Abstract
The mechanisms behind phytoplankton diversity patterns in natural ecosystems still remain elusive. In two shallow lakes with contrasting river connectivity, we first explored how diversity within each sampling (alfa diversity, α), among samplings (beta diversity, β1), and among hydrological seasons (β2) contributed to the diversity registered throughout the whole year (gamma diversity, γ). Then we estimated the importance of several environmental and temporal factors as structuring factors of these diversity patterns. To do this, we sampled the two lakes—one laterally isolated and other laterally connected lake with the Paraná River System—during a complete hydrological year. For the analyses, we considered both the species and the functional group level. At the species level, temporal variation (β1 + β2) made the main contribution for gamma diversity at the connected lake, possibly related to the constant species input from the river system. For the isolated lake, however, α was the main contributor. Regarding functional groups, α was the most important for both lakes, although no element of gamma diversity was different from the null model. Environmental factors like conductivity, turbidity, nutrient availability, and flood phases appeared as more relevant for the connected lake. Temporal processes (e.g., succession, ecological drift) were critical for the observed diversity patterns in both lakes. These results were consistent particularly considering the taxonomical approach. Our main findings are that the environment influences phytoplankton diversity patterns; however, other dynamics occurring on temporal scales may be more relevant for the phytoplankton community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Floodplain rivers differ from channeled rivers because of the periodic lateral exchange of energy and materials (Neiff 1990). The main feature of floodplains is that ecological attributes depend on the hydrological pulse, which, besides nutrients and climatic characteristics, is a primary driver of aquatic productivity (Junk et al. 1989; Thomaz et al. 2007). Likewise, the seasonality, flood frequency, duration, and intensity of the surface water connection between the river and its floodplain also influences the environmental conditions and consequently the biota of floodplains (Schagerl et al. 2009).
Temporal fluctuations in populations and the environment are universal in natural ecosystems. These fluctuations play a significant role in species coexistence and the stability of communities and ecosystem properties (Loreau et al. 2001; Gonzalez and Loreau 2009). Both species coexistence and ecosystem stability require some form of temporal niche differentiation by which distinct species respond differently to variations in their environment (Loreau and de Mazancourt 2008).
For phytoplankton, the effects of environmental fluctuation in floodplain ecosystems are highly documented for tropical and subtropical floodplain rivers (e.g., Divina de Oliveira and Calheiros 2000; Nabout et al. 2006; Mihaljević et al. 2009; 2013; Kraus et al. 2019); and particularly, for the Middle Paraná River System (García de Emiliani 1980, 1981, 1985, 1986, 1997; García de Emiliani and Anselmi de Manavella 1983; Zalocar de Domitrovic 1992, 1993, 2007; Devercelli 2006, 2010). However, when the focus is on diversity patterns of phytoplankton from floodplain ecosystems, the relationship between the distribution of species and environmental variation has been less explored. For instance, in lentic isolated lakes, the high-water retention time may benefit the establishment of a low number of species, favoring strong interspecific relationships, traits distribution, and environmental variation (Van der Gucht et al. 2007; Qu et al. 2018). Besides, the limited incoming of species could also relate to the low temporal variation of communities. In contrast, the hydrological pulse may increase similarity among lakes and the river system (Ward et al. 2002; Thomaz et al. 2007; Melack et al. 2009). The constant incoming of species can also favor the temporal variation of the communities due to the exchange of species between the lotic system and the lakes.
The term diversity, introduced by Whittaker (1972) more than forty years ago is a broadly used concept by ecologists to describe a hierarchical organization of species from local richness (alpha-α), between two comparable areas (beta-β) to a regional scale (gamma-γ). The mechanisms behind these diversity patterns and their levels (α, β, γ) are still highly debated and remain a central question in theoretical and applied ecology (Leibold et al. 2004; Cottenie 2005; Maloufi et al. 2016). For decades, it was assumed that communities were shaped by the environmental heterogeneity and niche differences among species (Niche Theory) (Hutchinson 1957; Padial et al. 2014). Then it was proposed that that interacting species are equivalent and that community structure results from random variation in births and deaths in populations and restricted dispersal (Neutral Theory) (Hubbell 2001). Nowadays, the tendency is to combine niches and neutrality approaches to explain community structure and test the relative importance of each component (de Mazancourt et al. 2013).
Few studies, however, have included the effect of temporal scales of observation that could affect the estimation of the relative influence of environment, space, and time (Castillo-Escrivà et al. 2020). At this point, partitioning of variance is useful to separate environmental and temporal fractions and examine the relative contribution of environmental control (Svenning and Skov 2005; Mykrä et al. 2007). This approach allowed to decrease the probability of considering spurious relationships between the community and environmental variation as being significant (Legendre and Gauthier 2014; Pineda et al. 2019; Castillo-Escrivà et al. 2020).
In this line, the gamma partitioning framework may be also helpful and a complementary analysis to variation partitioning to understand the processes behind community assemblage. For instance, gamma partitioning allows us to know the contribution of samples (i.e. local diversity-α) and the variation among them (i.e. beta diversity-β) to the total diversity (i.e. gamma diversity-γ). Besides, as gamma partitioning technique use null models to test the contribution of each gamma diversity component, it allows to know if the contribution is associated with an ecological process (if the observed pattern differs from the null model) or if, on the contrary, the contribution is associated to random dynamics (if the observed pattern is equal to the null model). These methods have been applied to study of several organisms communities (e.g., de Souza Nogueira et al. 2010; Angeler 2013; Castillo-Escrivà et al. 2020); however, studies using freshwater phytoplankton are still rare, with some recent contributions, like Pineda et al. (2019) and de Fátima Bomfim et al. (2021), who have shed light on the factors shaping phytoplankton diversity patterns mediated by temporal factors.
In this study, we explore how the differences in the hydrological connectivity of two shallow alluvial lakes (laterally connected and isolated, respectively) of the Middle Paraná River System may affect phytoplankton taxonomic and functional diversity throughout an entire hydrological year. For phytoplankton species and functional groups, we assessed the contribution of each sample (α diversity), the inter-sampling variation (β1 diversity), and among hydrological pulse phases (β2 diversity) to the total diversity registered (γ diversity) in one year at both lakes. We also explored the importance of the environmental and temporal factors for the diversity patterns observed. To do this, we tested two hypotheses: (1) the hydrological pulse is the primary source of temporal variation for both lakes. We expected (a) that the fractions β1–β2 will be more important in the partitioning of the connected lake, indicating a higher relevance of temporal factors, (b) a higher dominance of species in the isolated lake because they may have more time to colonize and develop under suitable conditions, thereby allowing species composition to reflect the differences in environmental conditions. (2) Environmental selection is more important in the isolated lake than in the connected one because the constant incoming of species in the connected lake can uncouple the relation environment–community. Thus, we expected (prediction c) a higher effect of the environment in the isolated lake and a higher effect of temporal factors in the connected one.
