Abstract
In myrmecophilous organisms, which live in symbiosis with ants, cuticular hydrocarbons (CHCs) play a pivotal role in interspecific communication and defense against chemical-oriented predators. Although these interactions form complex information webs, little is known about the influence of biotic environmental factors on the CHC profiles of myrmecophiles. Here, we analyzed the effect of different host plants and tending ants on the larval CHC profile of Synargis calyce (Lepidoptera: Riodinidae), a polyphagous species with facultative myrmecophily. Groups of caterpillars were fed individually with three host plant species (without tending ants), and with two tending ant species. Through gas chromatography analysis, we compared the cuticular profiles of treatments and found a high similarity between plants and caterpillars (65–82%), but a low similarity between caterpillars and their tending ants (30 − 25%). Cluster analysis showed that caterpillars, ants, and plants form distinct groups, indicating that S. calyce caterpillars have their own chemical profile. These results are similar to those observed for Lycaenidae caterpillars indicating that there is functional convergence in the chemical strategies used by myrmecophilous caterpillar species with similar ecology. Also, the results suggest that the cuticular compounds of S. calyce are primarily influenced by their host plants rather than their tending ants. Thus, we propose that these caterpillars present a trade-off between camouflage and directly informing their presence to ants, maintaining their unique chemical profile, though slightly affected by biotic environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ants are predominant in many terrestrial ecosystems in terms of abundance and biomass (von Beeren et al. 2012). These eusocial insects live in complex societies where communication plays a crucial role in their functioning (Hölldobler and Wilson 1990; Yamaoka 1990; Lenoir et al. 1999; Akino 2008). In ants and other social insects, communication and particularly the recognition of nestmates is primarily based on chemical cues and signals (Yamaoka 1990; Lenoir et al. 1999; Akino 2008; Blomquist and Bagnères 2010; Nunes et al. 2014). Cuticular hydrocarbons (CHCs), the main class of cuticular compounds in ants, are colony-specific and actively participate in nestmate recognition (Howard and Blomquist 2005; Hefetz 2007; Ferguson et al. 2021). The ecological success, wide distribution, and social organization of ants have contributed to the evolution of diverse associations with organisms from various kingdoms, including plants, fungi, and animals, particularly other insects (Casacci et al. 2019). Organisms that interact with ants during at least part of their lifecycle are called myrmecophiles (Hölldobler and Wilson 1990; Kronauer and Pierce 2011; Hölldobler and Kwapich 2022). There are about 10,000 species of myrmecophiles across various insect orders (Thomas et al. 2005; Parker 2016; Hölldobler and Kwapich 2022).
Myrmecophily in Lepidoptera is primarily observed in the families Lycaenidae and Riodinidae, with 75% of the species in these families having immatures stages that interact with ants (Pierce et al. 2002; Casacci et al. 2019). These families commonly exhibit facultative and unspecific relationships, involving interactions with various ant taxa. However, there are butterfly species that establish obligatory and specific relationships with specific ant taxa (Fiedler 1994, 2021; Kaminski 2008; Pierce and Dankowicz 2022). Interactions between butterflies and ants can range from mutually beneficial outcomes, such as mutualism, to interactions where butterflies benefit without harming ants in commensalism, and to antagonist interactions where butterflies may be preyed by ants or where ants may be harmed by butterflies, as seen in social parasitism and competition for resources (Fiedler 1995, 1996; Pierce and Dankowicz 2022). Both lycaenid and riodinid species exhibit various adaptations resulting from the pressures exerted by their association with ants (Pierce et al. 2002). These adaptations include highly specialized ant-organs involved in chemical and acoustic deception (Cottrell 1984; Fiedler et al. 1996; Pierce et al. 2002; Elmes et al. 2002; Barbero et al. 2012; Hill et al. 2022). For instance, nectary organs such as the dorsal nectary organ (DNO) in Lycaenidae and tentacular nectary organs (TNOs) in Riodinidae liquid secretions rich in sugar and amino acids (Newcomer 1912; Malicky 1970; DeVries 1988). Caterpillars of these two families are also equipped with a pair tentacular organs (TOs) in Lycaenidae and anterior tentacular organs (ATOs) in Riodinidae, which emit chemical signals or vibroacoustic signals that modify the ant behavior (Henning 1983; DeVries et al. 1986; DeVries 1988; Gnatzy et al. 2017; Schönrogge et al. 2017).
Chemical strategies mediated by cuticular compounds enable myrmecophiles to overcome the barrier of chemical recognition employed by ants (von Beeren et al. 2012). One such strategy is chemical camouflage, where organisms resemble their background and avoid detection by chemically oriented predators (Silveira et al. 2010). In herbivorous organisms, achieving chemical camouflage with their host plants is possible through diet (Espelie et al. 1991; Barbero 2016; Lima et al. 2024). In Lepidoptera, this strategy has already been demonstrated in both non-myrmecophilous and myrmecophilous caterpillars (Akino et al. 2004; Portugal and Trigo 2005; Lima et al. 2021). One of the most extensively studied strategies is chemical mimicry, where organisms possess chemical compounds that close resemble those of other organisms such as ants. This strategy has been observed in social parasitic species (Akino et al. 1999; Elmes et al. 2002; Schlick-Steiner et al. 2004; Schönrogge et al. 2004; Akino 2008). Additionally, some myrmecophilous caterpillars present low concentration of compounds on their surface, making their detection challenging - a strategy known as chemical insignificance (Inui et al. 2015; Barbero 2016). Recently, a new strategy has been proposed in myrmecophilous caterpillars called chemical conspicuousness. In this strategy, caterpillars that do not provide caloric rewards for ants exhibit a distinct cuticular profile compared to ants or host plants. However, their profile is similar to that of other caterpillars that offer caloric rewards to ants (Lima et al. 2021).
