Abstract
Environmental characteristics, spatial structures, and landscape features are ecological factors that drive beta diversity in stream communities, but the effects of these factors, considering multiple spatial scales on beta diversity in aquatic communities, still remain a goal of community ecology. Using the distance-based redundancy analysis (db-RDA) and variance partitioning, we evaluated the contribution of the local environment, regional, and spatial variables to total beta diversity and its components (i.e., species replacement and richness difference) for fish communities in 59 streams from the Brazilian Cerrado. The influence of local environmental, regional, and spatial variables on beta diversity was distinct along different spatial scales. Specifically, local environmental variables were the main drivers of dissimilarity between streams. We suggest that the environmental filter is the primary structuring mechanism of local communities in stream fishes in the Cerrado, regardless of the spatial scale. Together, spatial and regional variables may be considered complementary mechanisms to explain the variation in the beta diversity pattern. Thus, based on high beta diversity values and the number of unique species, our findings suggest that the preservation of stream structural features is necessary to maintain regional diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the effects of land use changes on natural communities is a significant challenge in the Anthropocene (Steffen et al. 2015; Newbold et al. 2016). Freshwater ecosystems, especially streams, are among the natural ecosystems most affected by land use changes (Reid et al. 2019; Dudgeon 2019). The effects of land use changes on stream ecosystems are scale-dependent and may vary across space (Townsend et al. 2003; Allan 2004; Petsch et al. 2021). Deforestation of native vegetation within a sub-basin to agricultural practice is associated with the input of sediment and nutrients into the streams (Roth et al. 1996; Burdon et al. 2013), leading to the loss of local microhabitats (Teresa and Casatti 2012). In turn, sediment input decreases depth and further substrate homogenization, reducing local habitat complexity (Schlosser 1991; Montag et al. 2019). Removing native vegetation increases luminosity on water bodies, favoring primary productivity and species that feed on periphyton (Bojsen and Barriga 2002). In this scenario, piscivorous fishes (with local habits in riffles) lose their habitats, while some environmental disturbance-tolerant generalist species are benefited (Casatti et al. 2009; Teresa and Casatti 2012). Previous studies have shown that fish taxonomic richness and functional diversity in stream communities are negatively affected by the removal of native vegetation (Teresa and Casatti 2012; Brejão et al. 2018).
Streams are organized in a hierarchically structured dendritic network system (Frissell et al. 1986; Altermatt 2013). Therefore, their structural characteristics can be influenced by the landscape surrounding this dendritic network and the human activities in the basin (Schlosser 1991). Divergent results have been shown in previous studies assessing the influence of ecological predictors at multiple scales on the structure of fish communities. Some studies have shown that local environmental conditions (i.e., depth, dissolved oxygen, and % of grass in margins) are the primary structuring drivers for stream fish communities (Bordignon et al. 2015; Montag et al. 2019). On the other hand, other studies have shown that stream fish communities are influenced by local environmental variables and spatial factors (i.e., watercourse distance) (Roa-Fuentes and Casatti 2017; Roa-Fuentes et al. 2019). These findings are evidence that environmental filtering and dispersal limitations are complementary mechanisms acting on the structure of communities (Carvalho and Cardoso 2014; López-Delgado et al. 2020).
Several studies have demonstrated how local communities exchange organisms and how the specification processes, extinction, dispersal, and environmental filtering events interact at various spatial and temporal scales to structure communities (Leibold et al. 2004; Presley et al. 2010; Erős 2017; Schmera et al. 2018). From a theoretical and empirical perspective, at least four models of metacommunity dynamics have been proposed (species sorting, mass effect, patch dynamics, and neutral model), each defined by the relative influences of environmental filtering, dispersal, habitat selection, habitat disturbance, biotic interactions, and stochastic factors (Leibold et al. 2004; Tonkin et al. 2018). The advances in metacommunity ecology have facilitated the understanding of how community composition varies in space and time (Baselga 2010; Legendre 2014).
Beta diversity can be defined as a change in the species composition of communities among sites (Gaston 2000; Cottenie 2005). Deterministic processes and stochastic factors have been identified as the main mechanisms driving dissimilarity patterns among communities (Ricklefs 1987; Dornelas et al. 2006; Chase et al. 2011). The deterministic factors are linked as species interact with abiotic conditions and biotic interactions (e.g., niche-based processes) that are reflected in the species sorting mechanism to determine variation in species communities (Chase and Leibold 2003; Chase 2007). On the other hand, the communities are influenced by stochastic factors such as colonization and extinction events, and the variation in species communities is not explained by niche species requirements but by rates of dispersal or ecological drift that reflect the mass effect in the structure of the communities (Hubbell 2001; Chase 2007). Furthermore, beta diversity may be partitioned into two additive sources of dissimilarity, species replacement (i.e., species substitution) and richness-difference components (Podani and Schmera 2011; Carvalho et al. 2012). Substitution of species among sites, often due to environmental filtering, biotic interactions, or historical factors, is described by species replacement (Baselga 2010; Perez Rocha et al. 2018). The species richness-difference component derives from the loss or gain of species along environmental gradients due to environmental changes or barriers to dispersal, reflecting niche diversity across spatial or temporal scales (Podani and Schmera 2011; Carvalho et al. 2012). Thus, the partitioning of beta diversity may provide valuable information about the processes and mechanisms of species distributions on community dynamics and how the species composition changes at different spatial scales (Carvalho et al. 2012; Baselga and Leprieur 2015).
The effects of land use changes on beta diversity patterns depend on the initial ecological conditions, the magnitude of the environmental disturbance, the species dispersion capacity, and the prevalence of stochastic events (Al-Shami et al. 2013; Zbinden and Matthews 2017). In general, landscape changes due to agricultural expansion could alter species composition, leading to rare species loss and common species predominance and contributing to biotic homogenization (i.e., increase in compositional similarity among fish stream communities) (Casatti et al. 2009; Petsch 2016). Biotic homogenization contributes to decreasing taxonomic beta diversity over time (Olden and Poff 2003; Petsch 2016) and may result in one particular cause of the richness-difference pattern where the site with smaller numbers of species is a subset of the species at a richer site (Baselga 2010; Baeten et al. 2012). Land use changes may also increase species substitution, mainly in communities where the dispersal process is predominant (Hawkins et al. 2015; Jamoneau et al. 2018). Dispersal processes are related to the ability of individuals to move among suitable habitats (Leibold et al. 2004). When these high dispersal rates occur, the local communities could be chiefly driven by mass effect events (Leibold et al. 2004; Heino et al. 2015b). However, environmental filters created due to changes in environmental conditions can hamper the movement of individuals among suitable habitats (Leibold and Chase 2017) and contribute to species replacement along the environmental gradient.
The relative influence of ecological predictors (e.g., environmental, land use, and space) on stream fish community structure may vary among networks (Sály et al. 2011; Montag et al. 2019). In each watershed, different land use forms in the surrounding streams together with the existing environmental and spatial characteristics in the drainage network can result in different stream fish community composition. Due to rapid conversion from native vegetation to agricultural activities (Strassburg et al. 2017; Latrubesse et al. 2019) and scarcity of studies that have addressed fish beta diversity, the streams from the Cerrado biome are good models for testing these questions. In addition, the metrics of beta diversity are essential to testing ecological theories, understanding regional biodiversity patterns, and guiding conservation policies (Socolar et al. 2016).
