Abstract
Artemisinin and its derivatives have now become essential antimalarial drugs for increasingly widespread drug-resistant malaria strains. Although artemisinin was first used for the treatment of malarial ailments, lots of subsequent studies have demonstrated it possesses other multiple pharmacological functions such as antitumor, antiarrhythmic, anti-fibrosis, as well as the activity against schistosomiasis. A wide array of the molecular mechanisms based on above-mentioned functions of artemisinin and its derivatives have also been explored. Experimental evidences suggest that artemisinin compounds may exert its functions via mechanisms like regulating key factors such as apoptosis-related BAX, FASL and caspase-3, multi-drug resistence genes, cytokines such as CD4+ and CD8+, inflammation-related NF-κB and COX2, telomerase, oxidative stress molecules, and so on. In this article, the proposed mechanisms of action of artemisinins are reviewed with the hope of gaining more insight into the multiple actions of these potent drugs and how they work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
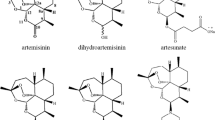
Artemisinin was first isolated from the plant Artemisia annua, sweet wormwood, an herb employed in traditional Chinese medicine. Its structure belongs to the category of sesquiterpene lactone containing an unusual endoperoxide bridge, which is generally thought to be the key player of biological activity. The endoperoxide bond could be activated by reduced heme or ferrous iron [20], leading to the production of cytotoxic carbon-centered radicals, which are highly potent alkylating agents (Meshnick et al. 1991). Radicals may target essential parasite macromolecules that causes parasite’s death. However, the precise mechanism of action and primary target of artemisinin (ART) remain unclear (Olliaro et al. 2001). Up to date, a series of active ART derivatives including dihydroartemisinin(DHA), artemether, arteether, and artesunate(ATS) were synthesized, universally with endoperoxide bridge as the key framework (Zhang et al. 2014). The strategy of ART reduction by potassium borohydride was used to produce DHA. Futhermore, artemether and arteether were made by the catalytic action of BF3-EtO2 on ART. And ATS was easily obtained by using the reduction reaction of acid anhydride on ART, which was dissolved in pyridine (Mei et al. 2008). Both SM934 and SM905 were synthesized on the basis of the structure modification of arteether, SM905 as well. And SM735 was synthesized from artemether (Hou and He 2009) (Fig. 1).
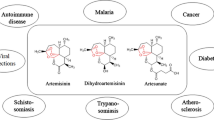
ART was discovered by the Chinese professor Youyou Tu in 1972, who was awarded the 2011 Lasker-DeBakey Clinical Medical Research Award and the 2015 Nobel Prize in Physiology or Medicine, respectively. ART is a colorless, crystalline substance with a molecular weight of 282 Da, a molecular formula of C15H22O5, and a melting point of 156–157 °C. Compared with other antimalarial drugs, ART and its derivatives have the most rapid action on malaria. Currently, ART-combination therapies have become the antimalarial standard treatment all over the world. Because of the effectiveness, low toxicity, and minimal side effects, ART and its derivatives are increasingly being used in Plasmodium vivax malaria. In addition to its certainly well antimalarial properties, compelling evidence had supported that ART-related compounds have potent biological activities in a variety of other human diseases, especially for cancers (Fig. 2).
Antiparasitism effect
Antimalaria
ART is the earliest found having antimalarial effect of all the ART compounds. Thereafter a series of ART derivatives were synthesized, including DHA, artemether, arteether, ATS, and so on. These derivatives all showstrong antimalarial actions to different degrees, even for the drug-resistant malaria. Interestingly, the potent anticancer action of ART can also be attributed to the endoperoxide bond. However, Lack of the endoperoxide moiety does not completely abrogate anticancer activity (Galal et al. 2002). Residual anticancer activity may be related to an alternative peroxide-independent mechanism (Beekman et al. 1998). Recent researches indicated that ART could effectively inhibit heme polymerization mediated by Plasmodium falciparum histidine-rich protein II and Plasmodium yoelii lysates. Furthermore, the interaction between ART and the purified malarial hemozoin could lead to malaria pigment concentration-dependent breakdown in vitro (Pandey et al. 1999).
Physiologically malaria pigment is stored in the food cavity of insect body. Chloroquine has a strong affinity for hemoglobin so that it exerts antimalarial action, but ART take effect via inhibiting the internalization of hemoglobin to block the parasite to use iron and protein (Hoppe et al. 2004). Ponmee et al. (2007) also found that heme and heme-containing proteins in the erythrocyte could notably reduce ART’s effectiveness, and may make for resistance of Plasmodium falciparum infected with α-thalassemic erythrocytes found in vitro. The antimalarial effect of ART could be increased by the extracellular hemin as a result of improving oxidative effect of hemin(Tangnitipong et al. 2012). Gupta et al. (2002) had done a study in the interactions of ART and mefloquine, quinine, and atovaquone respectively in three Plasmodium falciparum strains (K-1, FCR-3, and F-32)and the experimental results showed that the interaction of ART and quinine is most effective against malaria.
In addition, Conyers et al. (2014) tested eight new ART-derived trioxane dimer esters in mice infected with Plasmodium falciparum. With a single oral dose of 6 mg/kg combined with 18 mg/kg of mefloquine, five new ART derivatives had been shown to have a better effect than artemether. It is noteworthy that artecyclopentyl mether (CPM-1, a novel ART derivative) possesses a dose-dependent efficacy in mice infected with Plasmodium yoelii nigeriensis. In another study, CPM-1 had proved to have a potential antimalarial activity against asexual stages of the parasite (Agarwal et al. 2011).
To our knowledge, the death of Plasmodium may be the result of changes in a series of biochemical and physiological functions. Although lots of studies regarding various mechanisms of ART and/or ART derivertives have been conducted, their antimalarial mechanisms have not yet been elucidated completely.
Activity against pneumocystis carinii pneumonia
Li et al. (2001) tested the effect of DHA on apoptosis of alveolar macrophages (AMs) in pneumocystis carinii pneumonia (PCP)—infected mice, and the result showed that DHA therapy could decrease the apoptosis of AMs in PCP rats. It also showed that DHA therapy decreases the apoptosis in spleen cells of PCP rats (Li et al. 2003). AMs of PCP rats produce a high level of TNF-α, but the level of TNF-α is decreased after DHA therapy (Li et al. 2001). DHA could also alleviate inflammatory responses and kill Pc trophozoites and cysts in the lung tissues in PCP infected mice (Li et al. 2004). Previously, it had been reported that DHA and sodium ATS have therapeutic effects in treating Pneumocystis carinii pneumonia (Ye et al. 2001).
