Abstract
Although the endothelium is an extremely thin single-cell layer, it performs exceedingly well in preventing blood fluids from leaking into the surrounding tissues. However, specific pathological conditions can affect this cell layer, compromising the integrity of the barrier. Vascular leakage is a hallmark of many cardiovascular diseases and despite its medical importance, no specialized therapies are available to prevent it or reduce it. Small guanosine triphosphatases (GTPases) of the Rho family are known to be key regulators of various aspects of cell behavior and studies have shown that they can exert both positive and negative effects on endothelial barrier integrity. Moreover, extracellular matrix stiffness has now been implicated in the regulation of Rho-GTPase signaling, which has a direct impact on the integrity of endothelial junctions. However, knowledge about both the precise mechanism of this regulation and the individual contribution of the specific regulatory proteins remains fragmentary. In this review, we discuss recent findings concerning the balanced activities of Rho-GTPases and, in particular, aspects of the regulation of the endothelial barrier. We highlight the role of Rho-GTPases in the intimate relationships between biomechanical forces, microenvironmental influences and endothelial intercellular junctions, which are all interwoven in a beautiful filigree-like fashion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endothelium is a dynamic and metabolically active single layer of cells that lines the entire vasculature. As is now well established, endothelial cells (ECs) play a critical role in many physiological processes, including angiogenesis, permeability, control of vasomotor tone, the maintenance of blood fluidity, the trafficking of blood molecules and cells and both innate and adaptative immunity (Aird 2012). ECs act as gatekeepers and control the infiltration of cells and blood proteins into the vessel wall (Dejana 2004). During the last few decades, both intercellular junctions and cell matrix adhesions have been shown to be key elements in the barrier properties of both epithelial cells and ECs and the disruption of these junctions has been demonstrated to be a primary cause for the formation of intercellular gaps (Spindler et al. 2010). An impaired endothelial barrier function contributes to fluid movements and passage of macromolecules into the interstitial space (Dudek and Garcia 2001), resulting in a prolonged or uncontrolled vascular leak and edema (Weis 2008). Moreover, vascular leakage is a hallmark of several cardiovascular and inflammatory diseases (Mehta and Malik 2006). Impaired vascular barrier integrity is also directly related to other disorders, such as cancer, atherosclerosis and diabetes and leads to life-threatening conditions such as sepsis and acute lung injury (Weis and Cheresh 2005; Rolfe et al. 2005; Vandenbroucke et al. 2008; Bakker et al. 2009; Birukov 2009; Lee and Slutsky 2010). Despite the direct consequences of these conditions, no specific treatments and/or therapies are available to prevent or reduce them (Goldenberg et al. 2011).
Over the last decade, Ras-homolog (Rho) guanosine triphosphatases (GTPases), by virtue of their ability to control various aspects of cell behavior, particularly cell adhesion and dynamics of the cytoskeleton (Tapon and Hall 1997; Narumiya et al. 1997), have clearly been shown to be important regulators of the barrier function of the endothelium (Dudek and Garcia 2001; Wójciak-Stothard and Ridley 2002; Dejana 2004; Mehta and Malik 2006; Vandenbroucke et al. 2008). Rho-GTPases are activated by diverse vaso-active agents such as thrombin, vascular endothelial growth factor (VEGF), histamine, tumor necrosis factor-α (TNF-α) and platelet-derived growth factor, via G-protein-coupled receptors (GPCRs) or tyrosine kinase receptors that induce cell contractility or stabilization (for other recent reviews of endothelial permeability and Rho-GTPases, see Beckers et al. 2010; Spindler et al. 2010; Storck and Wójciak-Stothard 2013; Loirand et al. 2013). Moreover, bio-physical stimuli such as (blood) pressure and shear stress can also activate Rho-GTPases (Wójciak-Stothard and Ridley 2002; Lessey et al. 2012). As we have recently shown, Rho-GTPases can exert both positive and negative effects on the integrity of the vascular barrier (Szulcek et al. 2013). This balance of activities is a tight and thoroughly orchestrated mechanism. Control of Rho-GTPase activity involves more than 150 different regulatory proteins. However, knowledge about the individual contribution of these regulatory proteins to the spatio-temporal control of vascular barrier dynamics remains fragmentary (Beckers et al. 2010).
The endothelium senses subtle changes in its microenvironment, which in vivo is mechanically compliant and highly dynamic and has the ability to respond with appropriate alterations in barrier characteristics. Our blood vessels are known to stiffen with age (Redfield et al. 2005) and this stiffening is increased by risk factors associated with many common disorders such as diabetes, hypertension, atherosclerosis and renal disease. One consequence of stiffening is profound vascular remodeling, which exposes the EC barrier to abnormal hemodynamic forces, modified extracellular matrix (ECM) ligation and inflammatory cytokines (Wang and Fitch 2004; Conway and Schwartz 2013). In addition to low shear stress and cyclic stretch, enhanced arterial wall stiffness has been observed to be a precondition for the initiation and development of atherosclerosis (Ohayon et al. 2011). Recent data now indicate a critical role for ECM stiffness in the regulation of the Rho-GTPase signaling balance, with consequences for the intercellular force balance and integrity of endothelial intercellular junctions (Krishnan et al. 2011; Huynh et al. 2011; Birukova et al. 2013a).
In this review, we will discuss recent findings concerning the crucial role of Rho-GTPases in the maintenance, disruption and restoration of the integrity of the vascular barrier. We focus on the dual behavior of Rho-GTPases, as they can exert both positive and negative effects that directly influence the status of the endothelial barrier. Moreover, the precisely regulated balance between GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) is highlighted. Finally, we discuss the contribution of Rho-GTPases to biomechanical forces in the endothelium and the consequences for vascular permeability.
Permeability in the endothelium
The maintenance of a semi-permeable and size-selective barrier appears to play a crucial role in controlling the passage of macromolecules and fluid between the blood and the interstitial space. Loss of its proper function can lead to vascular leakage and subsequent edema and tissue inflammation, the hallmarks of several cardiovascular and inflammatory diseases (Mehta and Malik 2006). Transient permeability is thus necessary to ensure normal vascular homeostasis (Dejana et al. 2009; Monaghan-Benson and Wittchen 2011). Permeability to solutes and/or macromolecules can occur via two main routes: through channels or vesicles within an individual EC via the transcellular pathway, or between two adjacent ECs via the paracellular pathway (Mehta and Malik 2006; Vandenbroucke et al. 2008; Weis 2008). The transcellular pathway (also named transcytosis) is vesicle-mediated and specialized for the transport of macromolecules (e.g., plasma proteins) across the endothelial barrier. This process begins on the luminal side of the plasma membrane where macromolecules interact with specific macrodomains enriched with Caveolin-1 (Cav-1). This is followed by the scission of the vesicles into the cytosol before their final release through the basal membrane via exocytosis (Mehta and Malik 2006; Komarova and Malik 2010). In contrast, the paracellular permeability pathway is regulated by a complex network of adhesion molecules whose main function is to allow the diffusion of molecules within a 3-nm-diameter range and to restrict the passage of larger-sized molecules (Mehta and Malik 2006; Vandenbroucke et al. 2008; Wallez and Huber 2008; Weis 2008; Komarova and Malik 2010). However, in many pathological conditions, the regulation of the adhesion molecules at cell-cell contacts is misbalanced and results in the hyperpermeability of the endothelium. Regulation of endothelial junctions is thus of critical importance for all types of permeability and can be influenced by many pathways. For a better appreciation of the multiple roles of Rho-GTPases in its regulation, we will first discuss the major determinants of endothelial barrier function in more detail.
Endothelial junctions
ECs are connected by two main types of intercellular junctions, namely tight junctions (TJs) and adherens junctions (AJs). In both TJs and AJs, adhesion is mediated by transmembrane proteins that promote homophilic interactions and form a zipper-like structure along the cell border (Dejana 2004). The distribution of TJ and AJ proteins is, in ECs, highly intermingled, indicating the absence of distinct distribution patterns for their function as seen in other cell types (Dejana 2004; Vestweber et al. 2009; Popoff and Geny 2009). For transmitting signals between adjacent cells, a third type of junction is expressed, termed gap junctions (GJs). GJ complexes are channel-like structures that lie between two ECs and that allow the passage of water, ions and other small molecules for signaling along the cell layer (Mehta and Malik 2006; Komarova and Malik 2010). GJs, TJs and AJs function as specialized communication structures that relay cell position, growth and apoptosis and cell differentiation and that regulate homeostasis, however, all in their own unique way (Dejana 2004). To guide these diverse signaling pathways, cell junctions are highly regulated and dynamic structures whose main regulatory partners, among others, include the Rho family of GTPases and the Ras family, which is involved in cell proliferation and differentiation (Popoff and Geny 2009).
Adherens junctions
AJs are located at cell-cell contact areas. By virtue of their connection with the actin cytoskeleton, they participate in the maintenance of tissue integrity. The main molecular component of endothelial AJs is vascular endothelial cadherin (VE-cad), which is exclusively expressed in blood and lymph vessels (Dejana 2004; Gavard 2009; Giannotta et al. 2013). VE-cad deletion induces massive vascular defects leading to early mortality in embryonic mice, whereas in adults, loss of its function causes a hyperpermeability phenotype (Carmeliet et al. 1999; Crosby et al. 2005). VE-cad consists in five extracellular homologous cadherin-like repeats, a transmembrane domain and a cytoplasmic tail. The external domain mediates homophilic interactions (in trans) among cadherins of adjacent cells and confers Ca2+ dependency. Although the transmembrane domain and cadherins cooperate to promote parallel binding within the same cell (in cis), the cytoplasmic tail binds to intracellular proteins, i.e., β-catenin, p120-catenin and plakoglobin (γ-catenin), providing cadherin stability and the adhesive properties of AJs (Koch et al. 1999; Dejana 2004; Wallez and Huber 2008; Ebnet 2008; Komarova and Malik 2010).
Four main catenins that coordinate the dynamics and signaling of AJs have been identified: β-catenins, p120, plakoglobin and α-catenin. The first three contain homologous armadillo repeats, whereas α-catenin is homologous to vinculin, an actin-binding protein. Additionally, p120-catenin binds to the juxtamembrane domain of VE-cad and is involved in its retention at the cell surface and in preventing its degradation. The C-terminal domain binds with β-catenins and α-catenins, which in turn connect AJs to the actin cytoskeleton (Dejana 2004). This association to the actin cytoskeleton is crucial for junction assembly and maintenance. For instance, increased tension within the AJs has been shown to be associated with an increase in AJs size (Liu et al. 2010). Furthermore, growth, maintenance and remodeling can be hampered by the inhibition of RhoA/Rho kinase (ROCK) contractility (Huveneers and Danen 2010; Liu et al. 2010).
Since actin binding is crucial for AJ function, the linker proteins play an important role. A number of known actin-binding proteins are located at AJs, such as vinculin, EPLIN (epithelial protein lost in neoplasm), α-actinin, the previously mentioned α-catenin (Dejana and Orsenigo 2013) and other adhesive proteins including N-cadherin, platelet-endothelial cell adhesion molecule (PECAM-1) and junctional adhesion molecules (JAMs). However, the way that these proteins cooperatively interact and relate to the junctions and to actin dynamics is not yet fully understood (Dejana and Orsenigo 2013). For instance, α-catenin has been considered to link AJs to the actin cytoskeleton by direct interactions with both filamentous actin (F-actin) and β-catenin (Lampugnani et al. 1995). Nevertheless, this concept has been questioned because of observations showing that its binding to F-actin and β-catenin is actually mutually exclusive (Drees et al. 2005; Yamada et al. 2005). The bridge to the cytoskeleton might be mediated by other actin-binding proteins also located in the AJs, such as EPLIN and vinculin (Chervin-Pétinot et al. 2012). As described recently, α-catenin in combination with EPLIN (in an actomyosin-dependent manner) could cause the recruitment of vinculin and α-actinin to the AJs, leading to reinforcement of the actin cytoskeleton (Gulino-Debrac 2013). Moreover, the involvement of vinculin in the force-dependent remodeling of the AJs (Huveneers et al. 2012) and the critical role for Rho-GTPase in this response have previously been described (Braga 1999; Lampugnani et al. 2002).
