Abstract
Death receptor-dependent apoptosis is an important mechanism of growth control. It has been demonstrated that Ras association domain family protein 1A (RASSF1A) is a tumor suppressor protein involved in death receptor-dependent apoptosis. However, it is unclear how RASSF1A-mediated cell death is initiated. We have now detailed 14-3-3 dependent regulation of RASSF1A-mediated cell death. We demonstrate that basal association of RASSF1A with 14-3-3 was lost following stimulation with tumor necrosis factor alpha (TNFα) or TNFα related apoptosis inducing ligand (TRAIL). Subsequent to the loss of 14-3-3 association, RASSF1A associated with modulator of apoptosis (MOAP-1) followed by death receptor association with either TNFα receptor 1 (TNF-R1) or TRAIL receptor 1 (TRAIL-R1). 14-3-3 association required basal phosphorylation by the serine/threonine kinase, glycogen synthase kinase 3β (GSK-3β), on serine 175, 178, and 179. Mutation of these critical serines resulted in the loss of 14-3-3 association and earlier recruitment of RASSF1A to MOAP-1, TNF-R1, and TRAIL-R1. Furthermore, stable cells containing a triple serine mutant of RASSF1A [serine (S) 175 to alanine (A) [S175A], S178A, and S179A] resulted in increased basal cell death, enhanced Annexin V staining and enhanced cleavage of poly (ADP-ribose) polymerase (PARP) following TNFα stimulation when compared to stable cells containing wild type RASSF1A. RASSF1A-mediated cell death is, therefore, tightly controlled by 14-3-3 association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 14-3-3 family of proteins are a highly conserved group of acidic proteins involved in many aspects of maintaining cellular homeostasis. Seven isoforms of 14-3-3 have been identified in mammals (β, ε, γ, η, σ, τ, and ζ) and almost every known organism expresses multiple 14-3-3 isoforms [1]. Proteins that interact with the 14-3-3 family of proteins are involved in cell cycle regulation, intracellular trafficking/targeting, signal transduction, cytoskeletal structure, cell death and transcription [2–5]. Interaction is often regulated by phosphorylation of the interacting protein and/or the 14-3-3 isoform itself [6]. However, a number of phosphoserine-independent associations have been documented illustrating the complexity of 14-3-3 associations [7].
Several lines of evidence indicate that endogenous 14-3-3 proteins associate to form heterodimers within other family members [6, 8]. This has been observed for all 14-3-3 proteins, except for 14-3-3σ which is thought to form only homodimers [1, 9]. Mammalian 14-3-3σ is expressed primarily in epithelial cells and appears to play a unique role in cellular response to DNA damage and oncogenesis in humans and is frequently epigenetically silenced by promoter specific methylation in human cancers [9]. 14-3-3σ is now believed to be a tumor suppressor gene that is inactivated as frequently as RASSF1A and death associated protein kinase (DAPK) [10, 11]. Recently, it has been demonstrated that 14-3-3σ behaves differently than the other 14-3-3 isoforms in how it recognizes its phosphorylated targets. 14-3-3σ association requires the primary recognition sequence on the target protein, RSXpSXP or RXXXpSXP (R, arginine; S, serine; pS is a phosophorylated serine that 14-3-3 docks to; X, any amino acid; P, proline) [9]. In addition to the phospho-peptide requirement on the target protein for 14-3-3 associations, a separate binding site on 14-3-3 is required that is derived from the 14-3-3σ primary sequence composed of methionine-202, aspartate-204 and histidine-206. These residues mediate 14-3-3σ selectivity for ligand binding interactions [9].
The RASSF gene family consists of 10 related genes having several chromosomal locations [12, 13]. Some RASSF family members have been shown to be downregulated in cancer (such as RASSF1A, RASSF2, RASSF4, and RASSF6) [14, 15] and one may be involved in modulating NFκB (RASSF6) [16]. The RASSF1 locus contains two major splice variants (A and C) that arise by multiple promoter usage and alternative splicing. The longer isoform, 1A, consists of 340 amino acids and a unique 119 amino acid amino (N)-terminal region, encoded by exon 1Aα. RASSF1A gene silencing results from methylation of the promoter for exon 1Aα (and thus loss of 1A expression) without epigenetic loss of the other isoforms of RASSF1. RASSF1A co-localizes with microtubules and several reports indicate a role for RASSF1A in mitosis linked to its localization on microtubules [17, 18]. RASSF1A has also been shown to influence the anaphase promoting complex (APC) to promote the stabilization of the mitotic cyclins, A and B in HeLa cells [18], but not in 293T cells, a function that may involve modulation of βTrCP containing Skp1-Culin-Roc1/Rbx1/Hrt-1-F-box (SCF) ubiquitin E3 ligase activity (SCFβTrCP) [19]. Presumably, RASSF1A may carry out this function by localization to microtubules during interphase and to centrosomes and spindle body during mitosis [17]. All RASSF proteins contain a Ras binding domain, but direct association with K-Ras has been only observed for RASSF2, RASSF4, and RASSF5/Nore1 (novel Ras effector 1A) [13, 20]. However, this does not exclude the possibility the RASSF1A (or 1C) can associate with other yet to identified GTPase.