Considering this context, our goals were to answer the following questions: (i) What are the contributions of the replacement and richness differences to beta diversity in Cerrado stream fish communities? (ii) What is the best set of variables to predict spatial beta diversity patterns in Cerrado streams? We hypothesize that species sorting mechanisms are the primary structuring drivers acting on fish communities due to high environmental heterogeneity within the basins and intermediary rates of dispersal of species among sites (see Heino et al. 2015b). Another possibility is that the richness difference among streams is linked to mass effect mechanisms because of the adjustment of the fish communities by dispersal events due to changes in the landscapes surrounding the streams. Thus, we expect to find high values of beta diversity where the streams that have undergone less landscape modification may maintain their characteristics and evidence the replacement of species between sites.
Material and methods
Study area
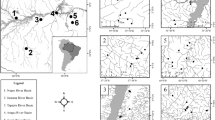
We sampled 59 stream stretches ranging from the first to fourth orders (Strahler 1957) inside the Upper Araguaia River (n = 30) and Middle Rio das Mortes basins (n = 29) in the Tocantins–Araguaia system (Fig. 1). The list containing the geographical coordinates for all sampling sites is available in the supplementary material (Online Resource Table S1). The study area lies within the Cerrado biome (Ribeiro and Walter 2008), and its landscape has undergone modification mainly because of native vegetation deforestation to pasture and agricultural areas (Latrubesse et al. 2019). The climate of the region is the Aw type according to the Köppen classification (Alvares et al. 2013), with two distinct periods: (i) rainy and hot (1301.11 ± 528.45 mm, mean ± SD; 26.2 ± 1.06 °C) from October to April and (ii) dry with a milder temperature (116.97 ± 15.96 mm; 24.2 ± 1.93 °C) from May to September (INMET 1979 to 2017).
Data collection
Local environmental variables
In each stream, we measured 23 environmental variables related to the limnologic conditions and habitat structure within the channel and the margins of the streams (Table 1). We measured the variables related to stream limnologic conditions (e.g., conductivity, dissolved oxygen, pH, turbidity, and water temperature) using a portable multiparametric probe (Horiba U-50) only once at the beginning of the sampled stretch before taking measurements inside the channel to prevent disturbance. We divided each sampled 50-m stretch into six equidistant cross-section transects. For each of the six cross-section transects, we make visual observation to calculate the mean values of the structural variables related to canal morphology (width and depth), substrate composition (sand, gravel, pebbles, rock, slabs, clay/silt), the margin composition (thin roots, thick roots, grass banks), and internal habitat structure (presence of the trunks and leaf litter bank). The mean width and depth were obtained from five measurements from one margin to the other in each of the six cross-section transects. Additionally, we determined the mean surface water velocity using the fluctuating material method (Teresa and Casatti 2012), from surface water velocity measured in each of the six cross-section transects. Finally, to represent the local riparian vegetation structure, we measured the forest width and visually assessed the proportions of shrubs, herbaceous plants, and trees along both banks within the sampled reach. Then we calculated the mean values based on these observations.
Regional variables
We used a geographic information system (GIS) to gathering the regional variables represented by catchment variables (i.e., area size, hill slope, bioclimatic, and land use variables within catchment area) and land use variables in the riparian zone (i.e., 60-m buffer zone around the drainage network upstream of the sampling sites; Online Resource Fig. S1). First, we delimited the upstream catchment of each sampling site and hydrographic network based on a digital elevation model (DEM) with 30-m spatial resolution (Topoda; www.webmapit.com.br/inpe/topodata/). Then, we built the catchment and hydrographic network using the r.water.outlet and r.stream.extract functions available in GRASS GIS 7.6 (GRASS Development Team 2019).
We estimated the average hill slope of each catchment from the average slope of all DEM pixels in the respective catchment. We extracted the bioclimatic variables from the raster images of high spatial resolution (30 arc-seconds ~ 1 km2) from WordClim (Fick and Hijmans 2017). We extracted the land use variables in the catchment and riparian zone from MapBiomas (MapBiomas 2021). We used the functions available in the raster package to extract the bioclimate and land use variables (Hijmans 2020).
We measured 10 catchment variables: the average hill slope, the catchment size upstream (km2), annual mean temperature, annual mean precipitation, seasonality precipitation, proportion of native vegetation, proportion of pasture, proportion of agriculture, proportion of silviculture, and proportion of urban infrastructure. We measured the following riparian zone variables: the proportion of native vegetation, the proportion of pastures, and the proportion of agriculture. Initially, we tested the correlation among catchment and land use riparian zone variables. We retained the catchment variables in the posterior analysis when the correlation was significant (Pearson correlation > 0.7; Online Resource Fig. S2).
Spatial variables
We built a pairwise distance matrix between all the sampled sites for each model (i.e., Global—model with all sites from both basins, Upper Araguaia River, and Middle Rio das Mortes) following the hydrographic network using the extension QGIS Network Analysis Toolbox 3 in QGIS 3.4 (QGIS Development Team 2023). Next, we generated our spatial variables for each model using distance-based Moran’s eigenvector maps (dbMEM) (Borcard and Legendre 2002; Dray et al. 2006). We retained only those dbMEMs that model a positive spatial correlation (Moran’s I is larger than E (I)). We calculated the dbMENs using the dbmem function from the R package adespatial (Dray et al. 2022).
Fish sampling
We sampled 59 streams between 2014 and 2017. We delimited a 50-m stretch once in each catchment based on the accessibility and relative independence of the catchment. We collected all samples during the diurnal period in the dry hydrologic cycle to increase our fish-catching efficiency (Ueida and Castro 1999). We blocked the 50-m stretch with seine nets (5.0-mm mesh size) to prevent the fish from escaping.
We used two distinct methods to collect the fish due to logistical issues. Thus, we sampled 35 stream stretches using seine nets (3.0 m width × 1.5 m height × 5.0 mm mesh size) and dipnets (0.5 m length × 0.45 m width × 5.0 mm mesh size) employing four collectors for approximately 1 hour. We collected 24 other stretches of streams using the electrofishing method (Honda EG1000 generator, 220 V, CA), and we employed three collectors for approximately 1 hour. For both basins and sampling methods, we analyzed the sample coverage estimation based on the Hill diversity series (Chao et al. 2014) with a confidence interval of 95%. The Hill diversity series showed that our sampling effort was sufficient to sample 93% of the estimated species (Online Resource Figs. S3–S4).
We anesthetized the sampled fish with benzocaine (CFMV 2012) and fixed them in a 10% formalin solution, and all individuals were conserved in 70% ethanol after 72 h. In the laboratory, we measured, weighed, and identified all individuals until lowest taxonomic level possible. The identification of taxa was based on the specialized bibliography as taxonomic reviews (Garutti and Langeani 2009; Malabarba and Jerep 2014; Terán et al. 2020; Tencatt et al. 2022), species descriptions (Garutti 1999; Petrolli et al. 2016), books (Buckup et al. 2007; Venere and Garutti 2011), or species list published on fish fauna from the Araguaia basin (Dagosta and Pinna 2019; Lima et al. 2021). We checked the validity of the species names using the Catalogue of Fishes (Fricke et al. 2023). The sampling was authorized by the Institute for Biodiversity Conservation (ICMBIO, SISBIO # 45,316–1) and by the Animal Use Ethics Committee from Universidade Federal do Mato Grosso (CEUA/UFMT – N° 23,108.152116).
