Abstract
Iron (Fe) is an abundant element found in the earth’s crust and is required by organisms to perform their metabolic activity. However, its toxicity in excess amounts in the aquatic environment can negatively impact the organism’s habitat. Therefore, the extra amount of iron in the aquatic environment needs to be checked or converted into a nontoxic form to sustain the well-being of the organisms. Biologically, this can be achieved by targeting iron-degrading microbes that can degrade iron in the environment. In this chapter, we present the role of various iron-degrading bacteria and their importance in the environment. Moreover, we highlight some essential mechanisms involved in the degradation of iron through microbes. Gallionellaceae sp. in the KS culture, for example, could oxidize iron in such a way that an electron is gained through iron oxidation and transmitted through the electron transport chain (ETC) where nitrate (NO2) is reduced step by step to nitric oxide (NO). Thus, this chapter explores the possibility of key microbial groups in remediating iron-toxic environments and the underlying mechanisms involved in the process to benefit the degrading environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaerobic photosynthetic
- Acidophilic
- Aquatic environment
- Iron-degrading microbes
- Neutrophilic
- Gallionellaceae
17.1 Introduction
Iron is one of the fourth most abundant key elements and also the redox-active metal in the earth’s crust. The iron requirement in the organisms is also remarkable; without it, the microorganisms cannot conduct their metabolic activity significantly. For instance, it is involved in cellular respiration and oxygen transport (Bury et al. 2001). Although it is imperative for organisms, excess of it affects the organisms. Most countries face the problem of excess iron in their environment, such as water bodies and sediment, which subsequently lead to iron toxicity to the organism. The ability of microbes to oxidize iron may convert the negative impact of iron to the positive impact in the environment that ultimately utilized by the microbes. Therefore, in this chapter, we discuss the key role of iron-degrading bacteria in nature and their importance in the aquatic environment. In addition, we also highlight some important mechanisms concerned in the degradation of iron by using microbes.
17.2 Toxicity of Iron
The toxicity of iron to organisms, particularly fish, can be broadly classified as acute or chronic depending on the time and strength of the toxic substance that the organisms are exposed to. While the first addresses a brief duration with a greater concentration of the toxic substance that causes adverse effects and leads to mortality in organisms, and the second addresses a prolonged exposure with a lower concentration of the agent that causes gradual adverse effects in the organism. Many researchers have studied iron toxicity to organisms, including fishes. For instance, according to Bury et al. (2001), the ferrous form (Fe2+) is more harmful to European plaice (Platichthys flesus) than the ferric form (Fe3+), and iron intake occurs largely in the latter section of the gut. The iron toxicity in the fish mainly affects the fish’s gills, subsequently causing respiratory problems (Zahedi et al. 2014). In addition, animals on iron-rich diets may experience stunted development, low feed conversion, food refusal, death, and histopathological injury in the liver cells, which retain excessive iron (Bury and Grosell 2003). Fish can be employed as ecological indicators, displaying physiological changes including opercular beating, abnormal swimming, gill modifications, and feeding changes or problems responding to changes in the aquatic ecosystem (Hundley et al. 2018). According to Gemaque et al. (2019), both the forms of iron ions (Fe2+ and Fe3+) are detrimental to the “piau” (Leporinus friderici), with Fe2+ being the most toxic.
17.3 Classification
Iron-degrading bacteria may be classified broadly into two groups: iron-reducing bacteria (IRB) and iron-oxidizing bacteria (IOB), based on their potential to reduce and oxidize iron, respectively, in the nature. While the first one applies to the ability of the bacteria to convert ferric (Fe3+) ions to ferrous (Fe2+) ions under both aerobic and anaerobic environments, the latter one is the ability of the bacteria to transform ferrous ions to ferric (Fe3+) ions under anaerobic and/or microaerobic conditions (Ebrahiminezhad et al. 2017). The majority of IOB are found in a variety of phyla within the bacteria domain, along with Firmicutes and Nitrospirae, the lion’s share being Proteobacteria (Hedrich et al. 2011). Moreover, IOB may be classified into four classes based on their physiological diversity: (a) acidophilic, aerobic iron oxidizers; (b) neutrophilic, aerobic iron oxidizers; (c) aerobic iron oxidizers; and (d) anaerobic photosynthetic iron oxidizers. With the exception of nitrate-dependent iron oxidizers, the majority of the species in this phylum belong to one of the Proteobacteria classes. Furthermore, the Zetaproteobacteria are iron-oxidizing neutrophilic chemolithoautotrophs found in estuarine and marine settings all over the world.
