Abstract
Despite the fact that we all know that melatonin plays a role and has some profound effects on animals, recent studies have shown that this biochemical can also be found in plants, microorganisms, and algae, and its effects can be seen in these organisms as well. Primarily, melatonin is considered a “sleep hormone”. In animals, it acts as an antioxidant, anti-inflammatory, and anti-carcinogenic agent and is used to treat several diseases. It is available in the market as a supplement. Melatonin has several functional roles in the plants, such as abiotic stress tolerance, as a secondary metabolite, synthesis of several phytohormones, defence mechanism, acts as a phytohormone, seedling growth, fruit development, root development, seed germination, flower development, crop and fruit yield, fruit storage, etc. Melatonin acts differently in different growth phases of the plants, viz., vegetative and reproductive phases. Also, it is found to have nutraceutical value. Here, in this chapter, we are dealing briefly with a historical perspective of this hormone, the isolation of this compound, how this hormone has importance in animals as well as in plants, how it was discovered, its biosynthetic pathways, the precursors and organelles involved in synthesis; altogether. Also, we are dealing with the comparative study of the mechanism of this newly discovered chemical in plants as well as animals, along with its current use in our day-to-day lives. Various studies have shown the abundance of this molecule and its benefits for humankind and plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Melatonin has also been studied for use in treating sleep disorders in addition to jet lag. Generally speaking, it decreases sleep latency and enhances sleep, particularly when circadian phasing is disrupted. Patients with neurological illnesses benefited the most from this in the latter situation (Hardeland 2005; Altaf et al. 2023). Several initiatives designed to lessen the effects of neurodegenerative illnesses such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis have been created or are being investigated to deal with these conditions (Altaf et al. 2022a, b). The effectiveness of this compound as a cancer-fighting agent has been extensively studied. More research should be focused on the potential anti-inflammatory effects of N1-acetyl-5-methoxykynuramine (AMK), particularly given that AMK is a natural downregulation and inhibitor of COX-2 (cyclooxygenase-2) (Hardeland 2005). The regulation of melatonin in the sleep/wake cycle, seasonal rhythms, and other circadian rhythms has already been observed, as well as its effect as an immunostimulator and cytoprotective agent. It has been observed that the substance is capable of safeguarding mitochondrial electron flux, antioxidant protection, and neuroprotection in various experimental systems. At night, melatonin levels are more significantly elevated, then information about “darkness” is passed on to the brain and light suppresses the mechanism by which it increases (Hardeland et al. 2006).
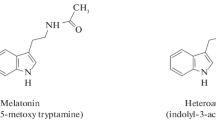
The chemical compound N-acetyl-5-methoxytryptamine, also referred to as melatonin, has been extensively researched in other parts of the world, and it is found in all living organisms, i.e., it is everywhere (Lerner et al. 1958; Zhang et al. 2023). In addition, it has been identified as a plant hormone that plays an important role in facilitating the regulation and development of plants (Arnao and Hernández-Ruiz 2019). A number of studies have shown that melatonin plays an essential role in maintaining a healthy circadian rhythm, sleep, mood, body temperature, appetite, and immune response in humans (Socaciu et al. 2020). Melatonin is a common indolamine that has received much research because the substance plays an essential role in controlling a wide range of physiological processes in animals and plants (Fig. 1.1). During the twentieth century, scientists discovered that certain plant species can synthesize large quantities of this chemical and store it in specialized tissues throughout the plant. As a result, it has been considered a ubiquitous molecule (Mannino et al. 2021).
Roles of melatonin in human physiology. Melatonin serves as a beneficial molecule/chemical in humans. Several functions of this hormone are depicted here entitled general actions, beneficial effects on sleep disorders, neurological disorders, other disorders, and anti-carcinogenic/anti-tumoral effects. ROS reactive oxygen species
It was originally thought that, when the substance was discovered, it was an antioxidant that had a wide range of positive effects on the various phases of plant growth and development, such as germination, root extension, photosynthesis, and leaf senescence (Arnao and Hernández-Ruiz 2019), as well as photosynthesis and leaf senescence (Wang et al. 2022). Among the many bioactive compounds present in vascular plants, it has been observed to be one of the most important (Ahmad et al. 2023) The compound can be found in a wide range of plant tissues, including those of seeds, roots, leaves, and fruits (Ahmad et al. 2023) Melatonin has been used extensively for disease pathogenesis and therapeutic development since it has been shown to modulate antioxidant, anti-inflammatory, and other biological properties (Zhang et al. 2023).
Melatonin is an artificially manufactured form of hormone present in animals, bacteria, plants, and fungi; apart from its antioxidant properties, melatonin has applications in beverages and food, dietary supplements, and pharmaceuticals. Bio-based “SPF” (spray polyurethane foam) is also synthesized from melatonin and having a role in generating insulation (Market analysis report 2019–2025). It is suggested by APA (American Psychiatric Association) reports that, throughout their lives, about one-third of adults experience sleep problems i.e., insomnia. Symptoms of this are persistent difficulties falling and persist in being asleep. Hence, it is obvious to observe the increased use of artificially synthesized melatonin (Lal et al. 2022; Mishra et al. 2022; Naz et al. 2022). It is expected that over the next 5 years, according to calculations, the Compound Annual Growth Rate (CAGR) for the melatonin market will be greater than 10%. The major companies which are functional in Melatonin Market are, viz., LLC, Natrol, Aspen Holdings, Pfizer Incorporation, Biotics Research Corporation, Nature’s Bounty (Market analysis report 2019–2025; Mordor Intelligence 2023–2028).