Anti-schistosoma
Tu et al. (2005) explored the therapeutic effect of ATS and artemether in Schistosoma mansoni—infected adult mice. The data showed that the efficacy of therapy with artemether and ATS on the mice were excellent and human Hgb could be degraded by the hemoglobinase of S.japoncium. Gong et al. (1997) reported that artemether had an inhibitory effect on hemoglobinase of S.japonicum. Moreover, it was also demonstrated that artemether had remarkably schistosomicidal effects on juvenile stages of S. mansoni Egyptian strain in vivo, thus preventing disease progression and morbidity (El-Beshbishi et al. 2013). In line with previous reports, recent studies have shown that artemether could be expected to protect the host from schistosomal infection or reduce the intensity of infection by using artemether in the early stage after infection (Xiao et al. 1994). Liu (2001) studied the therapeutic effect of DHA on experimental rats infected with Pneumocystis carinii. As a result, the survival rate increased and lung inflammation was alleviated. Thus it was concluded that DHA is one of the useful drugs to treat Pneumocystis carinii pneumonia. Xiao et al. (1995) also proved that artemether possesses an effect for prevention of schistosomiasis by reducing the infection rate and intensity of infection, as well as controlling acute schistosomiasis. Consistent with DHA, ATS also has significant anti-schistosoma action, it can kill schistosomula and reduce the fecundity of females effectively (Lu et al. 2004). No severe side effect was seen in animals treated with ATS (Le et al. 1983). As expected, arteether has a dramatic therapeutic effect on NIH mice infected with Schistosoma japonicum cercariae (Xiao et al. 1992). There is now a general consensus that ART-based combination therapies have beneficial actions against schistosomiasis.
Anti-leishmania
Want et al. (2015) tested ART-loaded poly lactic co-glycolic acid (ALPLGA) nanoparticles on mice with established visceral leishmaniasis infection. After in vitro treatment, ALPLGA nanoparticle can escalates lgG2a levels, increases lymphoproliferation, as well as enhances proinflammatory cytokines (IL-2 and IFN-γ) with effective inhibition of Th2 cytokines (IL-4v and IL-10) compared with ART treatment. Ghaffarifar et al. (2014) had also tested ART on leishmania both in vitro and in BALB/c mice. All of the results suggested that ART exerts an anti-leishmania effect in vivo and in vitro via apoptosis-related mechanism.
Anti-toxoplasma
Schultz et al. (2001) tested two thiazole derivatives of ART in Toxoplasma gondii (T. gondii) infected mice. Both derivatives showed moderate efficacy in a mouse model of acute toxoplasmosis, and one derivative CPH4-136 modestly but significantly decreased mouse brain cyst burden in a chronic T. gondii infected mouse model. These results suggest that ART derivatives have some action in treating Toxoplasma gondii. El Zawawy (2008) also tested ATS on T. gondii in vitro and in vivo. In vivo tests revealed that infected mice treated with ATS induced a remarkable reduction in the mortality rate and increased the survival time. Moreover, in vitro tests demonstrated a remarkable decrease in infectivity and viability of tachyzoites exposed to ATS compared with untreated controls. It is thus concluded that ATS would be a novel anti-toxoplasma drug in the future.
Activity against Clonorchis sinensis (C. sinensis)
Xiao et al. (2008) proved that artemether, artesunate, tribendimidine and praziquantel are active against C. sinensis, and these drugs combination possesses synergy. In addition, ATS, tribendimidine and praziquantel are also effective against Clonorchis sinensis, but ATS is only efficacious against adult worms (Xue et al. 2009). Previously, it had been reported that both artemether and tribendimidine rapidly destroy the tegument and the suckers of adult C. sinensis. However, the activity mechanisms of these drugs against C. sinensis have not to be fully defined (Xiao et al. 2009).
Activity against Trichomonas vaginalis
Tang et al. (2007) tested the efficacy of DHA on the ultrastructure of Trichomonas vaginalis trophozoites in vitro, and found DHA could result in decomposition and necrosis of the parasite via destroying membrane structure and organelles of Trichomonas vaginalis trophozoites.
Activity against Acanthamoeba spp
ATS can inhibit the growth of acanthamoeba trophozoites at a rate of 93.2% when treated with 100 μg/ml (after 2 days), and application at 500–700 μg/ml concentration can lead to an inhibition on the first day. These data showed that ATS was amebastatic rather than amebicidal in an axenic culture with trophozoites at the highest concentration of 100 μg/ml (Nacapunchai et al. 2002).
Anti-eimeria
Pop et al. (2015) used ART on Eimeria acervulina, E. tenella, and E. maxima in battery trials infected broiler chickens. The experimental results suggest that ART has little effect in single eimerian infections but could be used as a replacement for mixed coccidiosis.
Activity against gastrointestinal nematodes
Haemonchus contortus (H. contortus) is the leading pathogenic nematodes, a kind of parasites in abomasum, accidentally found in small intestine. This disease can result in digestive disorders, gastrointestinal tract inflammation, diarrhea, and angular. Serious cases will occur in mandibular interstitial edema. Temperature increases in a few cases, and breathing, pulse frequency and heart sounds are subdued, which eventually can lead to death due to the body extreme failure. Cala et al. (2014) studied the anthelmintic activity of A. annua crude extracts in vitro and compared the most valid extract with ART in H. contortus infected sheep. The data showed ART and A. annua extract had certain therapeutic effect on H. contortus. However, further studies are needed to determine the interactions of ART with commercial anthelmintics in infected animals.
Activity against giardia lamblia
To directly study the effect of DHA on Giardia lambia (G. lamblia) in vitro, Tian et al. had cultivated the trophozoites of G. lamblia with modified TYI-S-33 medium that contains DHA. The results conclusively indicated that DHA possesses a strong impairment on the plasma membrane and cytoskeleton of G. lamblia (Tian et al. 2005).
Anti-heterophyid
Fathy (2011) has tested the therapeutic effect of ATS in heterophyid infected mice. The results indicated that ATS has a notably action in treating experimental heterophyidiasis. Furthermore, surface tegumental damages of the adult worm was found in the form of bleb formation, disruption, erosion, and peeling. This drug would be a novel alternative therapy to treat human heterophyidiasis.
Antitumor
Although ART was first used against malaria, a wide range of research was later conducted indicating that ART and its derivatives have anticancer properties as well. Most of these agents exert their anticancer function via mechanisms like apoptosis, arrest of cell cycle at G0/G1, and so on. (Fig. 3 and Table 1)
Induce apoptosis of tumor cells
As an active death process involved in the specified procedures, apoptosis occurs after cells are induced by some kinds of signal molecules, characterized by a series of distinct genetic and biochemical and morphological changes. Induction of apoptosis currently becomes one of the most important strategy to treat cancer. Sheng et al. (2009) observed the action of ATS on tumor in murine transplanted hepatocarcinoma 22 (H22). As a result, daily intraperitoneal injection of ATS (15–60 mg/kg/day) caused a significant decrease of FasL expression in tumors. ATS can also enhance the radiosensitivity of HeLa cells. And its mechanism may be concerned with upregulation of cyclin B1 expression and downregulation of Weel expression (Gong et al. 2009). Zeng et al. (2009) examined the efficacy of ATS on the proliferation of Jurkat cells, Raji cells and acute lymphoblastic leukemia (ALL) primary cells. These were found that apoptosis occurred in Jurkat cells and Raji cells after exposure to ATS, and mitochondria transmembrane potential collapses and caspase-3 activities were increased in Jurkat, Raji, and ALL primary cells. In addition, ATS could induce apoptosis and suppress proliferation in K562 cells by activating caspase-3 and delivery of cyt-c (Xie et al. 2008; Lee et al. 2012) . Furthermore, Huang et al. (2012) treated HCT-8 human colon cancer cells with ATS at 10 ~ 30 μmol/L for 10 h, and found 41.4% of the cancer cells showed apoptosis when the concentration of ATS reached 30 μmol/L. Furthermore, ATS could inhibit the growth of HCT-8 cells and induce apoptosis of HCT-8 cells via regulating the expression of Bax genes. Dong et al. (2002) also demonstrated that ART can suppress the proliferation of HeLa cells and induce its apoptosis. It was also found that ATS may inhibit the growth of human liver cancer cell line (Bel-7402) via inducting cell apoptosis (Zhang et al. 1998) (daily oral administration of 100–324 mg/kg). The cytotoxic activity of artemether is correlated with its apoptosis induction (Guo et al. 2007). Fox et al. (2016) examined the ART-derived trioxane diphenylphosphate dimer 838 (ART-838) on 23 acute leukemia cell line. As a result, the cell proliferation and clonogenicity were decreased but the intracellular levels of reactive oxygen species were increased. Recently, Ooko et al. (2015) found that ART and its derivatives can induce iron-dependent apoptosis in cancer cells. This new form of apoptosis is called ferroptosis. ART’s powerful anticancer effect correlated with Fe2+-mediated cleavage of the endoperoxide bridge. Therefore, Chen et al. (2015) had also tried to replace Fe2+ with Mn2+ to react with ART, and the results suggest this way can produce better antitumor efficiency but more toxic products.
