (C, 3-C), 118.03 (CH, 6a-C), 117.98 (CH, 6b-C), 114.69 (CH, 5-C), 112.62 (C, 4a-C), 112.38 (C, 4b-C), 65.88 (CH2, –OCH2–), 17.70 (–CH3, 2a-CH3), 17.68 (-CH3, 2b-CH3), 14.31 (CH3, –CH3); HR-ESI-MS (m/z): 619.4814 [M + H]+.
N1, N3-bis (2, 4-dimethylphenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5h): White solid (The crude product was recrystallized by ethanol.)
Yield: 46.3%; m.p.: 186.5–187.6 °C; IR (KBr) cm−1: 3238.46 (υNH), 2910.28 (υCH), 1403.75, 1321.55 (υSO2), 1287.53, 1163.21 (υC-O-C), 954.26, 856.32, 725.14 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 9.67 (2H, s, 2 × -SO2NH-), 8.15 (1H, d, J = 2.6 Hz, H-2), 7.82 (1H, dd, J = 10.5, 2.2 Hz, H-6), 7.42 (1H, d, J = 7.3 Hz, H-5), 6.94 (2H, m, 5A-H, 5B-H), 6.69 (4H, m, 3A-H, 3B-H, 6A-H, 6B-H), 4.36 (2H, q, J = 7.1 Hz, -OCH2-), 2.33 (3H, s, 4a-CH3), 2.30 (3H, s, 4b-CH3), 2.20 (3H, s, 2a-CH3), 2.15 (3H, s, 2b-CH3), 1.32 (3H, t, J = 6.7 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 160.58 (C, 4-C), 138.77 (C, 4a-C), 138.25 (C, 4b-C), 134.60 (C, 1a-C), 134.11 (C, 1b-C), 132.77 (CH, 3a-C), 132.56 (CH, 3b-C), 130.12 (C, 1-C), 128.88 (CH, 6-C), 127.65 (2 × C, 2a-C, 2b-C), 125.66 (2 × CH, 5a-C, 5b-C), 124.23 (CH, 2-C), 122.01 (C, 3-C), 118.87 (CH, 6a-C), 118.45 (CH, 6b-C), 113.31 (CH, 5-C), 66.87 (CH2, –OCH2–), 20.78 (CH3, 4a-CH3), 20.31 (CH3, 4b-CH3), 17.52 (CH3, 2a-CH3), 17.03 (CH3, 2b-CH3), 14.34 (CH3, –CH3); HR-ESI-MS (m/z): 489.1542 [M + H]+.
N1, N3-bis (3, 4-difluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5i): White solid (The crude product was recrystallized by ethanol.)
Yield: 46.6%; m.p.: 205.1–206.3 °C; IR (KBr) cm−1: 3134.29 (υNH), 2981.59 (υCH), 1401.00, 1324.29 (υSO2), 1285.69, 1156.85 (υC-O-C), 973.66, 817.17, 744.11 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.48 (1H, s, -SO2NH-), 10.32 (1H, s, –SO2NH–), 8.39 (1H, d, J = 2.4 Hz, H-2), 7.96 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.43 (1H, d, J = 8.8 Hz, H-5), 7.29 (2H, m, 5A-H, 5B-H), 6.84 (2H, s, 2A-H, 2B-H), 6.45 (2H, m, 6A-H, 6B-H), 4.27 (2H, q, J = 7.6 Hz, -OCH2-), 1.30 (3H, t, J = 7.0 Hz, -OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.16 (C, 4-C), 144.70 (C,3a-C), 144.34 (C, 3b-C), 137.28 (C, 4a-C), 137.04 (C, 4b-C), 135.82 (C, 1a-C), 135.27 (C, 1b-C), 132.55 (C, 1-C), 127.59 (CH, 6-C), 124.47 (CH, 2-C), 122.87 (C, 3-C), 115.87 (CH, 5-C), 114.43 (CH, 5a-C), 114.07 (CH, 5b-C), 112.29 (CH, 6a-C), 112.09 (CH, 6b-C), 108.30 (CH, 2a-C), 108.10 (CH, 2b-C), 65.97 (CH2, –OCH2–), 14.34 (CH3, –CH3); HR-ESI-MS (m/z): 505.0511 [M + H]+.
N1, N3-bis (3-fluoro-4-chlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5j): White solid (The crude product was recrystallized by acetone.)
