Abstract
The aim of the present study was to investigate the impact of a multidimensional exercise intervention focusing on physical capacity; one-repetition maximum (1RM) and maximum oxygen uptake (VO2Max), activity level, general well-being and quality of life in cancer patients undergoing chemotherapy. The intervention comprised resistance and fitness training, massage, relaxation and body-awareness training. Eighty-two cancer patients, with or without evidence of residual disease, were included: 66 patients with 13 different types of solid tumours and 16 patients with 6 types of haematological malignancies. The patients trained in mixed groups for 9 h weekly for 6 weeks. Physical capacity, physical activity level and psychosocial well-being as measured by the Medical Outcomes Study 36-item Short-Form Health Survey and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 were assessed pre- and post-intervention. Highly significant increases were achieved in muscular strength (p<0.001), physical fitness (p<0.001) and physical activity levels (p<0.001). The patients reported significant reduction in treatment-related symptoms, i.e., fatigue (p=0.006) and pain (p=0.03). Highly significant improvements were observed in physical functioning (p<0.001) and role functioning (p<0.001). Even patients with advanced disease were able to improve their results after 6 weeks. It is concluded that a multidimensional exercise intervention, including resistance training, may be beneficial for cancer patients undergoing chemotherapy. This study indicates significant clinical meaningful improvements. The exact role of the intervention has to be defined in a randomized controlled design. A clinically controlled trial including 250 patients is currently being carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well documented that cancer patients who undergo chemotherapy experience treatment-related symptoms and side effects including nausea, insomnia, diarrhoea, etc. [30, 31]. Irrespective of their cancer diagnosis, patients report fatigue, diminished physical capacity [33, 36, 55] and declining quality of life (QoL) [22, 45].
There is growing evidence that exercise programmes can increase physical fitness, reduce fatigue and improve various quality-of-life indices [19, 39, 42, 52]. Women with breast cancer represent the largest group of patients who have participated in exercise studies [21]. According to recently published reviews, there is a need to test the feasibility and effectiveness of exercise for patients with other types of cancer than breast cancer [28, 56]. Very few studies investigated the potential impact of exercise on oncological or haematological cancer patients with mixed diagnoses who were undergoing cytostatic treatment [18, 19, 23, 24, 26]. Low to moderate exercise interventions of varying duration seem to be the standard across existing studies. Predominantly, studies have examined the effects of a single activity, e.g. cardiovascular training on stationary bicycles, rather than resistance exercise as the exercise modality [28]. Additional studies are needed to provide evidence of whether specific patient groups, i.e. at different stages of the disease and/or with different diagnoses and treatments, can benefit from exercise, to what extent, and in which forms. Due to the lack of evidence, no recommendations can be issued at present [10, 36].
In addition to exercise, clinical controlled trials demonstrated that intervention with relaxation training and massage can reduce a wide range of treatment-related symptoms, with considerable variance in the type of cancer, stage of the disease and/or treatment protocols [9, 44, 53, 57, 60]. These interventions have also proved to effect anxiety and depression [11, 37] and mood disturbances in cancer patients undergoing chemotherapy [13, 14, 44]. Similarly, sports science has shown that body awareness can be used to release tension and to control anxiety [46]. This body of research provides evidence that exercise, relaxation, massage and body awareness training each can impact positively on physical and/or psychosocial well-being.
We developed a structured and supervised exercise intervention composed of the following components: resistance and fitness training, massage, relaxation and body awareness training. The intention was to maximize the effect. The intervention was offered to a broad range of cancer patients undergoing chemotherapy to satisfy their general need for supportive care, irrespective of diagnosis. Our previous research documented physiological and psychological benefits of a group programme that included physical activity designed for male cancer patients with mixed diagnoses and poor prognoses [2]. Similarly, previous research with self-help groups, primarily for female cancer patients, showed positive psychosocial effects for patients with mixed diagnoses [3, 4]. No other study on the impact of a multidimensional intervention in cancer patients with mixed diagnosis undergoing cytostatic treatment was identified.
The patients participated in groups of seven to nine, 9 h weekly for 6 weeks. Feasibility, safety and preliminary results from the study’s pilot phase were previously published [5]. Based on the pilot findings, we hypothesized that cancer patients, with or without active disease, being treated with chemotherapy could increase their physical capacity, general well-being and quality of life. The aim of the present study was to investigate the impact of the exercise intervention focusing on physical capacity: one-repetition maximum (1RM) and maximum oxygen uptake (VO2Max), physical activity level, general well-being and quality of life in cancer patients undergoing cytostatic treatment.
Patients and methods
Design
This was a prospective study using a one-group design. Assessments were made at baseline (test 1) and repeated after 6 weeks (test 2). The study was approved by the scientific committees of the Copenhagen and Frederiksberg municipalities (j.no. 01-273/00) and by the Danish Data Protection Agency (j.no. 2000-41-0-149).
