Abstract
Sepsis is a leading cause of death worldwide. Increased vascular permeability is a major hallmark of sepsis. Dynamic alterations in actin fiber formation play an important role in the regulation of endothelial barrier functions and thus vascular permeability. Endothelial integrity requires a delicate balance between the formation of cortical actin filaments that maintain endothelial cell contact stability and the formation of actin stress fibers that generate pulling forces, and thus compromise endothelial cell contact stability. Current research has revealed multiple molecular pathways that regulate actin dynamics and endothelial barrier dysfunction during sepsis. These include intracellular signaling proteins of the small GTPases family (e.g., Rap1, RhoA and Rac1) as well as the molecules that are directly acting on the actomyosin cytoskeleton such as myosin light chain kinase and Rho kinases. Another hallmark of sepsis is an excessive recruitment of neutrophils that also involves changes in the actin cytoskeleton in both endothelial cells and neutrophils. This review focuses on the available evidence about molecules that control actin dynamics and regulate endothelial barrier functions and neutrophil recruitment. We also discuss treatment strategies using pharmaceutical enzyme inhibitors to target excessive vascular permeability and leukocyte recruitment in septic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a generalized inflammatory syndrome of the host in response to various infectious stimuli [1]. The most severe form, the septic shock, is accompanied by severe hypotension, impaired microvascular perfusion and organ damage [2]. This may finally lead to multiple organ failure [3]. Surprisingly, sepsis is still not fully recognized as an individual disease entity despite high morbidity and mortality rates in affected patients causing high socio-economic burdens for health care systems worldwide. The incidence of sepsis is reported to be in the range of 200–1000 sepsis patients per 100,000 inhabitants and year [4, 5]. The death toll from sepsis is assumed to be around 200,000 fatalities per year in the US alone [6].
Vascular inflammation during sepsis development is characterized by activation of immune cells and their recruitment to sites of inflammation where they are needed to combat exogenous pathogens [7]. However, this process also carries the risk of tissue damage if leukocyte recruitment is not controlled properly [3]. Furthermore, increased vascular endothelial permeability during inflammation leads to excessive fluid loss from the intravascular space which may cause vascular hypotension. Tissue edema formation can then severely compromise organ functions, thus causing, for example, the development of acute respiratory distress syndrome (ARDS) of the lung. While the term ARDS is used to describe the disease in human patients, the term acute lung injury (ALI) is used in the murine system to describe a condition of respiratory distress due to excessive pulmonary inflammation, leukocyte recruitment and edema formation.
Increased vascular permeability during sepsis is also associated with edema formation in other organs, e.g., kidney, liver and brain. For example, septic patients have an increased risk of acute kidney injury due to hypotension and hypovolemia that develop in response to increased vascular permeability [8]. The effusion of edema fluid into the kidney tissue can then directly impair organ function [9].
Under physiological conditions, vascular endothelial permeability is tightly regulated by the molecular interplay of intercellular junction molecules that span the gap between neighboring endothelial cells (EC). These junction molecules cooperate in maintaining endothelial integrity to limit endothelial permeability and to allow for just minimal leakage of plasma fluid into the interstitial compartment. The stability of EC contacts formed by tight junctions (TJ) and adherens junctions (AJ) depends on their connection to the actin cytoskeleton. Disturbed actin dynamics may shift the balance from stabilizing cortical actin to destabilizing contractile stress fibers, thus causing endothelial barrier dysfunction and increased vascular permeability [10]. Aberrant actin dynamics may also contribute to excessive immune cell recruitment into the sites of inflamed tissue areas [11].
Clinically, the treatment of sepsis and septic shock nowadays merely consists of supportive measures, e.g., administration of antibiotics, use of vasopressors and fluid resuscitation to stabilize hemodynamics, and organ replacement therapy (mechanical ventilation, dialysis). To date, no therapy exists that specifically targets the overshooting immune response including uncontrolled vascular permeability and neutrophil recruitment.
In this review, we summarize current knowledge on signaling pathways contributing to aberrant actin dynamics during sepsis leading to junction destabilization, vascular hyperpermeability and immune cell recruitment. We will highlight studies using pharmacological inhibitors of actin-modifying enzymes to ameliorate endothelial barrier dysfunction and discuss their usefulness in systemic inflammatory conditions such as sepsis.
Actin dynamics and endothelial barrier integrity
EC contacts are stabilized by the connection of transmembrane proteins of TJ such as claudins and occludins, and AJ such as vascular endothelial (VE)-cadherin to cortical actin fibers. This connection occurs via adaptor molecules such as members of the zonula occludens (ZO) family in the case of TJ and members of the catenin family in the case of AJ. The interaction of VE-Cadherin with the cortical actin cytoskeleton is further supported by other molecules, such as vinculin, epithelial protein lost in neoplasm (EPLIN) and afadin. For a detailed description of these interactions the interested reader is referred to another recent review [10]. Formation and stabilization of both junctions and cortical actin is controlled by small GTPases of the Rho family such as Rac1 and RhoA [12]. Other molecules such as vasodilator-stimulated phosphoprotein (VASP) are also known to control cortical actin formation and to enhance barrier stability [13].
On the other hand, pro-inflammatory agents such as thrombin or tumor necrosis factor-α (TNF-α) induce actin remodeling in a way that favors the formation of contractile stress fibers [14]. This process is induced by RhoA-mediated activation of Rho kinases (ROCK) and myosin light chain kinase (MLCK), and concomitant inhibition of myosin light chain phosphatase (MLCP) leading to hyperphosphorylation of MLC and actomyosin contractility [15]. Stress fibers connect to junctions and generate pulling forces within neighboring cells leading to cell contact destabilization and internalization of AJ and TJ molecules [16]. The shift from stabilizing endothelial cortical actin to contractile stress fibers during inflammation also supports leukocyte transendothelial migration [11, 17].
Kinases modulating actin dynamics are of clear clinical relevance as targets for the development of treatment strategies for disorders characterized by hyperpermeability and excessive leukocyte recruitment such as sepsis. Pharmacological inhibitors of ROCK1 and MLCK have been applied with various outcomes in EC, animal models of sepsis and clinical trials as discussed below.
The role of actin dynamics and actomyosin contractility in endothelial barrier destabilization during sepsis
Hyperpermeability is a hallmark of different inflammatory diseases such as ARDS [18] and sepsis [19, 20] and increased actomyosin contractility drives endothelial barrier dysfunction and vascular leakage. As discussed above, contractility is regulated in large parts by the RhoA/ROCK and MLCK signaling pathways that increase MLC2 phosphorylation at threonine-18 and serine-19 leading to disassembly of inter-endothelial contacts (Fig. 1). Therefore, the actomyosin contractility machinery has become the focus of many studies investigating sepsis.