We and others have recently defined some of the molecular mechanisms of apoptotic regulation by RASSF1A. RASSF1A has been recently demonstrated to modulate the activation of the mammalian sterile-20 kinase 2 (MST2) pathway by controlling p73 transcriptional activity linked to PUMA expression (a key pro-apoptotic effector) [21]. It can associate with the pro-apoptotic kinase, mammalian sterile-20 kinase 1 (MST1), and function with K-Ras to promote apoptosis [22, 23]. We have demonstrated the importance of the RASSF1A/MOAP-1 pathway in activating death receptor-evoked apoptosis [mainly through TNF-R1 and TRAIL but not CD95, ref 24] resulting in Bax conformational change, cytochrome c release, enhanced poly-ADP ribose polymerase (PARP) cleavage and cell death. Furthermore, we have recently demonstrated the dynamics of RASSF1A self-association and associations with death receptors and MOAP-1 following TNFα-stimulation and specific residues important for these associations [24, 25].
In this study, we have begun to elucidate how the apoptotic function of RASSF1A is initiated. We now demonstrate basal association of RASSF1A with 14-3-3σ that is rapidly lost following death receptor stimulation. Furthermore, we have identified S175, 178, and 179 as potential residues phosphorylated by GSK-3β allowing for 14-3-3 docking on RASSF1A. Our results demonstrate a novel mechanism of basal inhibition of RASSF1A-dependent cell death by the 14-3-3 family of proteins.
Materials and methods
Apoptosis assays
Human TNFα or TRAIL were added together with 10 μg/ml cycloheximide (CHX) for the indicated times and Annexin V staining analysis was carried out as previously described [24]. All apoptosis assays were performed at least six times. Data for all immunofluorescence and apoptosis assays were evaluated by Student’s t-test (two-tailed), unless otherwise stated.
GSK-3β in vitro kinase assay
Cells were lysed in 1 ml of GSK-3β lysis buffer containing 40 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100. Cell lysates were immunoprecipitated with 1.5 μg of anti-GSK-3β antibody overnight at 4°C, followed by Protein A Sepharose complexing for 1 h at 4°C. Immunoprecipitated samples were spun down, washed once with GSK-3β lysis buffer and twice with GSK-3β kinase buffer containing 25 mM Tris–HCl, pH 7.5, 10 mM MgCl2, 5 mM DTT. After the last wash, 40 μM ATP, 10 μCi 32-P-γ-ATP and 25 pmol of GST-RASSF1A was added in a total volume of 50 μl. In vitro kinase reaction was allowed to proceed for 30 min at 30°C and stopped by the addition of protein loading dye. The samples were boiled for 10 min and resolved using 10% SDS–PAGE. The gel was transferred to PVDF (polyvinylidene fluoride) blotting paper and autoradiographed overnight and X-ray film was developed using a Kodak X-OMAT 1000A developing system.
Other methods are available as “Supplemental Information”. All experiments were carried out at least three times.
Results
14-3-3 associated with RASSF1A
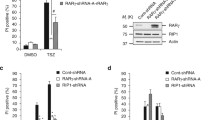
RASSF1A is a tumor suppressor gene involved in cell death utilizing TNF-R1 following TNFα stimulation. It is currently unclear how RASSF1A mediated-cell death is initiated. The present study investigated the potential association of RASSF family members with the 14-3-3 family of proteins. We first demonstrated endogenous association of RASSF1A with 14-3-3 using antibodies specific to RASSF1A (Fig. 1a). We observed basal association with 14-3-3 and a TNFα dependent release by 3 h following TNFα addition suggesting that 14-3-3 association may modulate RASSF1A-intitated cell death driven by TNFα. Furthermore, transient overexpression of HA-tagged RASSF1A (accession number AF102770), HA-tagged RASSF1C (accession number AF040703) and HA-RASSF7 (accession number NM_003475) in the human osteosarcoma cell line, U2OS, illustrated that RASSF1A specifically associated with 14-3-3 (Supplemental Fig. S1a). Similarly, using GST-tagged expression constructs, RASSF1A associated basally with 14-3-3 whereas RASSF1C and RASSF5A/novel Ras effector (Nore1A) (accession number NM_182663) associated weakly or not at all (Supplemental Fig. S1b). In addition, RASSF5B/Nore1B (also known as RAPL, regulator of adhesion and polarization enriched in lymphocytes, accession number NM_182665) and RASSF6B (accession number NM_201431) also demonstrated weak to no association with 14-3-3 (data not shown).
