Abstract
The most commonly known dementia, i.e., Alzheimer’s disease (AD) occurs in older age people and it is an irreversible neurodegenerative disorder of the brain that is primarily regarded as a decline in thinking and independence in daily activities. It is well documented that the disease development starts the years before the warning signs are evidenced and at that point most treatments become ineffective. Over a long time, vascular risk factors have been connected with cognitive decline and higher risk of AD through increased tau and cerebral amyloid β (Aβ) aggregates. Unlike programmed cell fate to death, ferroptosis is oxidative stress or aggregation of lipid peroxide and iron-reliant cell death associated with AD. The exact mechanism of action of ferroptosis in AD, related pathways, and differentially expressed genes need to be widely investigated. Ferroptosis hallmarks such as high levels of iron level, oxidative stress, and accumulation of lipid peroxides were investigated in the AD brain. Further, it was noticed that the development of neurofibrillary tangles and Aβ plaques are associated with iron burden in AD mice models. Here, we summarize the recent development of a ferroptosis-related molecular mechanisms, and chemical modulators of ferroptosis in order to control the progression of AD progression. The details of the existing potential targets for therapeutics in order to avert the ferroptosis in AD have been discussed. Ferroptosis-related studies for AD treatment in the future might be based on creating novel techniques and therapeutic agents to modulate ferroptosis in AD brain and construct animal models of AD in order to identify ferroptosis modulators to stop or reverse the neurodegeneration in AD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Alzheimer’s disease (AD) is a multifactorial progressive neurodegenerative disorder that normally occurs in elderly people. AD is one of the major causes of dementia globally where irreversible neuronal loss leads to loss of memory and reduced ability to carry out their daily routine independently. The first case of AD was reported by Alois Alzheimer, a German physician in 1907 who described particular changes in the cortical cell clusters after brain biopsy and accredited the behavioral changes in the patient due to these lesions. Alzheimer portrayed nerve fibrillary tangles and plaques which were identified as amyloid β (Aβ) plaques and NFTs (neurofibrillary tangles) in 1980. The diagnostic basis for AD was standardized in 1984 in the USA. These criteria were revised in 2011 and 2018 to distinguish the role of biomarkers in diagnosing AD and to create distinct diagnoses for the preclinical stages, cognitive impairment, and different phases of dementia in AD. This disease is usually diagnosed at a late stage when significant neurodegeneration has occurred. Diagnosis is based on clinical tests as no robust biomarkers have been identified so far.
1.1 Statistics of AD
The occurrence of dementia globally is approximately over 45 million where AD is the main reason of dementia and is accountable for approximately more than 70% of cases (Crous-Bou et al. 2017). It is estimated that globally approximately 35.6 million people are affected by AD. It is projected to rise up to 65.7 million by 2030 and by 2050 it will be around 115.4 million (Mcgirr et al. 2020). Whereas in India deaths due to AD were highest in three decades in 2019. AD-related deaths increased almost five times in India between 1990 and 2019. According to India Report 2010, 3.7 million people were living with AD in India which is now increased to 4 million. The actual figure of patients affected by dementia in India is expected to double by 2050 (Perianayagam et al. 2022).
1.2 Current Treatment for AD
Currently, available therapy for AD includes NMDA and cholinesterase inhibitors. Cholinesterase inhibitors and NMDA receptor antagonists are the two classes of medications used to treat AD. The acetylcholine neurotransmitter plays a crucial role in learning and memory, and cholinesterase enzyme hydrolyzes acetylcholine and converts it into acetic acid and choline whereas cholinesterase inhibitors like donepezil, rivastigmine, and galantamine prevent the hydrolysis of acetylcholine (Cai et al. 2018). By increasing the amount of acetylcholine in the brain, these medications can improve cognitive function and manage the symptoms of AD, including memory loss, confusion, and difficulties with language and thinking (Colović et al. 2013). Cholinesterase inhibitors are primarily used to treat mild to moderate AD, and they are generally well-tolerated. NMDA receptor antagonists, such as memantine, work by regulating the neurotransmitter glutamate, which is involved in learning and memory. In AD, excessive glutamate release can lead to neuronal damage and death. By blocking the overactivity of glutamate receptors, NMDA receptor antagonists can protect neurons from this damage and improve cognitive function (Liu et al. 2019). Memantine is primarily used to treat moderate to severe AD, although its side effects can include dizziness, confusion, and headaches (Khalaf et al. 2019). While cholinesterase inhibitors and NMDA receptor antagonists can help manage the symptoms of AD, they do not cure the disease, and their effectiveness varies from person to person. Moreover, they do not address the underlying causes of AD, which are complex and not fully understood. Ongoing research is focused on identifying new targets for drug development and developing more effective treatments that can slow or halt the progression of the disease.
The most recent drug approved for the treatment of Alzheimer’s disease (AD) is Aducanumab, which was approved by the “US Food and Drug Administration (FDA)” in June 2021 (Vaz et al. 2022). Aducanumab is a monoclonal antibody that targets amyloid beta, a protein that accumulates in AD patients’ brains and is believed to contribute in the development of the disease (Beshir and Aadithsoorya 2022). The approval of aducanumab was based on data from clinical trials that showed a reduction in the amount of amyloid beta in the brains of people with early-stage AD who received the drug (Tampi et al. 2021). However, the efficacy of aducanumab in slowing down cognitive decline and improving the overall function of people with AD remains controversial, with some experts questioning the clinical relevance of the trial data (Tampi et al. 2021).
Despite the approval of aducanumab, there is still a need for more effective treatments for AD, as the disease remains a significant public health challenge. Ongoing research is focused on identifying new targets for drug development, including tau protein and inflammation, as well as developing more personalized and targeted treatments that can address the complex nature of the disease. Moreover, other treatment options consist of escalating cognitive reserve and offering a nutritional approach to slow down and prevent the disease progression. AD progression starts years prior to the symptoms get visible and at this stage the available therapies become ineffective. The major proteins occupied through JAK2/STAT3 pathway in the hippocampus, like p-STAT3-Tyr705 and p-JAK2-Tyr1007 were overexpressed in several AD experimental models. It is well documented that glial cells like astrocytes, play a major part in the succession of AD. Devoid of showing a substantial result on amyloid pathologies and tau protein, the above pathway has shown a considerable impact on animal models of AD via tau and Aβ pathologies. Moreover, in AD, cholinergic atrophy that is necessary to the maintenance or survival of BFCN (basal forebrain cholinergic neurons) was outlined as a trophic failure during the NGF (nerve growth factor) metabolic pathway. In the case of AD, a modification in the alteration of the proNGF—NGF as well as augment in degradation of mature NGF occurs. Therefore, in the AD experimental studies, the function of exogenous mature NGF was exposed to recover the atrophic BFCN. In addition to this, it is also reported that the microRNA-107 mediated signaling pathway like FGF7/FGFR2/PI3K/Akt signaling pathway is also involved in the pathophysiology of AD. Several studies have suggested that dysregulation of miRNA expression could be associated with various human pathological conditions, including neurological disorders. Studies on miRNA therapies in neurodegenerative diseases are still in infant stage. Moreover, it is coming into the knowledge that, vascular dysfunction is connected to the reasonable turn down and higher threat of AD. Vascular risk factors were linked with elevated Aβ and tau protein burden, whereas collegially performing with Aβ to persuade reasonable turndown. For example, one of the main vascular risk factors like, apolipoprotein E4 polymorphism is genetically associated risk factor of AD. Various mechanisms of cell death were studied in AD pathology.
In recent decades, several reports suggest that besides apoptosis and necrosis, ferroptosis appears to be associated with AD (Dixon 2017; Hambright et al. 2017). Ferroptosis, which is increasing with aging, is genetically different from another form of cell death even though its biochemical and morphological properties are different (Dixon et al. 2012; Zhou et al. 2020).
