Abstract
When aphids parasitize plants with extrafloral nectaries (EFNs) and aphid colony size is small, ants frequently use EFNs but hardly tend aphids. However, as the aphid colony size increases, ants stop using EFNs and strengthen their associations with aphids. Although the shift in ant behavior is important for determining the dynamics of the ant–plant–aphid interaction, it is not known why this shift occurs. Here, we test two hypotheses to explain the mechanism responsible for this behavioral shift: (1) Extrafloral nectar secretion changes in response to aphid herbivory, or (2) plants do not change extrafloral nectar secretion, but the total reward to ants from aphids will exceed that from EFNs above a certain aphid colony size. To judge which mechanism is plausible, we investigated secretion patterns of extrafloral nectar produced by plants with and without aphids, compared the amount of sugar supplied by EFNs and aphids, and examined whether extrafloral nectar or honeydew was more attractive to ants. Our results show that there was no inducible extrafloral secretion in response to aphid herbivory, but the sugar concentration in extrafloral nectar was higher than in honeydew, and more ant workers were attracted to an artificial extrafloral nectar solution than to an artificial aphid honeydew solution. These results indicate that extrafloral nectar is a more attractive reward than aphid honeydew per unit volume. However, even an aphid colony containing only two individuals can supply a greater reward to ants than EFNs. This suggests that the ant behavioral shift may be explained by the second hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutualisms are reciprocal interspecific interactions in which interacting species provide net benefits to each other. Mutualisms are ubiquitous in nature (Boucher et al. 1982; Herre et al. 1999), and various types of mutualism are known, for example, those in which resources are exchanged (e.g., mycorrhizal fungi and plants) (Smith and Read 1997), those in which services are exchanged (e.g., sea anemones and anemonefish) (Allen 1972), and those in which services are exchanged for resources (e.g., ants and aphids) (Way 1963). The ecological and evolutionary importance of mutualisms is well accepted since mutualisms are strong driving forces for community organization (Wimp and Whitham 2001; Ohgushi et al. 2007) and may also play a role in speciation (Thompson 2005). However, mutualisms have been traditionally studied as pair-wise interactions, and our knowledge of how third-party species in a community influence the dynamics of mutualisms is limited. To investigate the effects of a third species on mutualisms is important because such knowledge deepens our understanding of temporal and spatial variation in interaction strength, which is necessary to elucidate mutualism dynamics (Strauss and Irwin 2004; Thompson 2005).
Some plants and some hemipteran insects have mutualistic associations with ants (Bentley 1977; Koptur 1992; Dixon 1998; Linsenmair et al. 2001). They provide sugar-based rewards (i.e., extrafloral nectar or honeydew) to ants in return for protective services by the ants (Bentley 1977; Koptur 1992; Katayama and Suzuki 2002, 2003b, 2004, 2005). Since hemipteran insects frequently parasitize plants with extrafloral nectaries (EFNs) (Becerra and Venable 1989), ant-mediated interactions occur between them (Buckley 1983; Gaume et al. 1998; Offenberg 2000; Engel et al. 2001). Ant–plant–hemipteran interactions are excellent systems to investigate the dynamics and complexity of multi-species interactions (Becerra and Venable 1989; Sakata and Hashimoto 2000; Engel et al. 2001) because the competition between plants and hemipteran insects for shared partners (i.e., ants) and their association with ants are related to the stability and fitness outcomes of these mutualistic interactions (Buckley 1987; Suzuki et al. 2004; Ueda et al. 2008; Katayama and Suzuki 2010).
In this context, several authors have found that ants shift their foraging pattern from the use of extrafloral nectar to that of honeydew as aphid (hemipteran insect) colony size increases on plants with EFNs (Sakata and Hashimoto 2000; Katayama and Suzuki 2003a; Suzuki et al. 2004). The shift in ant foraging pattern has different consequences for plants and aphids due to asymmetrical ant-mediated interactions (Offenberg 2001; Suzuki et al. 2004; Katayama and Suzuki 2010). When an aphid colony is small, ants frequently use EFNs and hardly use aphid honeydew (Katayama and Suzuki 2003a; Suzuki et al. 2004). In this case, the small aphid colony may gain indirect benefits from EFNs since ants attracted by EFNs protect the colony, which is too small to sufficiently attract ants by itself (Katayama and Suzuki 2010). On the other hand, when an aphid colony grows larger, ants frequently use aphid honeydew but hardly use EFNs (Katayama and Suzuki 2003a; Suzuki et al. 2004). In this case, although plants are directly damaged by sap feeding of aphids, the plants gain “indirect benefits from aphids” because ants attracted by aphids exclude other herbivores from the plants (Suzuki et al. 2004). Thus, the shift in ant foraging behavior is important for determining the dynamics of the ant–plant–aphid interaction.