17.3.1 Acidophilic, Aerobic Iron-Oxidizing Proteobacteria
These groups play an essential role in creating acidic and metal-fortified mine drainage waters. Acidophilic prokaryotes utilize the pH gradient across their cell membranes to drive ATP synthesis through F1F0 ATP synthase, but the accompanying proton influx is balanced by gaining electrons from the oxidation of ferrous iron (Hedrich et al. 2011). For example, Acidithiobacillus ferrooxidans, Acidithiobacillus ferrivorans, Acidiferrobacter thiooxydans, Thiobacillus prosperus, Mariprofundus ferrooxydans, Ferrovum myxofaciens, and Thiomonas spp are included in this group of proteobacteria (Colmer et al. 1950; Hallberg et al. 2010; Hedrich et al. 2011).
17.3.2 Neutrophilic, Aerobic Iron-Oxidizing Proteobacteria
These are the stalk-forming organisms where they divide at a limited partial pressure of oxygen in anti-gradients of oxygen and Fe2+, viz. Crenothrix, Ferritrophicales, Ferritrophicum radicicola, Gallionella, Leptothrix cholodnii, Leptothrix discophora, Leptothrix mobilis, Metallogenium, Siderocapsa, Sideroxydans spp., Sideroxydans, Sideroxydans sp. ES-1, Sideroxydans paludicola, and Sphaerotilus natans (Emerson and Moyer 1997). Transmission electron microscopy of Gallionella ferruginea or Mariprofundus ferrooxydans revealed that stalks are made up of certain fibrils, which accommodate few millimicrometer-sized iron oxyhydroxide crystals (Chan et al. 2011). In 1838, Gallionella ferruginea was first proclaimed by Ehrenberg as a leading neutrophilic iron oxidizer, where it can grow mixotrophically or autotrophically utilizing an electron donor (ferrous iron) (Hallbeck and Pedersen 1991). Mariprofundus ferrooxydans is an autotrophic, mesophilic, marine iron oxidizer, which has an immediate morphological resemblance to G. ferruginea, although phylogenetically it is amazingly isolated from the freshwater bacterium and is related to Zetaproteobacteria (Emerson et al. 2010). In addition, it was announced that such heterotrophic Pseudomonas or Pseudoalteromonas-like gammaproteobacteria separated from a seamount of volcanic sands under microaerobic conditions could catalyze ferrous iron oxidation and consequently lead to the development of iron mats in the deep seas (Sudek et al. 2009).
17.3.3 Neutrophilic, Anaerobic Iron Oxidizers
These groups have been created in freshwater, brackish, marine, and anaerobic sediments. Microbes that have a characteristic to oxidize iron and reduce nitrate under anaerobic conditions are classified as autotrophic and heterotrophic. These unique bacteria may thrive in organic acids when nitrate or oxygen served as an electron acceptor (Straub et al. 1996) such as Acidovorax sp., Aquabacterium, Dechlorosoma suillum strain PS, Dechloromonas spp., and Geobacter (Chaudhuri et al. 2001). In the presence of nitrate, Thiobacillus denitrificans also oxidized iron sulfide (FeS) (Straub et al. 1996). Comprehensive research is still being done to convert nitrate to nitrogen gas under anoxic conditions, in a process known as “nitrate-dependent anaerobic ferrous oxidization (NAFO)” with the help of ferrous iron as the electron donor (Su et al. 2017; Zhang et al. 2015). This is because iron is the most redox-active metal in organisms, sediment, and soil (Li et al. 2014).