1.2 Melatonin Discovery in Animals and Plants
Up until 1995, “melatonin” had been one of the compounds that had received the most attention in the scientific literature as well as specialized journals, such as the Melatonin Research and Journal of Pineal Research (founded in 1984), which was founded in 1985. There was a belief that an animal hormone might be the cause of this problem in particular. However, after that, the undeniable discovery of plant-based melatonin was eventually made available to the scientific community back in 1995 by a trio of different research groups (Hattori et al. 1995; Dubbels et al. 1995). Similarly, Dr. Saxena’s group in Canada has been developing and carrying out a line of research that is of particular interest. Various studies have suggested that melatonin may function as an auxin in in vitro cell cultures as a result of the structural similarities between IAA (indole-3-acetic acid) and melatonin. The researchers discovered phases of the phytomelatonin production pathway that were identical to the pathways already present in mammals (Murch et al. 2000, 2001; Murch and Saxena 2002) while they were studying the cells of St. John’s wort (Hypericum perforatum L.) culture.
There was an initial confirmation in 2004 that melatonin had a growth-stimulating effect in the hypocotyls of etiolated lupin (Lupinus albus L.), with an estimated stimulatory potential up to 63% when compared to IAA’s effects on lupin (Hernández-Ruiz et al. 2004). As previously mentioned, melatonin is the scientific name given to a hormone that is able to contract melanophores, which is implicated in the lightening of skin in frogs and fish melanocytes (skin-lightening molecule) (Hardeland et al. 2006). As a matter of fact, melatonin is now well known to exist in all kingdoms of life, from prokaryotes to eukaryotes, and even in plants (Lal et al. 2023; Kumar et al. 2023a, b). Phytomelatonin is the name given to a molecule that can be found in plants, known as melatonin (Arnao 2014). There is a distinction between phytomelatonin, which is derived from algae and plants, and animal or synthetic melatonin, which is derived from animals or synthetic materials. There are a number of studies that deal with plant-derived melatonin, such as those in food chemistry, plant physiology, phytochemistry, botany, and so on, but this term is often used in these studies. A pleiotropic chemical that has many roles in a variety of physiological reactions in plants is phytomelatonin (Arnao and Hernández-Ruiz 2018, 2020a, 2022; Aghdam et al. 2022).
This hormone, originally discovered as a hormone produced by the pineal gland of a cow (Lerner et al. 1958; Arnao and Hernández-Ruiz 2020b), is now produced by fungi, invertebrates, protozoa, bacteria, plants, the Harderian gland, skin, gut, leukocytes, and a number of extrapineal sites in vertebrates. As a result of the accumulation of melanin granules in the melanocytes, this active factor plays a crucial role in illuminating the skin colour of tadpoles, frogs, toads, and some fish, but does not affect animals (Fig. 1.2). Melatonin is the name of the compound, and it was scientifically identified as a N-acetylserotonin derivative in 1959 by Arnao and Hernández-Ruiz (Arnao and Hernández-Ruiz 2020a).
This figure illustrates the effects of melatonin on plant growth and development, including seed germination, root development, seedling growth, flower development, crop and fruit yield, and fruit storage, which are all important in the development and growth of plants. (Modified from Zhang et al. 2023)
In 1960, Lerner isolated and identified this compound as a small molecule with a molecular weight of 232 Daltons. It was discovered that this molecule aggregated pigment granules in both fish and frog skin; hence, it was named. Melatonin is an extensively dispersed chemical found in all kingdoms of life (Mannino et al. 2021; Arnao et al. 2022). There are several physiological properties of melatonin that contribute to its ability to combat oxidative stress, promote reproduction, and promote plant growth. Plant NPs (natural products) are considered to be hormones as well as plant hormones (Mangal et al. 2023; Watpade et al. 2023). In an indirect manner, melatonin is synthesized through the shikimate pathway, as it is a by-product of the shikimate pathway (Elshafie et al. 2023). Including its pleiotropic properties, melatonin is an important abiotic stress signalling molecule for plants as it makes them more resilient to both mild and severe conditions, and it affects many aspects of their development and function (Ahmad et al. 2023).
1.3 Melatonin Precursors and Organelle Involved
Melatonin was discovered to be a component of all vertebrates, to be rhythmically modulated by the pineal gland’s secretion, and to have a role in the circadian control and, occasionally, in the seasonal patterns (Hardeland et al. 2006). Several subcellular compartments, including the cytoplasm, endoplasmic reticulum, mitochondria, and chloroplasts, synthesize melatonin intermediates that control the following enzymatic pathways (Zhao et al. 2019; Arnao et al. 2023). It has been shown that rice plants contain up to four genes for histone DAC (deacetylases), enzymes that may reverse the conversions of 5-methoxytryptamine and serotonin into N-acetylserotonin and melatonin, respectively. Deacetylase activity of DAC is maximum for N-acetyltyramine. Also, the chloroplast-expressed DAC displayed enzyme activity towards melatonin, N-acetyltryptamine, and N-acetylserotonin (Lee et al. 2018; Arnao et al. 2023).
