Abstract
The partitioning of overall beta diversity into local contributions (LCBD; compositional uniqueness) and species contributions (SCBD) has enhanced the understanding of the organization of metacommunities. Here, we evaluated LCBD and SCBD of aquatic macrophyte metacommunities from 29 subtropical coastal wetlands, covering an extension of 640 km, in Southern Brazil. Furthermore, we assessed the environmental uniqueness, considering both the physical and chemical variables of water in wetlands, as well as climatic variables. We assessed the environmental and spatial correlates of LCBD and the environmental correlates of SCBD. We expected that (i) communities with greater compositional uniqueness would be found in environmentally more unique wetlands, (ii) the compositional uniqueness would also be related to climatic variables that determine the hydrological changes in wetlands, and (iii) the contribution of emergent species to beta diversity would be lower than of floating and submerged species. The compositional uniqueness did not present a clear spatial structure, being positively related to uniqueness in water chemistry and negatively to the mean air temperature during the driest quarter. The species contribution to beta diversity was higher for floating species than for emergent ones. Moreover, SCBD correlated to climatic variables and presented a positive relationship with site occupation, suggesting that more frequent species in terms of site occurrence contribute most to beta diversity. Our results show the importance of local environmental and broader climatic predictors in determining the compositional uniqueness of macrophyte communities and species contributions to overall beta diversity in the coastal wetlands of Southern Brazil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Untangling the variation in species composition among sites in a geographic area (beta diversity; Whittaker 1960; Anderson et al. 2011) into its components is fundamental to understand how biodiversity varies in space (Li et al. 2020; Panja et al. 2022; García-Girón et al. 2023) and time (Legendre and Gauthier 2014; Winegardner et al. 2017; Legendre and Condit 2019). In this context, partitioning overall beta diversity into the contribution of each local community (local contribution to beta diversity, LCBD) and of each species (species contribution to beta diversity, SCBD) (sensu Legendre and De Cáceres 2013) may shed light on the distinct factors affecting the organization of metacommunities (Tonkin et al. 2016; Schneck et al. 2022).
The contribution of each local community to beta diversity (LCBD) depends on its compositional uniqueness, that is, the degree of differentiation in species composition in relation to the regional typical community composition (Legendre and De Cáceres 2013). More unique species compositions can be related either to unique environmental characteristics or to dispersal limitation owing to geographic distance among sites (Heino et al. 2017; Landeiro et al. 2018). In freshwater ecosystems, compositional uniqueness was shown to be positively related to environmental uniqueness generated by stream physical characteristics for diatoms (Castro et al. 2019), insects (Schneck et al. 2022) and macrophytes (Bomfim et al. 2023). However, this relationship between compositional and environmental uniqueness is not explicitly considered in most studies (e.g., Tonkin et al. 2016; Bomfim et al. 2023; but see Heino et al. 2022). Moreover, most previous studies on freshwater systems have focused on evaluating local environmental correlates of compositional uniqueness (e.g., Heino and Grönroos 2017; Leão et al. 2020), so that the knowledge about the importance of regional-scale environmental gradients, such as climate, is scarce (Benito et al. 2020; Panja et al. 2022; Martínez-Román et al. 2023).
Species that contribute the most to beta diversity (SCBD) within a region of interest are those that vary more or less than average among sites (Legendre and De Cáceres 2013). For freshwater communities, it has been shown that species with high SCBD have either intermediate (Szabó et al. 2019; Pozzobom et al. 2020) or high occupancy (Vilmi et al. 2017a) and are regionally abundant (Vilmi et al. 2017a; Szabó et al. 2019). However, an alternative approach to better understand why certain species or groups of species may contribute more than others to beta diversity would be to relate SCBD to species traits (Li et al. 2020; Schneck et al. 2022) or functional groups (Heino and Grönroos 2017; Pozzobom et al. 2020) and environmental variables (Schneck et al. 2022). For example, in a study conducted in Brazilian floodplain lakes, functional groups of aquatic macrophytes partially explained SCBD (Pozzobom et al. 2020).
Wetlands are functionally complex ecosystems characterized by fluctuations in water level, which determine their physical, chemical, and biological conditions (Junk et al. 2014). Despite harboring a significant portion of biodiversity, these ecosystems are among the most vulnerable in the world (Junk et al. 2014), especially owing to the influence of factors, such as climate change, urban development, pollution, and invasive species, highlighting the urgency of protection and conservation actions (Maltchik et al. 2004). In Southern Brazil, palustrine wetlands represent a large proportion of wetlands, approximately 90%, and they can vary from permanently flooded to never flooded, but remain saturated for long periods during the annual cycle (Maltchik et al. 2004). They include a variety of formations, such as marshes, shallow lakes, meanders, and floodplains influenced by factors, such as precipitation, surface runoff, and groundwater discharge (Maltchik et al. 2004).