Yield: 43.3%; m.p.: 199.0−200.2 °C; IR(KBr) cm−1: 3253.33 (υNH), 2981.81 (υCH), 1383.91, 1333.20 (υSO2), 1288.11, 1155.44 (υC-O-C), 981.84, 812.25, 742.53 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.75 (1H, s, –SO2NH–), 10.34 (1H, s, –SO2NH–), 8.30 (1H, d, J = 4.0 Hz, H-2), 7.92 (1H, dd, J = 8.4, 3.6 Hz, H-6), 7.43 (1H, d, J = 11.6 Hz, H-5), 7.34 (2H, m, 5A-H, 5B-H), 6.92 (4H, m, 2A-H, 2B-H, 6A-H, 6B-H), 4.25 (2H, q, J = 7.3 Hz, –OCH2–), 1.29 (3H, t, J = 6.5 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 162.50 (C,3a-C), 162.07 (C, 3b-C), 159.79 (C, 4-C), 136.33 (C, 1a-C), 136.27 (C, 1b-C), 133.09 (C, 1-C), 130.25 (CH, 5a-C), 130.06 (CH, 5b-C), 127.47 (CH, 6-C), 122.99 (CH, 2-C), 120.20 (C, 3-C), 117.49 (CH, 6a-C), 117.24 (CH, 6b-C), 114.9 (CH, 5-C), 111.59 (CH, 4a-C), 111.35 (CH, 4b-C), 105.49 (CH, 2a-C), 105.29 (CH, 2b-C), 66.05 (CH2, –OCH2–), 14.35 (CH3, –CH3); HR-ESI-MS (m/z): 538.4102 [M + H]+.
N1, N3-bis (3-chloro-4-fluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5k): White solid (The crude product was recrystallized by ethanol.)
Yield: 45.5%; m.p.: 204.0–205.3 °C; IR (KBr) cm−1: 3158.20 (υNH), 2987.01 (υCH), 1400.68, 1326.62 (υSO2), 1107.21 (υC-O-C), 961.44, 774.67 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.49 (2H, s, 2 × –SO2NH–), 8.40 (1H, d, J = 2.4 Hz, H-2), 7.95 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.34 (1H, d, J = 8.8 Hz, H-5), 7.20–7.02 (4H, m, 2A-H, 2B-H, 5A-H, 5B-H), 6.76 (2H, m, 6A-H, 6B-H), 4.26 (2H, q, J = 6.7 Hz, -OCH2-), 1.29 (3H, t, J = 7.1 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.14 (C, 4-C), 146.93 (C, 4a-C), 146.29 (C, 4b-C), 136.00 (C, 1a-C), 135.88 (C, 1b-C), 131.21 (C, 1-C), 128.66 (CH, 6-C), 122.75 (CH, 2-C), 121.77 (C, 3-C), 120.57 (C,3a-C), 120.16 (C, 3b-C), 118.54 (CH, 2a-C), 118.21 (CH, 2b-C), 115.98 (CH, 6a-C), 115.78 (CH, 6b-C), 114.92 (CH, 5-C), 113.66 (CH, 5a-C), 113.25 (CH, 5b-C), 65.96 (CH2, –OCH2–), 14.30 (CH3, –CH3); HR-ESI-MS (m/z): 538.9937 [M + H]+.
N1, N3-bis (3, 4-dichlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5l): White solid (The crude product was recrystallized by ethanol.)
Yield: 45.5%; m.p.: 190.6–191.8 °C; IR (KBr) cm−1: 3241.30 (υNH), 2989.38 (υCH), 1401.04, 1316.00 (υSO2), 1156.30 (υC-O-C), 933.19, 859.67, 731.92 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 9.51 (1H, s, -SO2NH-), 9.35 (1H, s, –SO2NH–), 7.93 (1H, d, J = 2.4 Hz, H-2), 7.68 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.35 (1H, d, J = 8.8 Hz, H-5), 7.26–7.02 (6H, m, 2 × -C6H3), 4.27 (2H, q, J = 9.2 Hz, –OCH2–), 1.32 (3H, t, J = 6.4 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.94 (C, 4-C), 135.34 (C, 1a-C), 135.31 (C, 1b-C), 131.49 (C, 3a-C), 131.21 (C, 3b-C), 130.33 (C, 1-C), 129.76 (CH, 5a-C), 129.12 (CH, 5b-C), 128.84 (CH, 6-C), 123.66 (C, 4a-C), 123.14 (C, 4b-C), 122.65 (CH, 2-C), 121.54 (C, 3-C), 120.34 (CH, 6a-C), 120.02 (CH, 6b-C), 117.78 (CH, 2a-C), 117.15 (CH, 2b-C), 114.45 (CH, 5-C), 65.73 (CH2, –OCH2–), 14.42 (CH3, –CH3); HR-ESI-MS (m/z): 570.2911 [M + H]+.