Signaling pathways regulating endothelial barrier functions during sepsis. Several different effectors can activate receptor-mediated activation of small GTPases that induce actin remodeling. Myosin gets phosphorylated via RhoA/ROCK and MLCK, and interacts with actin filaments to form contractile actomyosin stress fibers. On the other hand, several ABP, such as cortactin, filamin A/B, cofilin and VASP, are getting phosphorylated and translocated to control inflammatory actin dynamics. Several substances have been identified that inhibit these pathways and counteract endothelial hyperpermeability during sepsis (bottom)
The role of RhoA and ROCK in endothelial hyperpermeability during sepsis
There is strong evidence for the involvement of ROCK in the regulation of vascular hyperpermeability after cecal ligation and puncture (CLP)-induced sepsis and lipopolysaccharide (LPS)-induced pulmonary inflammation because the increase in wet-to-dry ratio of septic lungs was reversed when rats were pre-treated with the specific ROCK inhibitor Y-27632 prior to CLP surgery [21, 22]. Additionally, lung histopathological scores from these animals were lower in the CLP group treated with Y-27632 when compared to non-treated CLP animals. Although the authors did not show direct evidence of actin cytoskeleton modulation in their experiments (e.g., phalloidin stainings, G/F-actin ratio, MLC2 phosphorylation), they observed overexpression of ROCK1 but not ROCK2 in the CLP group, and ROCK1 overexpression in EC has recently been shown to be sufficient to induce actomyosin contractility [23]. Similar results for ROCK1 were obtained in C57BL/6 mice in which ALI was induced by intravenous injection of LPS [22]. These results suggest that ROCK activation is a critical mechanism driving lung tissue damage and hyperpermeability during sepsis and ALI.
Thrombin is a serine-protease involved in coagulation and endothelial barrier hyperpermeability during inflammation by inducing RhoA activation [24], actin cytoskeleton remodeling and endothelial junction disassembly [25]. Thrombin production is increased during sepsis, thus further triggering vascular hyperpermeability [26]. Given the importance of ROCK as target of RhoA, these data suggest the existence of a feedback loop that triggers the inflammatory process during sepsis. Clearly, the RhoA/ROCK pathway is critical for early events driving sepsis progression leading to endothelial barrier dysfunction and organ damage. Thus, these molecules are potential therapeutic targets as discussed in more detail below. Of note, the compound Y-27632 inhibits both ROCK isoforms, ROCK1 and ROCK2 [27]. With the recent discovery of a new ROCK2-specific inhibitor termed SLx-2119 [28], it will now be possible to investigate isoform-specific effects on sepsis development.
The role of LIM-kinase-1 (LIMK) in endothelial hyperpermeability during sepsis
Another effector protein involved in cell contractility downstream of RhoA and ROCK is LIM-Kinase 1 (LIMK) which also participates in sepsis-induced barrier dysfunction in mouse lungs [29]. LPS-induced pulmonary inflammation in LIMK1-deficient animals resulted in less edema formation and mortality compared to wild type (WT) control mice. Depletion of LIMK1 in human umbilical vein EC (HUVEC) also resulted in improved transendothelial electrical resistance (TER) [30]. On the other hand, overexpression of LIMK1 in HUVEC led to more intercellular gap formation due to LIMK1-dependent phosphorylation of the actin depolymerizing factor cofilin [30]. These data demonstrate the importance of LIMK1-dependent actin dynamics for endothelial barrier functions during inflammation in vivo and in vitro.
The role of MLCK in endothelial hyperpermeability during sepsis
MLCK is another kinase capable of phosphorylating MLC2 to induce intracellular contractility and endothelial permeability [31]. To analyze whether MLCK has a role in LPS-induced endotoxemia, mice with a genetic deletion of the long isoform (MLCK210) were generated and subjected to i.p. injection of LPS. The effects on permeability observed in MLCK210-deficient animals were less pronounced than those observed in WT mice, suggesting that MLCK210 plays an important role during the onset of sepsis [32]. WT mice treated with a MLCK inhibitor generated in the same study [11-(3-chloro-6-imino-6H-pyridazin-1-yl)-undecanoic acid (6-phenyl-pyridazin-3-yl)-amide] showed similar protective effects 24 h after LPS injection as observed in MLCK210-deficient mice. By contrast, another study showed that endothelial-specific deletion of both the long (MLCK210) and the short (MLCK130) isoforms in C57BL/6 mice did not protect from LPS-induced sepsis [33]. When administered with a lethal dose of LPS, these mice had similar mortality rates and comparable inflammation-induced tissue injury in the lungs and kidneys when compared to LPS-injected WT mice. Permeability to Evans Blue in lungs and phosphorylation of MLC after intratracheal LPS instillation in these MLCK-deficient mice was comparable to WT mice [33]. Additionally, phosphorylation of MLC in EC freshly isolated from the aorta of these animals was also not affected by MLCK deletion when compared to WT controls [33]. The authors discussed that the discrepancies between the two mouse models may be a consequence of differential MLCK isoform expression. According to their study, the endothelium in vivo does not express MLCK210 so that the protection against LPS in MLCK210-deficient mice could be attributed to other cell types such as epithelial cells and smooth muscle cells. Thus, further studies are required to better define the role of MLCK isoforms in LPS-induced endothelial hyperpermeability. Unfortunately, the available evidence on the role of MLCK in human patients is very scarce. In a candidate gene approach, single polymorphisms and haplotypes of MLCK were associated with ARDS and concurrent endothelial barrier defects in patients [34, 35]. Further clinical studies are needed to clarify whether the data obtained from animal studies can be confirmed in humans.
The role of MLCP in endothelial hyperpermeability during sepsis
While MLCK increases MLC phosphorylation, MLCP de-phosphorylates MLC and reduces actomyosin contractility and endothelial permeability [36]. Very recently, MLCP was found to be involved in late CLP-induced sepsis in C57BL/6 mice after 5 days of surgery which, according to the authors, mimics late stages of human sepsis [36]. The authors found that ROCK-dependent inhibitory MLCP phosphorylation (Thr855) was reduced in isolated caudal arteries from CLP mice leading to more active MLCP, less contractility and less vascular permeability in late sepsis. Overactivation of MLCP 5 days after CLP also resulted in less vascular contraction after treatment of femoral arteries with the thromboxane A2 receptor agonist U46619 or the protein kinase C (PKC) activator phorbol-dibutyrate (PDBu) to induce vasoconstriction [37]. Recently, MLCK and MLCP expression levels were analyzed in artery biopsies (renal and aorta) from post-mortem patients in an age range of 63–78 years that suffered from severe sepsis [38]. MLCP expression in different arterial lysates (renal, dorsalis pedis, aorta and carotid) from all patients was increased, whereas MLCK expression was decreased. However, phosphorylation levels to determine their activation status were not analyzed. Of note, myosin heavy and light chain expression was unaffected in lysates of arterial and vascular smooth muscle cells.
A close relationship between RhoA/ROCK, MCLK and MCLP signaling pathways that result in MLC phosphorylation/dephosphorylation has also been demonstrated in human cell culture models in vitro. For example, MLC phosphorylation was MLCK- and ROCK2-dependent in human lung microvascular EC and controlled endothelial barrier function [39]. In HUVEC, Rho-GTPases and MLCK contributed to MLC phosphorylation followed by cell contraction [40]. In this study, LPS stimulation of HUVEC also led to decreased MLCP activity via increased intracellular cAMP levels, thus contributing to increased MLC phosphorylation.
These data imply that both MLCK and MLCP may be useful as targets in the treatment of sepsis. However, more efforts are needed to truly understand at which point in sepsis progression manipulation of these molecules will result in a better outcome.
Actin-binding proteins (ABP) regulating actin dynamics and endothelial barrier functions during septic conditions
Vascular permeability is in large part controlled by dynamic actin remodeling in EC [10]. Actin remodeling is directly influenced by kinases activating myosin motors and by ABP that modulate actin and myosin functions. Properties and functions of ABP have been reviewed recently [10]. Here, we focus on ABP that play a known role in actin dynamics and endothelial barrier functions during sepsis.