Although ant-plant-herbivore systems embrace complex communication networks involving multiple species (e.g., Lima et al. 2021, 2023), there is still a lack of information regarding the influence of phenotypic plasticity and biotic environmental factors on the cuticle compounds of generalist myrmecophilous species (Otte et al. 2018; Sprenger and Menzel 2020). Therefore, our aim was to investigate whether the CHC profile of a polyphagous caterpillar changes (1) according to their diet on different host plant species and (2) according to their interaction with different tending ant species. Considering that the caterpillar species used here were fed on their host plants, we hypothesized that their CHCs would be influenced primarily by their food source rather than the contact established with tending ants. Furthermore, due to the production of caloric rewards by caterpillars (trophobiosis) we predicted that caterpillars would exhibit a specific chemical profile distinct of both host plant and tending ants.
Methods and Materials
Study System
Synargis calyce C. Felder and R. Felder, 1862 (Lepidoptera: Riodinidae) (Fig. S1) is a Neotropical herbivorous and myrmecophilous butterfly whose caterpillars feed on several host plants in different families, including non-native species (Callaghan 1986; Beccaloni et al. 2008; Alves-Silva et al. 2018; Kaminski 2021). Female butterflies tend to lay their eggs on plants that are frequented by attendant ants and caterpillars are tended by ants during all instars (Callaghan 1986). Although it presents facultative myrmecophily, the caterpillars are almost always found with tending ants of several genera, but mainly Camponotus species (Callaghan 1986; Alves-Silva et al. 2018; Kaminski 2021). It is common to observe temporal turnover, with attendance by different species of ants during the day and night (LAK, personal observation). Due to the high degree of ecological plasticity of this butterfly in terms of both host plants and tending ants, it is an excellent model of a generalist myrmecophile.
Collection and Rearing of Study Species
Insects were collected at the Universidade de São Paulo (21.1637° S, 47.8592° W), Ribeirão Preto Campus, SP, Brazil, between January 2021 and April 2022. To conduct our chemical analysis, we collected ~ 90 eggs of S. calyce for rearing in the laboratory. Initially, ~ 20 field observations were conducted to identify plant species used by butterflies for oviposition and seven species were identified serving as host plants. Subsequently, eggs were collected from three of these host plants (Senegalia polyphylla (DC.) Britton and Rose (Fabaceae), Inga laurina (Sw.) Willd. (Fabaceae), and Terminalia catappa Linnaeus (Combretaceae)) at various study locations by harvesting branches where oviposition had been observed. In the field, three ant nests of Camponotus crassus Mayr, 1862 (Formicidae: Formicinae) were collected and transferred to the laboratory. Each nest was placed in two connected boxes measuring 9 × 26.6 × 26.6 cm. One box served as the nesting area and contained test tubes (15 cm long) filled with water, plugged with hydrophilic cotton. The other box served as the foraging area. The ant colonies were provided with a diet of Tenebrio molitor Linnaeus, 1758 larvae (Coleoptera: Tenebrionidae), diluted sugar solution (10%), and water ad libitum. Each colony consisted of approximately 150 workers, a queen, and some brood (eggs, larvae and pupae). Additionally, approximately, 600 workers of Paratrechina longicornis Latreille 1802 (Formicidae: Formicinae) were collected from three established colonies near the laboratory buildings. The insects were kept under controlled conditions at a temperature of 25 °C and a photoperiod of 12 h of light and 12 h of darkness.
Does the Chemical Composition of Caterpillars Change According to Their Food Sources?
As the caterpillars studied here are polyphagous, we selected three host plant species on which the caterpillars feed in the study area: two native, S. polyphylla, and I. laurina, and one non-native, T. catappa. Our aim was to investigate whether the CHCs of the caterpillar change according to its diet in the absence of tending ants. To conduct the experiment, we placed individually each egg in a plastic container (250 ml). Once the eggs hatched, we provided the caterpillars with shoots containing young leaves and extrafloral nectaries from the host plants. The shoots were replaced daily and kept in contact with moistened cotton to prevent them from drying. The S. calyce caterpillars were reared separately in plastic containers on three host plant species: caterpillar-S. polyphylla (n = 10), caterpillar-I. laurina (n = 10), caterpillar-T. catappa (n = 6). After reaching the fifth instar, we killed the caterpillars by freezing and kept them at -20 °C until CHC extractions were performed. Additionally, we collected leaves of S. polyphylla (n = 10), I. laurina (n = 10), and T. catappa (n = 10) for CHC extractions (See Table S1).
Does the Chemical Composition of Caterpillars Change According to Their Tending Ants?
In our field site, the caterpillars are attended by four different ant species: C. crassus, P. longicornis, Camponotus renggeri Emery, 1894, and Wasmannia auropunctata Roger, 1863 (AVCG, personal observation). Thus, to assess whether the caterpillar CHCs change according to their tending ants, we individually reared S. calyce caterpillars in a plastic container as previously described, along with a group of associated ants. Specifically, we reared the caterpillars with two experimental groups: (caterpillar-C. crassus) (n = 10), and (caterpillar-P. longicornis) (n = 10). These caterpillars were fed with the host plant S. polyphylla. Each caterpillar was placed together with 10 workers of C. crassus or 30 workers of P. longicornis. The number of ants was based on the average amount observed in the field. The ant workers were replaced every day until the caterpillars were frozen for chemical extraction, as previously mentioned. For chemical analysis, we also collected worker ants from colonies reared in the laboratory for C. crassus (n = 8 colonies; n = 20 ants for chemical analyses) and from colonies established near the laboratory for P. longicornis (n = 10 colonies; n = 300 ants for chemical analyses) (See Table S1).
Chemical Analyses
To perform the chemical analyses, we placed insects or plant shoots in glass vials (1.5 ml) and covered them with n-hexane (Macron Fine Chemicals, 95% n-hexane, USA) for 1 min (Lima et al. 2023). For each sample, a fifth-instar larva of S. calyce, two workers of C. crassus, 30 workers of P. longicornis, and one young shoot with two leaves from each plant species were used individually. External standards were exclusively employed and the samples were not weighed. Subsequently, we left each vial at room temperature in a flow chamber to allow for drying. Once completely dried, we resuspended the contents in 5 µl of hexane, of which 2 µl were manually injected. The samples were analyzed with gas chromatography coupled to a mass spectrometer (GC/MS; Shimadzu, model QP2010 Plus), using a 30 m Rxi-1ms column, with helium gas flow rate set at 1 ml/min. The oven temperature was initially set to 40 °C and then increased by 3 °C min- 1 until reaching 310 °C (held for 15min), following da Silva et al. (2021). The injector temperature was set to 250 °C. Data were analyzed by GC/MS Solutions for Windows (Shimadzu Corporation), and compounds were identified based on their mass spectra, including diagnostic and molecular ions (Carlson et al. 1998). Additionally, a retention index was calculated for each identified peak using a standard solution of different synthetic linear hydrocarbons (n-C21 to n-C40). We also consulted the Registry of Mass Spectral Data (Wiley) and National Institute of Standards and Technology (NIST) mass spectra search program (version 2.2) Libraries database for identification (Lima et al. 2023).