Prevent cancer invasion and metastasis
The basic characteristics of a malignant tumor include invasion and metastasis, which is a main cause of tumor treatment failure. It has been noted that about 30% of the patients with tumor at the time of diagnosis is metastasis stage. Therefore, how to find a drug that has an effective inhibition on invasion and metastasis of cancer is of great significance. Michaelsen et al. (2015) had also examined short-term bicalitumide combined with long-term treatment with A. annua capsules therapy that lead to important regression of advanced metastasized prostate cancer.
Inhibit tumor angiogenesis
Primary tumors have the capability of inducing new angiogenesis and metastasis process. Tumor angiogenesis process is controlled by many positive and negative factors and inhibition of tumor angiogenesis has become a novel antitumor treatment strategy. Wang et al. (2012) proved that DHA can induce the apoptosis of prostate cancer cell line PC-3M and the expression of VEGF mRNA and protein is decreased in a concentration-dependent way. DHA’s inhibitory effects on tumor angiogenesis were further confirmed by the experimental results that the growth of PC-3M cells was suppressed, together with the decreased expression of VEGF mRNA and protein. Wu et al. (2009) explored the antitumor effect of artemether in the growth of glioma and angiogenesis in brain cancer SD rats. The results showed artemether can remarkably inhibits the growth of brain glioma by penetrating the blood-brain barrier and inhibiting angiogenesis. Mondal and Chatterji (2015) studied the antitumor action of ART in HPV-39-infected human cervical cancer Me-180 cells, and the result indicated that ART can notably inhibit the expression of VEGF and ERα. In addition, ART can reduce the expression of the HPV-39 viral E6 and E7 components. All in all, to achieve an inhibitory effect on tumors via controlling angiogenesis becomes an important aspect of actions for artimisinin derivatives.
Regulate the body’s immune function
Killing tumor cells by regulating the body’s immune function has become one of the essential mechanisms of the therapy for cancer. ART was found to inhibit the growth of orthotopic tumor, which was related to a decrease in the percentage of immune T cells and the level of PGE2 in mice with cervical carcinoma. In addition, ART can decrease the expression of COX-2 and production of PEG2 in HeLa and CaSki cells. Thus, ART will be a potential drug for immunotherapy of cervical carcinoma (Zhang et al. 2014). Cui et al. (2006) tested ATS on mouse colon-rectal carcinoma cell line colon 26. The results showed that the ATS can suppress the colon 26 cells growth. In the supernatant fluid, ATS increased the expression of CD4+ T cells and reduced the expression of CD8+ T cells. It is thus concluded that ATS can inhibit colon 26 cells by regulating the cellular immune function. Additionally, recent researches showed that the concomitant immunosuppression cannot decrease the cytostatic and apoptotic effects of ATS (Ramacher et al. 2009). Wang et al. (2010) pointed out that gemcitabine-induced growth inhibition and apoptosis in BxPC-3 and PANC-1 cell lines is increased by DHA in vitro. It’s also demonstrated that gemcitabine can dramatically increase the anticancer effect of DHA, as manifested by remarkably increased apoptosis, as well as decreased NF-κB activity, Ki-67 index and its related gene products, accompanied by decreased cancer volume.
Inhibit telomerase activity
Telomerase, composed of RNA and protein body, belongs to a specific reverse transcriptase relying on its own RNA as a template, to make up the loss of telomere DNA due to cell division, making the cells immortalized. Tumor tissue can proliferate infinitely bacause of the increased telomerase. Thus inhibition of telomerase activity has been one of the elementary ways to treat tumor. Ai (2007) found that artesiminin can suppress the human HeLa cells growth, and reduce the telomerase activity. In Me-180 cervical cancer cells, the antiproliferative activity of ART was further confirmed by decreasing telomerase activity and reducing expression of hTERT and hTR subunits, and inducing apoptosis by nuclear chromatin condensation, FACS, and annexin V staining (Mondal and Chatterji 2015).
Block cell cycles
ART and its derivatives can block the cell cycle to exert anti-cancer action. ATS can inhibit the proliferation of tumor via making the cancer cells blocked at G0-G1 stage and reducing the CDC25A expression in esophageal cancer cells (Liu et al. 2008). Chen et al. (2005) treated rats with DHA at 100 mg/kg/day for 4 weeks and found the tumor volume in the treated rats on day 28 was about 54.3% of that of the controls. These data showed that DHA has dramatically antitumor activity on A549 human lung adenocarcinoma cell both in vitro and in vivo, and the inhibition in vitro may be associated to blockade of G0-G1 phases. Wang et al. (2007a) also showed that ATS can inhibit the mRNA, as well as protein expression of CDC25A in the Eca109 human esophageal carcinoma cells, and most of Eca 109 cells were arrested at the G2/M phase. Reichert et al. (2012) examined the radiation sensitizing properties of ATS on LN229 and U87MG cells. Glioma cells treated with ATS and irradiation led to an increased apoptosis rate, pronounced G2/M arrest and raised DNA damage as demonstrated by an elevated amount of γ-H2AX foci/nucleus. Incubation with ATS lowered survivin expression in a time and dose-dependent manner, but the expressions of XIAP, CIAP1, and CIAP2 were not affected. Li et al. (2009) also proved that ATS can increase the proportion of SP2/0 myeloma cells in G0/G1 phase and reduce the proportion of cells in S or G2/M phase.
Anti-inflammation and immunoregulation
Immunosuppressive activity
Zhou et al. (2005) investigated the inhibitory effect of SM 735 in the proliferation of splenocytes induced by ConA, MLR or LPS. The results showed SM 735 has a remarkable immunosuppressive action on both T-cell-mediated delayed-type hypersensitivity and B-cell-mediated QHS reactions in a dose-dependent manner. Combined with the previous in vitro experimental results, it is reasonable to think of SM 735 as a potential immunosuppressive agent. Aside from SM 735, it has been noted that SM 905 also suppressed T cell activation by inhibiting MAP kinases and Ras activation (Wang et al. 2007c). Recent researches also showed SM 905 inhibited NO and pro-inflammatory cytokine production in LPS-stimulated RAW 264.7 cells and the mechanism may relate to its inhibition on the MAPK and NF-κB signaling pathways (Wang et al. 2009). Consistent with SM 735 and SM 905, SM 934 also exerted significant immunosuppressive actions both in vitro and in vivo (Wang et al. 2007b). Artemether was also found to possess immunosuppressive action on T cells both in vivo and in vitro by lowering the activation of the Ras-Raf1-ERK1/2 protein kinase cascade in T cells (Wang et al. 2007b). In 2010, Ye et al. (2010) also reported DHA has an immunosuppressive effect on the proliferation of murine T-lymphocytes.
