Abstract
Cardiovascular diseases are the most frequent cause of morbidity and mortality worldwide. In order to discover novel compounds with anti-platelet aggregation activities, a series of novel 4-methoxy-1,3-phthalamidesamides (1a–1i) and a series of novel 4-methoxy-1,3-benzenedisulfon-amides (2a–2i) were synthesized and their anti-platelet aggregation activities were evaluated by the turbidimetric method in response to the following agonists: adenosine diphosphate (ADP), arachidonic acid (AA), and Collagen. Those compounds that have better in vitro activities were subjected to cell toxicity tests via cell counting kit-8 (CCK-8) assay. The inhibition rates of anti-platelet in vitro of five compounds 1g (39.45%), 2d (38.87%), 2g (38.55%), 2h (44.56%), and 2i (43.93%) were higher than that of two reference drugs picotamide (36.12%) and aspirin (38.45%) when ADP was selected as an inducer. The inhibition rates of seven compounds 1c (43.63%), 1d (40.02%), 1g (47.42%), 1i (40.45%), 2c (40.11%), 2d (40.45%), and 2i (49.05%) were higher than that of picotamide (34.89%) and aspirin (39.43%) when AA was selected as inducer. And the inhibition rates of five compounds 1d (47.22%), 1i (45.01%), 2d (38.74%), 2e (42.21%), and 2f (39.94%) were higher than picotamide (38.45%) and aspirin (37.08%) when collagen was selected as inducer. Moreover, the effect of cell toxicity exhibited that none of the compounds had obvious cell toxicity against L-929 cells. Therefore, 4-methoxy-1,3-phthalamidesamides (1a–1i) and 4-methoxy-1,3-benzenedisulfon-amides (2a–2i) have the potential to become a novel kind of anti-platelet drugs and deserve further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of cardiovascular and thromboembolic events has increased in the recent years and represents a major cause of death in western countries (Liu et al. 2016). Given platelet plays a pivotal role in the pathogenesis of thrombosis, anti-platelet drugs are used as the mainstream first-line drugs for preventing and treating thrombosis (Mirfazli et al. 2014; Lourenço et al. 2017). Currently, aspirin, which irreversibly inhibits the cyclooxygenase 1 enzyme in the arachidonic acid (AA) pathway, results in impaired synthesis of thromboxane A2 (TXA2) (Chelucci et al. 2014; Machlus et al. 2014).
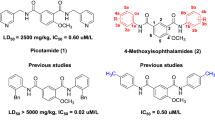
Picotamide (Fig. 1), that is N1,N3-bis(3-picolyl)-4-methoxyisophthalamide, is an anti-platelet drug sharing a dual inhibitory role: inhibition of TXA2 synthase and TXA2 receptor (Brandt et al. 2007). Picotamide can inhibit platelet aggregation in response to adenosine diphosphate (ADP), AA, and Collagen. Compared with aspirin, picotamide does not interfere with endothelial prostaglandin I2 production (Storey 2006). Moreover, picotamide has lower toxicity (LD50 = 2500 mg/kg) and an advantage in reducing total mortality in type II diabetes associated with peripheral arterial disease (Benimana et al. 2017; Patrono et al. 2001).
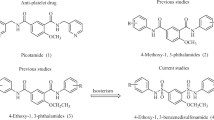
In our previous work, according to the anti-platelet aggregation mechanism and based on the principle of bioisosterism of picotamide, the 4-methoey group of diarylamides (Fig. 1) and disulfonamides (Fig. 1) was still maintained in the same 4-position of picotamide, and a sulfonyl group is an isostere of carbonyl group and the sulfonarmide is a bioisostere of the amide. With two meta-position monosubstituted phenyl group substitutes (-F, -Br, -CH3) replacing the two phenyl groups of diarylamides and disulfonamides, respectively, series of diarylamides and disulfonamides were synthesized, which provide an opportunity to research the influences of the steric factor alkyls (-CH3, O-CH3, O-CH2CH3) and electronic factor halides (-F, -Cl, -Br) on anti-platelet aggregation activities of two side phenyl rings (Liu et al. 2012; Wang et al. 2018).
On basis of these research work, in this study, in order to find out novel anti-platelet agents with potential activities and lower toxicity, we still keep the position of substituents in 1-, 3-, and 4-position of the parent phenyl ring and with two (o-, m-, or p-position) disubstituted phenyl group substitutes (-F, -Br, -CH3) replacing the hydrogen of different positions of two side phenyl rings, respectively. 4-Methoxyisophthalamides (series 1, Fig. 2) and 4-methoxy-1,3-benzenedisulfonamides (series 2, Fig. 2) were synthesized and their in vitro anti-platelet aggregation activities and cell toxicity were evaluated. This study aims to investigate the effects of new structural modifications of picotamide against platelet aggregation activity and cell toxicity. More importantly, it aims at exploring new anti-platelet drugs with higher activity and lower toxicity.
Meterials and methods
Synthetic routes and structural design
The general synthetic routes of target compounds are disclosed in Scheme 1. With 2,4-dimethyl phenol (3) as starting material, dimethylsulfate proceeded easily in the presence of sodium hydroxide in absolute ethanol at 30–45 °C for 6 h and then generated the compound (4). The process was continued to oxidize compound (4) with 6–8 equiv. of potassium permanganate in water to form compound (5). After recrystallization from dilute ethanol, drying compound (5) and reacting it with thionyl chloride gave another unstable intermediate compound (6), which could be used directly in next reaction without further purification (Bir et al. 2015). The yields of three reactions were 72.23, 49.56, and 98.78%, respectively. Finally, the desired compounds (1a–1i) are generated by reacting the compound (6) with different aromatic amines in anhydrous tetrahydrofuran (THF) and triethylamine (TEA). The preparation of other nine compounds (2a–2i) is with anisole (7) as starting material. Reaction of anisole (7) with chlorosulfonic acid produced compound (8), which reacted easily with the corresponding different aromatic amines at room temperature or under mild heat in anhydrous THF and TEA to produce the desired sulfonamide products in good yield. To obtain target compounds with high purity, crude products were recrystallized from appropriate solvents (Liu et al. 2017).
Referring to the structural design of these compounds, 4-methoxy group was still maintained at 4-position of parent benzene ring like picotaime. Besides, replacing the two 3-(aminomethyl) pyridine on the 1,3-position of picotamide with two 2-substituted phenyl rings, nine target compounds (1a–1i) were designed and prepared to elucidate the effect of different substituents (-CH3, -F, -Br) and different substituted positions (o, m or p) on anti-platelet activities in vitro. And in the series 2, these substituents as above were subjected to the same substitution, and the other nine target compounds (2a–2i) were designed and synthesized so as to compare the effects of the same substituents in two different series on the anti-platelet effect in vitro.
General experimental techniques
Eighteen novel target compounds were synthesized by the procedures mentioned above and their chemical structures were confirmed by infrared (IR) spectra, nuclear magnetic resonance spectra (1H-NMR and 13C-NMR), and electron spray ionization mass spectra (ESI-MS). Thin layer chromatography (TLC) was carried out on plate silica gel F254 (Qindao Haiyang Chemical, China). All chemical yields were not optimized, and they were generally the result of a single experiment.