Statistical Analyses
We used Morisita’s Similarity Index (SI) which ranges from 0% (indicating no similarity) to 100% (representing complete similarity) (Krebs 1999) to compare CHC profiles of different groups, following the methodology of Lima et al. (2021). This analysis was carried out using PAST software (Version 4.13) (Hammer et al. 2001). Furthermore, to assess the overall chemical similarity or dissimilarity between groups, we performed a permutation analysis (PERMANOVA). This analysis was performed using the adonis function from the vegan package (Oksanen et al. 2013) with 9999 permutations. In order to represent the multivariate chemical dataset and check for the cluster formation, we next performed a Principal Component Analysis (PCA). For this, we used the prcomp function of the stats package (R Core Team 2019). We also ran a multivariate similarity analysis (SIMPER) using the Bray-Curtis distance and adopting 999 permutations. The SIMPER analysis allowed us to determine the contribution of each chemical variable to the existing variation among samples. For this analysis, we used the simper function from the vegan package (Oksanen et al. 2013). For all tests, we determined the relative abundance percentages of each compound present in the cuticular extracts, treating the compounds as 100% and then analyzed the data. All these analyses were conducted using R version 4.0.2 (R Core Team 2019).
Results
Overall Chemical Information
A total of 78 peaks were identified in the cuticular extracts from the different groups studied (Table 1). Senegalia polyphylla exhibited 22 peaks, I. laurina had 29 peaks, T. catappa, C. crassus had 28 peaks each, P. longicornis had 22 peaks, and S. calyce caterpillars had 25–30 peaks. These peaks corresponded to various chemical compounds, including branched hydrocarbons (mono-, di-, and trimethylated), linear alkanes, alkenes, alcohols, and aldehydes. The carbon lengths of the identified compounds ranged from 18 to 36.
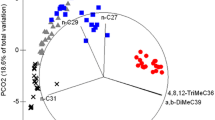
The cuticular profile of the three host plant species revealed a shared class of compounds, including linear alkanes, alcohols, and aldehydes. However, variations existed among them, particularly in the proportion and presence or absence of specific alcohols and aldehydes. For instance, 1-hexadecanol, 1-hexacosanol, and hexacosanal were exclusively present in I. laurina, while 1-triacontanol acetate was found only in T. catappa (Table 1). The SIs of the cuticular compounds of the plants varied according to the pairs of compared species. Terminalia catappa with S. polyphylla showed the highest SI of up to 77%, followed by T. catappa with I. laurina, which exhibited a SI of up to 65%, and I. laurina with S. polyphylla, which showed a SI of up to 62%. Senegalia polyphylla and I. laurina shared 20 compounds, representing 69% and 91% of their respective cuticles. Senegalia polyphylla shared 15 compounds with T. catappa, representing 52% and 68.2% of their respective cuticles. Terminalia catappa shared 18 compounds with I. laurina, representing 60% and 62.06% of their respective cuticles. Although post hoc pairwise comparisons did not reveal a significant difference based on relative abundance or chemical composition (Table 2), the PCA revealed that the three species form separate groups, in which the first and the second principal component explained 30% and 13.77%, respectively. (Fig. 1). In terms of major compounds, S. polyphylla had the n-C29, 1-triacontanol and triacontanal; I. laurina had the n-C29, Hexacosanol and n-C31, and T catappa had the n-C29 and n-C31.
Principal component analysis (PCA) of chemical compounds in S. calyce caterpillars (three groups reared on three plants species without ants and two groups reared with two ants species), the attendant ants (C. crassus and P. longicornis) and the host plants (S. polyphylla, I. laurina, and T. catappa)
Does the Chemical Composition of Caterpillars Change According to Their Food Sources?
When comparing the compounds found in caterpillars reared on three different host plants, we found the following similarity indices: The cuticular profiles of caterpillars reared on T. catappa showed a SI of up to 82% with T. catappa, up to 74% with I. laurina, and up to 61% with S. polyphylla. The cuticular profiles of caterpillars reared on I. laurina showed a SI of up to 76% with I. laurina and T. catappa, and up to 60% with S. polyphylla. Finally, caterpillars reared on S. polyphylla exhibited a SI up to 65% with S. polyphylla, and up to 64% with T. catappa, and I. laurina. Moreover, certain compounds were exclusively found in groups of caterpillars reared on specific plant species. For example, 1-hexadecanol was found only in the cuticular profile of caterpillars reared on I. laurina, and this particular compound was also identified in the chemical profile of this plant. Similarly, 1-docosanol, identified in T. catappa, was also detected in caterpillars reared on this plant but was absent in those reared on S. polyphylla (Table 1). The qualitative similarity varied according to the host plant. Specifically, caterpillars shared 19 compounds with S. polyphylla, representing 70.37% and 86.36% of their respective cuticles. Caterpillars and I. laurina shared 23 compounds, representing 76.66% and 79.31% of their respective cuticles, while caterpillars and T. catappa shared 17 compounds, representing 58.6% and 60.7% of their respective cuticles. However, there was a low degree of similarity in the relative abundance of compounds between caterpillars and their host plants. The post hoc pairwise comparisons revealed significant differences in relative abundance and in the chemical composition among caterpillars reared on different plants (Table 2). However, most of the compounds were shared in the three groups and we found an overlap among caterpillars in the PCA (Fig. 1). Specifically, caterpillar–T. catappa and caterpillar–I. laurina shared 27 compounds, representing 93.1% and 90% of their respective cuticles. Caterpillar-T. catappa and caterpillar–S. polyphylla shared 26 compounds, representing 89.7% and 92% of their respective cuticles. Caterpillar–I. laurina and caterpillar–S. polyphylla shared 25 compounds, representing 83.3% and 92.6% of their respective cuticles. In general, all caterpillar groups had n-alkanes (C29, C31, and C33) as their major compounds and they also showed a few methylated compounds, alcohols, and aldehydes, these last two also present in all plants.