1.3 Ferroptosis
It is a type of nonapoptotic cell death reliant on intracellular iron. The process of ferroptosis is one of the newest types of synchronized cell death associated with the overloading of iron species and lipid-based ROS (reactive oxygen species) accumulation, reduced glutathione levels, and inhibition of GPX4, resulting in disturbance in iron homeostasis and cell death (Stockwell et al. 2017; Dixon and Stockwell 2019). In 2012, the term ferroptosis was introduced by Dixon and his group, in order to describe the erastin-driven cell death, which mainly act by inhibiting the system Xc. The process ferroptosis can be incited by inhibiting system Xc, glutathione depletion, targeting excess iron, and directly inhibiting GPX4 enzyme. At the same time, ferroptosis inhibition is achieved by blocking excessive lipid peroxidation by targeting different pathways. These mechanisms are often related to the pathophysiology and development of diseases like cancer and AD. It is fundamentally different from other cell deaths like necrosis and apoptosis in biochemistry, functions, and morphology. The mechanism by which ferroptosis acts as a regulatory factor in many diseases remains elusive. The activation and inhibition of ferroptosis to alleviate different disease progression is a topic of great interest and has become a hotspot for etiological research and treatment. Here, we focus on systemically summarizing the various mechanisms implicated in the inhibition and initiation of ferroptosis. We have also discussed in depth various ferroptosis inducers and inhibitors.
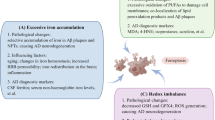
The overload of iron molecules may persuade ferroptosis in the AD mice model, and it could be exclusively prohibited by means of an iron chelator (Dixon et al. 2012; Wang et al. 2017). Rising evidences have recommended that ferroptosis might be revealed in cell death connected to neurons in several neurological diseases, like PD, Huntington’s and stroke escorted by mitochondrial dysfunction, lipid peroxidation, and GPX4 reduction (Alim et al. 2019; Wu et al. 2018; Tuo et al. 2017; Skouta et al. 2014). Moreover, ferroptotic inhibitors have been revealed as neuron protectors which also recover empirical function in stroke disease animal models (Alim et al. 2019; Tuo et al. 2017). GPX4 knockout mice directly showed significant neuronal loss and age-dependent neurodegenerative changes (Hambright et al. 2017). Taking into consideration that the iron burden in AD significantly generates elevated ROS level in the brain somehow strengthen the point that ferroptosis is implicated in the cognitive impairment and loss of neurons. However, the evidences are not enough to make a direct connection to it (Praticò et al. 2001; Praticò and Sung 2004; Castellani et al. 2007; Derry et al. 2020). Ferroptosis hallmarks, such as augmented oxidative stress and iron levels have been extensively noted in the AD brain. It is reported that formation of NFTs and Aβ plaques is connected with iron overload in AD mice model (Yamamoto et al. 2002; Peters et al. 2018). Moreover, iron level completely associated with cognitive decline in glutathione peroxidase (GPX4) and human subjects is protective in mice model of AD (Yoo et al. 2010; Ayton et al. 2017). After various efforts made by researcher globally on tau protein hyperphosphorylation and Aβ deposition, still an efficient and effective therapeutic option to decline the neurodegeneration in case of AD is missing. However, besides these rising evidences advocate that ferroptosis contributes to neurodegeneration. Therefore the involvement of ferroptosis and the mechanism connected with a classical pathway of ferroptosis during progression of AD became one of the major hurdles to deal with novel pathophysiological studies and therapeutics.
2 Mechanism Concerned to the Fundamental Ferroptotic Pathway
On the basis of available literature till date mechanism of ferroptosis can be separated into three fractions: (1) iron homeostasis, (2) GSH (glutathione) metabolism, and (3) lipid peroxidation or oxidative stress. Disturbance in any of these mechanisms either alone or in combination can induce ferroptosis.
2.1 Iron Homeostasis
It was reported that transferrin 1 receptor facilitates the entry of iron inside the cells where it get reduced from Fe3+ to Fe2+ in the endosome through STEAP3 (six-transmembrane epithelial antigen of the prostate 3) metalloreductase and plays a crucial role in ferroptosis (Zhang et al. 2012; Yan and Zhang 2020). In 2019, Gao et al. reported that Fe2+ accumulated in ferritin and ferroportin mediates its exportation from the cell. Further ferritin deprivation through NCOA4 (nuclear receptor coactivator 4) strengthen the ferroptotic process by accumulating the intracellular iron. In addition to this Fe2+ accumulation further leads to production of oxidative stress and ROS (Hou et al. 2016; Ward et al. 2014). Production of ROS such as O2− (superoxide anion) and hydroxyl free radical (.OH) due to excessive free intracellular iron accumulation results into oxidative damage to membrane or lipid enclosed molecules and lipid peroxidation which further lead to harm at cellular level and eventually to ferroptotic cell death (Aprioku 2013).
2.2 Glutathione Metabolism
Blocking of the glutamate/cystine antiporter can cause a decrease in intracellular GSH levels that ultimately results in the deactivation of GPX4, a critical enzyme involved in antioxidant defense and the regulation of ferroptosis (Seibt et al. 2019). These processes ultimately lead to ferroptosis cell death via ROS accumulation and enhanced lipid peroxidation (Wang et al. 2020). Further, it was reported that GPX4 reduces PL-PUFA(PE)-OOH to PL-PUFA(PE)-OH, where GPX4, also referred as GS-SG (glutathione disulfide), changes GSH into its oxidized form (Seibt et al. 2019; Cozza et al. 2017). Moreover, under oxidative stress despite Nrf2, xCT mRNA positively coordinated by ATF4 activation (transcription factor 4), whereas negative regulation of xCT mRNA via P53 leads to cysteine deprivation and susceptibility toward ferroptosis (Cai et al. 2018; Sato et al. 2004; Jiang et al. 2015; Habib et al. 2015).
2.3 Lipid Peroxidation and Oxidative Stress
It is well known that ROS-mediated lipid peroxidation that leads to oxidative stress is the main key to ferroptosis (Kuang et al. 2020). A decrease in GSH levels and GPX4 inhibition leads to 12/15 LOX (12/15-lipoxygenase) activation. Further the association of Fe2+ with 12/15-LOX results in the oxygenation of PUFA (polyunsaturated fatty-acids) and triggers lipid peroxidation- mediated ferroptosis (Kagan et al. 2017). Among the six isoforms of LOX, 12/15-LOX are most the abundant and considered as key ferroptotic cell death regulators (Kagan et al. 2017; Yang et al. 2016). Besides accumulated free intracellular iron-induced ROS generation, mitochondria also facilitate ROS production. Electron leakage from mitochondrial complexes I and III during the electron transport process gives rise to the production of ROS like superoxide (O2−) and hydrogen peroxide (H2O2). Consequently, this can lead to a reduction in mitochondrial membrane potential (Gao et al. 2019). Recently in 2020, Kuang et al. reported that reduction in mitochondrial membrane potential associated with ferroptosis and engaged regulatory mechanism different from apoptosis. Activation of 12/15-LOX due to GSH depletion can enhance cytosolic calcium ion (Ca2+) via importation from the mitochondrial extracellular compartment and by releasing from endoplasmic reticulum and mitochondria (Maher et al. 2018). GSH levels depletion may also induce the dysregulation of in and out Ca2+ transport from mitochondria via VDAC (voltage dependent anion channels) and MCU (mitochondrial Ca2+ uniporter) (Zorov et al. 2014; DeHart et al. 2018). Due to overload of Ca2+ and loss of mitochondrial function induce the Ca2+ dependent proteases (DeHart et al. 2018). Subsequently, ROS-mediated BID transactivation and induction of AIF (apoptosis-inducing factor) translocation to nucleus from mitochondria lead to cell death (Neitemeier et al. 2017). Reduction of MAMs (mitochondria-associated endoplasmic reticulum membranes) communication and activation of calcium-activated potassium channel have the potential to defence against ferroptosis dependent cell death (Krabbendam et al. 2020). The various pathways associated with ferroptosis are shown in Fig. 14.1.