Cancer patients were made aware of the project by posters and pamphlets in the out-patient clinic or ward at the departments of oncology and haematology. The exercise intervention included four components: physical exercise (resistance and cardiovascular/fitness training), relaxation training, body awareness training and massage. The intervention took place in a specially designed workout room located at the Copenhagen University Hospital and was carried out over a 6-week period, 9 h per week, in the mornings. Patients came in especially to participate in the exercise programme. On Mondays, Wednesdays and Fridays, the patients participated in physical exercise for 1.5 h followed by 0.5 h relaxation. On Tuesdays, the programme included 1.5 h of body awareness training followed by 0.5 h relaxation. Finally, on Mondays and Fridays, the patients received 0.5 h massage. The different components of the programme constituted a total package, which implied that the patients could not select one activity in preference of another. Seven to ten patients of mixed gender were included in each group. Physiotherapists and a cancer nurse specialist supervised the programme.
High-intensity physical training
Physical training was divided into three components: warm-up exercises, heavy resistance training and fitness. Warm-up exercise comprised dynamic actions with the large muscle groups, balance and coordination training. Three machines were used for heavy resistance training: a leg press, a chest press and a lat machine (Technogym, Gambettola, Italy). The practical goal in the training component was to accomplish three continuous series of five to eight repetitions at 85–95% of one-repetition maximum (1RM). This selection of heavy resistance training activities was made in an attempt to involve as many muscle groups in as few exercises as possible, allowing a noticeable effect to be accomplished in a short time span (10 min, two to three times weekly) [29, 49]. Cardiovascular/fitness training took the form of aerobic activities, which involved large muscle groups over longer periods and which aimed to positively affect circulation and overall energy levels [8, 27]. The exercise involved 10 min interval training on a stationary bicycle with a workload of 70–250 W, equivalent to 60–100% of each patient’s maximum heart rate, corresponding to 33 metabolic equivalent (MET; American College of Sports Medicine) hours weekly [5: p 708, 7].
Low-intensity physical training
Relaxation training
The relaxation training took place in the workout room, where patients lay on mats with pillows and rugs. Patients were instructed in the use of relaxation mechanisms, using principles of progressive relaxation. This involves switching from muscle tension to muscle relaxation motions in each of the muscle groups [17, 38, 58].
Body awareness training
Special emphasis was placed on physical movement and the purpose of exercise in increasing body acceptance, awareness and knowledge; training focused on balance/coordination grounding and integration of the senses (pp 708–709 in [5]) (in total 4 MET hours weekly [7]).
Massage
Massage was relaxing, facilitative or therapeutic, meeting the wish of each patient. Most patients preferred classic massage and/or scar tissue massage. In a few cases, patients requested venous pump massage, ultrasound or exercise therapy (pp 708–709 in [5]; see also [16, 60]).
On those days when the patients were offered high-intensity training, low-intensity training ended the training sessions. In this way, the cancer patients could maximize their physical capacity and afterward release stress and recover under guidance and observation. During the programme, the patients were advised to respect their own physical limitations as indicated by physical discomfort and uncontrollable exhaustion by participating to a level with which they felt comfortable. The programme is discussed by Quist et al. (unpublished data).
Screening and monitoring
In accordance with the guidelines and safety precautions defined by Winningham et al. [61] and Dimeo et al. [23], pre-exercise screening was performed every second day before the high-intensity physical training. If one of the following criteria were met, the patient was excluded from the physical training component of the programme on that specific day: diastolic blood pressure <45 or >95; pulse at rest >100; temperature >38°C; respiration frequency at rest >20; infection requiring treatment with antibiotics; ongoing bleeding; fresh petechiae; bruises; thrombocytes <50×109/l; leucocytes <1.0×109/l. Heart rate was continuously monitored and measured by means of a wireless heart rate transmitter worn by the patients [5: p 709].
Patients
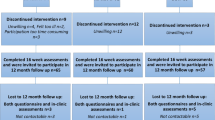
One hundred fifteen cancer patients undergoing chemotherapy gave written informed consent to enter the study. Thirty-three patients dropped out or were excluded from the study: 5 patients left because they felt that they did not belong to the group; 5 patients were excluded because of progressive acute illness; 9 patients were excluded because their cytostatic treatment terminated before completion of the exercise intervention; in 14 cases, the post-intervention measurements were not carried out. The reasons for the lack of post-intervention measurements were as follows: 6 cases were related to the patient’s work, 5 cases were related to leucopenia and 3 patients did not return the questionnaires.
The present report included 82 patients who received chemotherapy. Forty-two patients without evidence of disease received adjuvant treatment, and 40 patients received chemotherapy for advanced disease (lung, liver, lymph nodes).