Data analysis
Beta diversity
We built site-by-species matrices with either presence or absence for each model (i.e., global, Upper Araguaia River, and Middle Rio das Mortes). Next, we calculated the beta diversity components based on Jaccard dissimilarity coefficient following the approach devised by Podani and Schmera (2011) and Carvalho et al. (2012). As a measure of beta diversity (βtotal), the algebraic decomposition of the Jaccard dissimilarity index embedded general theoretical and methodological frameworks for analyzing patterns in presence–absence data (Podani and Schmera 2011; Carvalho et al. 2012). This approach consists in deriving from total beta diversity (βtotal) the species replacement (βrepl) and richness-difference (βrich) components: βtotal = βrepl + βrich (Podani and Schmera 2011; Carvalho et al. 2012; Podani et al. 2013). We know of the alternative approach proposed by Baselga (2010) that decomposes total beta diversity into turnover and nestedness components. However, in the present study, we are focused on the replacement and richness-difference components (Podani and Schmera 2011) because we are interested in any variation related to richness differences between sites instead of nestedness-related patterns (Carvalho et al. 2012; Legendre 2014). In addition, studies have showed this decomposition approach is conceptually and mathematically adequate for addressing complex issues in beta diversity (Carvalho et al. 2012; Legendre 2014). We calculated the beta diversity metrics using the beta.div.comp function of the R package adespatial (Dray et al. 2022).
We used the ternary plot called the SDR simplex to represent the contribution of each component to the total variation of the communities. Proposed by Podani et al. (2013), the SDR simplex plot is an intuitive triangular graph to show three indices resulting or derived from beta diversity partitioning: similarity (S), replacement (Repel), and richness difference (Richdiff). The total beta (βtotal) is calculated by summing Repel (βrepl) and Richdiff (βrich), while similarity is equal to 1 − βtotal. Thus, it is possible to represent each index in the ternary graph as a vertex of a triangle, allowing us to analyze the relative importance of each component (Podani et al. 2013). We built ternary plots using the R package ggtern (Hamilton and Ferry 2018).
Statistical analysis
We standardized (z transformation) all environmental local (except pH) and regional variables. We adopted a parsimonious approach (Dormann et al. 2013) to check for collinearity in the data. First, we performed Pearson pairwise correlations among the variables to each set of predictor variables (i.e., local and regional) inside each model. Variables with an absolute r coefficient > 0.7 were considered highly correlated (Online Resource Fig. S5). If two variables were highly correlated, we retained only a variable with a more biological sense in the posterior analysis. Then we performed distance-based redundancy analysis (db-RDA) (Legendre and Anderson 1999), where beta diversity components (βtotal, βrepl, and βrich) were response variables, and each predictor variable set (local and regional) was used as an explanatory variable. We performed variance inflation factor (VIF) analysis to control for multicollinearity and removed all those variables with VIF > 10 (Borcard et al. 2018). Finally, we performed predictor variable selection based on db-RDA for each model (global, Upper Araguaia River, and Middle Rio das Mortes) using the function ordiR2step (999 permutations) from the vegan package (Oksanen et al. 2018) with the double-stopping criterion (Blanchet et al. 2008). Our final set of predictor variables was composed only of those variables that substantially explained the model (Online Resource Tables S2–S4).
We evaluated the contribution of the local environment, regional variables, and spatial variables to beta diversity and our components (βtotal, βrepl, and βrich) in each model through db-RDA, together with the variance partitioning technique (Cottenie 2005; Peres-Neto and Legendre 2010). We used the sqrt.dist function in all db-RDA to correct problems of negative eigenvalues (Legendre and Anderson 1999). The significance of each fraction of the set of predictor variables and individual variables was tested through an analysis of variance (ANOVA). A total of 999 permutations and a significance level of 5% were considered in this analysis. We used the function anova with the option “by = term” to test the significance of each explanatory variable. A flowchart with all the steps used in this analysis is available in the supplementary material (Online Resource Fig. S6).
We performed analyses using the programming environment and statistical analysis R version 3.6.1 (R Core Team 2021). We used the capscale, varpart, and anova functions available in the vegan package (Oksanen et al. 2018) to perform the distance-based redundancy analyses, variance partitioning analysis, and analysis of variance, respectively.
Results
Fish communities
We collected 135 species distributed in six orders and 30 families. The Characidae, Loricariidae, and Cichlidae families were the most representative (38, 25, and 12 species, respectively) and contributed more than half of the captured species (Online Resource Table S5). The average species richness was 17.84 (SD = 10.51) species per catchment. Astyanax cf. goyacensis Eigenmann 1908 (n = 47), Knodus cf. breviceps (Eigenmann 1908) (n = 42), Characidium cf. zebra Eigenmann 1909 (n = 39), and Imparfinis mirini Haseman 1911 (n = 39) were the most frequent species. We collected 79 and 118 species in the Upper Araguaia River and Middle Rio das Mortes basins, respectively, considering the two basins. The basins shared 59 fish species. Seventeen species were exclusive to the Upper Araguaia River basin, whereas 59 species were exclusive to the Middle Rio das Mortes basin (Online Resource Table S5).
Environmental variable predictors
The water in the studied streams has low conductivity, is slightly acidic, has low turbidity, and has high dissolved oxygen levels (Table 1). The predominant substrate structure was composed of sand, gravel, and pebbles (Table 1). The catchment area size varied among streams (mean = 35.81 SD = 40.53 Km2), and the average hill slope ranged from 1.54º to 15.18º (Table 1). At the catchment scale, native vegetation, pastures, agriculture, silviculture, and urban infrastructure varied according to the following ranges: 8.83%–95.44%, 4.56%–91.17%, 0%–33.22%, 0%–15.47%, and 0%–0.83%, respectively (Table 1). In the riparian zone, the native vegetation, pastures, and agriculture varied according to the following ranges: 23.21%–100%, 0%–76.79%, and 0%–3.56% (Table 1), respectively. In general, an average of 55.51% of the catchment area of the streams was preserved and covered with native vegetation (Fig. 2), and most streams had riparian vegetation over 30 m.
Beta diversity patterns
We found high values of total beta diversity, ranging from 74.1% to 84% (Fig. 3). For all beta diversity analyses, fish community variation was primarily explained by the species replacement component rather than the richness difference (Figs. 3 and 4). As shown in the ternary plots, most site pairs were concentrated close to the left corner, with the centroid near to the species replacement side (Fig. 4).
Box plots of pairwise dissimilarities for the beta total (βtotal), species replacement (βrepl), and richness difference (βrich) of fish communities in different models. The central lines denote the median value, the box denotes the first (25th) and third (75th) percentiles, whiskers represent the smallest and largest value, and dots indicate outliers
Regarding the decomposition of βtotal into its two components, βrepl and βrich. To the global model, βrepl and βrich components contributed 41.5% and 40.2% to dissimilarity among fish stream communities, respectively (Fig. 4). In the Upper Araguaia River basin, βrepl and βrich components contributed 45.5% and 39% to the dissimilarity among fish stream communities, respectively (Fig. 4). Finally, in the Middle Rio das Mortes basin, βrepl and βrich components contributed 45.5% and 29.6% to the dissimilarity among fish stream communities, respectively (Fig. 4).