Molecular mechanisms involved in cell death due to ferroptosis. Ferroptosis pathway can be separated into three modes (1) iron metabolism, (2) glutathione metabolism, and (3) polyunsaturated fatty acid metabolism. Well-recognized ferroptosis inhibitors and their method of action are indicated in green
3 Role of Ferroptosis During AD Progression
In 2012, ferroptosis was first illustrated and stated that it differs from normal apoptosis corresponding to biochemical reaction and structural morphology, further ferroptosis is characterized by the accumulation of lipid peroxidation induced by free intracellular iron (Dixon et al. 2012; Stockwell et al. 2017). Erastin is considered as one of the major ferroptosis inducer that inhibits import of cystine from cystine-glutamate antiporter system Xc− and further leads to glutathione reduction (Dixon et al. 2012). A well-known selenoenzyme GPX4 uses glutathione as substrate in order to convert a potent toxic lipid hydroperoxides to non-toxic lipid alcohols (Yang et al. 2014; Ursini et al. 1982; Friedmann Angeli et al. 2014). Several reports suggest that tiny mitochondria with diminishing mitochondrial crista, solid mitochondrial membrane, transparent nuclei, and ruptured outer membrane of mitochondria are the morphology-related characteristic quality of ferroptosis (Mou et al. 2019; Miyake et al. 2020). Recently, it was reported that ferritinophagy facilitates ferroptosis regulation (Tang et al. 2018). It is reported that when ferroptosis occurs due to cysteine depletion, ferritinophagy comes into play and releases the free intracellular iron with the help of nuclear receptor coactivator 4 (Tang et al. 2018). A well-known modulator of ferritinophagy, i.e., HER2C regulates the FBXL5 turnover, which emerges to play a key function in the metabolism of iron and is basically a part of complex that aims proteasomal degradation via iron regulatory protein 2 (Tang et al. 2018; Moroishi et al. 2014). Further it is reported that HER2C deficiency may trigger neurodegeneration via the release of free iron, a damaging response to enhanced iron levels that may lead to ferritinophagy and damage of neurons (Tang et al. 2018). Nrf2 (Erythroid 2-related factor 2) is a well-known transcriptional regulator which control genes related to the mitochondrial function and involved in the lipid, glutathione, and iron metabolism (Abdalkader et al. 2018). Further Nrf2 can assist to defend against ferroptosis, and hence increasing Nrf2 signal may offer neuroprotective effects (Abdalkader et al. 2018). In recent decades, researchers demonstrate that ferroptosis may affect AD prognosis or pathogenesis, and elevated lipid peroxides level in brain is one of the characteristic quality of AD (Adibhatla and Hatcher 2010). GPX4 deletion in mice model of AD displays degeneration of hippocampus and cognitive impairment similar to ferroptosis-mediated neurodegeneration which includes neuroinflammation, lipid hydroperoxides, neuroinflammation, and ERK establishment which is improved by ferroptotic inhibitors (Hambright et al. 2017). Ferroptosis may intensify AD via heme oxygenase-1 pathways and targeting the Hif-1α (Tang et al. 2018). Reports suggest that it is altered by the interruption in lipid restoration involving GPX4 and glutathione (Stockwell et al. 2017). Cellular death via ferroptosis can be obscured by lipid peroxidation inhibitors, iron chelators, reduction of PUFAs, and lipophilic antioxidants (Stockwell et al. 2017). Therapeutic studies in AD models with potent anti-ferroptosis activity and experimentation with vitamin E supplements to treat AD have produced contradictory results or prevent cognitive decline (Lloret et al. 2019). Hybrids of donepezil-butylated hydroxytoluene used as potential anti-AD therapies (Cai et al. 2018). Moreover, cognitive improvement in AD has been achieved by idebenone, but irrefutable evidence for a clinically significant benefit is lacking (Thal et al. 2003; Senin et al. 1992; Weyer et al. 1997). Further vildagliptin showed significant biochemical changes, declined tau phosphorylation, memory improvement, amyloid-β attenuation, and enhanced expression of synaptic plasticity proteins in the hippocampus of AD rat models (Khalaf et al. 2019; Ma et al. 2018; Kosaraju et al. 2013). Linagliptin alleviated tau phosphorylation, amyloid-β attenuation, cognitive deficits, and neuroinflammation in rodent model of 3xTg-AD (Kosaraju et al. 2017). It is shown that linagliptin improved amyloid-β-activated intracellular ROS and dysfunction of mitochondria in cultured human neuronal cells. It was shown that baicalein prevents tau phosphorylation, declined amyloid-β and improved cognition in AD mouse models (Zhang et al. 2013; Gu et al. 2016). Certain reports demonstrated that in the case of AD, PD-146176 decreases tau pathology and amyloid-β, diminished autophagy and preserved memory in a 3xTg mouse model (Di Meco et al. 2017; Chu et al. 2015). It is reported that zileuton compound improves memory and lessens tau and Aβ pathophysiology in 3xTg AD mice model (Di Meco et al. 2014; Chu et al. 2013). A controlled and randomized use of sodium selenate reduce the raised concentration of selenium in CSF sample. This decrease in CSF selenium concentration correlated with changes in cognitive function as assessed by the Mini-Mental Status Examination (MMSE) (Cardoso et al. 2019).
4 Ferroptosis Inhibitors
4.1 Radical Trapping Antioxidants (RTAs)
These agents act by inhibiting the autoxidation of lipids, which is a crucial step in lipid peroxidation. The RTAs are also known as chain-breaking antioxidants that act by reacting with the chain that carries peroxyl radical, due to which the process of autoxidation of lipids does not occur (Ingold 1961; Ingold and Pratt 2014). The petroleum industry has widely used the RTAs for many years. It prevents the autoxidation of oil, fuels, rubber, and plastics; due to this property, they are widely used as excipients in petroleum (Burton and Ingold 1986). The role of RTA in suppressing lipid peroxidation was revealed after the characterization of α-tocopherol (Fig. 14.7), the highest biologically active form of vitamin E. It is one of the most potent RTA reported till date. The research on tocopherol has concluded that antioxidants have essential roles in treating cardiovascular and Alzheimer’s disease (Bowry and Stocker 1993). One of the primary disadvantages of antioxidants is that they frequently fail in in vivo tests. The activity that occurs in vitro does not cross over to the in vivo research. There are many misconceptions about antioxidants’ beneficial effects in disease prevention, and as a result, very little research has been done in this field (Angeli et al. 2017). Vitamin E deficiency leads to premature neurodegeneration onset, which is mainly related to ferroptosis-linked disease (Ulatowski and Manor 2015). So it is suggested that antioxidants carry beneficial therapeutic effects. Still, presently we have very little understanding regarding antioxidants, due to which fewer compounds have come into existence (Harding et al. 1982; Doba et al. 1985).
4.2 Vitamin E Analogs
After the identification of α-tocopherol, it has come to notice that vitamin analogs are one of the promising candidates that act as antioxidants. It is one of the naturally occurring lipid-soluble RTA which acts on the chain carrying peroxyl radical (Angeli et al. 2017). Much work has been done to enhance the reactivity of these analogs with peroxyl radicals. Many researchers have introduced different groups in naturally occurring vitamin E analogs. After synthesizing a large number of compounds and studying their SAR, the compound tetrahydronaphthyridinols (THNs) is identified as (Yang 2016) the promising lead with enhanced activity toward peroxyl radical. The SAR studies have suggested that the introduction of nitrogen group on the 3 and 5 positions in the ring relative to a hydroxyl group of classical phenolic RTA promisingly stabilizes electron-rich phenol against autoxidation. The substitution of N,N-dialkylamino (strongly electron-donating group) increased the rate of reaction toward the free radicals, mainly peroxyl radicals, by weakening the O–H bond and not compromising their resistance toward autoxidation (Wijtmans et al. 2003; Pratt et al. 2001). Considering these facts, the compound tetrahydronaphthyridinols THNs (Aza-Phenols) comes into existence, which possesses the best combination of stability and reactivity. The THNs showed 30-folds higher activity toward peroxyl radicals in organic solution and liposomes compared with α-tocopherol (Nam et al. 2007). After recognizing that oxidative lipid degradation is necessary for the execution of ferroptosis, the excellent activity of RTA toward the inhibition of lipid peroxidation makes them one of the most privileged candidates to inhibit ferroptosis, which is one of the key processes in many diseases like cardiac diseases, ischemia, and neurodegenerative disorders. Zilka et al. have shown that when THNs with different chain lengths were treated with alkyl chain ranging from 22 to 15, they exhibited excellent inhibitory properties toward ferroptosis and even showed more significant activity than ferrostatin and liproxstatin-1, well-known ferroptosis inhibitors (Zilka et al. 2017). THNs have also demonstrated higher tissue distribution because they efficiently bind with α-tocopherol transfer protein (αTPP) and protect themselves from liver metabolism (Li et al. 2013). These properties make THNs the essential candidates in the form of ferroptosis inhibitors.
