Abstract
Human genome has ten genes that are collectedly called Ras association domain family (RASSF). RASSF is composed of two subclasses, C-RASSF and N-RASSF. Both N-RASSF and C-RASSF encode Ras association domain-containing proteins and are frequently suppressed by DNA hypermethylation in human cancers. However, C-RASSF and N-RASSF are quite different. Six C-RASSF proteins (RASSF1–6) are characterized by a C-terminal coiled-coil motif named Salvador/RASSF/Hippo domain, while four N-RASSF proteins (RASSF7–10) lack it. C-RASSF proteins interact with mammalian Ste20-like kinases—the core kinases of the tumor suppressor Hippo pathway—and cross-talk with this pathway. Some of them share the same interacting molecules such as MDM2 and exert the tumor suppressor role in similar manners. Nevertheless, each C-RASSF protein has distinct characters. In this review, we summarize our current knowledge of how C-RASSF proteins play tumor suppressor roles and discuss the similarities and differences among C-RASSF proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
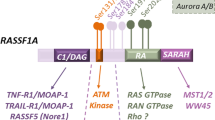
The human genome contains ten genes designated as Ras association (RA) domain family (RASSF) members [1, 2]. These genes encode proteins with one RA domain. Among them, RASSF1–6 harbor a coiled-coil motif in the C-terminal region (Fig. 1). As similar sequences are detected in the Drosophila proteins Salvador and Hippo, this motif is named the Salvador/RASSF/Hippo (SARAH) domain. RASSF7–10 lack this motif [3]. The SARAH domain is involved in cross-talk with the tumor suppressor Hippo pathway. Thereby, the presence of this domain distinguishes RASSF1–6 from RASSF7–10. Moreover, the RA domain resides in the N-terminus of RASSF7–10, whereas it is near the C-terminal region in RASSF1–6. Thus, RASSF7–10 are called N-RASSF proteins, whereas RASSF1–6 are known as C-RASSF proteins. Even though “C” generally denotes the C-terminus, it also indicates “classical.” Numerous reports have demonstrated that C-RASSFs are suppressed in human cancers and that the suppression of each C-RASSF is correlated with tumor progression [4]. Based on these reports, C-RASSFs, excluding RASSF1C, are regarded as tumor suppressors. Various underlying mechanisms have been proposed for C-RASSF-mediated tumor suppression. RASSF1 and RASSF5 are well researched, whereas other C-RASSF proteins have been less thoroughly investigated. Nevertheless, it has been noted that C-RASSF proteins share common mechanisms of tumor suppression. Even though some mechanisms depend on other tumor suppressors such as p53, pRb, and the Hippo pathway, C-RASSF proteins also utilize unique mechanisms for suppressing tumors. These findings highlight the importance of C-RASSFs as tumor suppressors in cancers, especially with the dysregulation of p53, pRb, and the Hippo pathway. Moreover, accumulating evidence supports that C-RASSF proteins play roles other than in tumor suppression. In this review, we attempt to summarize the current knowledge of C-RASSF (Table 1).
Summary of the Hippo pathway
C-RASSF proteins cross-talk with the Hippo pathway. As this cross-talk is one of the important properties of C-RASSF proteins, we will briefly summarize the Hippo pathway (Fig. 2). For details, readers are requested to refer to other reviews [5,6,7]. Genetic studies of Drosophila melanogaster revealed several mutants exhibiting cell overgrowth. One causative gene was named hpo, because the phenotype was reminiscent of the hippopotamus. Mutations of other three genes (sav, mats, and wts) resulted in the same phenotypes. hpo and wts encode the serine/threonine protein kinases Hippo and Warts, respectively. Hippo phosphorylates and activates Warts. The proteins encoded by sav and mats, namely Salvador and Mats, respectively, interact with Hippo and Warts and facilitate the Hippo-mediated activation of Warts. Yorkie, a protein that was identified as a Warts-interacting protein, cooperates with the transcription factor Scalloped and upregulates cell cycle-promoting and anti-apoptotic genes. However, activated Warts phosphorylates Yorkie, and subsequently, phosphorylated Yorkie is segregated in the cytoplasm and degraded. That is, the kinase cassette formed by Hippo, Salvador, Mats, and Warts negatively regulates Yorkie and Scalloped. Loss-of-function mutations of hpo, sav, mats, and wts lead to Yorkie and Scalloped hyperactivation and result in cell overproliferation and tissue overgrowth. The pathway composed of these genes was named the Hippo pathway. Humans have homologs of these genes as follows: mammalian Ste20-like kinase (MST) 1 and 2 (the human genes are STK4 and STK3, respectively) for Hippo; Sav1 (also called WW45) for Salvador; MOB1A and MOB1B for Mats; large tumor suppressor kinase (LATS) 1 and 2 for Warts; yes-associated protein 1 (YAP1) and transcriptional coactivator with PDZ-binding motif (TAZ) (also called WW domain-containing transcription regulator protein 1) for Yorkie; and TEA domain transcription factor (TEAD) 1–4 for Scalloped. Subsequent studies have continuously identified new components of the Hippo pathway. The entire picture of the Hippo pathway is complicated. It is currently obvious that YAP1/TAZ are regulated by other molecules than MST1/2 and LATS1/2. Regardless, the MST-LATS-YAP1/TAZ-TEAD axis is the core of the mammalian Hippo pathway, which is dubbed the canonical Hippo pathway.
Core architecture of Drosophila and the components of the mammalian Hippo pathway. The core components of the Drosophila Hippo pathway are depicted (left). Unphosphorylated Yorkie interacts with Scalloped in the nucleus. Hippo together with Mats and Salvador activates Warts. Activated Warts phosphorylates Yorkie. Phosphorylated Yorkie is trapped in the cytoplasm, where it undergoes degradation. Mammalian homologs are listed (right)
Human RASSF proteins
NORE1/RASSF5
Discovery of NORE1/RASSF5
As the first identified C-RASSF was mouse RASSF5 [8], with human RASSF5 reported later [9], we will start this review with this protein. In the pioneering study, the researchers screened for a protein that bound the active form of HRAS, obtaining mouse RASSF5, and named it novel Ras effector (NORE) 1 [8]. It is unsurprising that only RASSF5 was detected in that screening, as the affinity of RASSF5 for Ras proteins is high [10, 11]. The interaction between RASSF5 and Ras proteins is easily detected in vitro, whereas the detection of the interaction between Ras proteins and other C-RASSF proteins depends on the experimental conditions. Due to the historical background, RASSF5 is frequently described as NORE1. We also use NORE1 in this review. There are two major isoforms with different N-terminal sequences (NORE1A and NORE1B, which correspond to RASSF5 isoforms A and C, respectively). NORE1A is downregulated by hypermethylation in various cancers [4]. NORE1B is also suppressed in certain cancers [12]. DNA hypermethylation is not the only cause of this suppression. In oral cancer, miR-214 suppresses NORE1 [13]. NORE1 is also suppressed at the protein level by the E3 ligase ITCH [14]. NORE1B has been studied as a Rap1-binding protein in the field of immunology and described as RAPL [15]. RAPL determines the spatial localization of integrin subunit 2 and mediates Rap1-triggered integrin activation in T lymphocytes. RAPL regulates the localization of CDKN1B (P27KIP1), and its deficiency causes lymphoproliferative disorders [16]. These findings support that both NORE1A and NORE1B are tumor suppressors.
NORE1 as a target of Ras proteins
NORE1 binds Ras proteins (KRAS, HRAS, MRAS, and RRAS) and it is considered a typical target of RAS [8, 17]. The active form of Ras drives NORE1 to induce apoptosis and senescence [18, 19]. The SARAH domain of NORE1 (NORE1-SARAH) interacts with the SARAH domains of MST kinases (MST-SARAH) [10, 18, 20]. NORE1 attenuates the autophosphorylation at threonine 183 of MST1, which is essential for MST1 activity, and inhibits MST1 activation [20]. However, when KRAS G12V is coexpressed, MST1 is recruited via NORE1 to the plasma membrane and activated [20]. In this manner, KRAS induces apoptosis via NORE1-MST1. On the other hand, HRAS prompts NORE1 to bind to the SCFβ-TRCP–ubiquitin ligase complex and induces the degradation of β-catenin and MDM2 [21, 22]. HRAS also induces NORE1 to bind and stabilize homeodomain-interacting protein kinase 2 (HIPK2) [19]. HIPK2 phosphorylates p53 at Serine 46, induces p53 acetylation, and eventually upregulates pro-apoptotic genes. HRAS and KRAS trigger the formation of a complex including NORE1 and protein phosphatase 1A (PP1A), allowing NORE1 to stabilize and bridge PP1A to pRb [23]. Consequently, pRb remains in its dephosphorylated active form and promotes cellular senescence. These findings support that NORE1 is controlled by Ras signaling and that it is a target of Ras proteins.