14-3-3 modulates RASSF1A-dependent association with death receptors. a U2OS cells were stimulated as indicated and endogenous proteins associated with RASSF1A was recovered by immunoprecipitation (IP) with an anti-RASSF1A antibody (M304 from Dr. Gerd Pfeifer) followed by immunoblotting (IB) with the indicated antibodies. b Glutathione S-transferase (GST) tagged RASSF1A and hematagluttin (HA) tagged 14-3-3 isoforms were ectopically expressed in COS-1 cells. Associated GST-RASSF1A was recovered by immunoprecipitation with an anti-HA antibody followed by immunoblotting with the indicated antibodies. c HA tagged RASSF1A wild type (WT) or serine (S) to alanine (A) mutants of RASSF1A were ectopically expressed in U2OS cells and associated HA-RASSF1A was recovered by immunoprecipitation with an anti-14-3-3 antibody followed by immunoblotting with the indicated antibodies. The protein sequence of the potential 14-3-3 binding site is shown below Fig. 1d and the 3S to A mutant is a S175, 178, and 179A mutant. d HA tagged RASSF1A was ectopically expressed in U2OS cells. Forty-eight hours transfection, cells were stimulated with TNFα or TRAIL and associated HA-RASSF1A was recovered by immunoprecipitation with a rabbit anti-14-3-3 antibody followed by immunoblotting with the indicated antibodies. Rabbit anti-14-3-3 (sc-629) is a pan specific antibody that recognizes isoforms α, β, ε, ζ, γ, and σ. WCL, whole cell lysate
Subsequent studies examined the 14-3-3 isoform specificity for association with RASSF1A. Transient overexpression of GST-tagged RASSF1A and HA- tagged 14-3-3 in the monkey kidney cell line, COS-1, resulted in specific and robust association of RASSF1A with 14-3-3σ and, to a lesser extent, 14-3-3ε (Fig. 1b). 14-3-3 binding sites are often regulated by phosphorylation of the target site containing the recognition sequence, RSXpSXP or RXXXpSXP. RASSF1A has the sequence, 171VRPVSVPSSKKPPSL, with the underlined serine residues as potential sites for phosphorylation by a yet to be identified protein kinase. We hypothesized that this sequence may also be a potential docking site for 14-3-3 on RASSF1A. We proceeded to test our hypothesis by utilizing S175A, S178A, or S179A single mutants or a S175A/S178A/S179A triple mutant (3S → A) to explore 14-3-3 associations and RASSF1A self associations, two conditions that exist in the unstimulated state [25]. Interestingly, mutations to either the single serine mutants or the triple serine mutant resulted in the loss of basal associations with endogenous 14-3-3 (Fig. 1c) and a significant loss of RASSF1A self-association (Supplemental Fig. S2). These data would suggest that all three serine residues may be important for 14-3-3 basal association. This association may function to restrict RASSF1A-mediated cell death by maintaining RASSF1A self association.
Similar to the loss of endogenous 14-3-3 association with RASSF1A following TNFα stimulation, overexpressed HA-RASSF1A lost its association with 14-3-3 by 3 h of stimulation with either TNFα or TRAIL in U2OS cells (Fig. 1d). Previously, we have demonstrated association of RASSF1A with TNF-R1 or TRAIL-R1 by 3 h or death receptor stimulation [24, 25]. These data suggest that 14-3-3σ or 14-3-3ε basal associations with RASSF1A may function to inhibit RASSF1A mediated cell death. Death receptor stimulation may thus function to promote the loss of 14-3-3 association with RASSF1A.
Loss of 14-3-3/RASSF1A association resulted in earlier kinetics of TNF-R1, TRAIL-R1 and MOAP-1 complex formation
14-3-3 appears to be an important element as a basal inhibitor to RASSF1A. Our data supported an importance of S175, S178, and S179 for 14-3-3 docking to RASSF1A (Fig. 1c). We next proceeded to determine the functional importance of S175, S178, and S179 by carrying out co-immunoprecipitation analysis of the serine to alanine mutants of RASSF1A, the 3S → A triple mutant or the S175A, S178A, or S179A single mutants with death receptors and MOAP-1. Wild type RASSF1A associated with TNF-R1 and TRAIL-R1 by 3 h following cognate receptor activation (Fig. 2a, b, first panel). Not surprisingly, the 3S → A mutant of RASSF1A failed to associate with 14-3-3 and complexed with TNF-R1 (Fig. 2a) and TRAIL-R1 (Fig. 2b) much earlier than 3 h following cognate receptor activation. Similarly, the S175A mutant of RASSF1A failed to associate with 14-3-3 (Fig. 1c) and also resulted in an earlier recruitment to death receptors upon ligation with either TNFα (Fig. 2a, third panel) or TRAIL (Fig. 2b, third panel). In addition, we observed loss of 14-3-3 association with the S178A and S179A mutants of RASSF1A (Fig. 1c) and robust basal associations with TNF-R1 and TRAIL-R1 (Fig. 2a, b, fourth and fifth panel, respectively). These data would suggest that the serine mutants of RASSF1A can no longer associate with 14-3-3 and these mutants have gained the ability to complex basally or earlier with death receptors following cognate receptor activation.
14-3-3 binding site mutants of RASSF1A promoted earlier associations with death receptors and MOAP-1. a, b HA-RASSF1A WT or serine to alanine mutants were ectopically expressed in U2OS cells and associated HA-RASSF1A WT or serine to alanine mutants were recovered by immunoprecipitation with antibodies to TNF-R1 (a) or TRAIL-R1/DR4 (b) following TNFα (A) or TRAIL (b) stimulation. Immunoprecipitated proteins were immunoblotted with the indicated antibodies. c HA tagged RASSF1A wild type (WT) or serine to alanine mutants of RASSF1A were ectopically expressed with Myc-tagged MOAP-1 in U2OS cells and associated Myc-MOAP-1 was recovered by immunoprecipitation with an anti-HA antibody followed by immunoblotting with the indicated antibodies
We next proceeded to determine how wild type or the serine mutants of RASSF1A can associate with MOAP-1, a key RASSF1A-dependent downstream activator of Bax (Fig. 2c) [24–26]. Wild type RASSF1A associated with MOAP-1 upon TNFα stimulation with robust RASSF1A/MOAP-1 complex formation at 3 h. Surprisingly, we observed constitutive association of the serine mutants to MOAP-1 suggesting that the loss of 14-3-3 allows for uninhibited associations with its downstream target, MOAP-1.