In ant–plant–aphid interactions, an unsolved question has remained: Why does the ant foraging behavior shift from use of EFNs to that of honeydew? Here, we propose two scenarios to explain the ant foraging pattern. The first scenario is that extrafloral nectar secretion may change in response to aphid herbivory, as it is known to do in response to other kinds of herbivory damage (Heil et al. 2001; Ness 2003; Wäckers and Bonifay 2004). If plants gain some benefits by distracting ants away from the aphid colony (ant-distraction hypothesis: Becerra and Venable 1989), plants may increase the quality or quantity of extrafloral nectar. Such “ant distraction” may be more effective against small aphid colonies. However, when an aphid colony grows big enough to attract ants away from EFNs, the plants may stop extrafloral nectar secretion to reduce the costs of nectar secretion. In contrast, the second scenario supposes that plants do not change the quantity or quality of the extrafloral nectar in response to aphid herbivory, but that the ants may preferentially use aphid honeydew because the reward for ants from aphids may exceed that from EFNs at a given aphid colony size. Both scenarios assume that the quality of extrafloral nectar and its attractiveness to ants are superior to those of aphid honeydew; however, it remains unclear if this is indeed the case.
In this study, to judge which scenario is more likely, we examined the secretion pattern of extrafloral nectar in response to aphid herbivory, and compared the quality and attractiveness to ants of extrafloral nectar and aphid honeydew using Lasius japonicus ants, Aphis craccivora aphids, and Vicia faba plants with EFNs. We especially focused on the following three points: (1) Does quantity or quality of extrafloral nectar differ among plants attacked by different numbers of aphids? (2) Is extrafloral nectar or honeydew the more sugar-rich resource? and (3) Does extrafloral nectar or honeydew attract a greater number of ants? Our results show there is no inducible secretion of extrafloral nectar in response to aphid herbivory, but find that the sugar concentration in extrafloral nectar is higher than that in honeydew, and that the attractiveness to ants of extrafloral nectar is superior to that of aphid honeydew. Based on these results, we discuss the shift in ant foraging pattern depending on aphid colony size.
Materials and methods
Organisms
Vicia faba (broad bean) is a widely cultivated annual legume herb bearing EFNs. In Japan, this plant germinates in late winter, and begins to grow vigorously in early spring. It bears EFNs in late March and mid-May. Several ant species are often observed visiting the plants in spring to collect extrafloral nectar. To examine whether the secretion rate of extrafloral nectar depends on the frequency of its removal, we carried out a preliminary experiment in which we collected extrafloral nectar of V. faba every 2 h (12 times/day), every 4 h (6 times/day), and every 24 h (1 time/day), and compared the total volumes of extrafloral nectar secreted over a 24-h period. In a preliminary experiment, we had previously confirmed that total volume of extrafloral nectar during 24 h was not affected by the method used to collect it (ANOVA, F 2,11 = 0.161, p = 0.852, N. Katayama, unpublished data). Although we understand that this collection method does not mimic the foraging pattern of ants, we consider it likely that the removal of extrafloral nectar does not influence its secretion by V. faba. Vicia faba used for this study was cultured in a temperature-controlled experimental chamber at 22–25 °C under natural light conditions.
Aphis craccivora (cowpea aphid) is the most common ant-tended aphid on V. faba in Japan (Takizawa and Yasuda 2006). In the field, ants such as Lasius japonicus, Tetramorium tsushimae, and Formica japonica (Hymenoptera: Formicidae) visit colonies of this aphid and collect honeydew (Katayama and Suzuki 2003a). We collected one colony of A. craccivora from Vicia angustifolia L. (Leguminosae) growing on the campus of Saga University (Saga Prefecture: 33°17′N, 130°06′E). We reared the aphids on seedlings of V. faba grown in polyethylene pots (9 cm in diameter, 8 cm deep) in the laboratory at 20–25 °C under a 24 L photoperiod.