Interaction between NORE1 and MST kinases
MST kinases are mammalian homologs of yeast Ste20 kinases and core kinases of the Hippo pathway [24]. The interaction between NORE1 and MST kinases has been extensively studied [25,26,27]. The affinity of the homodimerization of MST-SARAH is weaker than that of heterodimerization between MST-SARAH and NORE1-SARAH. Thus, NORE1-SARAH blocks the homodimerization of MST-SARAH and inhibits the autoactivation of MST kinases, which is a prerequisite for MST kinase activity. Consistently, NORE1-SARAH fails to inhibit MST kinases once MST kinases are autophosphorylated and activated [28]. Moreover, both MST-SARAH and the N-terminal kinase domain (MST-N) bind NORE1-SARAH, and thus, NORE1-SARAH is sandwiched between MST-N and MST-SARAH [27, 29]. Moreover, the RA domain of NORE1 (NORE1-RA) binds to the region between MST-N and MST-SARAH, which is named the regulatory region (MST-RR). These findings indicate that NORE1 and MST kinases interact with each other at multiple sites. Hence, to understand the interaction between NORE1 and MST kinases, research using the whole molecules is important. Intriguingly, NORE1-SARAH enhances the phosphorylation of histone H2B by MST1 but attenuates the phosphorylation of FoxO [29]. This result implies that the effect of NORE1 on MST1 depends on the substrate. Therefore, to discuss the effect of NORE1 in the context of the Hippo pathway, it is essential to use Hippo pathway-related substrates such as MOB1 and LATS kinases.
By what mechanism does Ras signaling modulate the interaction between NORE1 and MST kinases? An experiment using mouse NORE1 revealed that NORE1-RA intramolecularly binds to the C1 domain (NORE1-C1), but that RAS releases NORE1-C1 from NORE1-RA [30]. It is presumed that Ras signaling triggers a conformational change of NORE1 and modulates the interaction between NORE1 and MST kinases.
RASSF1
Discovery of RASSF1
The human chromosome 3p21.3 frequently exhibits loss of heterozygosity in human cancers, which implies that a tumor suppressor is encoded in this region. Research to identify xeroderma pigmentosum A (XPA)-interacting proteins revealed a gene homologous to NORE1 in this region that was reported as RASSF1 [31]. In the first paper, three splicing variants were described, one of which was suppressed in human cancers through methylation of the CpG island promoter. This variant is the well-known tumor suppressor RASSF1A. Thereafter, numerous papers have reported that RASSF1A suppression is associated with tumor progression and poor prognosis in human cancers. Several splicing variants of RASSF1 are registered in the database of the National Center for Biotechnology Information, but most studies have focused on RASSF1A and RASSF1C.
RASSF1A stabilizes microtubules
Versatile mechanisms contribute to RASSF1A-mediated tumor suppression. Among them, microtubule stabilization is one of the most prominent mechanisms and one that is unique to RASSF1A. RASSF1A stabilizes microtubules via microtubule-associated proteins such as MAP1B and MAP1S (C19ORF5) [32,33,34,35]. The stabilization of microtubules by RASSF1A also depends on RAN [36]. RAN regulates nuclear transport, but it is also involved in the assembly of mitotic spindles. RASSF1A induces phosphorylation in the nuclear localization signal of regulator of chromosome condensation 1 (RCC1), the GDP/GTP exchanger of RAN, leading to RCC1 accumulation in the cytoplasm. As a result, RASSF1A increases the level of the GTP-bound form of RAN and stabilizes microtubules via RAN. Other proposed mechanisms underlying the stabilization of microtubules are the inhibition of histone deacetylase 6-mediated deacetylation of α-tubulin and the recruitment of protein arginine N-methyltransferase 5 to microtubules [37, 38]. An analysis of upregulated genes in the Rassf1a-deleted mouse liver revealed two categories of genes [39]. One group of genes is involved in microtubule polymerization, which underscores the importance of microtubule stabilization in the function of RASSF1A.
RASSF1A activates the Hippo pathway
The interaction between RASSF1A and MST kinases has long been recognized [10, 20]. Later, proteomic studies further identified RASSF1A as a component of the Hippo pathway [40]. The SARAH domain of RASSF1A (RASSF1-SARAH) binds MST1-SARAH similarly as NORE1-SARAH albeit with a slight difference [29]. Whether NORE1 activates or inhibits MST kinases depends on the context [28]. By contrast, RASSF1A activates MST kinases in vivo [41]. Several modes of activation have been proposed. RASSF1A stabilizes MST kinases [41]. RASSF1A prevents the dephosphorylation of MST kinases and maintains the active phosphorylated forms [42]. RASSF1A releases MST2 from inhibition by RAF1 [43]. Thus, RASSF1A activates MST kinases and drives the Hippo pathway. Earlier research revealed the role of RASSF1A in FAS-induced apoptosis [43]. FAS activates MST2 via RASSF1A and MST2 in turn activates LATS1. RASSF1A releases YAP1 from LATS1, induces the accumulation of YAP1 and p73 in the nucleus, and promotes the YAP1-p73–mediated transcription of pro-apoptotic genes. In this context, YAP1 is regarded as a tumor suppressor that cooperates with p73. A study using Sleeping Beauty transposase in Rassf1a-null mice demonstrated that YAP1 shifts from p73 to TEAD and RUNX2 in the Rassf1a-negative background and that additional Runx2 depletion further enhances YAP1-TEAD complex formation [44]. Moreover, RASSF1A inhibits the TGF-β-induced interaction between YAP1 and SMAD2 [45]. Although RASSF1A is degraded by ITCH in response to TGF-β, the remaining RASSF1A restricts the nuclear translocation of SMAD2 and promotes cooperation between YAP1 and p73. These findings may explain the mechanism by which YAP1 selects a binding partner among various transcription factors and behaves as a tumor suppressor or tumor promoter in a context-dependent manner. Another paper reported that under RASSF1A overexpression, YAP1 is phosphorylated by LATS kinases [46]. Consequently, AREG, which is a target of TEAD that encodes a member of the epidermal growth factor family, is suppressed. In all of these scenarios, RASSF1A drives the Hippo pathway as an upstream regulator and causes apoptosis irrespective of whether YAP1 behaves as an oncogene or tumor suppressor. However, in the Rassf1a-depleted mouse liver, the expression levels of total YAP1 and phosphorylated YAP1 are not significantly changed [39]. This finding suggests that RASSF1A functions as a tumor suppressor through a different mechanism from the canonical Hippo pathway. For instance, RASSF1A interacts with SAV1 independently of MST kinases and activates p73-driven gene transcription [47]. A recent paper reported that RHEB, the activator of mTOR kinase, interacts with RASSF1A and inactivates YAP1 through MST and LATS kinases [48]. Conversely, RASSF1A blocks REHB-mediated autophagy. Consequently, RASSF1A suppresses RHEB-mediated anchorage-independent cell growth of tumor cells.
RASSF1A as a target of Ras proteins
Active KRAS induces apoptosis through RASSF1A [49]. KRAS also enhances RASSF1A-mediated stabilization of microtubules [34]. These findings support that RASSF1A is a target of Ras protein. However, RASSF1A has lower affinity for Ras proteins than NORE1 [10]. It is argued that RASSF1A indirectly binds Ras proteins via NORE1 [50]. The connector enhancer of kinase suppressor Ras 1 (CNKR1), which is reported to interact with RASSF1A and induce apoptosis via MST kinases, is also a candidate that links Ras proteins to RASSF1A [51]. Ras signaling may regulate RASSF1A via NORE1 or CNKR1. In addition, RASSF1A dissociates the complex of RAF1 and MST2, decreases the inhibitory phosphorylation of RAF1, and increases MEK activities. This observation suggests that RASSF1A functions as a modifier of Ras signaling [52].
The relationship of RASSF1A with p53 and pRb
p53 and pRb are apparently involved in the tumor suppressor function of RASSF1A. However, unlike NORE1, there is no clear evidence that RASSF1A transduces Ras signaling to p53 and pRb. Ubiquitin-specific protease 7, DAAX, and MDM2 form a complex and enhance p53 degradation [53]. Upon DNA damage, RASSF1A disrupts the complex and enhances p53 expression [54]. However, the role of RASSF1A does not depend solely on p53. Rassf1a-null mice exhibit tumor susceptibility [55, 56]. Importantly, Rassf1a deletion enhances tumor development, generates more aneuploid cells, and shortens survival in p53-null mice. Thus, RASSF1A functions as a tumor suppressor even in the p53-negative background. Through what mechanism does RASSF1A suppress tumor independently of p53? To address this question, we are focusing on pRb, another major tumor suppressor. RASSF1A restricts G1 exit in H1299 cells expressing pRb, but when E7 papillomavirus protein or CCNA2 (cyclin A2) is overexpressed (the former inhibits the interaction between pRb and E2F, and the later directly activates CDK2 and bypasses the regulation by pRb), RASSF1A-induced arrest is canceled [57]. These findings suggest that pRb is implicated in RASSF1A-mediated G1/S arrest.
RASSF1A regulates the cell cycle
In addition to stabilizing microtubules, RASSF1A regulates cell cycle progression by increasing the expression of cyclin-dependent kinase inhibitors. RASSF1A upregulates p53 and thereby enhances CDKN1A. However, RASSF1A upregulates CDKN1A even in A549 cells expressing the HPV16 E6 protein, which destabilizes p53, and in H1299 cells lacking p53 [58]. Thus, RASSF1A can induce CDKN1A independently of p53. It is proposed that RASSF1A negatively regulates AKT, which suppresses CDKN1A [58]. Another group reported that RASSF1A suppresses HRAS-induced c-Jun N-terminal kinase (JNK) activation and blocks JNK-induced downregulation of CDKN1B [59]. Furthermore, RASSF1A decreases the expression of cyclin-dependent kinases and cyclins. RASSF1A reduces CDK4 expression by inducing miR-711, which targets CDK4 [60]. RASSF1A reduces CCNA2 and CCND1 (cyclin D1) expression. RASSF1A binds E4F1 and inhibits its association with the promoter of CCNA2 to induce its downregulation [61, 62]. RASSF1A affects the translation and stability of CCND1 mRNA and blocks the accumulation of CCND1 [57, 63].