Loss of 14-3-3/RASSF1A association resulted in enhanced cell death
Loss of 14-3-3 binding resulted in a constitutive association with MOAP-1 and earlier recruitment of RASSF1A to death receptors. Previously we demonstrated the requirement of MOAP-1 for RASSF1A-mediated cell death in H1299 cells, a non-small cell lung cancer cell line missing detectable endogenous RASSF1A and MOAP-1 [24]. Stable H1299 cells were generated containing Myc-MOAP-1 (H1299M) as previously described [24]. GFP-RASSF1A wild type or GFP-RASSF1A 3S → A were transiently transfected into H1299M cells and cell death was determined by Annexin V staining (Fig. 3a, b) and by the cleavage of PARP (Fig. 3c). Overexpression of GFP-RASSF1A 3S → A resulted in a significant increase in basal apoptosis in H1299M cells when compared to wild type cells and a significant difference between GFP-RASSF1A WT and GFP-RASSF1A 3S → A cells following TNFα stimulation (Fig. 3a, lower and upper right quadrants). In support of these data, PARP cleavage occurs by 7 h of stimulation with TNFα/cyclohexamide (CHX) (Fig. 3c) in both wild type cells (Fig. 3c, 1A WT) and control cells (containing empty vector). However, significant PARP cleavage can be observed by 3 h in cells containing HA-RASSF1A 3S → A mutant, a mutant that had lost the ability to associate with 14-3-3 but can be recruited earlier to death receptors. These data would suggest that the loss of 14-3-3 association with RASSF1A has a profound effect of molecular associations with death receptors and MOAP-1, and, more importantly, can result in enhanced death receptor dependent cell death following TNFα/cyclohexamide treatment.
14-3-3 binding site mutants of RASSF1A function to promote RASSF1A-mediated cell death. a, b H1299 stable cells containing Myc-MOAP-1 (H1299M) were transiently transfected with green fluorescent protein (GFP) tagged RASSF1A WT and the 3S → A mutant of RASSF1A. Annexin V/Propidium iodide staining of TNFα/cyclohexamide (CHX) treated H1299M cells was carried out for 16 h. A representative result in shown in (a) and a compiled result in (b). Right panel to Fig. 3a: expression of proteins used in Annexin V staining. Experiment was repeated six times and significance was evaluated by Student’s t-test (two-tailed) with the indicated P value (*) = 0.0002. CHX is a protein synthesis inhibitor that was used to inhibit survival signals and allow cell death to be observed in highly transformed cells such as the cancer cells used in this study. c HA-RASSF1A WT (1A WT) or the 3S → A mutant of RASSF1A were ectopically expressed in U2OS cells and associated TNFα/CHX-evoked PARP cleavage (see IB: Anti-PARP) was detected using an anti-PARP antibody in order to detect full length PARP (p116PARP) and cleaved PARP (p85 PARP). Also indicated are expression levels for RASSF1A expression and anti-Erk1/2 immunoblotting as loading controls
RASSF1A may be constitutively phosphorylated by GSK-3β that allows for basal 14-3-3 association
Our results so far demonstrate the importance of 14-3-3 association in modulating RASSF1A-mediated cell death. The 14-3-3 family of proteins predominantly associate with target proteins via phoshorylated residues [9, 11, 27]. Several kinases have been described that can phosphorylate RASSF1A on S197 and S203 [28, 29], but no reports have described a kinase for S175, S178, or S179 (the potential 14-3-3 binding site on RASSF1A). Motif aligorithms, such as Scansite, have predicted a potential 14-3-3 binding site for the sequence, 171VRPVSVPSSKKPPSL, and a potential kinase for this target sequence as glycogen synthase kinase (GSK) 3β. Furthermore, another putative 14-3-3 docking site was predicted, which is downstream of the GSK-3β at position S203, for the kinase protein kinase B (PKB)/Akt). If GSK-3β basally phosphorylated RASSF1A on S175, S178, and/or S179 (and thus facilitated 14-3-3 association), then inhibition of GSK-3β activity should result in the loss of S175, S178, and/or S179 phosphorylation and subsequent loss of 14-3-3 association with RASSF1A. A similar outcome may result if PKB/Akt governed the basal phosphorylation state of RASSF1A and subsequent association with 14-3-3. We proceeded to test both of these hypotheses.
In order to inhibit GSK-3β activity we utilized 20 mM lithium chloride (LiCl). LiCl has been demonstrated to inhibit GSK-3β activity in numerous cell lines [28–30]. However, it should be noted that LiCl can also interfere with the other biological pathways, such as the recycling of inositol in the phosphatidylinositol cycle [31, 32]. We do not think that this will affect the outcome of our analysis as we are mainly investigating membrane proximal events involving the TNF-R1 signaling, events upstream of phosphatidylinositol signaling. The initiation of the RASSF1A cell death pathway requires TNF-R1 stimulation and recruitment of MOAP-1 and RASSF1A to death receptors [25], events that do not require phosphatidylinositols or calcium for their initiation. As such, even though LiCl can inhibit the phosphatidylinositol cycle, we do not think it will affect the outcome of the use of LiCl to inhibit GSK-3β activity. Following treatment with LiCl in order to inhibit GSK-3β demonstrated, we observed a loss of basal RASSF1A association with 14-3-3 (Fig. 4a, + LiCl). However, treatment with a PKB/Akt inhibitor did not affect interaction of 14-3-3 and RASSF1A (Fig. 4a, + LY294002). Furthermore, mutation of the potential Akt phosphorylation site serine 203 to an alanine residue (S203A) of RASSF1A did not affect its basal association with 14-3-3 (Fig. 4b) nor did it affect the temporal kinetics of RASSF1A recruitment to TNF-R1 following ligation of the receptor (Fig. 4c). However, mutation at S175, S178, and/or S179 resulted in the loss of 14-3-3 association with RASSF1A, suggesting the importance of GSK-3β phosphorylation at S175, S178, and/or S179. These studies suggest that PKB/Akt phosphorylation may not influence RASSF1A signaling, but the potential GSK-3β phosphorylation site can and may be required for maintaining 14-3-3 association and initiation of RASSF1A-mediated cell death.
