Abstract
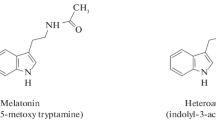
Melatonin (N-acetyl-5-methoxytryptamine) is a pineal gland hormone, relatively little research has been done on it in this area up until 1995. It can be found in several plant species in different concentrations. Melatonin has even been proposed as nature’s most adaptable biological signal molecule due to its widespread distribution throughout all kingdoms. Since Hattori first discovered melatonin in plants, Numerous studies have been released, expanding the field of phytomelatonin i.e. melatonin generated from plants. Plants biosynthesize phytomelatonin from the precursor tryptophan. Because of their powerful antioxidant properties, the majority of herbs with high melatonin content have been utilised for centuries to treat neurological problems linked to the production of free radicals. This brief summary aims to give a general understanding of phytomelatonin, including information on its distribution, biosynthesis, potential roles in the regulation and growth, and abiotic stress management of plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Melatonin has a long history and is a well-known companion in animal and human physiology, but it is new to plant physiology (Reiter et al. 2011). In 1958, Lerner and colleagues isolated melatonin for the first time from the pineal gland of cattle (Lerner et al. 1958) Melatonin was given that name because it can make the skin of several fish amphibians, and reptiles (Chava and Sirisha 2012). Melatonin is essential for controlling the circadian rhythm in mammals (Sahna et al. 2005). This powerful antioxidant boosts the gene expression of antioxidant enzymes while also protecting mitochondrial homeostasis. (Nitulescu et al. 2009; Carrillo et al. 2013; Fatma et al. 2013; Bhavini et al. 2009). Consequently, it is very helpful in treating neurological diseases like Alzheimer’s, etc. whose pathophysiology is linked to the cytotoxic effects of reactive oxygen species (Russel et al. 2010; Ayushi and Maheep 2007; Hardeland 2005; Jian and Ze 2006; Venkatramanujam 2011). For the first time, melatonin was independently discovered in plants by Dubbels et al. 1995; Hattori et al. 1995 (Dubbels et al. 1995; Hattori et al. 1995). Since then, research into phytomelatonin generated from plants has become one of the fastest-growing fields in plant physiology. Numerous scientific studies support the presence of melatonin is present in many plant species (Rudiger and Burkhard 2003). Melatonin is regarded as one of nature’s most adaptable biological signals due to its widespread dispersion and multidirectional activity. According to the current study, plants both produce and absorb this conventional indole derivative (Marino and Josefa 2006). The results of the experiments provide the clearest evidence for phytomelatonin’s functions as an antioxidant, free radical scavenger, and growth promoter (Russel et al. 2014). According to studies, excessive UV radiation increases the formation of indole compounds, which is strong evidence for phytomelatonin’s activity as an antioxidant that protects plants from strain related to oxidation and lessens macromolecule damage in a way that is comparable to that of animals (Katerova et al. 2012). It is essential for controlling plant reproductive physiology and protecting plant cells from apoptosis brought on by unfavorable environmental factors. Phytomelatonin has been identified to have a variety of physiological roles, including a probable role in blooming, regulating circadian rhythms and photoperiodicity, and serving as a growth regulator. Melatonin content varies by plant organ or tissue, with leaves and fragrant plants having higher levels than seeds (Dun 2015). It has auxin-like activity and regulates root, shoot, and explant growth. It also promotes seed germination and rhizogenesis, slows the start of induced leaf senescence, and regulates explant growth (Krystyna and Małgorzata 2013). Recently, a potential function in lupin rhizogenesis has also been suggested (Katarzyna et al. 2014).
Melatonin production in plants is well established and relatively little is known about its existence in organisms other than angiosperms. This is mostly owing to insufficient detection techniques and a lack of experimental protocols to look into phytomelatonin’s molecular and biochemical properties. To get quick, accurate results on phytomelatonin content, however, certain methodological protocols had been designed successfully. Tryptophan was discovered to be a common precursor for serotonin, melatonin, and indole-3-acetic acid (IAA) in investigations using radioisotope tracer techniques (Hernandez et al. 2004; Marino 2014; Russel et al. 2007). The whole biosynthetic pathways of phytomelatonin synthesis are yet to be explored vividly. According to certain accounts, plants may be able to absorb melatonin from the soil in which they are grown. According to the research, melatonin is also involved in the maintenance of chlorophyll and the promotion of photosynthesis (Van Tassel et al. 1995; Kolar and Machackova 2005). High-melatonin transgenic plants may significantly contribute to raising food yields and enhancing human health in general (Amit and Vinod 2014). This article’s goals include deepening our comprehension of the various physiological functions of phytomelatonin as well as discussing intriguing data about the substance.