Moreover, RASSF1A regulates cell cycle progression through E3 ligase complexes. Although whether RASSF1A directly interacts with CDC20 is controversial, one report argued that RASSF1 induces mitotic arrest by inhibiting CDC20 and anaphase-promoting complex (APC/C) [64, 65]. MAP1S, which bridges RASS1A to microtubules, is believed to augment the interaction between RASSF1A and CDC2 [66]. Paradoxically, RASSF1A depletion impairs cell proliferation in certain cells. This phenomenon is explained by the aberrant activation of APC/C during G1/S transition [63]. RASSF1A blocks β-TRCP-mediated degradation of Emi1, the inhibitor of APC/C, and allows the accumulation of CCNA and CCNB, which is necessary for the G1/S transition. Thus, RASSF1A is likely to positively and negatively regulate the cell cycle.
RASSF1A is required for DNA repair
As RASSF1A regulates the cell cycle and checkpoints [57, 64], it is unsurprising that RASS1A deletion impairs DNA repair. In this section, we present specific findings that directly link RASSF1A to DNA repair. RASSF1A reduces the CDK2-mediated phosphorylation of BRCA2, an essential component of the error-free DNA repair machinery of DNA double-strand breaks, through MST2 and LATS2, and blocks the disassembly of the recombinase RAD51 from BRCA2 to protect genome stability [67]. RASSF1A interacts with XPA and modulates the interaction between XPA and replication protein A [31, 68]. In this manner, RASSF1A is involved in homologous recombination and nucleotide excision repair.
RASSF1A regulates apoptosis
In addition to the Hippo pathway and p53, RASSF1A regulates apoptosis through modulator of apoptosis 1 (MOAP1) [69]. Tumor necrosis factor (TNF)-α and TNF-like apoptosis-inducing ligand (TRAIL) induce the recruitment of RASSF1A and MOAP1 to the receptor complexes [70]. RASSF1A binds to 14-3-3 in the basal state, but when stimulated by TNF-α and TRAIL, RASSF1A is dissociated from 14-3-3 and binds to MOAP1. Then, RASSF1A releases MOAP1 from intramolecular autoinhibition and triggers its association with BAX, resulting in the insertion of BAX into the mitochondrial membrane and cytochrome c release. RASSF1A also transduces signaling from FAS and the TNF-α receptor to MST1, MOB1, and NDR (STK38 and STK38L) kinases and induces apoptosis via NDR kinases [71].
RASSF1A is regulated by phosphorylation
RASSF1A has several phosphorylation sites. As expected, phosphorylation modulates the interaction between RASSF1A and its binding partners. Phosphorylation at serines 175, 178, and 179 by glycogen synthase kinase 3β is necessary for the binding of RASSF1A to 14-3-3 [72]. Phosphorylation at threonine 202 and serine 203 by MST1 is involved in the activation of NDR kinases in response to TNF-α [71]. Phosphorylation at serine 184 by checkpoint kinase 1 disrupts the association of RASSF1A with microtubules [73, 74]. Among numerous phosphorylation sites, serines 131 and 203 may be most important. Serine 131 is phosphorylated by ataxia telangiectasia mutated (ATM) in response to DNA damage [75]. Phosphorylation promotes the dimerization of RASSF1A and the association of MST2 and LATS1, stabilizes YAP1 and p73, and subsequently enhances CDKN1A expression. The polymorphism that converts alanine 133 to serine disrupts α helix-containing ATM recognition sites and compromises p53/p73 responses. Accordingly, RASSF1A A133S is associated with poor prognosis in patients with sarcoma and early onset breast cancer in BRCA1/2 mutation carriers [76, 77]. Serine 203 is phosphorylated by several kinases. RASSF1A activates Aurora A, and it is reciprocally phosphorylated at serine 203. Phosphorylation by Aurora A triggers the dissociation of RASSF1A from both microtubules and CDC20 [73, 78]. Serine 203 is also phosphorylated by Aurora B, the isoform of Aurora A, but in the late mitosis phase [79]. Subsequently, RASSF1A binds syntaxin 16, a component of t-SNARE, at the midzone/midbody. In this manner, RASSF1A is involved in membrane trafficking during cytokinesis. CDK4, protein kinase A (PKA), and protein kinase C (PKC) phosphorylate serine 203 [80,81,82]. Phosphorylation by CDK4 induces RASSF1A degradation through an interaction with Skp2, the subunit of the Skp1-Cul1-F-box ubiquitin ligase complex, and promotes G1/S progression [80]. PKC phosphorylates serine 197 in addition to serine 203 [82]. PKC-mediated phosphorylation at these sites likely prevents the regulation by RASSF1A of microtubules. These two findings imply that phosphorylation at serine 203 negatively regulates the tumor suppressor function of RASSF1A. However, inhibition of PKA-mediated phosphorylation compromises RASSF1A-mediated apoptosis and the upregulation of CDKN2A and BAX, meaning that PKA-mediated phosphorylation at serine 203 promotes the tumor suppressor function of RASSF1A [81]. It is difficult to elucidate the mechanism by which phosphorylation at the same site leads to different cellular consequences. We may need to consider the localization of RASSF1A and the combination of various phosphorylations. Temporal and spatial analyses of the phosphorylation of RASSF1A are essential for clarifying the mechanism by which RASSF1A orchestrates various cellular events in response to the pattern of phosphorylation.
The other mechanisms underlying the tumor suppressor roles of RASSF1A
RASSF1A regulates Rho signaling. The C-terminal region of RASSF1A binds active RHOA, whereas the N-terminal region interacts with Smad ubiquitin regulatory factor 1 and induces RHOA degradation [83]. Conversely, RASSF1A stimulates the cofilin/PP2A-mediated dephosphorylation of the guanine nucleotide exchange factor GEF-H1 and activates RHOB [84]. RHOB suppresses nuclear YAP1 and plays an anti-metastatic role. RASSF1A reduces estrogen receptor α expression through AKT and inhibits breast tumor growth [85]. Rac1 is activated in Rassf1-depleted mouse embryonic fibroblasts, suggesting that RASSF1A regulates Rac signaling [86]. MAP1S, which was previously described as a microtubule-associated protein, is involved in the biogenesis and degradation of autophagosomes [87]. As deregulation of autophagy is associated with tumorigenesis, it will be necessary to study the mechanism by which RASSF1A affects autophagy. RASSF1A blocks the SCFβ-RTRCP-mediated degradation of repressor element 1 silencing transcription factor and, consequently, downregulates the oncogenic factor miR-21, which targets various tumor suppressor genes such as PTEN [88]. All these properties may also contribute to the tumor suppressor function of RASSF1A.
RASSF1A may be implicated in non-cancer diseases
RASSF1A restricts Toll-like receptor-stimulated NFκB signaling [89]. RASSF1A interacts with and inhibits Tank binding kinase 1, the activator of NFκB signaling [39]. Correspondingly, Rassf1a-null mice displayed enhanced inflammatory reaction in a dextran sulfate sodium-induced colitis model [89]. RASSF1A and MST1 antagonize TNF-α signaling in cardiac myocytes and fibroblasts and block fibrosis [90, 91]. RASSF1A interacts with ATP2B4 (plasma membrane calmodulin-dependent calcium ATPase) in the heart [92]. RASSF1A depletion causes cardiac hypertrophy [90, 91]. Interestingly, the expression of genes related to the circadian clock is altered in the Rassf1a-deleted mouse liver [39]. RASSF1A may have additional roles other than tumor suppression. Investigation of the implication of RASSF1A in non-cancerous diseases will have clinical significance.
RASSF1C is an oncogene
Initially, RASSF1C was considered a tumor suppressor similarly as RASSF1A. It was reported that RASSF1C activates SAPK/JNK signaling, triggers senescence and apoptosis, and plays a tumor suppressor role in prostate cancer and renal carcinoma cells and that RASSF1C increases the sensitivity to CDDP in ovarian cancer cells [93,94,95]. Nevertheless, accumulating evidence has overturned this belief. RASSF1C is upregulated in breast, lung, esophageal, and pancreatic endocrine tumors [96, 97]. CpG islands are not hypermethylated in the promoter of RASSF1C. RASSF1C inhibits the β-TRCP-mediated degradation of β-catenin [98]. RASSF1C enhances the expression of genes implicated in cancer development, such as PIWIL1 [97, 99]. Furthermore, RASSF1A regulates lung cell transformation and tumorigenesis through modulating the expression of PIWI-interacting RNAs [100]. In cells with methylated RASSF1A, RASSF1C expression is alternatively enhanced, and the protein interacts with SRC and YES1 and increases the tyrosine phosphorylation of YAP1 [101]. Furthermore, RASSF1C promotes the SRC-dependent phosphorylation of E-cadherin and destabilizes cell junctions. As a result, RASSF1C increases nuclear β-catenin levels. Moreover, RASSF1C induces MYC. These findings strongly support that RASSF1C is an oncogene. It is known that RASSF1A and RASSF1C play opposite roles in cell proliferation and apoptosis in breast and lung cancer cells [102]. RASSF1C expression is high in breast and lung tumors, whereas RASSF1A expression is low. The expression ratio of RASSF1A and RASSF1C may be an important determinant of tumor properties.