Workers of L. japonicus are medium-sized ants (about 4 mm in body length). They prefer sugars and frequently feed on honeydew. A colony of L. japonicus (more than 2,000 workers) was collected on the campus of Saga University, and used in the below experiment.
Collection of extrafloral nectar
We examined extrafloral nectar secretion in response to aphid herbivory, using 24 seedlings of V. faba cultured for a month in a temperature-controlled experimental chamber. The seedlings of V. faba were about 20 cm in height, and bore 8–10 EFNs each. Before the experiment, all extrafloral nectar was removed from the EFNs by using 0.5-μl microcapillary tubes (Drummond Scientific Company, Broomall, PA, USA). We then assigned these seedlings to each of three treatments. Treatment 1 was that no aphids were released on the plant (control), and treatments 2 and 3 were that five and 50 adult aphids were released, respectively. Each seedling was put into a clear plastic cylinder with an iron lid in the bottom. The top of the cylinder was covered with fine mesh to prevent aphids from escaping. We placed each cylinder in a temperature-controlled chamber (23 °C under a 16L8D condition). After 24 h (day 1), we used 0.5-μl microcapillary tubes to collect extrafloral nectar secreted by the seedlings and measured the extrafloral nectar secretion per 24 h. To maintain constant aphid densities, we removed all newborn aphids from the plants every day. After 48 h (day 2) and 72 h (day 3) from when we first released aphids on the seedlings, we collected extrafloral nectar following the same procedure. All collected samples of extrafloral nectar were kept in a freezer at −30 °C until their sugar content could be analyzed.
In natural (and experimental) conditions, the evaporation of extrafloral nectar usually occurs. We consider that the evaporation is not a flaw of the experiment because ants use extrafloral nectar that has condensed after its water content has evaporated. To estimate sugar production from EFNs, we calculated the excretion rates of “sugars” in extrafloral nectar as described below.
Collection of honeydew
To compare what kinds of sugars were present in extrafloral nectar and honeydew, we collected honeydew from the aphids. We released five adult aphids each onto seven Vicia faba seedlings that had been cultured for about 3 weeks in the outdoor experimental chamber. Each seedling was put into a similar clear plastic cylinder as mentioned. We then reared these aphids under temperature-controlled conditions (23 °C under a 16L8D photoregime). After 7 days, we cut several leaves with aphids off each plant, and placed them in wet cotton on a Petri dish. While observing the aphids through a microscope, we collected honeydew excreted by the aphids, using 0.5-μl microcapillary tubes. Honeydew was collected until one microcapillary tube had been completely filled for each plant, for a total of seven tubes. All collected samples of honeydew were kept in a freezer at −30 °C until they were analyzed. While collecting the samples, we also measured the volume of one droplet of honeydew excreted by an aphid, using a 0.5-μl microcapillary tube.
Sugar analysis
The sugar concentrations of extrafloral nectar from days 1, 2, and 3, and aphid honeydew were analyzed by high-pressure liquid chromatography (HPLC), using a Cosmosil 5NH2-MS packed column (4.6 × 150 mm; Nacalai Tesque, Kyoto, Japan) and an 80 % acetonitrile mobile phase at room temperature. The flow rate was 1 ml min−1. Peak sizes for the various sugars present in the samples were calculated directly by a refractive index detector (RID; Shimadzu Corp., Kyoto, Japan) and used to calculate the concentrations of the sugars in samples. Samples were optimized using 11 sugar standards (fructose, glucose, sucrose, maltose, trehalose, melezitose, xylose, galactose, lactose, melibiose, raffinose), and the composition of each sample was determined by comparison of retention times with those from a standard sample measured on the same day.