Inclusion criteria were the following: residence in the Greater Copenhagen Council, age 18–65 years, both genders with a diagnosis of cancer (given at least 1 month previously) who were admitted to hospital for out-patient chemotherapy. The patients had received at least one series of chemotherapy and had a performance stage score of 0–1 (WHO). Patients who had previously undergone surgery and/or radiotherapy were included. Exclusion criteria were brain metastases, bone metastases and thrombocytopenia. Furthermore, the patients should be without cardiovascular symptoms, i.e. normal blood pressure, no signs of cardial insufficiency and no recent myocardial infarction. The patients’ characteristics and demographic information (mean age, sex, marital status and educational status) are described in Table 1.
The patients were first classified according to diagnosis and according to extent of disease at the beginning of the intervention. Forty-two of the patients had no evidence of disease (NED), which means that they received adjuvant treatment either after surgery or after having achieved complete remission with radiotherapy or chemotherapy. Forty patients had evidence of malignant disease (ED) while being treated with chemotherapy. Concerning patients with haematological malignancies, 4 patients with leukaemia were categorized as NED assessed by bone marrow investigation as well as normalized peripheral blood cell count; 11 patients with lymphoma and 1 patient with myeloma were categorized as ED based on radiology lesions and/or biological markers. Table 2 describes the characteristics of diagnoses and disease status and indicates the different regimens of treatment. No patients received immunotherapy at baseline. Thirteen patients had received antineoplastic treatment: 4 breast cancer patients received radiotherapy as well as chemotherapy [cyclophosphamide, methotrexate and 5-fluorouracil (CMF) or cyclophosphamide, epirubicin and fluorouracil (CEF)], 1 patient with cervical cancer received concomitant radiotherapy and cisplatinum, 2 patients (1 with cervical cancer and 1 with oesophageal cancer) received radiotherapy. Furthermore, 6 other patients (1 acute lymphoblastic leukemia, 1 acute myeloid leukemia, 1 non-Hodgkin’s lymphoma, 1 Ewing sarcoma and 2 ovary cancer patients) had previously been treated extensively with chemotherapy. At the beginning of the intervention, the patients had received one to ten series of treatments (median 3) at 3- or 4-week intervals. All patients were out-patients.
Assessment instruments
Disease and treatment variables were obtained through review of medical records. Repeated assessments were conducted at baseline (test 1) and after 6 weeks (test 2) and included two physiological tests (1RM, VO2Max), two questionnaires [the Medical Outcomes Study (MOS) 36-item Short-Form Health Survey (SF-36) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30)] and interviews (demographic data, leisure time physical activity level). On the first day, estimated 1RM tests were used to evaluate upper and lower body strength potential [15]. The tests involved performance on Technogym variable-resistance equipment and targeted the large muscle groups: chest press, leg press and pull down/lat machine. 1RM is stated in kilograms. To obtain an overall score for the strength, a new variable, weighted strength, was created as a weighted mean of capacity in chest press, leg press and lat machine. Maximum oxygen uptake (VO2Max) was indirectly estimated by use of a stepwise work capacity on an exercise bicycle (Monark Ergomedic 839E). The steady-rate test started with a workload of 67 W over 8 min, with the participants’ pulse rates being recorded during the final minute of the test. The watt max test started with 67 W, increasing by 20 W each consecutive minute until the patients did not wish to continue cycling. VO2Max was estimated using the formula VO2Max=0.16+(0.0117×MPO) (W) as described by Andersen [8].
General well-being was assessed by using the SF-36 (36 items) [59], which contains eight scales measuring general health concepts. Five scales (physical function, role physical, role emotional, social function, bodily pain) measure degree of dysfunction, and three scales (general health perceptions, vitality, mental health) consider the full range from negative to positive health conditions. Each scale comprises a total of all of the raw scores and is later converted to a 0–100 scale. It is possible to calculate two global measures, specifically physical component scale (PCS) and mental component scale (MCS).
Quality of life was assessed by using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30; 30 items) [1]. The QLQ-C30 comprises six functional scales (physical, role, cognitive, emotional, social functioning, and global QoL), symptom scales (fatigue, nausea/vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea). The raw scores are transformed linearly into scores ranging from 0 to 100. A high score on the global health status (QoL) represents a high QoL, a high score for a functional scale represents a high level of functioning, whereas a high score for a symptom scale indicates a high level of symptoms.
We combined two questionnaires because they focus on different aspects of physical and psychosocial well-being. The EORTC QLQ-C30 was developed specifically to measure health-related quality of life in cancer patients and includes assessment of symptoms and side effects. For this reason, it is suitable for cancer patients with mixed diagnoses undergoing cytostatic treatment. The SF-36 questionnaire is used for patients with different somatic diagnoses with focus on the patients’ health conditions in general. The period that is assessed by the two instruments is not identical. The SF-36 includes questions about the current period (15 items), the last 4 weeks (20 items) and the past year (1 item). QLQ-C30 focuses on the current period (5 items) and the last week (25 items). We have supplemented the questionnaires with our own methods (semistructured interview and patient diary) and thus investigated the patients’ feelings of fatigue [6].