Caveolin-1 (Cav-1)
Cav-1 is an ABP known to connect lipid rafts to the cytoskeleton and to regulate transcellular migration of lymphocytes [41]. Mice deficient for Cav-1 (Cav-1 KO) show less endothelial barrier dysfunction, lung edema formation and lower mortality rates during LPS-induced sepsis compared to WT mice [42, 43]. This protective effect in Cav-1 KO mice was associated with increased endothelial nitric oxide synthase (eNOS)-dependent NO production in the lungs. By contrast, studies using single bacterial infections of Cav-1 KO mice with either Klebsiella pneumoniae [44] or Pseudomonas aeruginosa [45], or multi-bacterial sepsis models such as CLP [46] revealed that Cav-1 deficiency was associated with increased pro-inflammatory cytokine production and higher mortality rates. Specifically, K. pneumoniae infection of Cav-1 KO mice showed that pro-inflammatory cytokine production was controlled by signal transducer and activator of transcription-5 (STAT5) and the glycogen synthase kinase-3β (GSK3β)/β-catenin/Akt signaling pathway in the lung [44]. P. aeruginosa infection of Cav-1 KO mice showed increased phosphorylation of janus kinase-2 (JAK2) levels and STAT3 activation with consequent nuclear factorkappa-light-chain-enhancer of activated B cells (NF-κB) nuclear translocation and increased pro-inflammatory cytokine production in the lung [45]. The observed differences between the LPS and the bacterial infection models cannot be clearly reconciled at this point but a possible explanation could be that bacterial infections represent a more complex scenario where many signaling pathways are implicated compared to LPS injection where only toll-like receptor (TLR)4 gets activated. This idea is supported by previous work where mutated TLR4, deficiency in LPS-binding protein (LBP, which is essential for LPS-TLR4 recognition) or induced sensitivity to LPS did not modify the outcome of lethal sepsis in mice [47, 48].
Cav-1 is phosphorylated at Tyr14 in response to LPS stimulation in a process dependent on endothelial CD14, an anchored glycoprotein essential for TLR4-dependent responses. This phosphorylation induces NF-ĸB signaling and pro-inflammatory cytokine production [49]. Upon LPS stimulation, TLR4 exposes a death domain on the intracellular portion of its Toll/interkeukin-1-receptor (TIR) to immediately recruit myeloid differentiation primary-response protein 88 (MyD88), and subsequently kinases such as IL-1-receptor-associated kinase 4 (IRAK4); a series of events that is fundamental for LPS-mediated responses [50].
Cav-1 also binds eNOS to prevent reactive oxygen species (ROS) formation [51]. Additionally, Cav-1 deletion leaves eNOS activated resulting in nitration of IRAK4, and subsequent TLR4 inhibition leads to impaired pro-inflammatory cytokine production. In HUVEC, Cav-1 is downregulated when challenged with serum of septic patients [52]. By contrast, LPS stimulation of liver EC induces Cav-1 expression and mitigates endothelial ROS production in vitro [53]. On the other hand, Cav-1 expression does not change in human dermal microvascular EC (HDMEC) in response to LPS indicating that caveolin-mediated effects on endothelial barrier integrity strongly depend on the challenged vascular bed [54].
Zonula Occludens-1 (ZO-1)
The adaptor molecule ZO-1 connects TJ transmembrane proteins to the actin cytoskeleton and is essential for endothelial barrier stability. TLR4 stimulation in brain EC monolayers induces vascular permeability due to relocation of β-catenin from the membrane to the cytosol; and EC co-cultured with astrocytes showed ZO-1 relocation from the membrane to perinuclear spaces [55].
Moesin
Moesin is an ABP involved in paracellular gap formation but also in wound healing by inducing cell migration in HUVEC [56]. Proteomic analysis of HUVEC after stimulation with LPS for 24 h revealed that moesin was secreted into supernatants [56]. Notably, moesin levels also increased in a time-dependent fashion in sera of mice with lethal sepsis and in humans with severe sepsis. Moesin levels in sera of septic patients correlated with the severity and outcome of sepsis [57]. Not only bacterial products such as LPS caused severe inflammatory responses but also damage-associated molecular patterns (DAMPs) which were released by injured tissues and recognized by TLR to promote acute inflammation [50]. Among DAMPs, high-mobility group box 1 (HMGB1) is a nuclear protein that was also secreted into extracellular spaces during sepsis where it could activate TLR2 and TLR4 to induce inflammation, actin cytoskeleton rearrangement and endothelial hyperpermeability. This response was dependent on moesin phosphorylation at Thr558 [57]. Since extracellular moesin was not phosphorylated, it was suggested that dephosphorylation was needed for its secretion [57]. HMGB1 is considered a late-onset mediator of sepsis as it is not immediately released by stressed cells into the peripheral circulation [58].
Vasodilator-stimulated phosphoprotein (VASP)
VASP is an ABP that has a central role in actin cytoskeleton dynamics as it participates in filament elongation, stabilization, branching inhibition and profilin/G-actin recruitment [11]. VASP together with filamin1 protected endothelial barrier functions as their inhibition during LPS stimulation of human lung microvascular EC augmented barrier dysfunction as manifested by reduced transendothelial electrical reesistance (TER) and diminished ZO-1 localization at cell contacts [59].
Cortactin
Cortactin is expressed in EC and plays a fundamental role in inflammation. Cortactin deficiency led to increased vascular permeability due to reduced endothelial Rap1 activity [60]. Recently, we discovered that cortactin deficiency augmented ROCK1 levels, phosphorylation of MLC and actomyosin contractility, and diminished adrenomedullin (ADM) secretion leading to enhanced endothelial permeability [23]. Downregulation of cortactin also attenuated sphingosine 1-phosphate (S1P)-induced endothelial barrier stability [61]. Consequences of cortactin deficiency for the progression of sepsis are currently being investigated in our laboratories.
Heat shock proteins (HSP)
HSP are highly conserved proteins that can be induced by a variety of stimuli. The most studied stress-inducible HSP are HSP90, HSP70 and HSP27 and they become upregulated upon noxious challenges to protect against apoptosis [62]. HSP27 and HSP90 were downregulated in HUVEC upon LPS exposure [63]. In experimental LPS-induced endotoxemia, overexpression of HSP27 confered protection against myocardial dysfunction, a risk factor in septic patients, leading to higher survival rates [64]. HSP27 phosphorylation levels significantly increased in lungs of rats after LPS-induced sepsis in a p38 mitogen-activated protein kinase (MAPK) activity-dependent fashion [65]. This was accompanied by diminished interaction of β-actin with HSP27.
Filamin A/B
Filamins are conserved, multi-domain actin cross-linking proteins that connect extracellular matrix (ECM) components to the actin cytoskeleton [66]. One of the most abundant ECM proteins is hyaluronan that binds to its receptor CD44. In LPS-induced pulmonary inflammation filamin expression was downregulated leading to vascular leakage [67]. High molecular weight hyaluronan (HMW-HA) protected the pulmonary endothelial barrier upon binding to its cognate receptor in a mechanism that involved annexin A2 and S100-A10-dependent filamin A/B recruitment to caveolin-enriched domains [67]. The authors also showed that silencing of both filamins prevented the protective effect of HMW-HA. In line with this, both filamin A and cofilin-1 were downregulated in human coronary artery EC as a result of Wnt5a stimulation. Wnt5a is produced by TLR-stimulated macrophages and high levels of this molecules were found in serum of patients with severe sepsis [68]. Wnt5a targeted ROCK to activate LIMK2 that in turn phosphorylated cofilin-1 leading to actin fiber bundling and stress fiber formation [68]. Inhibiting ROCK in LPS-stimulated liver EC prevented cofilin-1 phosphorylation [69]. These data again demonstrate the importance of different ABP for controlling endothelial actomyosin contractility and barrier integrity during septic conditions.