Secretion rates of sugars from EFNs and individual aphids
Secretion rates of sugars from EFNs and individual aphids per day were calculated using the following formula:
[secretion rate (μg day−1)] = [conc (μg μl−1)] × [volume (μl day−1)]
[conc (μg μl−1)] and [volume (μl day−1)] represent the concentration and the amount secreted per day of extrafloral nectar or honeydew, respectively. Average volume of extrafloral nectar per day collected from the above experiment was used as [volume (μl day−1)] of extrafloral nectar. Katayama and Suzuki (2002) reported that individual A. craccivora aphid excreted a droplet of honeydew approximately once per hour (0.94 h−1). Hence, in this study, [volume (μl day−1)] of honeydew excreted by individual aphids was estimated as follows:
[volume (μl day−1)] of honeydew = average honeydew droplet volume (0.047 μl, see “Results”) × number of honeydew droplets excreted per h (0.94 h−1) × 24 h.
Ant preference between artificial extrafloral nectar and honeydew
We compared the attractiveness to ants of extrafloral nectar and honeydew using artificial sugar solutions made to mimic extrafloral nectar and honeydew. A colony of L. japonicus was collected and transferred among 12 test tubes (1.2 cm in diameter and 18 cm in length) with 100 workers per tube (ant nest), to produce 12 experimental nests. The bottom of each tube was packed with wet cotton wool about 3 cm deep to maintain a suitable humidity. The tube was covered with aluminum foil to maintain darkness like that of a natural ant nest. Each test tube was connected to a vinyl chloride tube 6 mm in inner diameter and 10 cm long to form an entrance. The ants were fed 10 % sucrose solution from a test tube (1.2 cm in diameter, 12 cm in length) plugged with cotton wool. Before the experiment, the ants were starved for 4 days to increase the sensitivity of their reactions to sugar resources. We made two artificial sugar solutions, which were designed to mimic V. faba extrafloral nectar (sugar concentrations: 100 μg μl−1 fructose, 125 μg μl−1 glucose, 25 μg μl−1 sucrose) and aphid honeydew (sugar concentrations: 5 μg μl−1 fructose, 5 μg μl−1 glucose, 2.5 μg μl−1 sucrose, 35 μg μl−1 melezitose). We put 1 mg of cotton wool in each vial (1.2 cm in diameter, 12 cm in length), and added 2 ml of either artificial sugar solution into each vial.
The experiments were carried out at 25 °C under light conditions in a laboratory. We put an ant nest in the center of a plastic tray (length, 30 cm, width, 20 cm, height, 5 cm). The inner side of the tray wall was plastered with Fluon (Asahi Glass Company, Tokyo, Japan) to prevent the ants from escaping. One hour after putting the ant nest on the tray, we placed one vial containing artificial extrafloral nectar solution on one side in the tray, and placed one vial containing artificial honeydew solution on the other side. After 1 h had passed, the numbers of ants in the two artificial solution vials were counted ten times at 10-min intervals. The average numbers of ants across the ten counts of each trial were used for analysis. We replicated this trial 12 times using different ant nests.
Statistical analysis
To test for inducible extrafloral nectar secretion in response to aphid herbivory, repeated-measures ANOVA was used to examine the effects of time (during day 1–3) and aphid colony size on both extrafloral nectar secretion per 24 h and total sugar concentration in extrafloral nectar. We compared the concentration of each sugar between extrafloral nectar and honeydew using a t test. When comparing sugar concentrations between extrafloral nectar and honeydew, we used the same samples of extrafloral nectar that were collected to test for inducible extrafloral secretion. Because there were no significant differences in the sugar concentrations in extrafloral nectar among treatments (i.e., aphid colony size or time), the average concentration of sugars in the extrafloral nectar produced by each plant was used when comparing with the sugar concentrations in honeydew. A Wilcoxon test was used to compare the number of ants in the vials with artificial extrafloral nectar and in the vials with artificial honeydew in the sugar preference experiment.
Results
Effect of aphids on extrafloral nectar secretion
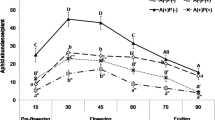
After we released aphids on the seedlings, we found no significant changes in extrafloral nectar secretion over the 3-day period, and no significant effect of aphids (aphid: F 2,21 = 1.194, p = 0.323; time, F 2,20 = 0.917, p = 0.416; aphid × time: F 4,40 = 0.805, p = 0.529; Fig. 1a). Three sugars, fructose, glucose, and sucrose, were detected in the extrafloral nectar of V. faba (Table 1). However, we found no significant effects of time or aphid numbers (i.e., among 0, 5, and 50 aphids) on the total sugar concentration in extrafloral nectar (aphid: F 2,21 = 0.598, p = 0.559; time: F 2,20 = 0.537, p = 0.593; aphid × time: F 4,40 = 1.411, p = 0.248; Fig. 1b).