Demographic data was obtained at baseline. Leisure time physical activity level was explored by interviews and included information from before diagnosis, at baseline and after 6 weeks. The patients are classified as (I) sedentary (completely inactive), (II) walking or cycling for pleasure, (III) regular physical exercise at least 3 h/week; or (IV) intense regular physical training at least 4 h/week [49].
Data were entered into Excel using Microsoft Office 2000 Professional for Windows 2000. Statistical analyses were carried out using SAS for Windows (Version 6.2) One-way analysis of variance (ANOVA) and standard cohort χ2 tests were applied to compare subjects who dropped out with those who completed the intervention on age, sex, level of education, NED/ED, and diagnosis. Paired t tests were applied to compare pre- and post-intervention scores in physical strength, VO2Max, EORTC QLQ-C30 and SF-36. A p value of 0.05 was set to indicate statistical significance. Values are expressed as mean±standard deviation.
Results
Physical capacity
Highly significant increases in the physical capacity were observed after 6 weeks. Paired sample t tests indicated a significant increase in aerobic capacity [t(81)=−7.412, p <0.001], chest press [t(81)=−14.70, p <0.001], leg press [t(81)=−20.5, p<0.001), pull down [t(81)=−14.7, p<0.001]. The patients’ average rate of VO2Max improvement was 16% (minimun −28.5%, maximum 61%). Fifteen percent of the patients experienced a decrease in their VO2Max and 7% had no change.
The patients’ average improvement in chest press was 41%, leg press, 46%, and pull down, 36%. Total increase in strength was 40% (minimum 0%, maximum 91%). Weighted strength measurement showed significance [t(81)=25.54, p<0.001]. Results are summarized in Table 3.
The patients experienced a highly significant decrease in leisure time physical activity level from pre-illness to when they began in the exercise intervention (baseline) (p<0.001), as seen in Table 4. Half of the patients had a level of physical activity of more than 3 h/week (groups III and IV) pre-illness compared with 4.4% when entering the exercise intervention. A significant increase in activity level was seen at week 6 (p<0.001); 82.5% of the patients achieved activity levels III and IV after 6 weeks.
Significant improvements were observed on all eight subscales, as well as on PCS and MCS from baseline to week 6, shown in Table 5. Three of the scales (bodily pain, general health perceptions and social function) revealed a significance level of <0.05, whereas the remaining seven scales showed a p value of <0.001 (physical functioning, role functioning, vitality, role emotional, mental health, PCS and MCS). In particular, role functioning and role emotional scale showed large changes of 21.0 points and 18.7 points, respectively, at week 6.
We observed significant improvements on 8 of the 15 measures assessing health-related quality of life (EORTC-QLQ-C30), as illustrated in Table 6 [global health status (p=0.017), physical functioning (p<0.001), role functioning (p<0.001) and emotional functioning (p= 0.022)]. Role functioning scale showed a 14.2-point difference from baseline to week 6. In addition, four scales measuring symptoms showed significant reductions: fatigue (p=0.006), pain (p=0.031), insomnia (p=0.002) and diarrhoea (p=0.013).
ANOVA was performed to find differences between NED patients and ED patients at baseline levels and changes of VO2Max, weighted strength, SF-36 scores, and EORTC QLQ-C30 scores. Patients with no evidence of disease (NED) had, at baseline, a significantly higher VO2Max (mean 2.35 l/min) than patients with evidence of disease (ED) (mean 2.08 l/min, p=0.043). Both groups showed equal improvement in aerobic capacity after the intervention. In contrast, there were no significant differences at baseline in weighted strength measurement (1RM) between the groups. However, there was a significantly greater advancement in the patients with NED at post-testing (p=0.03).
The data collected from the questionnaire showed a significant improvement in physical functioning as measured by the EORTC QLQ-C30 (p=0.001) and SF-36 (p=0.004) scales at post-testing, a constant pattern for both NED and ED patients. Differences between the two groups were observed. The NED patients reported a significantly higher physical functioning level at baseline as measured by both the EORTC QLQ-C30 (p=0.001) and the SF-36 (p=0.004). They also showed a significantly higher level of vitality (p=0.002), health perception (SF-36; p= 0.009) and fatigue (EORTC QLQ-C30; p=0.008) at baseline.
To determine which variables provide a significant impact on symptoms, Pearson correlation coefficients (PCC) between changes in symptoms and selected variables in SF-36 and EORTC QLQ-C30, along with weighted strength and VO2Max, were carried out. This study found a negative correlation (r=−0.232) between weighted strength and pain (p= 0.036). Those patients who significantly improved in strength were the same that significantly reduced their pain levels as measured by EORTC QLQ-C30. A similar correlation (r=−0.250) was found between VO2Max and pain (p=0.024). Moreover, a significant negative correlation (r=−0.288) was found between VO2Max and dyspnoea. No other significant correlations were found on treatment-related symptoms/side effects as measured by the EORTC QLQ-C30 scales and the SF-36 scales.
Discussion
The present study provided data on a multidimensional exercise intervention and included oncological and haematological patients, with or without residual disease (disease status ED/NED). As hypothesized, the patients showed significant improvements in physical capacity, physical activity level, general well-being and several quality-of-life indices, including treatment-related symptoms.