Does the Chemical Composition of Caterpillars Change According to Their Tending Ants?
The cuticular profiles of caterpillars reared with C. crassus, or P. longicornis ants, as well as caterpillars reared without attendant ants, had a high SI (> 90%). Moreover, the cuticular profiles of all caterpillar groups were qualitatively similar, with 24 shared compounds, representing 96% of their respective cuticles. Hence, we found an overlap among samples of caterpillars in the PCA (Fig. 1). The post hoc pairwise comparisons did not show differentiation based on chemical composition among caterpillars reared with different species of attendant ants (Table 2).
In contrast, the cuticular profiles of caterpillars and attendant ants showed low similarity indexes and varied according to the attendant ant (SI < 30% for C. crassus and SI < 25% for P. longicornis). Caterpillars and C. crassus shared 14 compounds, representing 54% and 50% of their respective cuticles. Caterpillars and P. longicornis shared four compounds, representing 16% and 18.2% of their respective cuticles. The post hoc pairwise comparisons showed a significant differentiation based on chemical composition between all groups of caterpillars and their respective attendant ants (Table 2). Moreover, the PCA revealed that ants and caterpillars form separate groups. In terms of major compounds, C. crassus had the n-C21, n-C30 and C33:1, and P. longicornis had the n-C29, 15-;13-;11-;9-MeC29, 15-;13-;11-MeC29 and 9.17-; 9.19-diMeC29 (Table 1).
In general, caterpillars, host plants, and attendant ants shared the n-C27, n-C29, and n-C31 compounds (Fig. 3). Only caterpillars and plants showed alcohols and aldehydes in their chemical profiles (hexadecanol, octadecanol, eicosanol, octacosanal, docosanol, and triacontanal). The most significant differentiating compounds among the groups, according to SIMPER analysis, were the n-C33, n-C31 and, n-C29, which were major compounds in caterpillars (Fig. 2; Table S2). Overall, we observed that all groups of caterpillars presented high similarity in composition and proportion of their cuticular compounds. Host plants and caterpillars had a higher number of compounds in common, with n-alkanes (n-C29 and n-C31) as their major compounds. Host plants also had various alcohols and aldehydes, which are also present in caterpillars, but in smaller proportions. The three plants exhibited qualitative similarities in their linear alkanes, with some variations in proportion. Additionally, there were qualitative differences in the alcohols and aldehydes among the three species. Thus, the cuticular profile of caterpillars was not influenced by attendant ants. Ant species showed a greater diversity of compounds compared to caterpillars, including various branched alkanes, alkanes, and alkenes (Fig. 3).
Box plots of the relative abundance of the most important chemical compounds (n-C29, n-C31 and n-C33) that contributed to differentiating the groups according to SIMPER. Groups 1 and 2 include attendant ants; 3–5 include host plants and 6–10 include caterpillars exposed to different conditions. Different letters represent p < 0.05
Comparison of CHC profiles among host plant, myrmecophilous caterpillar, and attendant ants. All compound identities can be found in Table 1
Discussion
We found that the cuticular profile of caterpillars are more similar to those of the host plants rather than to their tending ants. Moreover, caterpillars reared on different species of plants, without ants or in close contact with ants also had similar cuticular composition and proportion, meaning that in an overall perspective caterpillar cuticular composition is weakly affected by exogenous factors. However, some of their compounds varied depending on the host plants where they were reared, which suggests that they acquired part of these compounds through their diet. Thus, S. calyce caterpillars have a chemical profile that it is slightly altered by their food. Consequently, our hypothesis that the caterpillar cuticular composition is influenced by their food source was partially corroborated. Even though the caterpillars are not chemically identical to their host plants, there is a higher similarity between caterpillar-plant when compared to caterpillar-ant species. Given that some compounds, such as 1-hectacontanol, are present in one of the host plants and in caterpillars reared on that plant, but not in the other groups of caterpillars, this may suggest that at least part of the caterpillar chemical composition should derive from their food source. In this way, we suggest that the chemical composition of caterpillars is mainly genetically derived and slightly influenced by the environment. Acquiring compounds from host plants through diet and the usage of them when interacting with ants stands out as a promising strategy and it has also been observed in other plant-herbivorous insect systems (e.g., Silveira et al. 2010; Lima et al. 2021, 2024).
The three plant species used to feed the caterpillars exhibit qualitative similarities in their linear alkanes, as well as in certain alcohols and aldehydes. This suggests that S. calyce females likely tend to lay eggs on plants with similar chemical profiles, a similar pattern was observed in lycaenids (Lima et al. 2021). There is evidence that alkanes and alcohols can serve as signals for host plant selection (Li and Ishikawa 2006; Barbero 2016; Bertea et al. 2020). In a recent study involving S. calyce, it was observed that butterflies sometimes mistakenly lay eggs directly on treehoppers because the treehoppers have a cuticular profile similar to that of the host plant (Lima et al. 2023). The compounds found on the surface of leaves have been described to play a role in the chemical defense of plants (aliphatic hydrocarbons, fatty and phenolic acids derivatives) (Martemyanov et al. 2015: Bertea et al. 2020). Hence, there is a possibility that an evolutionary arms race between caterpillars and plants has driven caterpillars to develop mechanisms countering the chemical defenses of plants. This may involve detoxification of compounds through enzymes or the sequestration of such compounds. Consequently, caterpillars may exhibit a preference for specific chemical compounds present in various host plants (Zu et al. 2020).