Aquatic macrophytes are a key component in wetlands (De Magalhaes et al. 2016) and are characterized by a wide range of strategies that enables them to establish in distinct habitats (Santamaría 2002; Lacoul and Freedman 2006). Some species can grow through the water surface, while others live floating or submerged in the water column (Chambers et al. 2008; Murphy et al. 2019). Owing to such distinct architecture, the importance of local and regional environmental factors and spatial processes in determining the distribution of species (Alahuhta and Heino 2013; Gillard et al. 2020) differs among life forms (Schneider et al. 2018; Trindade et al. 2018; Garcia-Girón et al. 2023). For example, in subtropical coastal wetlands, it has been shown that the distribution of floating and submerged species was mainly influenced by water quality and spatial variables, respectively, while emergent species, characterized by a broader distribution, were influenced by climatic variables (Trindade et al. 2018). Moreover, it has been suggested that relating SCBD values to macrophyte life forms can improve our ability to detect species that contribute more to beta diversity (Pozzobom et al. 2020; Bomfim et al. 2023). Finally, identifying communities and species contributing the most to beta diversity would help understand key aspects of the organization of macrophyte metacommunities in wetlands.
We investigated the contribution of local communities and species to beta diversity of aquatic macrophytes in coastal wetlands, over c. 640 km in subtropical Brazil. We assessed the environmental and spatial correlates of LCBD and the environmental and biological correlates of SCBD. We expected that: (i) communities with greater compositional uniqueness would be found in environmentally more unique wetlands; (ii) given the large spatial scale of our study, compositional uniqueness would also be related to climatic variables that determine the hydrological changes in wetlands; and (iii) the contribution of emergent species to beta diversity would be lower than of floating and submerged species because the first ones are less dependent on water quality and are more widely distributed.
Material and methods
Study area
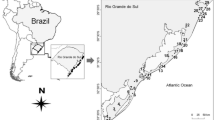
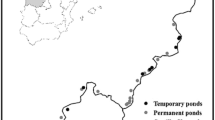
We conducted the study in 29 wetlands at the coastal plain of Rio Grande do Sul State, between coordinates (29º16′14.96″ S and 49º37′15.08″ W) and (33º37′25.15″ S and 53º13′46.56″ W), South Brazil (Fig. 1). The coastal plain extends approximately 640 km, covering 22,740 km2 of emerged lands and 14.260 km2 of water surfaces, totaling 37,000 km2 (Schwarzbold and Schäfer 1984). Originating from the Cenozoic, the coastal plain expanded primarily during the Quaternary period through alluvial fans and barrier–lagoon depositional systems, forming large bodies of water such as the Patos lagoon and lakes Mirim and Mangueira (Barboza et al. 2009). Currently, the landscape of the coastal plain consists of estuaries and deltas, marine beaches, rivers and streams, savannas, palm groves, flooded fields, and forests (seasonal, temporary, or permanent), lakes and lagoons, and permanently or temporarily wetlands (known in southern Brazil as banhados) (Becker et al. 2007). The climate of the region is humid subtropical (Maluf 2000). Annual precipitation varies between 1000 and 1500 mm, and the average annual temperature ranges between 16 and 20 ˚C, with average temperatures between 22 and 26 ˚C in the hottest months and between 10 and 15 ˚C in the coldest months (Nimer 1977).
To select the sampling sites, we chose areas with saturated soils or shallow waters, where vegetation is adapted to moist conditions and flooding, following the definition of wetlands by Mitsch and Gosselink (2000). On the basis of this criterion, we selected freshwater wetlands that allowed us to walk through their entire extent for sampling data. We checked all areas where it was possible to observe the surroundings to ensure they met the objectives of our study. We avoided large wetlands and those that did not have clearly defined border areas, which would be difficult to visually inspect; we also discarded wetlands clearly connected to other aquatic environments, as well as those near monocultures or other human activities. Despite being in a coastal plain, the sampled wetlands did not have any connection with the ocean and are characterized as freshwater (in situ measured salinity = 0 in all 29 sampled wetlands). Additionally, we used geographic coordinates to ensure that the chosen areas represented the coastal plain extensively. The wetlands’ area ranged from 0.19 to 5 hectares, with an average depth of 30 ± 10 cm. Each wetland was sampled once during the austral summer of 2016, a period characterized by low volume of rainfall. We used this dataset previously to evaluate the relative role of environmental and spatial factors on macrophyte species richness and assemblage composition (Trindade et al. 2018).
Aquatic macrophytes
To determine the richness and composition of aquatic macrophyte assemblages, we conducted a thorough visual survey of the vegetation. This study took place during a period of low precipitation, which may influence the size of wetland areas, thus facilitating our walk throughout each visited wetland area. The same researcher recorded the species in all sampled wetlands. The time spent searching for species varied among wetlands (from 60 to 90 min); always being interrupted after 20 min of walking without registering a new species. We identified the species on the basis of specialized literature (Cordazzo and Seeliger 1995; Irgang and Gastal 1996), as well as collaboration with specialists. We registered 114 aquatic macrophytes species: 90 emergent, 14 floating (including free-floating and rooted floating-leaved species), and 10 submerged (including fixed submerged and free-floating submerged species); detailed information is available in Trindade et al. (2018).