4.3 Lipoxygenase Inhibitor
Different studies have suggested that lipoxygenase is one of the key enzymes that plays a crucial role in the execution of ferroptosis by accelerating the process of lipid peroxidation (Fan and Fan 2018; Feng and Stockwell 2018). Lipoxygenase enzyme can activate the nuclear factor-кB (NF-кB) pathway, which expresses the transcription of genes like inducible nitric oxide synthase (iNOS), which is recognized as one of the critical factors in inflammatory responses (Mendes et al. 2017; Guo et al. 2019; Vallance and Leiper 2002). The iNOS accelerates the generation of NO radicals, which is an important parameter in many physiological processes. Excessive production of NO leads to the generation of reactive nitrogen species (RNOS), and excessive accumulation of RNOS shows a toxic effect and causes cell death and tissue damage (Blaise et al. 2005). The main drawback of lipoxygenase as a target is that it lacks specificity, as lipoxygenase exists in six isoforms in humans. So many researchers have studied the different isoforms to design isoform-specific inhibitors, which were of great medicinal effect (Gilbert et al. 2011). The other main obstacle regarding lipoxygenase was the lack of 3D structure information of human lipoxygenase. There has been no conclusive evidence that a particular isoform is entirely responsible for ferroptosis. One genetic study has suggested that the deficiency of Alox 15, the isoform of lipoxygenase, is capable of oxidizing esterified PUFAs and further suggested that it is responsible for cell death (Brütsch et al. 2015). Alox15 is an iron-containing enzyme without heme, which generates lipid peroxides from polyunsaturated acids like arachidonic acid and linoleic acid (LA) (Guo et al. 2019). Alox15 acts by oxidizing either AA or LA and converts them into their respective peroxides, which are 15-hydroxyeicosatetraenoic acid and 13-hydroperoxy-octadecadienoic acid (13-HpODE), respectively. Alox15 also generates lipoxins, which act as important inflammatory mediators (Solomon et al. 1997; Ivanov et al. 2010). Excessive generation of these lipid peroxides has toxic effects on the cell and causes death by ferroptotic cells. Although the cellular antioxidant system balances lipid peroxides, an imbalance of this process leads to ferroptotic cell death (Yang and Stockwell 2016). Several types of research have shown that the development of new lipoxygenase inhibitors can prevent ferroptosis. Based on their mechanism, the lipoxygenase inhibitors are divided into five groups: (1) redox inhibitors maintain the concentration of active site iron atom into its inactive form, which was reported as reduced form; (2) iron chelators remove the iron from the active sites due to which the enzyme losts its activity; (3) competitive inhibitors, (4) suicide substrates have a property to irreversibly bind with enzyme and inactivate the enzyme; and (5) allosteric inhibitors have a property to bind with a specific site which is responsible for the enzyme activity and inactivate that specific site (Haeggström and Funk 2011). Alox15 has been involved in a wide variety of illnesses, including asthma, diabetes, stroke, atherogenesis, cancer, Alzheimer’s disease, and Parkinson disease. This exerts the focus of many researchers to design Alox15 inhibitors for drug discovery. Rather than targeting a single lipoxygenase, several studies show that focusing on multiple lipoxygenase inhibition yields superior outcomes with greater ferroptosis suppression (Kagan et al. 2017; Friedmann Angeli et al. 2014). It remains elusive whether to develop specific lipoxygenase or pan-lipoxygenase inhibitors as novel drug which efficiently inhibits the execution of the ferroptosis process. Some compounds are reported as lipoxygenase inhibitors, as shown in Fig. 14.2.
4.4 Ferrostatin
Ferrostatin is an alkylamine derivative that came into existence by high-throughput screening of a large library of compounds targeting ferroptosis (Skouta et al. 2014; Hofmans et al. 2016). Ferrostatin mainly acts by inhibiting lipid peroxidation, but it does not inhibit the accumulation of mitochondrial ROS and does not inhibit lysosomal membrane permeability (Skouta et al. 2014). It has previously been reported that diarylamines and hindered dialkylamines effectively reduce agents and antioxidants. They are already employed as a preservative in the food industry to slow the oxidation process and prevent food spoilage. These antioxidants are widely used in the material sector to prevent oxidative deterioration of polymers, plastics, and other materials and in the chemical industry to prevent the autoxidation of rubber and plastics (DeHart et al. 2018). The diarylamines are exciting molecules and are under investigation as possible antioxidants that work as excellent free radical scavengers and have the therapeutic potential. The exploration of diarylamines has led to the discovery of ferrostatin-1, the first ferroptosis inhibitor. Ferrostatin-1 suppresses ferroptosis caused by erastin, although the specific site where ferrostatin-1 reacts is unclear. It is hypothesized that it works by preserving the levels of PUFA and their derivatives rather than changing the cysteine and glutathione levels. PUFAs are prone to oxidation. PUFAs are highly prone to oxidation, and it gets oxidized by both enzymatic (lipoxygenase mediated) and non-enzymatic (Fenton process mediated) processes (Loscalzo 2008). Ferrostain-1 acts by preventing the degradation of oxidized PUFA and their fragments by inhibiting their oxidative destruction. The ferrostatin-1 traps the free lipid peroxyl radical either by transferring the hydrogen atoms or by directly reducing lipid peroxide (Skouta et al. 2014). Ferrostatin-1 suffers from poor efficacy and metabolic stability. The ester group in this gets hydrolyzed into the inactive carboxylic acid group. It also exhibits weak microsomal activity and gets trapped into the microsomes due to its acidic nature (Hofmans et al. 2016; Devisscher et al. 2018).