Experimental details
Synthesis of 1-methoxy-2,4-dimethyl benzene (4)
To a solution the 2,4-di-methylphenol (3) (3.00 g, 24.59 mmol) in anhydrous THF at 30 °C was added sodium hydroxide (15% in water, 0.98 g, 24.59 mmol). Dimethylsulfate (3.10 g, 24.59 mmol) was added dropwise. The reaction was allowed to warm to 45 °C and maintained for an additional 6 h. The reaction was monitored by TLC (PE: EA = 5:1, Rf = 0.43). The reaction was partitioned between dichloromethane (CH2Cl2) (100 mL) and H2O (50 mL). The organic layer was washed with brine (50 mL) and dried over MgSO4. The resulting solution was recovered under vacuum to give 1-methoxy-2,4-dimethyl benzene (4) (2.41 g, yield 72.23%).
Synthesis of 4-methoxy-1,3-phthalic acid (5)
To a solution the 1-methoxy-2,4-dimethyl benzene (4) (2.41 g, 17.72 mmol) in H2O at 70 °C was added potassium permanganate (22.40 g, 0.14 mol). The reaction was allowed to warm to 80 °C until the disappearance of compound (4). Then the mixture was filtered and the solid washed with dichloromethane (CH2Cl2) (2 × 20 mL). The organic layer was washed with brine (20 mL) and dried over MgSO4. The resulting solution was recovered under vacuum to give 4-methoxy-1,3-phthalic acid (5) (1.72 g, yield 49.56%).
Synthesis of 4-methoxy-1,3-benzene dichloride (6)
4-Methoxy-1,3-phthalic acid (5) (1.00 g, 5.10 mmol) was dissolved in excess quantity of thionyl chloride (10 mL) and allowed to reflux for 8 h at 80 °C. The excess of thionyl chloride was recovered under vacuum to give 4-methoxy-1,3-benzenedichloride (6) (1.17 g, yield 98.78%).
Synthesis of N 1,N 3-bis(3-bromo-4-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1a)
The solution of 4-methoxy-1,3-benzenedichloride (6) (0.93 g, 4.0 mmol) in anhydrous THF (15 mL) was added to 3-bromine-4-methylaniline (1.48 g, 8.0 mmol). This solution was stirred rapidly at room temperature for about 1 h and then pyridine (1 mL) was added. The solution was refluxed for 4–8 h. The residue was recrystallized from methanol–water to terminal compound 1a (1.81 g, yield 85.2%).
The other compounds (1b–1i) were prepared with the similar method of preparing compound 1a.
N1,N3-bis(3-bromo-4-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1a)
White solid (The residue was recrystallized by 50% ethanol.); Yield = 85.2%; Rf: 0.38; m.p.: 157.2–158.4 °C; IR (KBr) υmax 3340.21, 2971.89, 1665.12, 1601.07, 1447.33, 1267.97, 982.92, 758.72 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.06 (s, 2H, 2× -CONH-), 8.72 (s, 1H, Ar-2-H), 8.62 (d, J = 6.8 Hz, 1H, Ar-6-H), 8.42 (d, J = 7.2 Hz, 1H, Ar-5-H), 8.15 (s, 2H, Ar-2’,2”-H), 7.86 (d, J = 4.8 Hz, 2H, Ar-6’, 6”-H), 7.49 (d, J = 7.8 Hz, 2H, Ar-5’, 5”-H), 3.78 (s, 3H, Ar-4-OCH3), 1.43 (s, 6H, Ar-4’, 4”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 164.49 (C, C = O), 163.42 (C, C = O), 142.57 (C, Ar-4-C), 141.97 (C, Ar-1”-C), 139.68 (C, Ar-1’-C), 133.63 (C, Ar-1-C), 133.13 (C, Ar-3-C), 132.29 (C, Ar-3’-C), 131.26 (C, Ar-3”-C), 130.28 (CH, Ar-2-C), 128.92 (CH, Ar-6-C), 127.83 (CH, Ar-5-C), 126.23 (CH, Ar-2’-C), 124.63 (CH, Ar-2”-C), 122.65 (CH, Ar-5”-C), 121.31 (CH, Ar-5’-C), 118.91 (CH, Ar-6’-C), 117.79 (CH, Ar-6”-C), 116.46 (C, Ar-4”-C), 115.36 (C, Ar-4’-C), 63.49 (O-CH3, Ar-4-O-CH3), 14.76 (2× CH3, Ar-4’,4”-CH3); MS (m/z): 532.9820 [M + H]+.
N1,N3-bis(3-fluoro-4-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1b)
Yellow solid (The residue was recrystallized by 50% ethanol.); Yield = 86.5%; Rf: 0.24; m.p.: 251.4–253.5 °C; IR (KBr) υmax 3445.45, 2360.34, 1657.65, 1552.34, 1338.45, 1240.64, 1036.34, 844.43 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.07 (s, 2H, 2× CONH-), 8.92 (s, 1H, Ar-2-H), 8.74 (d, J = 5.3 Hz, 1H, Ar-6-H), 8.32 (d, J = 8.2 Hz, 1H, Ar-5-H), 7.89 (s, 2H, Ar-2’,2”-H), 7.57 (d, J = 7.4 Hz, 2H, Ar-6’, 6”-H), 7.19 (d, J = 8.4 Hz, 2H, Ar-5’, 5”-H), 3.96 (s, 3H, Ar-4-OCH3), 1.69 (s, 6H, Ar-4’, 4“CH3); 13C-NMR (101 MHz, CDCl3): δ = 163.92 (C, C = O), 162.29 (C, C = O), 143.98 (C, Ar-4-C), 142.54 (C, Ar-1’-C), 141.63 (C, Ar-1-C), 139.13 (C, Ar-3-C), 138.99 (C, Ar-1”-C), 137.05 (d, J = 54 Hz, 2C, Ar-3’, 3”-C), 136.41 (CH, Ar-2-C), 135.43 (CH, Ar-6-C), 134.90 (CH, Ar-5-C), 132.55 (CH, Ar-2’-C), 131.12 (CH, Ar-2”-C), 128.43 (CH, Ar-5”-C), 126.64 (CH, Ar-5’-C), 124.19 (CH, Ar-6’-C), 122.64 (CH, Ar-6”-C), 121.36 (C, Ar-4”-C), 118.63 (C, Ar-4’-C), 64.44 (O-CH3, Ar-4-O-CH3), 14.83 (2× CH3, Ar-4’, 4”-CH3); MS (m/z): 411.0099 [M + H]+.
N1,N3-bis(2-fluoro-4-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1c)
Yellow solid (The residue was recrystallized by acetone.); Yield = 78.1%; Rf: 0.53; m.p.: 272.6–273.6 °C; IR (KBr) υmax 3358.12, 3067.65, 2984.53, 2223.76, 1658.56, 1264.56, 889.83, 830.25 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 9.69 (s, 2H, 2× -CONH-), 8.77 (s, 1H, Ar-2-H), 8.57 (d, J = 8.0 Hz, 1H, Ar-6-H), 8.34 (d, J = 8.0 Hz, 1H, Ar-5-H), 8.28 (s, 2H, Ar-3’, 3”-H), 8.34 (d, J= 7.0 Hz, 2H, Ar-6’, 6”-H), 7.69 (d, J = 5.0 Hz, 2H, Ar-5’, 5”-H), 3.86 (s, 3H, Ar-4-OCH3), 1.43 (s, 6H, Ar-4’, 4”-CH3); 13C-NMR (101 MHz, DMSO-d6): δ = 165.15 (C, C = O), 165.07 (C, C = O), 145.22 (C, Ar-4-C), 140.56 (C, Ar-1’-C), 139.68 (C, Ar-1-C), 137.23 (C, Ar-3-C), 136.99 (C, Ar-1”-C), 134.91 (d, J = 47 Hz, 2C, Ar-2’,2”-C), 132.12 (CH, Ar-2-C), 131.46 (CH, Ar-6-C), 130.89 (CH, Ar-5-C), 129.01 (CH, Ar-3’-C), 128.42 (CH, Ar-3”-C), 125.76 (CH, Ar-5”-C), 125.04 (CH, Ar-5’-C), 124.25 (CH, Ar-6’-C),122.46 (CH, Ar-6”-C), 120.53 (C, Ar-4”-C), 119.36 (C, Ar-4’-C), 44.94 (O-CH3, Ar-4-O-CH3), 13.66 (2× CH3, Ar-4’, 4”-CH3); MS (m/z): 411.0099 [M + H]+.