Regarding the influence of the local environment, regional variables, and spatial variables on βtotal, βrepl, and βrich in the global model, βtotal was explained by a set of all variables (Fig. 5a, Online Resource Table S6). Similarly, βrepl and βrich were explained mainly by local environmental variables (Fig. 5b, c, Online Resource Table S6). However, in the Upper Araguaia River basin, βtotal and its components (βrepl and βrich) were explained only by environmental variables (Fig. 5d, Online Resource Table S7). In the Middle Rio das Mortes River basin, βtotal was explained by the local, regional, and spatial environmental variables (Fig. 5g, Online Resource Table S8), while βrep was explained only by the local environmental variable (Fig. 5h, Online Resource Table S8), and βrich was explained by the local environment and regional variables (Fig. 5h).
Venn diagrams showing the influence of set predictor variables (environmental local, regional, and spatial variables) on the beta diversity components in stream fish communities. Values in bold indicate the set of variables with a significant influence on beta diversity, and asterisks indicate the significance level (*P < 0.05; **P < 0.005; ***P < 0.001). The result indicated by 0.000 corresponds to a negative fraction whose value was truncated to zero (Legendre 2008). Abbreviations: Local = local environmental variables; Regional = catchment variables and land use variables in the riparian zone; Spatial = spatial variables (dbMEM, distance-based Moran’s eigenvector maps); Not testable = not possible variance partitioning because only set environmental variables were selected during the variable selection process; βtotal = total beta diversity; βrepl = species replacement; βrich = richness difference
The db-RDA analyses showed that the relative importance of the predictor variables (i.e., local, regional, and spatial) to explain the beta diversity patterns differed between the models (Online Resource Table S9–S11). However, we did not find any influence of land use variables on the beta diversity components in our model (Online Resource Table S9–S11). In the global model, the βtotal component was influenced by the local environmental variables related to water physiological and chemical features (pH, dissolved oxygen, turbidity, and current velocity), morphology (width), substrate (trunks and leaf litter banks), margin structure (thin roots), regional variables (catchment size), and spatial variables (dbMEM1) (Online Resource Table S9). The βrepl component was influenced by local environmental variables related to the water physiological and chemical features (pH and current velocity) and stream structure (trunks, leaf litter banks, and thin roots). The regional variables were related to the available hydric features (annual mean precipitation; Online Resource Table S9). The βrich was influenced by local environmental variables related to stream structure (width, leaf litter banks, percentage of grasses) and spatial variables (dbMEM1) (Online Resource Table S9).
In the Upper Araguaia River basin, the db-RDA showed that the βtotal component was influenced only by local environmental variables related to the physiological and chemical characteristics of the water (conductivity, turbidity, current velocity) and stream morphology (depth; Online Resource Table S10). The βrepl component was only influenced by local environmental variables related to the physiological and chemical characteristics of the water (surface water velocity) and the mean width of the local riparian vegetation. Together, the βrich component was exclusively influenced by the local environmental variables related to the physiological and chemical characteristics of the water (conductivity) and the stream morphology (depth; Online Resource Table S10).
In the Middle Rio das Mortes River basin, the βtotal component was shown by the db-RDA to be influenced by local environmental variables related to water physiological and chemical characteristics (turbidity), morphology (depth), margin structures (percentage of grasses), and proportion of shrubs in the local riparian vegetation of the streams. The regional variables were represented by the hydric availability (annual mean precipitation) and the spatial variables that represented the spatial processes on a regional scale (dbMEM1) ( Online Resource Table S11). The βrepl component was influenced by local environmental variables related to water physiological and chemical characteristics (turbidity and water temperature), stream morphology (depth), substrate structure (trunks), and margin structure (thin roots), and by regional variables represented by the annual mean precipitation (hydric availability) and the spatial variables that represent the spatial processes on a regional scale (dbMEM3). In comparison, the βrich component was influenced by local environmental variables related to water physiological and chemical characteristics (pH), the margin structures (percentage of grasses), and regional variables represented by catchment size (Online Resource Table S11). We provided the separated significance test results for each explanatory variable in the supplementary material (Online Resource Tables S9–S11).
Discussion
Here, we found that beta diversity (βtotal) was mainly determined by species replacement (βrep), while richness difference (βrich) has secondary importance to the loss or gain of species along an environmental gradient. The prevalence of species replacement along environmental gradients could be related to the niche filtering process (Carvalho et al. 2012; Malumbres-Olarte et al. 2021; Frota et al. 2022), which refers to species-sorting metacommunity dynamics (Leibold et al. 2004; Heino et al. 2015a). Thus, environmental feature variation may be responsible for the difference in composition through niche differentiation (Ricklefs 1987; Siqueira et al. 2012). For example, some species, such as Brycon falcatus Müller & Troschel 1844 and Leporinus sp., were found only in streams at great depths. Simultaneously, fish species in the Gymnotiformes group are related to streams with higher conductivity and root meshes in the margins. Thus, this study reveals species-specific requirements for habitat occupation.
Local environmental, regional, and spatial variables distinctly influenced the dissimilarity of species between sites and their components (Fig. 5, Online Resource Tables S6–S8). Moreover, local environmental variables were the main drivers responsible for the dissimilarity among fish stream communities. On the other hand, regional and spatial variables secondarily explained the beta diversity patterns. This prevalence of the local environment in filtering and regulating the local communities (i.e., the species sorting process) has been shown in the literature to be the most important process structuring freshwater communities at various spatial extents (Perez Rocha et al. 2018; López-Delgado et al. 2020). For instance, López-Delgado et al. (2020) reported a strong species sorting process in structured fish communities in the Amazon Bita River at two spatial levels at the river and basin levels. This influence of the species sorting process on the dissimilarity among fish stream communities is more evident in headwater streams (Zbinden and Matthews 2017; Frota et al. 2022), as in our study. However, the mass effect and patch dynamics models can also be relevant in structuring the fish community at the basin level (López-Delgado et al. 2019). But the fact that our results showed the proportion explained by a set of the local environmental variables was larger than others set of the variables is clear evidence that niche-based processes (i.e., species sorting process) are the main mechanisms structuring fish communities in the streams studied.
Regional variables were less important in explaining variations in the beta diversity components. We suppose that the spatial scale of our study may be a possible explanation for the secondary importance of regional variables in explaining the beta diversity. The environmental factors that vary on a large spatial scale (i.e., climatic variables and land use) can have low variability on smaller spatial scales because these variables need a large amount of time or range to have noticeable variations (Leibold et al. 2004; Benone et al. 2020). However, we may not discard their importance in explaining aquatic variation patterns in communities because regional variables can interact with local environmental variables (Allan and Castillo 2007; Galbraith et al. 2008). For instance, surrounding land use, one regional variable, can affect fish communities directly through stream inputs of sediment organic matter and nutrients, and indirectly influence local variables such as substrate structure, and modify the physiological and chemical features of water streams (Allan 2004; Sweeney and Newbold 2014; Montag et al. 2019). In addition, the native vegetation cover in the catchment and in the riparian zone are the main sources of wood debris and leaf litter banks in streams (Paula et al. 2013; Sweeney and Newbold 2014), two important variables to explain the dissimilarity among fish communities in our study. Thus, the native and riparian vegetation within the catchments permit regional fish diversity maintenance through species niche requirements and environmental filters.