2 Biosynthesis of phytomelatonin
The precursor tryptophan has a phylogenetic widespread distribution and is the source of melatonin. It was long believed that only vertebrates’ pineal glands could produce this neuro-hormone (Ebels and Tommel 1972). Later, a new area of study on this substance was created when melatonin was discovered in photosynthesizing organisms. Animals cannot synthesis the necessary amino acid tryptophan, hence they must get it from other natural sources since it cannot be produced by them (Marino and Hernandez 2007). In addition to the hormone auxin, phytoalexins, glucosinolates, alkaloids, and indoleamines, tryptophan also serves as a precursor for phytomelatonin (Bandurski et al. 1995). In angiosperms, the rate of melatonin production varies rhythmically, peaking at night and seasonally during the flowering period (Katri et al. 2012). Chorismate and anthranilate are used in the shikimic acid pathway to biosynthesize tryptophan. The conversion of tryptophan to 5-hydroxytryptophan to serotonin is regulated by tryptophan hydroxylase. Arylalkylamine N-acetyl Transferase (AANAT) converts serotonin into N-acetyl serotonin, from which hydroxyindole-O-methyltransferase (HIOMT) produces melatonin. It should be emphasized that AANAT is yet to be identified, although plants can still produce melatonin on their own. As a result, the genetic features of the serotonin N-acetylating enzyme in plants may differ from those of animal AANAT. Tryptophan decarboxylase is the only enzyme in plants that makes indole-3-acetic acid (IAA) from tryptophan (Yeo et al. 2007; Bruno et al. 2005; Marcello et al. 2006) (Fig. 2.1).
3 Melatonin in Edible Plants
Humans have discovered the presence of melatonin in more than 140 distinct fragrant, medicinal, and food plants (Jan and Ivana 2005). To find melatonin in plant tissues, several advanced analytical methods were created. The most trustworthy sources among these include radioimmuno assays (RIA), enzyme-linked immunoadsorbent assays (ELISA), high-performance liquid chromatography (HPLC), and gas chromatography-mass spectrophotometry (GC-MS) (John et al. 2011; Kolar 2003; Marcello et al. 2012). According to reports, cereals from the family of Graminae, such as rice, barley, sweet corn, and oats, contain significant amounts of melatonin (Dun et al. 2007). Melatonin was found in bananas in a concentration of 0.655 ng/g, according to GC-MS analysis, but HPLC-MS suggested higher melatonin content (1 ng/g of plant tissue) (Badria 2002). In many fruits like berries, kiwis, etc., melatonin contents were reported. Both white and black mustard seeds contained melatonin (189 ng/g of plant tissue and 123 ng/g of plant tissue, respectively through RIA analysis) (Burkhardt et al. 2001; Manchester et al. 2000). Both green and roasted beans contained melatonin, with concentrations of 5.8 g/g dry weight and 8.0 g/g dry weight, respectively (Akula et al. 2012) (Table 2.1).
4 Role of phytomelatonin
4.1 Circadian Rhythm
Melatonin regulates the circadian rhythm in animals, peaking during the scotophase and remaining constant during the photoperiod (Kazutaka et al. 2013). Consequently, phytomelatonin was assumed to serve a similar purpose in plants. It produces a diurnal oscillation that increases at night and decreases during the day (Shiddamallayya et al. 2010). This showed how the photoperiod affects melatonin’s involvement in regulating circadian rhythm. Circadian alterations in melatonin levels have been seen in algae and dinoflagellates in addition to higher plants (Amod et al. 2005; Parvin et al. 2011; Atanu et al. 2014). Researchers looked at how exogenous melatonin treatment affected Chenopodium rubrum flowering. In comparison to the control plants, the data did not indicate any harmful effects or changes in the form, colour, or quantity of leaves. Thus, the role of melatonin in flowering remains unclear (Ackermann et al. 2006; Pasquale et al. 2003). Figure 2.2 elaborates on the multi-directional role of melatonin in plants.