Gelsolin
Gelsolin is another ABP that is involved in cytoskeletal rearrangements due to its capping and actin-monomer sequestering abilities [10]. Its deficiency led to severe actin stress fiber formation and endothelial barrier dysfunction [70]. Gelsolin is also known for its LPS-neutralizing capabilities [71]. It is also secreted into the blood and decreased levels correlated with disease severity and higher mortality in sepsis [72], and other critical conditions such as acute liver failure and myocardial infarction [73].
Actin dynamics and neutrophil recruitment during sepsis
In addition to endothelial hyperpermeability, excessive neutrophil recruitment is another hallmark of endotoxemia and sepsis. Neutrophil interactions with EC that precede extravasation during inflammation strongly depend on actin remodeling in both cell types [11]. Neutrophils can exploit endothelial adhesion molecules such as endothelial cell-selective adhesion molecule (ESAM) or junctional adhesion molecule-A (JAM-A) to facilitate their paracellular passage across endothelial monolayers and they can take advantage of endothelial barriers weakened by increased actomyosin contractility [74, 75]. However, neutrophil extravasation is a very complex process in which endothelial barrier dysfunction is not always accompanied by increased neutrophil extravasation [76]. We showed that cortactin-deficient mice have increased vascular permeability and weakened intercellular junctions but showed reduced neutrophil extravasation during inflammation [60]. These data clearly show that permeability and neutrophil extravasation are distinct processes that need to be studied independently. Several studies have been published recently using inhibitors of actomyosin contractility and ABP activation to target actin dynamics and reduce neutrophil accumulation in affected tissues during sepsis. For example, neutrophil counts were reduced in bronchoalveolar lavage fluids (BALF) of mice with CLP-induced sepsis that were co-treated with the ROCK inhibitor Y-27632 compared to non-treated septic mice [21, 22]. Additionally, administration of Y-27632 prior to CLP-induced sepsis in C57BL/6 mice reduced thrombin production and myeloperoxidase (MPO) activity indicating diminished leukocyte recruitment into the lung [26]. Another study demonstrated that inhibition of ROCK by Y-27632 in C57BL/6 mice with CLP-induced sepsis led to reduced neutrophil recruitment, MPO activity and tissue damage in the lungs as a consequence of reduced MAC-1 expression on the surface of peripheral blood neutrophils and reduced production of the chemokines CXCL1 and CXCL2 [77] (Fig. 2).
Actin-dependent mechanisms controlling the interactions of neutrophils with endothelial cells under septic conditions. In neutrophils, ROCK gets activated in response to CXCL12 to control actin dynamics that regulate integrin mobility and clustering, and expression of Mac1. In endothelial cells, ROCK-mediated NF-ĸB nuclear translocation induces expression of ICAM-1 to facilitate neutrophil adhesion. ROCK-mediated actin contractility contributes to endothelial junction destabilization and neutrophil transendothelial migration
Other neutrophil kinases that affect actomyosin contractility are also involved in the regulation of neutrophil transendothelial migration. For example, LIMK1-deficient neutrophils showed impaired chemotaxis to C5a and interleukin-8 (IL-8) gradients, presumably due to defects in actin polymerization [30], demonstrating the importance of LIMK1-dependent actin dynamics for neutrophil transmigration during inflammation. Neutrophils isolated from bone marrow and peripheral blood of MLCK-deficient mice infiltrated inflamed lungs to a lesser extent compared to WT neutrophils, and this was accompanied by reduced lung wet-to-dry ratios [78]. MLCK-deficient neutrophils could not efficiently adhere to and transmigrate across EC in vitro. Interestingly, MLCK-deficient lungs and neutrophils still showed increased MLC phosphorylation at serine-19 after LPS treatment, suggesting that other kinases, likely ROCK, can compensate for the loss of MLCK to maintain high MLC phosphorylation levels in response to LPS. Thus, it seems that pathways other than MLC phosphorylation mediate the protective effect in MLCK-deficient mice. Indeed, the authors showed that loss of MLCK in neutrophils was accompanied by less activation of β2-integrins in neutrophils as a consequence of less Src and Pyk2 activity. Furthermore, F-actin formation was disturbed in MLCK-deficient neutrophils in a Pyk2-dependent manner [78]. These data suggest that MLCK contributes to Src and Pyk2 activity and Pyk2-dependent actin polymerization leading to β2-integrin activation and excessive neutrophil recruitment during sepsis. Thus, it is reasonable to speculate that reduced neutrophil infiltration is responsible for at least some of the observed protective effects during septic conditions in MLCK-deficient mice.
In addition to increased endothelial barrier stability, lungs of Cav-1 KO mice showed lower intercellular adhesion molecule (ICAM)-1 levels, and consequently, diminished neutrophil adhesion and recruitment during LPS-induced sepsis [42, 43]. This was due to reduced NF-ĸB activity leading to reduced transcription of iNOS and ICAM-1. Administration of the NO synthase inhibitor nitro-L-arginine during LPS challenge increased NF-ĸB activity and restored neutrophil adhesion to EC in Cav-1 KO mice.
Taken together, aberrant actin dynamics in neutrophils clearly affect the disease outcome in murine models of sepsis.
Effects of pharmaceutical actin manipulation on the progression of endotoxemia and sepsis
In recent years, reinforcement of endothelial barrier stability to prevent excessive permeability and neutrophil influx during acute inflammatory responses has gained attention since these processes significantly contribute to organ failure during sepsis (Table 1). Several molecules that enhance barrier stability during acute inflammatory conditions have emerged as targets; however, no such therapeutic agent for treating sepsis is currently being used in the clinic [79]. In this chapter, we will summarize emerging treatment options directed at preventing actomyosin contractility to reduce vascular barrier dysfunction and neutrophil recruitment during experimental sepsis.
The inhibitor of ROCK1 and ROCK2, Y27632, ameliorated LPS-induced lethal sepsis in mice by preventing TNFα-induced vascular leakage, neutrophil extravasation and lung tissue damage [22]. LPS-induced reduction of endothelin-1and endothelial NO production, increase in ROCK2 expression and phosphorylation of moesin was attenuated by Y27632 [69]. Additionally, ROCK inhibition reduced peroxynitrite and ROS production, fragmentation of ROCK and caspase 3 cleavage in septic lungs [21]. The ROCK2 inhibitor fasudil prevented cofilin and dynamin-related protein-1 phosphorylation leading to endothelial barrier stabilization [90]. Fasudil increased eNOS and decreased iNOS during endotoxemia in rats leading to reduced vascular inflammation [91]. It also ameliorated LPS-induced acute lung injury in mice by inhibiting the RhoA/ROCK pathway [87]. Therefore, fasudil has been suggested as a promising therapeutic agent in sepsis [86].
Heparin is best known for its anticoagulant properties, but it also inhibits RhoA and ROCK and can ameliorate LPS-induced vascular permeability in the lung [82, 92].