N1,N3-bis(2-bromo-4-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1d)
Yellow solid (The residue was recrystallized by acetone.); Yield = 74.9%; Rf: 0.36; m.p.: 274.3–276.3 °C; IR (KBr) υmax 3398.08, 2954.53, 1677.03, 1604.15, 1280.02, 1072.78, 740.43 cm−1; 1H-NMR (400 MHz, DMSO-d6, TMS): δ = 10.72 (s, 2H, 2× -CONH-), 8.76 (s, 1H, Ar-2-H), 8.35 (d, J = 7.3 Hz, 1H, Ar-6-H), 8.06 (d, J = 5.2 Hz, 1H, Ar-5-H), 7.66 (s, 2H, Ar-3’, 3”-H), 7.66 (d, J = 5.3 Hz, 2H, Ar-6’, 6”-H), 7.13 (d, J = 6.3 Hz, 2H, Ar-5’, 5”-H), 4.17 (s, 3H, Ar-4-OCH3), 1.43 (s, 6H, Ar-4’, 4”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 163.49 (C, C = O), 162.39 (C, C = O), 142.57 (C, Ar-4-C), 140.16 (C, Ar-1’-C), 138.63 (C, Ar-1-C), 137.23 (C, Ar-3-C), 136.99 (C, Ar-1”-C), 135.59 (C, Ar-2’-C), 134.92 (C, Ar-2”-C), 132.52 (C, Ar-2-C), 131.56 (C, Ar-6-C), 130.89 (CH, Ar-5-C), 130.21 (CH, Ar-3’-C), 127.32 (CH, Ar-3”-C), 126.43 (CH, Ar-5”-C), 124.36 (CH, Ar-5’-C), 123.15 (CH, Ar-6’-C), 122.36 (CH, Ar-6”-C), 121.23 (C, Ar-4”-C), 119.52 (C, Ar-4’-C), 54.76 (O-CH3, Ar-4-O-CH3), 16.98 (2× CH3, Ar-4’, 4”-CH3); MS (m/z): 532.9820 [M + H]+.
N1,N3-bis(4-fluoro-2-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1e)
Yellow solid (The residue was recrystallized by acetone.); Yield = 70.7%; Rf: 0.36; m.p.: 157.5–158.6 °C; IR (KBr) υmax 3436.34, 3344.54, 2980.34, 1670.34, 1601.54, 1526.34, 1038.34, 809.54, 752.54 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.24 (s, H, 2× CONH-), 8.85 (s, 1H, Ar-2-H), 8.52 (d, J = 7.8 Hz, 1H, Ar-6-H), 8.22 (d, J = 8.6 Hz, 1H, Ar-5-H), 7.65 (s, 2H, Ar-3’, 3”-H), 7.06 (d, J = 5.6 Hz, 2H, Ar-5’, 5”-H), 7.06 (d, J = 7.5 Hz, 2H, Ar-6’, 6”-H), 4.03 (s, 3H, Ar-4-OCH3), 1.33 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 164.56 (C, C = O), 160.65 (C, C = O), 142.59 (C, Ar-4-C), 141.56 (C, Ar-1’-C), 137.65 (C, Ar-1-C), 135.33 (C, Ar-3-C), 134.53 (C, Ar-1”-C), 133.23 (C, Ar-2’-C), 132.22 (C, Ar-2”-C), 131.52 (C, Ar-2-C), 130.56 (C, Ar-6-C), 129.89 (CH, Ar-5-C), 127.41 (d, J = 43 Hz, 2C, Ar-4’, 4”-C), 126.21 (CH, Ar-3’-C), 125.32 (CH, Ar-3”-C), 123.43 (CH, Ar-5”-C), 121.36 (CH, Ar-5’-C), 118.15 (CH, Ar-6’-C), 116.36 (CH, Ar-6”-C), 54.86 (O-CH3, Ar-4-O-CH3), 18.76 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 411.0099 [M + H]+.
N1,N3-bis(4-bromo-2-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1f)
White solid (The residue was recrystallized by acetone.); Yield = 56.5%; Rf: 0.37; m.p.: 251.4–252.4 °C; IR (KBr) υmax 3445.34, 2360.54, 1657.56, 1552.23, 1509.76, 1338.56, 1240.75, 1036.34, 844.62, 781.63 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.07 (s, 2H, 2× -CONH-), 8.74 (s, 1H, Ar-2-H), 8.44 (d, J = 7.8 Hz, 1H, Ar-6-H), 8.22 (d, J = 5.8 Hz, 1H, Ar-5-H), 8.04 (s, 2H, Ar-3’,3”-H), 7.69 (d, J = 8.8 Hz, 2H, Ar-5’, 5”-H), 7.46 (d, J = 7.8 Hz, 2H, Ar-6’, 6”-H), 3.96 (s, 3H, Ar-4-OCH3), 1.63 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 163.92 (C, C = O), 162.29 (C, C = O), 142.98 (C, Ar-4-C), 141.99 (C, Ar-1’-C), 139.65 (C, Ar-1-C), 138.36 (C, Ar-3-C), 137.36 (C, Ar-1”-C), 136.56 (C, Ar-2’-C), 135.68 (C, Ar-2”-C), 134.29 (C, Ar-2-C), 133.56 (C, Ar-6-C), 132.19 (CH, Ar-5-C), 131.96 (C, Ar-4’-C), 130.95 (C, Ar-4”-C), 129.64 (CH, Ar-3’-C), 124.63 (CH, Ar-3”-C), 121.65 (CH, Ar-5”-C), 119.67 (CH, Ar-5’-C), 117.84 (CH, Ar-6’-C), 115.67 (CH, Ar-6”-C), 53.82 (O-CH3, Ar-4-O-CH3), 15.36 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 532.9820 [M + H]+.