The study population was composed of oncological (80%) and haematological (20%) patients and showed no statistically significant differences between its two patient groups with regard to physical capacity, physical activity level, general well-being and quality of life at baseline and post-intervention. In contrast to some of the earlier studies testing resistance training for oncological patients [26, 35] it was mandatory in the present study that the patients receive antineoplastic chemotherapy while participating in the intervention.
According to a recent review by Galvão and Newton [28], the present intervention is the first of its kind to incorporate a high-intensity training design. Only one study was found that tested resistance training in conjunction with cardiovascular training in comparable cancer patients who were undergoing cytostatic treatment. Kolden et al. [35] examined the effects of a supervised exercise intervention involving cardiovascular, resistance and flexibility training, 1 h three times a week over 16 weeks in 40 sedentary women with breast cancer. Sixty-five percent of the patients received chemotherapy [35]. Average increase in dynamic strength was 36 vs 40% in the present study, and cardiovascular capacity (VO2Max) was 15.4 vs 16% in the present study. We have analysed the different diagnostic groups (with n<5) individually and found no characteristic diagnosis-related pattern. As expected, however, the measures of physical capacity at baseline were significantly higher in male patients.
The results of the present study extend previous research showing that exercise can improve QoL in cancer patients undergoing chemotherapy [19, 25, 52, 56]. The present study used the EORTC QLQ-C30 questionnaire to measure quality of life, based on global health status scale (QoL). The mean baseline score of 59.62 on the global health status scale is somewhat higher than scores reported by Aaronson et al. [1] in a sample of 305 lung cancer patients (score 56.7 before and 55.2 during treatment). A study by Michelson et al. [41] of a large Swedish healthy population (n=3069) reported mean QoL scores of 74.7 and 78.1, respectively, in women and men, whereas Klee et al. [34] reported a mean QoL score of 71.8 among 608 healthy women.
The multidimensional intervention combined high-intensity physical activities, resistance and cardiovascular training, with low-intensity relaxation components. It is evident that the results of the objective physiological measurements (1RM, VO2Max) showed significant changes after 6 weeks. The combined resistance and cardiovascular training that the patients undertook within this intervention is a plausible explanation for the observed changes in physical capacity.
The changes in the symptoms scales in EORTC QLQ-C30 showed significant reductions in fatigue and pain. The significant reduction in the pain scales found in the present study may be related to other studies using relaxation component, for example, Sloman [54]. Furthermore, there is growing evidence that aerobic exercise programmes can reduce cancer-related fatigue in cancer patients undergoing chemotherapy [25, 36, 42, 43, 51]. The reduction of fatigue and pain found in the present study might be attributed to relaxation, exercise and/or a combination. Nevertheless, it is not possible to disentangle the effect of the diverse components in the programme.
Several issues must be considered when determining the meaningfulness of any change in questionnaire scores. According to the American scoring manual for SF-36 and the European EORTC QLQ-C30, a change in score of 3 to 5 points can be considered as a clinically significant change [32, 59]. Serious consideration should be given to the impact that a change in score from 3 to 5 could have on a person’s life situation (e.g., hospitalisation, disability/work limitations). In order not to overestimate the clinical significance of the score differences at pre- and post-testing, a change of 5 points was established as a moderate clinically significant change, whereas a difference of 10 points was considered a large clinically significant change.
The study results showed that 15 of the 25 SF-36 and EORTC QLQ-C30 subscales showed clinical significance. Three of these subscales showed greater than a 10-point score difference, whereas 12 subscales showed more than a 5-point difference. Results from the SF-36 scale indicated a large, clinically significant 21.3-point change in physical disability as measured by the role functioning subscale. At baseline (mean 24.7) on the role functioning subscale was two standard deviations below the normal population mean, and although the greatest advancement was found on this scale, the scores did not move into the normal range (mean 83.0, SD 31.4) [12] at week 6 (mean 45.73). However, the change indicates a 34% increase in functioning. Moreover, the role emotional subscale revealed a large, clinically significant 18.7-point improvement in emotional stability. In addition, the physical functioning, pain, vitality and mental health subscales all displayed moderate, clinically significant changes. Results from the EORTC QLQ-C30 scores showed a 14.3-point large clinically significant difference in role functioning, and seven other subscales, including treatment-related symptoms (fatigue, pain, insomnia, constipation, diarrhoea), demonstrated moderate clinical significance.
The literature shows that only few exercise interventions include patients at advanced disease stages, and these mainly involved haematological patients during or after high-dose chemotherapy and peripheral blood stem cell transplantation [20, 24]. The aim of these interventions was to reduce the deterioration of physical capacity caused by the intensive treatment. Generally our patients were subjected to less intensive treatment and we thus assume that comparison to this group is less relevant.