GSK-3β mediates the association of 14-3-3 with RASSF1A. a HA-RASSF1A was ectopically expressed in U2OS cells followed by overnight treatment with 20 mM LiCl or 20 μM LY294002 for 16 h. Associated HA-RASSF1A was recovered by RIPA buffer lysis of cells, followed by immunoprecipitation with rabbit anti-14-3-3 and immunobloted with the indicated antibodies. b, c HA-tagged mutants of RASSF1A were ectopically expressed in U2OS cells and associated HA-RASSF1A WT or serine to alanine mutants of RASSF1A were recovered by immunoprecipitation with antibodies to (b) 14-3-3 or (c) TNF-R1 and immunobloted against the indicated antibodies
Confirming an importance of basal GSK-3β phosphorylation, inhibition of GSK-3β activity with LiCl treatment resulted in a significant loss of 14-3-3 association with RASSF1A (Fig. 5a) and an earlier, robust recruitment of RASSF1A to TNF-R1 (Fig. 5b). Under LiCl treatment, expression levels of GSK-3β remained unchanged (Fig. 5b, bottom panel). Furthermore, we can observe a detectable association of RASSF1A with GSK-3β under basal conditions that is significantly lost upon TNFα stimulation (Fig. 5c). In agreement with Fig. 4b, RASSF1A 3S → A significantly lost its ability to associate with GSK-3β suggesting that prior phosphorylation of these residues was important in how RASSF1A associates with GSK-3β. Lastly, in order to directly investigate GSK-3β phosphorylation of RASSF1A, we carried out an in vitro kinase assay (IVK) using purified GST-RASSF1A as a substrate. GST-RASSF1A can be phosphorylated by GSK-3β, but this activity is lost upon GSK-3β inhibition with LiCl (Fig. 5d, lanes 2 and 3). Additionally, inhibition of PKB/Akt by LY294002 enhanced the ability of GSK-3β to phosphorylate GST-RASSF1A (Fig. 5d, lane 4). PKB/Akt has been demonstrated to negatively regulate GSK-3β activity via phosphorylation of serine-9 located at its N-terminus [30, 31]. As such, inhibition of PKB/Akt may lead to enhanced GSK-3β activity and thus enhanced phosphorylation of RASSF1A. These data suggest that GSK-3β can associate and phosphorylate RASSF1A and that the 14-3-3 docking site is also a GSK-3β phosphorylation site. GSK-3β may be responsible for 14-3-3 basal inhibition of RASSF1A in order to regulate RASSF1A-mediated cell death.
Basal GSK-3β phosphorylation of RASSF1A is important for 14-3-3 association with RASSF1A. a, b HA-RASSF1A was ectopically expressed in U2OS cells followed by overnight treatment with 20 mM LiCl. Associated HA-RASSF1A was recovered by RIPA lysis of cells, followed by immunoprecipitation (IP) with (a) 14-3-3 or (b) TNF-R1 and immunoblotting with the indicated antibodies. c HA tagged wild type or 3S → A mutant of RASSF1A were ectopically expressed in U2OS cells. Associated RASSF1A proteins were recovered by immunoprecipitation with an anti-GSK-3β antibody followed by immunoblotting with the indicated antibodies; d Top panel, GSK-3β in vitro kinase (IVK) assay was carried out by immunoprecipation with an anti-GSK-3β antibody, followed by protein A Sepharose complexing for 1 h. To the complex, purified GST-FKBP12 (lane 1) or GST-RASSF1A (lanes 2–4) was added and the IVK carried out as described in the “Materials and methods”. Additionally, cells were pre-treated with either 20 mM LiCl (to inhibit GSK-3β) (lane 3) or 20 μM LY294002 (to inhibit Akt/PKB) (lane 4) for 16 h prior to lysis. Bottom panel, coomassie blue gel of proteins used in the IVK assay in the top panel. GST-FKBP12 was used as control protein for GST-RASSF1A
Discussion
RASSF1A is a tumor suppressor protein that is a pro-apoptotic modulator of death receptor signaling. In the present study, we have illustrated the fundamental role of 14-3-3 family of proteins in the modulation of RASSF1A-mediated cell death. We can detect robust basal association of endogenous and overexpressed RASSF1A with 14-3-3 that is not observed for other RASSF isoforms investigated in this study (RASSF1C, RASSF7, and RASSF5A and 5B). The 14-3-3 binding site on RASSF1A (169KLVRPV175 SVPSSKKPPS184) is 100% conserved in RASSF1C, while 70% conserved in RASSF5A/Nore1A and RASSF5B/Nore1B (236KLRRPV249 TVPAGIRPQS258, sequence refers to RASSF5A/Nore1A). Curiously, RASSF5A/Nore1A and RASSF5B/Nore1B have a threonine residue at a similar position to S175 of RASSF1A and a conserved serine residue at position 184 of the RASSF1A sequence (S258 in RASSF5A/Nore1A). Robust associations with RASSF1A and weak associations with other RASSF family members would suggest that a secondary binding site either on 14-3-3 or RASSF1A may regulate specificity with RASSF1A in a similar way to that has been observed for 14-3-3σ interactions [9]. The basal association of 14-3-3 with RASSF1A appears to be regulated by the phosphorylation of putative serine residues mapped on positions 175, 178 and 179 on RASSF1A. The aforementioned serine residues are part of the 14-3-3 docking site that is constitutively phosphorylated by a kinase important to the canonical Wnt signaling pathway, the multifunctional serine/threonine kinase, GSK-3β. Upon TNFα stimulation, association with 14-3-3 is lost (Fig. 1), followed by the loss of RASSF1A self-association (Supplemental Fig. S2) and earlier complex formation with the BH3-like pro-apoptotic activator of Bax, MOAP-1 (Fig. 2c) and death receptors (Fig. 2a, b) and the promotion of cell death (Fig. 3) as depicted in Fig. 6. It is possible that after release of MOAP-1, RASSF1A may reassociate with 14-3-3 to remain inhibited again.