Pseudogulbenkiania strain 2002, an isolate from a sediment of a freshwater lake, could reduce nitrate and oxidize ferrous iron during the time of growing as an autotroph (Weber et al. 2006), but it can also grow under heterotrophic conditions on a variety of organic substances. 16S rRNA gene sequence analysis disclosed that this isolate was nearly linked to the beta proteobacterium, Pseudogulbenkiania subflava (99.3% sequence similarity) (Weber et al. 2009). Surprisingly, the discovery of iron-oxidizing proteobacteria explained nitrate-dependent iron (Fe2+, ferrous) oxidation by the stringent anaerobe Geobacter metallireducens (Finneran et al. 2002). Moreover, Raiswell and Canfield (2012) proposed that, anaerobically, ferrous form Fe2+ is oxidized with nitrate in the nitrate-containing oxygen minimum zones (OMZs) of present sea.
Anaerobic iron oxidation with nitrate (NO3) produces nitrite (NO2−), nitrogenous gases (nitrogen [N2] or nitrous oxide [N2O]), or ammonium (NH4+) (Carlson et al. 2013), and a range of authigenic Fe minerals, based on surrounding water chemistry and pH (Carlson et al. 2013), for example,
In the presence of nitrite, such as via heterotrophic nitrate reduction, iron oxidation accompanied by nitrite in Eq. (17.2) has been demonstrated to occur abiotically at around neutral pH, peculiarly when reactive Fe oxyhydroxide mineral surfaces are accessible to catalyze the process (Picardal 2012). The phrase “nitrate-dependent iron oxidation” refers to the microbial and partially abiotic mechanisms with the aid of ferrous iron oxidized using nitrate as the terminal electron acceptor (Picardal 2012; Klueglein et al. 2014).
Nitrate-dependent iron oxidation has been occurred in a diverse freshwater and marine sediments and also shown in laboratory cultures (Straub et al. 1996; Scholz et al. 2016). This might be a crucial mechanism in biogeochemical cycling, especially in OMZs. Despite the fact that sediments under the Peru OMZ discharge large quantities of iron into the anoxic column of water (Noffke et al. 2012), primary production in the eastern equatorial Pacific high-nitrate-low-chlorophyll (HNLC) zone off Peru has been demonstrated to be iron-restricted (Hutchins et al. 2002). Furthermore, ample of iron liberated from the sediments of Peru margin is restored and buried adjacent to its origin rather than being moved offshore within the OMZ (Scholz et al. 2014). Both of these surprising data might be explained by nitrate-dependent iron oxidation inside the anoxic column of water (Scholz et al. 2016).
17.3.4 Anaerobic Photosynthetic Iron (Fe) Oxidizers
The first proof that microbes might oxidize ferrous iron (Fe2+) in anaerobic settings came from phototrophic purple proteobacteria. The class Alphaproteobacteria is placed in the majority of iron-oxidizing phototrophs that have been discovered. Thiodictyon strain L7, a gammaproteobacterium, is the striking exception. This type of bacterium uses ferrous iron as a cause of carbon dioxide reductant, as revealed in the following equation, where CH2O represents fixed biomass carbon:
At the same time, the majority of photosynthetic microbes that oxidize ferrous iron consume such a reaction for the assimilation of carbon; it can further be utilized as a detoxification mode of action. Moreover, phototrophic iron oxidizers can employ soluble ferrous iron and other minerals, namely FeS or ferrous carbonate (FeCO3), as origins of reductants although they are not allowed to access ferrous iron in more crystalline minerals like magnetite (Fe3O4) or pyrite (FeS2) (Kappler and Newman 2004). So far, most of the identified and validated phototrophic iron oxidizers have been placed under the thoroughly varied family of Rhodobacteraceae within the class of Alphaproteobacteria including aerobic and anaerobic facultative heterotrophs, facultative methylotrophs, fermentative bacteria, and photoheterotrophs (Imhoff 2005).