Local environmental variables
In each wetland, we used a multiparameter water quality meter (Horiba-U50) to measure the following environmental variables at five random points: dissolved oxygen (DO; mg L−1), pH, and electrical conductivity (COND; µS cm−1; Supplementary Material, Table 1). We also collected three water samples for analyses of total phosphorus (TP; mg L−1; Valderrama 1981; Baumgarten and Rocha 1996) and total nitrogen (TN; mg L−1; Allen et al. 1974). We determined mean depth of the wet area (cm), using a graduated ruler at the same five points we measured the environmental variables. We recorded the altitude (m) and geographic coordinates at the central point of each wetland and used Google Earth Pro and Quantum Gis (QGis 3.22 Essen) to obtain wetland area (ha).
Climatic variables
We used 12 bioclimatic variables at a spatial resolution of a 1 km2 grid originated from the combination of temperature and precipitation from the global dataset of Worldclim2 (Fick and Hijmans 2017). For our purpose, we obtained the following data: annual mean temperature (AMT; mm), temperature seasonality (TS; standard deviation × 100), maximum temperature of warmest month (MAXTW; ºC), minimum temperature of coldest month (MINTC; ˚C), mean temperature of wettest quarter (MTWeQ; ˚C), mean temperature of driest quarter (MTDrQ; ˚C), annual precipitation (AP; mm), precipitation seasonality (PS; coefficient of variation), precipitation of wettest quarter (PWeQ; mm), precipitation of driest quarter (PDQ; mm), precipitation of warmest quarter (PWQ; mm) and precipitation of coldest quarter (PCQ; mm). These are the same bioclimatic variables selected by Trindade et al. (2018) because of their importance for the organization of macrophyte communities. However, here we opted not to include annual evapotranspiration because the available database is based on the previous version of Worldclim (Hijmans et al. 2005).
Data analyses
We calculated the total beta diversity (BDTotal) and partitioned it into the compositional uniqueness or local contribution (LCBD) and the species contribution to beta diversity (SCBD) following Legendre and De Cáceres (2013). For these analyses, we used presence-absence data and the Hellinger distance. LCBD and SCBD vary between 0 and 1, so that values closer to 1 indicate local communities and species that contribute more to beta diversity (Legendre and De Cáceres 2013).
We used the same approach to calculate local environmental uniqueness (LCEH or local contribution to environmental heterogeneity, as defined by Castro el al. 2019 and Schneck et al. 2022). We used Euclidean distance on two subsets of standardized environmental variables to obtain metrics on the wetlands physical (LCEHPhy; area, depth, and altitude) and water chemical uniqueness (LCEHWC; dissolved oxygen, pH, electric conductivity, total nitrogen, and total phosphorus). To verify which variables contributed more to environmental uniqueness, we correlated the LCEH values with environmental variables using Pearson’s correlation.
First, we used Pearson’s correlation to evaluate how LCBD relates to species richness. Next, we used beta regression (Cribari-Neto and Zeiles 2010) to model the relationship between LCBD and six predictors (LCEHPhy, LCEHWC and the climatic variables MTWeQ, MTDrQ, PS, and PCQ). This final model was defined after excluding the other eight climatic variables owing to multicollinearity (AMT, TS, MAXTW, MINTC, AP, PWeQ, PDQ and PWQ) using a variance inflation factor (VIF) criterion lower than 3. Then, we analyzed the spatial autocorrelation in the model residuals using Moran’s I correlograms. Residuals were spatially independent (P > 0.05, after Bonferroni’s correction for multiple tests; Oden 1984), and thus we did not include spatial variables in the LCBD regression model. Finally, we used Moran’s I correlograms to evaluate whether geographically closer wetlands showed more similar values of compositional uniqueness. The use of Moran’s I based correlograms to test for spatial autocorrelation in the residuals of the regression model and in the response variable (LCBD) followed Zuur et al. (2010) and Heino et al. (2017).
For SCBD we used Pearson’s correlation to explore how it relates to the occurrence of each species (the number of wetlands in which each species occurs). Following, we tested whether macrophyte life forms differ in their SCBD values and we used a beta regression with SCBD as the response variable and life form with three levels as the predictor. After that, we evaluated the pairwise comparisons through simultaneous tests and adjusted P values using the Holm correction. Finally, to advance our understanding on the species-environment relationship for those species that contribute the most to beta diversity, we used the fourth-corner analysis (Dray and Legendre 2008; see Schneck et al. 2022 for a similar approach). We used a matrix of sites and the six predictors, a second matrix of sites and species presence-absence, and a vector of SCBD values. Significance of correlations were assessed through 5999 permutations using model 6 from Dray et al. (2014) and corrected for multiple tests using the Holm correction. This analysis was repeated for the complete set of macrophyte species and separately for each life form.