The PMTR1, phytomelatonin receptor facilitates ROS signalling, controls homeostasis, and transmits a dark signal that stimulates night stomatal closure (preventing water loss during the night), which aids plant adaptation to dryland environments (Li et al. 2020). Melatonin is a multiregulatory molecule that controls the expression of genes related to abiotic stress resistance, the redox reactions and plant growth and development, sucrose metabolism {CWIN [cell wall invertase] and SUSY [sucrose synthase]}, and specialized metabolism {phenylpropanoid metabolism: DFR [dihydroflavonol reductase], CHI [chalcone isomerase] (Fig. 1.3). PAL [phenylalanine ammonia lyase], F3H [flavanone 3-hydroxylase], CHS [chalcone synthase], and ANS [anthocyanidin synthase]} (Weeda et al. 2014; Ahmad et al. 2023).
In plant cells, the location of the enzymes involved in the production of melatonin from tryptophan is varied. TDC is contained in the cytoplasm (Zhou et al. 2020). In chloroplasts, SNAT is expressed; in the endoplasmic reticulum, T5H is expressed (Back 2021; Rather et al. 2022), ASMT and COMT, however, are found in the cytoplasm (Mannino et al. 2021). The first and second of the four probable biosynthetic pathways of melatonin shown in Fig. 1.4 occur in the cytoplasm, while the third and fourth pathways lead to serotonin production in the endoplasmic reticulum (Back et al. 2016). Melatonin synthesis and accumulation can occur at a variety of ultimate subcellular sites; however, SNATs are exclusively found in the chloroplast and ASMTs/COMT in the cytoplasm. For instance, the serotonin SHT (N-hydroxycinnamoyl transferase) rapidly converts serotonin into phenylpropanoid amides in the cytoplasm, such as feruloylserotonin (Byeon and Back 2015). 2-OHM (2-hydroxymelatonin) is a product of the melatonin metabolism in chloroplasts; this reaction is catalysed by M2H (melatonin-2-hydroxylase). Conversely, melatonin is quickly transformed into cyclic 3-OHM (3-hydroxymelatonin) by M3H (melatonin-3-hydroxylase) (Lee et al. 2016; Ye et al. 2019).
1.4 Melatonin Biosynthetic Pathway in Animals and Plants
All vertebrates now have an enlarged mechanism for melatonin production, and other creatures, like insects, can also use this system (Herbert et al. 1960; Rahman et al. 2023; Thakur et al. 2023; Bairwa et al. 2023). The availability of the precursor, tryptophan, is a glaring variation in melatonin synthesis between animals and plants. Animals must consume tryptophan through diet because they cannot synthesize it on their own, unlike plants (Naz et al. 2023). The main melatonin-producing organelles and concentration centres are found to be animal mitochondria, similar to plants (Reiter 1991). In an isotope tracer investigation, the idea of melatonin produced by plants was initially suggested (Murch et al. 2000). Although there is a great deal of controversy surrounding this, it is believed that the biosynthetic pathway for phytomelatonin in vascular plants is comparable to that in animals (Murch et al. 2000; Tan et al. 2013; Zhao et al. 2019). Axelrod’s team first identified the mammalian melatonin biosynthesis route in 1960, and it is now well understood (Hardeland and Poeggeler 2003). The two functional groups of an indoleamine N-acetyl-5-methoxytryptamine (melatonin) have significance for the specificity of receptor binding, as well as for the molecule’s amphiphilicity, which allows it to enter any cell, compartment, or bodily fluid, and, intriguingly, for its oxidation chemistry (Hardeland et al. 2006).
Pathways for biosynthesis appear to be the same. Membrane and nuclear receptors, additional chemical interactions, or binding sites mediate these pleiotropic activities. Hepatic P450 monooxygenases mostly convert circulating melatonin to 6-hydroxyl and excrete it as 6-sulfatoxymelatonin. The relevance of pyrrole-ring cleavage is of the greater importance in other tissues, notably the brain. Photocatalytic, enzymatic, pseudoenzymatic, and multiple free-radical processes combine to produce the end product, N1-acetyl-N2-formyl-5-methoxykynuramine. Hydroxylation and nitrosation lead to the production of additional metabolites. N1-acetyl-5-methoxykynuramine, a secondary metabolite, promotes mitochondrial activity and suppresses cyclooxygenase-2 (Hardeland et al. 2006).
Tryptophan is assumed as the first substrate of the biosynthesis of melatonin and is engaged in four enzymatic steps that are catalysed by at least six enzymes, according to a number of research: including COMT (caffeic acid-O-methyltransferase), ASMT (N-acetylserotonin methyltransferase), SNAT (serotonin-N-acetyltransferase), T5H (tryptamine-5-hydroxylase), TPH (tryptophan hydroxylase), and TDC (tryptophan decarboxylase) (Back et al. 2016; Sun et al. 2021). For the synthesis of melatonin, the two reactions that contribute to tryptophan are hydroxylation and decarboxylation. They have been found in plants that are classified as herbivorous (Ahmad et al. 2023). There are four potential pathways for the biosynthesis of auxin, or IAA (indole-3-acetic acid), which is produced naturally in plants, that is, IAM (indole-3-acetamide), TAM (tryptamine), IAOx (indole-3-acetaldoxime), and IPyA (indole-3-pyruvic acid) (Fig. 1.5). There is still a need for more research into the synthesis of auxin from tryptophan in various crops under abiotic stress. N-acetylserotonin is produced by the catalysis of serotonin by SNATs, which is then methoxylated by ASMTs to produce melatonin (Ahmad et al. 2023).