N1,N3-bis(3-bromo-2-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1g)
White solid (The residue was recrystallized by acetone.); Yield = 64.7%; Rf: 0.22; m.p.: 211.3–212.3 °C; IR (KBr) υmax 3447.43, 3283.53, 1652.64, 1517.42, 1448.53, 1249.84, 1030.45, 767.32 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.57 (s, 2H, 2× -CONH-), 8.99 (s, 1H, Ar-2-H), 8.86 (d, J = 6.8 Hz, 1H, Ar-6-H), 8.24 (d, J = 5.4 Hz, 1H, Ar-5-H), 8.10 (d, J = 5.4 Hz, 2H, Ar-4’,4”-H), 7.70 (t, J = 6.2 Hz, 2H, Ar-5’,5”-H), 7.13 (d,J = 5.8 Hz, 2H, Ar-6’,6”-H), 4.04 (s, 3H, Ar-4-OCH3), 1.83 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, DMSO-d6): δ = 164.93 (C, C = O), 160.45 (C, C = O), 142.68 (C, Ar-4-C), 141.76 (C, Ar-1’-C), 140.67 (C, Ar-1-C), 139.63 (C, Ar-3-C), 138.35 (C, Ar-1”-C), 137.65 (C, Ar-3’-C), 136.67 (C, Ar-3”-C), 134.62 (C, Ar-2-C), 133.91 (C, Ar-6-C), 131.29 (CH, Ar-5-C), 130.15 (CH, Ar-4’-C), 128.49 (CH, Ar-4”-C), 127.48 (CH, Ar-2’-C), 126.64 (C, Ar-2”-C), 125.64 (C, Ar-5”-C), 120.62 (CH, Ar-5’-C), 118.63 (CH, Ar-6’-C), 117.95 (CH, Ar-6”-C), 62.82 (O-CH3, Ar-4-O-CH3), 17.36 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 532.9820 [M + H]+.
N1,N3-bis(3-fluoro-2-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1h)
White solid (The residue was recrystallized by acetone.); Yield = 67.1%; Rf: 0.22; m.p.: 275.5–277.6 °C; IR (KBr) υmax 3358.34, 3067.45, 2984.36, 2223.31, 1658.34, 1264.86, 1145.43, 889.25, 830.53 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.32 (s, 2H, 2× -CONH-), 8.77 (s, 1H, Ar-2-H), 8.54 (d, J = 6.0 Hz, 1H, Ar-6-H), 8.28 (d, J = 6.8 Hz, 1H, Ar-5-H), 7.99 (d, J = 7.0 Hz, 2H, Ar-4’,4”-H), 7.67 (t, J = 8.2 Hz, 2H, Ar-5’,5”-H), 7.35 (d, J = 5.6 Hz, 2H, Ar-6’,6”-H), 4.06 (s, 3H, Ar-4-OCH3), 1.53 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, DMSO-d6): δ = 165.15 (C, C = O), 165.07 (C, C = O), 143.37 (C, Ar-4-C), 142.36 (C, Ar-1’-C), 140.34 (C, Ar-1-C), 139.69 (C, Ar-3-C), 138.37 (C, Ar-1”-C), 136.83 (d, J = 58 Hz, 2C, Ar-3’,3”-C), 135.38 (C, Ar-2-C), 134.65 (C, Ar-6-C), 133.29 (CH, Ar-5-C), 131.26 (CH, Ar-4’-C), 129.37 (CH, Ar-4”-C), 127.34 (CH, Ar-2’-C), 125.67 (C, Ar-2”-C), 123.34 (C, Ar-5”-C), 121.35 (CH, Ar-5’-C), 119.64 (CH, Ar-6’-C), 116.57 (CH, Ar-6”-C), 61.37 (O-CH3, Ar-4-O-CH3), 14.38 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 411.0099 [M ± H]±+.
N1,N3-bis(4-bromo-3-methylphenyl)-4-methoxybenzene-1,3-isophthalamide (1i)
Brown solid (The residue was recrystallized by acetone.); Yield = 71.9%; Rf: 0.31; m.p.: 273.4–275.5 °C; IR (KBr) υmax 3398.08, 3258.45, 2984.45, 1677.03, 1604.15, 1280.02, 1072.78, 740.43 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.72 (s, 2H, 2× -CONH-), 8.87 (s, 1H, Ar-2-H), 8.55 (d, J = 7.0 Hz, 1H, Ar-6-H), 8.06 (d, J = 5.0 Hz, 1H, Ar-5-H), 7.83 (s, 2H, Ar-2’,2”-H), 7.56 (d, J = 6.5 Hz, 2H, Ar-5’,5”-H), 7.25 (d, J = 8.6 Hz, 2H, Ar-6’,6”-H), 4.07 (s, 3H, Ar-4-OCH3), 1.63 (s, 6H, Ar-3’,3”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 163.49 (C, C = O), 160.39 (C, C = O), 145.37 (C, Ar-4-C), 142.36 (C, Ar-1’-C), 140.34 (C, Ar-1-C), 138.69 (C, Ar-3-C), 137.37 (C, Ar-1”-C), 136.83 (d, J = 58 Hz, 2C, Ar-3’,3”-C), 135.38 (C, Ar-2-C), 134.65 (C, Ar-6-C), 133.29 (CH, Ar-5-C), 131.26 (CH, Ar-4’-C), 129.37 (CH, Ar-4”-C), 127.34 (CH, Ar-2’-C), 125.67 (C, Ar-2”-C), 123.34 (C, Ar-5”-C), 121.35 (CH, Ar-5’-C), 119.64 (CH, Ar-6’-C), 116.57 (CH, Ar-6”-C), 61.37 (O-CH3, Ar-4-O-CH3), 14.38 (2× CH3, Ar-2’,2”-CH3); MS: 532.9820 [M + H]+.
Synthesis of 4-methoxybenzene-1,3-disulfonyl dichloride (8)
Anisole (7) (0.88 g, 8.1 mmol) was added dropwise to a solution of chlorosulfonic acid (1.89 g, 16.2 mmol) in anhydrous dichloromethane (15 mL) at 0 °C under mechanical stirring, and the reaction mixture was stirred at 40 °C for about 5 h. Subsequently, thionyl chloride (1.93 g, 16.2 mmol) was added dropwise to the reaction mixture and the mixture was stirred at 50 °C for about 2 h. The reaction was monitored by TLC (PE: EA = 1:1, Rf = 0.67). After that, the mixture was poured into ice water with stirring. After standing under sufficient cooling, white solids precipitated; after vacuum filtration, white solids were dried. The crude product was obtained. After purification by recrystallization from cyclohexane five times, pure intermediate 4-methoxy-1, 3-benzenedisulfonyl chloride (8) was obtained (1.85 g, yield 75.2%).
Synthesis of N 1,N 3-bis(3-bromo-4-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2a)
The solution of 4-methoxybenzene-1,3-disulfonyldichloride (8) (0.87 g, 2.9 mmol) in anhydrous THF (15 mL) was added to pyridine (1 mL) and 3-bromine-4-methylanilin (1.07 g,5.8 mmol). This solution was stirred rapidly at room temperature for about 2 h and then was refluxed for 10 h. The resulting solution was concentrated in vacuo and the solvent was removed. The residue was recrystallized from methanol–water to terminal compound 2a (1.20 g, yield 68.5%).
The other compounds (2b–2i) were prepared with the similar method of preparing compound 2a.