Model for 14-3-3 regulation of RASSF1A-mediated cell death. GSK-3β phosphorylates RASSF1A on S175, 178, and 179 in order to promote 14-3-3 association. TNFα stimulation results in the simultaneous loss of 14-3-3 association and RASSF1A self-association, followed by membrane recruitment of MOAP-1 to receptor complexes, receptor internalization, and RASSF1A association with MOAP-1/TNF-R1. These events result in the promotion of the “open” conformation of MOAP-1 and subsequent activation of Bax resulting in apoptosis. See text for details
The 14-3-3 family of proteins are molecular scaffolds that function to restrict the localization, stability, phosphorylation state, activity and/or molecular interactions of their target proteins mainly via sequestration within cellular compartments [33]. 14-3-3 family members are found in plants and mammals with over 200 target proteins identified to date. The targets of 14-3-3 are diverse and include transcription factors, enzymes, cytoskeletal proteins, signaling molecules, apoptosis factors, and tumor suppressor proteins. 14-3-3 has been shown to associate with and regulate a number of BH-containing apoptotic proteins; including BAD [3, 34], Bax [35, 36] and apoptosis signal regulated kinase (ASK) 1 [37, 38]. Under basal conditions, ASK1 has been reported to form a complex with 14-3-3. Following TNFα stimulation, 14-3-3 association with ASK1 is lost allowing ASK1 to mediate cell death. Similarly, 14-3-3 is thought to interact with BAD preventing its translocation to the mitochondrial interface and prohibiting the release of Bax to undergo apoptosis. It is not surprising that RASSF1A, a key death receptor pro-apoptotic protein, can also be modulated by 14-3-3σ. Similar to BAD and ASK1, once released from 14-3-3 associations, RASSF1A can mediate Bax conformational change, cytochrome c release and ultimately cell death. Thus, it appears that 14-3-3 acts as a negative regulator for pro-apoptotic molecules.
Under basal conditions, RASSF1A is inhibited by complex formation with 14-3-3 that is potentially mediated by GSK-3β phosphorylation of serines 175, 178 and 179 on RASSF1A. Pre-treatment of U2OS cells with lithium chloride, a selective and potent GSK-3β inhibitor [39], hindered the association of 14-3-3 with RASSF1A, promoted constitutive MOAP-1/RASSF1A interaction and earlier recruitment with death receptors. In support of the present data it has been reported that pharmacological inhibition of GSK-3β kinase activity can also promote glioma cell death via c-Myc activation and subsequent induction of Bax conformational change [40]. In addition, a number of observations have suggested that the RASSF1 signaling pathway may intersect with the Wnt signaling pathway. Recent studies have demonstrated that both RASSF1A and 1C may directly modulate the E3-ligase, SCFβTRCP, resulting in increased β-catenin expression [19, 41]. RASSF1A was also demonstrated to inhibit SCFβTRCP to promote increased activity of anaphase promoting complex (APC) and subsequent levels of cyclin A and cyclin B [41]. β-catenin activity is predominantly inhibited by GSK-3β kinase via sequential phosphorylation events and SCFβTRCP ultimately triggers β-catenin degradation. In the present study, we demonstrate that death receptor stimulation may function to inhibit GSK-3β and allow for loss of 14-3-3 association (Fig. 5), RASSF1A self-association [25], and earlier recruitment to MOAP-1 and death receptors (Fig. 2). All of these events would result in increased PARP cleavage (Fig. 3) and cell death (Fig. 6).
The association with 14-3-3 requires the phosphorylation of specific serine residue(s) within the sequence 171VRPVSVPSSKKPPS of RASSF1A. Phosphorylation of RASSF1A has been investigated over the past several years and a number of potential phosphorylation sites have been identified for protein kinase C, protein kinase A, PKB/Akt, calmodulin kinase II, death associated protein kinase. However, the importance of all these sites are currently unknown. Recent data has demonstrated a potential importance of aurora kinase mediating threonine-202 and serine-203 phosphorylation of RASSF1A that may be involved in microtubule association and cell cycle progression [42–44]. Protein kinase C (PKC) phosphorylation at serine-197 and serine-203 appears to be fundamental for RASSF1A microtubule organization [27]. Interestingly, the S-phase kinase-associated protein 2 (Skp2) was demonstrated to promote the degradation of RASSF1A during the G1-S phase of the cell cycle. However, in order for this to occur cyclin dependent kinase 4 (cdk4) was required to phosphorylate serine-203 on RASSF1A mediating its interaction with a Skp2 complex to promote its ubiquitination and degradation [42]. Thus, it appears that cdk4 may be important to the function of RASSF1A as a regulator of cell cycle progression.