Tryptophan, a major ingredient in the production of melatonin in plants, is produced by a biosynthetic process. (1) DAHP synthase; (2) DHQ synthase; (3) DHQ dehydratase; (4) Shikimate dehydrogenase; (5) Shikimate kinase; (6) EPSP synthase; (7) Chorismate synthase; (8) Anthranilate synthase; (9) PRPP (phosphoribosyl pyrophosphate) transferase; (10) PRAI (PRA isomerase); (11) IGP synthase; (12) Tryptophan synthase. PEP 2-phosphoenolpyruvate, DAHP 3-deoxy-Darabinoheptulosonate-7phosphate, DHQ 3-dehydroquinic acid, DHS 3-dehydroshikimate, EPSP 5-enolpyruvylshikimate-3-phosphate, PRA Phosporibosyl antranilate
Tryptophan is the precursor of melatonin production and is an amino acid that plants can synthesize de novo through the shikimate pathway. All aromatic amino acids, including tryptophan, can be biosynthesized in plants using this process, which entails seven distinct stages. Briefly,
-
1.
The enzyme DAHP synthase (EC 2.5.1.54) is responsible for converting PEP (phosphoenol pyruvate) and erythrose-4-phosphate into DAHP (3-Deoxy-D-arabinoheptulosonate-7-phosphate). DHQ synthase (EC 4.2.3.4) is an enzyme that cyclizes DAHP into the 3-dehydroquinate form of DHQ by cyclizing DAHP into DHQ.
-
2.
DHQ dehydratase (EC 4.2.1.10) catalyses the dehydration which converts DHQ (3-dehydroquinic acid) into DHS (3-dehydroshikimate). Shikimate dehydrogenase (EC 1.1.1.25) further catalyses the dehydrogenation reaction, which converts DHS (3-dehydroshikimate) into Shikimate.
-
3.
The enzyme EPSP synthase (EC 2.5.1.19) transforms shikimate into EPSP (5-enolpyruvylshikimate-3-phosphate) after shikimate has been phosphorylated by the enzyme shikimate kinase (EC 2.7.1.71).
-
4.
The enzyme chorismate synthase (EC 4.2.3.5), the crucial stage in tryptophan biosynthesis, which transforms EPSP into chorismate, produces chorismite.
-
5.
Anthranilate synthase (EC 4.1.3.27) converts chorismate into anthranilate, which is then combined with PRPP (phosphoribosyl pyrophosphate) to produce PRA (phosphoribosyl anthranilate).
-
6.
To create indole-3-glycerol phosphate, which is then spontaneously transformed into the indole scaffold, the ribose ring added in this final process is first opened by PRAI (PRA isomerase; EC 5.3.1.24).
-
7.
The final step in the production of tryptophan is the action of TPS (tryptophan synthase; EC 4.2.1.20), which is responsible for the interaction of indole with serine (Mannino et al. 2021).
COMT, ASMT, and SNAT are three different enzymes, each of which may have several isoforms, which are required for two-step processes that produce melatonin from serotonin (Back et al. 2016). While the other two enzymes are methyltransferases, the first enzyme catalyses acetylation. Since serotonin, N-acetylserotonin, and 5-methoxytryptamine are substrates for all three enzymes, the order in which they function can also change in this situation (Park et al. 2013; Byeon et al. 2014; Lee et al. 2014). The conditions of plant growth determine which pathway is used for melatonin production (Fig. 1.6). In fact, the metabolic pathway from tryptophan to melatonin passes through the “tryptamine/serotonin/N-acetylserotonin intermediate” before arriving at melatonin under stressful or normal circumstances that do not create a substantial accumulation of serotonin (Byeon et al. 2015).
Melatonin biosynthesis mechanisms in microbes, humans, and plants. Green (plants), blue (animals), yellow (bacteria), and black (yeasts) are represented by various arrow colours. Unproven reactions are indicated by dashed lines. TPH tryptophan hydroxylase, TDC L-tryptophan decarboxylase, T5H tryptamine-5-hydroxylase, SNAT serotonin N-acetyltransferase, ASMT acetylserotonin O-methyltransferase, COMT caffeic acid 3-O-methyltransferase, DAC deacetylases
The initial step in the biosynthesis of melatonin in plants corresponds to the generation of serotonin from tryptophan. Two distinct routes might be implicated.
-
(a)
In the first route, tryptophan is first decarboxylated by TPH into tryptamine, which TDC subsequently hydroxylates into serotonin.
-
(b)
“TDC converts 5-hydroxytryptophan into serotonin by decarboxylation” follows the “TPH-mediated hydroxylation of tryptophan into 5-hydroxytryptophan, “ another alternative.