Materials and methods
Study area
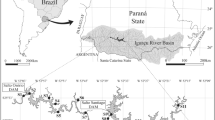
During the year 2010, we simultaneously sampled two shallow floodplain lakes (Irupé Lake and Mirador Lake), which have contrasting lateral connectivity to the Paraná River System. The Irupé Lake is a subtropical shallow lake connected to the Middle Paraná River System (31° 40′ 23.78" S, 60° 34′ 23.63" W, Argentina) throughout the Miní stream linked to the Colastiné River (a secondary channel of the Paraná River with a mean flow of 2,490 m3 s−1). Irupé Lake has an area of approximately 9.94 ha and had, during the study period, a maximum depth of 5.30 m in the limnetic area (Fig. 1). This lake was surrounded by a belt of Ludwigia peploides Raven (emergent vegetation), Eichhornia crassipes (Mart.) Solms (free-floating vegetation) and Ceratophyllum demersum Linnaeus (submerged vegetation) covering less than 30% of the total lake surface. On the contrary, Mirador Lake is a smaller subtropical shallow lake (31° 37' S, 60° 41' W, Argentina), with approximately 3.76 ha and a maximum registered depth of 3.3 m. The lake water is mainly supplied by groundwater infiltration and rainfall, without lateral connection to the fluvial system. For this lake, the alluvial plain has been highly modified for real estate purposes with several land modifications, like buildings, roads, and several human constructions in the area. These changes have permanently interrupted the lateral connection of this lake with the rest of the alluvial system. However, the lake is still immersed in a natural reserve where most uses are banned, and where only tourism contemplation and education activities are allowed. During the study period, the perimeter of this lake was lined with Panicum elephantipes Nees ex Trin. (rooted-floating vegetation) and Ludwigia peploides Raven without presenting free-floating or submerged macrophytes (Fig. 1).
Study area showing the section of the Middle Paraná River, the Colastiné River, directly connected to the Paraná, the Miní Stream, and the isolated (Mirador) and connected (Irupé) lakes. Image processing credits to Pekel et al. (2016)
Field samplings and laboratory analyses
Both lakes were sampled fortnightly during a full hydrological year (12 months from December 2009 to November 2010) with a sampling date difference between 1 and 3 days. We collected samples from different sites at each lake from a watercraft. For Irupé Lake, four samplings (one limnetic and three littoral) were chosen (n = 96), while we choose two littoral and one limnetic sampling point for Mirador Lake (n = 72). For each lake, we combined the samples within sampling dates for analyses because there was no difference in the total biovolume registered among sampling sites (p > 0.05 for each lake).
As a proxy of hydrological fluctuations of the lotic system (Colastiné River and Miní Stream), we used the hydrometric level of the Colastiné River (obtained from the Subsecretaría de Recursos Hídricos de la Nación, Argentina). Based on the mean hydrometric level for the whole year, we identified the three most frequent hydrological phases of a hydrological pulse: high water (HW): water level > 4.8 m (floods, from December to March), low water (LW): < 3.0 m (from September to December), and intermediate water (MW): 4.8 m < water level > 3.0 m based on Neiff (1990). The hydrometric level of the Colastiné River was highly correlated with the depth of both lakes (connected, R2 = 89%; isolated, R2 = 75%). In the case of the isolated lake, we considered the high correlation as a signal of a hyporheic connection between the lake and the fluvial system.
At each sampling, temperature (°C), pH, and conductivity (µS cm−1) were measured using HANNA portable electronic meters. Water transparency was measured with Secchi disc (m) and maximum depth (Zd) (m) with an ultrasonic sensor. The euphotic zone (Zeu) was estimated using the formula proposed by Koenings and Edmundson (1991) for turbid waters (Zeu = Secchi disc depth × 3.5). The ratio Zd:Zeu was also estimated. This ratio indicates the relative amount of time that phytoplankton spends in darkness, where high values indicate that phytoplankton spends most of the time at low light intensities (Reynolds 1994). Chlorophyll-a (Chl-a) concentration, turbidity, and two inorganic nutrient forms (nitrites + nitrates: NO2− + NO3−, and soluble reactive phosphorus: SRP) were estimated, by taking water samples of 2 L. Chl-a extraction was done by placing the GF/F filters with the filtrated sample with acetone (90%) through maceration in a glass grinder and stored at 4 °C for 6 to 12 h in darkness. The extracts obtained were clarified and measured with a spectrophotometer at 664 and 750 nm and after acidification with HCl 0.1 N at 665 and 750 nm (APHA 2005). Turbidity was measured at 450 nm with a HACH DR 2000 spectrophotometer. NO2− + NO3− concentration was determined by the reduction principle with metallic cadmium and SRP by the ascorbic acid method; both were determined using chemical kits from the HACH Company and measured at 400 and 880 nm, respectively using a HACH DR 5000 spectrophotometer.
Phytoplankton samples were collected from the subsurface water layer using 120 mL bottles and were immediately fixed with 1% acidified Lugol solution. The phytoplankton quantitative analysis was done following the Utermöhl (1958) method. The density obtained was expressed as ind mL−1 by counting at least 100 individuals of the most abundant species and accepting a counting error lower than 20% Venrick (1978). Algal biovolume was measured following the method proposed by Hillebrand et al. (1999) by measuring the dimension of at least ten individuals of each taxon. Biovolume data were expressed as mm3 L−1. Species identification was made by following keys and specific bibliography of each algal group, such as Komárek and Fott (1983), Tell and Conforti (1986), Krammer and Lange-Bertalot (1991), Zalocar de Domitrovic and Maidana (1997), Komárek and Anagnostidis (1999, 2005), and Komárek (2013). Species were classified in Reynolds Functional Groups (RFG) following Reynolds et al. (2002), Padisák et al. (2009), and the most recent revision made by Kruk et al. (2017). RFG classification includes phylogenetic affiliation, phenological, morphological, and physiological features with tolerances and sensitivities to temperature, ionic strength, light, nutrients, mixing of the water column, flushing, and grazing (Kruk et al. 2017). This classification has been widely used in other similar studies (e.g., Bovo-Scomparin et al. 2013; Devercelli et al. 2010; Pineda et al. 2017).
Data analyses
We performed a Principal Component Analysis (PCA) with the standardized and centered environmental variables for a general data description. Those variables with a low correlation with the ordination axes (not exceeding the central area delimited by the equilibrium contribution circle (ter Braak and Šmilauer 2012)) were removed. Each environmental variable was visually inspected using mean and standard deviation and considering the three identified water phases (HW, MW, and LW).
We run the Olmstead–Tukey association test expressed in a Cartesian coordinate system for phytoplankton to categorize species and Reynolds Functional Groups (RFG) according to their biovolume (Log10 (x + 1) transformed) in the x-axis and frequency (occurrence frequencies of each species or RFG) in the y-axis. The four quadrants were defined using the maximum value obtained for each axis divided by two. The classification was established according to the quadrant on which the species were located into Dominant (high biovolume and high-frequency occurrence), Occasional (high biovolume and low frequency of occurrence), Constant (low biovolume and high frequency of occurrence), and Rare (low biovolume and low frequency of occurrence). We also performed a Principal Coordinate Analyses (PCoA) using the relative abundance of each species or RFG. The ordination analyses obtained were validated by Permutational Analysis of Variance (PERMANOVA) to determine statistically significant differences, and it was used to evaluate the effect of water phases on the phytoplankton taxonomical and functional structure. The PCA and the PCoA analyses were performed using CANOCO v. 5.10 (ter Braak and Šmilauer 2012).