Stockwell et al. established structure–activity relationship and suggested that the replacement of the amine group (-NH2) of ferrostatin with a nitro group (NO2) leads to the drastically reduction of ferroptosis. The amine group acts as a radical trapper and helps to quench the free radical, which was an essential factor as an excellent radical trapping agent. The amino group acts as the tremendous hydrogen source and transfers one of the hydrogen atoms into free radicals to retard or inhibit the free radical chain reactions. Similarly, in phenolic-based RTA, the OH group of phenol acts as a hydrogen atom source. The N-substituted cycloalkyl (cyclohexyl) present in the ferrostatin is necessary for the activity. They also vary the number of carbon atom of the N-substituted cycloalkyl moiety and synthesized ten analogs. After studying these analogs, the conclusion comes that the compound with a large lipophilic bulky group, cycloalkyl moiety shows greater potency (Dixon et al. 2012; Dixon and Stockwell 2014). Skouta et al. also performed an SAR study of ferrostatin-1. They suggested that the introduction of heteroatoms in the N-substituted cycloalkyl moiety can reduce the potency and also studied the role of hydrophobicity in the interaction with the lipophilic membrane environment. The substitution of aryl group with electron withdrawing or donating group or containing heteroatom on central primary amine can ameliorate the potency of ferrostatin. The tertiary amines turned out to be less potent compared to secondary or primary amines. The replacement of ester with the amide group can decrease the activity by ten folds (SRS9–11, Fig. 14.3, IC50 = 950 nM) when compared to ferrostatin-1 (IC50 = 95 nM) (Skouta et al. 2014). The substitution of the benzyl group on the central amine leads to a potent compound (~6-fold) with higher activity (SRS11–92, Fig. 14.3, IC50 = 6 nm) when compared to ferrostain-1. These studies are mainly focused on ferrostatin-1 to improve its existing potency (Hofmans et al. 2016). Linkermann et al. designed a ferrostatin-1-derived compound with improved plasma and microsomal activity. In this compound, ethyl ester was replaced with the tertiary-butyl ester to increase microsomal stability. Also, the imine group was introduced to the central amine group to generate SRS16–84, with higher plasma stability (t1/2 ≥ 120 min), and to show more excellent metabolic stability toward mouse microsomes. Intriguingly, SRS16–84 turned out to be of poor potency (IC50 = 350 nm) when compared with ferrostatin-1. Further, the compound UMAC-2418 was designed by substituting the benzyl group on central amine and replacing ethyl ester with sulfonamide, which could inhibit erastin-induced ferroptotic cell death. In human and mouse microsomes, the compound UMAC-2418 showed better potency with IC50 = 4 nM and t/2 of 90 min and 180 min. With complete plasma stability up to 6 h (Hofmans et al. 2016). Further, the same research group reported UAMC-3203 (Jiang et al. 2015) with slightly less potency (12 nM) over UMAC-2418 with improved microsomal stability (with t1/2 (Human) = 20.5 h, t1/2 (Rat) = 16.5 h and t1/2 (Mouse) = 3.5 h), enhanced plasma stability up to 6 h and with improved water solubility. The compound UAMC-3203 (Fig. 14.3) also showed rapid redistribution into various tissues. It is worth mention that UAMC-3203 was found to be efficacious in the acute iron-induced poisoning injury in mice (Devisscher et al. 2018).
4.5 Liproxstatin
Liproxstatin is an anti-ferroptotic molecule discovered in high-throughput screening of a vast library of small molecules utilizing the cell-based assays (Zilka et al. 2017). The ferroptotic cell death was induced primarily through the deletion of the glutathione peroxidase-4 (GPX4) gene or by inhibiting the System Xc antiporter that allows the exchange of intracellular glutamate and extracellular cysteine, which is necessary for the generation or synthesis of glutathione (GSH). The liproxstatin suppresses the ferroptosis activity by inhibiting the accumulation of lipid hydroperoxide (LOOH). Liproxstatin was evaluated in an inducible GPX4 KO mouse cell line, and it inhibited the cell death in this model (Friedmann Angeli et al. 2014). The liproxstatin is a highly potent active spiroquinoxalinamine derivative that acts by scavenging the lipid peroxide by transferring hydrogen atom from the amine group (-NH) to the free radical and acts as radical trapping antioxidant, which differs from a well-known phenolic RTA, where phenolic OH group acts as the trapping free radicals (Sheng et al. 2017). Several efforts are underway further to improve the anti-ferroptosis activity of the ferroptosis inhibitors to optimize these inhibitors as better drug candidates for AD (Reichert et al. 2020), ischemia (Magtanong and Dixon 2018), and heart diseases (Xie et al. 2016). The underlying molecular mechanism of action of liproxstatin is still illusive (Sheng et al. 2017). It inhibits the cell death induced by ferroptosis by inactivating the 15-lipoxygenase (15-ALOX) enzyme activity (Kagan et al. 2017). In another study, Pratt et al. reported inhibition of autoxidation of lipid or lipid peroxidation process by liproxstatin (Zilka et al. 2017). The study also suggested that liproxstatin also acts as an excellent radical trapping antioxidant, which scavenges lipid peroxide by trapping free radicals. The computational studies revealed the intrinsic mechanism of liproxstatin activity using density functional theory (DFT). They used the methyl peroxyl radical (CH3OO−) model to carry this experiment to know how liproxstatin traps the free radicals (Tejero et al. 2007).
Further, a detailed SAR study on liproxstatin suggested the presence of the benzene group to be essential for the activity and its replacement, completely abolished activity (Friedmann Angeli et al. 2014). Similarly, amine (NH2) also plays a vital role in the activity because the amine group is essential for antioxidant properties for scavenging free radicals. The process of radicalization occurs due to the transfer of an electron from the HOMO of the liproxstatin to the LUMO of methyl peroxy radical (CH3OO−). The compounds with a higher value of HOMO energy exhibit potent antioxidant properties. Therefore, liproxstatin-1, -2, and -3 showed promising antioxidant properties with the HOMO value of −5.44, −5.36, and −5.34, respectively (Fig. 14.4). The compounds liproxstatin-4 and liproxstatin-5 are found to be weak antioxidants due to the lack of one of the amine groups in the structure of liproxstatin-4, and the substitution of methyl with chlorine further decreased the antioxidant property of liproxstatin-5 with HOMO values −5.50 eV, and 5.49 eV, respectively. Liproxstatin-6 showed less bioactivity with the lowest HOMO value of −6.17 eV. The main factor in reducing the HOMO value for liproxstatin-6 is the absence of the phenyl group, which plays a crucial role in the HOMO energy (Fig.14.4). This study further suggests that the aromatic amine group plays an important role and is an essential requirement for better activity. The aromatic amine is also necessary for the resonance effect. So, after studying a SAR by substituting and withdrawing different groups in the parent molecule (liproxstatin-1), we notice that the aromatic amine is crucial for an antioxidant property; by increasing the HOMO value, we can further improve the antioxidant property of the compounds (Sheng et al. 2017).
The SAR study by Sheng et al. has suggested that the amine group is essential for the activity; however, out of the three amines, the amine group participating in the radical trapping by donating a hydrogen atom to the free radicals is still illusive. All three -NH groups act as attacking sites for radicals carrying the radicalization process. Therefore, Sheng et al. carried out a study to find out the most reactive -NH site by using methyl peroxyl (CH3COO−) radical. Thus, the energy required to cross the barrier was calculated and found to be 13.45, 18.28, and 20.41 kcal mol−1 for 1-NH, 2-NH, and 3-NH, respectively. So, the smaller the energy required to cross the barrier, the better the reactive site. Comparing all three -NH sites, 1-NH and 2-NH (Fig. 14.5) sites required less energy to cross the energy barrier. Still, the 3-NH group showed a high energy barrier, so it might be possible that it does not show any antioxidant property. Therefore, the reactivity arrangement for -NH group in liproxstatin is 1-NH > 2-NH > 3-NH, based on the reaction energy barrier.
4.5.1 Role of Ubiquinol (UQH2) or Coenzyme Q10 (Antioxidant Synthesized by Body) in the Activity of Liproxtatin-1
UQH2 (Fig. 14.7) is one of the crucial components which acts as an antioxidant agent synthesized by the body. It is mainly a 2,3 dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, in which poly prenyl side chain contains 10 units with lipid-soluble properties (Dhanasekaran and Ren 2005; Saini 2011). CoQ 10 is converted into ubiquinol with the help of ferroptosis, thereby suppressing protein 1 (FSP1). During the radical trapping mechanism, liproxstatin itself gets oxidized into liproxstatin-1 radical, which acts as a new radical and causes damage to the bio-membrane and the DNA (Niki et al. 2005). So here, the role of UQH2 comes into existence, as it acts like an antioxidant system of the living organism and can trap the free radical generated by liproxstatin-1 and donates one hydrogen atom to the radical by which the radical again comes into its active form, the process is reported as regeneration of liproxstatin-1 and acts as a key factor to study the antioxidant property of liproxstatin in vivo (Wang and Hekimi 2013; Bowry and Stocker 1993). So, ubiquinol is the critical parameter in studying the regeneration process of liproxstatin-1. Therefore, ubiquinol has a phenolic OH group, which acts as a phenolic radical trapping antioxidant and transfers one hydrogen atom to the liproxstatin-1 radical. The energy required by the UQH2 hydrogen atom to cross the energy barrier is reported to be 15.65 kcal mol−1, and the process is exothermic (4.49 kcal mol−1) in nature (Sheng et al. 2017). The UQH2 radical is further reduced to its active form with the other redox reactions (Nohl et al. 2001). This free radical of tocopherol is recycled back to its active form by UQH2. The essential energy barrier required to transfer hydrogen atoms from UQH2 is reported as 12.1 kcal mol−1, which has been proved in an experiment (Ouchi et al. 2010). This study suggested that liproxstatin-1 acts as a good candidate as an antioxidant and inhibits the process of lipid peroxidation to a great extent by trapping a free radical (LOO) generated during the lipid peroxidation process. The free radical generated by the oxidation of liproxstatin-1 is recycled back by other antioxidants present in the body, like ubiquinol (Sheng et al. 2017). So ubiquinol shows a synergistic effect with liproxstatin and increases its antioxidant efficacy.