Here, catchment area size and variables related to hydric availability (annual mean precipitation and seasonality precipitation) were regional variables that influenced dissimilarity among fish stream communities. We highlight that greater catchments have more voluminous streams that should support more diverse fish communities (Angermeier and Schlosser 1989; Zbinden and Matthews 2017). The size of an area has been reported as a predictor with a potentially positive effect on species richness (MacArthur and Wilson 1967; Gooriah and Chase 2020) because there is a relationship that the larger the areas are, the greater the possibility of niches and habitat complexity, which can result in greater diversity (species richness). Thus, beta diversity is expected to increase with ecological heterogeneity in large areas (Ricklefs 1987; Bini et al. 2014; Heino et al. 2015a) because ecological heterogeneity allows the increase in species coexistence (i.e., contributing to the ecological specialization of species).
Hydric availability is related to habitat viability and environmental heterogeneity, favoring dissimilarity among fish stream communities (Oberdorff et al. 2019). For instance, the hydric availability that influences the water level in streams is an important driver of the mass effect process by creating the possibility of fish dispersion being active or passive through the net drainages (Tonkin et al. 2018). Additionally, hydric availability may influence local environmental variables (e.g., pH, turbidity, conductivity, and depth) by changing habitat features and species niche requirements, emphasizing the importance of the species sorting process in community assembly rules (Soininen 2014; Tonkin et al. 2016).
The influence of spatial processes driving metacommunity structure is linked to the spatial extent and capacity for movement of species or group dispersion (Landeiro et al. 2011; Astorga et al. 2012; Frota et al. 2022). The communities dominated by organisms with high dispersal rates and longest dispersal capabilities could be chiefly driven by mass effect events (Leibold et al. 2004; Heino et al. 2015a). High dispersal rates are associated with the homogenization of community composition, resulting in low beta diversity values. However, communities dominated by organisms with limited dispersal rates and short dispersal capabilities could be driven by species-sorting mechanics or dispersal limitations (Grönroos et al. 2013; Tonkin et al. 2018). Thus, species sorting prevails across multiple scales when dispersal rates are insufficient to overwhelm the environmental filtering process (Heino et al. 2015b; Tonkin et al. 2018). In addition, stronger dispersal limitations can be associated with the dendritic structure of stream networks. In contrast with dispersal in terrestrial landscapes, fish species are constrained to disperse through the watercourse stream network and are highly dependent on the degree of connectivity between branches of the network (Altermatt 2013; Tonkin et al. 2018). Here, we think the importance of spatial variables in the global model to explain the dissimilarity among fish stream communities is linked to dispersal limitations. The shortest distance among the watercourses in the studied streams in the Upper Araguaia River and the Middle Rio das Mortes basin is larger than 900 km, and most stream fish species are small (Online Resource Table S2). Thus, the distance among habitats represents a barrier to dispersion, contributing to the differentiation of fish communities among streams (Landeiro et al. 2011; Schmera et al. 2018). Therefore, biogeographic processes (e.g., speciation, extinction, and headwater capture) could result in ichthyofauna regionalization, as occurs in the interfluves of the Amazon, where great rivers represent a barrier to the smallest stream fish, leading to the isolation of these sites (Dambros et al. 2020).
In general, the local environmental variables contributed more to explaining the pattern of beta diversity. Indeed, despite the known strong influence of environmental variables in driving species distributions across intense habitat filters (Chase and Leibold 2003; Chase 2007), the dispersal rates are often found to mediate the relative importance of environmental versus spatial factors. It is important to consider that dispersion should be strong enough to allow individuals to reach sites that match their habitat requirements and therefore allow for environmental filters (Leibold and Chase 2017). In addition, the aquatic fauna of the stream's ecosystems is constantly extinct and recolonized due to waterspouts, suggesting that fish species have ecological adaptations to survive in extreme conditions (Taylor and Warren 2001). Thus, ecological strategies of species could be more important in explaining the variation in the aquatic community along the stream's gradient than dispersion limitation, since local sites are constantly recolonized.
Drivers of the species replacement component
In all models, species replacement was influenced only by local environmental variables (Fig. 5b, e, and h; Online Resource Tables S6–S8). These results provide evidence that βrepl favored species-sorting events. Local environmental variables related to the physiological and chemical parameters of water (e.g., pH, turbidity, water temperature, surface water velocity, and depth) and structural features (e.g., trunks and roots) were the main drivers of environmental filtering and were more prominent than spatial factors in structuring biological communities (Landeiro et al. 2012), leading us to believe that these factors were the drivers of community distribution. For instance, the water velocity could select species with the best swimming ability (Jackson et al. 2001), such as Characidium cf. zebra Eigenmann 1909, which was one of the few species occurring in a large number of streams. In turn, structural variables such as trunks and roots increase niche availability and provide opportunities for the establishment of several fish species. The ecological mechanisms associated with local environmental factors (e.g., environmental filtering) were shown by our results to be predominant over regional or spatial factors to explain species replacement among the studied stream fish communities.
Drivers of the richness-difference component
We found distinct patterns for each model regarding the influence of the sets of variables on the βrich component. There is a predominance of environmental factors that explain richness differences in the studied fish stream communities. Therefore, we propose that the hierarchical structure of rivers should be a significant driver of community change (Frissell et al. 1986; Tonkin et al. 2018). The importance of spatial variables in affecting species loss/gain depends mainly on the size of the geographical scale (Tonkin et al. 2018). The global model (the one with the greatest geographic range) was the only one in which spatial variables were more prominent than environmental variables while explaining the richness-difference patterns. Again, the importance of geographic isolation, as observed by the higher number of exclusive species found in both basins, is highlighted by this finding. On the other hand, we emphasize that among regional variables, only catchment size was important in explaining richness differences only in the Middle Rio das Mortes basin. This pattern can be explained in light of the hierarchical structure of fluvial systems, in which aquatic communities of small drainages are subsets of communities from larger drainages (Altermatt 2013; Tonkin et al. 2018), one particular case of the species richness pattern. Finally, the prevalence of the environmental factors in explanted the species loss/gain between sites is evidence of the intermediary dispersal rates among streams (Leibold et al. 2004; Tonkin et al. 2018). Therefore, species-sorting mechanisms are the main driver in shaping the structure of fish communities due to environmental filters (Heino et al. 2015b).