Through chemical analyses, we found that the chemical profile of caterpillars were not influenced by their attendant ants thus confirming our second hypothesis that S. calyce CHCs are not affected by the interactions that they establish with different ant species. The two ant species studied showed distinct chemical profiles, while the groups of S. calyce caterpillars reared with different ant species or without ants had similar cuticular profiles composition and proportion. This indicates that S. calyce caterpillars have cuticular profiles that are independent of their attendant ants, indicating that they do not use chemical mimicry as a strategy when interacting with them. This finding is supported by the dissimilarity in chemical composition between caterpillars and ants, compared to the similarity between caterpillars and plants. Additionally, S. calyce caterpillars have facultative association with several species of ants, and they do not exploit ant nests (Callaghan 1986; Alves-Silva et al. 2018; Kaminski 2021). Previous studies demonstrating chemical mimicry between caterpillars and ants have typically involved obligate interactions with a few specific ant species, where the CHCs of caterpillars closely resemble those of the ants (Henning 1983; Elmes et al. 1991, 2002; Dettner and Liepert 1994; Akino et al. 1999; Schönrogge et al. 2004; Hojo et al. 2009, 2014; Thomas et al. 2013; Witek et al. 2013; Barbero 2016; Casacci et al. 2019). Chemical mimicry with attendant ants is an effective strategy for social parasitic caterpillars, as they typically inhabit ant nests and benefit from being perceived as members of the colony, allowing them to exploit valuable resources within the nest such as ant larvae or trophallaxis (Fiedler 1991; Barbero 2016; Casacci et al. 2019). We also ruled out the possibility of a chemical insignificance strategy in S. calyce caterpillars as in previous studies where caterpillars and pupae used chemical insignificance, their cuticular profile consisted of only a few hydrocarbons in very small proportions (Lohman et al. 2006; Inui et al. 2015). In contrast, S. calyce caterpillars showed 27–30 cuticular compounds, with some of them in high proportions.
Thus, it seems that this species maintains its own chemical profile in a chemical conspicuousness strategy (sensu Lima et al. 2021). This strategy is the most likely since the chemical profiles of caterpillars exposed to various conditions remained mainly unchanged, with all groups exhibiting a high degree of similarity. Chemical conspicuousness becomes advantageous when interacting with attendant ants, increasing the likelihood that ants will associate the reward with specific cuticular profiles (Hojo et al. 2014). Studies conducted with Neotropical Lycaenidae species have demonstrated that these species possess conspicuous chemical profiles (Lima et al. 2021). Additionally, there are studies showing that caterpillars or pupae of facultative lycaenid butterflies from other regions have unique chemical profiles recognized by ants, which helps maintain their attending behavior (Ômura et al. 2009, 2012; Hojo et al. 2014; Mizuno et al. 2018). However, to our knowledge, this is the first study to explore cuticular hydrocarbons (CHCs) and investigate chemical strategies in a facultative species of the Riodinidae family.
In this study, caterpillars and plants shared several compounds when compared to ants. Notably, linear alkanes such as C29 and C31 are present in significant proportions across all groups, along with some alcohols and aldehydes. Therefore, we cannot rule out the possibility that in certain instances, caterpillars may employ chemical camouflage. Moreover, caterpillars reared on one host plant species exhibited a similarity exceeding 80%, which has been previously demonstrated as sufficient to serve as chemical camouflage strategy in other insect groups (Silveira et al. 2010). Host plants play a crucial role in providing an effective background for herbivorous organisms, allowing them to avoid detection by visually or chemically resembling their surroundings. This strategy is observed in various organisms that have close relationships with ants, enabling them to interact with ants without being attacked (von Beeren et al. 2012; Barbero 2016; Lima et al. 2024). Consequently, we propose that these caterpillars may employ a trade-off strategy between camouflage and informing their presence to ants, which could vary depending on the presence of predators or mutualist ants. Given the presence of numerous non-attendant ant species that visit the host plants, many of which have extrafloral nectaries, it is highly likely that chemical camouflage has been selected as an efficient strategy for S. calyce caterpillars to avoid attacks from different ants (Akino et al. 2004). On the other hand, informing their presence to attendant ants also is efficient for the caterpillars. The complexity of CHCs profiles is well-known, and it is likely that each chemical trait serves a distinct function (Sprenger and Menzel 2020). There may even be conflicts or trade-offs among the various functions of the chemical profile (Steiger and Stökl 2014; Ingleby 2015). Camouflage might be the protagonist during encounters with non-attendant ants, while directly informing their presence becomes more prominent with attendant ants, as it aims to induce ants to associate caterpillars’ chemical profile with the chemical reward, consequently securing ant protection (Hojo et al. 2014).
We suggest that specific compounds such as some aldehydes present in both plants and caterpillars or methylated alkanes present in caterpillars, play a role in making the caterpillars blend with the background or informing their presence to attendant ants respectively. For instance, in studies conducted on Lycaeides argyrognomon (Bergsträsser, 1779) (Lepidoptera: Lycaenidae), pupal cuticular lipids were found to contain various long-chain aliphatics aldehydes, including 1-octacosanal and 1-triacontanal, which were found to suppress ant aggression (Mizuno et al. 2018). Interestingly, these two compounds were found in all groups of S. calyce caterpillars and host plants, suggesting their potential importance in the interaction between S. calyce caterpillars and ants.
Studying tri-trophic relationships can present challenges when analyzing the results. For instance, in the PCA results, we observed a low percentage for PC1 and PC2. We suspect that these low percentages values may be linked to the number of groups included in the analysis, comprising five caterpillars, three plants, and two ant groups. The low existent variation within each main group (e.g. ant, caterpillar, and plant) may have contributed to an overall lower dissimilarity percentage when comparing all the groups at once. Thus, to elucidate our findings, we employed more than one type of analysis. Using multiple approaches to analyze the data stands out as a useful strategy when working with complex systems.
Ants exert strong selection pressure on myrmecophilous caterpillars, leading to the development of multimodal adaptations (Pierce and Dankowicz 2022; Marquis and Koptur 2022). These adaptations include morphophysiological, behavioral, chemical, and acoustic traits that caterpillars utilize to deceive, attract, alarm, or appease attending ants (Fiedler et al. 1996; Casacci et al. 2019). Synargis calyce caterpillars possess functional TNOs, which have been shown to contribute to the association with ants in other riodinids (DeVries 1988; Kaminski and Carvalho-Filho 2012; Kaminski et al. 2013; Mota et al. 2020; Kaminski et al. 2021). To further enhance our understanding of the multimodal signaling in myrmecophile systems, future studies should conduct behavioral assays to experimentally confirm the chemical strategy employed and investigate the products and effects of caterpillars’ ant-organs on attending ants, as well as compare them with the products of extrafloral nectaries from plants. This research will contribute to unraveling the specific role of these chemical strategies and organs in the complex interactions between myrmecophilous caterpillars and ants.