Both of these approaches are feasible since TDC exhibits strong in vitro affinities for tryptophan and 5-hydroxytryptophan. Decarboxylation has been shown to occur more frequently in plants than hydroxylation as a preliminary step, though (Back et al. 2016). An acetylated substance produced from serotonin is melatonin. The biosynthetic process in which the amino acid tryptophan produces indolic amines has been effectively examined in plants and mammals (Tan et al. 2015; Back et al. 2016).
In plants,
-
1.
The enzyme TDC (tryptophan decarboxylase) transforms tryptophan into tryptamine (Fig. 1.6).
-
2.
The enzyme T5H (tryptamine-5-hydroxylase), which has been widely researched in rice, converts tryptamine into serotonin (5-hydroxytryptamine) but has not been well investigated.
-
3.
SNAT (serotonin N-acetyltransferase) is N-acetylated serotonin. The hydroxyindole-O-methyltransferase i.e., ASMT (acetylserotonin methyltransferase) then methylates N-acetylserotonin to produce melatonin. COMT (caffeic acid-O-methyltransferase), an enzyme with a broad range of potential substrates, such as quercetin and caffeic acid, can methylate N-acetylserotonin in plants, as well (Byeon et al. 2014).
-
4.
After SNAT takes effect, serotonin may also be converted by ASMT/COMT into 5-methoxytryptamine to produce melatonin. In times of stress or senescence, this approach would take place (Back et al. 2016; Tan et al. 2016).
TPH (tryptophan hydroxylase) and TDC (tryptophan decarboxylase) operate in sequence to convert 5-hydroxytryptophan into serotonin in mammalian cells. The occurrence of 5-hydroxytryptophan revealed that certain enzymatic activities, including the action of TPH, operate with a reduced degree in the plant cells even though TPH was not identified in plants. Furthermore, according to several authors, 5-methoxytryptamine can be converted into melatonin under stress, suggesting that plant cells have greater potential for metabolic adaptation than animal cells do. This suggests that the melatonin biosynthesis pathway may take many different kinds of alternative routes (Arnao and Hernández-Ruiz 2014; Tan et al. 2016).
There are five enzymatic stages in the process (Fig. 1.7). Tryptophan is first hydroxylated by TPH to 5-hydroxytryptophan, which is then decarboxylated by the AADC (aromatic amino acid decarboxylase) to serotonin (5-hydroxytryptamine). For many years, the two last phases were ambiguous. In fact, neither the site of melatonin’s biosynthesis process nor the specific enzymes required for it were acknowledged to participate in the synthesis. When the mammalian melatonin biosynthetic route was found in 1960, it had been anticipated that only the pineal gland and liver are capable of acetylating serotonin to produce N-acetylserotonin (Pevet et al. 2017). It was incorrectly assumed that melatonin synthesis was not specialized in the liver, because ASMT was the first to be discovered in the pineal gland. Melatonin was first distinguished as a pineal-related neurohormone because of this. The production of melatonin by several organs and tissues in the periphery, including the skin, gut, hepatic cholangiocytes, lymphocytes, bone marrow, testis, ovary, Harderian gland, and retina, is currently well understood (Hardeland et al. 2011).
Because of the enzyme activity of ASMT for N-acetylserotonin was found to be around 14 times more potent, it was determined that this compound was the most suitable substrate of ASMT, as compared with the serotonin (Skene 2003). Based on these findings, it was hypothesized that firstly, AANAT acetylates serotonin in order to generate N-acetylserotonin, and that ASMT then converts the subsequent N-acetylserotonin into melatonin. It is generally acknowledged that AANAT is the enzyme that limits melatonin synthesis, i.e., a limiting factor. In fact, blue light (420–480 nm) is the primary regulating component in the melatonin synthesis process in animals (Ganguly et al. 2005). This type of daytime irradiation reduces the production of melatonin instantaneously by impairing AANAT’s ability to function both through protein dephosphorylation and downregulation of gene expression (Tan et al. 2011; Venegas et al. 2012). Other variables that could interfere with an animal’s ability to produce melatonin include fluctuations in temperature, food consumption, and various kinds of health conditions (Mannino et al. 2021). N-acetyltryptamine, which is produced by SNAT, may be transformed by T5H into N-acetylserotonin, which is subsequently manufactured as melatonin (Arnao et al. 2023).
1.5 Conclusion/Future Directions
Several recent studies have established the critical significance of melatonin in plant processes, notably its control of crop development and productivity. However, a full knowledge of melatonin, which affects crop development and production under abiotic stress conditions, is still inadequate. Other melatonin biosynthetic pathways, including ones independent of serotonin synthesis, may exist. The enzymes involved have yet to be discovered, and those that are known do not appear to be participating in this process. Aromatic and therapeutic plants have greater phytomelatonin levels than conventional veggies. Such botanical medicinal plants are perfect choices for future melatonin supplements. Controlling growing conditions might aid in the production of phytomelatonin-rich plants. Additional investigation on other species and varieties is required. The existence of phytomelatonin in all plant species studied so far suggests that it might be used as a nutraceutical ingredient. The discovery and research of phytomelatonin-rich species and variants should be prioritized. In terms of melatonin intake, we should focus on alternatives to synthetic melatonin and boost organically generated melatonin.