Differences in species richness and the number of functional groups between the isolated and connected lake were estimated with a Rarefaction analysis based on samples (i.e., incidence data) following Chao et al. (2014). We interpreted differences in diversity between lakes when, in the Rarefaction plots, the confidence interval (95%) of the curves (one curve for each lake) did not overlap. As a measure of diversity, we used Hill numbers (qD) to express the effective number of species (or Reynolds functional groups, RFG) for the total of the species/RFG (richness) (q = 0), the typical (q = 1), and the most frequent species/RFG (q = 2). The q term in the following equation defines each of the diversity measures (or Hill numbers):
where S is the number of species in the assemblage, πi is the probability that species i is detected in a sampling unit, and q defines the sensitivity to relative frequencies. When q = 0, the incidence probabilities of all species are identical, and 0D equals species richness. With q = 1, 1D is the exponential form of Shannon entropy based on the relative incidences in the assemblage. 1D can be interpreted as the number of “typical species” (Chao et al. 2014). When q = 2, 2D equals 1/(1– Simpson diversity), which gives more importance to frequent species while discounting the contribution of rare species (Gotelli and Chao 2013). It can be interpreted as the number of most frequent species. Rarefactions were calculated and plotted using the default values of the iNEXT package from R software (Hsieh et al. 2016).
To evaluate the contribution of each sample (α diversity) and the temporal variation (β diversity) to regional diversity (γ diversity), we performed Additive Partitioning of Diversity (Crist et al. 2003) at each lake separately. For both species and functional groups, we considered the within-sample diversity (α), the inter-sampling variation (β1), and the variation among water phases (β2). The regional diversity (γ) resulted from the sum of the α, β1, and β2 components. The beta diversity (β1 and β2) measured the adequate number of species (or groups) in a pool of samples not contained in an average community (Chao et al. 2012). The significance of each diversity component was tested through 999 randomizations according to a null model (samples randomly permuted across temporal scales). The derived null hypothesis states that gamma diversity components result from the random allocation of lower-level samples into higher-level ones (Crist et al. 2003). The observed diversity component was considered different from the null expectation when p values were less than 0.05. When the observed fraction was different from the null model, we interpreted it as some ecological process was responsible for the diversity pattern observed. With these results, it is difficult to determine the specific mechanism driving the species assemblage. However, when the importance of the observed diversity fraction was lower than expected (i.e., a negative Standardized Effect Size—SES) we interpreted that the establishment of species was limited at that particular scale. On the contrary, when the importance of the observed fraction was higher than expected (i.e., a positive SES) we interpreted as the establishment of species was favored.
The influence of the environment and time on the variation on phytoplankton species and RFG at each lake was tested using partial Redundancy Analyses (pRDA). As a response matrix, we used an occurrence and a biovolume matrix Hellinger transformed. As explanatory variables, we used one temporal and one environmental matrix (log-transformed except for pH and the Zd:Zeu ratio). We used asymmetric eigenvectors maps (AEM, Blanchet et al. 2008) since it allows us to model the directionality of time in which one sampling date influences future samplings (Legendre and Gauthier 2014). This method has been already used by other authors to describe temporal factors variations in the past (e.g., Baho et al. 2015; Pineda et al. 2019). Because our samplings were not made simultaneously the same day (samples from each lake were taken with a difference between 1 and 3 days), we included the number of days between samplings as weights. This analysis produced 15 temporal vectors (hereafter AEMs). For each lake and attribute (occurrence and biovolume), we used forward selection (999 permutations and p < 0.05) to select those environmental and temporal factors which significantly affected the phytoplankton assemblage. We investigated the collinearity of the environmental factors by using the variation inflation factor (VIF). Those variables with VIF > 10 were removed from the analysis (Legendre and Legendre 1998). With the selected environmental and temporal variables, we performed a pRDA. We kept the adjusted R2 as the value of importance of each fraction as it is not affected by the number of explanatory variables, making the results comparable (Peres-Neto et al. 2006). The pRDA with the two illustrative matrices originated five values: the shared fraction, the total environmental explanation, the total temporal explanation, the pure environmental explanation (in the absence of the interaction with time), and the pure temporal explanation (in the lack of the interaction with the environment). The significance of each fraction (except the shared one that cannot be tested) was tested at p < 0.05 with 999 permutations. For interpretation, we retained only the pure temporal and environmental fractions.
Results
Environmental variations
The first two axes of the PCA analysis explained 67.4% of total variability. The first axis defined a spatial gradient with the highest pH, Chl-a, SRP, and conductivity corresponding to the isolated lake. The highest turbidity values (FTU), nitrite–nitrates, and Zd:Zeu ratio were registered at the connected lake (Fig. 2). The second axis showed a temporal gradient with most of the environmental variables changing among water phases. Turbidity, Zd:Zeu, and nitrite–nitrate concentrations increased during the LW phase and decreased to MW and HW in the connected lake. In the disconnected lake Chl-a, conductivity and pH were higher during HW and LW, while SRP was higher during HW and MW phases. A visual inspection of data (Table 1) showed, for both lakes, that the temperature had the highest values during the HW phase and the lowest during the MW. For conductivity, both lakes showed an increasing trend from the HW to the LW phase; however, the values recorded in the isolated lake were more than one order of magnitude higher. The pH also showed a similar trend than conductivity, with an increment from HW to LW phases in both lakes, being the isolated more alkaline than the connected one. For SRP, the pattern differed, the connected lake had lower mean values while the highest values occurred during the MW period.
On the contrary, the isolated lake registered the maximum values of SRP during the HW phase. For the nitrate–nitrite concentrations, the connected lake increased from HW to LW. In contrast, in the isolated lake, the trend was more erratic across samplings. Both lakes showed similar values for this form of inorganic nitrogen. For the Chl-a concentration, an increased trend from HW to LW phase was also observed in the connected lake. In the isolated, however, similar concentrations among water phases was registered, being these values more than tenfold higher than the other. Regarding turbidity, the connected lake showed a similar pattern than the Chl-a concentration. This pattern, however, was inverted in the isolated lake; the latter also had lower values. Finally, the Zd:Zeu ratio showed similar values for both lakes. It increased from the HW to the LW in the connected lake. A change that was not noticeable in the isolated lake (Table 1, Supplementary material, Fig. S1).
Phytoplankton structure analyses
For the connected lake, two hundred and thirty taxa were registered throughout the study period, corresponding to a total of eighteen Reynold Functional Groups (RFG). Most of the species recorded in this lake were rare (97%), and only one (Cryptomonas ovata) was dominant. Most of the RFG were rare, and six (J, E, G, X3, P, and B) appeared in the Olmstead–Tukey test as occasional. Only the RFG Y (tolerant species to low light availability) was constant in the assemblage. No dominant RFG was registered during the study period (Fig. 3a, b).
For the isolated lake, ninety species belonging to thirteen RFG were registered. Most species were rare (64%). Protoperidinium sp. dominated in biovolume, and Cryptomonas ovata, Trachelomonas volvocina, Lepocinclis sp., Euglena rostrifera, Phacus longicauda, Coelomoron sp. and Microcystis aeruginosa were constant species. Most of the RFG structure appeared as occasional or rare, and only LO and W1 were dominant. No RFG was registered as constant (Fig. 3c, d).
The PCoA showed that the flooding phases (HW, MW, and LW) influenced the phytoplankton structure (relative abundance) for the connected lake by explaining 79.41% of total variation (PERMANOVA, F = 3.69; p = 0.0001). Tetraëdriella regularis, Mallomonas ovum, and Navicula sp. were dominant during the LW and part of the MW phases. Peridinium sp., Mallomonas sp., Eudorina elegans, Pandorina morum, and Cryptomonas curvata completed the MW assemblage and dominated the HW phase. Cryptomonas ovata, Lepocinclis ovum, and Chlorococcum sp. appeared as a group of well-represented species during the three water phases. About the RFG structure, the selected groups explained 62.31% of the total variability (PERMANOVA F = 4.08; p = 0.0001) with Y as dominant during HW and MW phases and X1, X2, MP, W1, and W2 better represented during part of the MW and the LW phase (Fig. 4a, b).