Treatment of EC with adenylate cyclase enhancers such as forskolin or the phosphodiesterase inhibitor IBMX increased levels of cAMP leading to improved endothelial barrier stability. The protein kinase A (PKA)-specific cAMP-analog N6-Benzoyl-cAMP and the exchange protein directly activated by cAMP (EPAC)-specific cAMP analog 8-(4-chlorophenylthio)-20-O-methyl-cAMP ameliorated LPS-induced vascular permeability in an additive fashion [59]. Moreover, a non-hydrolysable ATP analog stabilized the endothelial barrier in the lung during LPS-induced pulmonary inflammation in mice. Although the precise protective mechanisms are still unknown, the same authors showed that ATP enhances endothelial barrier stabilization via Rac1 and cortactin [85, 93].
Adrenomedullin (ADM) is an endogenous vasorelaxant peptide hormone [89, 94] that is capable of enhancing endothelial barrier function via regulation of the actin cystoskeleton [88, 95]. ADM acts through elevation of intracellular cAMP levels [96] leading to the activation of EPAC and the small GTPase Rap1 [97], enhanced cortical actin formation, strong VE-cadherin-mediated intercellular adhesion, and reduced actomyosin contractility [98]. We have recently reported that ADM was capable of counteracting increased vascular permeability induced by ROCK-dependent phosphorylation of MLC demonstrating that ADM can directly modulate actin dynamics to improve vascular barrier functions [23]. ADM was increased in plasma from patients suffering from systemic inflammatory response syndrome (SIRS) when compared to healthy control patients [99]. The authors found a correlation between plasma levels of ADM and disease severity so that ADM was postulated as a marker for sepsis progression. Continuous ADM blood infusion was beneficial in female C57BL/6 mice with Streptococcus pneumoniae-induced pneumonia that were subjected to mechanical ventilation 24 h post-infection to induce strong lung injury [100]. In this study, ADM-treated animals showed less lung permeability and pneumococcal pneumonia, and they were protected from tissue injury in liver and ileum.
On the other hand, there is evidence of detrimental effects caused by ADM during sepsis. Blocking ADM functions with the antagonist ADM (aa 22–52) or anti-adrenomedullin serum resulted in improved survival of rats treated with LPS. Cardiomyocytes isolated from these animals showed improved contractility after ADM inhibition which, in late sepsis phases, could improve blood pressure, tissue perfusion and oxygen delivery [101].
In summary, ADM is a promising therapeutic but more research is needed to better understand the mechanisms underlying the protective effects during early sepsis and the rather detrimental effects during late sepsis.
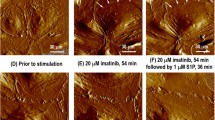
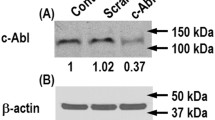
Abl-family kinases participate in the regulation of actin cytoskeleton dynamics by phosphorylation of MLCK, cortactin, vinculin and β-catenin. Imatinib was originally designed to inhibit the breakpoint cluster region-Abl fusion protein that causes chronic myeloid leukemia, but later it has been shown to inhibit vascular permeability induced by thrombin, histamine, VEGF, LPS and oxidative stress; thus making it also an interesting candidate for sepsis treatment [102]. However, the precise mechanisms for these effects remain elusive.
HSP90 is a chaperone protein involved in protein folding maturation and stabilization of kinases and transcription factors [83]. LPS-induced vascular leakage involved p120-catenin and β-catenin phosphorylation by pp60Src kinase [83]. This kinase is a target of HSP90, but it also phosphorylated HSP90 and induced its chaperone functions. HSP90 inhibitors such as radicicol and 17-allyamino demethoxygeldanamycin (17-AGG) bound to nucleotide-binding sites of HSP90 with high affinity leading to degradation of its targets, lower pro-inflammatory cytokine production, reduction of vascular permeability and better survival rates in mice that received intraperitoneal LPS injection and parallel administration of these HSP90 inhibitors [83, 84, 103].
Reduction of endothelial hyperpermeability during the course of sepsis is fundamental for the control of the disease. S1P was effective in the control of lung inflammation and vascular permeability [61]. FTY720 is a derivative of myriocin, a natural analog of S1P with immunosuppressive properties. Derivatives of FTY720 such as R-methoxy-FTY720, (R)/(S)-fluoro-FTY720 and β-glucuronide-FTY720 could enhance barrier stability of lung EC in vitro via cortactin translocation to the plasma membrane [80]. Thus, these S1P-derivatives could be promising therapeutic options.
Conclusions
The excessive increase in vascular permeability remains a major challenge in the treatment of sepsis as it causes severe intravascular hypovolemia and hypotonia that require extensive volume resuscitation and use of pharmacological vasopressors. Both the use of volume resuscitation and vasopressors may cause undesired complications and their application is associated with increased organ dysfunction and mortality. Thus, the identification of pharmacological inhibitors limiting the underlying clinical reason, i.e., endothelial barrier dysfunction, would be a milestone in the treatment of sepsis. Many of the aforementioned pharmacological agents have shown promising potential by reducing vascular permeability and improving the outcome of sepsis in rodents. Unfortunately, very little therapeutic approaches that were beneficial in rodents could improve disease outcome in clinical trials with human patients. The reasons for this phenomenon are manifold and include the fact that there are profound differences between rodents as model organisms and humans. Thus, it is of great importance to verify whether the molecular players and pathways involved in the regulation of endothelial barrier integrity and vascular permeability in rodents are also of importance in humans as this is a prerequisite for the transfer of treatment options into the human system.
Other important aspects are the specificity and safety of pharmacological inhibitors. Although they did not show any adverse effects in rodents during short term experiments, this does not necessarily exclude the possibility of undesired side effects on the long haul, or unspecific interactions with other regulatory pathways. Thus, basic research aiming at a better understanding of the pathophysiological pathways controlling actomyosin contractility, endothelial barrier dysfunction and increased vascular permeability in the human system is a necessity for the successful implementation of novel therapeutic strategies. Nevertheless, the modification of actin cytoskeleton dynamics in EC is a valuable approach in the development of treatment options for the detrimental disease sepsis. This is especially important taking into account that all available treatment options for sepsis are supportive measures that do not tackle the underlying molecular pathogenesis but merely aim at reducing the deleterious consequences of sepsis.