We performed all analyses in R environment (R Core Team 2023). We used package “adespatial” (Dray et al. 2020) to calculate LCBD, SCBD, and LCEH, “pgirmess” (Giraudoux 2018) for correlograms, “betareg” (Cribari-Neto and Zeileis 2010) for beta regressions, “multcomp” for pairwise comparisons (Hothorn et al. 2008) and “ade4” (Dray and Dufour 2007) for the fourth corner analysis.
Results
Total beta diversity of the macrophyte wetland communities was 0.65. Compositional uniqueness (LCBD) ranged from 0.023 to 0.056 (Fig. 2a) and no spatial structure was observed in LCBD values (Fig. 2b; none distance class was significant; P > 0.05). We found a negative correlation between LCBD and species richness (r = −0.394; P = 0.034; Fig. 3). The regression model showed that compositional uniqueness was positively related to water chemistry uniqueness (LCEHWC) and negatively related to the mean temperature of the driest quarter (MTDrQ) (model pseudo R2 = 0.435; Table 1). Finally, LCEHWC was positively correlated to total phosphorus and electrical conductivity (r = 0.73; r = 0.82, respectively; P < 0.0001).
The SCBD values ranged from 0.002 to 0.023 (0.009 ± 0.01). Of the 114 registered species, 47 species (or 41% of the total) contributed more than the average to beta diversity (Fig. 4a). The three species that most contributed to beta diversity were Schoenoplectus californicus (C. A. Mey) Soják (SCBD = 0.023; occurrence = 18 sites), Nymphoides humboldtiana (Kunth) Kuntze (SCBD = 0.021; occurrence = 20), and Cyperus esculentus L. (SCBD = 0.020; occurrence = 16) (Supplementary Material, Table 2). We found a positive correlation between SCBD values and species occurrence (r = 0.91; P < 0.0001) (Fig. 4b). When comparing SCBD values among life forms, we found that floating species had higher SCBD than emergent species (P = 0.002); however, submerged species did not differ from floating or emergent ones (P > 0.05; Fig. 4c). According to the fourth corner analysis, SCBD was positively correlated with mean temperature of the driest quarter (MTDrQ) and with precipitation of the coldest quarter (PCQ) (Table 2). However, when testing the SCBD-environment separately for each life form, it was clear that this pattern was driven by emergent species (Table 2).
Species contribution to beta diversity (SCBD) of macrophytes and its rank distribution (a), correlation between SCBD and the species occurrence in the wetlands (b) and boxplots of SCBD for the three life forms (c). The colors in plots (a) and (b) correspond to the life form in plot (c). In boxplots, the median is represented by a heavy horizontal line, while the first and third quartiles are depicted by the ends of the box. The whiskers indicate the minimum and maximum values
Discussion
Our findings indicate that the compositional uniqueness of macrophyte metacommunities from the studied coastal wetlands, which span approximately 640 km, is related not to wetland location but to local environmental uniqueness and climatic characteristics. Specifically, compositional uniqueness was positively related to local environmental uniqueness on the basis of water chemistry of the wetlands, and negatively related to the mean temperature of the driest quarter. Regarding the species contribution to beta diversity, the floating life form showed the highest SCBD values. Moreover, the species that most contributed to beta diversity were positively correlated with two climatic variables (precipitation of the coldest quarter and mean temperature of the driest quarter) but did not relate to local environmental uniqueness. Despite being well known that water chemistry (Akasaka et al. 2010; Alahuhta et al. 2013) and climate (Alahuhta et al. 2021; García-Girón et al. 2023) influence the diversity of aquatic plants, we showed here that the degree of uniqueness of macrophyte communities and the species contribution to overall beta diversity are also correlated with those variables.
Our results on LCBD showed that the most unique macrophyte communities had a reduced set of species and were found in wetlands characterized by low mean air temperature during the driest period of the year and that differed the most from the average local environmental characteristics. This indicates that regional climate and unique environmental water chemistry conditions played a role in selecting communities with a more unique species composition. Despite understanding beta diversity patterns is not an easy task, as numerous factors can influence the distribution of species locally and regionally (Alahuhta et al. 2013; Vilmi et al. 2017b; Gillard et al. 2020) some previous studies have also found clear correlates of compositional uniqueness from freshwater communities (e.g., Leão et al. 2020; Pozzobom et al. 2020; Schneck et al. 2022). Most of these studies focused on gradients of environmental degradation and found that more unique communities occurred in freshwater environments with reduced forest cover and more degraded conditions (e.g., diatoms and insects: Schneck et al. 2022; aquatic macrophytes: Bomfim et al. 2023). Our results, even without a sampling design focused on a predefined environmental gradient, showed a positive correlation between environmental uniqueness based on water chemistry (LCEHwc) and total water phosphorus or electrical conductivity, variables that are generally associated with increased degradation of aquatic environments (Wu et al. 2020; Liu et al. 2022). We thus suggest that the uniqueness in water chemistry conditions was driven by increased phosphorus concentrations and electrical conductivity, selecting a reduced set of species, and thus resulting in poorer and unique communities.