Principal coordinates analysis (PCoA) performed for the connected lake (a, b), and the isolated lake (c, d) for the species and the RFG, respectively. The plots only show those species or RFG that had a significant correlation (± 0.5) with each axis. High (H), Middle (M), and Low (L) water phases for each lake are indicated with dots while species and RFG with triangles. Key: Euglena rostrifera (Eros), Mucidosphaerium pulchellum (Mpull), Monoraphidium minutum (Mmin), Monoraphidium arcuatum (Marc), Amphora sp. (Amphr.), Lepocinclis ovum (Lovu), Protoperidinium sp. (Protop), Scenedesmus longicauda (Slong), Lepocinclis acus (Lacu), Oocystis sp. (Oocyst), Coelastrum pulchellum (Cpull), Planktothrix sp. (Plank.) Jaaginema gracile (Jgra), Desmodesmus brasiliensis (Dbra), Scenedesmus obtusus f. disciformis (Sobdis), Spirulina (Spi), Spirulina sp. 2 (Spi2), Tetraëdriella regularis (Treg), Euglena elastica (Eele), Navicula sp. (Navsp), Mallomonas sp. (Mallsp), Mallomonas ovum (Movu), Peridinium sp. (Peri), Cryptomonas ovata (Cova), Cryptomonas curvata (Ccur) Chlorococcum (Chloroc), Pandorina morum (Pmor)
For the isolated lake, the species selected in the PCoA analysis explained 98% (PERMANOVA F = 6.27; p = 0.001) of the total variation. However, the ordination analysis was less clear with most of the representative species were linked to the high water phase. Only Euglena rostrifera appeared as the best representative during LW phase. For the RFG, the groups selected accounted for 67.65% of total variation (PERMANOVA F = 5.81; p = 0.0001). Most of the RFG were well represented during the whole sampling year (Fig. 4c, d).
Phytoplankton diversity pattern analyses
The rarefaction analysis revealed a higher diversity at the connected lake for the total number of species and RFG (q = 0) as well as for the typical (q = 1) and most frequent species and RFG (q = 2). In all cases, the connected lake showed larger diversity for both the interpolated and extrapolated data, as evidenced by the non-overlapping of the confidence intervals (Fig. 5).
Sampled-size-based rarefaction (solid line segments) and extrapolation (dotted line segments) comparing the species and functional groups (RFG) diversity recorded at the isolated and connected lakes. Diversity is expressed as the number species (or RFG) for the total richness (q = 0), typical (q = 1), and for the most frequent species (or RFG) (q = 2). The shaded region represents a confidence interval of 95% obtained by a bootstrap method based on 200 replications
According to the Additive Partition Analysis, the contribution to gamma diversity of each sample (α), the inter-samplings variation (β1), and variation among water phases (β2) were variable for both lakes (Fig. 6). For species, α was more important in the isolated lake (47%) than in the connected one (30%), with fewer species than expected in the null model in both cases (isolated: SES = −4.53; connected: SES = −4.44, p < 0.001 for both). The total temporal variation (β1 + β2) was more important than α in both lakes. β1 was more important in the connected lake (38%) than in the isolated (28%) and lower than expected under a null distribution in both cases (connected SES = −6.22 and isolated SES = −4.82, p < 0.001 for both). β2 had the lowest contribution at both lakes (connected = 32%, isolated = 25%), but with a variation higher than expected in a null model of distribution (SES = 7.95 and SES = 7.41, respectively, p < 0.001 for both) (Fig. 6, Supplementary material, Table S1). For the RFG, α diversity had the highest contribution in both lakes (connected = 77% and isolated = 80%). The β1 was slightly more important in the connected lake (18% and 13% for isolated), while β2 showed similar values for both lakes (β2 = 6% and 7% for connected). In the RFG partitioning, no component of gamma diversity was statistically different from the null model (p > 0.05 for both) (Fig. 7).
Additive partitioning of the gamma diversity of phytoplankton species and functional groups at the connected and isolated lakes. The values of the gamma diversity components are expressed as a percentage. The α local diversity, β1 inter-samplings variation, and β2 variation among water phases. An asterisk indicates a component with a distribution statistically different from the null model
Partial Redundancy Analysis (pRDA) where are indicated the relative importance (% of explanation) of the environment (E) and temporal vectors (T) for the biovolume and occurrence of phytoplankton species and functional groups, in the connected and isolated lakes. Significant values are indicated with an asterisk. Values < 0 are not shown. The significance of the shared components is not testable. R represents the residuals of the analysis (it means, the variation that was not explained by the factors). The sum of the explanations and residuals may be slightly different from 100% due to the rounded or < 0 values
In the Partial Redundancy Analysis (pRDA), the forward procedure selected environmental and temporal factors as significant in all cases (Table 2). The hydrometric level of the river was the only factor always selected. More temporal variables (AEMs) were chosen in the case of the isolated lake. Variation partitioning better explained the phytoplankton community in the isolated lake than in the connected lake (lower residuals, Fig. 7). However, the importance of factors varied between lakes. In the connected lake, environmental and temporal factors significantly affected the community, except the environment for RFG. In contrast, only temporal factors were statistically significant in the isolated. The non-significance of the pure environmental fraction (p > 0.05), indicates that these variables are structured in time. As evidence, the shared fraction was larger in most cases (Fig. 7), indicating a high temporal autocorrelation of some environmental variables. Additional information regarding the significance of each fraction could be seen in the supplementary material (Table S2).
Discussion
In the laterally connected lake (Irupé Lake), the descriptive analyses, the Olmstead–Tukey association test and the PCoA analysis showed that the cryptophytes C. ovata and C. curvata were dominant, especially during the high water (HW) and middle water (MW) phases. These species can tolerate high turbulence and a low residence time through a high reproduction rate and small size compared to other algae groups (Litchman et al. 2010; Fraisse et al. 2015). Its dominance across the study period, indicates a marked influence of the river, with a constant input of water from the lotic system, especially during HW and part of MW phases, when the coupling with higher temperatures may also favor the development of these species. This pattern was already described for this and other river floodplains (García de Emiliani, 1997; Izaguirre et al. 2001; Reynolds et al. 2002; Descy et al. 2011).
For the isolated lake (Mirador Lake), the patterns observed in the Olmstead–Tukey association test and the PCoA analysis were utterly different. The PCoA analysis did not show a real pattern of species distribution according to the hydrological phases, and the assemblage tended to be dominated by Dinophyceae species like Protoperidinium sp. and Euglenophyceae like L. acus, L. ovum, and E. rostrifera. All of them develop better in stagnant waters. The appearance of Cyanobacteria like Planktothrix, Spirulina, and J. gracile is also remarkable in this lake. These species may find better conditions to develop when nutrients concentrations, pH, and water residence time are higher (Unrein et al. 2010; Paerl and Otten 2013; Paerl 2016). The patterns observed in this lake were also consistent with the mentioned by other authors in previous studies when they analyzed the impact of isolation in the phytoplankton assemblage structure from alluvial lakes (García de Emiliani 1997; Zalocar de Domitrovic 2003; Mihaljević et al. 2009; Stevic et al. 2013).