AJs can occur in two different forms: in a stable and linear form or in a dynamic punctuated form. The first form, also known as the zonula adherens, is supported by linear actin bundles that align along the cell-cell junctions and that are defined as circumferential actin bundles (CAB); the second form, the dynamic punctuated type, is connected by radial stress fibers (Millán et al. 2010; Hoelzle and Svitkina 2012; Huveneers et al. 2012). The switch between these two different AJ forms is controlled by many extracellular stimuli (shear stress, wound healing, inflammatory mediators and angiogenesis) and cell density (Lampugnani et al. 1995; Taha et al. 2014). Inflammatory mediators, such as thrombin or histamine, which induce the increase of permeability, prompt the formation of punctuate AJs (Millán et al. 2010; Huveneers et al. 2012). On the other hand, GPCR agonists, which are known to act as barrier integrity enhancers, induce the formation of linear AJs (Garcia et al. 2001; Fukuhara et al. 2005; Augustin et al. 2009). In addition, RacGTPase Rap1 has also recently been identified as a regulator of this switch of AJ forms (Ando et al. 2013); this will be discussed in more detail in the following section.
Moreover, a new mechanism that explains the capacity of cell junctions to respond to stimuli and concomitant junction dynamics and cell adhesion has recently been proposed. VE-cad dynamics and its subsequent plasticity are pushed by an interdependent control cycle between VE-cad-mediated cell adhesion and ARP2/3-controlled and actin-driven junction-associated intermitted lamelipodia (JAIL) formation (Taha et al. 2014).
Tight junctions
In the endothelium, TJs represent only one-fifth of the cell junctions but are essential for maintaining the integrity of the endothelial barrier (Mehta and Malik 2006). Considerable variety exists between the different areas of the vascular tree: large artery ECs, exposed to high flow, exhibit a well-developed TJ system, whereas in the microvasculature, TJs display lower complexity (Wallez and Huber 2008).
The main molecular components of TJs are transmembrane proteins, namely occludins, claudins and JAMs. TJs are known to have two mutually exclusive functions: a fence function that prevents the diffusion of apical and basolateral membrane components and a gate function that controls the paracellular passage of ions and solutes (Hartsock and Nelson 2008). In addition to these two classic functions, TJs have been considered to act as dynamic heteromeric signaling complexes in a bidirectional way: they can transmit information from the outside to the cell interior, thereby regulating gene expression and consecutive cellular responses and, the other way around, from the interior to the membrane on which new or existing TJs are located, thereby regulating their assembly and function (Terry et al. 2010). Moreover, TJs are also known to be linked to the actin cytoskeleton and are hence essential to the regulation of the endothelial barrier (Fanning 1998).
Despite this knowledge of the function and the characteristic signaling of TJs, their regulation in the endothelium is far less-well understood than the regulation of the AJs (Vandenbroucke et al. 2008). What has become clear is that, like the TJs, Rho-GTPases are crucial components of signaling mechanisms associated with their integrity and dynamics (Xiaolu et al. 2011; He et al. 2012; Stamatovic et al. 2012).
EC matrix adhesion
In addition to adhering to neighboring cells, the endothelial microenvironment is also characterized by the adhesion of the endothelial ventral/basolateral surface to the sub-endothelial ECM. This EC-ECM interaction is an important determinant of endothelial monolayer behavior. It involves more than 150 intracellular proteins and connects the cytoskeleton to the ECM via integrins (Zaidel-Bar and Geiger 2010). An important part of the local biochemical and mechanical niche of the cell is defined through this interaction complex. The interplay with the microenvironment is balanced within the cytoskeleton, which is able to handle various stimuli based on its enormous capability to disassemble, assemble and stabilize original interactions (Bursac et al. 2007). Through the matrix interaction, the endothelium experiences physical forces that are either endogenously or exogenously generated and that are responsible for the majority of the cytoskeleton tension (Fig. 1). This tension can be subsequently transmitted through the cytoskeleton, where it regulates the function of TJs and AJs to a great extent. A similar plasticity trait holds true for the cytoskeleton connection with endothelial junctions and the ECM. For example, the initial integrin linkage to the ECM, named nascent adhesion, is poorly connected to the cytoskeleton and hardly bears any force (Kim et al. 2000; Bayless et al. 2000; Zamir and Geiger 2001). Upon different force stimuli, force transmission can increase, which acts as a signal for the reinforcement of the adherent structures. In response, integrins (predominantly αvβ3 and α5β1), filamentous actin and various linker proteins are recruited to the nascent adhesions site (Schwartz 2010). This reinforcement gives rise to focal complexes that can subsequently be transformed into fully grown focal adhesions (FAs) when traction forces (TFs) are further increased (Galbraith et al. 2002). Interestingly, integrin activation itself can cause an increase in internal contractility via the RhoA/Rho kinase (ROCK) pathway and because cell contractility results in integrin activation, this process resembles a self-sustaining mechanical loop. Recently, CCM1 (an effector of Ras-related protein-1 small GTPase, Rap1) has been shown to be a possible regulatory protein controlling this process, as its absence destabilizes ICAP-1 (integrin cytoplasmic domain-associated protein-1), which in turn results in the activation of β1 integrins and additional binding to the ECM (Faurobert et al. 2013)
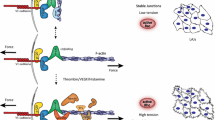
Representation of the biomechanical regulation of the endothelial barrier. Various types of mechanical forces are applied to these cells: these forces can be exogenous, i.e., externally applied, such as shear stress (left) and hydrostatic pressure (center), or they can be endogenously generated (i.e., by the cell or cytoskeleton itself), such as osmotic pressure (center) and actomyosin II contraction (right). The GTPase cycle (center). Rho-GTPases cycle between an active state (GTP-bound) and an inactive state (GDP-bound). This shift is mediated by three types of regulatory proteins: Rho guanine nucleotide exchange factors (Rho-GEF), Rho-GTPase-activating proteins (Rho-GAP) and Rho guanine-nucleotide dissociation inhibitors (Rho-GDI). Various types of stimuli can induce the actions of Rho-GEFs, Rho-GAPs, or Rho-GDIs: biomechanical stimuli (through mechanosensors such as cell adhesion molecules, e.g., platelet-endothelial cell adhesion molecule (PECAM), focal adhesion complexes, or junctional protein complexes) and biochemical stimuli such as cell membrane receptors. Once activated, Rho-GTPases interact with numerous effector proteins to mediate a response (JAM junctional adhesion molecule, F-actin filamentous actin)
However, when the FA stops being required, e.g., on the reduction in force transmission, the opposite path can be activated resulting in a reduced matrix-cytoskeleton link. The complex interplay of exogenous and endogenous applied forces and the ability of ECs to cope with these forces will determine the permeability status of the endothelial monolayer via the linkage to the endothelial junctions (Fig. 1). Nevertheless, we must bear in mind that this is a continuous process needing constant feedback and that the strength of the cell-matrix interaction is not the only parameter that determines force transmission.
Force transmission via the cell-matrix interaction is tightly regulated and predominately achieved by the Rho-GTPase balance. As has been known for more than two decades, Rho plays a significant role in the regulation of FA assembly (Ridley and Hall 1992). However, the specific molecular mechanism that translates mechanical cues into cellular signaling by Rho-GTPases is not yet fully understood, as recently reviewed by Ross et al. (2013). A first example that gives a hint of the specific molecular basis for cellular mechanotransduction and the coupling to Rho-GTPase family member Rac is the actin-binding protein filamin A. Recent studies have shown that filamin A can be mechanically deformed by its interaction with the tension-bearing actin cytoskeleton; this causes dissociation of FilGAP and the binding of β-integrins to the filamin A-actin complex (Shifrin et al. 2009; Ehrlicher et al. 2011). The release of FilGAP causes a negative feedback to Rac activation potentially explaining the inhibition of lamellipodia formation (Katsumi et al. 2002). A second example that links FA force-sensitivity to Rho-GTPase family activity is the work of Guilluy and colleagues. In their study, a feedback mechanism was shown to exist between the force-dependent stimulation of β-integrins and the increased activity of RhoA by the activation of two GEFs: leukemia-associated Rho-GEF (LARG) and microtubule-associated GEF (GEF-H1; Guilluy et al. 2011b).
Rho-GTPases and barrier function
Rho-family proteins are related to many different cell activities: they are known regulators of the actin cytoskeleton, cell-cycle progression and gene transcription and have been implicated in various cellular processes such as adhesion, migration, phagocytosis, cytokinesis, morphogenesis, polarization, growth and survival (Etienne-Manneville and Hall 2002; Rossman et al. 2005). Rho-GTPases are able to modulate the organization and dynamics of the actin cytoskeleton through the activation of downstream signaling. Thus, they can either induce cell contraction or enhance cell protrusion and/or expansion by the activation of a complex cascade of effectors. Moreover, this process requires a meticulous spatio-temporal balance (Wójciak-Stothard and Ridley 2002), which involves over 150 regulatory proteins, as we have recently reviewed (Beckers et al. 2010). However, knowledge regarding the contribution of each individual regulatory protein to the regulation of endothelial barrier integrity remains fragmental.
Rho-GTPases are one of five families of the Ras protein superfamily grouped according to sequence homologies (Rojas et al. 2012). To date, 22 are known in mammals (Fransson et al. 2003; Aspenström et al. 2004; Wennerberg and Der 2004; Jaffe and Hall 2005; Rossman et al. 2005; Ridley 2006; Rojas et al. 2012), of which 18 have been identified in the human genome: Rho (A, B, and C), Rac (1, 2, and 3), Cdc42, TC10, Rho-G, TCL, Rif, RhoD, RhoV/Chp, RhoU/Wrch, Rnd (1, 2, and 3) and RhoH/Tif (Rojas et al. 2012; Hall 2012). Rho family members act as molecular switches, oscillating between an active GTP-bound state and an inactive GDP-bound state (Fig. 1; Wójciak-Stothard and Ridley 2002; Etienne-Manneville and Hall 2002), thereby changing the conformation of the nucleotide-binding site (Cherfils and Zeghouf 2013). This swap induces conformational changes in switch I and switch II of the GTPase regions that allows small GTPases to bind to effectors and forward the upstream signals (Spindler et al. 2010). So far, three different types of regulatory proteins are known to affect the activation/inactivation state: GEFs, which catalyse the exchange of GDP for GTP; GAPs, which stimulate the intrinsic GTP hydrolysis; and guanine-nucleotide dissociation inhibitors (GDIs), which inhibit the dissociation of GDP (Fig. 1; Jaffe and Hall 2005). When in the active form (GTP-bound), these proteins transduce signals by interacting with downstream effectors. The activation process is mediated by many cell-surface receptors, including cytokines, tyrosine kinases, adhesion receptors and GPCRs (Bishop and Hall 2000; Rossman et al. 2005).
The three best-characterized members of this family of proteins are RhoA (Ras homolog gene family member A), Rac1 (Ras-related C3 botulinum toxic substrate 1) and Cdc42 (Cell division control protein 42). All three are key players in regulating the permeability of the endothelial barrier, as will be outlined below. In addition, recent evidence indicates an emerging role of RhoB in barrier function. Finally, we will briefly touch upon a related small GTPase, Rap1, involved in regulating endothelial vascular function.
RhoA
According to the original paradigm, RhoA is associated with loss of barrier integrity, Rac1 with maintenance and Cdc42 with barrier stabilization and recovery. During the late 1990s, stimulation with vaso-active substances such as endotoxin, VEGF and thrombin was shown to activate RhoA-induced (in vitro) loss of endothelial barrier integrity (Essler et al. 1998; van Nieuw Amerongen et al. 1998). This loss of barrier integrity was observed to be accompanied by the formation of prominent F-actin fibers and a contractile response of the endothelium (see The contraction process). In apparent contrast, RhoA was also observed to be involved in the maintenance of endothelial barrier function (Vouret-Craviari et al. 1998). We now know that basal activity of RhoA is important in the maintenance of inter-endothelial junctions (IEJs) by stimulating VE-cad to anchor at the membrane and by enhancing endothelial cortical actin via its effector diaphanous (Dia; Gavard et al. 2008). Recently, using live fluorescence resonance energy transfer (FRET) microscopy, we showed that RhoA activity, in addition to being associated with cell contractility, is also associated with barrier-protective effects (Szulcek et al. 2013). Activity hotspots associated with closure but not opening, of junctional spaces were observed in the vicinity of membrane protrusions (Szulcek et al. 2013).