Comparison of sugar concentrations and amounts between EFN and honeydew
The average volume of a honeydew droplet was 0.047 ± 0.003 μl (mean ± SE, n = 26). Four sugars (fructose, glucose, sucrose, and melezitose) were detected in honeydew (Table 1). Concentrations of fructose, glucose, sucrose, and total sugars were 14-, 27-, 13-, and 4.6-fold greater than those in honeydew, respectively (fructose: t 29 = 5.41, p < 0.001; glucose: t 29 = 6.21, p < 0.001; sucrose: t 29 = 2.21, p = 0.017; total sugars: t 29 = 4.65, p < 0.001; Table 1).
The estimated total sugar amount secreted from extrafloral nectar was 99.9 μg day−1, which was 1.9-fold greater than that excreted by individual aphids (Table 2).
Ant preference between artificial extrafloral nectar and artificial honeydew
When ants were given the opportunity to chose between the artificial extrafloral nectar solution and artificial honeydew solution, more workers visited the vial with the artificial extrafloral nectar solution than that with the artificial honeydew solution (z = −2.673, p < 0.001, Fig. 2).
Discussion
The present study demonstrates that the secretion of extrafloral nectar does not change in response to aphid herbivory. This indicates that the shift in ant foraging pattern from extrafloral nectar to honeydew at higher aphid densities (Sakata and Hashimoto 2000; Katayama and Suzuki 2003a; Suzuki et al. 2004) is not caused by the reduction of extrafloral nectar secretion, nor does the plant compensate for the costs of producing useless extrafloral nectar by reducing extrafloral nectar secretion. On the other hand, we found that the sugar concentration in extrafloral nectar is higher than that in honeydew. The ant preference experiment suggests that the attractiveness to ants of extrafloral nectar was superior to that of honeydew of aphids per unit volume. These results indicate that the shift in ant foraging behavior from the use of EFNs to that of honeydew may be explained by our second scenario; i.e., ants would selectively use aphid honeydew because the total reward for ants from aphids exceeded that from EFNs at a given aphid colony size.
Our experimental design (i.e., densities of 5-50 aphids and an experimental duration of 3 days) is based on previous research (Sakata and Hashimoto 2000; Katayama and Suzuki 2003a; Suzuki et al. 2004), which examined how ant foraging pattern changed depending on aphid colony size in field and laboratory experiments. These studies demonstrated that ants shifted their foraging pattern from the use of extrafloral nectar to that of honeydew one to a few days after 50–100 aphids were transferred to Vicia plants with extrafloral nectaries (EFNs). Based on previous studies and our present results, we consider that the shift in ant foraging pattern at higher aphid densities is not caused by the reduction of extrafloral nectar secretion. It is likely that more time and higher herbivory pressure by aphids results in the reduction of extrafloral nectar secretion because the plant is weakened by the aphid herbivory. However, investigating this possibility was not our aim in this study.
Under natural conditions, aphids gradually increase from a single or a few adult aphid(s) on a plant, and the herbivory pressure by the aphids gradually increases as the aphid colony grows. There is a possibility that the induced change of extrafloral nectar secretion may be elicited by such gradual growth of aphid colony size. It is not possible with existing data to evaluate this possibility at the present time; additional research to test this mechanism.
Why are aphids more attractive to ants?