In addition to water chemistry, the increase in compositional uniqueness with the decrease in mean air temperature during the driest period of the year suggests the importance of wetland hydroperiod in selecting unique communities of aquatic plants. This is because hydroperiod is known to influence species richness and community structure (Neiff 1996; Mitsch and Gosselink 2000; Maltchik et al. 2007; Schneider et al. 2018). During the driest period, high temperatures can cause increased desiccation of wetlands, which can favor the emergent life-form (Trindade et al. 2018). This can lead to some species becoming more common in the overall coastal plain (see Fig. 4b) and, as a result, reduce LCBD.
The absence of a defined spatial structure in LCBD indicates that the spatial location of the wetlands in the landscape was not a key factor in determining the compositional uniqueness of the macrophyte communities. The lack of a spatial pattern may reflect the high dispersal potential of aquatic plants (Viana et al. 2016), which, despite being considered passive dispersers, can reach long distances transported by different vectors (Soons 2006; Soons et al. 2016) such as water, wind, animals, and humans (Santamaría 2002; Lacoul and Freedman 2006; Chambers et al. 2008; Lobato‑de Magalhães et al. 2023). Therefore, our results indicate that niche processes exert more influence than dispersal limitation on the compositional uniqueness of wetland macrophyte communities, at least at a regional scale as the one studied here (~ 640 km).
The result that species with high SCBD values had intermediate to broad distributions was already shown for freshwater organisms (e.g., Pozzobom et al. 2020; Schneck et al. 2022). It could be understood that species with higher SCBD values have less dispersal limitation and greater colonization capacity. Moreover, 41% of the total species had SCBD values above the global average SCBD, indicating that a large number of species contributed to the variation in community compositions along the coastal plain. This is consistent with the high value of global beta diversity we found, suggesting that compositional differences between communities are mediated to a greater extent by the replacement of species with high SCBD than by species with restricted occurrences and low SCBD.
Floating species stood out as having the highest SCBD, similarly as found by Pozzobom et al. (2020). This life-form is strongly dependent on the presence of water and on the limnological characteristics of the wetland (Lacoul and Freedman 2006), being good competitors for resources (Henry-Silva et al. 2008). Despite the potential dependence of floating species from the water column’s nutrient concentrations, no correlation of SCBD with water chemistry uniqueness was observed either for floating or other life-forms. However, the positive correlation between SCBD and climate variables indicates that the species that most contributed to beta diversity occurred at wetlands characterized by high mean air temperature and precipitation at specific periods of the year. Temperature and precipitation can affect the species richness and composition of macrophyte communities (Lacoul and Freedman 2006; Maltchik et al. 2007). For example, temperature can strongly influence the distribution of emergent and floating species, as they remain exposed to the air, unlike submerged species that develop underwater in a more stable environment (Viana et al. 2016). High temperatures during the driest period can also affect the water balance of wetlands, causing the desiccation of environments and, consequently, influencing both the richness and composition of communities (Maltchik et al. 2007). Moreover, precipitation can be one of the main limiting factors for the diversity of aquatic plants owing to fluctuations in water levels (Lacoul and Freedman 2006; Maltchik et al. 2007). Intermittent wetlands, that remain only with saturated soil during a period of the year, limit the presence of submerged species and most floating species, favoring the colonization of emergent species, which can lead to a high contribution to beta diversity, as we found here.
Conclusion
When partitioning the total beta diversity of macrophyte communities, it was shown that local environmental factors and climate were the main correlates of compositional uniqueness. More unique communities had lower species richness and occurred in environmentally more unique sites with reduced mean air temperature at the driest period of the year. Furthermore, SCBD was related to climatic variables, and floating species stood out in their relative importance to overall beta diversity. These results enhance our understanding of the organization of macrophyte metacommunities in wetlands. Studies as the one here are essential to shed light on why certain local communities and some species contribute more to beta diversity than others, especially considering the increasing human impacts on wetlands around the world, mostly related to landscape modification for agriculture, livestock farming, and urbanization, in addition to ongoing climate change that may intensify the effects of these human activities.
Data availability
The data will be made available upon request to the corresponding author.