RASSF2
Identification of RASSF2 as a tumor suppressor
RASSF2 was reported as the third member of the RASSF protein family [103]. Enforced expression of RASSF2 causes cell cycle arrest and apoptosis. Deletion of RASSF2 enhances tumorigenicity and drug resistance in lung cancer cells. RASSF2 silencing via hypermethylation has been reported in various human cancers [4, 104, 105]. Cancer-associated fibroblasts produce miR-7, which suppresses RASSF2 expression [106]. Enhancer of zeste homolog 2, which is overexpressed in cancers, downregulates RASSF2 [107]. These data indicate that RASSF2 is a tumor suppressor.
The molecular mechanism underlying the tumor suppressor role of RASSF2
We do not yet fully understand the mechanism by which RASSF2 suppresses tumors. Several papers reported the inhibition of NFκB signaling, the activation of MST kinases and JNK, and the nuclear recruitment of prostate apoptosis response protein-4 (PAR-4) as the underlying mechanisms [108,109,110,111].
RASSF2 as a target of Ras proteins
Active KRAS enhances the interaction between RASSF2 and PAR-4 [110]. Furthermore, proteomics analysis revealed several molecules that bind RASSF2 in the KRAS-dependent manner [112]. These findings suggest that RASSF2 is a target of KRAS.
Other properties of RASSF2
Rassf2-null mice exhibit bone defects and hematopoietic abnormalities [108]. This phenotype indicates that RASSF2 physiologically plays a role other than tumor suppression. As each C-RASSF displays a distinct distribution in cells, regulation of the subcellular localization of C-RASSF is an important issue to study. In this light, the finding that the nuclear cytoplasmic shuttle of RASSF2 is regulated by extracellular regulated kinase 2 is interesting [113].
RASSF3
Our understanding of RASSF3 lags far behind that of other C-RASSFs. RASSF3 was reported as a homolog of RASSF1A [114]. Rassf3 causes resistance to mammary tumor development in neu-transgenic mice [115]. In humans, RASSF3 downregulation is detected in non-small cell lung cancer, and it is correlated with disease progression [116]. We reported that RASSF3 regulates apoptosis and the cell cycle via p53 and contributes to tumor suppression [117]. These findings support that RASSF3 is a tumor suppressor.
RASSF4
Whether RASSF4 is a tumor suppressor is elusive. RASSF4 suppression is observed in non-small cell lung cancer, nasopharyngeal carcinoma, and multiple myeloma [118,119,120]. RASSF4 overexpression induces apoptosis and inhibits proliferation in HEK293 cells [121]. RASSF4 reduces β-catenin, MYC, and CCND1 expression in osteosarcoma cells [122]. These properties support that RASSF4 is a tumor suppressor. Unexpectedly, however, RASSF4 is upregulated in alveolar rhabdomyosarcoma (aRMS), in which it activates YAP1 and promotes tumorigenesis [123]. The researchers explained that RASSF4 inhibits MST1 and suppresses the phosphorylation of YAP1. As we will expound upon in the section “RASSF6”, MST kinases and some, if not all, C-RASSF proteins inhibit each other under the basal condition. This mutual inhibition may be meaningful for avoiding excessive cell death. However, when cells are exposed to stresses such as DNA damage, MST kinases and C-RASSF are released from inhibition, after which they mediate apoptosis in a parallel manner. Suppose that the downstream tumor suppressive mechanism of C-RASSF proteins is impaired. In this situation, high expression of C-RASSF proteins may compromise the Hippo pathway and lead to tumorigenesis. If this scenario is correct, then the finding that RASSF4 activates YAP1 in aRMS is consistent with its original property as a tumor suppressor. However, a recent study revealed another possibility. RASSF4 interacts with the GDP-bound form of adenosine diphosphate ribosylation factor 6 (ARF6) and activates type I phosphatidylinositol phosphate kinase to enhance phosphatidylinositol 4,5-biphosphate (PI(4,5)P2) levels [124]. In response to this, the endoplasmic reticulum (ER) Ca2+ sensor stromal interaction molecule 1 is accumulated at ER–plasma membrane junctions, and store-operated Ca2+ entry is triggered. RASSF4 may contribute to tumorigenesis through phosphoinositide metabolisms and Ca2+ signaling.
RASSF6
Identification of RASSF6
RASSF6 was initially identified as a gene encoded in the bronchiolitis susceptibility locus [125]. Later, RASSF6 was characterized as a C-RASSF protein based on its sequence homology [126]. RASSF6 suppression by DNA hypermethylation is frequently observed in various human cancers [127,128,129]. In gastric cancer, miR-181a-5p suppresses RASSF6 [130]. Thereby, RASSF6 is considered a typical tumor suppressor. We identified RASSF6 in yeast two-hybrid screening using membrane-associated guanylate kinase inverted 1 (MAGI1) as bait [131]. The MAGI family consists of three members, MAGI1, MAGI2, and MAGI3 [132,133,134]. MAGI proteins have multiple PDZ, two WW, and one guanylate kinase domain. RASSF6 binds to the PDZ domains of MAGI proteins through the C-terminal PDZ-binding motif, the sequence of which distinguishes RASSF6 from other C-RASSF proteins. RASSF6 interacts with DLG1, which also has PDZ domains [135]. MAGI proteins and DLG1 are components of polarized epithelial junctions. MAGI2 is known as a tumor suppressor. Therefore, the interaction between RASSF6 and these proteins is intriguing, and its physiological significance must be clarified.
The molecular mechanism underlying the tumor suppressor role of RASSF6
RASSF6 causes apoptosis via caspase-dependent and caspase-independent mechanisms [131]. Conversely, RASSF6 depletion attenuates apoptosis caused by TNF-α, okadaic acid, high osmolarity, and ultraviolet radiation [131, 136,137,138]. These findings indicate that RASSF6 mediates apoptosis under various conditions. RASSF6 activates BAX via MOAP1 and promotes the release of cytochrome c, apoptosis-inducing factor, and endonuclease G from mitochondria [126, 131, 136]. Mechanistically, RASSF6 interacts with MDM2 and blocks the MDM2-mediated degradation of p53 [137]. KRAS augments the interaction between RASSF6 and MDM2 [139]. The RA domain (RASSF6-RA) binds to the C-terminal RING domain of MDM2. The SARAH domain (RASSF6-SARAH) intramolecularly binds to RASSF6-RA and inhibits the interaction between RASSF6-RA and MDM2. KRAS releases RASSF6-RA from this inhibition and shifts it toward binding to MDM2. In this manner, RASSF6 mediates Ras-induced p53-dependent apoptosis, supporting that RASSF6 is a target of Ras signaling. RASSF6 inhibits NFκB and MAPK signaling and promotes CDKN1A accumulation via JNK [126, 140]. These properties also contribute to tumor suppression.
RASSF6 cooperates with the Hippo pathway
RASSF6 is the highly pro-apoptotic protein. When RASSF6 is exogenously expressed, most cells do not survive for a long period, but when MST kinases are coexpressed, RASSF6-induced apoptosis is remarkably suppressed [136]. Contrarily, RASSF6 inhibits MST kinases. This mutual inhibition is mediated by the SARAH domains. Coexpression of full-length RASSF6 blocks the autophosphorylation of MST kinases in vivo and inhibits the phosphorylation of MOB1 in vitro. However, okadaic acid treatment dissociates RASSF6 and MST kinases from each other. Consequently, RASSF6-mediated apoptosis is triggered, and the Hippo pathway is simultaneously activated. Based on this finding, we speculate that RASSF6 and the Hippo pathway cooperatively function as tumor suppressors. In this respect, RASSF6 is different from RASSF1A in that it drives the Hippo pathway to suppress tumors.
C-RASSF proteins of non-mammalian organisms
C-RASSF of D. melanogaster
Drosophila melanogaster expresses one C-RASSF named dRASSF. Drosophila cells with Ras1 loss-of-function mutations exhibit growth defects, but additional mutation of dRASSF rescues the phenotype [141]. This implies that dRASSF antagonizes Ras1 and participates in Ras signaling. Likewise, the relationship with the Hippo pathway is conserved. dRASSF physically interacts with Hippo via the SARAH domain. dRASSF suppresses the overgrowth phenotype of the kinase-negative Hippo mutant but has no effect on the SARAH domain-lacking Hippo mutant [141]. This observation is comprehensible if dRASSF suppresses Hippo similarly as RASSF6 inhibits MST kinases via the SARAH domains. Proteomic analysis revealed that dRASSF interacts with the Drosophila striatin-interacting phosphatase and kinase (dSTRIPAK) complex and represses Hippo through dephosphorylation, thus functioning in contrast to RASSF1A, which that activates MST kinases through the inhibition of dephosphorylation [142]. Mutation of lethal (2) giant larvae, a regulator of apical-basal cell polarity, results in the mislocalization of Hippo, dRASSF, and dSTRIPAK [143]. This finding corroborates that these proteins form a complex. Given that NORE1 promotes pRb dephosphorylation via PP1A and that RASSF1A blocks of MST kinase dephosphorylation by PP2A, the regulation of dephosphorylation may be one of common functions of C-RASSF proteins [23, 42].