It is yet undefined as to how and whether phosphorylation of the serine residues by GSK-3β requires prior priming by an initiator kinase. A number of publications have reported that prior phosphorylation at specific sites is fundamental to initiate and/or enhance GSK-3β kinase activity. It was reported that serine-45 of β-catenin is initially phosphorylated by casein kinase I ε in order to promote the subsequent phosphorylation of serines 33 and 37 by GSK-3β [45]. Consequently, mutation of serine-45 inhibited GSK-3β phosphorylation at serines 33 and 37 and disrupted Wnt signaling [45]. Further studies illustrated the synergism of casein kinases (CK) and GSK-3β in the regulation of tumor suppressor phosphatase and tensin homologue (PTEN) function via initial phosphorylation of serine-370 by CK2 followed by phosphorylation at threonine-366 by GSK-3β at the C-terminus of PTEN [43]. Therefore, it is possible that prior to GSK-3β phosphorylation of RASSF1A at serine-175, an unknown initiator kinase is required to regulate the phosphorylation status of priming sites, such as that of serines-178 and 179, which would mediate GSK-3β activity and thus in turn modulate 14-3-3 association with RASSF1A. The investigation for kinases targeting RASSF1A are still in its infancy and future detailed analyses are required to address how GSK3-β phosphorylation of RASSF1A is initiated, maintained, and lost.
In this study, we have observed robust associations with GSK-β that is lost upon TNFα addition. Previously, we have demonstrated that death receptor stimulation resulted in the loss of RASSF1A self [25]. We speculate that death receptor stimulation may directly or indirectly inhibit GSK-β activity resulting in the loss of RASSF1A phosphorylation on S175, S178 or S179, loss of association with 14-3-3 and loss of RASSF1A self association. Following these events, RASSF1A is now free to associate with MOAP-1 and death receptors (such as TNF-R1) to promote apoptosis. This model cannot be tested until it is known how RASSF1A phosphorylation is regulated and maintained (an analysis that is beyond the scope of this study). Here, we report a novel role for 14-3-3 in the inhibition of RASSF1A-mediated cell death and the importance of a GSK-3β-dependent phosphorylation event on RASSF1A in order to restrict RASSF1A-dependent apoptosis. The findings reported in the present study and in our previous studies highlight the importance of the RASSF1A/MOAP-1 pathway to death receptor signaling and to the prevention of carcinogenesis.
References
Aitken A (2006) 14-3-3 Proteins: a historic overview. Semin Cancer Biol 16:162–172
Berg D, Holzmann C, Riess O (2003) 14-3-3 proteins in the nervous system. Nat Rev Neurosci 4:752–762
Rosenquist M (2003) 14-3-3 proteins in apoptosis. Braz J Med Biol Res 36:403–408
Tzivion G, Shen YH, Zhu J (2001) 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 20:6331–6338
Wilker EW, van Vugt MA, Artim SA et al (2007) 14-3-3 sigma controls mitotic translation to facilitate cytokinesis. Nature 446:329–332
Gardino AK, Smerdon SJ, Yaffe MB (2006) Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: a comparison of the X-ray crystal structures of all human 14-3-3 isoforms. Semin Cancer Biol 16:173–182
Borch J, Bych K, Roepstorff P, Palmgren MG, Fuglsang AT (2002) Phosphorylation-independent interaction between 14-3-3 protein and the plant plasma membrane H+-ATPase. Biochem Soc Trans 30:411–415
Aitken A, Baxter H, Dubois T et al (2002) Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem Soc Trans 30:351–360
Wilker EW, Grant RA, Artim SC, Yaffe MB (2005) A structural basis for 14-3-3 sigma functional specificity. J Biol Chem 280:18891–18898
Lal G, Padmanabha L, Provenzano M, Fitzgerald M, Weydert J, Domann FE (2008) Regulation of 14-3-3 sigma expression in human thyroid carcinoma is epigenetically regulated by aberrant cytosine methylation. Cancer Lett 267:165–174
Suzuki H, Itoh F, Toyota M, Kikuchi T, Kakiuchi H, Imai K (2000) Inactivation of the 14-3-3 sigma gene is associated with 5′ CpG island hypermethylation in human cancers. Cancer Res 60:4353–4357
Richter AM, Pfeifer GP, Dammann RH (2009) The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796:114–128
Avruch J, Xavier R, Bardeesy N et al (2009) Rassf family of tumor suppressor polypeptides. J Biol Chem 284:11001–11005
Dammann R, Schagdarsurengin U, Seidel C et al (2005) The tumor suppressor RASSF1A in human carcinogenesis: an update. Histol Histopathol 20:645–663
van der Weyden L, Adams DJ (2007) The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta 1776:58–85
Allen NP, Donninger H, Vos MD et al (2007) RASSF6 is a novel member of the RASSF family of tumor suppressors. Oncogene 26:6203–6211
Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS (2005) The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem 280:3920–3927
Song MS, Song SJ, Ayad NG et al (2004) The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol 6:129–137
Estrabaud E, Lassot I, Blot G et al (2007) RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of {beta}-catenin by interacting with {beta}TrCP. Cancer Res 67:1054–1061
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor BJ, Clark GJ (2003) RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J Biol Chem 278:28045–28051
Matallanas D, Romano D, Yee K et al (2007) RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27:962–975