Conclusion
Our results showed that local environmental variables were the main factors responsible for the variation patterns in the beta diversity components of stream fish communities. Simultaneously, the regional and spatial variables were the least useful in explaining the variation patterns of the fish communities. Therefore, these variables can be considered secondary mechanisms for the variation patterns of beta diversity. Although we did not find direct influence of land use variables on the beta diversity and its components, we may not discard their importance in explaining aquatic variation patterns in communities. Because land use variables can interact with local environmental variables, they constitute a set of important variables to explain the dissimilarity among fish communities in our study. Thus, the higher number of exclusive species in the basins shows the need to concentrate efforts on maintaining the remaining native and riparian vegetation within the catchments, which guarantees a variety of habitats and, consequently, regional fish diversity maintenance.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst 35:257–284. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122
Allan JD, Castillo MM (2007) Stream ecology, 2a. Springer, Dordrecht
Al-Shami SA, Heino J, Che Salmah MR et al (2013) Drivers of beta diversity of macroinvertebrate communities in tropical forest streams. Freshw Biol 58:1126–1137. https://doi.org/10.1111/fwb.12113
Altermatt F (2013) Diversity in riverine metacommunities: a network perspective. Aquat Ecol 47:365–377. https://doi.org/10.1007/s10452-013-9450-3
Alvares CA, Stape JL, Sentelhas PC et al (2013) Köppen’s climate classification map for Brazil. Meteorol Zeitschrift 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Angermeier PL, Schlosser IJ (1989) Species-area relationship for stream fishes. Ecology 70:1450–1462. https://doi.org/10.2307/1938204
Astorga A, Oksanen J, Luoto M et al (2012) Distance decay of similarity in freshwater communities: Do macro- and microorganisms follow the same rules? Glob Ecol Biogeogr 21:365–375. https://doi.org/10.1111/j.1466-8238.2011.00681.x
Baeten L, Vangansbeke P, Hermy M et al (2012) Distinguishing between turnover and nestedness in the quantification of biotic homogenization. Biodivers Conserv 21:1399–1409. https://doi.org/10.1007/s10531-012-0251-0
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A, Leprieur F (2015) Comparing methods to separate components of beta diversity. Methods Ecol Evol 6:1069–1079. https://doi.org/10.1111/2041-210X.12388
Benone NL, Leal CG, Santos LL et al (2020) Unravelling patterns of taxonomic and functional diversity of Amazon stream fish. Aquat Sci 82:75. https://doi.org/10.1007/s00027-020-00749-5
Bini LM, Landeiro VL, Padial AA et al (2014) Nutrient enrichment is related to two facets of beta diversity for stream invertebrates across the United States. Ecology 95:1569–1578. https://doi.org/10.1890/13-0656.1
Blanchet FG, Pierre L, Borcard D (2008) Forward selection of spatial explanatory variables. Ecology 89:2623–2632. https://doi.org/10.1890/07-0986.1
Bojsen BH, Barriga R (2002) Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshw Biol 47:2246–2260. https://doi.org/10.1046/j.1365-2427.2002.00956.x
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Modell 153:51–68. https://doi.org/10.1016/S0304-3800(01)00501-4
Borcard D, Gillet F, Legendre P (2018) Numerical ecology with R, 2a. Springer International Publishing, Cham
Bordignon CR, Casatti L, Pérez-Mayorga MA et al (2015) Fish complementarity is associated to forests in Amazonian streams. Neotrop Ichthyol 13:579–590. https://doi.org/10.1590/1982-0224-20140157
Brejão GL, Hoeinghaus DJ, Pérez-Mayorga MA et al (2018) Threshold responses of Amazonian stream fishes to timing and extent of deforestation. Conserv Biol 32:860–871. https://doi.org/10.1111/cobi.13061
Buckup PA, Menezes NA, Ghazzi MS (2007) Catálogo das espécies de peixes de água doce do Brasil. Museu Nacional, Rio de Janeiro
Burdon FJ, McIntosh AR, Harding JS (2013) Habitat loss drives threshold response of benthic invertebrate communities to deposited sediment in agricultural streams. Ecol Appl 23:1036–1047. https://doi.org/10.1890/12-1190.1
Carvalho JC, Cardoso P (2014) Drivers of beta diversity in Macaronesian spiders in relation to dispersal ability. J Biogeogr 41:1859–1870. https://doi.org/10.1111/jbi.12348
Carvalho JC, Cardoso P, Gomes P (2012) Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob Ecol Biogeogr 21:760–771. https://doi.org/10.1111/j.1466-8238.2011.00694.x
Casatti L, Ferreira CP, Carvalho FR (2009) Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: an assessment of two tropical pasture river basins. Hydrobiologia 632:273–283. https://doi.org/10.1007/s10750-009-9849-y
Chao A, Gotelli NJ, Hsieh TC et al (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Chase JM (2007) Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci 104:17430–17434. https://doi.org/10.1073/pnas.0704350104
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, Chicago
Chase JM, Kraft NJB, Smith KG, et al (2011) Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2:art24. https://doi.org/10.1890/ES10-00117.1
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x
Dagosta FCP, De PM (2019) The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Museum Nat Hist 431:1–163. https://doi.org/10.1206/0003-0090.431.1.1
Dambros C, Zuquim G, Moulatlet GM et al (2020) The role of environmental filtering, geographic distance and dispersal barriers in shaping the turnover of plant and animal species in Amazonia. Biodivers Conserv 29:3609–3634. https://doi.org/10.1007/S10531-020-02040-3/FIGURES/5
Dormann CF, Elith J, Bacher S et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography (cop) 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Dornelas M, Connolly SR, Hughes TP (2006) Coral reef diversity refutes the neutral theory of biodiversity. Nature 440:80–82. https://doi.org/10.1038/nature04534
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Modell 196:483–493. https://doi.org/10.1016/j.ecolmodel.2006.02.015
Dray S, Bauman D, Blanchet G, et al (2022) adespatial: multivariate multiscale spatial analysis. R Packag version 03–16
Dudgeon D (2019) Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr Biol 29:R960–R967
Erős T (2017) Scaling fish metacommunities in stream networks: synthesis and future research avenues. Community Ecol 18:72–86. https://doi.org/10.1556/168.2017.18.1.9
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Fricke R, Eschmeyer WN, Van der Laan R (2023) Catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Accessed 20 Oct 2023
Frissell CA, Liss WJ, Warren CE, Hurley MD (1986) A hierarchical framework for stream habitat classification: viewing streams in a watershed context. Environ Manage 10:199–214. https://doi.org/10.1007/BF01867358
Frota A, Ganassin MJM, Pacifico R et al (2022) Spatial distribution patterns and predictors of fish beta-diversity in a large dam-free tributary from a Neotropical floodplain. Ecohydrology 15:1–14. https://doi.org/10.1002/eco.2376
Galbraith HS, Vaughn CC, Meier CK (2008) Environmental variables interact across spatial scales to structure trichopteran assemblages in Ouachita Mountain rivers. Hydrobiologia 596:401–411. https://doi.org/10.1007/s10750-007-9124-z