4.6 ACSL4 Inhibitors
ACSL4 plays a crucial role in the execution of ferroptosis. During ferroptosis, it accelerates the esterification of polyunsaturated fatty acids (PUFA) such as arachidonic acid and adrenic acid to generate oxidized phosphatidylethanolamines (PE). It also accelerates the synthesis of AA-COA or AdA-CoA, which is required to form phospholipid hydroperoxide (PL-OOH), a key component in the lipid peroxidation process (Ivanov et al. 2010). The rosiglitazone, a peroxisome proliferator-activated receptor-γ (PPAR-γ) activator, inhibits ACSL4 activity and suppresses ferroptotic death (Askari et al. 2007). Rosiglitazone was used in in vivo studies to inhibit ACSL4 activity from finding its impact on ferroptosis during lung ischemia-reperfusion, it reduced the MDA production in ischemia reperfusion-injured lung tissues and attenuated ferroptosis (Xu et al. 2020). In the genetic model of ferroptosis, rosiglitazone (Fig. 14.7) can also alleviate and reduce the rate of mortality due to acute renal failure (Angeli et al. 2017; Doll 2017). Similarly, thiazolidinedione also inhibited ACSL4 and suppressed ferroptosis (Angeli et al. 2017). The above findings suggest that ACSL4 may be a potential therapeutic target. The pharmacological inhibition of ACSL4 by its inhibitors acts as the new paradigm in inhibiting ferroptosis and other lipid peroxidation-derived processes by limiting substrate availability. The ACSL4 inhibitors may offer opportunities for novel interventions for the treatment of neurodegenerative diseases caused due to lipid peroxidation.
4.7 Deuterated Phospholipids
The oxidative damage contributes to ferroptosis’s execution. The PUFA is more prone to oxidative damage because it contains bis-allylic protons, which are easily abstracted and produce alkyl radicals. These alkyl-radicals react with free oxygen to form peroxyl radicals, which then react with additional PUFAs to form a lipid peroxidation chain reaction. Malondialdehydes (MDA) and 4-hydroxynonenal, the two end products of lipid peroxidation, cause cell damage (Yang 2016). In 2007 Shchepinov et al. substituted linoleic acid (LA) with deuterated linoleic acid (D-LA). The 11,11-d2-LA (D-LA) experienced a propagation step of autoxidation, a 13-fold lesser than LA (Shchepinov 2007). Based on the findings, deuterated LA supplementation of cell medium was investigated and found to be beneficial in many cell models of lipid peroxidation linked to neurological illnesses such as Parkinson’s disease and Friedreich’s ataxia (Shchepinov et al. 2011; Cotticelli et al. 2013). In 2011, Shchepinov et al. did a study to find the role of PUFAs in ferroptosis. When H-PUFA is replaced with D-PUFA, which has deuterium in place of hydrogen at all bis-allylic positions, the initiation of deuterium abstraction and radical production gets decelerated as compared to H-PUFA (Shchepinov et al. 2011). Similarly in 2016, Yang et al. treated G-401 cell with D-linoleate (D-Lin), H-linoleate (H-lin), and vehicle (0.1% ethanol) for overnight and then exposed these cells to erastin and RSL3 to evaluate the impact of D-PUFA in the execution of ferroptosis. The D-PUFA showed a potential protective effect against erastin and RSL3 treated conditions and suppressed ferroptosis by inhibiting lipid peroxides generation which was measured by C11-BODIPY (Yang et al. 2016). In 2018, Shah et al. conducted a study to determine the role of LOX enzyme. In this study, they used deuterated arachidonic acid. All bis-allylic hydrogen atoms were replaced with their heavy isotope deuterium. The LOX did not abstract hydrogen to initiate lipid peroxidation and suppressed RSL3 induced cell death (Shah et al. 2018). The above finding suggested that deuterated phospholipids give important contribution to suppress ferroptosis by inhibiting lipid peroxidation process and can therefore be used as ferroptosis inhibitors to treat various neurodegenerative diseases (Figs. 14.6 and 14.7; Table 14.1).
5 Conclusion
The prognosis and pathogenesis of AD are multifactorial, and further continual discovery of novel signaling pathways on ferroptosis driven AD imitates the complexity of the disease. These complexity should be addressed in management of AD in patients for better therapeutic outcomes, and anti-ferroptotic drugs like iron chelators or inhibitors of better fit should be included as AD treatment options. This chapter sum up the evidences following the role of ferroptosis in AD pathogenesis and signifies what is known about the therapeutic targets for inhibition of AD progression. In addition to this ferroptosis-related differentially expressed genes in AD supported that the inhibition of ferroptosis might slow down the progression of disease and declination of memory, however this area is still uncovered and need to be widely investigated. Further development of new models for AD is needed to understand how ferroptosis affects cell-to-cell interaction and time duration of development of ferroptosis in AD. In future, studies must be focused on developing detection tools of ferroptosis and systematizing huge and randomized clinical trials of drugs related to ferroptosis inhibition in early or later stage of disease progression in AD models.