References
Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S, Tracey K, van der Poll T, Pelfrene E (2015) Sepsis: a roadmap for future research. Lancet Infect Dis 15(5):581–614. doi:10.1016/S1473-3099(15)70112-X
Gotts JE, Matthay MA (2016) Sepsis: pathophysiology and clinical management. BMJ 353:i1585. doi:10.1136/bmj.i1585
Rossaint J, Zarbock A (2015) Pathogenesis of multiple organ failure in sepsis. Crit Rev Immunol 35(4):277–291
Wilhelms SB, Huss FR, Granath G, Sjoberg F (2010) Assessment of incidence of severe sepsis in Sweden using different ways of abstracting International Classification of Diseases codes: difficulties with methods and interpretation of results. Crit Care Med 38(6):1442–1449. doi:10.1097/CCM.0b013e3181de4406
Flaatten H (2004) Epidemiology of sepsis in Norway in 1999. Crit Care 8(4):R180–R184. doi:10.1186/cc2867
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the US: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310
Phillipson M, Kubes P (2011) The neutrophil in vascular inflammation. Nat Med 17(11):1381–1390. doi:10.1038/nm.2514
Martensson J, Bellomo R (2015) Sepsis-induced acute kidney injury. Crit Care Clin 31(4):649–660. doi:10.1016/j.ccc.2015.06.003
Verma SK, Molitoris BA (2015) Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 35(1):96–107. doi:10.1016/j.semnephrol.2015.01.010
Garcia-Ponce A, Citalan-Madrid AF, Velazquez-Avila M, Vargas-Robles H, Schnoor M (2015) The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb Haemost 113(1):20–36. doi:10.1160/TH14-04-0298
Schnoor M (2015) Endothelial actin-binding proteins and actin dynamics in leukocyte transendothelial migration. J Immunol 194(8):3535–3541. doi:10.4049/jimmunol.1403250
Amado-Azevedo J, Valent ET, Van Nieuw Amerongen GP (2014) Regulation of the endothelial barrier function: a filum granum of cellular forces, Rho-GTPase signaling and microenvironment. Cell Tissue Res 355(3):557–576. doi:10.1007/s00441-014-1828-6
Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP (2002) Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 16(6):583–585
Marcos-Ramiro B, Garcia-Weber D, Millan J (2014) TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost 112(5). doi:10.1160/TH14-04-0299
Schnittler H (2016) Contraction of endothelial cells: 40 years of research, but the debate still lives. Histochem Cell Biol. doi:10.1007/s00418-016-1501-0
Dorland YL, Huveneers S (2016) Cell–cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci. doi:10.1007/s00018-016-2325-8
Hordijk PL (2016) Recent insights into endothelial control of leukocyte extravasation. Cell Mol Life Sci 73 (8):1591–1608. doi:10.1007/s00018-016-2136-y
Zarbock A, Distasi MR, Smith E, Sanders JM, Kronke G, Harry BL, von Vietinghoff S, Buscher K, Nadler JL, Ley K (2009) Improved survival and reduced vascular permeability by eliminating or blocking 12/15-lipoxygenase in mouse models of acute lung injury (ALI). J Immunol 183(7):4715–4722. doi:10.4049/jimmunol.0802592
Allison N, Yimu Y, Mario P, Lynelle S, Aneta G, Rubin MT, Eric PS (2014) Endothelial glycocalyx reconstitution influences pulmonary vascular permeability and is aberrant in sepsis. In: A98. EN.HELIAL BARRIER DYNAMICS: REGULATORS OF PERMEABILITY AND REPAIR. American Thoracic Society International Conference Abstracts. American Thoracic Society, pp A2208–A2208. doi:10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A2208
Lee WL, Slutsky AS (2010) Sepsis and endothelial permeability. N Engl J Med 363(7):689–691. doi:10.1056/NEJMcibr1007320
Cinel I, Ark M, Dellinger P, Karabacak T, Tamer L, Cinel L, Michael P, Hussein S, Parrillo JE, Kumar A, Kumar A (2012) Involvement of Rho kinase (ROCK) in sepsis-induced acute lung injury. J Thorac Dis 4(1):30–39. doi:10.3978/j.issn.2072-1439.2010.08.04
Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A (2005) Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol 32(6):504–510. doi:10.1165/rcmb.2004-0009OC
García Ponce A. CM, A.F., Vargas Robles H., Chánez Paredes S., Nava P., Betanzos, A., Zarbock A., Rottner K., Dietmar Vestweber D., Schnoor M. (2016) Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility. Sci Rep 2016(2016):srep29003
van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW (2000) Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87(4):335–340
Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E (1996) Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol 16(3):488–496
Wang Y, Braun OO, Zhang S, Norstrom E, Thorlacius H (2015) Thrombin generation in abdominal sepsis is Rho-kinase-dependent. Biochem Biophys Res Commun 460(3):691–696. doi:10.1016/j.bbrc.2015.03.091
Liao JK, Seto M, Noma K (2007) Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol 50(1):17–24. doi:10.1097/FJC.0b013e318070d1bd
Boerma M, Fu Q, Wang J, Loose DS, Bartolozzi A, Ellis JL, McGonigle S, Paradise E, Sweetnam P, Fink LM, Vozenin-Brotons MC, Hauer-Jensen M (2008) Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinol Int J Haemost Thromb 19 (7):709–718. doi:10.1097/MBC.0b013e32830b2891
Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K (2000) Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem 275(5):3577–3582
Gorovoy M, Han J, Pan H, Welch E, Neamu R, Jia Z, Predescu D, Vogel S, Minshall RD, Ye RD, Malik AB, Voyno-Yasenetskaya T (2009) LIM kinase 1 promotes endothelial barrier disruption and neutrophil infiltration in mouse lungs. Circ Res 105(6):549–556. doi:10.1161/CIRCRESAHA.109.195883
Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY (2013) Myosin light chain kinase signaling in endothelial barrier dysfunction. Med Res Rev 33(5):911–933. doi:10.1002/med.21270
Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM (2003) Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA 100(10):6233–6238. doi:10.1073/pnas.1031595100
Yu Y, Lv N, Lu Z, Zheng YY, Zhang WC, Chen C, Peng YJ, He WQ, Meng FQ, Zhu MS, Chen HQ (2012) Deletion of myosin light chain kinase in endothelial cells has a minor effect on the lipopolysaccharide-induced increase in microvascular endothelium permeability in mice. FEBS J 279(8):1485–1494. doi:10.1111/j.1742-4658.2012.08541.x
Garcia JG, Moreno Vinasco L (2006) Genomic insights into acute inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 291(6):L1113–L1117. doi:10.1152/ajplung.00266.2006
Kamp R, Sun X, Garcia JG (2008) Making genomics functional: deciphering the genetics of acute lung injury. Proc Am Thorac Soc 5(3):348–353. doi:10.1513/pats.200709-152DR
Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M (1998) Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 273(34):21867–21874
Reddi BA, Beltrame JF, Young RL, Wilson DP (2015) Calcium desensitisation in late polymicrobial sepsis is associated with loss of vasopressor sensitivity in a murine model. Intensiv Care Med Exp 3(1):36. doi:10.1186/s40635-014-0036-8
Zheng W, Kou Y, Gao FL, Ouyang XH (2016) Enzymatic changes in myosin regulatory proteins may explain vasoplegia in terminally ill patients with sepsis. Biosci Rep 36(2). doi:10.1042/BSR20150207