Rhodobacter sp. strain SW2, the foremost iron-oxidizing phototroph identified, oxidizes ferrous iron exclusively when supplied with an organic carbon origin and also uses hydrogen (H2) and organic molecules (Ehrenreich and Widdel 1994). Additional iron-oxidizing photosynthetic purple bacteria in the Rhodobacteraceae family comprise Rhodovulum iodosum and Rhodovulum robiginosum; both of them are found in marine environments and oxidize sulfide and ferrous iron when fed an organic cosubstrate like acetic acid (Hedrich et al. 2011). Heising and Schink (1998) revealed that a phototrophic isolate of Rhodomicrobium vannielii, a heterotrophic nonsulfur purple bacterium from the Hyphomicrobiaceae family, can oxidize ferrous iron, a trait that was later validated in the species’ type strain. Tentatively, Widdel et al. (1993) had already identified Rm. vannielii as the sole of the iron-oxidizing phototrophic isolates from freshwaters they had collected. Acetate or succinate was introduced to strain BS-1 as cosubstrates to promote growth when ferrous iron was present. According to Heising and Schink (1998), ferrous Fe oxidation is just a small act for Rm. Vannielii strain BS-1. Alternatively, the Hyphomicrobiaceae species, Rhodopseudomonas palustris strain TIE-1, segregated from an iron-rich mat was utilized as a model species for a genetic research by Jiao et al. (2005). The two photosynthetic iron-oxidizing gammaproteobacteria of Thiodictyon strains have been reported. One of the two, strain L7, was desegregated from the identical origin as Rhodobacter sp. SW2 (Ehrenreich and Widdel 1994), while the other Thiodictyon sp. strain f4 was from a marsh (Croal et al. 2004). In addition, among all the isolated phototrophic iron-oxidizing bacteria, Thiodictyon sp. strain f4 shows iron oxidation at elevated rates (Hegler et al. 2008). However, Widdel et al. (1993) observed no one at all of the confirmed Thiodictyon spp. they experimented with which could oxidize ferrous iron, implying that such a feature is unusual in this genus.
17.4 Iron-Reducing Bacteria’s Role
In the sediment column, the presence of anaerobic dissimilatory Fe(III) iron-reducing bacteria makes distinguishing the type of reactive iron challenging (Lovley and Phillips 1986). These organisms seem to be present in both bacteria and archaea and have a broad phylogenetic range (Kashefi and Lovley 2003). The Geobacteraceae, a wide phylogenetically coherent group that oxidizes acetate to carbon dioxide (CO2) using Fe(III) as the only electron acceptor (Lonergan et al. 1996), and also H2-oxidizing Fe(III) reducers including Shewanella putrefaciens and S. alga, are among them (Lonergan et al. 1996). It was proved that Fe(III) reducers may decrease Fe(III) sheet silicates and magnetite (Kostka et al. 1999). Because Fe sheet silicates and magnetite are resistant to inorganic acid leaching, they are normally allocated as unreactive or weakly reactive iron phases in sequential leaching systems. Numerous sulfate-reducing bacteria (SRB) are linked to Fe(III)-reducing bacteria (FeRB) on a phylogenetic level, and many Geobacteraceae species reduce S(0). The sulfate and iron reducers may be an element of a compact ecosystem as the FeRB uses acetate, which is a typical by-product of SRB metabolism. As a result, these organisms are expected to have a big influence on the iron in the sediment’s oxic and transition zones. The proportion of Fe(III) that is reduced by iron-reducing bacteria and reacting with the Fe(II) produced will be determined by the relative rates of the bacterial reduction reactions and sulfidation and the time spent by Fe(III) before being exposed to S(-II) in the bacterial iron-reducing subenvironment. Sequentially, this will change depending on sedimentation characteristics such as the degree of advection in the column of water, sediment grain size, the concentration of organic matter, and the velocity of sedimentation (Rickard 2012).