At present, there are few studies that directly compare sugar quality between extrafloral nectar and honeydew (but see Engel et al. 2001), however, it is likely that the sugar concentration is generally higher in extrafloral nectar than in honeydew (extrafloral nectar 25–35 %, honeydew 3–20 %) (Koptur 1979; Ruffner and Clark 1986; Helden et al. 1994; Kawano et al. 1999; Völkl et al. 1999; Engel et al. 2001; Yao and Akimoto 2001; Fischer et al. 2002). If so, why do ants shift their foraging pattern to use of honeydew? The reason, we suspect, is that at a given colony size, the total volume of honeydew output (and quantity of sugars therein) could be much higher than that of extrafloral nectar produced by the colony’s host plant. This study shows that the average honeydew droplet volume of A. craccivora aphids was 0.047 μl. In addition, Katayama and Suzuki (2002) examined the frequency of honeydew excretion by the same aphid species, and found that the aphids excreted about one droplet of honeydew per hour. From these results, we estimated the total volume of honeydew droplets produced per day by a single aphid (Table 2), and compared the sugar secretion rate between EFNs and aphids. The sugar secretion rate of one aphid appears inferior to that of EFNs, however, if the aphid population increases to two aphids, the order is reversed. Although not all droplets of honeydew produced by aphids may be provided to ants, aphids in a colony can still provide a greater quantity of sugar to ants than do EFNs.
Engel et al. (2001) reported that the quality of extrafloral nectar of V. faba was higher than that of honeydew of Aphis fabae (Scop.), and concluded that the presence of the aphids did not influence the attractiveness of EFNs due to the high quality of extrafloral nectar. However, this study did not compare total sugar quantity quantitatively. Our study is the first report which shows that the sugar-productive capacity of aphids in a colony outweighs that of EFNs.
Several studies have reported that the honeydew of ant-tended aphids contained melezitose, but that of non-ant-tended aphids did not (Stadler and Dixon 1998; Völkl et al. 1999; Engel et al. 2001; Fischer and Shingleton 2001; Fischer et al. 2001; Yao and Akimoto 2001). From these findings, melezitose tends to be considered a key substance for maintaining mutualisms with ants. In this study, we confirmed that the honeydew of A. craccivora contained melezitose but the extrafloral nectar of V. faba did not (Table 1). However, we found that ants preferred an artificial sugar solution mimicking extrafloral nectar (which did not contain melezitose) to one mimicking honeydew (which contained melezitose) when solution volume was held constant (Fig. 2). This result indicates that melezitose may be not so important in determining ant resource preference, or that the high sugar concentration in extrafloral nectar might outweigh the effect of melezitose in honeydew on ant resource preference.
Multi-species interactions among plants, aphids, and ants
Becerra and Venable (1989) proposed the interesting hypothesis that EFNs may function to defend plants from ant-hemipteran mutualisms (ant-distraction hypothesis). According to the ant-distraction hypothesis, EFNs provide an additional sugar resource for ants, so that the ants stop attending the hemipteran insects. Offenberg (2001) reported that Lasius niger (L.) ants stopped attending and started to prey on Aphis fabae aphids in the presence of acacia honey solution, supporting the ant-distraction hypothesis. We suspect this is because honey solution was provided in excess to the ants in that experiment. It is known that ants will prey on aphids in the presence of additional sugar resources (Sakata 1994, 1995), but we think that EFNs of Vicia cannot distract ants from aphids because aphids in a colony can provide a much greater amount of sugar to ants than can EFNs. In fact, ants hardly use EFNs at high aphid densities (Sakata and Hashimoto 2000; Katayama and Suzuki 2003a; Suzuki et al. 2004).
Furthermore, if plants have evolved the ability to distract ants as a counter-adaptation to ant-tended aphids, they would be expected to secrete more extrafloral nectar in response to sap feeding by aphids, especially since extrafloral nectar secretion is known to be inducible in many plants (Heil et al. 2001; Ness 2003; Wäckers and Bonifay 2004). This reaction may be more effective for the plants, especially at lower aphid density at which the aphids cannot attract ants adequately. However, V. faba did not react to sap feeding by aphids (Fig. 1). Thus, our results do not support the ant-distraction hypothesis. Suzuki et al. (2004) reported that when A. craccivora parasitized the EFN-bearing vetch V. angustifolia, the efficiency of herbivore exclusion by ants increased. This effect was a result of the fact that the aphids attracted ants effectively. That study also did not support the ant distraction hypothesis, but rather indicated that there are indirect benefits to plants with EFNs from the presence of aphids. In addition, when an aphid colony is small, the colony may gain indirect benefits from EFNs since ants attracted by EFNs also protect the colony (Katayama and Suzuki 2010). Therefore, we expect that an indirect mutualistic relationship can potentially occur between plants with EFNs and ant-tended aphids by synergistically attracting ant protection which benefits both parties. This shift in the foraging pattern of ants may be important for the stability and dynamics of ant–plant–aphid interactions, as well as for that of the larger arthropod community structure on the plants.