N1, N3-bis (3-methyl-4-fluorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5m): White solid (The crude product was recrystallized by ethanol.)
Yield: 50.6%; m.p.: 197.0–199.0 °C; IR (KBr) cm−1: 3253.81 (υNH), 2872.32 (υCH), 1383.24, 1331.11 (υSO2), 1288.64, 1153.93 (υC-O-C), 974.75, 853.40, 741.35 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.41 (2H, s, 2 × –SO2NH–), 8.26 (1H, d, J = 2.4 Hz, H-2), 7.85 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.29 (1H, d, J = 4.8 Hz, H-5), 7.06–6.78 (6H, m, 2 × -C6H3), 4.25 (2H, q, J = 9.1 Hz, -OCH2-), 2.09 (6H, d, J = 11.2 Hz, 3a-CH3, 3b-CH3), 1.31 (3H, t, J = 6.2 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.06 (C, 4-C), 151.51 (C, 4a-C), 151.34 (C, 4b-C), 134.62 (C, 1a-C), 134.17 (C, 1b-C), 131.93 (C, 1-C), 127.29 (CH, 6-C), 125.67 (C, 3a-C), 125.20 (C, 3b-C), 122.77 (CH, 2-C), 120.59 (C, 3-C), 117.88 (CH, 2a-C), 117.34 (CH, 2b-C), 116.66 (CH, 5a-C), 116.49 (CH, 5b-C), 115.21 (CH, 5-C), 113.40 (CH, 6a-C), 113.14 (CH, 6b-C), 65.89 (CH2, –OCH2–), 14.39 (CH3, –CH3), 13.94 (2 × CH3, 3a-CH3, 3b-CH3); HR-ESI-MS (m/z): 497.1052 [M + H]+.
N1, N3-bis (3-methyl-4-chlorophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5n): White solid (The crude product was recrystallized by isopropanol.)
Yield: 50.0%; m.p.: 202.2–203.7 °C; IR (KBr) cm−1: 3252.51 (υNH), 2892.91 (υCH), 1384.07, 1327.24 (υSO2), 1148.98 (υC-O-C), 945.81, 873.15, 820.07, 734.92 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.42 (2H, s, 2 × –SO2NH–), 8.26 (1H, d, J = 2.0 Hz, H-2), 7.85 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.31 (1H, d, J = 9.2 Hz, H-5), 7.29–6.94 (4H, m, 5A-H, 5B-H, 6A-H, 6B-H), 6.76 (2H, s, 2A-H, 2B-H), 4.25 (2H, q, J = 10.3 Hz, –OCH2–), 2.19 (6H, d, J = 11.2 Hz, 3a-CH3, 3b-CH3), 1.29 (3H, t, J = 7.2 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.05 (C, 4-C), 137.55 (C, 3a-C), 137.17 (C, 3b-C), 135.36 (C, 1a-C), 135.01 (C, 1b-C), 132.25 (C, 1-C), 130.92(CH, 5a-C), 130.73 (CH, 5b-C), 128.14 (CH, 6-C), 125.99 (C, 4a-C), 125.69 (C, 4b-C), 123.36 (CH, 2-C), 121.11 (C, 3-C), 119.74 (CH, 6a-C), 119.2 (CH, 6b-C), 117.96 (CH, 2a-C), 117.35 (CH, 2b-C), 114.97 (CH, 5-C), 65.89 (CH2, –OCH2–), 19.23 (2 × CH3, 3a-CH3, 3b-CH3), 14.36 (CH3, –CH3); HR-ESI-MS (m/z): 531.0417 [M + H]+.
N1, N3-bis (3-methyl-4-bromophenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5o): White solid (The crude product was recrystallized by isopropanol.)
Yield: 31.4%; m.p.: 209.0–210.3 °C; IR (KBr) cm−1: 3246.04 (υNH), 2881.73 (υCH), 1381.09, 1327.05 (υSO2), 1149.61 (υC-O-C), 1040.41, 936.84, 820.46, 722.81 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.49 (1H, s, –SO2NH–), 10.34 (1H, s, –SO2NH–), 8.26 (1H, d, J = 2.1 Hz, H-2), 7.96 (1H, dd, J = 7.2, 2.0 Hz, H-6), 7.31 (1H, d, J = 9.6 Hz, H-5), 7.27 (2H, m, 5A-H, 5B-H), 6.96 (4H, m, 2A-H, 2B-H, 6A-H, 6B-H), 4.25 (2H, q, J = 10.5 Hz, –OCH2–), 2.19 (6H, d, J = 8.4 Hz, 3a-CH3, 3b-CH3), 1.29 (3H, t, J = 5.8 Hz, –OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.06 (C, 4-C), 138.54 (C, 3a-C), 138.15 (C, 3b-C), 136.99 (C, 1a-C), 136.93 (C, 1b-C), 133.69 (CH, 5a-C), 133.11 (CH, 5b-C), 130.36 (C, 1-C), 128.79 (CH, 6-C), 123.54 (CH, 2-C), 120.42 (C, 3-C), 118.26 (CH, 2a-C), 118.03 (CH, 2b-C), 116.69 (CH, 6a-C), 116.39 (CH, 6b-C), 114.91 (C, 4a-C), 114.63 (C, 4b-C), 113.66 (CH, 5-C), 65.99 (CH2, –OCH2–), 22.02 (2 × CH3, 3a-CH3, 3b-CH3), 14.35 (CH3, –CH3); HR-ESI-MS (m/z): 619.9387 [M + H]+.