References
Aghdam MS, Mukherjee S, Flores FB, Arnao MB, Luo Z, Corpas FJ (2022) Functions of melatonin during postharvest of horticultural crops. Plant Cell Physiol 63(12):1764–1786. https://doi.org/10.1093/pcp/pcab175
Ahmad I, Song X, Ibrahim MEH, Jamal Y, Younas MU, Zhu G, Ali AYA (2023) The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front Plant Sci 14:1108507. https://doi.org/10.3389/fpls.2023.1108507
Altaf MA, Behera B, Mangal V, Singhal RK, Kumar R, More S, Naz S, Mandal S, Dey A, Saqib M, Kishan G, Kumar A, Singh B, Tiwari RK, Lal MK, Altaf MA, Behera B, Mangal V, Singhal RK, Lal MK (2022a) Tolerance and adaptation mechanism of Solanaceous crops under salinity stress. Funct Plant Biol. https://doi.org/10.1071/FP22158
Altaf MA, Mandal S, Behera B, Mangal V, Naz S, Kumar R, Kumar A, Ghorai M, Singh B, Dey A, Tiwari RK, Lal MK, Aftab T (2022b) Salinity stress tolerance in Solanaceous crops: current understanding and its prospects in genome editing. J Plant Growth Regul 1–17:4020. https://doi.org/10.1007/S00344-022-10890-0
Altaf MA, Sharma N, Singh J, Samota MK, Sankhyan P, Singh B, Kumar A, Naz S, Lal MK, Tiwari RK, Kumar R (2023) Mechanistic insights on melatonin-mediated plant growth regulation and hormonal cross-talk process in solanaceous vegetables. Sci Hortic 308:111570. https://doi.org/10.1016/J.SCIENTA.2022.111570
Arnao MB (2014) Phytomelatonin: discovery, content, and role in plants. Adv Botany 2014:1. https://doi.org/10.1155/2014/815769
Arnao MB, Cano A, Hernández-Ruiz J (2022) Phytomelatonin: an unexpected molecule with amazing performances in plants. J Exp Bot 73(17):5779–5800. https://doi.org/10.1093/jxb/erac009
Arnao MB, Giraldo-Acosta M, Castejón-Castillejo A, Losada-Lorán M, Sánchez-Herrerías P, El Mihyaoui A, Hernández-Ruiz J (2023) Melatonin from microorganisms, algae, and plants as possible alternatives to synthetic melatonin. Meta 13(1):72. https://doi.org/10.3390/metabo13010072
Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19(12):789–797. https://doi.org/10.1016/j.tplants.2014.07.006
Arnao BM, Hernández-Ruiz J (2018) The potential of phytomelatonin as a nutraceutical. Molecules 23(1):238. https://doi.org/10.3390/molecules23010238
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48. https://doi.org/10.1016/j.tplants.2014.07.006
Arnao MB, Hernández-Ruiz J (2020a) Is phytomelatonin a new plant hormone? Agronomy 10(1):95. https://doi.org/10.3390/agronomy10010095
Arnao MB, Hernández-Ruiz J (2020b) Melatonin in flowering, fruit set and fruit ripening. Plant Rep 33:77–87
Back K (2021) Melatonin metabolism, signaling and possible roles in plants. Plant J 105(2):376–391. https://doi.org/10.1111/tpj.14915
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res 61(4):426–437. https://doi.org/10.1111/jpi.12364
Bairwa A, Dipta B, Mhatre PH, Venkatasalam EP, Sharma S, Tiwari RK, Singh B, Thakur D, Naga KC, Maharana C, Sharma AK (2023) Chaetomium globosum KPC3: an antagonistic fungus against the potato cyst nematode, Globodera rostochiensis. Curr Microbiol 80(4):125. https://doi.org/10.1007/s00284-023-03228-w
Byeon Y, Back K (2015) Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J Pineal Res 58(3):343–351. https://doi.org/10.1111/jpi.12220
Byeon Y, Choi GH, Lee HY, Back K (2015) Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J Exp Bot 66(21):6917–6925. https://doi.org/10.1093/jxb/erv396
Byeon Y, Lee HY, Lee K, Back K (2014) Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J Pineal Res 57(2):219–227. https://doi.org/10.1111/jpi.12160
Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-áámass spectrometry. J Pineal Res 18(1):28–31. https://doi.org/10.1111/j.1600-079X.1995.tb00136.x
Elshafie HS, Camele I, Mohamed AA (2023) A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int J Mol Sci 24(4):3266. https://doi.org/10.3390/ijms24043266
Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC (2005) Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc Natl Acad Sci 102(4):1222–1227. https://doi.org/10.1073/pnas.0406871102
Hardeland R (2005) Antioxidative protection by melatonin— multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 27:119–130. https://doi.org/10.1385/ENDO:27:2:119
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93(3):350–384. https://doi.org/10.1016/j.pneurobio.2010.12.004
Hardeland R, Pandi-Perumal SR, Cardinali DP (2006) Melatonin. Int J Biochem Cell Biol 38(3):313–316. https://doi.org/10.1016/j.biocel.2005.08.020
Hardeland R, Poeggeler B (2003) Non-vertebrate melatonin. J Pineal Res 34(4):233–241. https://doi.org/10.1034/j.1600-079X.2003.00040.x
Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int 35(3):627–634. PMID: 7773197