The present study included patients with no evidence of disease (NED) (51.2%) and patients with evidence of the disease (ED) (48.8%). Overall, the NED patients scored higher at baseline and after 6 weeks on all scales (Table 7). With respect to changes post-intervention (week 6), there is no significant difference in improvement in either group apart from muscle strength (1RM) (p= 0.030), vitality (SF-36) (p=0.013) and pain level (SF-36) (p=0.027). NED patients showed significantly more advancement in strength (1RM) and more reduction in pain than the ED patients who excelled in vitality.
It can be concluded that NED as well as ED patients were able to improve their results after 6 weeks as estimated by their measured physical capacity, general well-being and quality of life.
The most strenuous components of the intervention programme were resistance and fitness/aerobic training, administered for 1.5 h three times weekly. The relationship between weighted strength and VO2Max was investigated, as well as the relationship of these factors to selected variables from the SF-36 and EORTC QLQ-C30 questionnaires. Increase in the PCS was significantly influenced by the physical strength results (p=0.023).
This study revealed significant correlation (0.282) between improvements in quality of life (global health status, EORTC QLQ-C30) and VO2Max (p=0.010). However, no significant correlation was found between weighted strength and the global health status.
The potential effect of the fitness training on fatigue is described in an earlier study by Schwartz [50] conducted on women with breast cancer. A positive relationship between advancement in physical capacity and fatigue was found; however, this could not be confirmed in the present study.
With regard to methodological considerations, the results confirmed that the oncological and haematological patients were capable of carrying out the exercise intervention.
The average attendance rate for included patients was 78%. This is comparable to attendance rates for clinical intervention studies with lesser dosage/intensity in cancer patients who had completed chemotherapy and during which physical activity was offered as part of their rehabilitation programme [47, 56].
The study sample has important selection biases. The patients were attracted to the intervention by posters displayed only in the out-patient clinic or in the ward. Thus, not all eligible patients were informed about the project. The study population was self-appointed and comprised cancer patients who pre-illness were motivated to undertake physical training.
Regarding patients who withdrew from the study before its completion, their reasons fall into two main categories: medical reasons (12%) and other reasons (17%). Age and sex were not significant factors in the drop-out rate. However, ED patients tended to drop out more frequently than did the NED patients (p=0.04). The question remains as to whether ED patients’ drop-out influence the study results as these proved more positive than earlier estimated. Since ED drop-outs were not tested, it cannot be dismissed that they may be representative of a group that may have scored low in some of the study parameters.
The results of this study gained impact by method triangulation, as various aspects of the intervention were examined by use of different methods. In general, detailing of data collection and well-recognized methodology strategies aimed to improve the quality of the research [40, 48]. Well-recognized and validated objective physiological measurements (1RM, estimated VO2Max) and subjective, standardized questionnaires (SF-36, EORTC QLQ-C30) were combined. The results of the objective physiological measurements and the two questionnaires show concurrent tendencies. Causal relationships between the objective measurements of physical capacity and the subjective dimensions of general health and quality of life reported by the cancer patients could not be ascertained within the study design. Neither was it possible to isolate the potential effect of the group involvement, which might have influenced the results. The small number of patients in the different diagnostic groups did not allow for valid cross-cancer comparisons.
Estimated VO2Max was used as one of the physiological tests. Standard measurement focuses on maximum concentration of oxygen consumption; however, estimated VO2Max was selected, as this method was most realizable in testing the patients. To compare the estimated VO2Max with the maximum oxygen consumption, it was necessary to measure seven patients with both tests. No significant difference was found between the tests.
Patients’ VO2Max and 1RM were measured on the first and last days of the intervention. As their protocols differed, some patients received treatment on days that fell between the pre- and post-tests. The planned tests were carried out on time to ensure similar conditions for the pre- and post-tests. In this study, we did not specifically carry out a cost–benefit analysis of adding the exercise intervention to the cancer patients’ treatment protocols. The staff charges were on a low level because, among other reasons, seven to nine patients participated in groups. The findings of previous exercise studies with cancer patients and survivors suggest that the cost/effectiveness ratio is likely to be very low, as argued in the study by Lucia et al. [36].
The patients’ substantial improvements in dynamic strength (1RM), physical functioning (SF36, EORTC QLQ-C30) and role functioning (SF36, EORTC QLQ-C30) identified in this study support the theory of resistance exercise as an additional beneficial intervention strategy to improve physical capacity, physical functioning and in the management of treatment-related symptoms. [5, 28].
Our first study [5] showed that the described intervention is feasible—this study indicates significant, clinical meaningful improvements after the intervention. Despite the demanding nature of the intervention, it was feasible even for those patients with advanced disease and reduced vitality. The exact role of the interventions in these patients, who are being treated with chemotherapy and, to some extent, change their disease status, has to be defined in a randomised controlled design. A clinically controlled trial including 250 patients with mixed diagnoses and who are undergoing chemotherapy is concurrently being carried out.