N1, N3-bis (3, 4-dimethylphenyl)-4-ethoxybenzene-1, 3-disulfonamide
(5p): White solid (The crude product was recrystallized by ethanol.)
Yield: 35.3%; m.p.: 182.3–183.9 °C; IR (KBr) cm−1: 3130.75 (υNH), 2980.86 (υCH), 1400.39, 1329.35(υSO2), 1148.86 (υC-O-C), 1018.94, 963.20, 815.27, 734.59 (υph-H); 1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 10.09 (1H, s, –SO2NH–), 9.91 (1H, s, –SO2NH–), 8.41 (1H, d, J = 2.0 Hz, H-2), 7.96 (1H, dd, J = 11.2, 2.4 Hz, H-6), 7.46 (1H, d, J = 8.8 Hz, H-5), 7.26 (2H, m, 6A-H, 6B-H), 6.84 (4H, m, 2A-H, 2B-H, 5A-H, 5B-H), 4.25 (2H, q, J = 6.6 Hz, -OCH2-), 2.64 (3H, s, 3a-CH3), 2.60 (3H, s, 3b-CH3), 2.43 (3H, s, 4a-CH3), 2.38 (3H, s, 4b-CH3), 1.32 (3H, t, J = 6.4 Hz, -OCH2CH3); 13C-NMR (101MHz, DMSO-d6, TMS) δ (ppm): 159.94 (C, 4-C), 137.39 (C, 3a-C), 137.20 (C, 3b-C), 135.34 (C, 1a-C), 135.31 (C, 1b-C), 131.11 (C, 1-C), 130.41 (CH, 5a-C), 130.29 (CH, 5b-C), 128.94 (CH, 6-C), 125.45 (C, 4a-C), 125.12 (C, 4b-C), 124.69 (CH, 2-C), 122.30 (C, 3-C), 116.98 (CH, 6a-C), 116.46 (CH, 6b-C), 114.54 (CH, 2a-C), 114.34 (CH, 2b-C), 113.80 (CH, 5-C), 65.73 (CH2, –OCH2–), 19.96 (2 × CH3, 3a-CH3, 3b-CH3), 19.04 (2 × CH3, 4a-CH3, 4b-CH3), 14.22 (–CH3); HR-ESI-MS (m/z): 489.1539 [M + H]+.
Bioassay
In vitro anti-platelet aggregation activity assay
The in vitro anti-platelet aggregation activities of the target compounds (5a–p) have been evaluated by Born’s turbidimetry (Born 1962; Barbosa et al. 2006; Liu et al. 2018).
Rabbit were anesthetized with 10% chloral hydrate, and blood was collected from the rabbit auricle vein. Whole blood was centrifuged at room temperature for 10 min (800–1000 rpm) with 3.8% citrate as anticoagulant (9:1 by volume), platelet-rich plasma (PRP) was attained from the supernatant. Platelet-poor plasma (PPP) was obtained by centrifugation of the remaining layer at room temperature for 10–15 min (3000 rpm), which was used to set the zero (confirm 100% luminousness).
Test compounds (at the concentration of 1.3 μM) previously dissolved in DMSO (5 μl) were added into PRP (20 μl). The same volume of DMSO without target compounds were added as control sample (The aim was to prove that DMSO seems to have no remarkable effect on the anti-platelet aggregation activity). After incubating for 5 min at 37 °C, ADP and AA were added as inducers and platelet aggregation were measured. The percentage inhibition of platelet aggregation of the target compounds was calculated according to the following formula:
S = the platelet aggregation in the presence of solvent. D = the platelet aggregation in the presence of test compounds.
The anti-platelet aggregation activity was denoted as percentage inhibition of platelet aggregation and the IC50 values of compounds with higher activities were calculated.