Moreover, the Fe-reducing bacteria may play a vital role in oil degradation. For instance, ferric iron is used by the hyperthermophile Ferroglobus placidus to consume benzene at 85 °C (Holmes et al. 2011; Waikhom et al. 2020). In oil field brine enrichment cultures, iron-reducing bacteria have been detected (Magot 2005); nevertheless, the availability of iron oxide surfaces may restrict the degree of oil biodegradation in the deep below. In sandstones, Fe(III) is found in the range of 0.4–4.0 oxide weight percent on pore filling or grain coating material (Pettijohn 1963). Although the energy gained by Fe(III) reduction is equivalent to aerobic respiration or NO3 reduction, the oxidant amount required is significantly more. According to the volume, around 10 L of hematite would be required to biodegrade 1 L of hydrocarbon approximately. Due to the inadequate biological availability of ferric iron in hematite, such a procedure would be extremely slow, made even more difficult by the fact that hematite is frequently destroyed in course of diagenesis, and the majority of the iron in sedimentary reservoir rocks is constrained in surprisingly less reactive silicates and sulfides (Prince and Walters 2016).
17.5 Mechanism of Iron Oxidation
17.5.1 Mechanisms
Mechanisms utilized by anaerobically nitrate-reducing iron oxidation (NRFeOx) bacteria to oxidize Fe(II) are yet to be more explored, even though it appears to have different mechanisms, even if the bacteria are autotrophs, chemodenitrifiers, or mixotrophs.
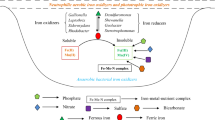
For autotrophic NRFeOx bacteria, three mechanisms have been put forward for Fe(II) oxidation (Fig. 17.1) such as (a) a devoted Fe(II) oxidoreductase, (b) a nonspecific performance of the nitrate reductase, or (c) the bc1 complex that receives electrons from Fe(II) and passages down the quinone pool (Bryce et al. 2018). The first scenario has dominated research in the recent years, intending to discover a particular outermost membrane Fe(II) oxidoreductase seen in these bacteria (Beller et al. 2013). Metagenomics investigation of the NRFeOx in the KS culture revealed homologs of the cytochrome c putative Fe(II) oxidase Cyc2, which have been detected in other identified and validated Fe(II) oxidizers in the draft genomes of Gallionellaceae sp. and Rhodanobacter sp. observed in KS culture (He et al. 2016).
The existing hypotheses on the mechanism of iron oxidation in (a) Gallionellaceae species, considered autotrophic nitrate reducers and Fe(II) oxidizers in the KS culture and (b) the reduction of nitrate might be mediated by Nap in place of Nar, and the reduction of nitric oxide could be aided by NorZ instead of NorC. Notably, NorZ utilizes electrons from quinols as opposed to cytochrome c (Reprinted from Bryce et al. (2018), copyright 2022 Society for Applied Microbiology, with permission from John Wiley and Sons). Abbreviations: OM, outer membrane; IM, inner membrane; Cyt c, cytochrome c; NO, nitric oxide; N2O, nitrous oxide; NAD, Nicotinamide adenine dinucleotide
Furthermore, the Gallionellaceae sp. and Dryobalanops aromatica RCB in the KS culture, which was suspected to be autotrophic but was not confirmed, both include homologs of the porin cytochrome c porin complex MtoAB (He et al. 2017). Figure 17.1a depicts a possible process for the autotrophic Fe(II) oxidizer to oxidize Fe(II) in the KS culture, in which an electron is gathered through Fe(II) oxidation and transmitted through the ETC, where nitrate is reduced step by step to nitric oxide (NO). NO has the ability to be absorbed by the surrounding population or to react with aqueous Fe(II) outside the cell.
