Abstract
Alzheimer’s disease is the most common forms of dementia. Dementia is the general term for cognitive decline severe enough to impede with daily activities. Early diagnosis of Alzheimer’s disease is important for slowing or stopping the disease’s development, and experts can start preventive treatment right away. The experts must be capable of identifying Alzheimer’s disease in its earliest and most challenging phases. The fundamental objective of this study is to create a machine learning model that can automatically diagnose disease using MRI, a widely used diagnostic tool. This research employed structural MRI to find out the difference between patients with Alzheimer’s disease (AD), stable mild cognitive impairment (sMCI), progressing mild cognitive impairment (pMCI), and normal cognitive functioning (CN). In this research paper, machine learning models, namely SVM, RF, DT, and CNN, are used for multi-class classification. CNN obtained the highest testing accuracy of 88.84% among the four models, with a precision of 80.42%, a recall of 73.17%, and an F1-score of 76.62% for the CN versus sMCI versus pMCI versus AD multi-class classification.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
28.1 Introduction
Alzheimer’s disease (AD), which causes cognitive impairment and behavioural difficulties, is the most common neurodegenerative disorder in older individuals, accounting for 60–80% of dementia cases. About 5–20% of the population, above the age of 65 suffers from mild cognitive impairment (MCI), which manifests as slight to moderate memory loss and other cognitive difficulties. MCI is a precursor to AD, with 10–15% of MCI patients developing AD each year [1]. Furthermore, over the next two to three decades, this number will rise steadily. Even though the aetiology of Alzheimer’s disease has not been fully elucidated and no treatment has been developed, early diagnosis of the AD is critical for both prevention and treatment [2]. There are about 50 million people living with Alzheimer’s disease (AD) across various regions of the world. It is predicted that the number of people diagnosed with Alzheimer’s disease would be more than double by the year 2050 as a result of the ageing of the world’s population [3]. There are several biomarkers for detection of Alzheimer’s disease, such as those discovered in neuroimaging, CSF, genetic, blood, urine biomarkers, and others. Additional categories for neuroimaging biomarkers include structural imaging biomarkers, functional imaging biomarkers, and molecular imaging biomarkers, in which the brain’s functions are determined by the brain's morphology and the molecules that are present. However, there is not yet sufficient evidence to determine which biomarker is the most accurate in detecting Alzheimer’s disease [4]. The diagnostic and therapeutic roles of dementia biomarkers have the potential to change to life an AD patients [5]. In recent years, non-invasive brain imaging has become more and more common for diagnosing Alzheimer’s disease. This imaging technique is completely safe for human brain tissues, making it a highly useful diagnostic tool. Non-invasive medical brain imaging is now one of the most preferred diagnostic tools by the physicians [6].
In recent years, machine learning algorithms for interpreting biological imaging and neuroimaging data have become increasingly popular. Deep learning, a machine learning framework based on “artificial neural networks,” has emerged in recent years”, due to the fact that it is capable of accurately predicting significant clinical outcomes. CNN models, which stand for “convolutional neural network,” are potential deep learning methods for detecting and classifying objects. These models are used rather extensively in the field of medical imaging. CNN models are able to recognize patterns in visual data sets and accurately predict outcomes for fundamental tasks such as categorizing. This ability has been demonstrated through extensive testing of CNNs as specialized artificial neural networks that perform image analysis by convolving several filters into a single input, each of which is about the size of a small patch of the image, to look for similar spatial characteristics across the whole image [7]. This study helps doctors make better decisions about neurodegenerative diseases, especially Alzheimer’s disease (AD).
The most important contributions that are made by this study are: To demonstrate an effective method for the multi-stage classification of AD. Texture feature extraction carried out by the application of the gray-level co-occurrence matrix (GLCM) for multi-stage categorization.
28.2 Related Work
This section provides a concise overview of the different structural MRI (sMRI) techniques used to diagnose Alzheimer’s disease. There are three main types of such methods: whole brain, region of interest, and landmark-based. Brain mapping methods, such as voxel-based morphometry (VBM), tensor-based morphometry (TBM) and deformation-based morphometry (DBM) track morphological changes to determine brain atrophy. Voxel-based methods measure tissue density in the human brain voxel by voxel. Tensor-based methods examine the local Jacobians of deformation fields in order to determine structural issues. DBM is utilized to accommodate nonlinear deformation fields to a standard anatomical template [1]. SVM classifiers and grey matter voxels were used to distinguish AD from CN in [8]. Firstly, the hippocampus areas of several subjects were segmented, and then, their form was quantified using spherical harmonics features extraction [9]. Traditional approaches for diagnosing Alzheimer's disease often have poor performance because they rely on manually produced visual information that are either difficult or impossible to optimize. As a result, these methods are typically ineffective.