The contraction process
The cross-linking activity of myosin light chain (MLC) is dependent on cytosolic Ca2+ and its binding to calmodulin (Wysolmerski and Lagunoff 1990). This interaction results in the phosphorylation of MLC by myosin light chain kinase (MLCK), which induces increased ATPase activity and myosin assembly into bipolar filaments and, as a consequence, results in the activation of myosin motor proteins (Lessey et al. 2012). The actomyosin-II-generated contraction is tightly controlled by the phosphorylation of the regulatory MLC domain but can also be hampered by phosphatase activity. The balance between MLCK and myosin light chain phosphatase (MLCP) determines the contractile status of the EC (Goeckeler and Wysolmerski 1995; Verin et al. 1998; Zeng et al. 2000; Kim et al. 2012). A prominent determinant in the interplay between MLCK and MLCP is RhoA and its downstream effector ROCK (Birukova et al. 2004). ROCK is capable of phosphorylating the regulatory subunit of MLCP at Thr695, Ser894 and Thr850 (Essler et al. 1998). This results in the inactivity of MLCP and, by the inhibition of MLC, dephosphorylation, which causes actomyosin II contraction and can cause endothelial barrier disruption (Fig. 2; van Nieuw Amerongen et al. 2000; Birukova et al. 2004). This mechanism can proceed even in the presence of relatively low levels of calcium (Connolly and Aaronson 2011). Pharmacological inhibition of ROCK decreases endothelial cytoskeletal contraction, reduces stress fiber formation and reduces permeability (van Nieuw Amerongen 2003; Birukova et al. 2004; Hardin et al. 2013).
Representation of the main actions of Rho-GTPases in the regulation of the endothelial barrier. A stable endothelial barrier is primarily mediated by Ras-related C3 botulinum toxic substrate 1 (Rac1) and cell division control protein 42 (Cdc42). Through various mechanisms and pathways, these Rho-GTPases promote adherens junction stabilization and the inhibition of Ras homolog gene family member A (RhoA), which decreases actomyosin contractility (left). Moreover, Rac1 is also considered to be a main mediator in barrier restoration through the Rap1-Tiam1 (T-cell lymphoma invasion and metastasis1) pathway (right). For the sake of simplicity, signaling pathways such as the phosphorylation of junctional proteins are not included here. Vaso-active agents such as thrombin or vascular endothelial growth factor (VEGF) can activate the RhoA signaling pathway and, through its effectors, induce endothelial contraction and consequent disassembly of endothelial cell junctions (center). In addition, VEGF can induce Rac1 activation, which in turns leads to p21-activated kinase (PAK) activation and consequent VE-cadherin endocytosis (PAR-1 protease-activated receptor-1, ROCK RhoA kinase, JAM junctional adhesion molecule, S1P sphingosine-1-phosphate, ZO zonula occludens, S1PR1 sphingosine-1-phosphate receptor1, F-actin filamentous actin)
Another effect of ROCK activity is the phosphorylation of LIM kinase, resulting in the inhibition of cofilin, which is an important actin-severing protein (Fig. 2; Sumi et al. 1999). As a consequence, the depolarization of actin is hampered leading to an indirect promotion of actin filaments and their propulsive force. Thus, because of the activation of the downstream effector ROCK and the consequent increased level of MLC and phosphorylation at the F-actin filaments, stress fiber formation occurs, which allows contractile forces to be set up and the pulling apart of the IEJs.
Rac1 and Cdc42
Similar to RhoA, Rac1 has a dual role in endothelial barrier function: it can mediate both permeability and adhesion of the endothelial barrier, depending on its context. Initially, its main function was shown to be related to junction stabilization and, thereby, to counteracting the permeability-increasing effects of RhoA activation (Wójciak-Stothard et al. 2001; Waschke et al. 2004). Rac1 activity increases during junction formation (Noren et al. 2001; Lampugnani et al. 2002) and has been associated with the re-annealing of junctions in response to GPCRs activation (Kouklis et al. 2004). Interestingly, its deletion has been observed to lead to an enhanced basal and thrombin-induced increased permeability in ECs, which emphasizes its direct connection to intercellular junctions and gap formation (Wójciak-Stothard et al. 2001). Thrombin-mediated permeability is accompanied by long-lasting Rac1 inactivation, which in consequence weakens the junctional complexes (Kouklis et al. 2004). In contrast, upon action by VEGF, the activity of Rac1 is increased, as is its downstream effector p21-activated kinase (PAK1; Garrett et al. 2007), which induces the phosphorylation and internalization of VE-cad and the consequent permeability of the endothelial barrier (Fig. 2; Gavard and Gutkind 2006). Similarly, as observed both in vitro and in vivo, platelet-activating-factor-induced hyperpermeability requires the activation and translocation of Rac1 (Knezevic et al. 2009). Moreover, the deletion of Rac1 in primary ECs in vitro has been shown to decrease VEGF-mediated endothelial permeability (Tan et al. 2008). Additionally, VEGF-induced Rac1 activation leads to the production of reactive oxygen species (ROS) resulting in the phosphorylation of VE-cad and the detachment of β-catenin and p120ctn, which increase permeability (Monaghan-Benson and Burridge 2009).
The maintenance of the endothelial barrier depends on the activation of RhoA, Rac1 and Cdc42. Moreover, RhoA activity decreases upon gap closure because of VE-cad and p120ctn engagement (Beckers et al. 2010). Rac1 and Cdc42, by inhibiting actomyosin II contractility and promoting the assembly of IEJs, promote tethering forces in the endothelium (Wójciak-Stothard and Ridley 2002; Waschke et al. 2006; Beckers et al. 2010; Spindler et al. 2010). Both Rac and Cdc42 appear to be equally relevant for barrier maintenance and enhancement; however, Cdc42 has different functions (Spindler et al. 2010). Cdc42 seems to be the most prominent GTPase for barrier restoration, because of its ability to regulate VE-cad, which is a prerequisite for AJ stability (Kouklis et al. 2004; Broman et al. 2007). During the recovery phase of AJs, post thrombin stimulation, Cdc42 activation is prolonged and Cdc42 is translocated from the cytosol to the membrane (Kouklis et al. 2004). After the loss of homotypic VE-cad interactions, the cytoplasmatic domain of VE-cad is responsible for Cdc42 activation by an as yet unknown Cdc42-specific GEF.
The coordinated activation of RhoA, Rac1 and Cdc42 secures effective vascular function (Storck and Wójciak-Stothard 2013).
RhoB
RhoB has been recently implicated in the regulation of vascular function. It is highly expressed in the lung and is 85 % homologous to RhoA (Storck and Wójciak-Stothard 2013). RhoB is activated by hypoxia (Kajimoto et al. 2007) and oxidative stress, tumor growth factor-β (TGFβ) and tyrosine kinases, among others (Vardouli et al. 2008; Wójciak-Stothard et al. 2012). Interestingly, both RhoA and RhoB can interact with the same downstream targets and under hypoxia conditions, RhoB actions seem to be complementary to RhoA with regard to actomyosin contractility and pulmonary endothelial permeability (Wójciak-Stothard et al. 2012). According to recent data, hypoxia-activated RhoB enhances actin filament formation by interacting with forming homolog protein mDia1 (mDia), whereas RhoA promotes the phosphorylation of MLC mediated by ROCK. However, both RhoA and RhoB induce cell contractility and therefore affect endothelial permeability (Wójciak-Stothard et al. 2012). This study has shown that the inhibition of either RhoA or RhoB in human pulmonary arterial endothelial cells prevents the hypoxia-induced increase of endothelial permeability. Furthermore, the deletion of RhoB in human umbilical vein endothelial cells (HUVECs) reduces cell migration, sprouting and capillary morphogenesis but increases VEGF-induced RhoA activation (Howe and Addison 2012). As previously observed in mice, the activation of RhoB in human pulmonary vascular cells induces cell growth (Adini et al. 2003).
Rap1
Not only Rho members are involved in permeability control. Ras-related protein-1 small GTPase (Rap1) is known to be a critical regulator of cell-cell junctions and endothelial permeability. Initially described in vitro (Fukuhara et al. 2005; Cullere et al. 2005; Wittchen et al. 2005; Baumer et al. 2008), Rap1 activation by cyclic adenosine monophosphate (cAMP)-activated Epac promotes cell-cell junction formation and endothelial barrier enhancement. This signaling pathway (Epac-Rap1) has also been demonstrated in vivo, where its protective effects on the lung endothelium in a ventilator-induced lung injury mice model have been observed (Birukova et al. 2009). Similarly, Rap1 has recently been shown to enhance EC junctions (Fig. 2). Rap1 induces dynamic punctuated AJs to shift into stable linear AJs by regulating non-muscle myosin II activity and by differentially regulating two Rho-GTPases pathways: it suppresses the Rho-ROCK-myosin II pathway and therefore inhibits the formation of stress fibers connecting to punctuate AJs and it enhances, at cell-cell junctions, Cdc42 activation, which, through the myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) that activates myosin II, promotes CAB formation (Ando et al. 2013). Furthermore, the signaling cascade through which Rap1 regulates Rho and actomyosin-induced tension has recently been identified. Ras-interacting protein 1 (Rasip1) is a Rac-effector that is involved in endothelial barrier function through its interaction with Rho-GAP ArhGAP29 (Xu et al. 2011). Recent data show that the axis Rap1-Rasip1-ArhGAP29 induces endothelial barrier function (Post et al. 2013).
Interestingly, another group has shown that Rap1 and its family member Rap2 have antagonistic actions with regard to the control of endothelial barrier resistance (Pannekoek et al. 2013). Whereas the presence of Rap1 is related to barrier resistance induction, the absence of Rap2 results in an increased barrier resistance. Moreover, the deletion of both isoforms of Rap1 (Rap1a and Rap1b) has been observed to lead to partial embryonic lethality, showing its importance in angiogenesis and the maintenance of vascular stability (Chrzanowska-Wodnicka et al. 2008; Lakshmikanthan et al. 2011).
Regulation of Rho-GTPases by GEFs, GAPs and GDIs
When activated, Rho-GTPases excel in binding to numerous effector proteins leading to a cascade-like activation of downstream signals. In order to regulate so many different cellular functions, these highly dynamic proteins are tightly and spatio-temporally regulated (Pertz 2010; Thumkeo et al. 2013). Moreover, as a repercussion of this universality, Rho proteins regularly cooperate or antagonize each other to control many diverse cellular processes. Indeed, Rho proteins crosstalk and interact at various levels, being involved in the regulation of activity, the regulation of protein expression and the regulation of downstream effectors (Guilluy et al. 2011a). These coordinated actions of Rho-GTPases are required for various biological processes, including the regulation of endothelial barrier function.
As previously mentioned, three classes of regulatory proteins control the activation state of small GTPases: GEFs, GAPs and GDIs (recently reviewed in Cherfils and Zeghouf 2013). Here, we provide recent examples of the specific contribution of the members of each class (GDIs/GAPs/GEFs) to particular aspects of the regulation of permeability.
RhoGDIs
Rho-GTPases, in the inactive form (GDP-bound), can be sequestrated in the cytoplasm by Rho-GDIs (Fukumoto et al. 1990; Olofsson 1999). Rho-GDIs consist, so far, in three members: Rho-GDI1 (also known as Rho-GDIα), Rho-GDI2 (also known as Rho-GDIβ, LY-GDI and D4-GDI) and Rho-GDI3 (also known as Rho-GDIγ; Dransart et al. 2005; Garcia-Mata et al. 2011). Rho-GDI-1 is the most abundant, best-characterized member, is ubiquitously expressed and has been shown to bind to Rho-GTPases in vitro (Qiao et al. 2003, 2008; Knezevic et al. 2007). However, whether Rho-GDI itself can affect endothelial barrier function in vivo is unknown. An elegant approach by Gorovoy and colleagues showed an increased basal permeability in lungs of Rho-GDI-1 knockout mice and increased RhoA activity; these findings suggest that by inhibiting RhoA activation, Rho-GDI-1 regulates lung micro vessel endothelial barrier function in vivo (Gorovoy et al. 2007). To the best of our knowledge, no information regarding the influence of Rho-GDI2 or Rho-GDI3 on barrier function is available at the moment.
RhoGAP
Compared with Rho-GDIs, Rho-GAPs represent a much larger group of proteins that regulate Rho-GTPases. As mentioned before, Rho-GAPs promote the hydrolysis of GTP-bound Rho-GTPases causing their inactivation. To date, more than 70 Rho-GAPs have been identified, of which 67 are found in the human genome and at least one of them, p73GAP, has preferential endothelial expression (Su et al. 2004). Rho-GAPs are regulated by extracellular stimuli, such as growth factors and vaso-active agents (Fig. 1) (Tcherkezian and Lamarche-Vane 2007). For example, as recently reviewed, thrombin can induce the inactivation of RhoA through p190Rho-GAP, as can it inactivate Rac1 through ArhGAP22 or FilGAP (Beckers et al. 2010). Similarly, VEGF stimulation can promote the inactivation of Cdc42 by Rich1 and induce the inactivation of Rac1 through ArhGAP22, p73Rho-GAP, or p68Rho-GAP (Beckers et al. 2010). However, solid evidence of a direct role in the regulation of the endothelial barrier exists only for p190Rho-GAP (Siddiqui et al. 2011; Ponik et al. 2013; Zebda et al. 2013).