Conclusions
Over the course of a multidimensional intervention, including resistance training, cancer patients undergoing chemotherapy could significantly increase muscular strength, physical fitness and physical activity levels. The patients experienced a significant reduction in essential, treatment-related symptoms and significantly improved their general well-being and quality of life in numerous aspects. The observed changes in questionnaire scores from pre- to post-testing were substantial and reached clinical significance in several dimension scores. The results of the study indicate that oncological and haematological cancer patients with and without residual disease undergoing chemotherapy may benefit from multidimensional exercise interventions.
References
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Adamsen L, Rasmussen JM, Pedersen LS (2001) ‘Brothers in arms’: how men with cancer experience a sense of comradeship through group intervention which combines physical activity with information relay. J Clin Nurs 10:528–537
Adamsen L, Rasmussen JM (2001) Sociological perspectives on self-help groups: reflections on conceptualization and social processes. J Adv Nurs 35:909–917
Adamsen L (2002) ‘From victim to agent’: the clinical and social significance of self-help group participation for people with life-threatening diseases. Scand J Caring Sci 16:224–231
Adamsen L, Midtgaard J, Rorth M, Borregaard N, Andersen C, Quist M, Moller T, Zacho M, Madsen JK, Knutsen L (2003) Feasibility, physical capacity, and health benefits of a multidimensional exercise programme for cancer patients undergoing chemotherapy. Support Care Cancer 11:707–716
Adamsen L, Midtgaard J, Andersen C, Quist M, Moeller T, Roerth M (2004) Transforming the nature of fatigue through exercise: qualitative findings from a multidimensional exercise program in cancer patients undergoing chemotherapy. Eur J Cancer Care 13:362–370
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, Jacobs DR Jr, Leon AS (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498–S504
Andersen LB (1995) A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports 5:143–146
Arakawa S (1997) Relaxation to reduce nausea, vomiting, and anxiety induced by chemotherapy in Japanese patients. Cancer Nurs 20:342–349
Ballard-Barbash R, Blair A, Blair SN, Byers T, Hoffman-Goetz L, Lee I-M, Troiano R, Westerlind K (2002) Physical activity across the cancer continuum: report of a workshop: review of existing knowledge and innovative designs for future research. Cancer 95:1134–1143
Bindemann S, Soukop M, Kaye SB (1991) Randomised controlled study of relaxation training. Eur J Cancer 27:170–174
Bjorner JB, Damsgaard MT, Watt T, Groenvold M (1998) Tests of data quality, scaling assumptions, and reliability of the Danish SF-36. J Clin Epidemiol 51:1001–1011
Bredin M, Corner J, Krishnasamy M, Plant H, Bailey C, A’Hern R (1999) Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. BMJ 318:901–904
Bridge LR, Benson P, Pietroni PC, Priest RG (1988) Relaxation and imagery in the treatment of breast cancer. BMJ 297:1169–1172
Brzycki M (1993) Strength testing: predicting a one-rep max from reps to fatigue. J Phys Educ Recreat Dance 64:88–90
Bunkan BH, Schultz CM (1998) Medisinsk massasje (Medical massage), 2nd edn. Universitetsforlaget, Oslo, Norway
Burish TG, Lyles JN (1981) Effectiveness of relaxation training in reducing adverse reactions to cancer chemotherapy. J Behav Med 4:65–78
Coleman EA, Coon S, Hall-Barrow J, Richards K, Gaylor D, Stewart B (2003) Feasibility of exercise during treatment for multiple myeloma. Cancer Nurs 26:410–419
Courneya KS, Friedenreich CM (1999) Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med 21:171–179
Courneya KS, Keats MR, Turner AR (2000) Physical exercise and quality of life in cancer patients following high dose chemotherapy and autologous bone marrow transplantation. Psychooncology 9:127–136
Courneya KS (2003) Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc 35:1846–852
Curt GA (2000) Impact of fatigue on quality of life in oncology patients. Semin Hematol 37(Suppl 6):14–17
Dimeo FC, Stieglitz R-D, Novelli-Fischer U, Fetscher S, Keul J (1999) Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 85:2273–2277
Dimeo F, Schwartz S, Fietz T, Wanjura T, Boning D, Thiel E (2003) Effects of endurance training on the physical performance of patients with hematological malignancies during chemotherapy. Support Care Cancer 11:623–628
Dimeo FC (2001) Effects of exercise on cancer-related fatigue. Cancer 92(6 Suppl):1689–1693
Durak EP, Lilly PC (1998) The application of an exercise and wellness program for cancer patients: a preliminary outcomes report. J Strength Cond Res 12:3–6
Fentem PH (1994) ABC of sports medicine: benefits of exercise in health and disease. BMJ 308:1291–1295
Galvão DA, Newton RU (2005) Review of exercise intervention studies in cancer patients. J Clin Oncol 23:899–909
Green S (1994) A definition and systems view of anaerobic capacity. Eur J Appl Physiol Occup Physiol 69:168–173
Greene D, Nai LM, Fieler VK, Dudgeon D, Jones LS (1994) A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Pract 2:57–62