Attending to the diversity patterns, we predicted a higher temporal turnover contribution (β1 and β2) to gamma diversity in the connected lake than in the isolated one (prediction a). At this point, the partition of the gamma diversity (Fig. 6) confirmed this prediction. Indeed, the constant exchange of species between the lake and the river favors species substitution. In this way, the connectivity with the river may also favor the higher number of species and functional groups registered in the connected lake by favoring the incoming species from the regional pool. In comparison, in the isolated lake, where lateral connectivity with the lotic systems is restricted, alpha diversity had a higher contribution to gamma diversity. The rarefaction analysis also showed that the total species richness (q = 0) was significantly higher in the connected lake, as expected. Similarly, the most frequent (q = 1) and the most typical species (q = 2) and RFG showed a similar trend with higher values observed in the connected lake than in the isolated, as expected.
We also anticipated a higher dominance of species and RFG in the isolated lake (lower values of q = 2) where more estable conditions could allow better competitors to exclude other species (prediction b). Indeed, interspecific dynamics in planktonic organisms seem to be an essential driver of the structure of communities in more stable environments (Margalef 1978). These results align with our prediction, showing that q = 2 values tend to be lower in the isolated lake than in the connected one. On the contrary, we found a higher species richness and a lower dominance in the connected lake. Indeed, the recurrent instability of the water column, continuous water flow, and increased turbulence may impose limits upon colonization, establishment, and development of phytoplankton species (Bovo-Scomparin et al. 2013; Jati et al. 2017; Lansac-Tôha et al. 2019). A higher number of species and RFG could be advantageous by increasing the resilience and resistance of the ecosystem to several disturbances, and by favoring ecosystem renewal and reorganization following change (Elmqvist et al. 2003; Downing and Leibold 2010; Mori et al. 2013). In the isolated lake, the lateral disconnection with the fluvial system had become permanent, and this seems to be traduced in a low incoming of species and RFG.
Notably, when RFGs were considered in the gamma diversity partition, the observed diversity patterns were not different from the null model. In other words, at the functional group level, the observed pattern did not appear to arise from ecological processes. The functional group approximation assumes that several species can be grouped by affinity and functional response to environmental changes (Lavorel et al. 1997; Díaz and Cabido 2001). However, these approximations may mask some diversity patterns because functional diversity uses average trait values for species by assuming that the species of the same group are entirely redundant and equally different among other. Nevertheless, as Cianciaruso et al. (2009) has pointed out, the classification of functional groups does not always capture the aspects of biodiversity most relevant to ecosystem stability and functionality. In this regard, the functional traits approach is increasingly used to understand ecological processes (Petchey and Gaston 2002; Cianciaruso et al. 2009; Pavoine and Bonsall 2011; Carmona et al. 2016).
Results suggest that, for the laterally connected lake, species distribution within the lake and among samplings (α local diversity and β) were controlled, partially, by environmental variation (prediction c), with fewer species than expected in the null model. This indicates that although the connected lake had a high species exchange with the lotic system across the year, a low number of species became established, limited, at least in part, by environmental factors as the pRDA indicated. For the isolated lake, the analysis also suggests a lower number of species than that expected in an null model. However, as we will see later, none of the environmental variables considered in this study appeared as relevant; hence, other dynamics such as competition, predation, or facilitation may have an important role in shaping the community. We lack data to test the effect of these interactions, with the signal of such ecological dynamics probably hidden in the high values of the pRDA residues. In fact, these dynamics could also be significant in the connected lake, as it also had high residual values.
For both lakes the observed α and β1 components were lower than expected, suggesting that a low number of species are established and that variation on finer temporal scales does not favor the diversity, at least in our study. On the other hand, the variation associated to seasonal flood (β2) was greater than expected (see partitioning analysis), indicating a positive effect on the biodiversity of the environmental variation associated with the flood pulse.
Among the controlling environmental factors found in the pRDA analysis for the connected lake, major drivers included temperature, SRP, changes in the light availability (estimated by the Zd:Zeu ratio and turbidity), and the river water level (used here as a proxy of the hydrological influence on these lakes). Among these variables, the hydrological influence could be considered the most relevant factor. Indeed, as several studies have shown, the hydrological pulse is considered a significant factor shaping other more local environmental variables across the alluvial system (like conductivity, turbidity, or nutrients availability) (Junk and Wantzen 2004). For instance, we showed that the species variation in the connected lake was influenced by the flood pulse phases (PCoA analyses) at least. These changes were also significant for gamma diversity, being a favorable factor especially in the connected lake. Our results are consistent with those found by other authors, who have already recognized the environmental factors mentioned above, in particular the importance of the flooding pulse, as relevant in structuring phytoplankton assemblages in shallow alluvial lakes (e.g., Izaguirre et al. 2001; Zalocar de Domitrovic et al. 2007; Sinistro 2010; Frau et al. 2015).
For both lakes, the pRDA analysis showed that the effect of temporal factors was more important than the environment. Indeed, the explanation of the environment at the connected lake was lower than 6%, while in the isolated lake the effect was null. This partially contradicts our third prediction (prediction c) since we assumed that the environmental variation would be more important in the isolated lake. In contrast, in the connected lake, the constant transport of organisms would determine that temporal changes, not related to the environment, would be more relevant as we found here.
The temporal signal could be related to several processes and scales (Castillo-Escrivà et al. 2020). However, determining the specific process associated with several temporal scales is complicated. In this study, we have some evidence of the influence of processes acting at finer and broader temporal scales. For instance, in the connected lake, those AEMs linked to broader temporal scales (e.g., AEM1, 2, 3) were significant drivers of communities. These kinds of AEMs can be linked to higher dissimilarity among more distant samples (Blanchet et al. 2008; Legendre and Gauthier 2014). A phenomenon probably related to local extinctions without replacement from the regional pool (Pineda et al. 2019) or autogenic processes unrelated to environmental changes, like species succession or rapid changes in trophic interactions (Honti et al. 2007; Lotter and Anderson 2012) which can modify phytoplankton structure.
On the other hand, AEMs related to finer scales (e.g., AEM10, 12, 14, 15) were also significant community drivers, especially in the isolated lake. These AEMS indicate high similarity between near samples on time (Blanchet et al. 2008; Legendre and Gauthier 2014). This result confirm what we found in the PCoA analysis showing a most homogeneous assemblage throughout the year. We are aware that both broader and finer AEMs were significant in both lakes, but we need more evidence in this regard to achieving more precise conclusions.
The substantial temporal influence in the partitioning would also be explained first by considering the resolution level of the analysis. Indeed, when patterns are analyzed at a low aggregation level (such as the presence-absence species resolution), things seem less predictable. However, if we look at a more aggregated level, such as certain groups of algae (e.g., classes like Chlorophyceae and Euglenophyceae), the patterns observed tend to be better explained by the environment (Hastings et al. 1993; Smale 1998; Scheffer 1999). A pattern already confirmed in a previous study perfomerd in the isolated lake (Frau et al. 2015) where we work with phytoplankton phyla. Secondly, variation in the order that species colonize a habitat may be amplified by initial differences in the size of population species (Zhou and Ning 2017). In other words, more abundant species would have a greater probability of dispersal than those less abundant; however, this effect could be diluted by differences among traits and adaptative fitness, which could determine less expected assemblage associations.