Some NRFeOx bacteria may undergo an enzymatic process to oxidize Fe(II), but whether or not these organisms are real mixotrophs is still up for debate. Many people assume that Fe(II) oxidation is powered by denitrification (Brons et al. 1991). It has been hypothesized by Carlson et al. (2013) that all heterotrophic denitrifiers can increase nitrate-dependent Fe(II) oxidation. Several nitrate reducers, including Escherichia coli, can oxidize an organic molecule when it is combined with Fe(II) (Brons et al. 1991).
Although the rate of dissolved Fe(II)-mediated abiotic nitrate reduction by nitrite to N2O is a slow process, the rate of Fe(II)-mediated nitrite reduction to N2O is kinetically advantageous under environmental conditions if reactive chemical substrates act as catalysts (Colman et al. 2008). Heterogeneous surface catalysis, for instance, can decrease nitrogen species by Fe(II) on viable surfaces such as cell surfaces, green rust, crystalline Fe(III) oxyhydroxides, and pyrite (Bryce et al. 2018). Many researchers have shown that microbially driven NRFeOx, heterotrophic nitrate reduction, and Fe(III)-coupled ammonium oxidation, that is, iron-ammox can result in the development of reactive nitrogen species (nitrite, NO2−, or nitric oxide [NO]) as a metabolic intermediate (Picardal 2012), providing a sufficient stock of compounds that could react rapidly with Fe(II) (Bryce et al. 2018).
17.5.2 Mechanisms of Ferrous Iron Oxidation by Photoferrotrophs
The insight mechanisms engaged in Fe(II) oxidation by phototrophic anoxygenic Fe(II)-oxidizing bacteria are yet a mystery. However, Rp. palustris TIE-1 has been the subject of the most scientific research for the process of phototrophic bacterial Fe(II) oxidation. Besides, the pioABC operon, which stands for “photosynthetic Fe(II) oxidation,” is assumed to be essential for electron transport by Fe(II) oxidation in this species. This three-gene operon encodes three proteins: PioA, PioB, and PioC (Jiao and Newman 2007), where PioA is a periplasmic decaheme c-type cytochrome, PioB is an outer membrane beta-barrel protein, and PioC is a periplasmic, strong potential iron–sulfur cluster protein. MtrA and MtrB, which are expressed by the Fe(III) reducer Shewanella oneidensis MR-1, are the same as PioA and PioB. (Jiao and Newman 2007). Iro, the putative Fe(II) oxidoreductase of A. ferrooxidans, is linked to PioC. Rp. palustris TIE-1 carries electrons from PioA to PioC, which gives them to the bc1 complex. The electrons may be shifted to the phototrophic reaction center in the inner membrane, according to some studies (Bird et al. 2014). The pioABC operon in Rhodomicrobium vannielii acts similarly to the operon in Rp. palustris TIE-1 (Bryce et al. 2018).
In Rp. palustris TIE-1, the deletion of pioA consequences in nearly full loss of Fe(II)-oxidizing capacity, although the deletion of PioB and PioC consequences in only partial loss when correlated to the wild type (Bose and Newman 2011). When Fe(II) is utilized as an electron donor, the pio genes are expressed at their highest levels, but they are transcribed and translated under all anoxic growth conditions. The global regulator FixK controls the expression of the pio operon (Bose and Newman 2011). Supplementary clue in what way Fe(II) influences cellular activities in Rp. palustris TIE-1 has come from transcriptome and proteome analyses. These findings reveal that high levels of Fe(II) cause stress reactions unexpectedly in anoxic settings, despite the fact that Fe(II) toxicity caused by oxidative stress created by the Fenton’s reaction is not be a concern. The induction of many metal efflux pathways characterizes the cellular response, which was observed throughout both short- and long-term periods of time (Bird et al. 2013). The evidence of PioA’s location in the cell is currently contradictory. Based on the sequencing results, Jiao and Newman (2007) suggested that PioA appears to be a periplasmic protein. Nevertheless, Bose et al. (2014) revealed that Rp. palustris TIE-1 can oxidize the crossed-valent Fe(II)–Fe(III) mineral magnetite (Fe3O4) in the solid phase and utilize electrons straight from positioned electrodes. PioA can oxidize Fe(II) with the association of its electron transport mechanism, which must thus be existing on the cell’s outer membrane, according to this discovery. Furthermore, Rp. palustris TIE-1 can only approach surface-bound Fe(II) in magnetite, emphasizing the necessity for a straight surface–mineral interaction mechanism (Byrne et al. 2015, 2016). Rhodobacter ferrooxidans SW2 is another well-studied “anoxygenic phototrophic Fe(II) oxidation” pathway. This organism’s foxEYZ operon permits it to oxidize Fe(II) (Croal et al. 2007).