C-RASSF of Caenorhabditis elegans
In the first paper that reported NORE1, the researchers identified C. elegans T24F1.3 as a homolog of NORE1 [8]. Later, we studied T24F1.3 in C. elegans, characterized the mutant, and named the gene rsf-1 [144]. RSF-1 interacts with CST-1/2, which are homologs of MST kinases. However, as CST-1/2 are not involved in the regulation of Wts, the homolog of LATS kinases, RSF-1 is irrelevant to the Hippo pathway, and the loss-of-function mutant of rsf-1 does not exhibit a phenotype related to cell proliferation and apoptosis. Importantly, rsf-1 mutation suppresses the multivulva phenotype of active mutants of let-60, the homolog of RAS. This observation suggests that RSF-1 is implicated in Ras signaling. Moreover, RSF-1 interacts with Rab-39, a small GTP-binding protein [145]. rsf-1 silencing and rab-39 mutation make worms more sensitive to oxidative stress. As RASSF1A binds RHOA and RASSF4 interacts with ARF6, we can assume that C-RASSF proteins interact with several GTP-binding proteins other than Ras proteins.
Perspective
The number of research papers concerning C-RASSF proteins has continuously grown, but the progress has not been equal for all C-RASSFs. For example, regulation by phosphorylation is well researched for RASSF1A but not for other C-RASSF proteins. Nonetheless, we currently know several mechanisms instrumental for the tumor suppressor functions of C-RASSF proteins. It is difficult to believe that all mechanisms work simultaneously. Moreover, it is unlikely that all C-RASSF proteins equally contribute to tumor suppression in all tissues. Research to determine the mechanism by which each C-RASSF functions in various tissues, cells, and subcellular compartments and under various conditions will be indispensable for clarifying the whole picture of C-RASSF. We also need to raise a very naïve question. Why do mammals have so many C-RASSF proteins? It is unquestionable that C-RASSF proteins can interact with each other when they are colocalized in cells [135]. MST1 and MST2 form a heterodimer with lower kinase activity than their homodimers [146]. Accordingly, we can hypothesize that heterodimers of C-RASSF proteins have different activities and that mammals require the fine-tuning of C-RASSF proteins to maintain tissue homeostasis. Research to dissect the relationships among C-RASSF proteins is important. From the clinical viewpoint, reactivation of C-RASSF expression via epigenetic reactivation is a reasonable strategy for cancer therapy. The simple idea is to inhibit DNA methylation. However, if we identify a certain mechanism that is pivotal for C-RASSF proteins to function as tumor suppressors, we can develop a surrogate method to compensate for the function of C-RASSF in cancer cells with C-RASSF downregulation. Such perspectives motivate us to study C-RASSF.
Abbreviations
- APC/C:
-
Anaphase-promoting complex
- ARF6:
-
Adenosine diphosphate ribosylation factor 6
- ATM:
-
Ataxia telangiectasia mutated
- CNKR1:
-
Connector enhancer of kinase suppressor Ras 1
- ER:
-
Endoplasmic reticulum
- HIPK2:
-
Homeodomain-interacting protein kinase 2
- JNK:
-
c-Jun N-terminal kinase
- LATS:
-
Large tumor suppressor kinase
- MAGI:
-
Membrane-associated guanylate kinase inverted
- MOAP1:
-
Modulator of apoptosis 1
- MST:
-
Mammalian Ste20-like kinase
- NORE:
-
Novel Ras effector
- PAR-4:
-
Prostate apoptosis response protein-4
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- RA:
-
Ras association
- RCC1:
-
Regulator of chromosome condensation 1
- SARAH:
-
Salvador/RASSF/Hippo
- STRIPAK:
-
Striatin-interacting phosphatase and kinase
- TAZ:
-
Transcriptional coactivator with PDZ-binding motif
- TEAD:
-
TEA domain transcription factor
- TNF-α:
-
Tumor necrosis factor-α
- TRAIL:
-
Tumor necrosis factor-like apoptosis-inducing ligand
- XPA:
-
Xeroderma pigmentosum A
- YAP1:
-
Yes-associated protein 1
References
Avruch J, Xavier R, Bardeesy N, Zhang XF, Praskova M, Zhou D, Xia F (2009) Rassf family of tumor suppressor polypeptides. J Biol Chem 284:11001–11005
Volodko N, Gordon M, Salla M, Ghazaleh HA, Baksh S (2014) RASSF tumor suppressor gene family: biological functions and regulation. FEBS Lett 588:2671–2684
Sherwood V, Recino A, Jeffries A, Ward A, Chalmers AD (2010) The N-terminal RASSF family: a new group of Ras-association-domain-containing proteins, with emerging links to cancer formation. Biochem J 425:303–311
Richter AM, Pfeifer GP, Dammann RH (2009) The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796:114–128
Harvey K, Tapon N (2007) The Salvador–Warts–Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7:182–191
Pan D (2010) The hippo signaling pathway in development and cancer. Dev Cell 19:491–505
Meng Z, Moroishi T, Guan KL (2016) Mechanisms of Hippo pathway regulation. Genes Dev 30:1–17
Vavvas D, Li X, Avruch J, Zhang XF (1998) Identification of Nore1 as a potential Ras effector. J Biol Chem 273:5439–5442
Hesson L, Dallol A, Minna JD, Maher ER, Latif F (2003) NORE1A, a homologue of RASSF1A tumour suppressor gene is inactivated in human cancers. Oncogene 22:947–954
Avruch J, Praskova M, Ortiz-Vega S, Liu M, Zhang XF (2006) Nore1 and RASSF1 regulation of cell proliferation and of the MST1/2 kinases. Methods Enzymol 407:290–310
Chan JJ, Flatters D, Rodrigues-Lima F, Yan J, Thalassinos K, Katan M (2013) Comparative analysis of interactions of RASSF1-10. Adv Biol Regul 53:190–201
Macheiner D, Heller G, Kappel S, Bichler C, Stättner S, Ziegler B, Kandioler D, Wrba F, Schulte-Hermann R, Zöchbauer-Müller S, Grasl-Kraupp B (2006) NORE1B, a candidate tumor suppressor, is epigenetically silenced in human hepatocellular carcinoma. J Hepatol 45:81–89
Li TK, Yin K, Chen Z, Bao Y, Zhang SX (2017) MiR-214 regulates oral cancer KB cell apoptosis through targeting RASSF5. Genet Mol Res 16(1). https://doi.org/10.4238/gmr16019327
Suryaraja R, Anitha M, Anbarasu K, Kumari G, Mahalingam S (2013) The E3 ubiquitin ligase Itch regulates tumor suppressor protein RASSF5/NORE1 stability in an acetylation-dependent manner. Cell Death Dis 4:e565
Katagiri K, Maeda A, Shimonaka M, Kinashi T (2003) RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol 4:741–748
Katagiri K, Ueda Y, Tomiyama T, Yasuda K, Toda Y, Ikehara S, Nakayama KI, Kinashi T (2011) Deficiency of Rap1-binding protein RAPL causes lymphoproliferative disorders through mislocalization of p27kip1. Immunity 34:24–38
Oertli B, Han J, Marte BM, Sethi T, Downward J, Ginsberg M, Hughes PE (2000) The effector loop and prenylation site of R-Ras are involved in the regulation of integrin function. Oncogene 19:4961–4969
Khokhlatchev A, Rabizadeh S, Xavier R, Nedwidek M, Chen T, Zhang XF, Seed B, Avruch J (2002) Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol 12:253–265
Donninger H, Calvisi DF, Barnoud T, Clark J, Schmidt ML, Vos MD, Clark GJ (2015) NORE1A is a Ras senescence effector that controls the apoptotic/senescent balance of p53 via HIPK2. J Cell Biol 208:777–789
Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J (2004) Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J 381:453–462
Schmidt ML, Calvisi DF, Clark GJ (2016) NORE1A regulates MDM2 Via β-TrCP. Cancers (Basel) 8:E39
Schmidt ML, Donninger H, Clark GJ (2014) Ras regulates SCF(β-TrCP) protein activity and specificity via its effector protein NORE1A. J Biol Chem 289:31102–31110
Barnoud T, Donninger H, Clark GJ (2016) Ras Regulates Rb via NORE1A. J Biol Chem 291:3114–3123
Rawat SJ, Chernoff J (2015) Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem Sci 40:149–156
Hwang E, Ryu KS, Pääkkönen K, Güntert P, Cheong HK, Lim DS, Lee JO, Jeon YH, Cheong C (2007) Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc Natl Acad Sci USA 104:9236–9241
Makbul C, Constantinescu Aruxandei D, Hofmann E, Schwarz D, Wolf E, Herrmann C (2013) Structural and thermodynamic characterization of Nore1-SARAH: a small, helical module important in signal transduction networks. Biochemistry 52:1045–1054