Deep learning algorithms aid in the early diagnosis of Alzheimer’s disease (AD) symptoms, and the monitoring of the progression from mild cognitive impairment (MCI) to Alzheimer’s disease is discussed in [1]. Existing methods, even though they are popular, use unrelated brain areas or less precise landmarks to diagnose AD. Densely connected neural networks were employed to extract multi-scale information from pre-processed images. In addition, a connection-wise attention mechanism was used to the process of diagnosing Alzheimer’s disease [2]. A large number of studies make use of convolutional neural networks (CNNs) to record deep-level medical image data in an effort to diagnose Alzheimer’s disease (AD) and forecast clinical scores [3]. 2D anatomical slices were used in the advanced diagnostic learning process for Alzheimer’s disease (AD) [10]. Alzheimer’s disease was diagnosed utilizing the lateral ventricles, periventricular white matter, and cortical grey matter [11]. Several statistical methods, such as support vector machines, logistic regression, decision trees, and random forests, were used in the process of AD diagnostic prediction [12]. A fuzzy hyperplane-based least square twin support vector machine for feature categorization and early AD detection using sagittal plane slices from 3D MRI imaging data are described in [13]. Researchers are trying to improve their ability to detect Alzheimer’s disease by using subject-level, patch-based, and slice-based computational neuroimaging methods [4]. Clinical data and a hybrid texture, edge, color, and density feature extraction were utilized to diagnose AD [14]. The use of deep learning-based multiclass classification to distinguish between the stages of Alzheimer’s disease for early diagnosis is discussed in [15]. Axial brain images from a three-dimensional MRI were fed into a convolutional neural network (CNN) for multiclass categorization [16]. Voxel-based morphometry was used to extract grey matter from the brain and then correlated clinical cognitive scores in MCI with grey matter in order to diagnose Alzheimer’s disease [17]. Multi-modal medical imaging has the potential to improve Alzheimer’s disease (AD) categorization and diagnosis [6]. Multimodal adaptive-similarity feature selection for AD diagnosis is discussed in [18].
28.3 Material and Methods
28.3.1 Data Collection
ADNI, which stands for the Alzheimer’s disease neuroimaging initiative, data is used in this research. https://ida.loni.usc.edu. ADNI has different phases like ADNI-1, ADNI-GO, ADNI-2, and ADNI-3. The ADNI-1 baseline dataset used in this work comprises of 342 subjects including 89 CN, 100 sMCI, 110 pMCI, and 43 AD. Cognitive normal (CN) class includes healthy controls with no conversion into any other stage of Alzheimer’s disease within 36 months of baseline follow up. Mild cognitively impaired individuals who were nevertheless capable of carrying out everyday tasks were kept in MCI class. Stable MCI (sMCI) subjects were those who had been diagnosed with MCI and had not changed or returned to any other class after at least 36 months from baseline. Progressive MCI (pMCI) class included subjects who were initially diagnosed with MCI but later developed AD within 36 month. Patients in the AD class were those who had been first diagnosed with Alzheimer's disease and had no signs of reversal within 24 months.
All the T1weighted-sMRI data is acquired with a 1.5 T T1-weighted 3D MP-RAGE sequence. The main reason for choosing structural MRI in this work is that these scans are more detailed and cheaper as compared to other imaging modalities like SPECT, PET, and DTI. sMRI is mainly used to capture the pathology and anatomy of brain. Other Imaging Protocol details include Acquisition Type: 3D; Field Strength: 1.5 T; Slice Thickness: 1.2 mm; Flip Angle: 8.0 degrees; X, Y, and Z each have 256.0 pixels; Visit—baseline and screening. In order to assess cognitive impairment, the 30-point MMSE questionnaire is often used in clinical and research contexts. MMSE can assist the clinicians to assess the level of dementia and also amenable to patients and their families. The subjects details are provided in Table 28.1.
The proposed methodology of AD classification is shown in Fig. 28.1. Detailed descriptions of the steps are provided in the subsections that follow.