Data Availability
Data was deposited in the supplementary section.
References
Akino T (2008) Chemical strategies to deal with ants: a review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol News 11:173–181
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea Rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond B Biol Sci 266:1419–1426. https://doi.org/10.1098/rspb.1999.0796
Akino T, Nakamura KI, Wakamura S (2004) Diet-induced chemical phytomimesis by twig-like caterpillars of Biston Robustum Butler (Lepidoptera: Geometridae). Chemoecology 14:165–174. https://doi.org/10.1007/s00049-004-0274-4
Alves-Silva E, Bächtold A, Del-Claro K (2018) Florivorous myrmecophilous caterpillars exploit an ant–plant mutualism and distract ants from extrafloral nectaries. Austral Ecol 43:643–650. https://doi.org/10.1111/aec.12609
Barbero F (2016) Cuticular lipids as a cross-talk among ants, plants and butterflies. Int J Mol Sci 17:1966. https://doi.org/10.3390/ijms17121966
Barbero F, Patricelli D, Witek M, Balletto E, Casacci LP, Sala M, Bonelli S (2012) Myrmica ants and their butterfly parasites with special focus on the acoustic communication. Psyche 2012:1–11. https://doi.org/10.1155/2012/725237
Beccaloni GW, Viloria AL, Hall SK, Robinson GS (2008) Catalogue of the hostplants of the neotropical butterflies/Catálogo De las plantas huésped de las mariposas neotropicales. Monografias Tercer Milenio, Saragosa
Bertea C, Casacci P, Bonelli S, Zampollo A, Barbero F (2020) Chemical, physiological, and molecular responses of host plants to lepidopteran egg-laying. Front Plant Sci 10:1768. https://doi.org/10.3389/fpls.2019.01768
Blomquist GJ, Bagnères AG (2010) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press
Callaghan CJ (1986) Restinga butterflies: biology of Synargis brennus (Stichel) (Riodinidae). J Lepid Soc 40:93–96
Carlson DA, Bernier UR, Sutton BD (1998) Elution patterns from capillary GC for methyl-branched alkanes. J Chem Ecol 24:1845–1865
Casacci LP, Bonelli S, Balletto E, Barbero F (2019) Multimodal signaling in myrmecophilous butterflies. Front Ecol Evol 7:454. https://doi.org/10.3389/fevo.2019.00454
Cottrell CB (1984) Aphytophagy in butterflies: its relationship to myrmecophily. Zool J Linn Soc 80:1–57. https://doi.org/10.1111/j.1096-3642.1984.tb02318.x
da Silva RC, Brown RL, do Nascimento FS, Wenseelers T, Oi CA (2021) Cuticular hydrocarbons as cues of caste and sex in the German wasp Vespula Germanica. Insect Soc 68:261–276. https://doi.org/10.1007/s00040-021-00817-5
Dettner K, Liepert C (1994) Chemical mimicry and camouflage. Annu Rev Entomol 39:129–154. https://doi.org/10.1146/annurev.en.39.010194.001021
DeVries PJ (1988) The larval ant-organs of Thisbe Irenea (Lepidoptera: Riodinidae) and their effects upon attending ants. Zool J Linn Soc 94:379–393. https://doi.org/10.1111/j.1096-3642.1988.tb01201.x
DeVries PJ, Harvey DJ, Kitching IJ (1986) The ant associated epidermal organs on the larva of the lycaenid butterfly Curetis regula Evans. J Nat Hist 20:621–633. https://doi.org/10.1080/00222938600770421
Elmes G, Akino T, Thomas J, Clarke R, Knapp J (2002) Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525–535. https://doi.org/10.1007/s00442-001-0857-5
Elmes G, Thomas JA, Wardlaw JC (1991) Larvae of Maculinea rebeli, a large-blue butterfly, and their Myrmica host ants: wild adoption and behaviour in ant‐nests. J Zool 223:447–460. https://doi.org/10.1111/j.1469-7998.1991.tb04775.x
Espelie KE, Bernays EA, Brown JJ (1991) Plant and insect cuticular lipids serve as behavioral cues for insects. Arch Insect Biochem Physiol 17:223–233
Ferguson ST, Bakis I, Zwiebel LJ (2021) Advances in the study of olfaction in eusocial ants. Insects 12:252. https://doi.org/10.3390/insects12030252
Fiedler K (1991) European and North West African Lycaenidae (Lepidoptera) and their associations with ants. J Res Lepid 28:239–257
Fiedler K (1994) Lycaenid butterflies and plants: is myrmecophily associated with amplified hostplant diversity? Ecol Entomol 19:79–82. https://doi.org/10.1111/j.1365-2311.1994.tb00393.x
Fiedler K (1995) Associations of lycaenid butterflies with ants in Turkey. Die tagfalter der türkei unter Berücksichtigung der angrenzenden Länder 1:437–450
Fiedler K (1996) Host-plant relationships of lycaenid butterflies: large-scale patterns, interactions with plant chemistry, and mutualism with ants. Entomol Exp Appl 80:259–267
Fiedler K (2021) The ant associates of Lycaenidae butterfly caterpillars–revisited. Nota Lepidopterologica 44:159–174. https://doi.org/10.3897/nl.44.68993
Fiedler K, Hölldobler B, Seufert P (1996) Butterflies and ants: the communicative domain. Experientia 52:14–24. https://doi.org/10.1007/BF01922410
Gnatzy W, Jatho M, Kleinteich T, Gorb S, Hustert R (2017) The eversible tentacle organs of Polyommatus caterpillars (Lepidoptera, Lycaenidae): morphology, fine structure, sensory supply and functional aspects. Arthropod Struct Develop 46:788–804. https://doi.org/10.1016/j.asd.2017.10.003
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hefetz A (2007) The evolution of hydrocarbon pheromone parsimony in ants (Hymenoptera: Formicidae)-interplay of colony odor uniformity and odor idiosyncrasy. Myrmecol News 10:59–68
Henning SF (1983) Chemical communication between Iycaenid larvae (Lepidoptera: Lycaenidae) and ants (Hymenoptera: Formicidae). J Entomol Soc South Afr 46:341–366