Cytotoxicity assay on L-929 cells
According to the results of in vitro anti-platelet aggregation activity, compounds with higher activities were chosen to further conduct in vitro cytotoxicity study. Mouse fibroblast cells (L-929) were used to assess the in vitro cytotoxicity by Cell Counting Kit-8 (CCK-8) assays (Abe et al. 2000; Xiong et al. 2007).
L-929 were added into sterilized the 96-well microplates (1 × 104 cells per well immersed in RPMI-1640 medium) and incubated in a humid 5% CO2 atmosphere at 37 °C for 24 h. Afterwards, the cells were exposed to target compounds at a specific concentration, continued to incubate in a humid 5% CO2 atmosphere at 37 °C for 48 h. After cultivation for 48 h, the medium was removed and replaced with fresh RPMI-1640 medium (100 mL). Ultimately, CCK-8 solution was added into the 96-well microplates (10 μL, per/well) and cultivated at 37 °C for 2 h. The absorbance was monitored and recorded on a microplate reader (Bio-Tek FLx800 fluorescence microplate reader) at 450 nm.
The cell viability rate on L-929 was calculated by the following formula:
Docking studies
The crystal structure of P2Y12 receptor (PDB code 4PXZ) was obtained from the RCSB Protein Data Bank. The 3D structures of target compounds (5a and 5h) were drawn in mol2 format through ChemBio 3D. The docking studies were performed with AutoDock Vina in PyRx software. The best docked pose with the lowest energy calculated by AutoDock version 4.2 was selected and analyzed with PyMol software.
Results and discussion
In vitro anti-platelet aggregation activity
With picotamide, aspirin, and clopidogrel as positive control drugs, the in vitro anti-platelet activities of target compounds were assessed induced by ADP and AA. The primary screening data for target compounds (1.3 μM) in vitro activities on anti-platelet aggregation were given in Table 2 (with ADP as inducer) and Table 3 (with AA as inducer).
As were shown in Fig. 2, we surprisingly found that most 2, 4-disubstituted compounds (5a, 5b, 5c, 5d, 5g, 5h) exhibited superior inhibitory activity than positive control agent picotamide while 3, 4-disubstituted compounds (5i–p) have no remarkable inhibitory activity induced by ADP at the concentration of 1.3 μM. The order of inhibition rate induced by ADP was: 5h (44.5%) > clopidogrel (39.1%) > 5c (34.86%) > 5a (34.5%) > aspirin (32.52%) > 5b (31.67%) > 5d (30.45%) > 5g (27.8%) > picotamide (26.56%). The IC50 values of compounds with higher activities were further calculated when ADP was used as inducer. The order of anti-platelet aggregation activity was: 5h (0.32 μM) > Clopidogrel (0.41 μM) > 5c (0.49 μM) > 5a (0.51 μM) > Aspirin (0.53 μM) > 5b (0.66 μM) > 5g (0.73 μM) > Picotamide (0.76 μM) > 5d (0.82 μM). Moreover, the IC50 value of compounds 5h (0.32 μM), 5c (0.49 μM), 5a (0.51 μM) were lower than that of two positive control drugs aspirin (0.53 μM) and picotamide (0.76 μM) induced by ADP. Especially, compound 5h revealed the highest inhibition rate (44.5%) and the lowest IC50 value (0.32 μM) than three positive control drugs clopidogrel (39.1%, 0.41 μM), aspirin (32.52%, 0.53 μM), picotamide (26.56%, 0.76 μM) against ADP induced platelet aggregation.
On the other hand, as were shown in Fig. 3, 2, 4-disubstituted compounds (5a, 5b, 5g, 5h) and 3, 4-disubstituted compounds (5k, 5l, 5p) had higher inhibition rate than positive control agent clopidogrel induced by AA at the concentration of 1.3 μM. The order of inhibition rate induced by AA was: 5h (40.9%) > Picotamide (32.7%) > 5b (30.56%) > 5p (30.4%) > 5a (29.6%) > 5g (29.4%) > Aspirin (28.9%) > 5l (28.5%) > 5k (27.8%) > Clopidogrel (23.5%). Simultaneously, compounds 5h (40.9%), 5b (30.56%), 5p (30.4%), 5a (29.6%) and 5g (29.4%) exhibited higher inhibition rates than that of two positive control agents aspirin (28.9%) and clopidogrel (23.5%). It was worth noting that compound 5h (40.9%) displayed highest inhibition rates than three positive control drugs picotamide (32.7%), aspirin (28.9%), and clopidogrel (23.5%).