The summary of multi directional actions of melatonin in plant growth, metabolism and redox balance (Bhattacharjee and Kumar 2018)
4.2 Antioxidant and Free Radical Scavenger
Melatonin is a well-known antioxidant in mammals (Russel 2001). This prompted the scientists to speculate that the indole molecule would behave similarly in plants. According to reports, Lycopersicon esculentum Mill. (cultivated tomato) melatonin concentration is five times higher than that of Lycopersicon pimpinellifolium Mill. (wild tomato), making the first one more tolerant in higher ozone levels. Melatonin protects against photography (Fuhrberg et al. 1996). Large amounts of free radicals, reactive nitrogen species (RNS), are evolved during photosynthesis. Additionally, as photophase exposure to light increases, the violaxanthin cycle becomes hindered, resulting in reduced plastidial photo-protection. Eichhornia crassipes had a perfect diurnal rhythm, with melatonin metabolites peaking in the late-night section of the light-dark cycle. This suggested melatonin metabolites may protect against harmful ROS and RNS damages (Balzer and Hardeland 1991). Additionally, it has been suggested higher plants and algae can benefit from melatonin’s photoprotective actions against UV radiation (Wolf et al. 2001). Alpine and Mediterranean plants subjected to high UV levels in their native habitat contain more melatonin than the same species exposed to lower UV levels, lending support to this notion. (Tettamanti et al. 2000).
4.3 Growth Promoter
Melatonin and IAA, a powerful plant growth stimulant, are structurally similar. Melatonin is thus recommended to imitate auxin and promote vegetative development in a wide range of plant species (Kolar et al. 2003). Changes in endogenous melatonin levels, according to research, impeded auxin and cytokinin-induced root and shoot organogenesis respectively. This demonstrates the role of melatonin as a plant growth regulator (Russel and Manchester 2005). Later, Hernandez-Ruiz et al., incubated etiolated hypocotyls from Lupinus albus L. to further explore the function of melatonin with various melatonin and IAA concentrations (Hernandez and Arnao 2008). Both chemicals were scattered in plant tissues along a concentration gradient, with lower concentrations stimulating growth and higher concentrations inhibiting growth in both intact and de-rooted plant tissues. Melatonin produced the most roots and hypocotyls in this study, with values for root length that were nearly identical to those of the IAA concentrations studied (Hernandez et al. 2005).
Further, the growth-promoting effects of melatonin were demonstrated in various monocots, including oat, wheat, canary grass, and barley. Studies showed that melatonin, as opposed to IAA, stimulated development in coleoptiles by about 10, 20, 31, and 55%, respectively (Dubbels et al. 1995).
4.4 Defense Against Herbivores
Melatonin possesses a bitter and unpleasant palate, and offers defense against herbivores (Kolar and Machackova 2001). Additionally, because melatonin tends to accumulate in animal bodies, ingestion of plants like walnut (3.5–1.0 ng/g) that contain high levels of the hormone can disrupt the physiology of herbivores. According to studies, feeding rats a diet high in melatonin elevated their blood levels from 11.51.9 pg/ml to 38.04.3 pg/ml (Rudiger 2015). This finding might be relevant to how plants defend themselves from herbivores. White fly reproduction in tobacco is reduced by tryptophan decarboxylase overexpression, an enzyme that converts 5-hydroxytryptophan to 5-hydroxytryptamine (Thomas et al. 1995). Melatonin inhibits white fly reproduction, but the exact mechanism by which it does so is still unknown.
4.5 Abiotic Stress Tolerance
The use of melatonin can help to mitigate the detrimental consequences of abiotic stressors. The manufacture of phytomelatonin, which generally occurs within chloroplasts, and the accompanying metabolic processes have been widely researched. Melatonin controls stress responses by reducing ROS and RNS species buildup and modulating stress response pathways. Phytomelatonin role in abiotic stress is mentioned below.
4.5.1 Drought Stress
Drought inhibits plant growth and development. Drought aggravates ROS and RNS species via activation of stress signalling pathways. Drought stress activates transcription factors such as NACs, MYBs, AP2/EREBPs, bZIPs, HDs, and bHLHs (Zhang et al. 2014). Melatonin production is often stimulated by a lack of water (Shi et al. 2015). Endogenous melatonin levels alter when melatonin biosynthesis genes (e.g., TDC, ASMT, COMT, and SNAT) are activated during a water shortage. Drought also stimulates melatonin generation in Graminae species. (Moustafa et al. 2020).