Recent studies of Rho-GAP p190Rho-GAP-A have shed light on a long-standing question of the correlation between the transcellular and paracellular pathway. These two permeability mechanisms are now known to be interrelated, with molecule crosstalk between them. Cav-1 has been shown to be necessary for transcellular protein routes (Michel 1996) but mice lacking this molecule display permeable AJs, suggesting that Cav-1 probably helps to seal these junctions (Schubert et al. 2002). Indeed, recent work has shown that the loss of Cav-1 activates the enzyme endothelial nitric oxide synthase, which generates nitric oxide that, by reaction, forms peroxynitrite. As a consequence, the peroxynitrite modifies the AJ protein p190Rho-GAP-A, preventing it from inhibiting RhoA. As a further consequence, activated RhoA interferes with the AJ structure, disassembles it and makes it leaky (Siddiqui et al. 2011). In cav-1-deficient mice, the tyrosine nitration of p190Rho-GAP increases RhoA activation and thrombin-induced endothelial permeability (Nuno et al. 2012). Moreover, Cav-1 has also been shown to regulate Rac1 activity in rat pulmonary microvascular endothelial cells. Its down-regulation significantly increases the activity of Rac1, which enhances AJs and therefore induces barrier protection. However, if stimulated with TNFα, Cav-1 down-regulation decreases Rac1 activity, which leads to barrier disruption effects (Shao et al. 2013).
Finally, as mentioned in the previous section, Rap1 is a Rac GTPase involved in the regulation of cell-cell adhesion, as it is known to be a key regulator of junctional formation (Kooistra et al. 2007). Two Rap1 effectors, namely Rasip1 and Ras-association and dilute domain-containing protein (Radil), by interacting with a Rho-GAP (ArhGAP29), have recently been shown to form a complex that mediates Rap1-induced endothelial barrier function (Post et al. 2013). Through this ArhGAP29 interaction, the RhoA signaling pathway is impaired and therefore, stress fiber formation is inhibited but junctional tightening is enhanced (Post et al. 2013).
RhoGEFs
Rho-GEFs represent the functional connection between the various extracellular stimuli and the activation of Rho-family GTPases and, as consequence, regulate numerous cellular responses (Zheng 2001; Rossman et al. 2005). Rho-GEFs are essential for the regulation of the cytoskeleton and thus influence the status of cell-cell junctions. Based on their homology, over 80 GEF members are known (Erickson and Cerione 2004; Hall 2012). However, small differences occur between them (either in the domains or flanking sequences) determining their role in the regulatory process. Like Rho-GAPs, Rho-GEFs are mainly regulated by external stimuli such as vaso-active agents or even exogenous forces. Upon thrombin stimulation, RhoA can be activated by several Rho-GEFs, such as LARG, PDZ-Rho-GEF, p115Rho-GEF, or GEF-H1. Similarly, Cdc42 can be thrombin-activated through the action of Rho-GEF Px-RICS. In contrast, VEGF can induce RhoA activation through other Rho-GEFs, namely ECT2 or Syx and it can also activate Rac1 through Vav2 or even Rap1 via RapGEF1 (Beckers et al. 2010). Here, we highlight a few recent examples of Rho-GEFs that influence the endothelial barrier directly, revealing exciting novel insights into the regulation of vascular function.
The cell cytoskeleton is composed of actin filaments, microtubules and intermediate filaments. Disturbance of these elements can lead to changes in cell shape and also an increase in vascular permeability. The key role of microtubule-associated GEF-H1 in the microtubule-mediated regulation of Rho activity, cytoskeletal remodeling, and endothelial permeability has been reported (Birukova et al. 2006). Moreover, GEF-H1 is associated with microtubule disassembly, which in turn is associated with the disruptive effects of vaso-active molecules such as thrombin, which activate the Rho signaling cascade, disrupting the endothelial barrier. Additionally, the depletion of GEF-H1 in a specific mice model attenuates vascular leakage induced by lung ventilation (Birukova et al. 2010). This study has also shown the importance of microtubules, the cytoskeleton and forces in vascular function. In a recent publication, the same group revealed the way that the modulation of GEF-H1 activity via the Rac1 effector PAK1 and the consequent suppression of Rho signaling protect the atrial natriuretic-peptide-mediated endothelial barrier in an acute lung injury model (Tian et al. 2013).
Upon stimulation of lysophosphatidic acid (LPA), protease-activated receptor-1 (PAR-1), angiotensin-II and sphingosine-1-phosphate (S1P), GPCRs can induce the activation of the heterotrimeric G proteins Gα12 and Gα13. Once activated, Gα13 can bind to p115Rho-GEF initiating the activation cycle of RhoA and inducing its downstream signaling cascade via ROCK and mDia, which impair the barrier function (Mehta and Malik 2006). However, in addition to being a GEF for Rho-GTPase, p115Rho-GEF also acts as a GAP for Gα12 and Gα13 (Hart et al. 1998; Kozasa et al. 1998).
TNF-α, a common proinflammatory factor, is known to disrupt the endothelial barrier and thus to increase permeability (Ferro et al. 2000). Although the activation of the small GTPase Rac and the production of ROS have been previously associated with TNF-α-induced endothelial barrier dysfunction (Gertzberg et al. 2004; Papaharalambus et al. 2005), the inter-relationship between these two separate entities was unknown. In a recent study, a Rac-specific GEF that mediates TNF-α-induced vascular permeability was identified (Naikawadi et al. 2012). Phosphatidylinositol (3,4,5)-triphosphate–dependent Rac exchanger-1 (P-Rex1), which is highly expressed in vascular ECs, is a key mediator in this process, which involves Rac activation and ROS production in a phosphoinositide 3-kinase (PI3K)-dependent manner. Moreover, P-Rex1 deletion has been shown to protect junction integrity and significantly reduce intercellular adhesion molecule-1 expression, thus impairing leukocyte transendothelial migration and sequestration (Naikawadi et al. 2012). In addition, another junctional Rho-GEF has recently been identified. Tumor endothelial marker (TEM4) has been demonstrated to be crucial for cell junction integrity, cell-cell adhesion and barrier function. Its depletion disrupts HUVEC cell junctions and affects negatively endothelial barrier function (Ngok et al. 2013). Moreover, it has also been shown to regulate EC migration and to be an essential regulator of the actin cytoskeleton (Mitin et al. 2013).
Evidence has accumulated that Rap1 activation, in addition to enhancing VE-cad interaction with p120 catenin, is crucial for the down-regulation of Rho signaling and the dissolution of actin stress fibers. Moreover, Rap effector Afadin (known to regulate epithelial cell-cell junctions) mediates and stimulates AJ reannealing by the translocation of the Rac1-specific GEF Tiam1 (T-cell lymphoma invasion and metastasis1), which leads to the resealing of intercellular gaps (Birukova et al. 2013b). Interestingly, Tiam1 has also recently been reported to be involved in VEGF-induced permeability. An increased recruitment of Tiam1 to AJs and VE-cad in the absence of nitric oxide and reduced Rho activation and stress fiber formation was observed (Di Lorenzo et al. 2013).
Similarly, another Rho-GEF has been reported to be involved in Rap1- and VEGF-induced permeability. Facial-genital dysplasia-5 (FGD5) was identified in a genome-wide profile analysis of specific ECs genes (Ho et al. 2003) and some of its family members have been characterized as being GEFs for Cdc42 (Zheng et al. 1996; Hayakawa et al. 2008; Huber et al. 2008). FGD5 expressed in ECs is involved in vascular function as a regulator of vascular pruning (Cheng et al. 2012) and endothelial adhesion (Nakhaei-Nejad et al. 2012) and is known to be partially localized at cell-cell junctions (Kurogane et al. 2012). FGD5 has recently been identified as a Rap1 downstream signaling molecule inducing Cdc42 activation at EC junctions and therefore stimulating the formation of linear AJs (Ando et al. 2013)
Forces and barrier function
ECs interact with their environment and with neighboring ECs to form a monolayer structure with unique barrier properties. Both cell-cell and cell-matrix interactions are mediated by adhesion molecules, which, through specialized linker proteins, are coupled to the actin cytoskeleton. These adhesion complexes are of pivotal importance as force-bearing structures. Via these adhesions, external forces can be transmitted to the ECs and vice-versa. The transmission of forces can also be used in an opposite direction for endogenously generated forces. Hence, the actin cytoskeleton is of great importance, since this is the main framework on which all forces are exerted (Wessells et al. 1971; Wang et al. 1993; Goeckeler and Wysolmerski 1995). Coupled to this structure are various force-bearing proteins that have the ability to sense mechanical forces through their force-sensitive elements (Chen 2008; Leckband et al. 2011; Ross et al. 2013). The interaction of Rho-GTPases and forces can activate the stretch-sensitive FA protein talin and the intercellular signaling cascade that follows. By the application of force over the integrin-cytoskeleton-binding protein talin, molecular conformation changes occur that expose cryptic binding sites for vinculin (Del Rio et al. 2009; Roca-Cusachs et al. 2009). As a consequence, vinculin becomes activated in the vinculin-paxillin-focal-adhesion kinase complex, which is essential for the transmission of high traction (Plotnikov et al. 2012).
This enables the EC to translate mechanical forces into biochemically relevant signaling, a mechanism that is called mechanotransduction (Orr et al. 2006; Eyckmans et al. 2011). The sensing of forces via mechanotransduction is of critical importance for balancing the highly dynamic forces that are applied on the endothelium, which, despite this, can maintain proper cell-cell interactions. Balancing the forces within the endothelial monolayer is, in this perspective, predominantly achieved by the modulation of the FA contacts and by the fine-tuning of internal contractile forces. As a consequence, the disruption of cell-cell contacts and, thereby, the formation of inter-endothelial gaps can only result following the application of too high contractile forces or too low adhesive forces (Dudek and Garcia 2001). In both processes, Rho-GTPases play a major role and are therefore seen as the main regulators of endothelial permeability. As an example of the link between TFs and vascular permeability, Faurobert and colleagues recently connected RhoA-mediated β1 integrin activation, ROCK-dependent stress fiber formation and cell contractility to reduce VE-cad cell-cell junction stability and increase vascular permeability in an ICAP-1-deficient mouse model (Faurobert et al. 2013).
In vivo, the balance of basal forces can be disturbed by the influence of many external physical and mechanical cues, such as shear stress, cyclic strain and osmotic and hydrostatic pressure (Fig. 1). As a result of these stimuli, Rho-GTPase balance is disturbed (Shiu et al. 2004; Shikata et al. 2005; Kaunas et al. 2005; Tzima 2006) and a new equilibrium needs to be formed quickly to prevent barrier dysfunction (Schwartz and DeSimone 2008; Califano and Reinhart-King 2010; Weber et al. 2011). Furthermore, this is a complex enterprise to manage within a monolayer of approximately 1–6 × 1013 ECs (Augustin et al. 1994). Fortunately, despite the marked difference between the origin of the forces (internal or external), the involved molecular transduction mechanisms are analogous to a great extent (Delanoë-Ayari et al. 2004; Discher et al. 2005; Hoffman et al. 2011). This enables the ECs to filter all input signals to a certain degree and to elicit one general response. As is well known, both force-sensing and ECM-rigidity-sensing are critically important for controlling many aspects of cell behavior, such as cell growth, differentiation and cancer malignant progression and also for rigidity-sensing of the ECM (Chen 2008; Dupont et al. 2011).