Cytotoxicity on L-929 cells
According to the results of in vitro anti-platelet activities, nine compounds were chosen to further conduct in vitro cytotoxicity study. The results analysis of in vitro cytotoxicities were shown in Table 4. The results revealed that the cytotoxicities of the target compounds were positively correlated with the drug concentration and were negatively correlated with the IC50 values. The order of cytotoxicity was: 5h (86.46 μM) < 5l (74.73 μM) < Picotamide (72.54 μM) < 5k (67.46 μM) < 5a (62.13 μM) < 5g (53.48 μM) < 5b (52.79 μM) < 5p (37.56 μM) < 5c (37.30 μM) < 5d (14.30 μM/L). Among them, the IC50 values of two target compounds 5h (86.46 μM), 5l (74.73 μM) were higher than picotamide (72.54 μM). The results displayed that compounds 5h and 5l had the lower cytotoxicity than picotamide.
As were shown in Fig. 4, we known that compound 5h had the highest anti-platelet activity (44.5, 28.5 %) induced by ADP and AA and the lowest cytotoxicity (86.46 μM). What’s more, other eight compounds were no significant relationship between the anti-platelet activities and cytotoxicities.
Docking studies
In order to confirm target compounds can interact with the P2Y12 receptor sites on the platelet membrane, target compounds with higher activities (5a and 5h) were selected to docked and analyzed the binding interaction. The molecular docking results shown that compounds 5a had a good binding affinity represented by a total docking score of −8.93 kcal/mol (Fig. 5a). In the docking pose, compound 5a forms four H-bonds to the binding-sites residues which including the polar and uncharged Gln-263, Asn-159, Asn-191, and the polar and positively charged Arg-256. Similarly, the docking results for compound 5h also exhibited higher binding affinity in the total score of −11.55 kcal/mol (Fig. 5b). In the docking pose, compound 5h forms eight H-bonds to the binding-sites residues which including the polar and uncharged Gln-263, Asn-159, Asn-191, Tyr-105, and the polar and positively charged Arg-256, Lys-179, Lys-280, and His-187. Moreover, analyzing the chemical nature of the amino acid residues, it was revealed that compound 5a and 5h bind to hydrophilic residues which form multiple H-bonds network.
Structure activity relationship (SAR) exploration
In order to investigate the probable correlation between the physicochemical parameters of the target compounds and their anti-platelet activity, the structure activity relationship (SAR) analysis was performed with various molecular descriptors. The hydrophobic effect has been described by mean of the octanol–water partition coeffificient (Clog P). The surface area and molecular volume have been used as descriptors for the steric effect. The polarizability and refractivity have been considered as descriptor for both electronic state (London dispersive forces) properties and volume of the molecules. The physicochemical parameters of the target compounds are listed in Table 5. Analysis of the general molecular parameters does not show a significant relationship between the anti-platelet activities and physicochemical parameters of the target compounds.
However, we think that anti-platelet activity of target compounds could be influenced by the numbers and positions of the different substituents attached to the benzene ring.
-
1.
The effect of anti-platelet aggregation activity when the two substitutions were introduced into the 2-position and 4-position of phenyl rings.
Effects of the 2, 4-disubstituted compounds on the anti-platelet activity induced by ADP and AA shows similar pattern. The order of anti-platelet aggregation activity was: 4a* (2-fluoro) > 5a (2, 4-difluoro) > 5b (2-fluoro-4-chloro) > picotamide; 4b* (2-chloro) > 5c (2-chloro-4-fluoro) > 5d (2, 4-dichloro) > picotamide. It was manifested that the activity of 2, 4-dihalogenated compounds were lower than that of 2-halogenated compounds, while their activity were higher than that of picotamide.
The order of anti-platelet aggregation activity was: 5h (2, 4-dimethyl) > 5g (2-methyl-4-bromo) > picotamide > 4d* (2-methyl) > 4f* (4-methyl) > 5f (2-methyl-4-chloro) > 5e (2-methyl-4-fluoro). That meant the activity of 2, 4-dialkyl compounds can be significantly enhanced than that of 2- or 4-alkyl. Moreover, when the derivatives bearing an electron-donating group and an electron-withdrawing group are concurrently introduced into the 2-position and 4-position of phenyl rings, bromo-substituted compounds are more potent than the fluoro- and chloro-substituted analogs.
-
2.
The effect of anti-platelet aggregation activity when the two substitutions were introduced into the 3-position and 4-position of the phenyl rings.