Increased melatonin levels improve the stability of drought-stressed plants (Meng et al. 2014; Ding et al. 2018). Melatonin treatment can promote seed germination and lateral root formation, process (Hosseini et al. 2021; Sun et al. 2021). Melatonin also suppresses ROS-induced oxidative damage and increases antioxidative enzyme levels during drought in plants (Li et al. 2015; Antoniou et al. 2017; Alharby and Fahad 2020; Sadak and Bakry 2020).
4.5.2 Waterlogging Stress
Crop survival, growth, and productivity can all be harmed by waterlogging. This checks gas diffusion, resulting in hypoxic stress in the roots, and promotes ROS accumulation (Zhang et al. 2019; Gu et al. 2020; Wu et al. 2021). Waterlogging causes a rise in endogenous melatonin levels (Moustafa et al. 2020). Exogenous melatonin treatment significantly improves the seedling vitality of many plants (Zheng et al. 2017; Zhang et al. 2019; Gu et al. 2020). It reduces stomatal closure, chlorophyll and photosynthesis reduction, and leaf senescence (Zhang et al. 2019; Gu et al. 2020; Zheng et al. 2017). Melatonin treatment also reduces the oxidative damage caused by waterlogging. To maintain redox homeostasis under waterlogging stress, melatonin activates antioxidant enzymes and minimized H2O2 levels in both leaves and roots of peach seedlings (Gu et al. 2020).
4.5.3 Salt Stress
Melatonin improves salt tolerance in many plants such as maize, wheat, etc. (Liang et al. 2015; Zhou et al. 2016; Chen et al. 2018; Ke et al. 2018; Zhang et al. 2021). Salt stress alters the expression of essential biosynthetic enzyme patterns resulting increase in endogenous melatonin levels (Arnao and Hernández 2009). Furthermore, overexpression of SNAT can improve plant salt tolerance considerably (Wu et al. 2021). SNAT inhibition, on the other hand, lowers endogenous melatonin levels, making rice more sensitive to salt stress (Byeon and Back 2016). Exogenous melatonin protects plants from salt stress by regulating antioxidant enzyme expression (Zhan et al. 2019) resulting in suppression of ROS and H2O2 caused level by salinity. Furthermore, melatonin-NO crosstalk regulates redox equilibrium via differential expression of copper/zinc-SOD and manganese-SOD under salt stress (Arora and Bhatla 2017; Kaya et al. 2020).
4.5.4 Cold Stress
Melatonin protects plants from cold-induced stress. Plant cold tolerance can be improved by increasing endogenous melatonin levels. SNAT transgenic rice showed better stability than wild-variety (Kang et al. 2010). Melatonin improves cold tolerance in grafted watermelons (Tan et al. 2007; Li et al. 2021a).
4.5.5 Heat Stress
Heat stress has physiological, transcriptional, post-transcriptional, and epigenetic effects on plants (Zhao et al. 2020). Melatonin improves thermal tolerance in plants. COMT1 and TDC silencing in tomatoes resulted in a decline in melatonin biosynthesis causing temperature-induced stress (Ahammed et al. 2019).
Tolerance to potassium deficiency is a critical issue in crop production. Melatonin increases potassium levels in Malus and wheat (Li et al. 2016, Li 2021a). TaNAC71-regulated TaHAK1 is a vital factor to cope with MT-mediated potassium deficiency in wheat (Li et al. 2021b). Furthermore, melatonin reduces nano-plastic uptake by roots and translocation to shoots by regulating the expression of aquaporin-related genes (TIP2-9, PIP2, PIP3, PIP1-5, and PIP1.2) (Li et al. 2021c).
5 Conclusion & Future Aspects
Melatonin regulates various physiological functions in plants, including the circadian rhythm, cytoprotection and growth promotion, antioxidant defence, and free radical scavenging (Xiaoyuan et al. 2014). Additionally, it encourages rhizogenesis, cellular growth, and protects from environmental stress conditions (Chandana et al. 2014). Uses of phytomelatonin in agriculture; and humans have gained momentum at present. The first, exogenous melatonin administration to plants promotes improved growth and development as well as greater response to a variety of environmental stressors, including radiation, heat, cold, and drought.