Other alternatives to explain the low environmental influence could be differences in growth rates, delayed response of phytoplankton to environmental changes, or the effect of important drivers that we did not measure, such as zooplankton grazing that occasionally could be a major controlling factor in floodplain lakes (Frau 2021). At least for these two lakes we can assume that grazing was not a relevant controlling factor during the study period. Indeed, in a previous work made in the isolated lake (Mirador Lake), Frau et al. (2015) showed that zooplankton was dominated by microphagous rotifers and did not affect phytoplankton structure. In Frau et al. (2017), we also found that high abundances of planktivorous fish control zooplankton and these in turn cannot control phytoplankton. Unpublished data for the connected lake (Irupé Lake) also suggests a similar pattern considering that microphagous rotifers also dominated the zooplankton assemblage during the study period (M.F. Gutierrez, personal communication).
The evidence compiled by other authors about the factors that control phytoplankton composition and beta diversity still requires intensive study considering that the results obtained may depend on the spatial extent (Heino et al. 2015; Bortolini et al. 2017). Some studies have shown that the phytoplankton composition is controlled by only pure environmental effects (Vanormelingen et al. 2008; Padial et al. 2014; Bortolini et al. 2017), or both environmental and spatial effects (Soininen et al. 2007; Teittinen et al. 2016; Devercelli et al. 2016). In comparison, others have shown that neither environmental nor spatial effects structured phytoplankton communities (Beisner et al. 2006; Nabout et al. 2009). While most studies approach the effect of environment or environment and space, few studies have included the temporal influence as another proxy of processes that depend on time. In this study, we found that temporal changes unrelated to environmental variations were more critical for phytoplankton structuring than the environment. These results are consistent with Pineda et al. (2019) and de Fátima Bomfim et al. (2021), which also analyzed the dynamics of connected and disconnected lakes and found a relatively high relevance of temporal changes on the structure of phytoplankton communities.
Conclusions
In this study, we described the phytoplankton diversity patterns in two shallow lakes from a subtropical region with contrasting connectivity to a fluvial system, as well as environmental and temporal drivers. As we expected, alfa diversity was more relevant for gamma diversity in the isolated lake. At the same time, the change of species among samplings could be more relevant in the connected lake. We also found that even though environmental factors are suitable descriptors of phytoplankton diversity, other temporal factors could be more important drivers. Indeed, although the temporal variation of the phytoplankton community associated with changes in environmental conditions (i.e., temporal succession) has been studied for a long time, our results suggest that other temporal factors not related to environmental changes may have relevance as controlling factors of phytoplankton diversity, at least in these two shallow lakes. We are aware that our results may be limited in their generalization because we only considered two lakes from a vast alluvial plain which is the Paraná River System. However, a highlight of this approach is the role that temporal factors may play in the structuration of the phytoplankton assemblages. All of this suggests novel and exciting new research avenues to follow in plankton research.
Data availability
Data available on request from the authors.
References
Angeler DG (2013) Revealing a conservation challenge through partitioned long-term beta diversity: increasing turnover and decreasing nestedness of boreal lake metacommunities. Diversity Distrib 19:772–781
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. Am Public Health Assoc, USA
Baho DL, Futter MN, Johnson RK, Angeler DG (2015) Assessing temporal scales and patterns in time series: comparing methods based on redundancy analysis. Ecol Complex 22:162–168
Beisner BE, Peres-Neto PR, Lindström ES, Barnett A, Longhi ML (2006) The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87:2985–2991
Blanchet FG, Legendre P, Borcard D (2008) Modelling directional spatial processes in ecological data. Ecol Model 215:325–336. https://doi.org/10.1016/j.ecolmodel.2008.04.001
Bortolini JC, Pineda A, Rodrigues LC, Jati S, Velho LFM (2017) Environmental and spatial processes influencing phytoplankton biomass along a reservoirs-river-floodplain lakes gradient: a metacommunity approach. Freshw Biol 62:1756–1767. https://doi.org/10.1111/fwb.12986
Bovo-Scomparin VM, Train S, Rodrigues LC (2013) Influence of reservoirs on phytoplankton dispersion and functional traits: a case study in the upper paraná river, Brazil. Hydrobiologia 702:115–127
Carmona CP, de Bello F, Mason NWH, Lepš J (2016) Traits without borders: integrating functional diversity across scales. Trends Ecol Evol 31:5
Castillo-Escrivà A, Mesquita-Joanes F, Rueda J, (2020) Effects of the temporal scale of observation on the analysis of aquatic invertebrate metacommunities. Front Ecol Evol 8:561838
Chao A, Chiu CH, Hsieh TC, Inouye BD (2012) Proposing a resolution to debates on diversity partitioning. Ecology 93:2037–2051. https://doi.org/10.1890/11-1817.1
Chao A, Gotelli NJ, Hsieh TC et al (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Cianciaruso MV, Batalha MA, Gaston KJ, Petchey OL (2009) Including intraspecific variability in functional diversity. Ecology 90:81–89
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x
Crist TO, Veech JA, Gering JC, Summerville KS (2003) Partitioning species diversity across landscapes and regions: a hierarchical analysis of alpha, beta, and gamma diversity. Am Nat 162:734–743. https://doi.org/10.1086/378901
de Fátima BF, Miranda Lansac-Tôha F, Costa Bonecker C, Lansac-Tôha F (2021) Determinants of zooplankton functional dissimilarity during years of El Niño and La Niña in floodplain shallow lakes. Aquat Sci 83:41
de Mazancourt C, Forest I, Larocque A et al (2013) Predicting ecosystem stability from community composition and biodiversity. Ecol Lett 16:617–625
de Souza NI, Nabout JC, Rodrigues Ibañez MS, Maurice Bourgoin L (2010) Determinants of beta diversity: the relative importance of environmental and spatial processes in structuring phytoplankton communities in an amazonian floodplain. Acta Limnol Bras 22:3
Descy JP, Leitao M, Everbecq E, Smitz JS, Frank J (2011) Phytoplankton of the river loire france: a biodiversity and modeling study. J Plankton Res 0:1–16
Devercelli M (2006) Phytoplankton of the middle paraná river during an anomalous hydrological period: a morphological and functional approach. Hydrobiologia 563:465–478
Devercelli M (2010) Changes in phytoplankton morpho-functional groups induced by extreme hydroclimatic events in the middle paraná river (argentina). Hydrobiologia 639:5–19
Devercelli M, Scarabotti P, Mayora G, Schneider B, Giri F (2016) Unravelling the role of determinism and stochasticity in structuring the phytoplanktonic metacommunity of the Paraná River Floodplain. Hydrobiologia 764:1–18
Díaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Divina de Oliveira M, Fernades Calheiros D (2000) Flood pulse influence on phytoplankton communities of the south pantanal floodplain, Brazil. Hydrobiologia 427:101–112