N1,N3-bis(3-bromo-4-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2a)
Brown solid (The residue was recrystallized by acetone.); Yield = 68.5%; Rf: 0.42; m.p.: 185.1–186.2 °C; IR (KBr) υmax 3340.21, 2971.89, 1665.12, 1601.07, 1447.33, 1267.97, 982.92, 758.72 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.06 (s, 2H, 2× -SO2NH-), 8.72 (s, 1H, Ar-2-H), 8.62 (d, J = 6.4 Hz, 1H, Ar-6-H), 8.23 (d, J = 7.6 Hz, 1H, Ar-5-H), 7.96 (s, 2H, Ar-2’,2”-H), 7.68 (d, J = 8.5 Hz, 2H, Ar-6’,6”-H), 7.28 (d, J = 7.4 Hz, 2H, Ar-5’,5”-H), 3.78 (s, 3H, Ar-4-OCH3), 1.43 (s, 6H, Ar-4’,4”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 142.13 (C, Ar-4-C), 141.35 (C, Ar-1”-C), 135.37 (C, Ar-1’-C), 134.35 (C, Ar-1-C), 132.19 (C, Ar-3-C), 130.59 (C, Ar-3’-C), 128.16 (C, Ar-3”-C), 126.37 (CH, Ar-2-C), 125.46 (CH, Ar-6-C), 123.28 (CH, Ar-5-C), 120.86 (CH, Ar-2’-C), 118.67 (CH, Ar-2”-C), 117.26 (CH, Ar-5”-C), 115.38 (CH, Ar-5’-C), 114.69 (CH, Ar-6’-C), 113.24 (CH, Ar-6”-C), 112.57 (C, Ar-4”-C), 110.53 (C, Ar-4’-C), 58.36 (O-CH3, Ar-4-O-CH3), 16.38 (2× CH3, Ar-4’,4”-CH3); MS (m/z): 604.9160 [M + H]+.
N1,N3-bis(3-fluoro-4-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2b)
Orange solid (The residue was recrystallized by acetone.); Yield = 84.6%; Rf: 0.38; m.p.: 231.6–232.5 °C; IR (KBr) υmax 3445.76, 2360.34, 1657.43, 1552.53, 1338.65, 1240.76, 1036.45, 844.75, 781.54 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.07 (s, 2H, 2× -SO2NH-), 8.89 (s, 1H, Ar-2-H), 8.64 (d, J = 4.6 Hz, 1H, Ar-6-H), 8.22 (d, J = 6.5 Hz, 1H, Ar-5-H), 7.89 (s, 2H, Ar-2’,2”-H), 7.57 (d, J = 7.4 Hz, 2H, Ar-5’,5”-H), 7.19 (d, J = 6.8 Hz, 2H, Ar-6’,6”-H), 3.96 (s, 3H, Ar-4-OCH3), 1.69 (s, 6H, Ar-4’,4”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 155.37 (C, Ar-4-C), 144.18 (C, Ar-1’-C), 143.21 (C, Ar-1-C), 139.13 (C, Ar-3-C), 138.27 (C, Ar-1”-C), 137.28 (d, J = 54 Hz, 2C, Ar-3’,3”-C), 135.41 (CH, Ar-2-C), 135.03 (CH, Ar-6-C), 134.90 (CH, Ar-5-C), 132.55 (CH, Ar-2’-C), 131.12 (CH, Ar-2”-C), 128.43 (CH, Ar-5”-C), 127.75 (CH, Ar-5’-C), 126.38 (CH, Ar-6’-C), 125.38 (CH, Ar-6”-C), 123.35 (C, Ar-4”-C), 119.65 (C, Ar-4’-C), 61.27 (O-CH3, Ar-4-O-CH3), 15.83 (2× CH3, Ar-4’,4”-CH3); MS (m/z): 483.0782 [M + H]+.
N1,N3-bis(2-fluoro-4-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2c)
Orange solid (The residue was recrystallized by acetone.); Yield = 67.1%; Rf: 0.38; m.p.: 232.6–233.6 °C; IR (KBr) υmax 3342.45, 3007.34, 2952.35, 2921.64, 2202.64, 1645.46, 1289.56, 1165.26, 869.59, 810.45 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 10.32 (s, 2H, 2× -SO2NH-), 8.84 (s, 1H, Ar-2-H), 8.67 (d, J = 5.8 Hz, 1H, Ar-6-H), 8.47 (d, J = 7.5 Hz, 1H, Ar-5-H), 8.08 (s, 2H, Ar-3’,3”-H), 7.89 (s, 2H, Ar-6’,6”-H), 7.45 (d, J = 8.4 Hz, 2H, Ar-5’,5”-H), 7.13 (d, J = 7.4 Hz, 2H, Ar-6’,6”-H), 3.96 (s, 3H, Ar-4-OCH3), 1.53 (s, 6H, Ar-4’,4”-CH3); 13C-NMR (101 MHz, DMSO-d6): δ = 138.68 (C, Ar-4-C), 136.35 (C, Ar-1’-C), 135.18 (C, Ar-1-C), 132.29 (C, Ar-3-C), 131.36 (C, Ar-1”-C), 129.46 (d, J = 47 Hz, 2 C, Ar-2’,2”-C), 128.15 (CH, Ar-2-C), 127.46 (CH, Ar-6-C), 125.69 (CH, Ar-5-C), 123.49 (CH, Ar-3’-C), 121.27 (CH, Ar-3”-C), 118.64 (CH, Ar-5”-C), 114.59 (CH, Ar-5’-C), 113.28 (CH, Ar-6’-C), 111.41 (CH, Ar-6”-C), 110.68 (C, Ar-4”-C), 109.38 (C, Ar-4’-C), 67.52 (O-CH3, Ar-4-O-CH3), 16.35 (2× CH3, Ar-4’,4”-CH3); MS (m/z): 483.0782 [M + H]+.
N1,N3-bis(2-bromo-4-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2d)
Orange solid (The residue was recrystallized by acetone.); Yield = 86.5%;Rf: 0.38; m.p.: 214.5–215.3 °C; IR (KBr) υmax 3395.23, 2786.64, 1664.32, 1502.35, 1456.02, 1365.56, 1313.57, 1220.95, 1012.26, 763.21 cm−1; 1H-NMR (400 MHz, DMSO-d6, TMS): δ = 9.92 (s, 2H, 2× -SO2NH-), 8.83 (s, 1H, Ar-2-H), 8.53 (d, J = 8.6 Hz, 1H, Ar-6-H), 8.16 (d, J = 6.5 Hz, 1H, Ar-5-H), 7.86 (s, 2H, Ar-3’,3”-H), 7.63 (d, J = 8.6 Hz, 2H, Ar-6’,6”-H), 7.13 (d, J = 6.7 Hz, 2H, Ar-5’,5”-H), 4.17 (s, 3H, Ar-4-OCH3), 1.43 (s, 6H, Ar-4’,4”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 142.35 (C, Ar-4-C), 139.35 (C, Ar-1’-C), 137.18 (C, Ar-1-C), 136.28 (C, Ar-3-C), 135.27 (C, Ar-1”-C), 134.29 (C, Ar-2’-C), 132.94 (C, Ar-2”-C), 131.49 (C, Ar-2-C), 130.59 (C, Ar-6-C), 128.27 (CH, Ar-5-C), 127.09 (CH, Ar-3’-C), 126.38 (CH, Ar-3”-C), 125.45 (CH, Ar-5”-C), 123.16 (CH, Ar-5’-C), 121.08 (CH, Ar-6’-C), 120.16 (CH, Ar-6”-C), 119.65 (C, Ar-4”-C), 117.35 (C, Ar-4’-C), 64.26 (O-CH3, Ar-4-O-CH3), 15.35 (2× CH3, Ar-4’,4”-CH3); MS (m/z): 604.9160 [M + H]+.