The genomic sequencing of Chlorobium phaeoferrooxidans reveals that these green sulfur bacteria have yet another mechanism. In Mariprofundus ferrooxidans PV-1, which is a microaerophilic Fe(II) oxidizer, the outer membrane cytochrome (Cyc2PV-1) encoded by this genome is thought to be engaged in Fe(II) oxidation (Crowe et al. 2008). Cyc2PV-1 is a far relative of Cyc2, a lithotrophic Fe(II)-oxidizing bacteria found in a broad range of obligatory lithotrophic bacteria (He et al. 2017). Besides, Cyc2 is encoded in the genome of C. ferrooxidans DSM13031 (He et al. 2017). The electron transport pathways and proteins involved in Fe(II) oxidation are likely varied, and no one mechanism exists across every single one of the physiological groups of Fe(II) oxidizers, neither within a single group of Fe(II) oxidizers, like phototrophs (Bryce et al. 2018).
17.6 Environmental Significance
The NRFeOx mechanism aids biochemically and microbially driven redox shifts of iron and nitrate species (Liu et al. 2019). The potency and remodeling of pollutants like chlorinated organic compounds and heavy metals (arsenic [As], antimony [Sb], and uranium [U]) are created by the “Fe III–Fe II redox wheel.”
Nitrate reduction is intimately linked to N pollution in numerous natural and anthropogenic systems of waters, and the generation of N2O (greenhouse gas) has the ability to affect global climate change.
As a result, an in-depth investigation of the redox reactions that may occur in the middle of reactive Fe and N species will assist in an improved insight into the Fe–N cycle in nature. It has long been understood that immobilizing these pollutants requires the absorption of heavy metals and radioactive nuclides onto Fe and manganese (Mn) oxides (Means et al. 1978a, b). A practical bioremediating technique for heavy metals including iron and radioactive nuclide fixation in reducing settings might be devised depending on the reported anaerobic NRFeOx employing Dechlorosoma species. The findings show that the oxidation of Fe(II) by D. suillum is a unique strategy for heavy metal and radionuclide stabilization and immobilization in the ecosystem. Senn and Hemond (2002) revealed that nitrate altered the arsenic (As) cycling in anoxic circumstances in an urban Upper Mystic Lake located in Massachusetts, the United States, by oxidizing Fe(II) to generate arsenic-adsorbing particulate hydrous iron oxides and creating the oxidation of As(III) to As(V).