Downing AL, Leibold MA (2010) Species richness facilitates ecosystem resilience in aquatic food webs. Freshw Biol 55:2123–2137
Elmqvist T, Folke C, Nystrom M et al (2003) Response diversity, ecosystem change, and resilience. Front Ecol Environ 1:488–494. https://doi.org/10.2307/3868116
Fraisse S, Bormans M, Lagadeuc Y (2015) Turbulence effects on phytoplankton morpho-functional traits selection. Limnol Oceanogr 60:872–884
Frau D (2021) Grazing impacts on phytoplankton from South American water ecosystems: a synthesis. Hydrobiologia. https://doi.org/10.1007/s10750-021-04748-x
Frau D, Devercelli M, José de Paggi S, Scarabotti P, Mayora G, Battauz Y, Senn M (2015) Can top-down and bottom-up forces explain phytoplankton structure in a subtropical and shallow groundwater connected lake? Mar Freshw Res 66:1106–1115
Frau D, Battauz Y, Sinistro R (2017) Why predation is not a controlling factor of phytoplankton in a neotropical shallow lake: a morpho-functional perspective. Hydrobiologia 788:115–130
García de Emiliani MO (1980) Fitoplancton de una laguna del valle aluvial del río Paraná (‘Los Matadores’), Santa Fe, Argentina. estructura y distribución en relación a factores ambientales. Ecología 4:127–140
García de Emiliani MO (1981) Fitoplancton de los principales cauces y tributarios del valle aluvial del río Paraná: tramo Goya-Diamante. I Rev Asoc Cienc Nat Litoral 12:112–125
García de Emiliani MO (1985) Fitoplancton de los principales cauces y tributarios del valle aluvial del río Paraná: tramo Goya-Diamante. III Rev Asoc Cienc Nat Litoral 16:95–112
García de Emiliani MO (1986) Fitoplancton de los principales cauces y tributarios del valle aluvial del río Paraná (tramo Goya-Diamante). IV Rev Asoc Cienc Nat Litoral 17:51–61
García de Emiliani MO (1997) Effects of water level fluctuations on phytoplankton in a river-floodplain lake system (Paraná River, Argentina). Hydrobiologia 357:1–15
García de Emiliani MO, Anselmi de Manavella MI (1983) Fitoplancton de los principales cauces y tributarios del valle aluvial del Río Paraná: tramo Goya-Diamante. III Rev Asoc Cienc Nat Litoral 14:217–237
Gonzalez A, Loreau M (2009) The Causes and Consequences of Compensatory dynamics in ecological communities. Annu Rev Ecol Evol Syst 40:393–414
Gotelli NJ, Chao A (2013) Measuring and estimating species richness, species diversity, and biotic similarity from sampling data. In: Levin SA (ed) Encyclopedia of Biodiversity, 2nd edn. Academic Press, Waltham, pp 195–211
Hastings A, Hom CL, Ellner S, Turchin P, Godfray HCJ (1993) Chaos in ecology: is mother nature a strange attractor? Annu Rev Ecol Evol Syst 24:1–33
Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015) Metacommunity organization, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60:845–869. https://doi.org/10.1111/fwb.12533
Honti H, Istvánovics V, Osztoics A (2007) Stability and change of phytoplankton communities in a highly dynamic environment—the case of large, shallow Lake Balaton (Hungary). Hydrobiologia 581:225–240
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Hubbell SP (2001) A unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Hutchinson GE (1957) Concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427. https://doi.org/10.1101/SQB.1957.022.01.039
Izaguirre I, O’ Farrell I, Tell G, (2001) Variation in phytoplankton composition and limnological features in a water–water ecotone of the lower Paraná basin (Argentina). Freshw Biol 46:63–74
Jati S, Bortolini JC, Moresco GA et al (2017) Phytoplankton community in the last undammed stretch of the Paraná River: considerations on the distance from the dam. Acta Limnol Bras 29:e112
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-flood plain systems. Can J Fish Aquat Sci 106:110–127
Junk WJ, Wantzen KM (2004) The flood pulse concept: new aspects approaches and applications—an update. In: Welcomme RL, Petr T (eds) Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries. Bangkok: Food and Agriculture Organization and Mekong River Commission, FAO Regional Office for Asia and the Pacific, pp 117–149
Koenings JP, Edmundson JA (1991) Secchi disk and photometer estimates of light regimes in Alaskan lakes: effects of yellow color and turbidity. Limnol Oceanogr 36:91–105
Komárek J (2013) Cyanoprokaryota Teil/3rd part: heterocytous genera. In: Gärtner L, Krienitz M, Chagerl M (eds) Süswasserflora von Mitteleuropa (Freshwater flora of Central Europe). Springer Spektrum, Berlin
Komárek J, Anagnostidis K (1998) Cyanoprokaryota. Teil 1: Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa 19/1. Gustav Fisher Verlag, Jena
Komárek J, Anagnostidis K (2005) Cyanoprokaryota. Teil 2: Oscillatoriales. In: Büdel B, Gärtner G, Krienitz L, Schagerl M (eds) Süsswasserflora von Mitteleuropa 19/2. Elsevier, Germany
Komárek J, Fott B (1983) Chlorophyceae chlorococcales. In: Huber-Pestalozzi G (ed) Das Phytoplankton des Sdwasswes Die Binnenggewasser, vol 16(5). Schweizerbart’sche Verlagsbuchhandlung Germany
Krammer K, Lange-Bertalot H (1991) Bacillariophyceae 3 Teil Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa. Gustav Fischer Verlag, Germany
Kraus CN, Bonnet MP, de Souza NI et al (2019) Unraveling flooding dynamics and nutrients’ controls upon phytoplankton functional dynamics in amazonian floodplain lakes. Water 11:154
Kruk C, Devercelli M, Huszar VLM et al (2017) Classification of reynolds phytoplankton functional groups using individual traits and machine learning techniques. Freshw Biol 62:1681–1692
Lansac-Tôha FM, Heino J, Quirino BA et al (2019) Differently dispersing organism groups show contrasting beta diversity patterns in a dammed subtropical river basin. Sci Total Environ 691:1271–1281
Lavorel S, McIntyre S, Landsberg J, Forbes TDA (1997) Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol Evol 12:474–478. https://doi.org/10.1016/S0169-5347(97)01219-6
Legendre P, Gauthier O (2014) Statistical methods for temporal and space-time analysis of community composition data. Proc r Soc B Biol Sci 281:20132728. https://doi.org/10.1098/rspb.2013.2728
Legendre P, Legendre L (1998) Numerical Ecology. Elsevier, Elsevier, Amsterdam
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Litchman E, de Tezanos PP, Klausmeier CA, Thomas MK, Yoshiyama K (2010) Linking traits to species diversity and community structure in phytoplankton. Hydrobiologia 653:15–28
Loreau M, de Mazancourt C (2008) Species synchrony and its drivers: neutral and nonneutral community dynamics in fluctuating environments. Am Nat 172:E48-66
Loreau M, Naeem S, Inchausti P et al (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808
Lotter AF, Anderson NJ (2012) Limnological responses to environmental changes at inter-annual to decadal time-scales. In: Birks HJB, Lotter AF, Juggins S, Smol JP (eds) Tracking Environmental Change using Lake Sediments: Data Handling and Numerical Techniques. Springer, Dordrecht, pp 557–578
Maloufi S, Catherine A, Mouillot D, Louvard Cout A, Bernard C, Troussellier M (2016) Environmental heterogeneity among lakes promotes hyper b-diversity across phytoplankton communities. Freshw Biol 61:633–645
Margalef R (1978) Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta 1:493–509
Melack JM, Novo EMLM, Forsberg BR, Piedade MTF, Maurice L (2009) Floodplain ecosystem processes. In: Gash J, Keller M, Silva-Dias P (eds) Amazonia and global change Geophysical Monograph Series 186. American Geophysical Union, Washington, pp 525–541