Hill GM, Trager MD, Lucky A, Daniels JC (2022) Protective benefits of tending ants to a critically endangered butterfly. J Insect Sci 22:9. https://doi.org/10.1093/jisesa/ieac068
Hojo MK, Wada-Katsumata A, Akino T, Yamaguchi S, Ozaki M, Yamaoka R (2009) Chemical disguise as particular caste of host ants in the ant inquiline parasite Niphanda fusca (Lepidoptera: Lycaenidae). Proc R Soc Lond B Biol Sci 276:551–558. https://doi.org/10.1098/rspb.2008.1064
Hojo MK, Yamamoto A, Akino T, Tsuji K, Yamaoka R (2014) Ants use partner specific odors to learn to recognize a mutualistic partner. PLoS ONE 9:e86054. https://doi.org/10.1371/journal.pone.0086054
Hölldobler B, Kwapich (2022) The Lycaenidae: mutualists, predators, and parasites. The guests of ants: how myrmecophiles interact with their hosts. Harvard University Press, Massachusetts, pp 149–219
Hölldobler B, Wilson EO (1990) The Ants. The Belknap Press of Harvard University Press, Cambridge, p 732
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Ann Rev Entomol 50:371–393. https://doi.org/10.1146/annurev.ento.50.071803.130359
Ingleby FC (2015) Insect cuticular hydrocarbons as dynamic traits in sexual communication. Insects 6:732–742. https://doi.org/10.3390/insects6030732
Inui Y, Shimizu-kaya U, Okubo T, Yamsaki E, Itioka T (2015) Various chemical strategies to deceive ants in three Arhopala species (Lepidoptera: Lycaenidae) exploiting Macaranga. PLoS ONE 10:e0120652. https://doi.org/10.1371/journal.pone.0120652
Kaminski LA (2021) Ant-butterfly interactions - Borboletas formigueiras. iNaturalist. https://www.inaturalist.org/projects/ant-butterfly-interactions-borboletas-formigueiras . Accessed 26 Apr 2023
Kaminski LA (2008) Polyphagy and obligate myrmecophily in the butterfly Hallonympha paucipuncta (Lepidoptera: Riodinidae) in the Neotropical Cerrado savanna. Biotropica 40:390–394. https://doi.org/10.1111/j.1744-7429.2007.00379.x
Kaminski LA, Carvalho-Filho FS (2012) Life history of Aricoris propitia (Lepidoptera: Riodinidae)—A myrmecophilous butterfly obligately associated with fire ants. Psyche 2012:1. https://doi.org/10.1155/2012/126876
Kaminski LA, Mota LL, Freitas AV, Moreira GR (2013) Two ways to be a myrmecophilous butterfly: natural history and comparative immature-stage morphology of two species of Theope (Lepidoptera: Riodinidae). Biol J Linn Soc 108:844–870. https://doi.org/10.1111/bij.12014
Kaminski LA, Volkmann L, Callaghan CJ, DeVries PJ, Vila R (2021) The first known riodinid ‘cuckoo’butterfly reveals deep-time convergence and parallelism in ant social parasites. Zool J Linn Soc 193:860–879. https://doi.org/10.1093/zoolinnean/zlaa150
Krebs CJ (1999) Ecological Methodology. Addison Wesley Longman, Menlo Park
Kronauer DJ, Pierce NE (2011) Myrmecophiles. Curr Biol 21:R208–R209
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) The individuality and the colonial identity in ants: the emergence of the social representation concept. In: Information Processing in Social Insects, Basel, pp 219–37
Li GQ, Ishikawa Y (2006) Leaf epicuticular wax chemicals of the Japanese knotweed Fallopia japonica as oviposition stimulants for Ostrinia latipennis. J Chem Ecol 32:595–604. https://doi.org/10.1007/s10886-005-9022-7
Lima LD, Ceballos-González AV, Prato A, Cavalleri A, Trigo JR, Nascimento FS (2024) Chemical camouflage induced by diet in a pest treehopper on host plants. Plants 13:216. https://doi.org/10.3390/plants13020216
Lima LD, Ceballos-González AV, Prato A, Kaminski LA, Nascimento FS (2023) Plant-treehopper convergence may trick butterflies into trophic oviposition mistakes. Biotropica 55:292–298. https://doi.org/10.1111/btp.13194
Lima LD, Trigo JR, Kaminski LA (2021) Chemical convergence between a guild of facultative myrmecophilous caterpillars and host plants. Ecol Entomol 46:66–75. https://doi.org/10.1111/een.12941
Lohman DJ, Liao Q, Pierce NE (2006) Convergence of chemical mimicry in a guild of aphid predators. Ecol Entomol 31:41–51. https://doi.org/10.1111/j.0307-6946.2006.00758.x
Malicky H (1970) New aspects of the association between lycaenid larvae (Lycaenidae) and ants (Formicidae, Hymenoptera). J Lepid Soc 24:190–202
Marquis RJ, Koptur S (2022) Caterpillars in the middle: Tritrophic interactions in a changing world. Springer, pp 319–391
Martemyanov VV, Pavlushin SV, Dubovskiy IM, Belousova IA, Yushkova YV, Morosov SV, Glupov VV (2015) Leaf surface lipophilic compounds as one of the factors of silver birch chemical defense against larvae of gypsy moth. PLoS ONE 10:e0121917. https://doi.org/10.1371/journal.pone.0121917
Mizuno T, Hagiwara Y, Akino T (2018) Chemical tactic of facultative myrmecophilous lycaenid pupa to suppress ant aggression. Chemoecology 28:173–182. https://doi.org/10.1007/s00049-018-0270-8
Mota LL, Kaminski LA, Freitas AV (2020) The tortoise caterpillar: carnivory and armoured larval morphology of the metalmark butterfly Pachythone xanthe (Lepidoptera: Riodinidae). J Nat Hist 54:309–319. https://doi.org/10.1080/00222933.2020.1759720
Newcomer EJ (1912) Some observations on the relations of ants and lycaenid caterpillars, and a description of the relational organs of the latter. J N Y Entomol Soc 20:31–36
Nunes TM, Mateus S, Favaris AP, Amaral MF, von Zuben LG, Clososki GC, Lopes NP (2014) Queen signals in a stingless bee: suppression of worker ovary activation and spatial distribution of active compounds. Sci Rep 4:7449. https://doi.org/10.1038/srep07449