A key determinant for force-balancing and force transduction in ECs is the physical microenvironment. The local endothelial niche is highly variable and is mainly determined by the compliance of the surrounding substrate. Within the human body, a wide range of substrate stiffnesses is observed (Yeung et al. 2005; Discher et al. 2005): from ∼1 kPa in the brain (Flanagan et al. 2002; Georges et al. 2006), to ∼10 kPa in striated muscle (Engler et al. 2004, 2006), to ∼30 kPa in precalcified bone (Engler et al. 2006), or to ∼100 kPa in calcified sites of atherosclerotic rabbit thoracic artery (Matsumoto et al. 2002; Liu et al. 2010). Furthermore, high local variation can be attributable inter alia to constriction (Mao et al. 2009) or local injury of the vasculature (Klein et al. 2009; Liu et al. 2010). ECs, because of their diverse distribution, have to cope with the whole range of substrate stiffnesses and, as a consequence, have to adapt their functions to their local surroundings. Previous studies have shown that substrate stiffness is a strong determinant of endothelial behavior, such as cell morphology (Yeung et al. 2005; Kumar et al. 2006; Ghosh et al. 2008; Byfield et al. 2009; Stroka and Aranda-Espinoza 2011), locomotion (Pelham and Wang 1997; Gray et al. 2003), spreading (Yeung et al. 2005; Reinhart-King et al. 2005) and growth (Yeung et al. 2005). In all these responses, remodeling of the force-bearing actin cytoskeleton plays a central role. By the formation of F-actin stress fibers and the maturation of FAs, the cell-matrix interaction is reinforced in response to stiffer substrates (Reinhart-King et al. 2005; Peyton et al. 2007; Byfield et al. 2009; Califano and Reinhart-King 2010). Recently, Krishnan and co-workers established the importance of the endothelial response to increased substrate stiffness for the regulation of endothelial barrier properties, showing that this is accompanied by increased TFs (Krishnan et al. 2011). The group of Birukov recently provided a mechanistic explanation for this observation by demonstrating a stiffness-dependent activation of myosin phosphatase subunit 1 and MLC phosphorylation in combination with stress fiber formation (Birukova et al. 2013a). Moreover, evidence of FA maturation on stiffer substrates has been provided by the observation of an increasing surface area (Yeung et al. 2005; Saez et al. 2005; Ghibaudo et al. 2008; Han et al. 2012) and the recruitment of unbound integrins by focal adhesion kinase and PI3K activation (Schwartz 2010). Furthermore, Stroka and Aranda-Espinoza ( 2011) linked this increased cell-substrate interaction to higher mechanical loading and showed the significance of the cell-cell junctions in the balancing of these physical forces. Together, these studies reveal that, upon increased substrate stiffness, the cell-matrix interaction is reinforced and is associated with higher cytoskeleton tension and thus a higher probability of cell-cell contact disruption leading to barrier dysfunction. These findings might have importance for various pathological conditions, such as diabetes, hypertension, cancer and atherosclerosis, which are all associated with increased substrate stiffness (Zieman et al. 2005; Kass 2005; Saez et al. 2005; Mitchell et al. 2008; Levental et al. 2009; Conway and Schwartz 2013). Moreover, substrate stiffening has also been associated with aging (Redfield et al. 2005) and has been shown to enhance endothelial permeability via Rho-dependent cellular contractile responses (Huynh et al. 2011).
Intermezzo on force measurements
Ever since it became clear that the endothelium itself can contract, a tremendous interest has developed in setting up experiments to prove it. One of the first groups to link the importance of endothelial contraction to an increase permeability of blood vessels was that of Majno and colleagues (1969). Electron microscopy revealed that, upon stimulation of histamine-type mediators, ECs showed cytoplasmic changes that suggested contraction. The visualization of endothelial contractions was elegantly studied by Morel and colleagues, who developed a wrinkling assay with single pulmonary microvascular endothelial cells (Morel et al. 1989). They observed that cell-generated tension created wrinkles in the underlying deformable substrate and that the same wrinkles would disappear upon relaxation without loss of cell adhesion. Subsequently, new methods were developed that made it possible to study the contraction of whole endothelial monolayers. First, isometric tension measurements were performed on HUVECs via an isometric force monitory apparatus (Kolodney and Wysolmerski 1992). This technique allowed quantitative measurements of isometric tension, which were strongly correlated to those obtained after the activation of the actomyosin-II-based contractile system (Goeckeler and Wysolmerski 1995). Moreover, the actin cytoskeleton was shown to be essential for contraction and upon stimulation with the vaso-active agent thrombin, a rapid increase of 2- to 2.5-fold in isometric contraction was observed (Goeckeler and Wysolmerski 1995). Second, a technique for measuring forces more locally was developed to analyze the mechanical properties of the endothelium. With the introduction of atomic force microscopy, local structural and mechanical information about individual stress fibers (Stroka and Aranda-Espinoza 2011) and VE-cad (Baumgartner et al. 2000; Baumgartner 2003) could be studied. Topographical information obtained from ECs showed an increase in stress fiber stiffness upon contraction (Lu et al. 2008), of which the micromechanical properties and endothelial permeability were strongly related (Arce et al. 2008). Recently developed possibilities for measuring local forces include tension-sensitive FRET sensors for measurements of intracellular tensions (Hur et al. 2012; Conway et al. 2013) and the tension-gauge-tether assay developed to evaluate molecular forces within the cell-matrix interactions of single ligand receptors (Wang and Ha 2013). All in all, the previously described methods are able to measure endothelial contractility. However, they lacked the ability to produce force maps of an endothelial monolayer as a whole. This changed with the introduction of traction force microscopy (TFM), which has now established itself as a powerful tool for studying EC monolayer contractility (Dembo et al. 1996; Balaban et al. 2001; Beningo and Wang 2002). This technique has clearly demonstrated that TFs underneath a monolayer are characterized by a diverse heterogenic force landscape with punctuated hotspots having a magnitude many times greater than the mean TF. Interestingly, thrombin-induced-force hotspots are associated with paracellular gap formation (Fig. 3). Nevertheless, this does not necesseraly represent a direct correlation.
Traction force microscopy (TFM) of endothelial cells. TFM maps of a confluent monolayer of human umbilical cord vein endothelial cells (HUVECs) grown on 4-kPa polyacrylamide substrate coated with collagen (a–d). Phase contrast image of the HUVEC monolayer under baseline conditions (a) and after stimulation with 1U/ml of the vaso-active agent thrombin (c). The heterogenic traction-force landscape was mapped for the baseline (b) and thrombin-stimulated (d) conditions. After a 30-min stimulation with thrombin, cellular contractions were observed in the phase contrast image, which were accompanied by paracellular gap formation (c, blue arrows). Traction forces increased considerably after stimulation and punctuated-force hot spots appeared (d, blue arrows)
To study this process in more detail, new TFM experiments were designed to examine the local force transduction of the cell-matrix interaction. These studies inter alia showed that an increase in TFs was linearly correlated to an increase in the number of FAs (Han et al. 2012). Moreover, nowadays, with the use of distinct knowledge over FAs in combination with the TF characteristics, forces at individual FAs can be calculated. Other studies have revealed mean TFs of 0.16 ± 0.08 kPa per mm2 of FA footprint area and mean peak forces of 0.8 ± 0.3 kPa (Stricker et al. 2011; Plotnikov et al. 2012). These differences between mean and peak forces indicate enormous time- and location-dependent variations, which are an indication of force-balancing in single ECs. Within the endothelial monolayer, this mechanism is of critical importance for maintaining not only cell-matrix contacts but also cell-cell contacts and is, in that sense, crucial for proper barrier function.
Force propagation via cell-cell contacts is called intercellular stress and triggers cytoskeleton rearrangements of the influenced cells. Via this interplay of forces, the endothelium behaves as a coordinated cellular collective, rather than a simple assembly of many individual cells (Hardin et al. 2013). Within this tightly connected monolayer sheet, force needs to be balanced according to Newton’s laws within the cytoskeleton. This principle in combination with the ability to measure TF has resulted in an elegant approach used by the group of Fredberg to modulate monolayer stress (Tambe et al. 2011, 2013). Monolayer stress microscopy shows increased intercellular stress with higher TF in a similar heterogeneous pattern to TFM. Interestingly, the force landscape of many closely opposed peaks and valleys has a long transduction length scale of multiple cell diameters, which even increases upon treatment with vaso-active agents when the separation of cell-cell interactions is not complete (Hardin et al. 2013). Maruthamuthu and colleagues also demonstrated the strong correlation between forces transmitted through cell–cell adhesions and cell-matrix adhesions and showed a constant fractional relationship of 0.5 (Maruthamuthu et al. 2011). These results pinpoint the great importance of the physical microenvironment for the integrity of the endothelial monolayer and indicate the prominent role for the cell-matrix in this relationship.
Concluding remarks
All in all, it has become clear that the integrity of the endothelial barrier both in vitro and in vivo is regulated by distinct and opposing Rho-GTPases activities. Of 22 known Rho-GTPases, only robust information about three and an emerging fourth member are available, whereas for the remaining family members hardly any information or studies are known, although most of them have profound effects on the EC cytoskeleton (Sorokina and Chernoff 2005). Similarly to RhoB with its recent implication in vascular function, other Rho-GTPases, such as RhoC, which share downstream targets with RhoA, will also be implicated in the regulation of the endothelial barrier. The combined actions of Rho-GTPases require a crucial spatio-temporal regulation and this process is thoroughly orchestrated. However, the fundamental question regarding the means by which the two contractile and adhesive forces are driven remains unanswered. We hypothesize that Rho-GTPases are driven by as yet unknown distinct combinations of regulatory proteins and expect that, in the near future, detailed information from systematic analysis resulting from loss-of-function screens will be revealed.
What we do know is that the cell-matrix interaction plays a key role in this relationship and that this interaction displays high local variation and is enormously dynamic. To study this process, high spatial resolution microscopy is used to examine force dynamics up to single FAs (Plotnikov and Waterman 2013). Plotnikov and colleagues have shown that single FAs work autonomously and can only be found in two distinct states: either expressing stable or dynamic fluctuating TFs (Plotnikov et al. 2012). In single mouse embryonic fibroblasts, both observations have been linked to rigidity sensing of the matrix; however, whether this is also the case in other cell types and cell clusters, such as endothelial monolayers, is unknown. To achieve a better understanding of the involvement of forces in endothelial function and barrier disruption, in-depth knowledge of mechanotransduction is essential. Hence, new single-molecular FRET imaging techniques can provide additional information about local force transmission (Kim and Ha 2013; Wang and Ha 2013). Moreover, Dubrovskyi and colleagues recently published a method that enables new studies aimed at mapping changes in local trans-monolayer permeability in an EC monolayer over time (Dubrovskyi et al. 2013). Such novel methods will allow, in the near future, the simultaneous mapping of forces and monolayer integrity; this is essential for a better understanding of the relationship between forces and monolayer integrity (Hardin et al. 2013).
To obtain more insight into the in vivo situation, the role of fluid shear stress on the dynamics and regulation of TFs in ECs needs to be examined. The recent study of Hur and co-workers has shown that cytoskeletal remodeling, changes in cell-matrix and cell–cell interactions and increased intracellular tensions are effects of chemo-mechanical responses rather than a direct result of mechanical loading attributable to the shear stress (Hur et al. 2012). The effect of soluble factors on endothelial barrier function is unknown and should be addressed in new investigations.