Mihaljević M, Stevic F, Horvatic J, Kutuzovic BH (2009) Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (Kopacki Rit Nature Park, Croatia). Hydrobiologia 618:77–88
Mihaljević M, Špoljarić D, Stević F, Pfeiffer T (2013) Assessment of flood-induced changes of phytoplankton along a river–floodplain system using the morpho-functional approach. Environ Monit Assess 185:8601–8619
Mori AS, Furukawa T, Sasaki T (2013) Response diversity determines the resilience of ecosystems to environmental change. Biol Rev 88:349–364
Mykrä H, Heino J, Muotka T (2007) Scale-related patterns in the spatial and environmental components of stream macroinvertebrate assemblage variation. Global Ecol Biogeogr 16:149–159
Nabout JC, Nogueira IS, Oliveira LG (2006) Phytoplankton community of floodplain lakes of the Araguaia River, Brazil, in the rainy and dry seasons. J Plankton Res 28:181–193
Nabout JC, Siqueira T, Bini LM, Nogueira IDS (2009) No evidence for environmental and spatial processes in structuring phytoplankton communities. Acta Oecol 35:720–726
Neiff JJ (1990) Ideas para la interpretación ecológica del Paraná. Interciencia 15:424–441
Padial AA, Ceschin F, Declerck SAJ et al (2014) Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9:e111227
Padisák J, Crossetti LO, Naselli-Flores L (2009) Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621:1–19
Paerl HW, Otten TG (2013) Harmful Cyanobacterial bloom: causes, consequences and controls. Microb Ecol 65:995–1010
Paerl HW, Gardner WS, Havens KE, Joyner AR, McCarthy MJ, Newell SE, Qin B, Scott JT (2016) Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 54:213–222
Pavoine S, Bonsall MB (2011) Measuring biodiversity to explain community assembly: a unified approach. Biol Rev 86:792–812
Pekel JF, Cottam A, Gorelick N, Belward AS (2016) High-resolution mapping of global surface water and its long-term changes. Nature 540:418–422
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625. https://doi.org/10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2
Petchey OL, Gaston KJ (2002) Functional diversity (FD), species richness, and community composition. Ecol Lett 5:402–411
Pineda A, Moresco GA, Magro de Paula AC et al (2017) Rivers affect the biovolume and functional traits of phytoplankton in floodplain lakes. Acta Limnol Bras 29:e113
Pineda A, Peláez Ó, Días JD et al (2019) The El Niño Southern Oscillation (ENSO) is the main source of variation for the gamma diversity of plankton communities in subtropical shallow lakes. Aquat Sci 81:49. https://doi.org/10.1007/s00027-019-0646-z
Qu Y, Wu N, Guse B, Fohrer N (2018) Riverine phytoplankton shifting along a lentic-lotic continuum under hydrological, physiochemical conditions and species dispersal. Sci Total Environ 619–620:1628–1636. https://doi.org/10.1016/j.scitotenv.2017.10.139
Reynolds CS (1994) The long, the short and the stalled: on the attributes of phytoplankton selected by physical mixing in lakes and rivers. Hydrobiologia 289:9–21
Reynolds CS, Huszar V, Kruk C, Naselli-Flores L, Melo S (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:417–428
Schagerl M, Drozdowski I, Angeler DG, Hein T, Preiner S (2009) Water age – a major factor controlling phytoplankton community structure in a reconnected dynamic floodplain (Danube, Regelsbrunn, Austria). J Limnol 68:274–287
Scheffer M (1999) Searching explanations of nature in the mirror world of math. Conservation Ecol 3:11
Sinistro R (2010) Top-down and bottom-up regulation of planktonic communities in a warm temperate wetland. J Plankton Res 32:109–220
Smale S (1998) Finding a horseshoe on the beaches of rio. The Math Intell 20:39–44
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30:3–12
Stevic F, Mihaljević M, Spoljaric D (2013) Changes of phytoplankton functional groups in a floodplain lake associated with hydrological perturbations. Hydrobiologia 709:143–158
Svenning JC, Skov F (2005) The relative roles of environment and history as controls of tree species composition and richness in Europe. J Biogeogr 32:1019–1033
Teittinen A, Kallajoki L, Meier S, Stigzelius T, Soininen J (2016) The roles of elevation and local environmental factors as drivers of diatom diversity in subarctic streams. Freshwat Biol 61:1509–1521
Tell G, Conforti V (1986) Euglenophyta pigmentadas de Argentina. Bibl Phycol 75:1–301
ter Braak CJ, Šmilauer P (2012) Canoco reference manual and users guide software for ordination, version 5.0 Ithaca: Microcomputer Power
Thomaz SM, Bini M, Bozelli R (2007) Floods increase similarity among aquatic habitats in river–floodplain systems. Hydrobiologia 579:1–13
Unrein F, O’Farrell I, Izaguirre I, Sinistro R, dos Santos AM, Tell G (2010) Phytoplankton response to pH rise in a N-limited floodplain lake: relevance of N2-fixing heterocystous cyanobacteria. Aquat Sci 72:179–190
Utermöhl H (1958) Zur Vervollkommnung der quantitative phytoplankton: methodik. Mitt Int Verein Theor Angew 9:1–38
Van der Gucht K, Cottenie K, Muylaert K et al (2007) The power of species sorting: local factors drive bacterial community composition over a wide range of spatial scales. Proc Natl Acad Sci U S A 104:20404–20409. https://doi.org/10.1073/pnas.0707200104
Vanormelingen P, Cottenie K, Michels E, Muylaert K, Vyverman W, de Meester L (2008) The relative importance of dispersal and local processes in structuring phytoplankton communities in a set of highly interconnected ponds. Freshwat Biol 53:2170–2183
Venrick EL (1978) How many cells to count? In: Sournia A (ed) Phytoplankton manual. UNESCO, Paris
Ward JV, Tockner K, Arscott DB, Claret C (2002) Riverine landscape diversity. Freshwat Biol 47:517–540
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21(213):251
Zalocar de Domitrovic Y (1992) Fitoplancton de ambientes inundables del río Paraná (Argentina). Rev Hydrobiol Trop 25:177–188
Zalocar de Domitrovic Y (1993) Fitoplancton de una laguna vegetada por Eichhornia crassipes en el valle de inundación del río Paraná (Argentina). Amb Subtrop 3:39–67
Zalocar de Domitrovic Y (2003) Effect of fluctuations in the water level on phytoplankton development in three lakes of the Paraná River floodplain (Argentina). Hydrobiologia 510:175–193
Zalocar de Domitrovic Y, Maidana NI (1997) Taxonomic and ecological studies of the Parana River diatom flora (Argentina). In: Kociolek P (ed) Lange-Bertalot F. Bibliotheca Diatomologica. J. Cramer, Berlin
Zalocar de Domitrovic Y, Poide ASG, Casco SL (2007) Abundance and diversity of phytoplankton in the Paraná River (Argentina) 220 km downstream of the Yacyretá reservoir. Braz J Biol 67:53–63
Zhou J, Ning D (2017) Stochastic community assembly: does it matter in microbial ecology? Microbiol Mol Biol Rev 81:1–32
Acknowledgements
We thank to S. José de Paggi, F. Rojas Molina, E. Creus, and C. De Bonis who contributed to the samplings of these two lakes. This project was supported by Universidad Nacional del Litoral (CAI+D 14-78) and Agencia Nacional de Promoción Científica y Tecnológica (PICT 2010 n° 2350). The authors also thank anonymous reviewers who improved the quality of this manuscript with their suggestions and to P. de Tezanos Pinto who kindly revised language of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Frau, D., Pineda, A., Mayora, G. et al. Phytoplankton taxonomic and functional diversity in two shallow alluvial lakes with contrasting river connectivity. Aquat Sci 84, 26 (2022). https://doi.org/10.1007/s00027-022-00857-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00857-4