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MHH Szoecs E + 1 more (2013) Package ‘vegan’. Community ecology package. 1-295 version 2
Ômura H, Watanabe M, Honda K (2009) Cuticular hydrocarbons of larva and pupa of Reverdin’s blue, Lycaeides argyrognomon (Lycaenidae) and its tending ants. Lepid Sci 60:203–210. https://doi.org/10.18984/lepid.60.3_203
Ômura H, Watanabe M, Honda K (2012) Cuticular hydrocarbon profiles of Lycaeides subsolanus larvae and their attendant ants. Lepid Sci 63:186–190. https://doi.org/10.18984/lepid.63.4_186
Otte T, Hilker M, Geiselhardt S (2018) Phenotypic plasticity of cuticular hydrocarbon profiles in insects. J Chem Ecol 44:235–247. https://doi.org/10.1007/s10886-018-0934-4
Parker J (2016) Myrmecophily in beetles (Coleoptera): evolutionary patterns and biological mechanisms. Myrmecological news 22:65–108
Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47:733–771. https://doi.org/10.1146/annurev.ento.47.091201.145257
Pierce NE, Dankowicz E (2022) Behavioral, ecological and evolutionary mechanisms underlying caterpillar-ant symbioses. Curr Opin Insect Sci 52:100898. https://doi.org/10.1016/j.cois.2022.100898
Portugal AHA, Trigo JR (2005) Similarity of cuticular lipids between a caterpillar and its host plant: a way to make prey undetectable for predatory ants? J Chem Ecol 31:2551–2561. https://doi.org/10.1007/s10886-005-7613-y
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 16 Feb 2023
Schlick-Steiner BC, Steiner FM, Höttinger H, Nikiforov A, Mistrik R, Schafellner C, Baier P, Christian E (2004) A butterfly’s chemical key to various ant forts: Intersection-odour or aggregate-odour multi-host mimicry? Naturwissenschaften 91:209–214. https://doi.org/10.1007/s00114-004-0518-8
Schönrogge K, Barbero F, Casacci LP, Settele J, Thomas JA (2017) Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim Behav 134:249–256. https://doi.org/10.1016/j.anbehav.2016.10.031
Schönrogge K, Wardlaw J, Peters A, Everett S, Thomas J, Elmes G (2004) Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J Chem Ecol 30:91–107. https://doi.org/10.1023/B:JOEC.0000013184.18176.a9
Silveira HC, Oliveira PS, Trigo JR (2010) Attracting predators without falling prey: chemical camouflage protects honeydew-producing treehoppers from ant predation. Am Nat 175:261–268. https://doi.org/10.1086/649580
Sprenger PP, Menzel F (2020) Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: how and why they differ among individuals, colonies, and species. Myrmecol News 30:1–6. https://doi.org/10.25849/myrmecol.news_030:013
Steiger S, Stökl J (2014) The role of sexual selection in the evolution of chemical signals in insects. Insects 5:423–438. https://doi.org/10.3390/insects5020423
Thomas JA, Elmes G, Sielezniew M, Stankiewicz-Fiedurek A, Simcox DJ, Settele J, Schönrogge K (2013) Mimetic host shifts in an endangered social parasite of ants. Proc R Soc Lond B Biol Sci 280:20122336. https://doi.org/10.1098/rspb.2012.2336
Thomas JA, Schönrogge K, Elmes GW (2005) Specialisations and host associations of social parasites of ants. Evolutionary ecology. In: Rolff J, Fellowes M, Holloway G (eds) Symposium of the Royal Entomological Society XXI, pp 475–514. https://doi.org/10.1079/9780851998121.047
von Beeren C, Pohl S, Witte V (2012) On the use of adaptive resemblance terms in chemical ecology. Psyche 2012:1. https://doi.org/10.1155/2012/635761
Witek M, Casacci LP, Barbero F, Patricelli D, Sala M, Bossi S, Bonelli S (2013) Interspecific relationships in co-occurring populations of social parasites and their host ants. Biol J Linn Soc 109:699–709. https://doi.org/10.1111/bij.12074
Yamaoka R (1990) Chemical approach to understanding interactions among organisms. Physiol Ecol Jap 27:31–52
Zu P, Boege K, Del-Val E, Schuman MC, Stevenson PC, Zaldivar-Riverón A, Saavedra S (2020) Information arms race explains plant-herbivore chemical communication in ecological communities. Science 368:1377–1381. https://doi.org/10.1126/science.aba2965
Acknowledgements
We thank Paulo Roberto Barbosa for host plant identification.
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grants to AVCG (140313/2020-6) and FSN (05082/2018-5 and 307702/2018-9). This study was financially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 to AVCG and RCS. Additional support was given by São Paulo Research Foundation (FAPESP) to RCS (grant 2018/22461-3), LDL (2021/00984-7) and to FSN (2021/05598-8 and 2018/10996-0). RCS currently holds a Fyssen Postdoctoral Fellowship (Fyssen Fondation, France).
Author information
Authors and Affiliations
Contributions
AVCG and LAK conceived, designed and conducted field experiments. AVCG, RCS, ICCT, FSN and NPL conducted chemical analysis. AVCG, RCS and LDL conducted data analysis. AVCG and RCS wrote the first version of the manuscript. All the authors read, wrote the final version and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 656 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ceballos-González, A.V., da Silva, R.C., Lima, L.D. et al. Influence of Host Plants and Tending Ants on the Cuticular Hydrocarbon Profile of a Generalist Myrmecophilous Caterpillar. J Chem Ecol 50, 222–236 (2024). https://doi.org/10.1007/s10886-024-01477-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-024-01477-y