Ni L, Li S, Yu J, Min J, Brautigam CA, Tomchick DR, Pan D, Luo X (2013) Structural basis for autoactivation of human Mst2 kinase and its regulation by RASSF5. Structure 21:1757–1768
Liao TJ, Tsai CJ, Jang H, Fushman D, Nussinov R (2016) RASSF5: an MST activator and tumor suppressor in vivo but opposite in vitro. Curr Opin Struct Biol 41:217–224
Bitra A, Sistla S, Mariam J, Malvi H, Anand R (2017) Rassf proteins as modulators of Mst1 kinase activity. Sci Rep 7:45020
Harjes E, Harjes S, Wohlgemuth S, Müller KH, Krieger E, Herrmann C, Bayer P (2006) GTP-Ras disrupts the intramolecular complex of C1 and RA domains of Nore1. Structure 14:881–888
Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP (2000) Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 25:315–319
Liu L, Tommasi S, Lee DH, Dammann R, Pfeifer GP (2003) Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene 22:8125–8136
Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, Clark GJ, Downward J, Maher ER, Latif F (2004) RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res 64:4112–4116
Vos MD, Martinez A, Elam C, Dallol A, Taylor BJ, Latif F, Clark GJ (2004) A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res 64:4244–4250
Liu L, Vo A, McKeehan WL (2005) Specificity of the methylation-suppressed A isoform of candidate tumor suppressor RASSF1 for microtubule hyperstabilization is determined by cell death inducer C19ORF5. Cancer Res 65:1830–1838
Dallol A, Hesson LB, Matallanas D, Cooper WN, O’Neill E, Maher ER, Kolch W, Latif F (2009) RAN GTPase is a RASSF1A effector involved in controlling microtubule organization. Curr Biol 19:1227–1232
Sakai N, Saito Y, Fujiwara Y, Shiraki T, Imanishi Y, Koshimizu TA, Shibata K (2015) Identification of protein arginine N-methyltransferase 5 (PRMT5) as a novel interacting protein with the tumor suppressor protein RASSF1A. Biochem Biophys Res Commun 467:778–784
Jung HY, Jung JS, Whang YM, Kim YH (2013) RASSF1A suppresses cell migration through inactivation of HDAC6 and increase of acetylated α-tubulin. Cancer Res Treat 45:134–144
Zhang X, Guo C, Wu X, Li AX, Liu L, Tsark W, Dammann R, Shen H, Vonderfecht SL, Pfeifer GP (2016) Analysis of liver tumor-prone mouse models of the hippo kinase scaffold proteins RASSF1A and SAV1. Cancer Res 76:2824–2835
Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP (2007) RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol 17:700–705
Oh HJ, Lee KK, Song SJ, Jin MS, Song MS, Lee JH, Im CR, Lee JO, Yonehara S, Lim DS (2006) Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res 66:2562–2569
Guo C, Zhang X, Pfeifer GP (2011) The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J Biol Chem 286:6253–6261
Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O’neill E (2007) RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27:962–975
van der Weyden L, Papaspyropoulos A, Poulogiannis G, Rust AG, Rashid M, Adams DJ, Arends MJ, O’Neill E (2012) Loss of RASSF1A synergizes with deregulated RUNX2 signaling in tumorigenesis. Cancer Res 72:3817–3827
Pefani DE, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S, O’Neill E (2016) TGF-β targets the hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation. Mol Cell 63:156–166
Ahn EY, Kim JS, Kim GJ, Park YN (2013) RASSF1A-mediated regulation of AREG via the Hippo pathway in hepatocellular carcinoma. Mol Cancer Res 11:748–758
Donninger H, Allen N, Henson A, Pogue J, Williams A, Gordon L, Kassler S, Dunwell T, Latif F, Clark GJ (2011) Salvador protein is a tumor suppressor effector of RASSF1A with hippo pathway-independent functions. J Biol Chem 286:18483–18491
Nelson N, Clark GJ (2016) Rheb may complex with RASSF1A to coordinate Hippo and TOR signaling. Oncotarget 7:33821–33831
Vos MD, Dallol A, Eckfeld K, Allen NP, Donninger H, Hesson LB, Calvisi D, Latif F, Clark GJ (2006) The RASSF1A tumor suppressor activates Bax via MOAP-1. J Biol Chem 281:4557–4563
Ortiz-Vega S, Khokhlatchev A, Nedwidek M, Zhang XF, Dammann R, Pfeifer GP, Avruch J (2002) The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene 21:1381–1390
Rabizadeh S, Xavier RJ, Ishiguro K, Bernabeortiz J, Lopez-Ilasaca M, Khokhlatchev A, Mollahan P, Pfeifer GP, Avruch J, Seed B (2004) The scaffold protein CNK1 interacts with the tumor suppressor RASSF1A and augments RASSF1A-induced cell death. J Biol Chem 279:29247–29254
Romano D, Nguyen LK, Matallanas D, Halasz M, Doherty C, Kholodenko BN, Kolch W (2014) Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat Cell Biol 16:673–684
Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W (2002) Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416:648–653
Song MS, Song SJ, Kim SY, Oh HJ, Lim DS (2008) The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2-DAXX-HAUSP complex. EMBO J 27:1863–1874
Tommasi S, Dammann R, Zhang Z, Wang Y, Liu L, Tsark WM, Wilczynski SP, Li J, You M, Pfeifer GP (2005) Tumor susceptibility of Rassf1a knockout mice. Cancer Res 65:92–98
Tommasi S, Besaratinia A, Wilczynski SP, Pfeifer GP (2011) Loss of Rassf1a enhances p53-mediated tumor predisposition and accelerates progression to aneuploidy. Oncogene 30:690–700
Shivakumar L, Minna J, Sakamaki T, Pestell R, White MA (2002) The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol 22:4309–4318
Thaler S, Hähnel PS, Schad A, Dammann R, Schuler M (2009) RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf–MEK–ERK pathway and inhibition of Akt. Cancer Res 69:1748–1757
Yoo YA, Na AR, Lee MS, Yoon S, Kim JS, Yoo YD (2006) RASSF1A suppresses oncogenic H-Ras-induced c-Jun N-terminal kinase activation. Int J Oncol 29:1541–1547
Liao A, Tan G, Chen L, Zhou W, Hu H (2016) RASSF1A inhibits gastric cancer cell proliferation by miR-711-mediated downregulation of CDK4 expression. Oncotarget 7:5842–5851
Fenton SL, Dallol A, Agathanggelou A, Hesson L, Ahmed-Choudhury J, Baksh S, Sardet C, Dammann R, Minna JD, Downward J, Maher ER, Latif F (2004) Identification of the E1A-regulated transcription factor p120 E4F as an interacting partner of the RASSF1A candidate tumor suppressor gene. Cancer Res 64:102–107
Ahmed-Choudhury J, Agathanggelou A, Fenton SL, Ricketts C, Clark GJ, Maher ER, Latif F (2005) Transcriptional regulation of cyclin A2 by RASSF1A through the enhanced binding of p120E4F to the cyclin A2 promoter. Cancer Res 65:2690–2697
Whitehurst AW, Ram R, Shivakumar L, Gao B, Minna JD, White MA (2008) The RASSF1A tumor suppressor restrains anaphase-promoting complex/cyclosome activity during the G1/S phase transition to promote cell cycle progression in human epithelial cells. Mol Cell Biol 28:3190–3197
Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, Lee H, Choi N, Kim J, Kim H, Kim JW, Choi EJ, Kirschner MW, Lim DS (2004) The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol 6:129–137
Liu L, Baier K, Dammann R, Pfeifer GP (2007) The tumor suppressor RASSF1A does not interact with Cdc20, an activator of the anaphase-promoting complex. Cell Cycle 6:1663–1665
Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS (2005) The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem 280:3920–3927
Pefani DE, Latusek R, Pires I, Grawenda AM, Yee KS, Hamilton G, van der Weyden L, Esashi F, Hammond EM, O’Neill E (2014) RASSF1A-LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat Cell Biol 16(962–971):961–968
Donninger H, Clark J, Rinaldo F, Nelson N, Barnoud T, Schmidt ML, Hobbing KR, Vos MD, Sils B, Clark GJ (2015) The RASSF1A tumor suppressor regulates XPA-mediated DNA repair. Mol Cell Biol 35:277–287
Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP, Latif F, Downward J, Neel BG (2005) The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell 18:637–650
Foley CJ, Freedman H, Choo SL, Onyskiw C, Fu NY, Yu VC, Tuszynski J, Pratt JC, Baksh S (2008) Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol Cell Biol 28:4520–4535