The order of anti-platelet aggregation activity induced by ADP was: 5a (2, 4-difluoro) > 5b (2-fluoro-4-chloro) > 5j (3-fluoro-4-chloro) > 5i (3, 4-difluoro); 5c (2-chloro-4-fluoro) > 5d (2, 4-dichloro) > 5k (3-chloro-4-fluoro) > 5l (3, 4-dichloro); 5h (2, 4-dimethyl) > 5p (3, 4-dimethyl); 5g (2-methyl-4-bromo) > 5f (2-methyl-4-chloro) > 5e (2-methyl-4-fluoro) > 5o (3-methyl-4-bromo), 5n (3-methyl-4-chloro), 5m (3-methyl-4-fluoro). In general, the result indicated that the activity of 3, 4-disubstituted compounds were lower than that of 2, 4-disubstituted compounds induced by ADP.
The order of anti-platelet aggregation activity induced by AA was similar as ADP, except four compounds 5l (3, 4-dichloro), 5k (3-chloro-4-fluoro), 5d (2, 4-dichloro), and 5c (2-chloro-4-fluoro). The order of activity of these four compounds is: 5l > 5k > 5d > 5c. The result revealed that some 3, 4-disubstituted compounds possibly help to promote the anti-platelet aggregation activity than that of 2, 4-disubstituted compounds induced by AA.
Conclusion
Sixteen target compounds (5a-p), which are the picotamide analogs, were synthesized and evaluated to the anti-platelet aggregation activity in vitro. According to the results of anti-platelet activities, compounds with higher activities were selected to continue in vitro cytotoxicity study. It has been identified that compound 5h had the highest anti-platelet activity and the lowest cytotoxicity. The SAR study indicated that the anti-platelet activity can be changed by the introduction of different substituents in different positions of the phenyl rings. Among them, the compound bearing two methyl groups at 2- and 4-positions of the phenyl rings have remarkable in vitro anti-platelet activity than others. To sum up, N, N’-disubstitutedphenyl-4-ethoxylbenzene-1, 3-disulfonamides (5a–p) may help in developing novel anti-platelet agents and have the crucial significance.
Abbreviations
- ADP:
-
Adenosine diphosphate
- AA:
-
Arachidonic acid
- SAR:
-
Structure activity relationship
- COX-I:
-
Cyclooxygenase-I
- TXA2 :
-
Thromboxane A2
- PGI2 :
-
Prostaglandin I2
- TLC:
-
Thin layer chromatography
- CCK-8:
-
Cell Counting Kit-8
References
Abe S, Ochi H, Takahashi Y, Ishijima SA, Osumi M, Yamaguchi H (2000) Characteristic biological effects of itraconazole on L929 fibroblasts and their cell membrane. J Infect Chemother 6(1):35–40
Barbosa Jr F, Sertorio JT, Gerlach RF, Tanus-Santos JE (2006) Clinical evidence for lead-induced inhibition of nitric oxide formation. Arch Toxicol 80(12):811–816
Born GV (1962) Aggregation of platelets by ADP and its reversal. Nature 194(4832):927–928
Brito FCF, Kummerle AE, Lugnier C, Fraga CAM, Barreiro EJ, Miranda ALP (2010) Novel thienylacylhydrazone derivatives inhibit platelet aggregation through cyclic nucleotides modulation and thromboxane A2 synthesis inhibition. Eur J Pharmacol 638(1-3):5–12
Celestini A, Violi F (2007) A review of picotamide in the reduction of cardiovascular events in diabetic patients. Vasc Health Risk Manag 3(1):93–98
Eskandariyan Z, Zadeh ME, Tehrani KHME, Mashayekhi V, Kobarfard F (2014) Synthesis of thioether derivatives of quinazoline-4-one-2-thione and evaluation of their antiplatelet aggregation activity. Arch Pharm Res 37(3):332–339
Guthrie R (2011) Review and management of side effects associated with antiplatelet therapy for prevention of recurrent cerebrovascular events. Adv Ther 28(6):473–482
Jayakumar T, Yang CH, Geraldine P, Yen TL, Sheu JR (2016) The pharmacodynamics of antiplatelet compounds in thrombosis treatment. Expert Opin Drug Met 12(6):615–632
Khalid W, Badshah A, Khan AU, Nadeem H (2018) Synthesis, characterization, molecular docking evaluation, antiplatelet and anticoagulant actions of 1,2,4 triazole hydrazone and sulphonamide novel derivatives. Chem Cent J 12(1):11
Li GL, Wang X, Meng X, Lin YB, Li X, Liu XJ (2015) Design, synthesis of novel N, N'-bis-(halogenophenyl)-4-methoxybenzene-1, 3-disulfonamides and evaluation of their anti-platelet aggregation activity. Acta Pharmaceutica Sinica 50(2):185–190