N1,N3-bis(4-fluoro-2-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2e)
Orange solid (The residue was recrystallized by acetone.); Yield = 72.5%; Rf: 0.38; m.p.: 117.4–118.5 °C; IR (KBr) υmax 3445.43, 3356.35, 2998.65, 1656.42, 1608.74, 1574.42, 1013.45, 885.93, 746.63 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 9.24 (s, H, 2× -SO2NH-), 8.75 (s, 1H, Ar-2-H), 8.62 (d, J = 7.8 Hz, 1H, Ar-6-H), 8.32 (d, J = 8.6 Hz, 1H, Ar-5-H), 7.95 (s, 2H, Ar-3’,3”-H), 7.52 (d, J = 8.1 Hz, 2H, Ar-5’,5”-H), 7.12 (d, J = 7.6 Hz, 2H, Ar-6’,6”-H), 4.03 (s, 3H, Ar-4-OCH3), 1.33 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 140.36 (C, Ar-4-C), 139.31 (C, Ar-1’-C), 138.29 (C, Ar-1-C), 137.46 (C, Ar-3-C), 136.41 (C, Ar-1”-C), 134.26 (C, Ar-2’-C), 133.16 (C, Ar-2”-C), 131.26 (C, Ar-2-C), 128.67 (C, Ar-6-C), 126.58 (CH, Ar-5-C), 124.36 (d, J = 43 Hz, 2C, Ar-4’,4”-C), 123.16 (CH, Ar-3’-C), 121.35 (CH, Ar-3”-C), 126.18 (CH, Ar-5”-C), 123.27 (CH, Ar-5’-C), 120.36 (CH, Ar-6’-C), 118.68 (CH, Ar-6”-C), 62.18 (O-CH3, Ar-4-O-CH3), 15.38 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 483.0782 [M + H]+.
N1,N3-bis(4-bromo-2-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2f)
Orange solid (The residue was recrystallized by acetone.); Yield = 62.2%; Rf: 0.58; m.p.: 221.6–222.7 °C; IR (KBr) υmax 3445.42, 2360.15, 1657.36, 1552.83, 1509.36, 1338.45, 1240.73, 1036.51, 844.74, 781.58 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 9.67 (s, 2H, 2× -SO2NH-), 8.84 (s, 1H, Ar-2-H), 8.54 (d, J = 8.6 Hz, 1H, Ar-6-H), 8.02 (d, J = 7.5 Hz, 1H, Ar-5-H), 7.89 (s, 2H, Ar-3’,3”-H), 7.59 (d, J = 7.8 Hz, 2H, Ar-5’,5”-H), 7.36 (d, J = 6 Hz, 2H, Ar-6’,6”-H), 3.86 (s, 3H, Ar-4-OCH3), 1.53 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 142.35 (C, Ar-4-C), 141.65 (C, Ar-1’-C), 140.23 (C, Ar-1-C), 135.16 (C, Ar-3-C), 134.26 (C, Ar-1”-C), 131.48 (C, Ar-2’-C), 129.67 (C, Ar-2”-C), 128.36 (C, Ar-2-C), 126.53 (C, Ar-6-C), 124.25 (CH, Ar-5-C), 123.14 (C, Ar-4’-C), 122.67 (C, Ar-4”-C), 121.39 (CH, Ar-3’-C), 120.26 (CH, Ar-3”-C), 118.43 (CH, Ar-5”-C), 116.57 (CH, Ar-5’-C), 115.76 (CH, Ar-6’-C), 113.29 (CH, Ar-6”-C), 62.57 (O-CH3, Ar-4-O-CH3), 14.36 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 604.9160 [M + H]+.
N1,N3-bis(3-bromo-2-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2g)
Orange solid (The residue was recrystallized by acetone.); Yield = 75.3%; Rf: 0.54; m.p.: 261.5–262.4 °C; IR (KBr) υmax 3447.43, 3283.45, 1652.83, 1517.75, 1448.23, 1249.14, 1030.65, 767.56 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 9.57 (s, 2H, 2× -SO2NH-), 8.89 (s, 1H, Ar-2-H), 8.66 (d, J = 6.8 Hz, 1H, Ar-6-H), 8.34 (d, J = 6.8 Hz, 1H, Ar-5-H), 7.89 (d, J = 8.6 Hz, 2H, Ar-4’,4”-H), 7.56 (t, J = 7.8 Hz, 2H, Ar-5’,5”-H), 7.10 (t, J = 6.3 Hz, 2H, Ar-6’,6”-H), 4.04 (s, 3H, Ar-4-OCH3), 1.83 (s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, DMSO-d6): δ = 142.68 (C, Ar-4-C), 141.27 (C, Ar-1’-C), 138.39 (C, Ar-1-C), 137.43 (C, Ar-3-C), 136.34 (C, Ar-1”-C), 135.46 (C, Ar-3’-C), 134.16 (C, Ar-3”-C), 133.27 (C, Ar-2-C), 131.05 (C, Ar-6-C), 129.37 (CH, Ar-5-C), 127.67 (CH, Ar-4’-C), 126.54 (CH, Ar-4”-C), 125.13 (CH, Ar-2’-C), 123.35 (C, Ar-2”-C), 121.06 (C, Ar-5”-C), 118.56 (CH, Ar-5’-C), 116.54 (CH, Ar-6’-C), 114.38 (CH, Ar-6”-C), 58.67 (O-CH3, Ar-4-O-CH3), 14.56 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 604.9160 [M + H]+.
N1,N3-bis(3-fluoro-2-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2h)
Orange solid (The residue was recrystallized by acetone.); Yield = 65.2%; Rf: 0.18; m.p.: 245.5–247.6 °C; IR (KBr) υmax 3358.67, 3067.45, 2984.63, 2223.42, 1658.83, 1264.84, 1145.13, 889.52, 830.85 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 9.32 (s, 2H, 2× -SO2NH-), 8.84 (s, 1H, Ar-2-H), 8.67 (d, J = 5.0 Hz, 1H, Ar-6-H), 8.21 (d, J = 6.8 Hz, 1H, Ar-5-H), 7.79 (d, J = 6.6 Hz, 2H, Ar-4’,4”-H), 7.46 (t, J= 6.8 Hz, 2H, Ar-5’,5”-H), 7.15 (d, J = 5.4 Hz, 2H, Ar-6’,6”-H), 4.06(s, 3H, -OCH3), 1.73(s, 6H, Ar-2’,2”-CH3); 13C-NMR (101 MHz, DMSO-d6): δ = 142.37 (C, Ar-4-C), 141.36 (C, Ar-1’-C), 138.34 (C, Ar-1-C), 137.76 (C, Ar-3-C), 135.29 (C, Ar-1”-C), 133.16 (d, J = 58 Hz, 2C, Ar-3’,3”-C), 131.06 (C, Ar-2-C), 128.46 (C, Ar-6-C), 126.35 (CH, Ar-5-C), 131.26 (CH, Ar-4’-C), 129.37 (CH, Ar-4”-C), 127.34 (CH, Ar-2’-C), 125.67 (C, Ar-2”-C), 123.34 (C, Ar-5”-C), 120.35 (CH, Ar-5’-C), 118.64 (CH, Ar-6’-C), 115.57 (CH, Ar-6”-C), 56.37 (O-CH3, Ar-4-O-CH3), 16.37 (2× CH3, Ar-2’,2”-CH3); MS (m/z): 483.0782 [M + H]+.