Vichalkovski A, Gresko E, Cornils H, Hergovich A, Schmitz D, Hemmings BA (2008) NDR kinase is activated by RASSF1A/MST1 in response to Fas receptor stimulation and promotes apoptosis. Curr Biol 18:1889–1895
Ghazaleh HA, Chow RS, Choo SL, Pham D, Olesen JD, Wong RX, Onyskiw C, Baksh S (2010) 14-3-3 mediated regulation of the tumor suppressor protein, RASSF1A. Apoptosis 15:117–127
Rong R, Jiang LY, Sheikh MS, Huang Y (2007) Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene 26:7700–7708
Jiang L, Rong R, Sheikh MS, Huang Y (2014) Mitotic arrest by tumor suppressor RASSF1A is regulated via CHK1 phosphorylation. Mol Cancer Res 12:119–129
Hamilton G, Yee KS, Scrace S, O’Neill E (2009) ATM regulates a RASSF1A-dependent DNA damage response. Curr Biol 19:2020–2025
Yee KS, Grochola L, Hamilton G, Grawenda A, Bond EE, Taubert H, Wurl P, Bond GL, O’Neill E (2012) A RASSF1A polymorphism restricts p53/p73 activation and associates with poor survival and accelerated age of onset of soft tissue sarcoma. Cancer Res 72:2206–2217
Gao B, Xie XJ, Huang C, Shames DS, Chen TT, Lewis CM, Bian A, Zhang B, Olopade OI, Garber JE, Euhus DM, Tomlinson GE, Minna JD (2008) RASSF1A polymorphism A133S is associated with early onset breast cancer in BRCA1/2 mutation carriers. Cancer Res 68:22–25
Song SJ, Song MS, Kim SJ, Kim SY, Kwon SH, Kim JG, Calvisi DF, Kang D, Lim DS (2009) Aurora A regulates prometaphase progression by inhibiting the ability of RASSF1A to suppress APC-Cdc20 activity. Cancer Res 69:2314–2323
Song SJ, Kim SJ, Song MS, Lim DS (2009) Aurora B-mediated phosphorylation of RASSF1A maintains proper cytokinesis by recruiting Syntaxin16 to the midzone and midbody. Cancer Res 69:8540–8544
Song MS, Song SJ, Kim SJ, Nakayama K, Nakayama KI, Lim DS (2008) Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition. Oncogene 27:3176–3185
Richter AM, Schagdarsurengin U, Rastetter M, Steinmann K, Dammann RH (2010) Protein kinase A-mediated phosphorylation of the RASSF1A tumour suppressor at Serine 203 and regulation of RASSF1A function. Eur J Cancer 46:2986–2995
Verma SK, Ganesan TS, Parker PJ (2008) The tumour suppressor RASSF1A is a novel substrate of PKC. FEBS Lett 582:2270–2276
Lee MG, Jeong SI, Ko KP, Park SK, Ryu BK, Kim IY, Kim JK, Chi SG (2016) RASSF1A directly antagonizes RhoA activity through the assembly of a Smurf1-mediated destruction complex to suppress tumorigenesis. Cancer Res 76:1847–1859
Dubois F, Keller M, Calvayrac O, Soncin F, Hoa L, Hergovich A, Parrini MC, Mazières J, Vaisse-Lesteven M, Camonis J, Levallet G, Zalcman G (2016) RASSF1A suppresses the invasion and metastatic potential of human non-small cell lung cancer cells by inhibiting YAP activation through the GEF-H1/RhoB pathway. Cancer Res 76:1627–1640
Thaler S, Schmidt M, Schad A, Sleeman JP (2012) RASSF1A inhibits estrogen receptor alpha expression and estrogen-independent signalling: implications for breast cancer development. Oncogene 31:4912–4922
Dallol A, Agathanggelou A, Tommasi S, Pfeifer GP, Maher ER, Latif F (2005) Involvement of the RASSF1A tumor suppressor gene in controlling cell migration. Cancer Res 65:7653–7659
Xie R, Nguyen S, McKeehan K, Wang F, McKeehan WL, Liu L (2011) Microtubule-associated protein 1S (MAP1S) bridges autophagic components with microtubules and mitochondria to affect autophagosomal biogenesis and degradation. J Biol Chem 286:10367–10377
Ram RR, Mendiratta S, Bodemann BO, Torres MJ, Eskiocak U, White MA (2014) RASSF1A inactivation unleashes a tumor suppressor/oncogene cascade with context-dependent consequences on cell cycle progression. Mol Cell Biol 34:2350–2358
Gordon M, El-Kalla M, Zhao Y, Fiteih Y, Law J, Volodko N, Anwar-Mohamed A, Mohamed A, El-Kadi AO, Liu L, Odenbach J, Thiesen A, Onyskiw C, Ghazaleh HA, Park J, Lee SB, Yu VC, Fernandez-Patron C, Alexander RT, Wine E, Baksh S (2013) The tumor suppressor gene, RASSF1A, is essential for protection against inflammation -induced injury. PLoS One 8:e75483
Mohamed TM, Zi M, Prehar S, Maqsood A, Abou-Leisa R, Nguyen L, Pfeifer GP, Cartwright EJ, Neyses L, Oceandy D (2014) The tumour suppressor Ras-association domain family protein 1A (RASSF1A) regulates TNF-α signalling in cardiomyocytes. Cardiovasc Res 103:47–59
Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, Sadoshima J (2010) Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J Clin Investig 120:3555–3567
Armesilla AL, Williams JC, Buch MH, Pickard A, Emerson M, Cartwright EJ, Oceandy D, Vos MD, Gillies S, Clark GJ, Neyses L (2004) Novel functional interaction between the plasma membrane Ca2+ pump 4b and the proapoptotic tumor suppressor Ras-associated factor 1 (RASSF1). J Biol Chem 279:31318–31328
Li J, Wang F, Protopopov A, Malyukova A, Kashuba V, Minna JD, Lerman MI, Klein G, Zabarovsky E (2004) Inactivation of RASSF1C during in vivo tumor growth identifies it as a tumor suppressor gene. Oncogene 23:5941–5949
Kitagawa D, Kajiho H, Negishi T, Ura S, Watanabe T, Wada T, Ichijo H, Katada T, Nishina H (2006) Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation. EMBO J 25:3286–3297
Lorenzato A, Martino C, Dani N, Oligschläger Y, Ferrero AM, Biglia N, Calogero R, Olivero M, Di Renzo MF (2012) The cellular apoptosis susceptibility CAS/CSE1L gene protects ovarian cancer cells from death by suppressing RASSF1C. FASEB J 26:2446–2456
Amaar YG, Minera MG, Hatran LK, Strong DD, Mohan S, Reeves ME (2006) Ras association domain family 1C protein stimulates human lung cancer cell proliferation. Am J Physiol Lung Cell Mol Physiol 291:L1185–L1190
Reeves ME, Baldwin SW, Baldwin ML, Chen ST, Moretz JM, Aragon RJ, Li X, Strong DD, Mohan S, Amaar YG (2010) Ras-association domain family 1C protein promotes breast cancer cell migration and attenuates apoptosis. BMC Cancer 10:562
Estrabaud E, Lassot I, Blot G, Le Rouzic E, Tanchou V, Quemeneur E, Daviet L, Margottin-Goguet F, Benarous R (2007) RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of beta-catenin by interacting with betaTrCP. Cancer Res 67:1054–1061
Reeves ME, Baldwin ML, Aragon R, Baldwin S, Chen ST, Li X, Mohan S, Amaar YG (2012) RASSF1C modulates the expression of a stem cell renewal gene, PIWIL1. BMC Res Notes 5:239
Reeves ME, Firek M, Jliedi A, Amaar YG (2017) Identification and characterization of RASSF1C piRNA target genes in lung cancer cells. Oncotarget 8:34268–34282
Vlahov N, Scrace S, Soto MS, Grawenda AM, Bradley L, Pankova D, Papaspyropoulos A, Yee KS, Buffa F, Goding CR, Timpson P, Sibson N, O’Neill E (2015) Alternate RASSF1 transcripts control SRC activity, E-cadherin contacts, and YAP-mediated invasion. Curr Biol 25:3019–3034
Reeves ME, Firek M, Chen ST, Amaar Y (2013) The RASSF1 gene and the opposing effects of the RASSF1A and RASSF1C isoforms on cell proliferation and apoptosis. Mol Biol Int 2013:145096
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, Clark GJ (2003) RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem 278:28045–28051
Hesson LB, Wilson R, Morton D, Adams C, Walker M, Maher ER, Latif F (2005) CpG island promoter hypermethylation of a novel Ras-effector gene RASSF2A is an early event in colon carcinogenesis and correlates inversely with K-ras mutations. Oncogene 24:3987–3994
Cooper WN, Dickinson RE, Dallol A, Grigorieva EV, Pavlova TV, Hesson LB, Bieche I, Broggini M, Maher ER, Zabarovsky ER, Clark GJ, Latif F (2008) Epigenetic regulation of the ras effector/tumour suppressor RASSF2 in breast and lung cancer. Oncogene 27:1805–1811
Shen Z, Qin X, Yan M, Li R, Chen G, Zhang J, Chen W (2017) Cancer-associated fibroblasts promote cancer cell growth through a miR-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget 8:1290–1303
Yu P, Guo Y, Yusufu M, Liu Z, Wang S, Yin X, Peng G, Wang L, Zhao X, Guo H, Huang T, Liu C (2016) Decreased expression of EZH2 reactivates RASSF2A by reversal of promoter methylation in breast cancer cells. Cell Biol Int 40:1062–1070
Song H, Kim H, Lee K, Lee DH, Kim TS, Song JY, Lee D, Choi D, Ko CY, Kim HS, Shin HI, Choi J, Park H, Park C, Jeong D, Lim DS (2012) Ablation of Rassf2 induces bone defects and subsequent haematopoietic anomalies in mice. EMBO J 31:1147–1159