References
Akasaka M, Takamura N, Mitsuhashi H, Kadono Y (2010) Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshw Biol 55:909–922. https://doi.org/10.1111/j.1365-2427.2009.02334.x
Alahuhta J, Heino J (2013) Spatial extent, regional specificity and metacommunity structuring in lake macrophytes. J Biogeo 40:1572–1582. https://doi.org/10.1111/jbi.12089
Alahuhta J, Kanninen A, Hellsten S, Vuori K, Kuoppala M, Hämäläinen H (2013) Environmental and spatial correlates of community composition, richness and status of boreal lake macrophytes. Ecol Ind 32:172–181. https://doi.org/10.1016/j.ecolind.2013.03.031
Alahuhta J, Lindholm M, Baastrup-Spohr L, García-Girón J, Toivanen M, Heino J, Murphy K (2021) Macroecology of macrophytes in the freshwater realm: patterns, mechanisms and implications. Aquat Bot. https://doi.org/10.1016/j.aquabot.2020.103325
Allen S, Grimshaw M, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications, London
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Barboza EG, Tomazelli LJ, Dillenburg SR, Rosa MLCC (2009) Planície costeira do Rio Grande do Sul. Erosão em longo período. Sociedad Uruguaya De Geología 15:94–97
Baumgarten MGZ, Rocha JMB (1996) Manual de Análises em Oceanografia Química. Editora da FURG, Rio Grande
Becker FG, Ramos RA, Moura LA. (2007). Biodiversidade. regiões da lagoa do casamento e dos butiazais de tapes, planície costeira do Rio Grande do Sul.Ministério do Meio Ambiente. – Brasília: MMA.SBF
Benito X, Vilmi A, Luethje M, Carrevedo ML, Lindholm M, Fritz SC (2020) Spatial and temporal ecological uniqueness of andean diatom communities are correlated with climate, geodiversity and long-term limnological change. Front Ecol Evol 8:263. https://doi.org/10.3389/fevo.2020.00260
Bomfim FF, Fares ALB, Melo DGL, Vieira E, Michelan TS (2023) Land use increases macrophytes beta diversity in amazon streams by favoring amphibious life forms species. Community Ecol 24:159–170. https://doi.org/10.1007/s42974-023-00139-5
Castro E, Siqueira T, Melo AS, Bini LM, Landeiro VL, Schneck F (2019) Compositional uniqueness of diatoms and insects in subtropical streams is weakly correlated with riffle position and environmental uniqueness. Hydrobiologia 842:219–232. https://doi.org/10.1007/s10750-019-04037-8
Chambers PA, Lacoul P, Murphy KJ, Thomaz SM (2008) Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595:9–26. https://doi.org/10.1007/s10750-007-9154-6
Cordazzo CV, Seeliger U (1995) Guia Ilustrado da Vegetação Costeira no Extremo Sul do Brasil, 2nd edn. Editora da FURG, Rio Grande
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Statist Software 34:1–24. https://doi.org/10.18637/jss.v034.i02
De Magalhaes TL, Da Costa Bortoluzzi RL, Mantovani A (2016) Plant distribution in freshwater wetlands of the Brazilian subtropical highland grasslands. Brazilian Journal of Botany 39:239–249. https://doi.org/10.1007/s40415-015-0226-y
Dray S, Dufour A (2007) The ade4 package: implementing the duality diagram for ecologists. J Statist Software 22:1–20. https://doi.org/10.18637/jss.v022.i04
Dray S, Legendre P (2008) Testing the species traits-environment relationships: the fourth-corner problem revisited. Ecology 89:3400–3412. https://doi.org/10.1890/08-0349.1
Dray S, Choler P, Doledec S, Peres-Neto PR, Thuiller W, Pavoine S, ter Braak CJ (2014) Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95:14–21. https://doi.org/10.1890/13-0196.1
Dray S, Bauman D, Blanchet G, Borcard D, Clappe S, Guenard G, Jombart T, Larocque G, Legendre P, Madi N, Wagner HH (2020) adespatial: multivariate multiscale spatial analysis. R package version 0.3–8. https://CRAN.R-project.org/package=adespatial
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1 km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
García-Girón J, Heino J, Baastrup-Spohr L, Clayton J, deWinton M, Feldmann T, Fernández-Aláez C, Ecke F, Grillas P, Hoyer MV, Kolada A, Kosten S, Lukács BA, Mjelde M, Mormul RP, Rhazi L, Rhazi M, Sass L, Xu J, Alahuhta J (2023) Compositional breakpoints of freshwater plant communities. Limnetica 42:291–301. https://doi.org/10.23818/limn.42.21
Gillard MB, Aroviita J, Alahuhta J (2020) Same species, same habitat preferences? The distribution of aquatic plants is not explained by the same predictors in lakes and streams. Freshw Biol 65:878–892. https://doi.org/10.1111/fwb.13470
Giraudoux P (2018) pgirmess: spatial analysis and data mining for field ecologists. R package version 1.6.9. https://CRAN.R-project.org/package=pgirmess
Heino J, Grönroos M (2017) Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia 183:151–160. https://doi.org/10.1007/s00442-016-3754-7