References
Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR (2018) Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci 12:466
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12(1):125–169
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262–79.e25
Angeli JPF, Shah R, Pratt DA, Conrad M (2017) Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci 38(5):489–498
Aprioku JS (2013) Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil 14(4):158–172
Arbiser JL, Bonner MY, Ward N, Elsey J, Rao S (2018) Selenium unmasks protective iron armor: a possible defense against cutaneous inflammation and cancer. Biochim Biophys Acta Gen Subj 1862(11):2518–2527
Askari B, Kanter JE, Sherrid AM, Golej DL, Bender AT, Liu J et al (2007) Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-gamma-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 56(4):1143–1152
Ayton S, Lei P, Duce JA, Wong BX, Sedjahtera A, Adlard PA et al (2013) Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol 73(4):554–559
Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY et al (2017) Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain 140(8):2112–2119
Beshir SA, Aadithsoorya AM (2022) Aducanumab therapy to treat Alzheimer’s disease: a narrative review. Int J Alzheimers Dis 2022:9343514
Blaise GA, Gauvin D, Gangal M, Authier S (2005) Nitric oxide, cell signaling and cell death. Toxicology 208(2):177–192
Bowry VW, Stocker R (1993) Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc 115(14):6029–6044
Brigelius-Flohé R (2009) Vitamin E: the shrew waiting to be tamed. Free Radic Biol Med 46(5):543–554
Brütsch SH, Wang CC, Li L, Stender H, Neziroglu N, Richter C et al (2015) Expression of inactive glutathione peroxidase 4 leads to embryonic lethality, and inactivation of the Alox15 gene does not rescue such knock-in mice. Antioxid Redox Signal 22(4):281–293
Burton GW, Ingold KU (1986) Vitamin-E—application of the principles of physical organic-chemistry to the exploration of its structure and function. Acc Chem Res 19:194
Cai P, Fang SQ, Yang HL, Yang XL, Liu QH, Kong LY et al (2018) Donepezil-butylated hydroxytoluene (BHT) hybrids as anti-Alzheimer’s disease agents with cholinergic, antioxidant, and neuroprotective properties. Eur J Med Chem 157:161–176
Cao JY, Dixon SJ (2016) Mechanisms of ferroptosis. Cell Mol Life Sci 73(11–12):2195–2209
Cardoso BR, Roberts BR, Malpas CB, Vivash L, Genc S, Saling MM et al (2019) Supranutritional sodium selenate supplementation delivers selenium to the central nervous system: results from a randomized controlled pilot trial in Alzheimer’s disease. Neurotherapeutics 16(1):192–202
Castellani RJ, Moreira PI, Liu G, Dobson J, Perry G, Smith MA et al (2007) Iron: the Redox-active center of oxidative stress in Alzheimer disease. Neurochem Res 32(10):1640–1645
Chu J, Li JG, Praticò D (2013) Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS One 8(8):e70991
Chu J, Li JG, Giannopoulos PF, Blass BE, Childers W, Abou-Gharbia M et al (2015) Pharmacologic blockade of 12/15-lipoxygenase ameliorates memory deficits, Aβ and tau neuropathology in the triple-transgenic mice. Mol Psychiatry 20(11):1329–1338
Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11(3):315–335
Cotticelli MG, Crabbe AM, Wilson RB, Shchepinov MS (2013) Insights into the role of oxidative stress in the pathology of Friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox Biol 1(1):398–404
Cozza G, Rossetto M, Bosello-Travain V, Maiorino M, Roveri A, Toppo S et al (2017) Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: the polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic Biol Med 112:1–11
Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL (2017) Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther 9(1):71
DeHart DN, Fang D, Heslop K, Li L, Lemasters JJ, Maldonado EN (2018) Opening of voltage dependent anion channels promotes reactive oxygen species generation, mitochondrial dysfunction and cell death in cancer cells. Biochem Pharmacol 148:155–162
Derry PJ, Hegde ML, Jackson GR, Kayed R, Tour JM, Tsai AL et al (2020) Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog Neurobiol 184:101716
Devisscher L, Van Coillie S, Hofmans S, Van Rompaey D, Goossens K, Meul E et al (2018) Discovery of novel, drug-like ferroptosis inhibitors with in vivo efficacy. J Med Chem 61(22):10126–10140
Dhanasekaran M, Ren J (2005) The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Curr Neurovasc Res 2(5):447–459
Di Meco A, Lauretti E, Vagnozzi AN, Praticò D (2014) Zileuton restores memory impairments and reverses amyloid and tau pathology in aged Alzheimer’s disease mice. Neurobiol Aging 35(11):2458–2464
Di Meco A, Li JG, Blass BE, Abou-Gharbia M, Lauretti E, Praticò D (2017) 12/15-Lipoxygenase inhibition reverses cognitive impairment, brain amyloidosis, and tau pathology by stimulating autophagy in aged triple transgenic mice. Biol Psychiatry 81(2):92–100
Dixon SJ (2017) Ferroptosis: bug or feature? Immunol Rev 277(1):150–157
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10(1):9–17
Dixon SJ, Stockwell BR (2019) The hallmarks of ferroptosis. Annu Rev Cancer Biol 3(1):35–54
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Doba T, Burton GW, Ingold KU (1985) Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta Lipids Lipid Metab 835:298
Doll S (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13:91
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98
Fan EKY, Fan J (2018) Regulation of alveolar macrophage death in acute lung inflammation. Respir Res 19(1):50
Feng H, Stockwell BR (2018) Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol 16(5):e2006203
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB et al (2019) Role of mitochondria in ferroptosis. Mol Cell 73(2):354–63.e3
Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, Brash AR et al (2011) The structure of human 5-lipoxygenase. Science (New York, NY) 331(6014):217–219
Gu XH, Xu LJ, Liu ZQ, Wei B, Yang YJ, Xu GG et al (2016) The flavonoid baicalein rescues synaptic plasticity and memory deficits in a mouse model of Alzheimer’s disease. Behav Brain Res 311:309–321
Guo H, Verhoek IC, Prins GGH, van der Vlag R, van der Wouden PE, van Merkerk R et al (2019) Novel 15-lipoxygenase-1 inhibitor protects macrophages from lipopolysaccharide-induced cytotoxicity. J Med Chem 62(9):4624–4637
Habib E, Linher-Melville K, Lin HX, Singh G (2015) Expression of xCT and activity of system xc(−) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol 5:33–42
Haeggström JZ, Funk CD (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev 111(10):5866–5898
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17
Harding AE, Muller DP, Thomas PK, Willison HJ (1982) Spinocerebellar degeneration secondary to chronic intestinal malabsorption: a vitamin E deficiency syndrome. Ann Neurol 12(5):419–424
Hofmans S, Vanden Berghe T, Devisscher L, Hassannia B, Lyssens S, Joossens J et al (2016) Novel ferroptosis inhibitors with improved potency and ADME properties. J Med Chem 59(5):2041–2053
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ III et al (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12(8):1425–1428
Ingold KU (1961) Inhibition of the autoxidation of organic substances in the liquid phase. Chem Rev 61:563
Ingold KU, Pratt DA (2014) Advances in radical-trapping antioxidant chemistry in the 21st century: a kinetics and mechanisms perspective. Chem Rev 114:9022
Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H et al (2010) Molecular enzymology of lipoxygenases. Arch Biochem Biophys 503(2):161–174
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–62
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90
Khalaf SS, Hafez MM, Mehanna ET, Mesbah NM, Abo-Elmatty DM (2019) Combined vildagliptin and memantine treatment downregulates expression of amyloid precursor protein, and total and phosphorylated tau in a rat model of combined Alzheimer’s disease and type 2 diabetes. Naunyn Schmiedebergs Arch Pharmacol 392(6):685–695
Kontoghiorghe CN, Kontoghiorghes GJ (2016) Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des Dev Ther 10:465–481
Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK et al (2013) Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J Pharm Pharmacol 65(12):1773–1784
Kosaraju J, Holsinger RMD, Guo L, Tam KY (2017) Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer’s disease. Mol Neurobiol 54(8):6074–6084
Krabbendam IE, Honrath B, Dilberger B, Iannetti EF (2020) SK channel-mediated metabolic escape to glycolysis inhibits ferroptosis and supports stress resistance in C. elegans. Cell Death Dis 11(4):263
Kuang F, Liu J, Tang D, Kang R (2020) Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol 8:586578
Kwon M-Y, Park E, Lee S-J, Chung SW (2015) Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget 6(27):24393–24403
Li B, Harjani JR, Cormier NS, Madarati H, Atkinson J, Cosa G et al (2013) Besting vitamin E: sidechain substitution is key to the reactivity of naphthyridinol antioxidants in lipid bilayers. J Am Chem Soc 135(4):1394–1405
Liu J, Chang L, Song Y, Li H, Wu Y (2019) The role of NMDA receptors in Alzheimer’s disease. Front Neurosci 13:43
Lloret A, Esteve D, Monllor P, Cervera-Ferri A, Lloret A (2019) The effectiveness of vitamin E treatment in Alzheimer’s disease. Int J Mol Sci 20(4):879