Rheumatoid arthritis (RA)
RA is a kind of chronic systemic inflammatory disease with unknown etiology that can lead to joint deformity and loss of function. In 2007, Smith et al. (2007) reported that ATS exerts a therapeutic effect for RA via inhibiting the inflammatory responses in RA fibroblast-like synoviocytes. In 2009, another study (He et al. 2011) found that ATS can down-regulate angiogenic factor expression in RA fibroblast-like synoviocytes. In addition, Wang et al. (2008) also found ATS has anti-inflammatory effect in collagen-induced arthritis rats by inhibiting inflammatory factors. As with ATS, a novel aretmisinin derivative SM905 is also capable of decreasing the inflammatory and pathogenic Th17 responses in collagen-induced arthritisin DBA/1 mice (Wang et al. 2008). In order to ascertain the anti-inflammatory mechanism of ART and its derivatives, Li et al. (2010) compared the effects of different concentrations of ATS and methotrexate on the apoptosis and proliferation of fibroblast-like synoviocytes (FLS). The results indicated that the anti-inflammatory mechanism is inhibiting FLS proliferation and advancing FLS apoptosis.
Systemic lupus erythematosus (SLE)
ART and its derivatives have a significant therapeutic effect in SLE in addition to their roles in treating malaria and cancer. SLE is a systemic autoimmune disease that may affect many internal tissues and/or organs such as heart, lungs, joints, skin, blood vessels, liver, kidneys, and nervous system in the body. Wu et al. (2015) showed SM 934 (a chemosynthetic ART derivative) is able to significantly prolong the life-span of MRL/lpr rats, improve the lymph adenopathy symptoms and reduce the levels of serum anti-nuclear antibodies as well as the pathogenic cytokines IL-6, IL-10, and IL-21. Additionally, SM 934 treatment can restore the B cell compartment in the spleen of MRL/lpr mice. It was also found that SM 934 has inhibitory effects on plasma cell formation and B-cell activation in MRL/lpr mice. Huang et al. (2014) also demonstrated that DHA can suppress LPS-induced cell activation by blocking the TLR4/IRF/IFN pathway in spleen cells of MRL/lpr mice. Moreover, Hou et al. (2011) showed that ART analog SM 934 can remarkably prolong the life-span and relieve the symptoms of glomerulonephritis in the lupus-prone female MRL/lpr mice by down-regulating both Th1 cell and Th17 cell responses. Ouyang et al. (2009) also examined the immunomodulatory property of ATS in systemic lupus erythematosus by using a MRL/lpr murine model. The results indicated that ATS can reduce monocyte chemotactic protein-1(MCP-1), a major pro-inflammationary cytokine, in serum, urine and kidney. Moreover, the expression of B cell activating factor was reduced in MRL/lpr mice treated with ATS. It was thought that the therapeutic effect of ATS on MRL/lpr nephritis mice is related to its inhibition on ICAM-1 expression (Wang et al. 2010). ART has an anti-inflammatory effect in lupus nephritis mice by regulating the expression of the mRNA of GR alpha and GR beta in peripheral blood mononuclear cells and the transcriptional coactivator P300/CBP protein in renal tissue (Wu et al. 2012). Wu et al. (2010) found that ART can remarkably reduce the serum levels of TNF-α and IL-6 and reduce the expression of the NF-κB p65 protein, and NF-κB and TGF-β1 mRNA in renal tissue.
Treating neuroinflammation
Neuroinflammation in the central nervous system is often generated after the prompt response of the CNS by activating resident immune cells (especially microglia cells) to infection, trauma and stroke, and many other stimuli. It is noteworthy that uncontrolled neuroinflammation may lead to various neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis and multiple sclerosis. Professor Okorji (2015) investigated the effects of ART and its derivatives in LPS-activated BV2 microglia. The results indicated that ART, artemether and ATS could suppress neuroinflammation in LPS-activated BV2 microglia via interfering with NF-κB and p38MAPK signaling.
Antiviral activity
Currently, the treatment to HHV-6 infection still remains unsatisfactory so new effective drugs are urgently needed. Romero et al. (2005) proved that treatment of HHV-6-infected cells with ATS notably decreased viral protein synthesis at both early and late stages without apparent necrotic cytotoxicity or drug-induced apoptosis, and HHV-6A genome replication was remarkably reduced as well. Furthermore, ATS also possesses unique activity against human cytomegalovirus (Chou et al. 2011). Compared with ART monomers, ART-derived dimers had a better anti-cytomegalovirus activity (Arav-Boger et al. 2010). It was previously reported that several natural products including ATS derived from medicinal herbs used in traditional Chinese medicine possess anti-HBV effect. It was also found that HBV DNA release was suppressed when treated with ATS with an IC50 of 0.5 mM and a synergistic action was generated when used together with lamivudine (Romero et al. 2005). Previous studies had indicated that ART and its analogs may possibly have anti-HCV effect by the induction of reactive oxygen species (Obeid et al. 2013). In addition, hemin can potentiate anti-HCV activity of ART (Paeshuyse et al. 2006). Further, 12-n-butyldeoxoART, an ART-related trioxane, has been shown to have a great antiviral activity against HIV-1 in a recent study (Jung and Schinazi 1994). In general, ART and its derivatives can inhibit a variety of viruses, such as human cytomegalovirus, hepatitis B virus, hepatitis C virus, and HIV-1. Further study of the complete profile of the pharmacological activities and molecular modes of action of ART and its derivatives as well as their performance in clinical trials will helpful to reveal the full antiviral potential of these versatile pharmacological tools.
Treating sepsis
Sepsis is a systematic inflammatory response syndrome with high mortality and no specifically effective drug on the market today. Endotoxin is one of main pathogenic molecules that induce sepsis. It was early reported that ART drugs antagonized the inflammation induced by endotoxin. Zhang et al. (2005) studied the effects of ATS on free calcium ion concentration in peritoneal macrophages of endotoxemia mouse induced by hyperthermia and found that ATS can remarkably decrease the level of intracellular free calcium. A study conducted by Liang et al. (2002) proved that ATS can dramatically inhibit endotoxin-induced production of inflammatory mediators from macrophages both in vivo and in vitro, indicating that ATS can inhibit the inflammatory responses caused by endotoxin. Li et al. (2008) also tested the effect of ATS in sepsis mouse model and the result showed that ATS can protect rats against a lethal challenge with heat-killed E. Coli in a dose-dependent manner by decreasing the expression of NF-κB and TLR4, TLR9 mRNA. Cao et al. (2007) observed the therapeutic action of ART on lung injury in septic mice, and demonstrated that ART might reduce the concentration of LPS, and suppress the expression and the releasing of proinflammatory cytokines in lung tissues, alleviating the pathological lung injury in septic mice as a result.