N1,N3-bis(4-bromo-3-methylphenyl)-4-methoxybenzene-1,3-disulfonamide (2i)
Orange solid (The residue was recrystallized by acetone.); Yield = 81.2%; Rf: 0.23; m.p.: 263.4–265.6 °C; IR(KBr) υmax 3378.08, 3045.45, 2945.84, 1645.03, 1623.15, 1245.02, 1046.78, 735.43 cm−1; 1H-NMR (400 MHz, CDCl3, TMS): δ = 9.72 (s, 2H, 2× -SO2NH-), 8.85 (s, 1H, Ar-2-H), 8.57 (d, J = 6.0 Hz, 1H, Ar-6-H), 8.26 (d, J = 5.0 Hz, 1H, Ar-5-H), 7.86 (s, 2H, Ar-2’,2”-H), 7.45 (d, J = 8.2 Hz, 2H, Ar-5’, 5”-H), 7.15 (d, J = 7.2 Hz, 2H, Ar-6’, 6”-H), 3.97 (s, 3H, Ar-4-OCH3), 1.83 (s, 6H, Ar-3’, 3”-CH3); 13C-NMR (101 MHz, CDCl3): δ = 146.35 (C, Ar-4-C), 145.35 (C, Ar-1’-C), 144.38 (C, Ar-1-C), 143.21 (C, Ar-3-C), 142.06 (C, Ar-1”-C), 140.21 (d, J = 58 Hz, 2C, Ar-3’, 3”-C), 138.56 (C, Ar-2-C), 137.65 (C, Ar-6-C), 135.29 (CH, Ar-5-C), 131.26 (CH, Ar-4’-C), 129.37 (CH, Ar-4”-C), 127.34 (CH, Ar-2’-C), 125.67 (C, Ar-2”-C), 123.34 (C, Ar-5”-C), 121.35 (CH, Ar-5’-C), 119.64 (CH, Ar-6’-C), 116.57 (CH, Ar-6”-C), 57.23 (O-CH3, Ar-4-O-CH3), 15.38 (2× CH3, Ar-2’,2”-CH3); MS: 604.9160 [M + H]+.
Biological assays
In vitro anti-platelet aggregation assays
In vitro anti-platelet aggregation activities of target compounds were tested and assessed by using the method of Born test (Born 1962). Rabbit plasma used to measure the derivatives of anti-platelet aggregation activities. Fresh venous blood was taken from the ear vein of rabbits (weight: 2–3 kg, male), with 3.8% sodium citrate as anticoagulant (9:1 by volume). Samples were centrifuged at 800 rpm/min for 10 min at room temperature to obtain the platelet-rich plasma (PRP). The PRP supernatant was removed and the residue was centrifuged at 3000 rpm/min for 10 min at room temperature to obtain platelet-poor plasma (PPP), which was used as the blank. Target compounds were previously dissolved in dimethylsulfoxide (DMSO) (1.3 × 10−6 M/L), and the solution (5 µL) was added to PRP (200 µL) and incubated for 2 min. Aggregation was induced by adding 20 µL ADP (5 mM/L), AA (20 µM/L), or Collagen (1 mg/mL). DMSO (0.5% v/v) was used as negative control (according to the pre-experiment, 5 µL DMSO showed no significant effect on the platelet aggregation). Platelet aggregation inhibition rates were calculated by the following formulas and those compounds that exhibited higher inhibitory activities were further diluted to calculate IC50 when the inducer was ADP and AA.
where S = platelet aggregation in the presence of solvent and D = platelet aggregation in the presence of test compounds.
The compounds that had higher anti-platelet aggregation activity at the concentration of 1.3 × 10−6 M/L were diluted to 0.65 × 10−6 and 0.325 × 10−6 M/L. And then the above procedure was repeated to calculated the IC50 value. The calculation formula is as follows:
where Xm = corresponds to l g (maximum dose); I = l g (maximum dose/adjacent dose); P = sum of the positive reaction rates; Pm = maximum positive response rate; Pn = minimum positive response rate.
Picotamide and aspirin were used as standard drugs. Those compounds that had better inhibitory activities were chosen to calculate IC50 when the inducer was ADP and AA. All the results are summarized in Table 1.
In vitro cell toxicity assay
The effect of test compounds on cell toxicity was evaluated on mouse fibroblast cells (L-929). Cells were cultivated at 37 °C in a humidified atmosphere of 5% CO2 on 96-well micro-plates (1 × 104 cell/well) for 24 h. Test compounds were dissolved in DMSO and were used for the treatment of cells. In control group, cells were treated with 0.1% DMSO. And then the cells were exposed to test compounds at the concentrations of 10 and 100 µM/L and incubated at 37 °C for 48 h. After incubation for 48 h, the medium was removed and replaced with 100 µL of fresh complete medium of RPMI-1640. Then CCK-8 solution was added to the 96-well plates at 10 µL per well and incubated for 1 h. The absorbance of the solution was recorded at 450 nm on micro-plate reader (Bio-Tek Flx800 fluorescence micro-plate reader) and the relative cell viability (%) was calculated according to the following formulas and the cell morphology was recorded by fluorescence microscopy (Nikon eclipse Ti). The relative cell viability (%) is set out in Table 2.
Results and Discussion
In Fig. 3, the derivatives 1a–1d and 2a–2d displayed an anti-platelet activity: among these eight derivatives, with AA and Collagen as inducer: in series 1, 1c (43.63%) > 1d (40.02%) > Aspirin (39.43%) > Picotamide (34.89%) > 1a (33.75%) > 1b (30.87%) and 1d (47.22%) > Picotamide (38.45%) > Aspirin (37.08%) > 1c (37.05%) > 1b (25.92%) > 1a (21.94%), respectively; in series 2, 2d (40.54%) > 2c (40.11%) > Aspirin (39.43%) > Picotamide (34.89%) > 2b (34.15%) > 2a (20.68%) and 2d (38.74%) > Picotamide (38.45%) > Aspirin (37.08%) > 2c (32.37%) > 2b (27.86%) > 2a (25.90%), respectively. Based on the activity date, it could be suggested that the derivatives with (2-Br, 4-CH3) 1d and 2d were better among other compounds and superior than both aspirin and picotamide. However, with ADP as an inducer: in series 1, 1d (39.52%) > Aspirin (38.45%) > 1c (37.62%) > Picotamide (36.12%) > 1b (25.61%) > 1a (18.01%); in series 2, 2c (43.67%) > Aspirin(38.45%) > 2d (37.62%) > 2a(36.51%) > Picotamide(36.12%) > 2b (19.18%). The results showed that compounds 1d and 2d have the advantage to improve the activities on anti-platelet aggregation.
In Fig. 4, the compounds 1c–1f and 2c–2f exhibited promising inhibitory activity against both platelet aggregation agonists ADP and AA. In series 1, 1d (39.52%) > Aspirin (38.45%) > 1c (37.62%) > Picotamide (36.12%) > 1e (24.99%) > 1f (21.49%) and 1c (43.63%) > 1d (40.02%) > Aspirin (39.43%) > 1e (36.43%) > Picotamide (34.89%) > 1f (31.63%), respectively; in series 2, 2c (43.67%) > Aspirin (38.45%) > 2d (37.62%) > 2f (36.87%) > Picotamide (36.12%) > 2e (27.59%) and 2d (40.54%) > 2c (40.11%) > Aspirin (39.43%) > Picotamide (34.89%) > 2e (25.93%) > 2f (20.91%), respectively. The derivatives 1d and 2d were the most active compounds and higher than aspirin and picotamide. Moreover, with Collagen as an inducer: in series 1, 1d (47.22%) > Picotamide (38.45%) > Aspirin (37.08%) > 1c (37.05%) > 1e (34.15%) > 1f (32.02%); in series 2, 2e (42.1%) > 2f (39.94%) > 2d (38.74 %) > Picotamide (38.45%) > Aspirin (37.08%) > 2c (32.37%). The results showed that: compound 1d was most active against aggregation induced by Collagen.