Additionally, melatonin speeds up plant germination, development, and productivity. It slows the senescence of leaves brought on by stress. These cumulative findings suggest that treating farmed plants with exogenous melatonin or overproducing plants with greater melatonin levels may aid crops in more readily resisting various harmful environmental situations that they typically experience throughout their growth (Pandi et al. 2006). The latter parts deal with the potential introduction of melatonin-rich plant foods or dietary supplements because of the enormous health benefits it offers, especially in the fight against neurodegenerative diseases like Alzheimer’s. According to studies, persons who take up to 1 gram of melatonin orally each day experience no negative side effects. Melatonin is additionally quickly absorbed through the digestive system. As a result, the use of melatonin as a nutraceutical appears to have a bright future in promoting a better lifestyle (Charanjit et al. 2008; Jemima et al. 2011).
References
Ackermann K, Bux R, Rub U, Horst-Werner K, Gerold K, Jörg HS (2006) Characterization of human melatonin synthesis using autoptic pineal tissue. Endocrin 7:3235–3242
Ahammed GJ, Xu W, Liu A, Chen S (2019) Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot 161:303–311
Akula R, Parvatam G, Kadimi US, Gokare AR (2012) Melatonin and serotonin profile in beans of Coffea species. J Pineal Res 4:470–476
Alharby HF, Fahad S (2020) Melatonin application enhances biochar efficiency for drought tolerance in maize varieties: modifications in physio-biochemical machinery. Agron J 112:2826–2847
Amit KT, Vinod K (2014) Melatonin: an integral signal for daily and seasonal timing. Indian J Exp Biol 52:425–437
Amod PK, Laurie AK, Girish JK (2005) Herbal complement inhibitors in the treatment of neuroinflammation. Ann N Y Acad Sci 1056:413–429
Antoniou C, Chatzimichail G, Xenofontos R, Pavlou JJ, Panagiotou E, Christou A, Vasileios F (2017) Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J Pineal Res 62:e12401
Arnao MB, Hernández RJ (2009) Chemical stress by different agents affects the melatonin content of barley roots. J Pineal Res 46:295–299
Arora D, Bhatla SC (2017) Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of cu/Zn SOD and Mn SOD. Free Radic Biol Med 106:315–328
Atanu B, Shastry CS, Santanu S (2014) Isolation, purification and structural elucidation of N-Acetyl-5- Methoxytryptamine (Melatonin) from Crataeva nurvala Buch-Ham stem bark. AJPCT 3:301–309
Ayushi J, Maheep B (2007) Melatonin–a “magic biomolecule”. Ann Neurosci 4:1–5
Badria FA (2002) Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J Med Food 3:153–157
Balzer B, Hardeland R (1991) Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra. Science 5021:795–797
Bandurski RS, Cohen JD, Slovin J, Reinecke DM (1995) Auxin biosynthesis and metabolism. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer Academic Publishers, Dordrecht
Bhattacharjee A, Kumar B (2018) Phytomelatonin: a comprehensive literature review and recent advance on medicinal meadow. Int J Hydro 2(3):396–403
Bhavini B, Muhammad UF, Archit B (2009) The therapeutic potential of melatonin in neurological disorders. Rec Patents End Metabol Imm Drug Discov 1:60–64
Bruno C, Jocelyne B, Guy C (2005) The basic physiology and pathophysiology of melatonin. Sleep Med Rev 1:11–24
Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (2001) Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J Agri Food Chem 10:4898–4902
Byeon Y, Back K (2016) Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions. J Pineal Res 60:348–359
Carrillo VA, Patricia JL, Alvarez SN, Rodríguez-Rodríguez A, Juan MG (2013) Melatonin: buffering the immune system. Int J Mol Sci 4:8638–8683
Chandana H, Somenath G, Amaresh KS (2014) Phyto-melatonin: a novel therapeutic aspect of melatonin in nature’s way. Therapeutic value and neuroprotection. CRC Press, Melatonin
Charanjit K, Sivakumar V, Ling EA (2008) Melatonin and its therapeutic potential in neuroprotection. CNS Agents Med Chem CRC Publications 8(4):260–266
Chava VK, Sirisha K (2012) Melatonin: A novel indolamine in oral health and disease. Int J Dent 2012:1–9