References
Adini I, Rabinovitz I, Sun JF, Prendergast GC, Benjamin LE (2003) RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev 17:2721–32. doi:10.1101/gad.1134603
Aird WC (2012) Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2:a006429. doi:10.1101/cshperspect.a006429
Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N (2013) Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol 202:901–16. doi:10.1083/jcb.201301115
Arce FT, Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MF, Lal R, Garcia JGN, Dudek SM (2008) Regulation of the micromechanical properties of pulmonary endothelium by S1P and thrombin: role of cortactin. Biophys J 95:886–94. doi:10.1529/biophysj.107.127167
Aspenström P, Fransson A, Saras J (2004) Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J 377:327–37. doi:10.1042/BJ20031041
Augustin HG, Kozian DH, Johnson RC (1994) Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. Bioessays 16:901–6. doi:10.1002/bies.950161208
Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10:165–77. doi:10.1038/nrm2639
Bakker W, Eringa EC, Sipkema P, van Hinsbergh VWM (2009) Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 335:165–89. doi:10.1007/s00441-008-0685-6
Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B (2001) Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3:466–72. doi:10.1038/35074532
Baumer Y, Drenckhahn D, Waschke J (2008) cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol 129:765–78. doi:10.1007/s00418-008-0422-y
Baumgartner W (2003) Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. J Cell Sci 116:1001–1011. doi:10.1242/jcs.00322
Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D (2000) Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci U S A 97:4005–10. doi:10.1073/pnas.070052697
Bayless KJ, Salazar R, Davis GE (2000) RGD-Dependent Vacuolation and Lumen Formation Observed during Endothelial Cell Morphogenesis in Three-Dimensional Fibrin Matrices Involves the αvβ3 and α5β1 Integrins. Am J Pathol 156:1673–1683. doi:10.1016/S0002-9440(10)65038-9
Beckers CML, van Hinsbergh VWM, Van Nieuw Amerongen GP (2010) Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 103:40–55. doi:10.1160/TH09-06-0403
Beningo KA, Wang Y-L (2002) Flexible substrata for the detection of cellular traction forces. Trends Cell Biol 12:79–84
Birukov KG (2009) Small GTPases in mechanosensitive regulation of endothelial barrier. Microvasc Res 77:46–52. doi:10.1016/j.mvr.2008.09.006
Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD (2004) Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67:64–77. doi:10.1016/j.mvr.2003.09.007
Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD (2006) GEFH1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 290:L540–8. doi:10.1152/ajplung.00259.2005
Birukova AA, Fu P, Xing J, Birukov KG (2009) Rap1 mediates protective effects of iloprost against ventilator-induced lung injury. J Appl Physiol 107:1900–10. doi:10.1152/japplphysiol.00462.2009
Birukova AA, Fu P, Xing J (2010) Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol 298:837–48. doi:10.1152/ajplung.00263.2009
Birukova AA, Tian X, Cokic I, Beckham Y, Gardel ML, Birukov KG (2013a) Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc Res 87:50–7. doi:10.1016/j.mvr.2012.12.006
Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG (2013b) Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 24:2678–88. doi:10.1091/mbc.E13-02-0098
Bishop A, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348(Pt 2):241–55
Braga VM (1999) Small GTPases and regulation of cadherin dependent cell-cell adhesion. Mol Pathol 52:197–202
Broman MT, Mehta D, Malik AB (2007) Cdc42 regulates the restoration of endothelial adherens junctions and permeability. Trends Cardiovasc Med 17:151–6. doi:10.1016/j.tcm.2007.03.004
Bursac P, Fabry B, Trepat X, Lenormand G, Butler JP, Wang N, Fredberg JJ, An SS (2007) Cytoskeleton dynamics: fluctuations within the network. Biochem Biophys Res Commun 355:324–30. doi:10.1016/j.bbrc.2007.01.191
Byfield FJ, Reen RK, Shentu T-P, Levitan I, Gooch KJ (2009) Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech 42:1114–9. doi:10.1016/j.jbiomech.2009.02.012
Califano JP, Reinhart-King CA (2010) Exogenous and endogenous force regulation of endothelial cell behavior. J Biomech 43:79–86. doi:10.1016/j.jbiomech.2009.09.012
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–57
Chen CS (2008) Mechanotransduction - a field pulling together? J Cell Sci 121:3285–92. doi:10.1242/jcs.023507
Cheng C, Haasdijk R, Tempel D, van de Kamp EHM, Herpers R, Bos F, Den Dekker WK, Blonden LAJ, de Jong R, Bürgisser PE, Chrifi I, Biessen EAL, Dimmeler S, Schulte-Merker S, Duckers HJ (2012) Endothelial cell-specific FGD5 involvement in vascular pruning defines neovessel fate in mice. Circulation 125:3142–58. doi:10.1161/CIRCULATIONAHA.111.064030
Cherfils J, Zeghouf M (2013) Regulation of Small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 93:269–309. doi:10.1152/physrev.00003.2012
Chervin-Pétinot A, Courçon M, Almagro S, Nicolas A, Grichine A, Grunwald D, Prandini M-H, Huber P, Gulino-Debrac D (2012) Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem 287:7556–72. doi:10.1074/jbc.M111.328682
Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, Vansluys J (2008) Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1bdeficient mice. Blood 111:2647–56. doi:10.1182/blood-2007-08-109710
Connolly MJ, Aaronson PI (2011) Key role of the RhoA/Rho kinase system in pulmonary hypertension. Pulm Pharmacol Ther 24:1–14. doi:10.1016/j.pupt.2010.09.001
Conway DE, Schwartz MA (2013) Flow-dependent cellular mechanotransduction in atherosclerosis. J Cell Sci 126:5101–9. doi:10.1242/jcs.138313
Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA (2013) Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol 23:1024–30. doi:10.1016/j.cub.2013.04.049
Crosby CV, Fleming PA, Argraves WS, Corada M, Zanetta L, Dejana E, Drake CJ (2005) VE-cadherin is not required for the formation of nascent blood vessels but acts to prevent their disassembly. Blood 105:2771–6. doi:10.1182/blood-2004-06-2244
Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN (2005) Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105:1950–5. doi:10.1182/blood-2004-05-1987
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–70. doi:10.1038/nrm1357
Dejana E, Orsenigo F (2013) Endothelial adherens junctions at a glance. J Cell Sci 126(Pt 12):2545–9. doi:10.1242/jcs.124529
Dejana E, Tournier-Lasserve E, Weinstein BM (2009) The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16:209–21. doi:10.1016/j.devcel.2009.01.004
Del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP (2009) Stretching single talin rod molecules activates vinculin binding. Science 323:638–41. doi:10.1126/science.1162912
Delanoë-Ayari H, Al Kurdi R, Vallade M, Gulino-debrac D, Riveline D (2004) Membrane and acto-myosin tension promote clustering of adhesion proteins. Proc Natl Acad Sci 101:2229–2234
Dembo M, Oliver T, Ishihara A, Jacobson K (1996) Imaging the traction stresses exerted by locomoting cells with the elastic substratum method. Biophys J 70:2008–22. doi:10.1016/S0006-3495(96)79767-9
Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL, Sessa WC (2013) eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci 126:5541–52. doi:10.1242/jcs.115972
Discher DE, Janmey P, Wang Y-L (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–43. doi:10.1126/science.1116995, 80-
Dransart E, Olofsson B, Cherfils J (2005) RhoGDIs revisited: novel roles in Rho regulation. Traffic 6:957–66. doi:10.1111/j.1600-0854.2005.00335.x
Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI (2005) Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123:903–15. doi:10.1016/j.cell.2005.09.021
Dubrovskyi O, Birukova AA, Birukov KG (2013) Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93:254–63. doi:10.1038/labinvest.2012.159
Dudek SM, Garcia JGN (2001) Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91:1487–1500
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–83. doi:10.1038/nature10137
Ebnet K (2008) Organization of multiprotein complexes at cell-cell junctions. Histochem Cell Biol 130:1–20. doi:10.1007/s00418-008-0418-7
Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP (2011) Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 478:260–3. doi:10.1038/nature10430
Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher DE (2004) Substrate compliance versus ligand density in cell on gel responses. Biophys J 86:617–28. doi:10.1016/S0006-3495(04)74140-5
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–89. doi:10.1016/j.cell.2006.06.044
Erickson JW, Cerione RA (2004) Structural elements, mechanism, and evolutionary convergence of Rho protein-guanine nucleotide exchange factor complexes. Biochemistry 43:837–42. doi:10.1021/bi036026v
Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M (1998) Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem 273:21867–74. doi:10.1074/jbc.273.34.21867
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–35. doi:10.1038/nature01148
Eyckmans J, Boudou T, Yu X, Chen CS (2011) A hitchhiker’s guide to mechanobiology. Dev Cell 21:35–47. doi:10.1016/j.devcel.2011.06.015
Fanning AS (1998) The Tight Junction Protein ZO-1 Establishes a Link between the Transmembrane Protein Occludin and the Actin Cytoskeleton. J Biol Chem 273:29745–29753. doi:10.1074/jbc.273.45.29745
Faurobert E, Rome C, Lisowska J, Manet-Dupé S, Boulday G, Malbouyres M, Balland M, Bouin A-P, Kéramidas M, Bouvard D, Coll J-L, Ruggiero F, Tournier-Lasserve E, Albiges-Rizo C (2013) CCM1-ICAP-1 complex controls β1 integrin-dependent endothelial contractility and fibronectin remodeling. J Cell Biol 202:545–61. doi:10.1083/jcb.201303044
Ferro T, Neumann P, Gertzberg N, Clements R, Johnson A (2000) Protein kinase Calpha mediates endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol 278:L1107–17
Flanagan LA, Ju Y, Marg B, Osterfield M, Paul A (2002) Neurite branching on deformable substrates. Neuroreport 13:2411–2415
Fransson A, Ruusala A, Aspenström P (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem 278:6495–502. doi:10.1074/jbc.M208609200
Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N (2005) Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25:136–46. doi:10.1128/MCB.25.1.136-146.2005
Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y (1990) Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene 5:1321–8
Galbraith CG, Yamada KM, Sheetz MP (2002) The relationship between force and focal complex development. J Cell Biol 159:695–705. doi:10.1083/jcb.200204153
Garcia JGN, Liu F, Verin AD, Birukova AA, Dechert MA, Gerthoffer WT, Bamberg JR, English D (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108:689–701. doi:10.1172/JCI12450
Garcia-Mata R, Boulter E, Burridge K (2011) The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 12:493–504. doi:10.1038/nrm3153
Garrett TA, Van Buul JD, Burridge K (2007) VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res 313:3285–97. doi:10.1016/j.yexcr.2007.05.027
Gavard J (2009) Breaking the VE-cadherin bonds. FEBS Lett 583:1–6. doi:10.1016/j.febslet.2008.11.032
Gavard J, Gutkind JS (2006) VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8:1223–34. doi:10.1038/ncb1486
Gavard J, Patel V, Gutkind JS (2008) Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14:25–36. doi:10.1016/j.devcel.2007.10.019
Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA (2006) Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J 90:3012–8. doi:10.1529/biophysj.105.073114
Gertzberg N, Neumann P, Rizzo V, Johnson A (2004) NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol 286:L37–48. doi:10.1152/ajplung.00116.2003
Ghibaudo M, Saez A, Trichet L, Xayaphoummine A, Browaeys J, Silberzan P, Buguin A, Ladoux B (2008) Traction forces and rigidity sensing regulate cell functions. Soft Matter 4:1836. doi:10.1039/b804103b
Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE (2008) Tumorderived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A 105:11305–10. doi:10.1073/pnas.0800835105
Giannotta M, Trani M, Dejana E (2013) VE-Cadherin and Endothelial Adherens Junctions: Active Guardians of Vascular Integrity. Dev Cell 26:441–54. doi:10.1016/j.devcel.2013.08.020
Goeckeler ZM, Wysolmerski RB (1995) Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol 130:613–27
Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL (2011) Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 3:88ps25. doi:10.1126/scitranslmed.3002011
Gorovoy M, Neamu R, Niu J, Vogel S, Predescu D, Miyoshi J, Takai Y, Kini V, Mehta D, Malik AB, Voyno-Yasenetskaya T (2007) RhoGDI-1 modulation of the activity of monomeric RhoGTPase RhoA regulates endothelial barrier function in mouse lungs. Circ Res 101:50–8. doi:10.1161/CIRCRESAHA.106.145847
Gray DS, Tien J, Chen CS (2003) Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. J Biomed Mater Res A 66:605–14. doi:10.1002/jbm.a.10585
Guilluy C, Garcia-Mata R, Burridge K (2011a) Rho protein crosstalk: another social network? Trends Cell Biol 21:718–726
Guilluy C, Swaminathan V, Garcia-Mata R, Brien ETO, Superfine R, Burridge K (2011b) The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 13:724–729. doi:10.1038/ncb2254