In Fig. 5, the compounds 1e–1h and 2e–2h have obvious anti-platelet aggregation activities using corresponding ADP and AA. The order of different disubstituted is: in series 1, 1g (38.45%) = Aspirin (38.45%) > Picotamide (36.12%) > 1h (25.28%) > 1e (24.99%) > 1f (21.49%) and 1g (47.42%) > Aspirin (39.43%) > 1h (37.19%) > 1e (36.43%) > Picotamide (34.89%) > 1f (31.63%), respectively; in series 2, 2h (44.56%) > 2g (38.55%) > Aspirin (38.45%) > 2f (36.87%) > Picotamide (36.12%) > 2e (27.59%) and 2g (49.05%) > Aspirin (39.43%) > Picotamide (34.89%) > 2h (26.09%) > 2e (25.93%) > 2f (20.91%), respectively. The derivatives 1g and 2g were two of the most powerful anti-platelet agents. For collagen-induced platelet aggregation: in series 1, 1f (42.02%) > Picotamide (38.45%) > Aspirin (37.08%) > 1e (34.15%) > 1h (30.47%) > 1g (28.15%); in series 2, 2e (42.21%) > 2f (39.94%) > Picotamide (38.45%) > 2g (37.42%) > Aspirin (37.08%) > 2h (34.61%). The derivatives 1f and 2f were acceptable and higher than other derivatives. This may be attributed to the steric, lipophilic, or position effects of -F and -Br. In order to find out which one of the mentioned effects is the cause of the decrease in the activity, further complementary studies on the new derivatives of indole will be under taken in our laboratory.
As illustrated in Table 1, for ADP-induced platelet aggregation, compounds 1c (IC50: 0.30 µM/L), 1d (IC50: 0.39 µM/L), 2d (IC50: 0.14 µM/L), and 2i (IC50: 0.26 µM/L) have higher activities than positive control drugs picotamide (IC50: 0.53 µM/L) and aspirin (IC50: 0.56 µM/L). And compound 2d, that is N1,N3-bis(3-bromo-4-methylphenyl)-4-methoxybenzene-1,3-disulfonamide exhibited the most activity when the agonist was ADP.
As illustrated in Table 1, for AA-induced platelet aggregation, compounds 1d (IC50: 0.21 µM/L), 1e (IC50: 0.26 µM/L), 1g (IC50: 0.28 µM/L), and 2d (IC50: 0.12 µM/L) have the significant anti-platelet aggregation activities superior to the positive control drugs picotamide (IC50: 0.52 µM/L) and aspirin (IC50: 0.41 µM/L). And compound 2b, namely, N1,N3-bis(3-bromo-4-methyl phenyl)-4-methoxybenzene-1,3-disulfonamide, possessed the most potent inhibition of platelet aggregation induced by AA.
As illustrated in Table 2, the results demonstrated that some compounds exhibited lower cell toxicities against L-929. Herein, compounds 1a–1c, 1e–1i, 2a, 2d, and 2i had higher cell survival rate than picotamide at the dose of 10 μM/L.
Conclusion
Eighteen novel 4-methoxy-1,3-phthalamides (1a–1i) and 4-methoxy-1,3-benzenedisulfonamides (2a–2i) were synthesized and evaluated for anti-platelet aggregation activities and cell toxicity. The results indicated that, compared with picotamide and aspirin, some compounds exhibited moderate anti-platelet aggregation activities and none of these compounds had obvious cell toxicity against L-929. Compound 2d (IC50: 0.12 µmol/L), that is, N1,N3-bis(3-bromo-4-methylphenyl)-4-methoxy benzene-1,3-disulfonamide showed significant activities on anti-platelet aggregation induced by ADP, AA, and Collagen and had lower cell toxicities at the dose of 10 and 100 µM/L. Thus compound 2d has the potential to become a new anti-platelet drug with elevated activity and lower toxicity. This study may provide valuable information for future design and development of electron-donating or electron-withdrawing groups on anti-platelet aggregation activities of the two series.
References
Brandt JT, Payne CD, Wiviott SD, Weerakkody G, Farid NA, Small DS (2007) A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J 153(1):0-2147483647
Benimana O, Zhao L, Kong Y, Li Z, Xie Z (2017) The progress in the research of anti-platelet agents (1995–2017). Future Med Chem 9(10):1087–1110
Bir SC, Maiti TK, Bollam P, Nanda A (2015) Felix platter and a historical perspective of the meningioma. Clin Neurol Neurosurg 134(21):75–78
Born GV (1962) Aggregation of blood platelets by adenosine diphosphate and its reveasal. Nature 194:927–929
Chelucci RC, Dutra LA, Pires ME, Melo TR, Bosquesi PL, Chung MC (2014) Anti-platelet and antithrombotic activities of non-steroidal anti-inflammatory drugs containing an n-acyl hydrazone subunit. Molecules 19(2):2089
Liu XJ, Wang CQ, Wang X, Zhang QX, Liu K (2016) Synthesis and antiplatelet aggregation activities in vitro of N,N-di(3-substitutedphenyl)-4-methoxyl benzene-1,3-disulfonamides. J Pharm Pharm Sci 4:72–76
Lourenço AL, Rrs S, Silva LA, Saito MS, Jfr M, Cabral LM (2017) Synthesis and mechanistic evaluation of novel n’-benzylidene-carbohydrazide-1h-pyrazolo[3,4-b]pyridine derivatives as non-anionic anti-platelet agents. Eur J Med Chem 135:213–229
Liu XJ, Shi XX, Zhong YL, Liu N, Liu K (2012) Design, synthesis and in vitro activities on anti-platelet aggregation of 4-methoxybenzene-1,3-isophthalamides. Bioorg Med Chem Lett 22(21):6591–6595
Liu XJ, Wang CQ, Meng J, Shi XX, Yan YN, Liu XG (2017) Design, synthesis and biological evaluation of 4-methoxy diaryl isophthalates as anti-platelet agents. Med Chem Res 1:1–9
Mirfazli SS, Kobarfard F, Firoozpour L, Asadipour A, Esfahanizadeh M, Tabib K (2014) N-substituted indole carbohydrazide derivatives: synthesis and evaluation of their anti-platelet aggregation activity. Daru 22(1):65
Machlus KR, Thon JN, Italiano JE (2014) Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol 165(2):227–236
Patrono C, Coller B, Dalen JE, Fitzgerald GA, Fuster V, Gent M (2001) Platelet-active drugs. The relationship among dose, effectiveness and side effects. Chest 119(1):39S–63S
Storey RF (2006) Biology and pharmacology of the platelet P2Y12 receptor. Curr Pharm Des 12(10):1255–1259
Wang Y, Wang X, Chen X, Liu XJ (2018) Synthesis and in vitro anti-platelet aggregation activities of 2-methoxy-5-arylamido-N-(pyridin-3-yl-methyl)benzamides. Arch Pharm (Weinheim) 352(1):e1800257
Acknowledgements
The authors are grateful to the National Science Foundation of China (11341014) and the Committee of Science and Technology of Tianjin of China (15JCZDJC33100) for the financial supports and Shenyang Pharmaceutical University of China for running platelet aggregation assays.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, G., Wang, C., Zhang, Z. et al. Synthesis and in vitro activities on anti-platelet aggregation of 4-methoxy-1,3-phthalamidesamides and benzenedisulfonamides. Med Chem Res 28, 1413–1424 (2019). https://doi.org/10.1007/s00044-019-02381-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02381-x