Herbert W, Betty GR, Julius A (1960) Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. Biochim Biophys Acta 43:352–353. https://doi.org/10.1016/0006-3002(60)90453-4
Hernández-Ruiz J, Cano A, Arnao MB (2004) Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220:140–144. https://doi.org/10.1007/s00425-004-1317-3
Kumar R, Kaundal P, Tiwari RK, Lal MK, Kumari H, Kumar R, Naga KC, Kumar A, Singh B, Sagar V, Sharma S (2023a) Development of reverse transcription recombinase polymerase amplification (RT-RPA): a methodology for quick diagnosis of potato Leafroll viral disease in potato. Int J Mol Sci 24:2511. https://doi.org/10.3390/ijms24032511
Kumar A, Lal MK, Sahoo U, Sahoo SK, Sah RP, Tiwari RK, Kumar R, Sharma S (2023b) Combinatorial effect of heat processing and phytic acid on mineral bioavailability in rice grain. Food Chem Adv 2:100232. https://doi.org/10.1016/j.focha.2023.100232
Lal P, Behera B, Yadav MR, Sharma E, Altaf MA, Dey A, Kumar A, Tiwari RK, Lal MK, Kumar R (2023) A bibliometric analysis of groundwater access and its management: making the invisible visible. Water 15(4):806. https://doi.org/10.3390/w15040806
Lal MK, Tiwari RK, Kumar A, Dey A, Kumar R, Kumar D, Jaiswal A, Changan SS, Raigond P, Dutt S, Luthra SK, Mandal S, Singh MP, Paul V, Singh B (2022) Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants 11:2857
Lee HY, Byeon Y, Lee K, Lee HJ, Back K (2014) Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J Pineal Res 57(4):418–426. https://doi.org/10.1111/jpi.12181
Lee K, Lee HY, Back K (2018) Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J Pineal Res 64(2):e12460. https://doi.org/10.1111/jpi.12460
Lee K, Zawadzka A, Czarnocki Z, Reiter RJ, Back K (2016) Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J Pineal Res 61(4):470–478. https://doi.org/10.1111/jpi.12361
Lerner A, Case J, Takahashi Y, Lee T, Mori W (1958) Isolation of melatonin, a pineal factor that lightens melanocytes. J Am Chem Soc 80:2587. https://doi.org/10.1021/ja01543a060
Li D, Wei J, Peng Z, Ma W, Yang Q, Song Z et al (2020) Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in arabidopsis. J Pineal Res 68:e12640. https://doi.org/10.1111/jpi.12640
Mangal V, Lal MK, Tiwari RK, Altaf MA, Sood S, Gahlaut V, Bhatt A, Thakur AK, Kumar R, Bhardwaj V, Kumar V (2023) A comprehensive and conceptual overview of omics-based approaches for enhancing the resilience of vegetable crops against abiotic stresses. Planta 257(4):80. https://doi.org/10.1007/s00425-023-04111-5
Mannino G, Pernici C, Serio G, Gentile C, Bertea CM (2021) Melatonin and phytomelatonin: chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—an overview. Int J Mol Sci 22(18):9996. https://doi.org/10.3390/ijms22189996
Market Analysis Report (2019–2025) Melatonin Market Size, Share & Trends Analysis Report By Application, Regional Outlook, Competitive Strategies, And Segment Forecasts, 2019 to 2025. Melatonin Market Size, Share & Trends|Global Industry Report, 2025. grandviewresearch.com. Accessed 30 Mar 2023
Mishra UN, Jena D, Sahu C, Devi R, Kumar R, Jena R, Irondi EA, Rout S, Tiwari RK, Lal MK, Baig MJ, Kumar A (2022) Nutrigenomics: an inimitable interaction amid genomics, nutrition and health. Innovative Food Sci Emerg Technol 82:103196. https://doi.org/10.1016/J.IFSET.2022.103196
Mordor Intelligence (2023–2028) Melatonin Market—Growth, Trends, COVID-19 Impact, and Forecasts. Melatonin Market Size & Share Analysis—Industry Research Report—Growth Trends. mordorintelligence.com. Accessed 30 Mar 2023
Murch SJ, Campbell SS, Saxena PK (2001) The role of serotonin and melatonin in plant morphogenesis: regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John's wort (Hypericum perforatum L.). In Vitro Cell Dev Biol Plant 37:786–793. https://doi.org/10.1079/IVP2001235
Murch S, Krishnaraj S, Saxena P (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. john's wort (Hypericum perforatum l. cv. Anthos) plants. Plant Cell Rep 19:698–704. https://doi.org/10.1007/s002990000206
Murch SJ, Saxena PK (2002) Melatonin: a potential regulator of plant growth and development? In Vitro Cell Dev Biol Plant 38:531–536. https://doi.org/10.1079/IVP2002333
Naz S, Bilal A, Saddiq B, Ejaz S, Ali S, Tul S, Haider A, Sardar H, Nasir B, Ahmad I, Tiwari RK, Lal MK, Shakoor A, Alyemeni MN, Mushtaq N, Altaf MA (2022) Foliar application of salicylic acid improved growth, yield, quality and photosynthesis of pea (Pisum sativum L.) by improving antioxidant defense mechanism under saline conditions. Sustainability 14:14180. https://doi.org/10.3390/SU142114180
Naz S, Jamshed S, Nisar QA, Nasir N (2023) Green HRM, psychological green climate and proenvironmental behaviors: An efficacious drive towards environmental performance in China. Curr Psychol 42(2):1346–1361