Bogatcheva NV, Zemskova MA, Poirier C, Mirzapoiazova T, Kolosova I, Bresnick AR, Verin AD (2011) The suppression of myosin light chain (MLC) phosphorylation during the response to lipopolysaccharide (LPS): beneficial or detrimental to endothelial barrier? J Cell Physiol 226(12):3132–3146. doi:10.1002/jcp.22669
Essler M, Staddon JM, Weber PC, Aepfelbacher M (2000) Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol 164(12):6543–6549
Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ (2006) Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol 8(2):113–123. doi:10.1038/ncb1356
Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB (2006) Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol (Baltimore, Md : 1950) 177(7):4853–4860
de Almeida CJ, Witkiewicz AK, Jasmin JF, Tanowitz HB, Sotgia F, Frank PG, Lisanti MP (2011) Caveolin-2-deficient mice show increased sensitivity to endotoxemia. Cell cycle (Georgetown, Tex) 10 (13):2151–2161. doi:10.4161/cc.10.13.16234
Guo Q, Shen N, Yuan K, Li J, Wu H, Zeng Y, Fox J 3rd, Bansal AK, Singh BB, Gao H, Wu M (2012) Caveolin-1 plays a critical role in host immunity against Klebsiella pneumoniae by regulating STAT5 and Akt activity. Eur J Immunol 42(6):1500–1511. doi:10.1002/eji.201142051
Yuan K, Huang C, Fox J, Gaid M, Weaver A, Li G, Singh BB, Gao H, Wu M (2011) Elevated inflammatory response in caveolin-1-deficient mice with Pseudomonas aeruginosa infection is mediated by STAT3 protein and nuclear factor kappaB (NF-kappaB). J Biol Chem 286(24):21814–21825. doi:10.1074/jbc.M111.237628
Feng H, Guo L, Song Z, Gao H, Wang D, Fu W, Han J, Li Z, Huang B, Li XA (2010) Caveolin-1 protects against sepsis by modulating inflammatory response, alleviating bacterial burden, and suppressing thymocyte apoptosis. J Biol Chem 285(33):25154–25160. doi:10.1074/jbc.M110.116897
Feng H, Guo W, Han J, Li XA (2013) Role of caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life Sci 93(1):1–6. doi:10.1016/j.lfs.2013.05.016
Echtenacher B, Freudenberg MA, Jack RS, Mannel DN (2001) Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect Immun 69(12):7271–7276. doi:10.1128/IAI.69.12.7172-7276.2001
Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G, Minshall RD, Malik AB, Hu G (2013) Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J Immunol (Baltimore, Md : 1950) 191 (12):6191–6199. doi:10.4049/jimmunol.1300873
Vadillo E, Pelayo R (2012) [Toll-like receptors in development and function of the hematopoietic system]. Rev Invest Clin 64(5):461–476
Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, Tiruppathi C, Zhao YY (2010) Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol 176(5):2344–2351. doi:10.2353/ajpath.2010.091088
Huang X, Pan L, Pu H, Wang Y, Zhang X, Li C, Yang Z (2013) Loss of caveolin-1 promotes endothelial-mesenchymal transition during sepsis: a membrane proteomic study. Int J Mol Med 32(3):585–592. doi:10.3892/ijmm.2013.1432
Kwok W, Clemens MG (2010) Targeted mutation of Cav-1 alleviates the effect of endotoxin in the inhibition of ET-1-mediated eNOS activation in the liver. Shock (Augusta, Ga) 33(4):392–398. doi:10.1097/SHK.0b013e3181be3e99
Schlegel N, Leweke R, Meir M, Germer CT, Waschke J (2012) Role of NF-kappaB activation in LPS-induced endothelial barrier breakdown. Histochem Cell Biol 138(4):627–641. doi:10.1007/s00418-012-0983-7
Cardoso FL, Kittel Á, Veszelka S, Palmela I, Tóth A, Brites D, Deli MA, Brito MA (2012) Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PloS One 7(5):e35919. doi:10.1371/journal.pone.0035919
Kwon OK, Lee W, Kim SJ, Lee YM, Lee JY, Kim JY, Bae JS, Lee S (2015) In-depth proteomics approach of secretome to identify novel biomarker for sepsis in LPS-stimulated endothelial cells. Electrophoresis 36(23):2851–2858. doi:10.1002/elps.201500198
Lee W, Kwon OK, Han MS, Lee YM, Kim SW, Kim KM, Lee T, Lee S, Bae JS (2015) Role of moesin in HMGB1-stimulated severe inflammatory responses. Thromb Haemost 114(2):350–363. doi:10.1160/th14-11-0969
Riedemann NC, Guo RF, Ward PA (2003) Novel strategies for the treatment of sepsis. Nat Med 9(5):517–524. doi:10.1038/nm0503-517
Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, Verin AD (2009) Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol 221(3):750–759. doi:10.1002/jcp.21913
Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D (2011) Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med 208(8):1721–1735 pii]10.1084/jem.20101920
Abbasi T, Garcia JG (2013) Sphingolipids in lung endothelial biology and regulation of vascular integrity. Handb Exp Pharmacol (216):201–226. doi:10.1007/978-3-7091-1511-4_10
Wang X, Chen M, Zhou J, Zhang X (2014) HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (review). Int J Oncol 45(1):18–30. doi:10.3892/ijo.2014.2399
Liu Z, Zhong T, Zheng D, Cepinskas I, Peng T, Su L (2016) Heat stress pretreatment decreases lipopolysaccharide-induced apoptosis via the p38 signaling pathway in human umbilical vein endothelial cells. Mol Med Rep 14(1):1007–1013. doi:10.3892/mmr.2016.5303
You W, Min X, Zhang X, Qian B, Pang S, Ding Z, Li C, Gao X, Di R, Cheng Y, Liu L (2009) Cardiac-specific expression of heat shock protein 27 attenuated endotoxin-induced cardiac dysfunction and mortality in mice through a PI3K/Akt-dependent mechanism. Shock (Augusta, Ga) 32(1):108–117. doi:10.1097/SHK.0b013e318199165d
Hirano S, Rees RS, Yancy SL, Welsh MJ, Remick DG, Yamada T, Hata J, Gilmont RR (2004) Endothelial barrier dysfunction caused by LPS correlates with phosphorylation of HSP27 in vivo. Cell Biol Toxicol 20(1):1–14
Razinia Z, Makela T, Ylanne J, Calderwood DA (2012) Filamins in mechanosensing and signaling. Annu Rev Biophys 41:227–246. doi:10.1146/annurev-biophys-050511-102252
Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG (2010) High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 299(5):L639–L651. doi:10.1152/ajplung.00405.2009
Skaria T, Bachli E, Schoedon G (2016) Wnt5A/Ryk signaling critically affects barrier function in human vascular endothelial cells. Cell Adhes Migr:1–15. doi:10.1080/19336918.2016.1178449
Kwok W, Clemens MG (2014) Rho-kinase activation contributes to Lps-induced impairment of endothelial nitric oxide synthase activation by endothelin-1 in cultured hepatic sinusoidal endothelial cells. Shock (Augusta, Ga) 42(6):554–561. doi:10.1097/shk.0000000000000252
Becker PM, Kazi AA, Wadgaonkar R, Pearse DB, Kwiatkowski D, Garcia JG (2003) Pulmonary vascular permeability and ischemic injury in gelsolin-deficient mice. Am J Respir Cell Mol Biol 28(4):478–484. doi:10.1165/rcmb.2002-0024OC
Bucki R, Georges PC, Espinassous Q, Funaki M, Pastore JJ, Chaby R, Janmey PA (2005) Inactivation of endotoxin by human plasma gelsolin. BioChemistry 44(28):9590–9597. doi:10.1021/bi0503504
Lee PS, Patel SR, Christiani DC, Bajwa E, Stossel TP, Waxman AB (2008) Plasma gelsolin depletion and circulating actin in sepsis: a pilot study. PloS One 3(11):e3712. doi:10.1371/journal.pone.0003712