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersall MH (1996) On the receiving end. V. Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7:189–195
Groenvold M, Klee MC, Sprangers MA, Aaronson NK (1997) Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient–observer agreement. J Clin Epidemiol 50:441–450
Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L (1994) The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs 17:367–378
Klee M, Groenvold M, Machin D (1997) Quality of life of Danish women: population-based norms of the EORTC QLQ-C30. Qual Life Res 6:27–34
Kolden GG, Strauman TJ, Ward A, Kuta J, Woods TE, Schneider KL, Heerey E, Sanborn L, Burt C, Millbrandt L, Kalin NH, Stewart JA, Mullen B (2002) A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology 11:447–456
Lucia A, Earnest C, Perez M (2003) Cancer-related fatigue: can exercise physiology assist oncologists? Lancet Oncol 4:616–625
Luebbert K, Dahme B, Hasenbring M (2001) The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology 10:490–502
Lyles JN, Burish TG, Krozely MG, Oldham RK (1982) Efficacy of relaxation training and guided imagery in reducing the aversiveness of cancer chemotherapy. J Consult Clin Psychol 50:509–524
MacVicar MG, Winningham ML, Nickel JL (1989) Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res 38:348–351
Malterud K (2001) The art and science of clinical knowledge: evidence beyond measures and numbers. Lancet 358:397–400
Michelson H, Bolund C, Nilsson B, Brandberg Y (2000) Health-related quality of life measured by the EORTC QLQ-C30-reference values from a large sample of Swedish population. Acta Oncol 39:477–484
Mock V, Pickett M, Ropka ME, Muscari E, Stewart KJ, Rhodes VA, McDaniel R, Grimm PM, Krumm S, McCorkle R (2001) Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract 9:119–127
Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, Stewart KJ, Cameron L, Zawacki K, Podewils LJ, Cohen G, McCorkle R (2005) Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology 14:464–477
Molassiotis A, Yung HP, Yam BMC, Chan FYS, Mok TSK (2002) The effectiveness of progressive muscle relaxation training in managing chemotherapy-induced nausea and vomiting in Chinese breast cancer patients: a randomised controlled trial. Support Care Cancer 10:237–246
Nail LM, Jones LS (1995) Fatigue as a side effect of cancer treatment: impact on quality of life. Qual Life—A Nursing Challenge 4:8–13
Pensgaard AM, Ursin H (1998) Stress, control, and coping in elite athletes. Scand J Med Sci Sports 8:183–189
Pinto BM, Eakin E, Maruyama NC (2000) Health behavior changes after a cancer diagnosis: what do we know and where do we go from here? Ann Behav Med 22:38–52
Polit DF, Beck CT (2003) Nursing research: principles and methods, 7th edn. Lippincott Williams & Wilkins, Philadelphia
Saltin B, Gollnick PD (1983) Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, Adrian PH, Geiger SR (eds) Handbook of physiology—skeletal muscle, American Physiological Society, Washington, DC, pp 555–631
Schwartz AL (1999) Fatigue mediates the effects of exercise on quality of life. Qual Life Res 8:529–538
Schwartz AL, Mori M, Gao R, Nail LM, King ME (2001) Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc 33:718–723
Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, Woodard S, Wells G, Reid R (2001) Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol 19:657–665
Sloman R, Brown P, Aldana E, Chee E (1994) The use of relaxation for the promotion of comfort and pain relief in persons with advanced cancer. Contemp Nurse 3:6–12
Sloman R (1995) Relaxation and the relief of cancer pain. Nurs Clin North Am 30:697–709
Smets EM, Garssen B, Schuster-Uitterhoeve AL, de Haes JC (1993) Fatigue in cancer patients. Br J Cancer 68:220–224
Stricker CT, Drake D, Hoyer K-A, Mock V (2004) Evidence-based practice for fatigue management in adults with cancer: exercise as an intervention. Oncol Nurs Forum 31:963–976
Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE (1995) Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain 63:189–198
Troesch LM, Rodehaver CB, Delaney EA, Yanes B (1993) The influence of guided imagery on chemotherapy-related nausea and vomiting. Oncol Nurs Forum 20:1179–1185
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36) I: conceptual framework and item selection. Med Care 30:473–483
Weinrich SP, Weinrich MC (1990) The effect of massage on pain in cancer patients. Appl Nurs Res 3:140–145
Winningham ML, MacVicar MG, Burke CA (1986) Exercise for cancer patients: guidelines and precautions. Phys Sportsmed 14:125–134
Acknowledgements
This research was supported by grants from The Egmont Foundation, The Lundbeck Foundation, The Novo Nordic Foundation, The Svend Andersen Foundation, The Danish Cancer Society, The Becket Foundation, The Åse & Ejnar Foundation, and The Copenhagen Hospital Cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adamsen, L., Quist, M., Midtgaard, J. et al. The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer 14, 116–127 (2006). https://doi.org/10.1007/s00520-005-0864-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-005-0864-x