28.3.2 MRI Image Pre-processing
To standardize the data into the appropriate shape and format, preprocessing is conducted for each brain structural MRI. It is a preparatory process that is intended to remove undesired non-brain tissues such as the skull, eyes, fat, and other things that are present in brain MRI images [19]. In this work, skull stripping is conducted by using brain extraction tool (BET) of FSL 6.0 from https://fsl.fmrib.ox.ac.uk/. The outcome of skull stripping is shown in Fig. 28.2. Once BET has reduced the extraneous material, the brain itself can be isolated from the whole brain scan. 50 middle slices of each subject that contain useful information about Alzheimer’s disease are selected and remaining images are discarded.
28.3.3 Feature Extraction
As shown in Fig. 28.1, after preprocessing, 3D images are transformed to many groups of 2D images along sagittal direction. In the process of classification, the use of texture information reduced the amount of noise in the optical picture pixels. Gray-level co-occurrence matrix (GLCM), which is a common statistical method, was used for feature extraction for T1w-sMRI images. The features extraction from images described in Table 28.2.
28.3.4 Machine Learning Techniques
Even it is widely acknowledged that machine learning falls under the umbrella term of artificial intelligence, there are several points of view within the academic community that place more emphasis on its status as a subfield of computer science rather than AI. Machine learning can be thought of as both multidisciplinary and interdisciplinary field due to its usage of ideas and methods adopted from a wide range of disciplines. Machine learning classifies and diagnoses Alzheimer’s disease severity. Three classifiers, namely SVM, RF, and DT are applied in this work.
Support Vector Machine: The support vector machine, more commonly known as SVM, is a well-known method of supervised learning that is applicable for classification and regression analysis. However, its most common use is in machine learning classification tasks. The SVM method figures out the best line or decision boundary to divide n-dimensional space into classes. This makes it easier to put future data points into the right category.
Decision Tree: Decision tree is a supervised learning technique. It looks like a tree, with fundamental nodes that show the features of a dataset, branches that show how to make decisions, and leaf nodes that show the final classes. It is possible to “learn” a tree by first dividing the source set into multiple subsets, each of which is evaluated using an attribute value test. Decision tree classifiers are excellent for knowledge discovery’s exploratory phase as they do not require domain knowledge or parameters.
Random Forest: Feature randomization, often called feature bagging, provides a random set of characteristics to reduce decision tree correlation. This is one of the most significant differences between decision trees and random forests. While decision trees take into account all possible feature splits, random forests select only a fraction of the possible combinations.
28.3.5 Deep Learning
Machine learning has employed artificial neural networks and representation learning, a subset of which is deep learning. Deep learning applications in speech recognition, computer vision, machine translation, natural language processing, bioinformatics, medical imaging, climate research, board game programming, and material inspection have utilized architectures such as deep belief networks, deep neural networks, recurrent neural networks, deep reinforcement learning, convolutional neural networks, transformers, and others.
Convolutional neural networks are a component of deep neural networks. In one layer, these networks perform convolution instead of matrix multiplication.
CNNs are a type of deep learning architecture typically employed for image classification and recognition tasks. It has numerous layers, including convolutional, pooling, and fully linked layers. The convolutional layer uses image filtering to pull out features from the input image. The pooling layer reduces sample sizes, which in turn reduces processing time, and the fully connected layer offers the final forecast. Backpropagation and gradient descent are used to train the network to acquire the most efficient filters.
28.4 Results and Discussion
In this work, multiclass categorization of Alzheimer’s disease is being accomplished by the application of machine learning and deep learning models. For this purpose, the total dataset is divided 80:10:10 between training, validation, and testing, respectively. All the experiments are carried out using python 3.5 and i5 processor with 8 GB of RAM. Keras, a deep learning package, is used in conjunction with Tensorflow as the backend to construct the neural network. The performance of the model is evaluated using the parameters, precision, recall, accuracy, and F1-score. The results of performing multiclass classification (AD vs. CN vs. pMCI vs. sMCI) using a machine learning and deep learning techniques are presented in Table 28.3.
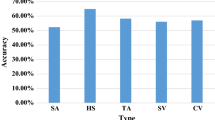
Out of the four models, CNN (scratch) achieved the highest accuracy of 88.84%, precision of 80.42%, recall of 73.17%, and F1 score of 76.62%. It is clear from the above results that deep learning model performed better than traditional machine learning models. The bar graph of evaluation metrics of all four models is shown in Fig. 28.3.