Anti-fibrosis
Fibrosis which occurs in a variety of organs is a serious threat to human health and life. Xu et al. (2009) reported that ATS could decrease the expression of TGF-β1 and TNF-α in pulmonary fibrosis mice, significantly reducing the degree of pulmonary fibrosis similar to the therapeutic effect of methylprednisolone. Lai et al. (2015) found that ART can relieve hepatic fibrosis induced by various pathogenic factors and inflammation via inhibiting LPS/TLR4/NF-κB signaling pathway in mice. Li et al. (2009) proved the inhibition effect of ATS on mastocyte in a rabbit ear hypertrophic scar model. The results showed ATS can suppress fibroblast proliferation by reducing mastocyte and collagen synthesis. In addition, ATS also has a notably inhibitory activity on the liver fibrosis. A recent study showed it can suppress the activated proliferation of hepatic stellate cells by increasing the expression of apoptosis genes, leading to the rebalance of apoptosis and proliferation and finally the hepatic fibrosis is reduced (Yang and Liu 2009).
Antiarrhythmia
ART was shown to has an obvious inhibitory action on arrhythmia in rat and guinea pig models induced by aconitine and uabain. Studies have proven that aconitine-induced arrhythmia in rats is associated to its promotion to INa, ICa and prolongation of action potential duration, while uabain-induced arrhythmia in guinea pigs mainly involves the regulation on ICa, IKr and shortening of the action potential duration. These results suggest ART may have the potential to be further developed as a anti-arrhythmic agent in the future (Du and Liu 2003).
Antibacterial action
The anti-bacterial effect of ART had been studied in a wide range of bacteria, such as Escherichia coli (Sack 1975), Pseudomonas aeruginosa, Staphylococcus aureus, and Mycobacterium intracellulare (Slade et al. 2009). Combination-therapy research was also performed. It was found that ATS could increase the antibacterial effect of various β-lactam antibiotics against E. coli, which might be related to the inhibition of a major multidrug efflux pump system, AcrAB-TolC (Li et al. 2011). Interestingly, Appalasamy et al. (2014) demonstrated that ART and a precursor had an inhibitory effect on the growth of Gram-positive and Gram-negative bacteria but not Candida albicans. Their antibacterial activity was similar to that of antibiotic streptomycin.
Concluding remarks
More than 40 years has passed since artermisinin’ discovery by professor Tu Youyou in China in 1972. Artermisinin currently saves millions of patients suffering from malignant malaria worldwide annually. Cancer is another life-threatening disease and current cancer therapeutic strategies have been reported to cause many serious side effects in patients. Tumor heterogeneity further devaluates the effectiveness of a given therapeutic strategy. There is, thus, a strong demand for therapeutic agents that are efficacious against a specific cancer type and yet have a minimal to no side effects. Besides the therapeutic effect against malaria, cancer therapy has been proved to be the most potentially clinically applied action of ART and its derivatives among all their biological functions. ART is an already approved antimalarial drug in humans and therefore uncovering its anticancer properties and underlying mechanisms will be critical to determine which cancer phenotype can best be treated with this phytochemical ingredient or its derivatives. This is the precondition for the realization of accurate treatment of ART compounds to malignant tumors.
Abbreviations
- ART (page 1, line 1):
-
artemisinin
- DHA (page 2, line 10):
-
dihydroartemisinin
- ATS (page 2, line 11):
-
artesunate
- NF-Κb (page 1, line 11):
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- COX-2 (page 1, line 11):
-
cyclooxygenase-2
- SM934 (page 2, line 9):
-
2-aminoarteether (β)maleate
- SM905 (page 2, line 9):
-
1-(12-β-dihydroartemisinoxy) -2-hydroxy-3-tert-butylaminopropane maleate
- SM735 (page 2, line 10):
-
3-(12-β-artemisininoxy) phenoxyl succinic acid
- Da (page 3, line 4):
-
Dalton
- TNF-α9 (page 4, line 36):
-
tumor necrosis factor α
- Hgb (page 5, line 7):
-
hemoglobin
- G0/G1 (page 8, line 1):
-
resting phase or Gap 1 phase(cell cycle)
- VEGF (page 13, line 5):
-
vascular endothelial growth factor
- Erα (page 13, line 15):
-
estrogen receptor α
- PGE2 (page 13, line 22):
-
prostaglandin E2
- COX-2 (page 13, line 23):
-
cyclooxygenase-2
- CDC25A (page 14, line 18):
-
cell division cycle 25 homolog A gene
- ConA (page 15, line 6):
-
concanavalin A
- LPS (page 15, line 6):
-
lipopolysaccharides
- TLR4/IRF/IFN (page 16, line 21):
-
Toll-like receptor 4/Interferon regulatory factors/ Interferons
- TGF-β1 (page 17, line 8):
-
transforming growth factor beta 1
- CNS (page 17, line 10):
-
central nervous system
- HHV-6 (page 17, line 18):
-
human herpes virus 6
- HBV (page 17, line 27):
-
hepatitis B virus
- HCV (page 18, line 3):
-
hepatitis C virus
- HIV-1 (page 18, line 7):
-
human immunodeficiency virus-1
- TLR9 (page 18, line 26):
-
Toll-like receptor 9
- INa(page 19, line 14):
-
the “rapid” delayed rectifier current that conducts sodium(Na+) ions out of the muscle cells of the heart
- ICa (page 19, line 14):
-
the “rapid” delayed rectifier current that conducts calcium (Ca+) ions out of the muscle cells of the heart
- IKr (page 19, line 16):
-
the “rapid” delayed rectifier current that conducts potassium (K+) ions out of the muscle cells of the heart
References
Agarwal J, Singh SP, Chanda D, Bawankule DU, Bhakuni RS, Pal A (2011) Antiplasmodial activity of artecyclopentyl mether a new artemisinin derivative and its effect on pathogenesis in plasmodium yoelii nigeriensis infected mice. Parasitol Res 109(4):1003–1008
Ai J (2007) Inhibiting effect of artemisinin on hela cell growth. J Shanxi Med Univ 3:5–9
Appalasamy S, Lo KY, Ch’Ng SJ, Nornadia K, Othman AS, Chan LK (2014) Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of artemisia annua l. Biomed Res Int 2014(31):226–235
Arav-Boger R, He R, Chiou CJ, Liu J, Woodard L, Rosenthal A (2010) Artemisinin-derived dimers have greatly improved anti-cytomegalovirus activity compared to artemisinin monomers. Plos One 5(4):e10370
Beekman AC, Wierenga PK, Woerdenbag HJ (1998) Artemisinin-derived sesquiterpene lactones as potential antitumour compounds: cytotoxic action against bone marrow and tumour cells. Planta Med 64(7):615–619