Suhler E, Lin W, Yin HL, Lee WM (1997) Decreased plasma gelsolin concentrations in acute liver failure, myocardial infarction, septic shock, and myonecrosis. Crit Care Med 25(4):594–598
Daniel AE, van Buul JD (2013) Endothelial junction regulation: a prerequisite for leukocytes crossing the vessel wall. J Innate Immun 5(4):324–335. doi:10.1159/000348828
Vestweber D (2015) How leukocytes cross the vascular endothelium. Nat Rev Immunol 15(11):692–704. doi:10.1038/nri3908
Muller WA (2014) How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol 184(4):886–896. doi:10.1016/j.ajpath.2013.12.033
Hasan Z, Palani K, Rahman M, Zhang S, Syk I, Jeppsson B, Thorlacius H (2012) Rho-kinase signaling regulates pulmonary infiltration of neutrophils in abdominal sepsis via attenuation of CXC chemokine formation and Mac-1 expression on neutrophils. Shock 37(3):282–288. doi:10.1097/SHK.0b013e3182426be4
Xu J, Gao XP, Ramchandran R, Zhao YY, Vogel SM, Malik AB (2008) Nonmuscle myosin light-chain kinase mediates neutrophil transmigration in sepsis-induced lung inflammation by activating beta2 integrins. Nat Immunol 9(8):880–886. doi:10.1038/ni0.1628
Han S, Lee SJ, Kim KE, Lee HS, Oh N, Park I, Ko E, Oh SJ, Lee YS, Kim D, Lee S, Lee DH, Lee KH, Chae SY, Lee JH, Kim SJ, Kim HC, Kim S, Kim SH, Kim C, Nakaoka Y, He Y, Augustin HG, Hu J, Song PH, Kim YI, Kim P, Kim I, Koh GY (2016) Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med 8(335):335ra355. doi:10.1126/scitranslmed.aad9260
Camp SM, Chiang ET, Sun C, Usatyuk PV, Bittman R, Natarajan V, Garcia JG, Dudek SM (2016) “Pulmonary endothelial cell barrier enhancement by novel FTY720 analogs: methoxy-FTY720, fluoro-FTY720, and beta-glucuronide-FTY720”. Chem Phys Lipids 194:85–93. doi:10.1016/j.chemphyslip.2015.10.004
Feng Y, Hu L, Xu Q, Yuan H, Ba L, He Y, Che H (2015) Cytoprotective role of alpha-1 antitrypsin in vascular endothelial cell under hypoxia/reoxygenation condition. J Cardiovasc Pharmacol 66(1):96–107. doi:10.1097/fjc.0000000000000250
Han J, Ding R, Zhao D, Zhang Z, Ma X (2013) Unfractionated heparin attenuates lung vascular leak in a mouse model of sepsis: role of RhoA/Rho kinase pathway. Thromb Res 132(1):e42–e47. doi:10.1016/j.thromres.2013.03.010
Chatterjee A, Snead C, Yetik-Anacak G, Antonova G, Zeng J, Catravas JD (2008) Heat shock protein 90 inhibitors attenuate LPS-induced endothelial hyperpermeability. Am J Physiol Lung Cell Mol Physiol 294(4):L755–L763. doi:10.1152/ajplung.00350.2007
Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, Venema RC, Catravas JD (2007) Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med 176(7):667–675. doi:10.1164/rccm.200702-291OC
Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD (2008) Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol 294(2):L319–L324. doi:10.1152/ajplung.00283.2007
Preau S, Delguste F, Yu Y, Remy-Jouet I, Richard V, Saulnier F, Boulanger E, Neviere R (2016) Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, mitochondrial fission, and autophagy. Antioxid Redox Signal 24(10):529–542. doi:10.1089/ars.2015.6421
Li Y, Wu Y, Wang Z, Zhang XH, Wu WK (2010) Fasudil attenuates lipopolysaccharide-induced acute lung injury in mice through the Rho/Rho kinase pathway. Med sci Monit Int Med J Exp Clin Res 16(4):BR112–B118
Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krull M, Seybold J, Seeger W, Rascher W, Schutte H, Suttorp N (2002) Adrenomedullin reduces endothelial hyperpermeability. Circ Res 91(7):618–625
Roh J, Chang CL, Bhalla A, Klein C, Hsu SY (2004) Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 279(8):7264–7274. doi:10.1074/jbc.M305332200
Shah S, Savjani J (2016) A review on ROCK-II inhibitors: from molecular modelling to synthesis. Bioorg Med Chem Lett 26(10):2383–2391. doi:10.1016/j.bmcl.2016.03.113
McGown CC, Brown NJ, Hellewell PG, Brookes ZL (2011) ROCK induced inflammation of the microcirculation during endotoxemia mediated by nitric oxide synthase. Microvasc Res 81(3):281–288. doi:10.1016/j.mvr.2011.02.003
Mu E, Ding R, An X, Li X, Chen S, Ma X (2012) Heparin attenuates lipopolysaccharide-induced acute lung injury by inhibiting nitric oxide synthase and TGF-beta/Smad signaling pathway. Thromb Res 129(4):479–485. doi:10.1016/j.thromres.2011.10.003
Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG (2006) Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Physiol Lung Cell Mol Physiol 291(2):L289–L295. doi:10.1152/ajplung.00343.2005
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T (1993) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192(2):553–560. doi:10.1006/bbrc.1993.1451
Aslam M, Pfeil U, Gunduz D, Rafiq A, Kummer W, Piper HM, Noll T (2012) Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin-induced barrier failure in endothelial cell monolayers. Br J Pharmacol 165(1):208–222. doi:10.1111/j.1476-5381.2011.01540.x
Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, Matsuo H (1995) Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem 270(9):4412–4417
Bos JL (2003) Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 4(9):733–738. doi:10.1038/nrm1197
Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN (2005) Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105(5):1950–1955. doi:10.1182/blood-2004-05-1987
Ueda S, Nishio K, Minamino N, Kubo A, Akai Y, Kangawa K, Matsuo H, Fujimura Y, Yoshioka A, Masui K, Doi N, Murao Y, Miyamoto S (1999) Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med 160(1):132–136. doi:10.1164/ajrccm.160.1.9810006
Muller-Redetzky HC, Will D, Hellwig K, Kummer W, Tschernig T, Pfeil U, Paddenberg R, Menger MD, Kershaw O, Gruber AD, Weissmann N, Hippenstiel S, Suttorp N, Witzenrath M (2014) Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: protection by adrenomedullin. Crit care 18(2):R73. doi:10.1186/cc13830
Hyvelin JM, Shan Q, Bourreau JP (2002) Adrenomedullin: a cardiac depressant factor in septic shock. J Card Surg 17(4):328–335
Rizzo AN, Aman J, van Nieuw Amerongen GP, Dudek SM (2015) Targeting Abl kinases to regulate vascular leak during sepsis and acute respiratory distress syndrome. Arterioscler Thromb Vasc Biol 35(5):1071–1079. doi:10.1161/atvbaha.115.305085
Barabutis N, Handa V, Dimitropoulou C, Rafikov R, Snead C, Kumar S, Joshi A, Thangjam G, Fulton D, Black SM, Patel V, Catravas JD (2013) LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol 304(12):L883–L893. doi:10.1152/ajplung.00419.2012
Acknowledgements
This work was supported by an international bilateral grant from the Mexican Council for Science and Technology (Conacyt, 207268 to MS) and the German Ministry for Education and Research (BMBF, 01DN14039 to AZ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schnoor, M., García Ponce, A., Vadillo, E. et al. Actin dynamics in the regulation of endothelial barrier functions and neutrophil recruitment during endotoxemia and sepsis. Cell. Mol. Life Sci. 74, 1985–1997 (2017). https://doi.org/10.1007/s00018-016-2449-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2449-x