Gulino-Debrac D (2013) Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 1:e24180. doi:10.4161/tisb.24180
Hall A (2012) Rho family GTPases. Biochem Soc Trans 40:1378–82. doi:10.1042/BST20120103
Han SJ, Bielawski KS, Ting LH, Rodriguez ML, Sniadecki NJ (2012) Decoupling Substrate Stiffness, Spread Area, and Micropost Density: A Close Spatial Relationship between Traction Forces and Focal Adhesions. Biophys J 103:640–8. doi:10.1016/j.bpj.2012.07.023
Hardin C, Rajendran K, Manomohan G, Tambe DT, Butler JP, Fredberg JJ, Martinelli R, Carman CV, Krishnan R (2013) Glassy Dynamics, Cell Mechanics, and Endothelial Permeability. J Phys Chem B. doi:10.1021/jp4020965
Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G (1998) Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280:2112–4
Hartsock A, Nelson WJ (2008) Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–9. doi:10.1016/j.bbamem.2007.07.012
Hayakawa M, Matsushima M, Hagiwara H, Oshima T, Fujino T, Ando K, Kikugawa K, Tanaka H, Miyazawa K, Kitagawa M (2008) Novel insights into FGD3, a putative GEF for Cdc42, that undergoes SCF(FWD1/beta-TrCP)-mediated proteasomal degradation analogous to that of its homologue FGD1 but regulates cell morphology and motility differently from FGD1. Genes Cells 13:329–42. doi:10.1111/j.1365-2443.2008.01168.x
He F, Yin F, Omran A, Yang L, Xiang Q, Peng J (2012) PKC and RhoA signals crosstalk in Escherichia coli endotoxin induced alterations in brain endothelial permeability. Biochem Biophys Res Commun 425:182–8. doi:10.1016/j.bbrc.2012.07.063
Ho M, Yang E, Matcuk G, Deng D, Sampas N, Tsalenko A, Tabibiazar R, Zhang Y, Chen M, Talbi S, Ho YD, Wang J, Tsao PS, Ben-Dor A, Yakhini Z, Bruhn L, Quertermous T (2003) Identification of endothelial cell genes by combined database mining and microarray analysis. Physiol Genomics 13:249–62. doi:10.1152/physiolgenomics.00186.2002
Hoelzle MK, Svitkina T (2012) The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol Biol Cell 23:310–23. doi:10.1091/mbc.E11-08-0719
Hoffman BD, Grashoff C, Schwartz MA (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475:316–23. doi:10.1038/nature10316
Howe GA, Addison CL (2012) RhoB controls endothelial cell morphogenesis in part via negative regulation of RhoA. Vasc Cell 4:1. doi:10.1186/2045-824X-4-1
Huber C, Mårtensson A, Bokoch GM, Nemazee D, Gavin AL (2008) FGD2, a CDC42- specific exchange factor expressed by antigen-presenting cells, localizes to early endosomes and active membrane ruffles. J Biol Chem 283:34002–12. doi:10.1074/jbc.M803957200
Hur SS, del Ãlamo JC, Park JS, Li Y-S, Nguyen HA, Teng D, Wang K-C, Flores L, Alonso-Latorre B, Lasheras JC, Chien S (2012) Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proc Natl Acad Sci U S A 109:11110–5. doi:10.1073/pnas.1207326109
Huveneers S, Danen EHJ (2010) The interaction of SRC kinase with beta3 integrin tails: a potential therapeutic target in thrombosis and cancer. Sci World J 10:1100–5. doi:10.1100/tsw.2010.114
Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J (2012) Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol 196:641–52. doi:10.1083/jcb.201108120
Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA (2011) Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3:112ra122. doi:10.1126/scitranslmed.3002761
Jaffe AB, Hall A (2005) Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–69. doi:10.1146/annurev.cellbio.21.020604.150721
Kajimoto H, Hashimoto K, Bonnet SN, Haromy A, Harry G, Moudgil R, Nakanishi T, Rebeyka I, Thébaud B, Michelakis ED, Archer SL (2007) Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species: a newly recognized mechanism for sustaining ductal constriction. Circulation 115:1777–88. doi:10.1161/CIRCULATIONAHA.106.649566
Kass DA (2005) Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 46:185–93. doi:10.1161/01.HYP.0000168053.34306.d4
Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA (2002) Effects of cell tension on the small GTPase Rac. J Cell Biol 158:153–64. doi:10.1083/jcb.200201105
Kaunas R, Nguyen P, Usami S, Chien S (2005) Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A 102:15895–900. doi:10.1073/pnas.0506041102
Kim H, Ha T (2013) Single-molecule nanometry for biological physics. Rep Prog Phys 76:016601. doi:10.1088/0034-4885/76/1/016601
Kim S, Bell K, Mousa SA, Varner JA (2000) Regulation of Angiogenesis in Vivo by Ligation of Integrin α5β1 with the Central Cell-Binding Domain of Fibronectin. Am J Pathol 156:1345–1362. doi:10.1016/S0002-9440(10)65005-5
Kim K-M, Csortos C, Czikora I, Fulton D, Umapathy NS, Olah G, Verin AD (2012) Molecular characterization of myosin phosphatase in endothelium. J Cell Physiol 227:1701–8. doi:10.1002/jcp.22894
Klein EAE, Castagnino P, Kothapalli D, Yin L, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK (2009) Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19:1511–1518. doi:10.1016/j.cub.2009.07.069.Cell
Knezevic N, Roy A, Timblin B, Konstantoulaki M, Sharma T, Malik AB, Mehta D (2007) GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol Cell Biol 27:6323–33. doi:10.1128/MCB.00523-07
Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN (2009) Tiam1 and Rac1 are required for platelet-activating factorinduced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem 284:5381–94. doi:10.1074/jbc.M808958200
Koch AW, Bozic D, Pertz O, Engel J (1999) Homophilic adhesion by cadherins. Curr Opin Struct Biol 9:275–81. doi:10.1016/S0959-440X(99)80038-4
Kolodney MS, Wysolmerski RB (1992) Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol 117:73–82
Komarova Y, Malik AB (2010) Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72:463–93. doi:10.1146/annurev-physiol-021909-135833
Kooistra MRH, Dubé N, Bos JL (2007) Rap1: a key regulator in cell-cell junction formation. J Cell Sci 120:17–22. doi:10.1242/jcs.03306
Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB (2004) Cdc42 regulates the restoration of endothelial barrier function. Circ Res 94:159–66. doi:10.1161/01.RES.0000110418.38500.31
Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC (1998) p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280:2109–11
Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VWM, Carman CV, Brain JD, Fredberg JJ, Butler JP, Van Nieuw Amerongen GP (2011) Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 300:C146–54. doi:10.1152/ajpcell.00195.2010
Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE (2006) Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J 90:3762–73. doi:10.1529/biophysj.105.071506
Kurogane Y, Miyata M, Kubo Y, Nagamatsu Y, Kundu RK, Uemura A, Ishida T, Quertermous T, Hirata K, Rikitake Y (2012) FGD5 mediates proangiogenic action of vascular endothelial growth factor in human vascular endothelial cells. Arterioscler Thromb Vasc Biol 32:988–96. doi:10.1161/ATVBAHA.111.244004
Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M (2011) Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 118:2015–26. doi:10.1182/blood-2011-04-349282
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E (1995) The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129:203–17
Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E (2002) VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13:1175–89. doi:10.1091/mbc.01-07-0368
Leckband DE, le Duc Q, Wang N, de Rooij J (2011) Mechanotransduction at cadherinmediated adhesions. Curr Opin Cell Biol 23:523–30. doi:10.1016/j.ceb.2011.08.003
Lee WL, Slutsky AS (2010) clinical implications of basic research Sepsis and Endothelial Permeability. N Engl J Med 363:689–691
Lessey EC, Guilluy C, Burridge K (2012) From mechanical force to RhoA activation. Biochemistry 51:7420–32. doi:10.1021/bi300758e
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906. doi:10.1016/j.cell.2009.10.027
Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ (2010) Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190:693–706. doi:10.1083/jcb.201004082
Loirand G, Sauzeau V, Pacaud P (2013) Small g proteins in the cardiovascular system: physiological and pathological aspects. Physiol Rev 93:1659–720. doi:10.1152/physrev.00021.2012
Lu L, Oswald SJ, Ngu H, Yin FC-P (2008) Mechanical properties of actin stress fibers in living cells. Biophys J 95:6060–71. doi:10.1529/biophysj.108.133462
Majno G, Shea SM, Leventhal M (1969) Endothelial contraction induced by histaminetype mediators. J Cell Biol 42:647–672
Mao Y, Sun Q, Wang X, Ouyang Q, Han L, Jiang L, Han D (2009) In vivo nanomechanical imaging of blood-vessel tissues directly in living mammals using atomic force microscopy. Appl Phys Lett 95:013704. doi:10.1063/1.3167546
Maruthamuthu V, Sabass B, Schwarz US, Gardel ML (2011) Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A 108:4708–13. doi:10.1073/pnas.1011123108
Matsumoto T, Abe H, Ohashi T, Kato Y, Sato M (2002) Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas 23:635–48
Mehta D, Malik AB (2006) Signaling Mechanisms Regulating Endothelial Permeability. Physiol Rev 86:279–367. doi:10.1152/physrev.00012.2005
Michel CC (1996) Transport of macromolecules through microvascular walls. Cardiovasc Res 32:644–53
Millán J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernández-Martín L, Correas I, Ridley AJ (2010) Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol 8:11. doi:10.1186/1741-7007-8-11
Mitchell GF, Conlin PR, Dunlap ME, Lacourcière Y, Arnold JMO, Ogilvie RI, Neutel J, Izzo JL, Pfeffer MA (2008) Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension 51:105–11. doi:10.1161/HYPERTENSIONAHA.107.099721
Mitin N, Rossman KL, Currin R, Anne S, Marshall TW, Bear JE, Bautch VL, Der CJ (2013) The RhoGEF TEM4 Regulates Endothelial Cell Migration by Suppressing Actomyosin Contractility. PLoS One 8:e66260. doi:10.1371/journal.pone.0066260
Monaghan-Benson E, Burridge K (2009) The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 284:25602–11. doi:10.1074/jbc.M109.009894
Monaghan-Benson E, Wittchen ES (2011) In vitro analyses of endothelial cell permeability. Methods Mol Biol 763:281–90. doi:10.1007/978-1-61779-191-8_19
Morel NM, Dodge AB, Patton WF, Herman IM, Hechtman HB, Shepro D (1989) Pulmonary microvascular endothelial cell contractility on silicone rubber substrate. J Cell Physiol 141:653–9. doi:10.1002/jcp.1041410325
Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, Ye RD (2012) A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res 111:1517–27. doi:10.1161/CIRCRESAHA.112.273078
Nakhaei-Nejad M, Haddad G, Zhang Q-X, Murray AG (2012) Facio-genital dysplasia-5 regulates matrix adhesion and survival of human endothelial cells. Arterioscler Thromb Vasc Biol 32:2694–701. doi:10.1161/ATVBAHA.112.300074
Narumiya S, Ishizaki T, Watanabe N (1997) Rho effectors and reorganization of actin cytoskeleton. FEBS Lett 410:68–72. doi:10.1016/S0014-5793(97)00317-7
Ngok SP, Geyer R, Kourtidis A, Mitin N, Feathers R, Der C, Anastasiadis PZ (2013) TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J Cell Sci 126:3271–7. doi:10.1242/jcs.123869
Noren NK, Niessen CM, Gumbiner BM, Burridge K (2001) Cadherin engagement regulates Rho family GTPases. J Biol Chem 276:33305–8. doi:10.1074/jbc.C100306200
Nuno DW, England SK, Lamping KG (2012) RhoA localization with caveolin-1 regulates vascular contractions to serotonin. Am J Physiol Regul Integr Comp Physiol 303:R959–67. doi:10.1152/ajpregu.00667.2011
Ohayon J, Gharib AM, Garcia A, Heroux J, Yazdani SK, Malvè M, Tracqui P, Martinez M-A, Doblare M, Finet G, Pettigrew RI (2011) Is arterial wall-strain stiffening an additional process responsible for atherosclerosis in coronary bifurcations?: an in vivo study based on dynamic CT and MRI. Am J Physiol Heart Circ Physiol 301:H1097–106. doi:10.1152/ajpheart.01120.2010
Olofsson B (1999) Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal 11:545–54
Orr AW, Helmke BP, Blackman BR, Schwartz MA (2006) Mechanisms of mechanotransduction. Dev Cell 10:11–20. doi:10.1016/j.devcel.2005.12.006
Pannekoek W-J, Linnemann JR, Brouwer PM, Bos JL, Rehmann H (2013) Rap1 and Rap2 antagonistically control endothelial barrier resistance. PLoS One 8:e57903. doi:10.1371/journal.pone.0057903
Papaharalambus C, Sajjad W, Syed A, Zhang C, Bergo MO, Alexander RW, Ahmad M (2005) Tumor necrosis factor alpha stimulation of Rac1 activity. Role of isoprenylcysteine carboxylmethyltransferase. J Biol Chem 280:18790–6. doi:10.1074/jbc.M410081200
Pelham RJ, Wang YI (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94:13661–5
Pertz O (2010) Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci 123:1841–50. doi:10.1242/jcs.064345
Peyton SR, Ghajar CM, Khatiwala CB, Putnam AJ (2007) The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys 47:300–320. doi:10.1007/s12013-007-0004-y
Plotnikov SVV, Waterman CM (2013) Guiding cell migration by tugging. Curr Opin Cell Biol 25:619–26. doi:10.1016/j.ceb.2013.06.003
Plotnikov SVV, Pasapera AM, Sabass B, Waterman CM (2012) Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151:1513–27. doi:10.1016/j.cell.2012.11.034
Ponik SM, Trier SM, Wozniak MA, Eliceiri KW, Keely PJ (2013) RhoA is down-regulated at cell-cell contacts via p190RhoGAP-B in response to tensional homeostasis. Mol Biol Cell 24(1688–99):S1–3. doi:10.1091/mbc.E12-05-0386