Cala AC, Ferreira JF, Chagas AC, Gonzalez JM, Rodrigues RA, Foglio MA (2014) Anthelmintic activity of artemisia annua l. extracts in vitro and the effect of an aqueous extract and artemisinin in sheep naturally infected with gastrointestinal nematodes. Parasitol Res 113(6):2345–2353
Cao H, Guo Y, Wei L, Wang N, Hai Q, Zhou H (2007) Effect of artemisinin on lung injury in septic rats. A Acad Med Mil Tert 29(10):951–954
Chen J, Zhang W, Zhang M, Guo Z, Wang H, He M (2015) Mn(ii) mediated degradation of artemisinin based on fe3o4@mnsio3-fa nanospheres for cancer therapy in vivo. Nanoscale 7(29):12542–12551
Chen W, Qi H, Wu C, Cui Y, Liu B, Yan L (2005) Effect of dihydroartemisinin on proliferation of human lung adenocarcinoma cell line a549. Chin J Lung Cancer 8(2):85–88
Chou S, Marousek G, Auerochs S, Stamminger T, Milbradt J, Marschall M (2011) The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir Res 92(2):364–368
Conyers RC, Mazzone JR, Tripathi AK, Sullivan DJ, Posner GH (2014) Antimalarial chemotherapy: orally curative artemisinin-derived trioxane dimer esters. Bioorg Med Chem Lett 25(2):245–248
Cui C, Wang R, Tong H, Wang Z, Ding J, Zhang Z (2006) Influence of artesunate and oxymatrine on the immunosuppressive effect of mouse colon-rectal carcinoma cell line colon 26. Curr Immuno 26(2):152–156
Deng X, Yu H, Wang K, Li X (2007) Inhibitory effect of artemisinin on hepatoma h22 cells. Prac Clin Med 11:3–8
Dong H, Song W, Sun J (2002) Effect of artemisinin on the growth of hela cells. J Harbin Med Univ 36(6):423–424. 427
Du Z, Liu Y (2003) The anti-arrhythmic action research of artemisinin. J Chin Pharm 38(5):372
El-Beshbishi SN, Taman A, El-Malky M, Azab MS, El-Hawary AK, El-Tantawy DA (2013) In vivo, effect of single oral dose of artemether against early juvenile stages of schistosoma mansoni, egyptian strain. Exp Parasitol 135(2):240–245
Fathy FM (2011) Anthelmintic effect of artesunate in experimental heterophyid infection. J Egypt Soc Parasitol 41(2):469–483
Fox JM, Moynihan JR, Mott BT, Mazzone JR, Anders NM, Brown PA (2016) Artemisinin-derived dimer art-838 potently inhibited human acute leukemias, persistedin vivo, and synergized with antileukemic drugs. Oncotarget 7(6):7268–7279
Galal AM, Ross SA, ElSohly MA, ElSohly HN, El-Feraly FS, Ahmed MS, McPhail AT (2002) Deoxyar-temisinin derivatives from photo oxygenation of anhydrodeo-xydihydroartemisinin and their cytotoxic evaluation. J Nat Prod 65(2):184–188
Ghaffarifar F, Esavand HF, Dalimi A, Hassan ZM, Delavari M, Mikaeiloo H (2014) Evaluation of apoptotic and antileishmanial activities of artemisinin on promastigotes and balb/c mice infected with leishmania major. Iran J Parasitol 10(2):258–267
Gong P, Li Y, Feng Y, Ruppel A, Caffrey C (1997) Inhibitory effect of artemether on proteinase of schistosoma japonicum. J Chin Pharm 18(3):198–201
Gong X, Cao J, Fan S, Shen F, Lu H, Zhu W (2009) Study on the radiosensitizing effect of artemisinin on human cervical cancer and corresponding mechanisms. Tumor 29(10):950–954
Guo Y, Wang J, Zhang B, Chen Z (2007) Effect of artemether on the poliferation of human lung adenocarcinoma cell line a549. Postgrad Med J 3:2–9
Gupta S, Thapar MM, Wernsdorfer WH, Bjorkman A (2002) In vitro interactions of artemisinin with atovaquone, quinine, and mefloquine against plasmodium falciparum. Antimicrob Agents Chemother 46(5):1510–1515
He Y, Fan J, Lin H, Yang X, Ye Y, Liang L (2011) The anti-malaria agent artesunate inhibits expression of vascular endothelial growth factor and hypoxia-inducible factor-1α in human rheumatoid arthritis fibroblast-like synoviocyte. Rheumatol Int 31(1):53–60
Hoppe HC, Ui VSD, Meredith SA, Egan J, Weber BW (2004) Antimalarial quinolines and artemisinin inhibit endocytosis in plasmodium falciparum. Antimicrob Agents Chemother 48(7):2370–2378
Hou L, He S (2009) Sm934, a water-soluble derivative of arteminisin, exerts immunosuppressive functions in vitro and in vivo. Int Immunopharmacol 9(13):1509–1517
Hou L, He S, Li X, Yang Y, He P, Zhou Y (2011) Oral administration of artemisinin analog sm934 ameliorates lupus syndromes in mrl/ lpr, mice by inhibiting th1 and th17 cell responses. Arthritis Rheumatol 63(8):2445–2455
Huang W, Liu N, Niu H (2012) Effect of artesunate on cell apoptosis and cell cycle of human colon cancer cell hct-8. Chin J Expt Tradit Med For 11:225–228
Huang X, Xie Z, Liu F, Han C, Zhang D, Wang D (2014) Dihydroartemisinin inhibits activation of the toll-like receptor 4 signaling pathway and production of type i interferon in spleen cells from lupus-prone mrl/lpr mice. Int Immunopharmacol 22(1):266–272
Jung M, Schinazi RF (1994) Synthesis and in vitro, anti-human immunodeficiency virus activity of artemisinin (qinghaosu)-related trioxanes. Bioorg Med Chem Lett 4(7):931–934
Karpelmassler G, Westhoff MA, Kast RE, Dwucet A, Nonnenmacher L, Wirtz C (2014) Artesunate enhances the antiproliferative effect of temozolomide on u87mg and a172 glioblastoma cell lines. Anti Cancer Agent Me 14(2):313–318
Lai L, Chen Y, Tian X, Li X, Zhang X, Lei J (2015) Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of lps/tlr4/nf-κb signaling pathway in rats. Eur J Pharmacol 765:234–241
Le W, You J, Mei J (1983) Chemotherapeutic effect of artesunate in experimental schistosomiasis. Acta Pharm Sin 18(8):619–621
Lee J, Zhang G, Wu X, Xu F, Zhou J, Zhang X (2012) Growth inhibitory effect of dihydroartemisinin on bcr/abl+ chronic myeloid leukemia k562 cells involve akt, erk and nf-κb modulation. J Cancer Res Clin 138(12):2095–2102
Li B, Yao Q, Pan X, Wang N, Zhang R, Li J (2011) Artesunate enhances the antibacterial effect of β-lactam antibiotics against escherichia coli by increasing antibiotic accumulation via inhibition of the multidrug efflux pump system acrab-tolc. J Antimicrob Chemoth 66(4):769–777
Li B, Zhang R, Li J, Zhang L, Ding G, Luo P (2008) Antimalarial artesunate protects sepsis model mice against heat-killed escherichia coli challenge by decreasing tlr4, tlr9 mrna expressions and transcription factor nf-κb activation. Int Immunopharmacol 8(3):379–389
Li J, Yang B, Chen Y (2008) Experimental study on the effect of artesunate(art) on hepg-2 cells in vitro. Lishizhen Med Mate Med Res 4:22
Li S, Xue F, Cheng Z, Yang X, Wei S, Gao F (2009) Improving effectiveness of business operations risk inspections. Int J Hematol 90(4):513–521
Li W, Chen Y, Liu C (2001) Effects of dihydroartemisinin on the level of tnf-α in rats infected with pneumocystis carinii pneumonia. J Immunol 4:009