It is clear from Fig. 28.3 that CNN outperformed machine learning techniques in terms of precision, recall and F1-score. The overall performance of CNN is better than machine learning models.
The results of the proposed method are compared with two state-of-art methods: (1) deep multi-task multi-channel learning (DM2L) [20] (2) deep residual neural networks (ResNet) [21]. In the first method, a landmark-based approach is used to automatically generate discriminative image patches from MRI images which were further utilized for multi-class classification [20]. In the second method, a deep residual neural networks are applied for predicting progression from mild cognitive impairment (MCI) to Alzheimer’s disease (AD) [21]. The comparison results are presented in Table 28.4.
It is clear from the above results that deep learning techniques performed better than landmark-based approach. The proposed convolutional network outperformed deep residual network.
Machine learning techniques have one main drawback that they require manual feature extraction which makes these techniques time consuming. On the other side, deep learning algorithms have in-built potential for feature extraction and feature selection which makes them more efficient.
28.5 Conclusions
AD is a neurodegenerative disease that gets worse over time and affects people’s health, society, and the economy all over the world. As there is no medication or drug to cure or reverse AD, many researchers in numerous fields have been focusing on early detection and prognosis using various approaches. In particular, neuroimaging data provides quantitative and qualitative measures on which computational models can be built to predict AD progression. In this paper, an attempt is made for multi-class categorization of Alzheimer’s disease. The structural MRI scans are preferred over PET, DTI, and SPECT because they are clearer, more detailed, and less expensive. In this work, machine learning and deep learning models are proposed to perform multi-class classification of Alzheimer’s disease using T1w-sMRI. The deep learning model performed better than machine learning techniques. However, deep learning techniques require large data and computation as compared to machine learning approaches. Also, the results confirmed that sMRI is a potential biomarker for early diagnosis of AD. In future, the model will be trained with more data. Other modalities like PET, CSF, and SPECT can be explored to obtain better results for prediction of Alzheimer’s disease.
References
Chen, Y., Xia, Y.: Iterative sparse and deep learning for accurate diagnosis of Alzheimer’s disease. Pattern Recognit. 116, 107944 (2021). https://doi.org/10.1016/j.patcog.2021.107944
Zhang, J., Zheng, B., Gao, A., et al.: A 3D densely connected convolution neural network with connection-wise attention mechanism for Alzheimer’s disease classification. Magn Reson Imaging 78, 119–126 (2021). https://doi.org/10.1016/j.mri.2021.02.001
Lao, H., Zhang, X.: Regression and classification of Alzheimer’s disease diagnosis using NMF-TDNet features from 3D brain MR Image. IEEE J Biomed. Heal. Inform. 26, 1103–1115 (2022). https://doi.org/10.1109/JBHI.2021.3113668
Goenka, N., Tiwari, S.: AlzVNet: a volumetric convolutional neural network for multiclass classification of Alzheimer’s disease through multiple neuroimaging computational approaches. Biomed. Signal Process Control 74, 103500 (2022). https://doi.org/10.1016/j.bspc.2022.103500
Vishnu, V.Y., Modi, M., Garg, V.K., et al.: Role of inflammatory and hemostatic biomarkers in Alzheimer’s and vascular dementia—a pilot study from a tertiary center in Northern India. Asian J Psychiatr 29, 59–62 (2017). https://doi.org/10.1016/j.ajp.2017.04.015
Kong, Z., Zhang, M., Zhu, W., et al.: Multi-modal data Alzheimer’s disease detection based on 3D convolution. Biomed. Signal Process Control 75, 103565 (2022). https://doi.org/10.1016/j.bspc.2022.103565
Qiu, S., Chang, G.H., Panagia, M., et al.: Fusion of deep learning models of MRI scans, mini-mental state examination, and logical memory test enhances diagnosis of mild cognitive impairment. Alzheimer’s Dement Diagnosis, Assess Dis. Monit. 10, 737–749 (2018). https://doi.org/10.1016/j.dadm.2018.08.013