Cooper WN, Hesson LB, Matallanas D, Dallol A, von Kriegsheim A, Ward R, Kolch W, Latif F (2009) RASSF2 associates with and stabilizes the proapoptotic kinase MST2. Oncogene 28:2988–2998
Donninger H, Hesson L, Vos M, Beebe K, Gordon L, Sidransky D, Liu JW, Schlegel T, Payne S, Hartmann A, Latif F, Clark GJ (2010) The Ras effector RASSF2 controls the PAR-4 tumor suppressor. Mol Cell Biol 30:2608–2620
Song H, Oh S, Oh HJ, Lim DS (2010) Role of the tumor suppressor RASSF2 in regulation of MST1 kinase activity. Biochem Biophys Res Commun 391:969–973
Barnoud T, Wilkey DW, Merchant ML, Clark JA, Donninger H (2016) Proteomics analysis reveals novel RASSF2 interaction partners. Cancers (Basel) 8:E37
Kumari G, Mahalingam S (2009) Extracellular signal-regulated kinase 2 (ERK-2) mediated phosphorylation regulates nucleo-cytoplasmic shuttling and cell growth control of Ras-associated tumor suppressor protein, RASSF2. Exp Cell Res 315:2775–2790
Tommasi S, Dammann R, Jin SG, Zhang XF, Avruch J, Pfeifer GP (2002) RASSF3 and NORE1: identification and cloning of two human homologues of the putative tumor suppressor gene RASSF1. Oncogene 21:2713–2720
Jacquemart IC, Springs AE, Chen WY (2009) Rassf3 is responsible in part for resistance to mammary tumor development in neu transgenic mice. Int J Oncol 34:517–528
Fukatsu A, Ishiguro F, Tanaka I, Kudo T, Nakagawa K, Shinjo K, Kondo Y, Fujii M, Hasegawa Y, Tomizawa K, Mitsudomi T, Osada H, Hata Y, Sekido Y (2014) RASSF3 downregulation increases malignant phenotypes of non-small cell lung cancer. Lung Cancer 83:23–29
Kudo T, Ikeda M, Nishikawa M, Yang Z, Ohno K, Nakagawa K, Hata Y (2012) The RASSF3 candidate tumor suppressor induces apoptosis and G1–S cell-cycle arrest via p53. Cancer Res 72:2901–2911
Han Y, Dong Q, Hao J, Fu L, Han X, Zheng X, Wang E (2016) RASSF4 is downregulated in nonsmall cell lung cancer and inhibits cancer cell proliferation and invasion. Tumour Biol 37:4865–4871
Chow LS, Lo KW, Kwong J, Wong AY, Huang DP (2004) Aberrant methylation of RASSF4/AD037 in nasopharyngeal carcinoma. Oncol Rep 12:781–787
De Smedt E, Maes K, Verhulst S, Liu H, Kassambara A, Maes A, Robert N, Heirman C, Cakana A, Hose D, Breckpot K, van Grunsven LA, De Veirman K, Menu E, Vanderkerken K, Moreaux J, De Bruyne E (2017) Loss of RASSF4 expression in multiple myeloma promotes RAS-driven malignant progression. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-17-1544
Eckfeld K, Hesson L, Vos MD, Bieche I, Latif F, Clark GJ (2004) RASSF4/AD037 is a potential ras effector/tumor suppressor of the RASSF family. Cancer Res 64:8688–8693
Zhang M, Wang D, Zhu T, Yin R (2017) RASSF4 overexpression inhibits the proliferation, invasion, EMT, and Wnt signaling pathway in osteosarcoma cells. Oncol Res 25:83–91
Crose LE, Galindo KA, Kephart JG, Chen C, Fitamant J, Bardeesy N, Bentley RC, Galindo RL, Chi JT, Linardic CM (2014) Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J Clin Investig 124:285–296
Chen YJ, Chang CL, Lee WR, Liou J (2017) RASSF4 controls SOCE and ER-PM junctions through regulation of PI(4,5)P2. J Cell Biol 216:2011–2025
Hull J, Rowlands K, Lockhart E, Sharland M, Moore C, Hanchard N, Kwiatkowski DP (2004) Haplotype mapping of the bronchiolitis susceptibility locus near IL8. Hum Genet 114:272–279
Allen NP, Donninger H, Vos MD, Eckfeld K, Hesson L, Gordon L, Birrer MJ, Latif F, Clark GJ (2007) RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene 26:6203–6211
Hesson LB, Dunwell TL, Cooper WN, Catchpoole D, Brini AT, Chiaramonte R, Griffiths M, Chalmers AD, Maher ER, Latif F (2009) The novel RASSF6 and RASSF10 candidate tumour suppressor genes are frequently epigenetically inactivated in childhood leukaemias. Mol Cancer 8:42
Liang YY, Chen MY, Hua YJ, Chen S, Zheng LS, Cao X, Peng LX, Xie P, Huang BJ, Sun R, Wang L, Xiang YQ, Guo X, Qian CN (2014) Downregulation of Ras association domain family member 6 (RASSF6) underlies the treatment resistance of highly metastatic nasopharyngeal carcinoma cells. PLoS One 9:e100843
Ye HL, Li DD, Lin Q, Zhou Y, Zhou QB, Zeng B, Fu ZQ, Gao WC, Liu YM, Chen RW, Li ZH, Chen RF (2015) Low RASSF6 expression in pancreatic ductal adenocarcinoma is associated with poor survival. World J Gastroenterol 21:6621–6630
Mi Y, Zhang D, Jiang W, Weng J, Zhou C, Huang K, Tang H, Yu Y, Liu X, Cui W, Zhang M, Sun X, Zhou Z, Peng Z, Zhao S, Wen Y (2017) miR-181a-5p promotes the progression of gastric cancer via RASSF6-mediated MAPK signalling activation. Cancer Lett 389:11–22
Ikeda M, Hirabayashi S, Fujiwara N, Mori H, Kawata A, Iida J, Bao Y, Sato Y, Iida T, Sugimura H, Hata Y (2007) Ras-association domain family protein 6 induces apoptosis via both caspase-dependent and caspase-independent pathways. Exp Cell Res 313:1484–1495
Nagashima S, Kodaka M, Iwasa H, Hata Y (2015) MAGI2/S-SCAM outside brain. J Biochem 157:177–184
Feng X, Jia S, Martin TA, Jiang WG (2014) Regulation and involvement in cancer and pathological conditions of MAGI1, a tight junction protein. Anticancer Res 34:3251–3256
Van Itallie CM, Anderson JM (2014) Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36:157–165
Iwasa H, Jiang X, Hata Y (2015) RASSF6; the putative tumor suppressor of the RASSF family. Cancers (Basel) 7:2415–2426
Ikeda M, Kawata A, Nishikawa M, Tateishi Y, Yamaguchi M, Nakagawa K, Hirabayashi S, Bao Y, Hidaka S, Hirata Y, Hata Y (2009) Hippo pathway-dependent and -independent roles of RASSF6. Sci Signal 2:59
Iwasa H, Kudo T, Maimaiti S, Ikeda M, Maruyama J, Nakagawa K, Hata Y (2013) The RASSF6 tumor suppressor protein regulates apoptosis and the cell cycle via MDM2 protein and p53 protein. J Biol Chem 288:30320–30329
Withanage K, Nakagawa K, Ikeda M, Kurihara H, Kudo T, Yang Z, Sakane A, Sasaki T, Hata Y (2012) Expression of RASSF6 in kidney and the implication of RASSF6 and the Hippo pathway in the sorbitol-induced apoptosis in renal proximal tubular epithelial cells. J Biochem 152:111–119
Sarkar A, Iwasa H, Hossain S, Xu X, Sawada T, Shimizu T, Maruyama J, Arimoto-Matsuzaki K, Hata Y (2017) Domain analysis of Ras-association domain family member 6 upon interaction with MDM2. FEBS Lett 591:260–272
Liang YY, Zheng LS, Wu YZ, Peng LX, Cao Y, Cao X, Xie P, Huang BJ, Qian CN (2014) RASSF6 promotes p21(Cip1/Waf1)-dependent cell cycle arrest and apoptosis through activation of the JNK/SAPK pathway in clear cell renal cell carcinoma. Cell Cycle 13:1440–1449
Polesello C, Huelsmann S, Brown NH, Tapon N (2006) The Drosophila RASSF homolog antagonizes the hippo pathway. Curr Biol 16:2459–2465
Ribeiro PS, Josué F, Wepf A, Wehr MC, Rinner O, Kelly G, Tapon N, Gstaiger M (2010) Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell 39:521–534
Parsons LM, Grzeschik NA, Richardson HE (2014) lgl regulates the hippo pathway independently of Fat/Dachs, Kibra/Expanded/Merlin and dRASSF/dSTRIPAK. Cancers (Basel) 6:879–896
Iwasa H, Kuroyanagi H, Maimaiti S, Ikeda M, Nakagawa K, Hata Y (2013) Characterization of RSF-1, the Caenorhabditis elegans homolog of the Ras-association domain family protein 1. Exp Cell Res 319:1–11
Takenaka M, Inoue H, Takeshima A, Kakura T, Hori T (2013) C. elegans Rassf homolog, rasf-1, is functionally associated with rab-39 Rab GTPase in oxidative stress response. Genes Cells 18:203–210
Rawat SJ, Araiza-Olivera D, Arias-Romero LE, Villamar-Cruz O, Prudnikova TY, Roder H, Chernoff J (2016) H-ras inhibits the hippo pathway by promoting Mst1/Mst2 heterodimerization. Curr Biol 26:1556–1563
Acknowledgements
S.H. is supported by the MEXT scholarship. This work was supported by research grants from Japan Society for the Promotion of Science (26460359, 26293061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Iwasa, H., Hossain, S. & Hata, Y. Tumor suppressor C-RASSF proteins. Cell. Mol. Life Sci. 75, 1773–1787 (2018). https://doi.org/10.1007/s00018-018-2756-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2756-5