Li W, Chen Y, Liu C (2003) Effects of dihydroartemisinin on apoptosis of spleen cells in rats infected with pneumocystis carinii pneumonia. Chin J Zoonoses 19(02):55–59
Li W, Chen Y, Liu C, Yu D (2004) Pathological changes in lungs of rats infected with pneumocystis carinii pneumonia after therapy with dihydroartemisinin. Chin J Zoonoses 20(3):223–227
Li W, Shi C, Huang C, Yi W (2009) Inhibiting effect of artesunate on mastocyte in rabbit ear hypertrophic scar. Chin J Aesth Med 1(2):33
Li Y, Zhou J, Zhang Y (2010) Effect of different concentrations of artesunate and methotrexate on synoviocytes of rheumatoid arthritis. Chin Pharma 13:10
Liang A, Xue B, Wang J, Li C (2002) Study on inhibitory effect of artesunate on endotoxin-induced production of inflammatory mediators. Chin J Expt Tradit Med For 2002:S1
Liu C (2001) A primary observation of therapeutic effect of dihydroartemisinin on experimental rats infected with pneumocystis carinii. Acta Univ Sci Med 3:11–18
Liu L, Wang J, Liu J, Guo J, Zuo L (2008) Inhibitory effect of artesunate on human esophageal squamous carcinoma and its related mechanism. J Chin Med Univ 4:37–39
Lu S, Yan X, Li S, Wu L, Fan S, Xu L (2004) Prophylactic effect of artesunate against experimental infection of schistosoma mansoni. Chin J Parasitol Paras Dis 22(1):20–23
Mei L, Shi K, Su J, Zha Z, Liu L (2008) Progress in domestic study of artemisinin. J Laser 29(2):95–96
Meshnick SR, Thomas A, Ranz A, Xu C, Pan H (1991) Artemisinin (qinghaosu): the role of intracellular heminin its mechanism of antimalarial action. Mol Biochem Parasit 49(2):181–189
Michaelsen FW, Saeed MEM, Schwarzkopf J, Efferth T (2015) Activity of artemisia annua, and artemisinin derivatives, in prostate carcinoma. Phytomedicine 22(14):1223–1231
Mondal A, Chatterji U (2015) Artemisinin represses telomerase subunits and induces apoptosis in hpv‐39 infected human cervical cancer cells. J Cell Biochem 116(9):1968–1981
Nacapunchai D, Phadungkul K, Kaewcharus S (2002) In vitro effect of artesunate against acanthamoeba spp. Se Asian J Trop Med 33:49–52
Obeid S, Alen J, Nguyen VH, Pham VC, Meuleman P, Pannecouque C (2013) Artemisinin analogues as potent inhibitors of in vitro hepatitis c virus replication. Plos One 8(12):324–324
Ohgami Y, Elstad CA, Chung E, Shirachi DY, Quock RM, Lai HC (2010) Effect of hyperbaric oxygen on the anticancer effect of artemisinin on molt-4 human leukemia cells. Anticancer Res 30(11):4467–4470
Okorji UP (2015) Inhibition of neuroinflammation by artemisinin and its derivatives. Doctoral thesis, University of Huddersfield
Olliaro PL, Haynes RK, Meunier B, Yuthavong Y (2001) Possible modes of action of the artemisinin-type compounds. Trends Parasitol 17(3):122–126
Ooko E, Saeed MEM, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K (2015) Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 22(11):1045–1054
Ouyang J, Zhang H, Gu Z, Zhao S, Xu T, Zhou K (2009) A pilot study of the therapeutic efficacy and mechanism of artesunate in the mrl/lpr murine model of systemic lupus erythematosus. Chin J Immun 6(6):461–467
Paeshuyse J, Coelmont L, Vliegen I, Hemel JV, Vandenkerckhove J, Peys E (2006) Hemin potentiates the anti-hepatitis c virus activity of the antimalarial drug artemisinin. Biochem Biophys Res Commun 348(1):139–144
Pandey AV, Tekwani BL, Singh RL, Chauhan VS (1999) Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J Biol Chem 274(27):19383–19388
Ponmee N, Chuchue T, Wilairat P, Yuthavong Y, Kamchonwongpaisan S (2007) Artemisinin effectiveness in erythrocytes is reduced by heme and heme-containing proteins. Biochem Pharmacol 74(1):153–160
Pop L, Györke A, Tǎbǎran AF, Dumitrache MO, Kalmár Z, Magdaş C (2015) Effects of artemisinin in broiler chickens challenged with eimeria acervulina, e. maxima, and e. tenella, in battery trials. Vet Parasitol 214(3):264–271
Qin W, Mao W, Yuan L, Yi Z, Ping W (2001) Experimental studies of antitumor effect of artesunate on liver cancer. Chin J Chin Mat Med 26(10):707–708. 720
Ramacher M, Umansky V, Efferth T (2009) Effect of artesunate on immune cells in ret-transgenic mouse melanoma model. Anti Cancer Drug 20(10):910–917
Reichert S, Reinboldt V, Hehlgans S, Efferth T, Rödel C, Rödel F (2012) A radiosensitizing effect of artesunate in glioblastoma cells is associated with a diminished expression of the inhibitor of apoptosis protein survivin. Radiother Oncol 103(3):394–401
Romero MR, Efferth T, Serrano MA, Castaño B, Macias RIR, Briz O (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis b virus production in an “in vitro” replicative system. Antivir Res 68(2):75–83
Sack RB (1975) Human diarrheal disease caused by enterotoxigenic escherichia coli. Annu Rev Microbiol 29(1):333–53
Schultz TL, Hencken CP, Woodard LE, Posner GH, Yolken RH, Jones-Brando L (2001) A thiazole derivative of artemisinin moderately reduces toxoplasma gondii cyst burden in infected mice. J Parasitol 100(4):516–521
Sheng Q, Song J, Zhang H (2009) Effect of artesunate on tumor fasl expression in mice of transplanted hepatocarcinoma 22. Pharmacol Clin Chin Mate Med 100(4):516–521
Sheng Q, Zhang H, Nong H, Song J (2008) Effect of artesunate on tumor growth in mice of transplanted hepatocarcinoma 22 (h_(22)) and tumor vegf expression. Pharmacol Clin Chin Mate Med 6:10
Slade D, Galal AM, Gul W, Radwan MM, Ahmed SA, Khan SI (2009) Antiprotozoal, anticancer and antimicrobial activities of dihydroartemisinin acetal dimers and monomers. Bioorg Med Chem 17(23):7949–7957
Smith SE, Toledo AA, Massey JB, Kort HI (2007) Anti-malarial agent artesunate inhibits tnf-alpha-induced production of proinflammatory cytokines via inhibition of nf-kappab and pi3 kinase/akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. J Rheumatol 46(6):920–926
Tang Z, Zhou X, Gao X (2007) Effect of dihydroartemisinin on the ultrastructure of trichomonas vaginalis trophozoites in vitro. Chin J Parasitol Paras Dis 25(1):41–44
Tangnitipong S, Thaptimthong T, Srihirun S, Unchern S, Kittikool D, Udomsangpetch R (2012) Extracellular heme enhances the antimalarial activity of artemisinin. Biol Pharm Bull 35(1):29–33
Tian X, Lu S, Liu Y, Wang F, Song H (2005) Effect of dihydroartemisinin on ultrastructure of giardia lamblia in vitro. Chin J Parasitol Paras Dis 23(5):292–295
Tu Z, Utzinger J, Chollet J, Xiao S, Tanner M (2005) Therapeutic effect of artemether and artesunate in mice infected with schistosoma mansoni. Chin J Schi Contl 5:17–22
Wang H, Jiang B, Zhang H, Liu B, Sun L (2010) Artesunate relieves lupus nephritis by inhibiting the expression of icam-1. J Clin Med Prac 23(5):292–295