Garutti V (1999) Descrição de Astyanax argyrimarginatus sp. n. (Characiformes, Characidae) procedente da bacia do Rio Araguaia. Brasil Rev Bras Biol 59:585–591. https://doi.org/10.1590/S0034-71081999000400008
Garutti V, Langeani F (2009) Redescription of Astyanax goyacensis Eigenmann, 1908 (Ostariophysi: Characiformes: Characidae). Neotrop Ichthyol 7:371–376. https://doi.org/10.1590/S1679-62252009000300003
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227. https://doi.org/10.1038/35012228
Gooriah LD, Chase JM (2020) Sampling effects drive the species–area relationship in lake zooplankton. Oikos 129:124–132. https://doi.org/10.1111/OIK.06057
GRASS Development Team (2019) Geographic Resources Analysis Support System (GRASS GIS) Software. Version 7:6
Grönroos M, Heino J, Siqueira T et al (2013) Metacommunity structuring in stream networks: roles of dispersal mode, distance type, and regional environmental context. Ecol Evol 3:4473–4487. https://doi.org/10.1002/ece3.834
Hamilton NE, Ferry M (2018) ggtern : ternary diagrams using ggplot2. J Stat Softw 87:1–17. https://doi.org/10.18637/jss.v087.c03
Hawkins CP, Mykrä H, Oksanen J, Vander Laan JJ (2015) Environmental disturbance can increase beta diversity of stream macroinvertebrate assemblages. Glob Ecol Biogeogr 24:483–494. https://doi.org/10.1111/geb.12254
Heino J, Melo AS, Bini LM (2015a) Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw Biol 60:223–235. https://doi.org/10.1111/fwb.12502
Heino J, Melo AS, Siqueira T et al (2015b) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60:845–869. https://doi.org/10.1111/fwb.12533
Hijmans RJ (2020) raster: geographic data analysis and modeling
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton, New Jersey
INMET (2020) Instituto Nacional de Meteorologia. http://www.inmet.gov.br/portal/. Accessed 17 Aug 2020
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities—the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170. https://doi.org/10.1139/f00-239
Jamoneau A, Passy SI, Soininen J et al (2018) Beta diversity of diatom species and ecological guilds: response to environmental and spatial mechanisms along the stream watercourse. Freshw Biol 63:62–73. https://doi.org/10.1111/fwb.12980
Landeiro VL, Magnusson WE, Melo AS et al (2011) Spatial eigenfunction analyses in stream networks: do watercourse and overland distances produce different results? Freshw Biol 56:1184–1192. https://doi.org/10.1111/j.1365-2427.2010.02563.x
Landeiro VL, Bini LM, Melo AS et al (2012) The roles of dispersal limitation and environmental conditions in controlling caddisfly (Trichoptera) assemblages. Freshw Biol 57:1554–1564. https://doi.org/10.1111/j.1365-2427.2012.02816.x
Latrubesse EM, Arima E, Ferreira ME et al (2019) Fostering water resource governance and conservation in the Brazilian Cerrado biome. Conserv Sci Pract 1:1–8. https://doi.org/10.1111/csp2.77
Legendre P (2008) Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. J Plant Ecol 1:3–8. https://doi.org/10.1093/jpe/rtm001
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334. https://doi.org/10.1111/geb.12207
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Leibold MA, Chase JM (2017) Metacommunity patterns in space. In: Metacommunity Ecology, Volume 59. Princeton University Press, pp 90–130
Lima LB, Oliveira FJM, Borges FV et al (2021) Streams fish from Upper Araguaia and Middle Rio da Mortes basin, Brazil: generating subsidies for preservation and conservation of this critical natural resource. Biota Neotrop 21:2021. https://doi.org/10.1590/1676-0611-bn-2021-1205
López-Delgado EO, Winemiller KO, Villa-Navarro FA (2019) Do metacommunity theories explain spatial variation in fish assemblage structure in a pristine tropical river? Freshw Biol 64:367–379. https://doi.org/10.1111/fwb.13229
López-Delgado EO, Winemiller KO, Villa-Navarro FA (2020) Local environmental factors influence beta-diversity patterns of tropical fish assemblages more than spatial factors. Ecology 101:1–12. https://doi.org/10.1002/ecy.2940
MacArthur RH, Wilson EO (1967) The theory of island biogeography, vol 1. Princeton University Press
Malabarba LR, Jerep FC (2014) Review of the species of the genus Serrapinnus Malabarba, 1998 (Teleostei: Characidae: Cheirodontinae) from the rio Tocantins-Araguaia basin, with description of three new species. 3847:57–79
Malumbres-Olarte J, Rigal F, Girardello M, et al (2021) Habitat filtering and inferred dispersal ability condition across-scale species turnover and rarity in Macaronesian island spider assemblages. J Biogeogr 1–14. https://doi.org/10.1111/jbi.14271
MapBiomas (2021) Coleção 6 da Série Anual de Mapas de Cobertura e Uso de Solo do Brasil. www.mapbiomas.org/pages/downloads. Accessed 17 Nov 2021
Montag LFA, Winemiller KO, Keppeler FW et al (2019) Land cover, riparian zones and instream habitat influence stream fish assemblages in the eastern Amazon. Ecol Freshw Fish 28:317–329. https://doi.org/10.1111/eff.12455
Newbold T, Hudson LN, Arnell AP, et al (2016) Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science (80- ) 353:288–291. https://doi.org/10.1126/science.aaf2201
Oberdorff T, Dias MS, Jézéquel C, et al (2019) Unexpected fish diversity gradients in the Amazon basin. Sci Adv 5:eaav8681. https://doi.org/10.1126/sciadv.aav8681
Oksanen J, Blanchet FG, Friendly M, et al (2018) vegan: community ecology package. R Packag. version 2.5–2
Olden JD, Poff NL (2003) Toward a mechanistic understanding and prediction of biotic homogenization. Am Nat 162:442–460. https://doi.org/10.1086/378212
Paula FR, Gerhard P, Wenger SJ et al (2013) Influence of forest cover on in-stream large wood in an agricultural landscape of southeastern Brazil: a multi-scale analysis. Landsc Ecol 28:13–27. https://doi.org/10.1007/s10980-012-9809-1
Peres-Neto PR, Legendre P (2010) Estimating and controlling for spatial structure in the study of ecological communities. Glob Ecol Biogeogr 19:174–184. https://doi.org/10.1111/j.1466-8238.2009.00506.x
Perez Rocha M, Bini LM, Domisch S et al (2018) Local environment and space drive multiple facets of stream macroinvertebrate beta diversity. J Biogeogr 45:2744–2754. https://doi.org/10.1111/jbi.13457
Petrolli MG, Azevedo-Santos VM, Benine RC (2016) Moenkhausia venerei (Characiformes: Characidae), a new species from the rio Araguaia, Central Brazil. Zootaxa 4105:159–170. https://doi.org/10.11646/zootaxa.4105.2.4
Petsch DK (2016) Causes and consequences of biotic homogenization in freshwater ecosystems. Int Rev Hydrobiol 101:113–122. https://doi.org/10.1002/iroh.201601850
Petsch DK, Blowes SA, Melo AS, Chase JM (2021) A synthesis of land use impacts on stream biodiversity across metrics and scales. Ecology 0:1–12. https://doi.org/10.1002/ecy.3498
Podani J, Schmera D (2011) A new conceptual and methodological framework for exploring and explaining pattern in presence—absence data. Oikos 120:1625–1638. https://doi.org/10.1111/j.1600-0706.2011.19451.x
Podani J, Ricotta C, Schmera D (2013) A general framework for analyzing beta diversity, nestedness and related community-level phenomena based on abundance data. Ecol Complex 15:52–61. https://doi.org/10.1016/j.ecocom.2013.03.002
Presley SJ, Higgins CL, Willig MR (2010) A comprehensive framework for the evaluation of metacommunity structure. Oikos 119:908–917. https://doi.org/10.1111/j.1600-0706.2010.18544.x
QGIS Development Team (2023) QGIS Geographic Information System