Loscalzo J (2008) Membrane redox state and apoptosis: death by peroxide. Cell Metab 8(3):182–183
Ma QH, Jiang LF, Mao JL, Xu WX, Huang M (2018) Vildagliptin prevents cognitive deficits and neuronal apoptosis in a rat model of Alzheimer’s disease. Mol Med Rep 17(3):4113–4119
Magtanong L, Dixon SJ (2018) Ferroptosis and brain injury. Dev Neurosci 40(5–6):382–395
Maher P, van Leyen K, Dey PN, Honrath B, Dolga A, Methner A (2018) The role of Ca(2+) in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium 70:47–55
Mcgirr S, Venegas C, Swaminathan A (2020) Alzheimers disease: a brief review. J Exp Neurol 1(3):89–98
Mendes KL, Lelis DF, Santos SHS (2017) Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev 38:98–105
Miyake S, Murai S, Kakuta S, Uchiyama Y, Nakano H (2020) Identification of the hallmarks of necroptosis and ferroptosis by transmission electron microscopy. Biochem Biophys Res Commun 527(3):839–844
Moroishi T, Yamauchi T, Nishiyama M, Nakayama KI (2014) HERC2 targets the iron regulator FBXL5 for degradation and modulates iron metabolism. J Biol Chem 289(23):16430–16441
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C et al (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12(1):34
Nam TG, Rector CL, Kim HY, Sonnen AF, Meyer R, Nau WM et al (2007) Tetrahydro-1,8-naphthyridinol analogues of alpha-tocopherol as antioxidants in lipid membranes and low-density lipoproteins. J Am Chem Soc 129(33):10211–10219
Neitemeier S, Jelinek A, Laino V, Hoffmann L, Eisenbach I, Eying R et al (2017) BID links ferroptosis to mitochondrial cell death pathways. Redox Biol 12:558–570
Niki E, Yoshida Y, Saito Y, Noguchi N (2005) Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun 338(1):668–676
Nohl H, Kozlov AV, Staniek K, Gille L (2001) The multiple functions of coenzyme Q. Bioorg Chem 29(1):1–13
Ouchi A, Nagaoka S-i, Mukai K (2010) Tunneling effect in regeneration reaction of vitamin E by ubiquinol. J Phys Chem B 114(19):6601–6607
Perianayagam A, Bloom D, Lee J, Parasuraman S, Sekher TV, Mohanty SK et al (2022) Cohort profile: the longitudinal ageing study in India (LASI). Int J Epidemiol 51(4):e167–e176
Peters DG, Pollack AN, Cheng KC, Sun D, Saido T, Haaf MP et al (2018) Dietary lipophilic iron alters amyloidogenesis and microglial morphology in Alzheimer’s disease knock-in APP mice. Metallomics 10(3):426–443
Praticò D, Sung S (2004) Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis 6(2):171–175
Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VM (2001) Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci 21(12):4183–4187
Pratt DA, DiLabio GA, Brigati G, Pedulli GF, Valgimigli L (2001) 5-Pyrimidinols: novel chain-breaking antioxidants more effective than phenols. J Am Chem Soc 123(19):4625–4626
Probst L, Dächert J, Schenk B, Fulda S (2017) Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol 140:41–52
Reichert CO, de Freitas FA, Sampaio-Silva J, Rokita-Rosa L, Barros PL, Levy D et al (2020) Ferroptosis mechanisms involved in neurodegenerative diseases. Int J Mol Sci 21(22):8765
Saini R (2011) Coenzyme Q10: the essential nutrient. J Pharm Bioallied Sci 3(3):466–467
Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S (2004) Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun 325(1):109–116
Seibt TM, Proneth B, Conrad M (2019) Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 133:144–152
Senin U, Parnetti L, Barbagallo-Sangiorgi G, Bartorelli L, Bocola V, Capurso A et al (1992) Idebenone in senile dementia of Alzheimer type: a multicentre study. Arch Gerontol Geriatr 15(3):249–260
Shah R, Shchepinov MS, Pratt DA (2018) Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci 4(3):387–396
Shchepinov MS (2007) Reactive oxygen species, isotope effect, essential nutrients, and enhanced longevity. Rejuvenation Res 10(1):47–59
Shchepinov MS, Chou VP, Pollock E, Langston JW, Cantor CR, Molinari RJ et al (2011) Isotopic reinforcement of essential polyunsaturated fatty acids diminishes nigrostriatal degeneration in a mouse model of Parkinson’s disease. Toxicol Lett 207(2):97–103
Sheng X, Shan C, Liu J, Yang J, Sun B, Chen D (2017) Theoretical insights into the mechanism of ferroptosis suppression via inactivation of a lipid peroxide radical by liproxstatin-1. Phys Chem Chem Phys 19(20):13153–13159
Shimada K, Stockwell BR (2015) tRNA synthase suppression activates de novo cysteine synthesis to compensate for cystine and glutathione deprivation during ferroptosis. Mol Cell Oncol 3(2):e1091059
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K et al (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136(12):4551–4556
Solomon EI, Zhou J, Neese F, Pavel EG (1997) New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem Biol 4(11):795–808
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285
Tampi RR, Forester BP, Agronin M (2021) Aducanumab: evidence from clinical trial data and controversies. Drugs Context 10:2021-7-3
Tang M, Chen Z, Wu D, Chen L (2018) Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. J Cell Physiol 233(12):9179–9190
Tejero I, González-García N, González-Lafont À, Lluch JM (2007) Tunneling in green tea: understanding the antioxidant activity of catechol-containing compounds. A variational transition-state theory study. J Am Chem Soc 129(18):5846–5854
Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E et al (2003) Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology 61(11):1498–1502
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530
Ulatowski LM, Manor D (2015) Vitamin E and neurodegeneration. Neurobiol Dis 84:78–83
Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C (1982) Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta 710(2):197–211
Vallance P, Leiper J (2002) Blocking NO synthesis: how, where and why? Nat Rev Drug Discov 1(12):939–950
Vaz M, Silva V, Monteiro C (2022) Role of aducanumab in the treatment of Alzheimer’s disease: challenges and opportunities. Clin Interv Aging 17:797–810
Wang Y, Hekimi S (2013) Molecular genetics of ubiquinone biosynthesis in animals. Crit Rev Biochem Mol Biol 48(1):69–88
Wang H, An P, Xie E, Wu Q, Fang X, Gao H et al (2017) Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66(2):449–465
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M et al (2020) ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ 27(2):662–675
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10):1045–1060
Weyer G, Babej-Dölle RM, Hadler D, Hofmann S, Herrmann WM (1997) A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology 36(2):73–82
Wijtmans M, Pratt DA, Valgimigli L, DiLabio GA, Pedulli GF, Porter NA (2003) 6-Amino-3-pyridinols: towards diffusion-controlled chain-breaking antioxidants. Angew Chem Int Ed Engl 42(36):4370–4373
Wu JR, Tuo QZ, Lei P (2018) Ferroptosis, a recent defined form of critical cell death in neurological disorders. J Mol Neurosci 66(2):197–206
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23:369
Xu Y, Li X, Cheng Y, Yang M, Wang R (2020) Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J 34(12):16262–16275
Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F et al (2002) Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem 82(5):1137–1147
Yan N, Zhang J (2020) Iron metabolism, ferroptosis, and the links with Alzheimer’s disease. Front Neurosci 13:1443
Yang WS (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113:E4966
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26(3):165–176
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2):317–331
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113(34):E4966–E4975
Yoo MH, Gu X, Xu XM, Kim JY, Carlson BA, Patterson AD et al (2010) Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer’s disease. Antioxid Redox Signal 12(7):819–827
Zhang F, Tao Y, Zhang Z, Guo X, An P, Shen Y et al (2012) Metalloreductase Steap3 coordinates the regulation of iron homeostasis and inflammatory responses. Haematologica 97(12):1826–1835
Zhang SQ, Obregon D, Ehrhart J, Deng J, Tian J, Hou H et al (2013) Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer’s disease transgenic mouse model. J Neurosci Res 91(9):1239–1246
Zhou RP, Chen Y, Wei X, Yu B, Xiong ZG, Lu C et al (2020) Novel insights into ferroptosis: implications for age-related diseases. Theranostics 10(26):11976–11997
Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M et al (2017) On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci 3(3):232–243
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950
Acknowledgement
GM is thankful to Indian Council of Medical Research (ICMR) for providing research grant sanctioned under ICMR 5/4-5/3/24/Neuro/2022-NCD-I (ICMR-EMR-2021-10363).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, G., Rana, N.K., Mishra, I., Modi, G.P. (2023). Ferroptosis Modulators: A Potential Therapeutic Target in Alzheimer’s Disease. In: Sharma, A., Modi, G.P. (eds) Natural Product-based Synthetic Drug Molecules in Alzheimer's Disease. Springer, Singapore. https://doi.org/10.1007/978-981-99-6038-5_14
Download citation
DOI: https://doi.org/10.1007/978-981-99-6038-5_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6037-8
Online ISBN: 978-981-99-6038-5
eBook Packages: MedicineMedicine (R0)