Wang J, Hou L, Yang Y, Tang W, Li Y, Zuo J (2009) Sm905, an artemisinin derivative, inhibited no and pro-inflammatory cytokine production by suppressing mapk and nf-kappab pathways in raw 264.7 macrophages. Acta Pharmacol Sin 30(10):1428–1435
Wang J, Liu L, Li J, Liu J, Guo J, Zuo L (2007a) Inhibitory effect of artesunate on human esophageal carcinoma is associated with cdc25a modulation. Acta Acad Med Mil Tert 5:428–431
Wang J, Tang W, Shi L, Wan J, Zhou R, Ni J (2007b) Investigation of the immunosuppressive activity of artemether on t-cell activation and proliferation. Brit J Pharmacol 150(5):652–661
Wang J, Tang W, Zhou R, Wan J, Shi L, Zhang Y (2008) The new water-soluble artemisinin derivative sm905 ameliorates collagen-induced arthritis by suppression of inflammatory and th17 responses. Brit J Pharmacol 153(6):1303–1310
Wang J, Wei T, Yang Z, Jin W, Shi L, Yu Z (2007c) Suppressive effect of a novel water-soluble artemisinin derivative sm905 on t cell activation and proliferation in vitro and in vivo. Eur J Pharmacol 564(1):211–218
Wang L, Mo H, Zhang L (2012) Study on the mechanisms of artesunate on anti-inflammatory in collagen-induced arthritis in rats. Chin J Clin Pharm 28(1):46–48
Wang S, Yue G, Hua C, Rui K, Jiang H, Pan S (2010) Dihydroartemisinin inactivates nf-κb and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro, and in vivo. Cancer Lett 293(1):99–108
Wang X, Zhang L, Ding G (2012) Inhibitory effect of dihydroartemisinin on the growth of human prostate cancer pc-3m cells and its mechanism. Int J Androl 18(7):590–594
Want MY, Islamuddin M, Chouhan G, Ozbak HA, Hemeg HA, Dasgupta AK (2015) Therapeutic efficacy of artemisinin-loaded nanoparticles in experimental visceral leishmaniasis. Colloid Surface B 130:215–221
Wu X, Sun W, Shi X, Wang Z, An P, Qiao C (2012) effect of artemisinin on the expressions of gralpha mrna, grbeta mrna and p300/cbp protein in lupus nephritis mice. J Chin Med Mat 35(4):608–612
Wu X, Zhang W, Shi X, Wan S (2010) Therapeutic effect of artemisinin on lupus nephritis mice and its mechanisms. Acta Bioch Bioph Sin 42(12):916–923
Wu X, Zhang Y (2008) Study on the anti-tumor effect of artesunate. Chin J Drug App Monitor 6:19
Wu Y, He S, Bai B, Zhang L, Lu X, Lin Z (2015) Therapeutic effects of the artemisinin analog sm934 on lupus-prone mrl/lpr mice via inhibition of tlr-triggered b-cell activation and plasma cell formation. Cell Mol Immunol 13:379–390
Wu Z, Gao C, Wu Y, Zhu Q, Chen Y, Liu X (2009) Inhibitive effect of artemether on tumor growth and angiogenesis in the rat c6 orthotopic brain gliomas model. Integr Cancer Ther 8(1):88–92
Xiao S, Keiser J, Xue J, Tanner M, Morson G, Utzinger J (2009) Effect of single-dose oral artemether and tribendimidine on the tegument of adult clonorchis sinensis in rats. Parasitol Res 104(3):533–541
Xiao S, Xue J, Tanner M, Zhang Y, Keiser J, Utzinger J (2008) Effect of tribendimidine, artesunate, artemether and praziquantel, administered intragastrically at single, multiple or combined doses, to rats infected with clonorchis sinensis. Chin J Parasitol Paras Dis 26(5):321–326
Xiao S, Yin J, Mei J, You J, Li Y (1992) Effect of arteether on schistosoma japonicum. Acta Pharm Sin 27(3):161–165
Xiao S, You J, Jiao P, Mei J (1994) Effect of early treatment of artemether against schistosomiasis in mice. Chin J Parasitol Paras Dis 12(1):7–12
Xiao S, You J, Mei J, Jiao P, Guo H (1995) Experimental studies on the preventive effect of artemether against schistosomal infection. Chin J Parasitol Paras Dis 13(4):241–248
Xie H, Yao L, Chen L, Hu W (2008) Effect of artemisinin on caspase-3 and cytochrome c of leuvemia cell k562. Chin Arch Tradit Chin Med 12:51–53
Xu G, Chen P, Liang J (2009) The study on effect and mechanism of artesunate in treating experimental pulmonary fibrosis in rats. Clin Med Eng 9:4–6
Xue J, Xu L, Qiang H, Zhang Y, Xiao S (2009) Therapeutic effect of tribendimidine,artesunate and praziquantel administered to hamsters infected with clonorchis sinensis. Chin J Parasitol Paras Dis 27(3):215–218
Yang D, Liu J (2009) Effect of artesunate on apoptosis and proliferation of hepatic stellate cellls of hepatic fibrosis mice. Henan TCM 5:19–21
Ye B, Chen Y, Liu C (2001) The therapeutic effect of dihydroarteminsinin and sodium artesunate on pneumocystis carinii pneumonia of rat model. Chin J Zoonoses 17(4):43–45
Ye Y, Zeng Y, Huang X, Lu X, Zeng X, Wang X (2010) Immunosuppressive effect of dihydroartemisinin on murine t lymphocytes. Chin J Pathophys 26(3):417–423
Youns M, Efferth T, Reichling J, Fellenberg K, Bauer A, Hoheisel JD (2009) Gene expression profiling identifies novel key players involved in the cytotoxic effect of artesunate on pancreatic cancer cells. Biochem Pharmacol 78(3):273–283
El Zawawy LA (2008) Effect of artesunate on toxoplasma gondii: in vitro and in vivo studies. J Egypt Soc Parasitol 38(1):185–201
Zeng Y, Xun N, Meng W, Wen Q (2009) Inhibitive effect of artesunate on human lymphoblastic leukemia/lymphoma cells. J Sichuan Univ 40(6):1038–1043
Zhang L, Liu Z, Ye J, Sha M, Qian H, Bu X (2014) Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting pge 2, production and foxp3 expression. Cell Biol Int 38(5):639–646
Zhang M, Tao L, Zhao F, Lu X, Xin Z, Zhou X, Li X (2014) Extraction of Arteannuin from Herba Artemisiae Annuae and synthesis of Artemether. J Tradit Chin Vet Med 5:35–36
Zhang P, Luo B, Tan Q, Zou F, Wan W, Guo J (2005) Effects of artesunate on free calcium concentration in peritoneal macrophages of endotoxemia mice induced by hyperthermia. Chin J Indust Med 2:14–17
Zhang X, Yang X, Pan Q (1998) Studies on the antitumor effect and apoptosis induction in human liver cancer cell line (bel-7402) by sodium artesunate. Chin Tradit Herb Drug 7:17–20
Zhou W, Zhou R, Zhang Y (2005) A novel artemisinin derivative, 3-(12-β-artemisininoxy) phenoxyl succinic acid (sm735), mediates immunosuppressive effects in vitro and in vivo. Acta Pharmacol Sin 26(11):1352–1358
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81274112; 81373986; 81473372; 81403322; 81403125), Beijing Municipal Natural Science Foundation (7152106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dai, YF., Zhou, WW., Meng, J. et al. The pharmacological activities and mechanisms of artemisinin and its derivatives: a systematic review. Med Chem Res 26, 867–880 (2017). https://doi.org/10.1007/s00044-016-1778-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1778-5