Chen Z, Gu Q, Yu X, Huang L, Xu S, Wang R, Wei S, Wenbiao S (2018) Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann Bot 121:1127–1136
Ding F, Wang G, Wang M, Zhang S (2018) Exogenous melatonin improves tolerance to water deficit by promoting cuticle formation in tomato plants. Molecules 23:1605
Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 1:28–31
Dun XT (2015) Melatonin and plants. J Exp Bot 3:625–626
Dun XT, Lucien CM, Pat H, Russel JR (2007) Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav 6:514–516
Ebels MGMB, Tommel DKJ (1972) Separation of pineal extracts on sephadex G-10. Anal Biochem 1:234–244
Fatma PK, Alper K, Arzu UT, Arzu BY (2013) Antibacterial and antitumor activities of melatonin. Spatula DD 2:33–39
Fuhrberg B, Balzer I, Hardeland R, Astrid W, Klaus L (1996) The vertebrate pineal hormone melatonin is produced by the brown alga Pterygophora californica and mimics dark effects on growth rate in the light. Planta 1:125–131
Gu X, Xue L, Lu L, Xiao J, Song G, Xie M, Huiqin Z (2020) Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J Plant Growth Regul 40:2178–2190
Hardeland R (1997) New actions of melatonin and their relevance to biometeorology. Int J Biometeorol 41:47–57
Hardeland R (2005) Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2:119–130
Hardeland R, Cardinali DP, Srinivasan V, Warren Spence D, Gregory MB, Seithikurippu RPP (2011) Melatonin-a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 3:350–384
Hattori A, Migitaka H, Iigo M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Bio Int 3:627–634
Hernandez RJ, Arnao MB (2008) Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J Agric Food Chem 22:10567–10573
Hernandez RJ, Cano A, Arnao MB (2004) Melatonin: A growth-stimulating compound present in lupin tissues. Planta 1:140–144
Hernandez RJ, Cano A, Arnao MB (2005) Melatonin acts as a growth-stimulating compound in some monocot species. J Pin Res 2:137–142
Hosseini MS, Samsampour D, Zahedi SM, Zamanian K, Rahman MM, Mostofa MG, Lam-Son PT (2021) Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol Plant 172:1363–1375
Jan K, Ivana M (2005) Melatonin in higher plants: occurrence and possible functions. J Pineal Res 4:333–341
Jemima J, Bhattacharjee P, Singhal RS (2011) Melatonin – a review on the lesser known potential nutraceutial. IJPSR 8:1975–1987
Jian ZW, Ze FW (2006) Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin 1:41–49
John TY, Christian B, Miriam W, David WP, Meertsc WL, Michael S (2011) Rotationally resolved electronic spectroscopy of biomolecules in the gas phase melatonin. J Mol Spectrosc 268:115–122
Kang K, Lee K, Park S, Kim YS, Back K (2010) Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J Pineal Res 49:176–182
Katarzyna S, Rafał S, Krystyna MJ (2014) Involvement of melatonin applied to Vigna radiata L. seeds in plant response to chilling stress. Cent Eur J Biol 11:1117–1126
Katerova Z, Todorova D, Tasheva K, Sergiev I (2012) Influence of ultraviolet radiation on plant secondary metabolite production. Gen Plant Physiol 3–4:113–144
Katri P, Nora S, Riitta K (2012) Dietary factors and fluctuating levels of melatonin. Food Nutri Res 56:1–9
Kaya C, Higgs D, Ashraf M, Alyemeni MN, Ahmad P (2020) Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant 168:256–277
Kazutaka S, Meaghan S, Borlongan CV (2013) Melatonin-based therapeutics for neuroprotection in stroke. Int J Mol Sci 5:8924–8947
Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Shiwen W (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9:914
Kolar J (2003) Effects of melatonin on circadian rhythms and photoperiodism in higher plants, PhD thesis. Faculty of Natural Sciences, Charles University, Prague
Kolar J, Machackova I (2001) Occurrence and possible function of melatonin in plants a review. Endocytobiosis Cell Res 14:75–84
Kolar J, Machackova I (2005) Melatonin in higher plants: occurrence and possible functions. J Pineal Res 4:333–341
Kolar J, Johnson CH, Machackova I (2003) Exogenously applied melatonin affects flowering of the short-day plant Chenopodium rubrum. Physiol Plant 118:605–612
Krystyna MJ, Małgorzata MP (2013) Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol Plant 12:3285–3292