Heino J, Bini LM, Andersson J, Bergsten J, Bjelke U, Johansson F (2017) Unravelling the correlates of species richness and ecological uniqueness in a metacommunity of urban pond insects. Ecol Ind 73:422–431. https://doi.org/10.1016/j.ecolind.2016.10.006
Heino J, García Girón J, Hämäläinen H, Hellsten S, Ilmonen J, Karjalainen J, Mäkinen T, Nyholm K, Ropponen J, Takolander A, Tolonen KT (2022) Assessing the conservation priority of freshwater lake sites based on taxonomic, functional and environmental uniqueness. Divers Distrib 28:1966–1978. https://doi.org/10.1111/ddi.13598
Henry-Silva GG, Camargo AFM, Pezzato MM (2008) Growth of freefloating aquatic macrophytes in different concentrations of nutrients. Hydrobiologia 610:153–160. https://doi.org/10.1007/s10750-008-9430-0
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. https://doi.org/10.1002/joc.1276
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Irgang BE, Gastal CVS Jr (1996) Macrófitas aquáticas da planície costeira do RS. UFRGS, Porto Alegre
Junk WJ, Piedade MTF, Lourival R, Wittmann F, Kandus P, Lacerda LD, Bozelli RL, Esteves FA, Nunes da Cunha C, Maltchik L, Schöngart J, Schaeffer-Novelli Y, Agostinho AA (2014) Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquat Conserv Mar Freshwat Ecosyst 24:5–22. https://doi.org/10.1002/aqc.2386
Lacoul P, Freedman B (2006) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14:89–136. https://doi.org/10.1139/a06-001
Landeiro VL, Franz B, Heino J, Siqueira T, Bini LM (2018) Species-poor and low-lying sites are more ecologically unique in a hyperdiverse Amazon region: evidence from multiple taxonomic groups. Divers Distrib 24:966–977. https://doi.org/10.1111/ddi.12734
Leão H, Siqueira T, Torres NR, Montag LFA (2020) Ecological uniqueness of fish communities from streams in modified landscapes of Eastern Amazonia. Ecol Ind 111:106039. https://doi.org/10.1016/j.ecolind.2019.106039
Legendre P, Condit R (2019) Spatial and temporal analysis of beta diversity in the barro colorado island forest dynamics plot. Panama Forest Ecosyst 6:7. https://doi.org/10.1186/s40663-019-0164-4
Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol Lett 16:951–963. https://doi.org/10.1111/ele.12141
Legendre P, Gauthier O (2014) Statistical methods for temporal and space–time analysis of community composition data. Proc R Soc B 281:20132728. https://doi.org/10.1098/rspb.2013.2728
Li F, Tonkin JD, Haase P (2020) Local contribution to beta diversity is negatively linked with community wide dispersal capacity in stream invertebrate communities. Ecol Ind 108:105715. https://doi.org/10.1016/j.ecolind.2019.105715
Liu C, Du Y, Zhong J, Zhang L, Huang W, Han C, Chen K, Gu X (2022) From macrophyte to algae: differentiated dominant processes for internal phosphorus release induced by suspended particulate matter deposition. Water Res 1:119067. https://doi.org/10.1016/j.watres.2022.119067
Lobato-de Magalhães T, Murphy K, Efremov A et al (2023) How on Earth did that get there? natural and human vectors of aquatic macrophyte global distribution. Hydrobiologia 850:1515–1542. https://doi.org/10.1007/s10750-022-05107-0
Maltchik L, Rolon AS, Guadagnin DL, Stenert C (2004) Wetlands of Rio Grande do Sul, Brazil: a classification with emphasis on plant communities. Acta Limnologica Brasiliensia 16:137–151. https://app.periodikos.com.br/article/627b1134782aad05cd1891c8/pdf/alb-16-2-137.pdf
Maltchik L, Rolon AS, Schott P (2007) Effects of hydrological variation on the aquatic plant community in a floodplain palustrine wetland of southern Brazil. Limnology 8:23–28. https://doi.org/10.1007/s10201-006-0192-y
Maluf JRT (2000) A new climatic classification for the state of Rio Grande do Sul, Brazil. Revista Brasileira De Agrometeorologia 8:141–150
Martínez-Román N, Epele LB, Manzo LM, Grech MG, Archangelsky M (2023) Beetle mania: Understanding pond aquatic beetles diversity patterns through a multiple-facet approach. Heliyon 9:e19666. https://doi.org/10.1016/j.heliyon.2023.e19666
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. Wiley, New York
Neiff JJ (1996) Large rivers of South America: toward the new approach. SIL Proceedings 26:167–180. https://doi.org/10.1080/03680770.1995.11900701
Nimer E (1977) Clima. In: IBGE - Geografia do Brasil Região Sul. SERGRAF-IBGE, Rio de Janeiro
Oden NL (1984) Assessing the significance of a spatial correlogram. Geogr Anal 16:1–16. https://doi.org/10.1111/j.1538-4632.1984.tb00796.x
Panja S, Podder A, Chakrabarty M, Homechaudhuri S (2022) Spatial pattern of freshwater habitats and their prioritization using additive partitions of beta diversity of inhabitant piscine assemblages in the Terai-Dooars ecoregion of Eastern Himalayas. Limnology 23:57–72. https://doi.org/10.1007/s10201-021-00666-y