Popoff MR, Geny B (2009) Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta 1788:797–812. doi:10.1016/j.bbamem.2009.01.011
Post A, Pannekoek W-J, Ross SH, Verlaan I, Brouwer PM, Bos JL (2013) Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci U S A 110:11427–32. doi:10.1073/pnas.1306595110
Qiao J, Huang F, Lum H (2003) PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284:L972–80. doi:10.1152/ajplung.00429.2002
Qiao J, Holian O, Lee B-S, Huang F, Zhang J, Lum H (2008) Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA. Am J Physiol Cell Physiol 295:C1161–8. doi:10.1152/ajpcell.00139.2008
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA (2005) Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112:2254–62. doi:10.1161/CIRCULATIONAHA.105.541078
Reinhart-King CA, Dembo M, Hammer DA (2005) The dynamics and mechanics of endothelial cell spreading. Biophys J 89:676–89. doi:10.1529/biophysj.104.054320
Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16:522–9. doi:10.1016/j.tcb.2006.08.006
Ridley AJ, Hall A (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–99
Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP (2009) Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci U S A 106:16245–50. doi:10.1073/pnas.0902818106
Rojas AM, Fuentes G, Rausell A, Valencia A (2012) The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 196:189–201. doi:10.1083/jcb.201103008
Rolfe BE, Worth NF, World CJ, Campbell JH, Campbell GR (2005) Rho and vascular disease. Atherosclerosis 183:1–16. doi:10.1016/j.atherosclerosis.2005.04.023
Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA (2013) Integrins in mechanotransduction. Curr Opin Cell Biol 25:613–8. doi:10.1016/j.ceb.2013.05.006
Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–80. doi:10.1038/nrm1587
Saez A, Buguin A, Silberzan P, Ladoux B (2005) Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J 89:L52–4. doi:10.1529/biophysj.105.071217
Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow C-W, Lisanti MP (2002) Microvascular hyperpermeability in caveolin-1(−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem 277:40091–8. doi:10.1074/jbc.M205948200
Schwartz MA (2010) Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol 2:a005066. doi:10.1101/cshperspect.a005066
Schwartz MA, DeSimone DW (2008) Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol 20:551–6. doi:10.1016/j.ceb.2008.05.005
Shao M, Yue Y, Sun G-Y, You Q-H, Wang N, Zhang D (2013) Caveolin-1 regulates Rac1 activation and rat pulmonary microvascular endothelial hyperpermeability induced by TNF-α. PLoS One 8:e55213. doi:10.1371/journal.pone.0055213
Shifrin Y, Arora PD, Ohta Y, Calderwood DA, Mcculloch CA (2009) The Role of FilGAPFilamin A Interactions in Mechanoprotection. Mol Biol Cell 20:1269–1279. doi:10.1091/mbc.E08
Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JGN, Birukov KG (2005) Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304:40–9. doi:10.1016/j.yexcr.2004.11.001
Shiu Y-T, Li S, Marganski WA, Usami S, Schwartz MA, Wang Y-L, Dembo M, Chien S (2004) Rho mediates the shear-enhancement of endothelial cell migration and traction force generation. Biophys J 86:2558–65. doi:10.1016/S0006-3495(04)74311-8
Siddiqui MR, Komarova YA, Vogel SM, Gao X, Bonini MG, Rajasingh J, Zhao Y-Y, Brovkovych V, Malik AB (2011) Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability. J Cell Biol 193:841–50. doi:10.1083/jcb.201012129
Sorokina EM, Chernoff J (2005) Rho-GTPases: new members, new pathways. J Cell Biochem 94:225–31. doi:10.1002/jcb.20327
Spindler V, Schlegel N, Waschke J (2010) Role of GTPases in control of microvascular permeability. Cardiovasc Res 87:243–53. doi:10.1093/cvr/cvq086
Stamatovic SM, Sladojevic N, Keep RF, Andjelkovic AV (2012) Relocalization of junctional adhesion molecule A during inflammatory stimulation of brain endothelial cells. Mol Cell Biol 32:3414–27. doi:10.1128/MCB.06678-11
Storck EM, Wojciak-Stothard B (2013) Rho GTPases in pulmonary vascular dysfunction. Vascul Pharmacol 58:202–10. doi:10.1016/j.vph.2012.09.004
Stricker J, Aratyn-Schaus Y, Oakes PW, Gardel ML (2011) Spatiotemporal constraints on the force-dependent growth of focal adhesions. Biophys J 100:2883–93. doi:10.1016/j.bpj.2011.05.023
Stroka KM, Aranda-Espinoza H (2011) Effects of Morphology vs. Cell-Cell Interactions on Endothelial Cell Stiffness. Cell Mol Bioeng 4:9–27. doi:10.1007/s12195-010-0142-y
Stroka KM, Aranda-Espinoza H (2012) Effects of Morphology vs. Cell–Cell Interactions on Endothelial Cell Stiffness. Cell Mol Bioeng 4:9–27. doi:10.1007/s12195-010-0142-y.Effects
Su Z-J, Hahn CN, Goodall GJ, Reck NM, Leske AF, Davy A, Kremmidiotis G, Vadas MA, Gamble JR (2004) A vascular cell-restricted RhoGAP, p73RhoGAP, is a key regulator of angiogenesis. Proc Natl Acad Sci U S A 101:12212–7. doi:10.1073/pnas.0404631101
Sumi T, Matsumoto K, Takai Y, Nakamura T (1999) Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol 147:1519–32
Szulcek R, Beckers CML, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJP, Minshall RD, van Hinsbergh VWM, Van Nieuw Amerongen GP (2013) Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc Res 99:471–82. doi:10.1093/cvr/cvt075
Taha AA, Taha M, Seebach J, Schnittler H-J (2014) ARP2/3-mediated junctionassociated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Mol Biol Cell 25:245–56. doi:10.1091/mbc.E13-07-0404
Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, Fredberg JJ, Trepat X (2011) Collective cell guidance by cooperative intercellular forces. Nat Mater 10:469–75. doi:10.1038/nmat3025
Tambe DT, Croutelle U, Trepat X, Park CY, Kim JH, Millet E, Butler JP, Fredberg JJ (2013) Monolayer stress microscopy: limitations, artifacts, and accuracy of recovered intercellular stresses. PLoS One 8:e55172. doi:10.1371/journal.pone.0055172
Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS (2008) An essential role for Rac1 in endothelial cell function and vascular development. FASEB J 22:1829–38. doi:10.1096/fj.07-096438
Tapon N, Hall A (1997) Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol 86–92
Tcherkezian J, Lamarche-Vane N (2007) Current knowledge of the large RhoGAP family of proteins. Biol Cell 99:67–86. doi:10.1042/BC20060086
Terry S, Nie M, Matter K, Balda MS (2010) Rho signaling and tight junction functions. Physiology 25:16–26. doi:10.1152/physiol.00034.2009
Thumkeo D, Watanabe S, Narumiya S (2013) Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol 92:303–15. doi:10.1016/j.ejcb.2013.09.002
Tian X, Tian Y, Gawlak G, Sarich N, Wu T, Birukova AA (2013) Control of vascular permeability by atrial natriuretic peptide via GEF-H1-dependent mechanism. J Biol Chem. doi:10.1074/jbc.M113.493924
Tzima E (2006) Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 98:176–85. doi:10.1161/01.RES.0000200162.94463.d7
Van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VWM (1998) Transient and Prolonged Increase in Endothelial Permeability Induced by Histamine and Thrombin: Role of Protein Kinases, Calcium, and RhoA. Circ Res 83:1115–1123. doi:10.1161/01.RES.83.11.1115
Van Nieuw Amerongen GP, Van Delft S, Vermeer MA, Collard JG, van Hinsbergh VWM (2000) Activation of RhoA by Thrombin in Endothelial Hyperpermeability: Role of Rho Kinase and Protein Tyrosine Kinases. Circ Res 87:335–340. doi:10.1161/01.RES.87.4.335
Van Nieuw Amerongen GP, Koolwijk P, Versteilen AMG, van Hinsbergh VWM (2003) Involvement of RhoA/Rho Kinase Signaling in VEGF-Induced Endothelial Cell Migration and Angiogenesis In Vitro. Arterioscler Thromb Vasc Biol 23:211–217. doi:10.1161/01.ATV.0000054198.68894.88
Vandenbroucke E, Mehta D, Minshall R, Malik AB (2008) Regulation of endothelial junctional permeability. Ann N Y Acad Sci 1123:134–45. doi:10.1196/annals.1420.016
Vardouli L, Vasilaki E, Papadimitriou E, Kardassis D, Stournaras C (2008) A novel mechanism of TGFbeta-induced actin reorganization mediated by Smad proteins and Rho GTPases. FEBS J 275:4074–87. doi:10.1111/j.1742-4658.2008.06549.x
Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JGN (1998) Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol 19:767–76. doi:10.1165/ajrcmb.19.5.3126
Vestweber D, Winderlich M, Cagna G, Nottebaum AF (2009) Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol 19:8–15. doi:10.1016/j.tcb.2008.10.001
Vouret-Craviari V, Boquet P, Pouysségur J, Van Obberghen-Schilling E (1998) Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell 9:2639–53
Wallez Y, Huber P (2008) Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778:794–809. doi:10.1016/j.bbamem.2007.09.003
Wang Y-X, Fitch RM (2004) Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol 2:379–84
Wang X, Ha T (2013) Defining single molecular forces required to activate integrin and notch signaling. Science 340:991–4. doi:10.1126/science.1231041
Wang N, Butler JP, Ingber DE, Donald E (1993) Mechanotransduction Across the Cell Surface and Through the Cytoskeleton. Science 260:1124–1127, 80-
Waschke J, Baumgartner W, Adamson RH, Zeng M, Aktories K, Barth H, Wilde C, Curry FE, Drenckhahn D (2004) Requirement of Rac activity for maintenance of capillary endothelial barrier properties. Am J Physiol Heart Circ Physiol 286:H394–401. doi:10.1152/ajpheart.00221.2003
Waschke J, Burger S, Curry F-RE, Drenckhahn D, Adamson RH (2006) Activation of Rac-1 and Cdc42 stabilizes the microvascular endothelial barrier. Histochem Cell Biol 125:397–406. doi:10.1007/s00418-005-0080-2
Weber GF, Bjerke MA, DeSimone DW (2011) Integrins and cadherins join forces to form adhesive networks. J Cell Sci 124:1183–93. doi:10.1242/jcs.064618
Weis SM (2008) Vascular permeability in cardiovascular disease and cancer. Curr Opin Hematol 15:243–9. doi:10.1097/MOH.0b013e3282f97d86
Weis SM, Cheresh DA (2005) Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437:497–504. doi:10.1038/nature03987
Wennerberg K, Der CJ (2004) Rho-family GTPases: it’s not only Rac and Rho (and I like it). J Cell Sci 117:1301–12. doi:10.1242/jcs.01118
Wessells NK, Spooner BS, Ash JF, Bradley MO, Luduena MA, Taylor EL, Wrenn JT, Yamada K (1971) Microfilaments in cellular and developmental processes. Science 171:135–43
Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K (2005) Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280:11675–82. doi:10.1074/jbc.M412595200
Wójciak-Stothard B, Ridley AJ (2002) Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 39:187–199
Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ (2001) Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114:1343–55
Wójciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, Louise H, Prendergast GC, Wilkins MR (2012) Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 110:1423–34. doi:10.1161/CIRCRESAHA.112.264473
Wysolmerski RB, Lagunoff D (1990) Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci U S A 87:16–20
Xiaolu D, Jing P, Fang H, Lifen Y, Liwen W, Ciliu Z, Fei Y (2011) Role of p115RhoGEF in lipopolysaccharide-induced mouse brain microvascular endothelial barrier dysfunction. Brain Res 1387:1–7. doi:10.1016/j.brainres.2011.02.059
Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O (2011) Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 20:526–39. doi:10.1016/j.devcel.2011.02.010
Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ (2005) Deconstructing the cadherin-catenin-actin complex. Cell 123:889–901. doi:10.1016/j.cell.2005.09.020
Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60:24–34. doi:10.1002/cm.20041
Zaidel-Bar R, Geiger B (2010) The switchable integrin adhesome. J Cell Sci 123:1385–8. doi:10.1242/jcs.066183
Zamir E, Geiger B (2001) Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci 114:3583–90
Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG (2013) Interaction of p190RhoGAP with C-terminal domain of p120- catenin modulates endothelial cytoskeleton and permeability. J Biol Chem 288:18290–9. doi:10.1074/jbc.M112.432757
Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Côté G, Wysolmerski R (2000) Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci 113(Pt 3):471–82
Zheng Y (2001) Dbl family guanine nucleotide exchange factors. Trends Biochem Sci 26:724–732
Zheng Y, Fischer DJ, Santos MF, Tigyi G, Pasteris NG, Gorski JL, Xu Y (1996) The faciogenital dysplasia gene product FGD1 functions as a Cdc42Hs-specific guanine-nucleotide exchange factor. J Biol Chem 271:33169–72
Zieman SJ, Melenovsky V, Kass DA (2005) Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25:932–43. doi:10.1161/01.ATV.0000160548.78317.29
Acknowledgements
The authors thank Dr. Ramaswamy Krishnan for all the support and advice given for the traction force microscopy set-up at the VU University Medical Center.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Dutch Heart Foundation grant NHS2011T072.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amado-Azevedo, J., Valent, E.T. & Van Nieuw Amerongen, G.P. Regulation of the endothelial barrier function: a filum granum of cellular forces, Rho-GTPase signaling and microenvironment. Cell Tissue Res 355, 557–576 (2014). https://doi.org/10.1007/s00441-014-1828-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-014-1828-6