R Core Team (2021) R: A language and environment for statistical computing. R Found. Stat. Comput. https://www.R-project.org
Reid AJ, Carlson AK, Creed IF et al (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do Bioma Cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Embrapa Cerrados, Planaltina, pp 151–212
Ricklefs RE (1987) Community diversity: relative roles of local and regional processes. Science (80- ) 235:167–171. https://doi.org/10.1126/science.235.4785.167
Roa-Fuentes CA, Casatti L (2017) Influence of environmental features at multiple scales and spatial structure on stream fish communities in a tropical agricultural region. J Freshw Ecol 32:281–295. https://doi.org/10.1080/02705060.2017.1287129
Roa-Fuentes CA, Heino J, Cianciaruso MV et al (2019) Taxonomic, functional, and phylogenetic β-diversity patterns of stream fish assemblages in tropical agroecosystems. Freshw Biol 64:447–460. https://doi.org/10.1111/fwb.13233
Roth NE, Allan JD, Erickson DL (1996) Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landsc Ecol 11:141–156. https://doi.org/10.1007/BF02447513
Sály P, Takács P, Kiss I et al (2011) The relative influence of spatial context and catchment- and site-scale environmental factors on stream fish assemblages in a human-modified landscape. Ecol Freshw Fish 20:251–262. https://doi.org/10.1111/j.1600-0633.2011.00490.x
Schlosser IJ (1991) Stream fish ecology: a landscape perspective. Bioscience 41:704–712. https://doi.org/10.2307/1311765
Schmera D, Árva D, Boda P et al (2018) Does isolation influence the relative role of environmental and dispersal-related processes in stream networks? An empirical test of the network position hypothesis using multiple taxa. Freshw Biol 63:74–85. https://doi.org/10.1111/fwb.12973
Siqueira T, Bini LM, Roque FO et al (2012) Common and rare species respond to similar niche processes in macroinvertebrate metacommunities. Ecography (cop) 35:183–192. https://doi.org/10.1111/J.1600-0587.2011.06875.X
Socolar JB, Gilroy JJ, Kunin WE, Edwards DP (2016) How should beta-diversity inform biodiversity conservation? Trends Ecol Evol 31:67–80. https://doi.org/10.1016/j.tree.2015.11.005
Soininen J (2014) A quantitative analysis of species sorting across organisms and ecosystems. Ecology 95:3284–3292. https://doi.org/10.1890/13-2228.1
Steffen W, Richardson K, Rockstrom J, et al (2015) Planetary boundaries: guiding human development on a changing planet. Science (80- ) 347:1259855–1259855. https://doi.org/10.1126/science.1259855
Strahler AN (1957) Quantitative analysis of watershed geomorphology. Eos, Trans Am Geophys Union 38:913–920. https://doi.org/10.1029/TR038i006p00913
Sweeney BW, Newbold JD (2014) Streamside forest buffer width needed to protect stream water quality, habitat, and organisms: a literature review. J Am Water Resour Assoc 50:560–584. https://doi.org/10.1111/jawr.12203
Taylor CM, Warren ML (2001) Dynamics in species composition of stream fish assemblages: environmental variability and nested subsets. Ecology 82:2320–2330. https://doi.org/10.1890/0012-9658(2001)082[2320:DISCOS]2.0.CO;2
Tencatt LFC, Britto MR, Isbrücker IJH, Pavanelli CS (2022) Taxonomy of the armored catfish genus Aspidoras (Siluriformes: Callichthyidae) revisited, with the description of a new species. Neotrop Ichthyol 20:. https://doi.org/10.1590/1982-0224-2022-0040
Terán GE, Benitez MF, Mirande JM (2020) Opening the Trojan horse: phylogeny of Astyanax, two new genera and resurrection of Psalidodon (Teleostei: Characidae). Zool J Linn Soc 190:1217–1234. https://doi.org/10.1093/zoolinnean/zlaa019
Teresa FB, Casatti L (2012) Influence of forest cover and mesohabitat types on functional and taxonomic diversity of fish communities in Neotropical lowland streams. Ecol Freshw Fish 21:433–442. https://doi.org/10.1111/j.1600-0633.2012.00562.x
Tonkin JD, Stoll S, Jähnig SC, Haase P (2016) Contrasting metacommunity structure and beta diversity in an aquatic-floodplain system. Oikos 125:686–697
Tonkin JD, Altermatt F, Finn DS et al (2018) The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshw Biol 63:141–163. https://doi.org/10.1111/fwb.13037
Townsend CR, Dolédec S, Norris R et al (2003) The influence of scale and geography on relationships between stream community composition and landscape variables: description and prediction. Freshw Biol 48:768–785. https://doi.org/10.1046/j.1365-2427.2003.01043.x
Ueida VS, Castro RMC (1999) Coleta e fixação de peixes de riachos. In: Caramaschi EP, Mazzoni R, Peres-Neto PR (eds) Ecologia de peixes de riachos. Oecologia Brasiliensis, Rio de Janeiro, Brasil, pp 1–22
Venere PC, Garutti V (2011) Peixes do Cerrado: Parque Estadual da Serra Azul, rio Araguaia, MT. RiMa, São Carlos
Zbinden ZD, Matthews WJ (2017) Beta diversity of stream fish assemblages: partitioning variation between spatial and environmental factors. Freshw Biol 62:1460–1471. https://doi.org/10.1111/fwb.12960
Acknowledgements
The Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT) for granting the PhD Scholarship to the first author (FAPEMAT Notice 002/2015, Process 155509/2015). We are grateful to the UNEMAT Graduate Program in Ecology and Conservation (UNEMAT/PPGEC) and FAPEMAT (“Projeto Veredas” 227925/2015) for partial financial support. We thank Eddie Lenza, Edson Fontes, Jean Ortega, Fabiano Corrêa, and Renato Dala-Corte for their valuable suggestions that contributed to the improvement of the manuscript. We are also grateful to colleagues at the Laboratório de Ecologia e Conservação de Ecossistemas Aquáticos (LECEA/UFMT) for help in field collection. We would like to thank all the landowners for their permission to collect samples from their properties. L. B. Lima and F. J. M. Oliveira were partially financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) Finance Code 001; L. B. Lima was supported by Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT/CNPq Notice 001/2022, Process FAPEMAT-PRO.000073/2023); P. De Marco-Junior and D. P. Lima-Junior are supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (308694/2015-5 and 305923/2020-0 grants, respectively).
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT Notice 002/2015, Process155509/2015), the Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT/CNPq Notice 001/2022, Process FAPEMAT-PRO.000073/2023), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance Code 001), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (308694/2015–5 and 305923/2020–0 grants, respectively).
Author information
Authors and Affiliations
Contributions
LBL, DPLJ, and PDJ contributed to the conception and design of the study. LBL, FJMO, and DPLJ conducted fieldwork and material preparation. LBL performed the statistical analyses. LBL wrote the first draft of the manuscript. All authors contributed to the previous manuscript versions' writing, review, and ideas. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
The work conforms to the legal requirements of the country in which it was carried out.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lima, L.B., Oliveira, F.J.M., De Marco Júnior, P. et al. Local environmental variables are the best beta diversity predictors for fish communities from the Brazilian Cerrado streams. Aquat Sci 86, 19 (2024). https://doi.org/10.1007/s00027-023-01032-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01032-z