Klöppel, S., Stonnington, C.M., Chu, C., et al.: UKPMC funders group automatic classification of MR scans in Alzheimer’s disease. Brain 131, 681–689 (2008). https://doi.org/10.1093/brain/awm319.Automatic
Gerardin, E., Chételat, G., Chupin, M., Cuingnet, R., Desgranges, B., Kim, H.S., Niethammer, M., Dubois, B., Lehéricy, S., Garnero, L., Eustache, F.: Multidimensional classification of hippocampal shape features discriminates Alzheimer's disease and mild cognitive impairment from normal aging. Neuroimage 47(4), 1476–1486 (2009). https://doi.org/10.1016/j.neuroimage.2009.05.036
Al-Khuzaie, F.E.K., Bayat, O., Duru, A.D.: Diagnosis of Alzheimer disease using 2D MRI slices by convolutional neural network. Appl. Bionics. Biomech. 6690539 (2021). https://doi.org/10.1155/2021/6690539
Ocasio, E., Duong, T.Q.: Deep learning prediction of mild cognitive impairment conversion to Alzheimer’s disease at 3 years after diagnosis using longitudinal and wholebrain 3D MRI. PeerJ. Comput. Sci. 7, 1–21 (2021). https://doi.org/10.7717/PEERJ-CS.560
Ghosh, M., Raihan, M.M.S., Raihan, M., et al.: A comparative analysis of machine learning algorithms to predict liver disease. Intell. Autom. Soft Comput. 30, 917–928 (2021). https://doi.org/10.32604/iasc.2021.017989
Sharma, R., Goel, T., Tanveer, M., Murugan, R.: FDN-ADNet: fuzzy LS-TWSVM based deep learning network for prognosis of the Alzheimer’s disease using the sagittal plane of MRI scans. Appl. Soft. Comput. 115, 108099 (2022). https://doi.org/10.1016/j.asoc.2021.108099
Raghavaiah, P., Varadarajan, S.: A CAD system design for Alzheimer’s disease diagnosis using temporally consistent clustering and hybrid deep learning models. Biomed. Signal Process Control 75,103571 (2022). https://doi.org/10.1016/j.bspc.2022.103571
Khan, R., Qaisar, Z.H., Mehmood, A., et al.: A practical multiclass classification network for the diagnosis of Alzheimer’s disease. Appl. Sci. 12 (2022). https://doi.org/10.3390/app12136507
Lim, B.Y., Lai, K.W., Haiskin, K., et al.: Deep learning model for prediction of progressive mild cognitive impairment to Alzheimer’s disease using structural MRI. Front Aging Neurosci. 14, 1–10 (2022). https://doi.org/10.3389/fnagi.2022.876202
Huang, H., Zheng, S., Yang, Z., et al.: Voxel-based morphometry and a deep learning model for the diagnosis of early Alzheimer ’s disease based on cerebral gray matter changes. 1–10 (2022). https://doi.org/10.1093/cercor/bhac099
Shi, Y., Zu, C., Hong, M., et al.: ASMFS: adaptive-similarity-based multi-modality feature selection for classification of Alzheimer’s disease. Pattern. Recognit. 126, 108566 (2022). https://doi.org/10.1016/j.patcog.2022.108566
Wen, J., Thibeau-Sutre, E., Diaz-Melo, M., et al.: Convolutional neural networks for classification of Alzheimer’s disease: overview and reproducible evaluation. Med. Image Anal. 63, 101694 (2020). https://doi.org/10.1016/j.media.2020.101694
Liu, M., Zhang, J., Adeli, E., Di, S.: Joint classification and regression via deep multi-task multi-channel learning for Alzheimer’s disease diagnosis. IEEE Trans. Biomed. Eng. 66, 1195–1206 (2019). https://doi.org/10.1109/TBME.2018.2869989
Abrol, A., Bhattarai, M., Fedorov, A., et al.: Deep residual learning for neuroimaging: an application to predict progression to Alzheimer’s disease. J. Neurosci. Methods 339, 108701 (2020). https://doi.org/10.1016/j.jneumeth.2020.108701
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Kishore, N., Goel, N. (2024). Automated Classification of Alzheimer’s Disease Stages Using T1-Weighted sMRI Images and Machine Learning. In: Jha, P.K., Tripathi, B., Natarajan, E., Sharma, H. (eds) Proceedings of Congress on Control, Robotics, and Mechatronics. CRM 2023. Smart Innovation, Systems and Technologies, vol 364. Springer, Singapore. https://doi.org/10.1007/978-981-99-5180-2_28
Download citation
DOI: https://doi.org/10.1007/978-981-99-5180-2_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5520-6
Online ISBN: 978-981-99-5180-2
eBook Packages: EngineeringEngineering (R0)