Oh HJ, Lee KK, Song SJ et al (2006) Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res 66:2562–2569
Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J (2004) Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J 381:453–462
Baksh S, Tommasi S, Fenton S et al (2005) The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol Cell 18:637–650
Foley CJ, Freedman H, Choo SL et al (2008) Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol Cell Biol 28:4520–4535
Tan KO, Fu NY, Sukumaran SK et al (2005) MAP-1 is a mitochondrial effector of Bax. Proc Natl Acad Sci U S A 102:14623–14628
Verma SK, Ganesan TS, Parker PJ (2008) The tumour suppressor RASSF1A is a novel substrate of PKC. FEBS Lett 582:2270–2276
Feyt C, Kienlen-Campard P, Leroy K et al (2005) Lithium chloride increases the production of amyloid-beta peptide independently from its inhibition of glycogen synthase kinase 3. J Biol Chem 280:33220–33227
Korur S, Huber RM, Sivasankaran B et al (2009) GSK3beta regulates differentiation and growth arrest in glioblastoma. PloS one 4:e7443
Al-Assar O, Crouch DH (2005) Inactivation of MAP kinase signalling in Myc transformed cells and rescue by LiCl inhibition of GSK3. Molecular cancer 4:13
Laurenz JC, Smith SB (1998) Lithium chloride does not inhibit the proliferation of L6 myoblasts by decreasing intracellular free inositol. J Anim Sci 76:66–73
Tritsaris K, Gromada J, Jorgensen TD, Nauntofte B, Dissing S (2001) Reduction in the rate of inositol 1,4,5-trisphosphate synthesis in rat parotid acini by lithium. Arch Oral Biol 46:365–373
Dougherty MK, Morrison DK (2004) Unlocking the code of 14-3-3. J Cell Sci 117:1875–1884
Adachi M, Zhang YB, Imai K (2003) Mutation of BAD within the BH3 domain impairs its phosphorylation-mediated regulation. FEBS Lett 551:147–152
Nomura M, Shimizu S, Sugiyama T et al (2003) 14-3-3 Interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278:2058–2065
Samuel T, Weber HO, Rauch P et al (2001) The G2/M regulator 14-3-3sigma prevents apoptosis through sequestration of Bax. J Biol Chem 276:45201–45206
Liu Y, Yin G, Surapisitchat J, Berk BC, Min W (2001) Laminar flow inhibits TNF-induced ASK1 activation by preventing dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest 107:917–923
Zhang H, Zhang H, Lin Y, Li J, Pober JS, Min W (2007) RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem 282:14788–14796
Klein PS, Melton DA (1996) A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 93:8455–8459
Chin PC, Majdzadeh N, D’Mello SR (2005) Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res Mol Brain Res 137:193–201
Whitehurst AW, Ram R, Shivakumar L, Gao B, Minna JD, White MA (2008) The RASSF1A tumor suppressor restrains anaphase-promoting complex/cyclosome activity during the G1/S phase transition to promote cell cycle progression in human epithelial cells. Mol Cell Biol 28:3190–3197
Song MS, Song SJ, Kim SJ, Nakayama K, Nakayama KI, Lim DS (2008) Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition. Oncogene 27:3176–3185
Rong R, Jiang LY, Sheikh MS, Huang Y (2007) Mitotic kinase Aurora-A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene 26:7700–7708
Liu L, Guo C, Dammann R, Tommasi S, Pfeifer GP (2008) RASSF1A interacts with and activates the mitotic kinase Aurora-A. Oncogene 27:6175–6186
Sakanaka C (2002) Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J Biochem 132:697–703
Acknowledgments
We would like to thank Christina Onyskiw for her excellent technical assistance with our experiments. We would like to thank Dr. Victor Yu and NaiYang Fu for the MOAP-1 expression construct; Dr. Gerd Pfeifer for the GST expression constructs for RASSF1A, 1C, and RASSF5; Dr. Yutaka Hata for human Myc-tagged RASSF6B; Dr. Andrew Chalmers for human HA-tagged RASSF7; and Dr. Michael Yaffe for the HA-14-3-3 expression constructs. Grant support for this study was provided by the University of Alberta, Faculty of Medicine and Department of Pediatrics (N031000425) (SB); by the Women and Children’s Health Research Institute (WCO14) (SB, HA-G, JDO), Canadian Institutes of Health Research (MOP-79494) (CO), Alberta Heritage Foundation for Medical Research (G220170170) (SB), and Canadian Foundation for Innovation/Alberta Small Equipment Grants Program (13118); and by the Office of the Provost and VP Academic Award (SLC, RCC, DP and RXW).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2009_451_MOESM2_ESM.pdf
Supplemental Fig. S1 Specificity of RASSF1A/14-3-3 associations. a and b, HA (a) or GST (b) tagged RASSF proteins were ectopically expressed in U2OS cells. Associated RASSF isoforms were recovered by 14-3-3 immunoprecipitation (IP) followed by immunoblotting (IB) with the indicated antibodies. (PDF 119 kb)
10495_2009_451_MOESM3_ESM.pdf
Supplemental Fig. S2 Loss of RASSF1A self association with 14-3-3 binding site mutants. Green fluorescent protein (GFP) tagged RASSF1A and HA tagged RASSF1A WT or serine mutants were ectopically expressed in U2OS cells. Associated GFP-RASSF1A was recovered by immunoprecipitation with an anti-HA antibody followed by immunoblotting with the indicated antibodies. (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Ghazaleh, H.A., Chow, R.S., Choo, S.L. et al. 14-3-3 Mediated regulation of the tumor suppressor protein, RASSF1A. Apoptosis 15, 117–127 (2010). https://doi.org/10.1007/s10495-009-0451-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0451-6