Park S, Byeon Y, Back K (2013) Functional analyses of three ASMT gene family members in rice plants. J Pineal Res 55(4):409–415. https://doi.org/10.1111/jpi.12088
Pevet P, Klosen P, Felder-Schmittbuhl MP (2017) The hormone melatonin: animal studies. Best Pract Res Clin Endocrinol Metab 31(6):547–559. https://doi.org/10.1016/j.beem.2017.10.010
Rahman M, Borah SM, Borah PK, Bora P, Sarmah BK, Lal MK, Tiwari RK, Kumar R (2023) Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2 and Trichoderma harzianum NBG. Front Plant Sci 14:353. https://doi.org/10.3389/fpls.2023.1141506
Rather AA, Natrajan S, Lone AS, Tiwari RK, Lal MK, Kumar R (2022) Exogenous application of salicylic acid improves growth and yield of black gram Vigna mungo L. by improving antioxidant defense mechanism under saline conditions. Russian. J Plant Physiol 69(7):151
Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 12(2):151–180. https://doi.org/10.1210/edrv-12-2-151
Skene DJ (2003) Optimization of light and melatonin to phase-shift human circadian rhythms. J Neuroendocrinol 15(4):438–441. https://doi.org/10.1046/j.1365-2826.2003.01006.x
Socaciu AI, Ionut R, Socaciu MA, Ungur AP, Bârsan M, Chiorean A et al (2020) Melatonin, an ubiquitous metabolic regulator: functions, mechanisms and effects on circadian disruption and degenerative diseases. Rev Endocr Metab Disord 21:465–478. https://doi.org/10.1007/s11154-020-09570-9
Sun C, Liu L, Wang L, Li B, Jin C, Lin X (2021) Melatonin: a master regulator of plant development and stress responses. J Integr Plant Biol 63:126–145. https://doi.org/10.1111/jipb.12993
Tan DX, Hardeland R, Back K, Manchester LC, Alatorre Jimenez MA, Reiter RJ (2016) On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J Pineal Res 61(1):27–40. https://doi.org/10.1111/jpi.12336
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20(10):18886–18906. https://doi.org/10.3390/molecules201018886
Tan DX, Manchester LC, Fuentes-Broto LPSD, Paredes SD, Reiter RJ (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev 12(3):167–188. https://doi.org/10.1111/j.1467-789X.2010.00756.x
Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J Pineal Res 54(2):127–138. https://doi.org/10.1111/jpi.12026
Thakur R, Devi R, Lal MK, Tiwari RK, Sharma S, Kumar R (2023) Morphological, ultra structural and molecular variations in susceptible and resistant genotypes of chickpea infected with botrytis grey mould. PeerJ 11:e15134. https://doi.org/10.7717/peerj.15134
Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, Acuña-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 52(2):217–227. https://doi.org/10.1111/j.1600-079X.2011.00931.x
Wang K, Xing Q, Ahammed GJ, Zhou J (2022) Functions and prospects of melatonin in plant growth, yield, and quality. J Exp Bot 73(17):5928–5946. https://doi.org/10.1093/jxb/erac233
Watpade S, Naga KC, Pramanick KK, Tiwari RK, Kumar R, Shukla AK, Mhatre PH, Lal MK, Pal D, Manjunatha N (2023) First report of powdery mildew of pomegranate (Punica granatum) caused by Erysiphe punicae in India. J Plant Dis Prot 130:1–6. https://doi.org/10.1007/s41348-023-00718-8
Weeda S, Zhang N, Zhao X, Ndip G, Guo Y, Buck GA et al (2014) Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS One 9:e93462. https://doi.org/10.1371/journal.pone.0093462
Ye T, Yin X, Yu L, Zheng SJ, Cai WJ, Wu Y, Feng YQ (2019) Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-ñmass spectrometry. J Pineal Res 66(1):e12531. https://doi.org/10.1111/jpi.12531
Zhang J, Pan Y, Xu X, Li L, Sun Q, Wang Q, Tong Z (2023) Melatonin-mediated development and abiotic stress tolerance in plants. Front Plant Sci 14:17. https://doi.org/10.3389/fpls.2023.1100827
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R et al (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol 10:249. https://doi.org/10.3389/fendo.2019.00249
Zhou Y, Liao L, Liu X, Liu B, Chen X, Guo Y, Zeng Z (2020) Crystal structure of Oryza sativa TDC reveals the substrate specificity for TDC-mediated melatonin biosynthesis. J Adv Res 24:501–511. https://doi.org/10.1016/j.jare.2020.06.004
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Suresh, T.R. et al. (2023). Melatonin Discovery and Divergent Biosynthetic Pathways in Plants. In: Kumar, R., Altaf, M.A., Lal, M.K., Tiwari, R.K. (eds) Melatonin in Plants: A Regulator for Plant Growth and Development. Springer, Singapore. https://doi.org/10.1007/978-981-99-6745-2_1
Download citation
DOI: https://doi.org/10.1007/978-981-99-6745-2_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6744-5
Online ISBN: 978-981-99-6745-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)