References
Allen GR (1972) The anemonefishes: their classification and biology. T.F.H. Publications, Neptune City
Becerra JXI, Venable DL (1989) Extrafloral nectaries: a defense against ant-Homoptera mutualisms? Oikos 55:276–280
Bentley BL (1977) Extrafloral nectaries and protection by pugnacious bodyguards. Annu Rev Ecol Evol Syst 8:407–427
Boucher DH, James S, Keeler KH (1982) The ecology of mutualism. Annu Rev Ecol Evol Syst 13:315–347
Buckley R (1983) Interaction between ants and membracid bugs decreases growth and seed set of host plant bearing extrafloral nectarines. Oecologia 58:132–136
Buckley RC (1987) Interactions involving plants, Homoptera, and ants. Annu Rev Ecol Evol Syst 18:111–135
Dixon AFG (1998) Aphid ecology. Chapman and Hall, London
Engel V, Fischer MD, Wäckers FL, Völkl W (2001) Interactions between extrafloral nectaries, aphids and ants: are there competition effects between plant and homopteran sugar sources? Oecologia 129:577–584
Fischer MK, Shingleton AW (2001) Host plant and ants influence the honeydew sugar composition of aphids. Funct Ecol 15:544–550
Fischer MK, Hoffmann KH, Völkl W (2001) Competition for mutualists in an ant-homopteran interaction mediated by hierarchies of ant attendance. Oikos 92:531–541
Fischer MK, Völkl W, Schopf R, Hoffmann KH (2002) Age-specific patterns in honeydew production and honeydew composition in the aphid Metopeurum fuscoviride: implications for ant-attendance. J Insect Physiol 48:319–326
Gaume L, McKey D, Terrin S (1998) Ant-plant-homopteran mutualism: how the third partner affects the interaction between a plant-specialist ant and its myrmecophyte host. Proc R Soc Lond B 265:569–575
Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE (2001) Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA 98:1083–1088
Helden MV, Tjallingii WF, van Beck TA (1994) Phloem sap collection from lettuce (Lactuca sativa L.): chemical comparison among collection methods. J Chem Ecol 20:3191–3206
Herre EA, Knowlton N, Mueller UG, Rehner SA (1999) The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol 14:49–53
Katayama N, Suzuki N (2002) Cost and benefit of ant attendance for Aphis craccivora (Hemiptera: Aphididae) with reference to aphid colony size. Can Entomol 134:241–249
Katayama N, Suzuki N (2003a) Changes in the utilization of extrafloral nectaries of Vicia faba (Leguminosae) and honeydew of aphids by ants with increasing aphid density. Ann Entomol Soc Am 96:579–584
Katayama N, Suzuki N (2003b) Bodyguard effects for aphids of Aphis craccivora Koch (Homoptera: Aphididae) as related to the activity of two ant species, Tetramorium caespitum Linnaeus (Hymenoptera: Formicidae) and Lasius niger L. (Hymenoptera: Formicidae). Appl Entomol Zool 38:427–433
Katayama N, Suzuki N (2004) Role of extrafloral nectaries of Vicia faba for attraction of ants and herbivores exclusion by ants. Entomol Sci 7:117–122
Katayama N, Suzuki N (2005) The importance of the encounter rate between ants and herbivores and of ant aggressiveness against herbivores in herbivore exclusion by ants on Vicia angustifolia L. (Leguminosae) with extrafloral nectaries. Appl Entomol Zool 40:69–76
Katayama N, Suzuki N (2010) Extrafloral nectaries indirectly protect small aphid colonies via ant-mediated interactions. Appl Entomol Zool 45:505–511
Kawano S, Azuma H, Ito M, Suzuki K (1999) Extrafloral nectaries and chemical signals of Fallopia japonica and Fallopia sachalinensis (Polygonaceae), and their roles as defense systems against insect herbivory. Plant Species Biol 14:167–178
Koptur S (1979) Facultative mutualism between weedy vetches bearing extrafloral nectaries and weedy ants in California. Am J Bot 66:1016–1020
Koptur S (1992) Extrafloral nectary-mediated interactions between insects and plants. In: Bernays E (ed) Insect–plant interaction Vol, IV. CRC press, Boca Raton, pp 81–129