Lerner AB, James DC, Yoshiyata T, Lee TH, Wataru M (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc 80(10):2587
Li C, Tan DX, Liang D, Chang C, Jia D, Ma F (2015) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot 66:669–680
Li C, Liang B, Chang C, Wei Z, Zhou S, Ma F (2016) Exogenous melatonin improved potassium content in Malus under different stress conditions. J Pineal Res 61:218–229
Li GZ, Liu J, Chen SJ, Wang PF, Liu HT, Dong J, Yong-Xing Z, Ying-Xin X, Chen-Yang W, Tian-Cai G, Guo-Zhang K (2021a) Melatonin promotes potassium deficiency tolerance by regulating HAK1 transporter and its upstream transcription factor NAC71 in wheat. J Pineal Res 70:e12727
Li S, Guo J, Wang T, Gong L, Liu F, Brestic M, Shengqun L, Fengbin S, Xiangnan L (2021b) Melatonin reduces nanoplastic uptake, translocation, and toxicity in wheat. J Pineal Res 71:e12761
Li H, Guo Y, Lan Z, Xu K, Chang J, Ahammed GJ, Jianxiang M, Chunhua W, Xian Z (2021c) Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic Res 8:57
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Hongkai W, Yangwen Q, Xin-Guang Z, Dun-Xian T, Shou-Yi C, Chengcai C (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res 59:91–101
Manchester LC, Tan DX, Reiter RJ, Won P, Kanishka M, Wenbo Q (2000) High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci 25:3023–3029
Marcello I, Mara R, Franco F (2006) Melatonin content in grape: myth or panacea? J Sci Food Agric 10:1432–1438
Marcello I, Sara V, Mara R (2012) Occurrence and analysis of melatonin in food plants. Hand book of analysis of active compounds in functional foods. CRC Press, pp 651–662
Marino BA (2014) Phytomelatonin: discovery, content, and role in plants. Adv Bot 2014:1–11
Marino BA, Hernandez RJ (2007) Melatonin in plants: more studies are necessary. Plant Sig Behav 5:381–382
Marino BA, Josefa HZ (2006) The physiological function of melatonin in plants. Plant Signal Behav 3:89–95
Meng J, Xu T, Wang Z, Fang Y, Xi Z, Zhang Z (2014) The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pineal Res 57:200–212
Moustafa FM, Mahmoud A, Arnao MB, Sheteiwy MS, Dafea M, Soltan M, Amr E, Mirza H, Shaoying A (2020) Melatonin-induced water stress tolerance in plants: recent advances. Antioxidants 9:809
Nitulescu AAL, Niculina M, Aurelia C, Cristina MD (2009) Experimental research on mice regarding the implication of melatonin in pain management. Farmacia 2:223–228
Pandi PSR, Srinivasan V, Maestroni GJM, Cardinali D, Poeggeler B, Hardeland R (2006) Melatonin: nature’s most versatile biological signal? FEBS J 13:2813–2838
Parvin S, Abdul K, Abdul M, Md Ekramul H, Ashik M, IIW M (2011) Triterpenoids and phytosteroids from stem bark of Crataeva nurvala Buch Ham. J Appl Pharm Sci 9:47–50
Pasquale A, Giuseppe A, David B, Leopoldo C, Felice F, Maria CN, Maria AL, Luisa T (2003) Melatonin: structural characterization of its non-enzymatic mono-oxygenate metabolite. J Pineal Res 35:269–275
Reiter RJ, Coto MA, Boga JA, Russel JR, Coto-Montes A, Jose AB, Fuentes-Broto L, Rosales-Corral SA, Du-Xian T (2011) Melatonin: new applications in clinical and veterinary medicine, plant physiology and industry. Neuro Endocrinol Lett 5:575–587
Rudiger H (2015) Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J Exp Bot 3:627–646
Rudiger H, Burkhard P (2003) Non-vertebrate melatonin. J Pineal Res 4:233–241
Russel JR (2001) Melatonin in plants. Nutri Rev 9:286–290
Russel JR, Manchester LC (2005) Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 9:921–924
Russel JR, Dun XT, Lucien CM, Artemis PS, Maria DM, Luis JF, Terron PM (2007) Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed function. World Rev Nutri Diab 97:211–230
Russel JR, Reyes GM, Fuentes-Broto L, Dun-Xian T (2010) Melatonin reduces oxidative catastrophe in neurons and glia. Act Nerv Super Rediviva 2:93–103
Russel JR, Dun XT, Annia G (2014) Melatonin reduces lipid peroxidation and membrane viscosity. Front Physiol 5:1–4
Sadak MS, Bakry BA (2020) Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol Mol Biol Plants 26:907–919
Sahna E, Parlakpinar H, Turkoz Y, Acet A (2005) Protective effects of melatonin on myocardial ischemia- reperfusion induced infarct size and oxidative changes. Physiol Res 5:491–495