Liu XJ, He X, Shi CL, Meng J, Shao YL, Si HQ, Hu T (2011) Synthesis and in vitro activities on anti-platelet aggregation of N, N’-di(2-substituted-phenyl)-4-methoxyisophthalamides and benzene-1, 3-disulfonamides. Chin Chem Lett 22(10):1139–1142
Liu XJ, Wang CQ, Meng J, Shi XX, Yan YN, Liu XG (2017) Design, synthesis and biological evaluation of 4-methoxy diaryl isophthalates as antiplatelet agents. Med Chem Res 1:1–9
Liu XJ, Wang SQ, Zhang J, Zhang FX, Li GZ, Wang BJ, Shao YL, Zhang LG, Fang L, Cheng MS (2006) Design, synthesis, and activities of novel derivatives of isophthalamide and benzene-1,3-disulfonamide. Chem Res Chin U 22(3):356–359
Liu XJ, Wang Y, Liu LL, Chen GL (2018) Synthesis and in vitro activities on anti-platelet aggregation of 4-methoxyisophthalamides. Med Chem Res 27(8):1971–1983
Liu XJ, Shi TE, Wang X, Wei TT, Meng X (2015) Synthesis and the evaluations in vitro antiplatelet aggregation activitiesof 4-ethoxyisophthalamides. Cardiovasc Hematol Agents Med Chem 13:124–128
Liu XJ, Shi XX, Zhong YL, Liu N, Liu K (2012) Design, synthesis and in vitro activities on anti-platelet aggregation of 4-methoxybenzene-1,3-isophthalamides. Bioorg Med Chem Lett 22(21):6591–6595
Mclewee N, Archer T, Wills R, Mackin A, Thomason J (2017) Effects of aspirin dose escalation on platelet function and urinary thromboxane and prostacyclin levels in normal dogs. Pharmacol Therapeut 41(1):1–8
Metil DS, Sampath A, Reddy CVR, Bandichhor R (2018) Synthesis and characterization of potential related substances of the antiplatelet agent clopidogrel bisulfate. Chemistryselect 3(1): 100–104
Mirfazli SS, Kobarfard F, Firoozpour L, Asadipour A, Esfahanizadeh M, Tabib K, Shafiee A, Foroumadi A (2014) N-Substituted indole carbohydrazide derivatives: synthesis and evaluation of their antiplatelet aggregation activity. Daru 22(1):65
Peng JJ, Zhao LF, Wang LL, Chen H, Qiu YG, Wang J, Yang HY, Liu J, Liu H (2018) Design, synthesis, and biological evaluation of 2-(phenoxyaryl)-3-urea derivatives as novel P2Y1 receptor antagonists. Eur J Med Chem 158:302–310
Pogliani E, Milani M (1996) Safety and efficacy of picotamide, a dual anti-thromboxane agent, in patients with thrombocytosis and a previous thromboembolic event: a 1-year observational study. J Int Med Res 24(3):311
Reddy MVB, Tsai WJ, Qian K, Lee KH, Wu TS (2011) Structure–activity relationships of chalcone analogs as potential inhibitors of ADP-and collagen-induced platelet aggregation. Bioorg Med Chem 19(24):7711–7719
Sharma V, Jaiswal PK, Kumar S, Mathur M, Swami AK, Yadav DK, Chaudhary S (2018) Discovery of Aporphine analogues as potential antiplatelet and antioxidant agents: design, synthesis, structure–activity relationships, biological evaluations, and in silico molecular docking studies. Med Chem 13(17):1817–1832
Siwek A, Staczek P, Stefanska J (2011) Synthesis and structure–activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur J Med Chem 46(11):5717–5726
Wang Y, Wang X, Chen X, Liu XJ (2018) Synthesis and in vitro anti-platelet aggregation activities of 2-methoxy-5-arylamido-N-(pyridin-3-yl-methyl)benzamides. Arch Pharm 352:1–12
Xiong JW, Xiao H, Zhang ZX (2007) An experimental research on different detection conditions between MTT and CCK-8. Acta Laser Biol Sin 16(5):559–562
Yip S, Benavente O (2011) Antiplatelet agents for stroke prevention. Neurotherapeutics 8(3):475–487
Acknowledgements
The authors grate to the National Science Foundation of China (11341014) & the Committee of Science and Technology of Tianjin of China (15JCZDJC33100) for the financial supports and Shenyang Pharmaceutical University of China for running platelet aggregation assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Liu, X., Qiu, k. et al. N, N’-disubstitutedphenyl-4-ethoxyl benzene-1, 3-disulfonamides: design, synthesis, and evaluation of anti-platelet aggregation activity. Med Chem Res 28, 1388–1401 (2019). https://doi.org/10.1007/s00044-019-02379-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02379-5