Linsenmair KE, Heil M, Kaiser WM, Fiala B, Koch T, Boland W (2001) Adaptations to biotic and abiotic stress: Macaranga-ant plants optimize investment in biotic defence. J Exp Bot 52:2057–2065
Ness JH (2003) Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 134:210–218
Offenberg J (2000) Correlated evolution of the association between aphids and ants and the association between aphids and plants with extrafloral nectaries. Oikos 91:146–152
Offenberg J (2001) Balancing between mutualism and exploitation: the symbiotic interaction between Lasius ants and aphids. Behav Ecol Sociobiol 49:304–310
Ohgushi T, Craig TP, Price PW (2007) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge
Ruffner GA, Clark WD (1986) Extrafloral nectar of Ferocactus acanthodes (Cactaceae): composition and its importance to ants. Am J Bot 73:185–189
Sakata H (1994) How an ant decides to prey on or to attend aphids. Res Popul Ecol 36:45–51
Sakata H (1995) Density-dependent predation of the ant Lasius niger (Hymenoptera: Formicidae) on two attended aphids Lachnus tropicalis and Myzocallis kuricola (Homoptera: Aphididae). Res Popul Ecol 37:159–164
Sakata H, Hashimoto Y (2000) Should aphids attract or repel ants? Effect of rival aphids and extrafloral nectaries on ant-aphid interactions. Popul Ecol 42:171–178
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, San Diego
Stadler B, Dixon AFG (1998) Costs of ant attendance for aphids. J Anim Ecol 67:454–459
Strauss Y, Irwin RE (2004) Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu Rev Ecol Evol Syst 35:435–466
Suzuki N, Ogura K, Katayama N (2004) Efficiency of herbivore exclusion by ants attracted to aphids on the vetch Vicia angustifolia L. (Leguminosae). Ecol Res 19:275–282
Takizawa T, Yasuda H (2006) The effects of attacks by the mutualistic ant, Lasius japonicus Santschi (Hymenoptera: Formicidae) on the foraging behavior of the two aphidophagous ladybirds, Coccinella septempunctata brucki Mulsant (Coleoptera: Coccinellidae) and Propylea japonica (Thunberg) (Coleoptera: Coccinellidae). Appl Entomol Zool 41:161–169
Thompson JN (2005) The geographic mosaic of coevolution. The University of Chicago Press, Chicago
Ueda S, Quek S-P, Itioka T, Inamori K, Sato Y, Murase K, Itino T (2008) An ancient tripartite symbiosis of plants, ants and scale insects. Proc R Soc Lond B 275:2319–2326
Völkl W, Woodring J, Fischer M, Lorenz MW, Hoffmann KH (1999) Ant-aphid mutualisms: the impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 118:483–491
Wäckers FL, Bonifay C (2004) How to be sweet? Extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85:1512–1518
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Wimp GM, Whitham TG (2001) Biodiversity consequences of predation and host plant hybridization on an aphid—ant mutualism. Ecology 82:440–452
Yao I, Akimoto S (2001) Ant attendance changes the sugar composition of the honeydew of the drepanosiphid aphid Tuberculatus quercicola. Oecologia 128:36–43
Acknowledgments
We are deeply grateful to Ryohei Yamaoka for permission to use equipment at Kyoto Institute of Technology and for his helpful advice and support during this study. We also thank Hideki Kagata, Tetsuhiro Kawagoe at Kyoto University, and two reviewers for their helpful comments in this research. This study was partly supported by a JSPS Research Fellowship for Young Scientists to N. Katayama.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nobuhiko Suzuki: Deceased.
About this article
Cite this article
Katayama, N., Hembry, D.H., Hojo, M.K. et al. Why do ants shift their foraging from extrafloral nectar to aphid honeydew?. Ecol Res 28, 919–926 (2013). https://doi.org/10.1007/s11284-013-1074-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-013-1074-5