Pozzobom UM, Heino J, Brito MTS, Landeiro VL (2020) Untangling the determinants of macrophyte beta diversity in tropical floodplain lakes: insights from ecological uniqueness and species contributions. Aquat Sci 82:56. https://doi.org/10.1007/s00027-020-00730-2
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Santamaría L (2002) Why are most aquatic plants widely distributed? dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica 23:137–154. https://doi.org/10.1016/S1146-609X(02)01146-3
Schneck F, Bini LM, Melo AS, Petsch DK, Saito VS, Wengrat S, Siqueira T (2022) Catchment scale deforestation increases the uniqueness of subtropical stream communities. Oecologia 199:671–683. https://doi.org/10.1007/s00442-022-05215-7
Schneider B, Cunha ER, Marchese M, Thomaz SM (2018) Associations between macrophyte life forms and environmental and morphometric factors in a large sub-tropical floodplain. Front Plant Sci 9:195. https://doi.org/10.3389/fpls.2018.00195
Schwarzbold A, Schäfer A (1984) Gênese e morfologia das lagoas costeiras do Rio Grande do Sul, Brasil. Amazoniana 9:87–104. https://archive.org/details/amazoniana-9-001-087-104/page/n9/mode/2up
Soons MB (2006) Wind dispersal in freshwater wetlands: knowledge for conservation and restoration. Appl Veg Sci 9:271–278. https://doi.org/10.1111/j.1654-109X.2006.tb00676.x
Soons MB, Brochet AL, Kleyheeg E, Green AJ (2016) Seed dispersal by dabbling ducks: an overlooked dispersal pathway for a broad spectrum of plant species. J Ecol 104:443–455. https://doi.org/10.1111/1365-2745.12531
Szabó B, Lengyel E, Padisák J, Stenger-Kovács C (2019) Benthic diatom metacommunity across small freshwater lakes: driving mechanisms, β-diversity and ecological uniqueness. Hydrobiologia 828:183–198. https://doi.org/10.1007/s10750-018-3811-9
Tonkin JD, Heino J, Sundermann A, Haase P, Jähnig S (2016) Context dependency in biodiversity patterns of central German stream metacommunities. Freshw Biol 61:607–620. https://doi.org/10.1111/fwb.12728
Trindade CRT, Landeiro VL, Schneck F (2018) Macrophyte functional groups elucidate the relative role of environmental and spatial factors on species richness and assemblage structure. Hydrobiologia 823:217–230. https://doi.org/10.1007/s10750-018-3709-6
Valderrama JC (1981) The simultaneous analysis of total nitrogen and phosphorus in natural waters. Mar Chem 10:109–122. https://doi.org/10.1016/0304-4203(81)90027-X
Viana DS, Figuerola J, Schwenk K, Manca M, Hobæk A, Mjelde M, Preston CD, Gornall RJ, Croft JM, King RA, Green AJ, Santamaría L (2016) Assembly mechanisms determining high species turnover in aquatic communities over regional and continental scales. Ecography 39:281–288. https://doi.org/10.1111/ecog.01231
Vilmi A, Alahuhta J, Hjort J, Kärnä OM, Leinonen K, Rocha MP, Tolonen KE, Tolonen KT, Heino J (2017a) Geography of global change and species richness in the North. Environ Rev 25:184–192. https://doi.org/10.1139/er-2016-0085
Vilmi A, Karjalainen SM, Heino J (2017b) Ecological uniqueness of stream and lake diatom communities shows different macroecological patterns. Divers Distrib 23:1042–1053. https://doi.org/10.1111/ddi.12594
Whittaker RH (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr 30:279–338. https://doi.org/10.2307/1943563
Winegardner AK, Legendre P, Beisner BE, Gregory-Eaves I (2017) Diatom diversity patterns over the past c. 150 years across the conterminous United States of America: identifying mechanisms behind beta diversity. Glob Ecol Biogeogr 26:1303–1315. https://doi.org/10.1111/geb.12640
Wu T, Zhu G, Zhu M, Xu H, Zang Y, Qin B (2020) Use of conductivity to indicate long-term changes in pollution processes in Lake Taihu, a large shallow lake. Environ Sci Pollut Res 27:21376–21385. https://doi.org/10.1007/s11356-020-08590-x
Zuur AF, Leno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
The Conselho Nacional de Desenvolvimento Científico e Tecnológico funded part of this study (CNPq; process 474279/2013-8). We thank Sonia Hefler for helping in the identification of botanic material, Maria Gabriela Junqueira for creating the map of the study area, and Priscilla de Carvalho, Antonio Camargo and Leonardo Maltchik for suggestions in a previous version of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by C.R.T.T. Analyses were performed by C.R.T.T. and F.S. Funding acquisition and supervision were performed by F.S. The first version of the manuscript was written by C.R.T.T. and both authors edited previous versions of the manuscript, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trindade, C.R.T., Schneck, F. Compositional uniqueness and species contribution to beta diversity of aquatic macrophyte metacommunities from coastal wetlands. Aquat Sci 86, 97 (2024). https://doi.org/10.1007/s00027-024-01115-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-024-01115-5