Sergio DP, Carmen B, Russel JR, Rodríguez B (2009) Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: streptopelia Risoria as a model. Int J Tryptophan Res 2:23–36
Shi H, Jiang C, Ye T, Tan DX, Reiter R, Zhang H, Renyi L, Zhulong C (2015) Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in Bermuda grass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J Exp Bot 66:681–694
Shiddamallayya N, Azara Y, Gopakumar K (2010) Hundred common forest medicinal plants of Karnataka in primary healthcare. Indian J Trad Knowl 1:90–95
Sun C, Liu L, Wang L, Li B, Jin C, Lin X (2021) Melatonin: a master regulator of plant development and stress responses. J Integr Plant Biol 63:126–145
Tan DX, Manchester LC, Di MP, Martinez GR, Prado FM, Reiter RJ (2007) Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. FASEB J 21:1724–1729
Tettamanti C, Cerabolini B, Gerola P (2000) Melatonin identification in medicinal plants. Acta Phytother 3:137–144
Thomas JC, Adams DG, Nessler CL, Brown JK, Bohnert HJ (1995) Tryptophan decarboxylase, tryptamine, and reproduction of the whitefly. Plant Physiol 2:717–720
Van Tassel D, Roberts N, Neill S (1995) Melatonin from higher plants: isolation and identification of N-acetyl-5- methoxytryptamine. Plant Physiol 2:101–112
Venkatramanujam S (2011) Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection. Ther Adv Neurol Disord 5:297–317
Wolf K, Kolar J, Witters WD, Harryvan O, Ivana M (2001) Daily profile of melatonin levels in Chenopodium rubrum depends on photoperiod. J Plant Physiol 11:1491–1493
Wu Q, Su N, Huang X, Cui J, Shabala L, Zhou M, Min Y, Sergey S (2021) Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun 2:100188
Xiaoyuan F, Meng W, Yanyun Z, Ping H, Yinn D (2014) Melatonin from different fruit sources, functional roles, and analytical methods. Trends Food Sci Tech 1:21–31
Yeo JK, Young NL, Young JO, In-yong H, Woong JP (2007) What is the role for melatonin in plants? Review on the current status of phytomelatonin research. JNTB 1:9–14
Zhan H, Xiaojun N, Ting Z, Shuang L, Xiaoyu W, Xianghong D, Wei T, Weining S (2019) Melatonin: a small molecule but important for salt stress tolerance in plants. Int J Mol Sci 20(3):709
Zhang JY, Cruz CMH, Torres JI, Kang Y, Allen SN, Huhman DV, Yuhong T, Jeremy M, Lloyd WS, Michael KU (2014) Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ 37:2553–2576
Zhang Q, Liu X, Zhang Z, Liu N, Li D, Hu L (2019) Melatonin improved waterlogging tolerance in alfalfa (Medicago sativa) by reprogramming polyamine and ethylene metabolism. Front Plant Sci 10:44
Zhang Y, Fan Y, Rui C, Zhang H, Xu N, Dai M, Xiugui C, Xuke L, Delong W, Junjuan W, Jing W, Qinqin W, Shuai W, Chao C, Lixue G, Lanjie Z, Wuwei Y (2021) Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front Plant Sci 12:693690
Zhao J, Lu Z, Wang L, Jin B (2020) Plant responses to heat stress: physiology, transcription, noncoding RNAs, and epigenetics. Int J Mol Sci 22:117
Zheng X, Zhou J, Tan DX, Wang N, Wang L, Shan D, Jin K (2017) Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. Seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front Plant Sci 8:483
Zhou X, Zhao H, Cao K, Hu L, Du T, Baluska F, Zhirong Z (2016) Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front Plant Sci 7:1823
Acknowledgements
We sincerely acknowledge The Assam Royal Global University, Guwahati, Pratiksha Institute of Pharmaceutical Sciences, Guwahati and Assam Downtown University, Guwahati for providing necessary infrastructure to prepare the manuscript.
Conflict of interest
The author declares there is no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bhattacharjee, A., Debnath, S., Sikdar, P., Bhattacharya, K., Chanu, N.R. (2023). Melatonin in Plants: Biosynthesis, Occurrence and Role in plants. In: Mukherjee, S., Corpas, F.J. (eds) Melatonin: Role in Plant Signaling, Growth and Stress Tolerance. Plant in Challenging Environments, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-031-40173-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-40173-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40172-5
Online ISBN: 978-3-031-40173-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)