After large-scale Fe(II) oxidation started, arsenate was discarded from the water adjacent to the injection at the U.S. Geological Survey (USGS) test site on Cape Cod, Massachusetts, the United States, by integration into hydrous Fe oxide, which was precipitated in the iron-reduction zone in a sandy aquifer under an anoxic condition (Senn and Hemond 2002; Höhn et al. 2006), revealing the significance of NRFeOx to arsenic immobilization. The inclusion of nitrate in paddy field soils decreased the Fe(II) concentration in the soil solution while increasing NRFeOx bacterial density, resulting in considerably reduced dissolved Fe(II) concentrations in the rhizosphere soil solution (Liu et al. 2019). It was proposed that NRFeOx bacteria activation might result in arsenic coprecipitation with or adsorption to Fe(III) ions in the soil, followed by reduced arsenic absorption utilizing rice plants (Chen et al. 2008). The findings of continuous-flow sand-filled columns revealed that microbial oxidation of arsenate and Fe(II) associated with denitrification boosted aqueous As immobilization in anaerobic conditions by creating hydrous iron oxides–coated sands with adsorbed pentavalent arsenic (Sun et al. 2009). Despite the fact that it does not affect the anaerobic NRFeOx bacteria metabolism, arsenate efficiently immobilized arsenic during Fe(II) oxidation (efficient for more than 96%), lowering the residual dissolved arsenic concentrations to levels approaching or even below the acceptable limit of drinking water of 10 μg/L (Hohmann et al. 2010). The ability of a single anaerobic NRFeOx Citrobacter freundii strain PXL1 to reduce and remove As(III) and nitrate from water synchronously was investigated, and it was disclosed that these bacteria are potential microorganisms for in situ remediation of arsenite- and nitrate-contaminated groundwater in China (Li et al. 2015). The concurrent usage of Fe(II) and nitrate successfully reduced the inflation of arsenic (As) in rice paddy plants by intensifying arsenic oxidation or immobilization intervened by biotic or abiotic iron redox transformation and mineralization (Wang et al. 2018), and the conclusions delivered an understanding about the As/Fe/N biogeochemical cycles, which are essential from the perspective of agricultural crop management activity of arsenic toxicity and its mitigation in arsenic-affected area.
Even though nitrate respiration has long been established as a general process in microbes (Ducluzeau et al. 2009), little has been written about the effects of iron oxidation on nitrate reduction and releasing of a greenhouse gas (N2O) due to a lack of understanding of nitrogen cycle through abiotic and biotic procedures. However, these abiotic N2O generation mechanisms have been recognized since the nineteenth century; they are typically disregarded in contemporary environmental studies, making it critical to analyze their importance (Zhu-Barker et al. 2015). Following an early idea (the ferrous wheel theory) for abiotic nitrate immobilization in forest soils, reactive Fe(II) species might convert nitrate to nitrite. Nitrite was then combined alongside dissolved organic materials to form dissolved organic nitrogen (Davidson et al. 2003). Despite the fact that the abiotic and biotic interactions were unclear, the data highlighted the realness activity and significance of NRFeOx in the renowned N cycle scenario. The potential of iron-dependent nitrogen cycling in riparian forest sediments also discussed the Fe–N interaction (Clement et al. 2005). Under a variety of environmental circumstances, dual (N and O) isotope structures were used to investigate the reduction of abiotic nitrite by Fe(II). The findings showed that Fe(II)-mediated nitrite reduction could be a significant origin of abiotic environmental N2O, particularly in an iron-rich environmental condition with potent redox fluctuations (Buchwald et al. 2016).
17.7 Bioremediation
Various biological approaches, like bioremediation including phytoremediation, have been used to remediate environmental toxins, and they are not only cost-effective but also environmentally beneficial. Bioremediation is a broad word that refers to the transformation of harmful or complicated pollutants into simpler, less toxic forms, or even full degradation, using biological mechanisms including bacteria or microbial products (Datta et al. 2020; Singh et al. 2021). The process of eliminating pollutants from the environment in the absence of the use of chemicals is known as intrinsic bioremediation (Lovley 2003). By altering the environment in which the microorganisms are grown at the polluted location, microbial activity can be increased to achieve higher clean-up rates. The bioremediation technique is regarded as designed in these circumstances (Mishra et al. 2021).
Iron oxidation occurs in most aquatic habitats that include elemental or reduced forms of iron, that is, ferrous iron (Eggerichs et al. 2020). Microorganisms that accelerate iron oxidation gain the energy they need to multiply when they use either nitrate or oxygen as an electron acceptor (Emerson et al. 2010). Iron oxidation of microorganisms can be divided into four categories that have been already mentioned above somewhere in the content. Zagury et al. (1994) demonstrated heavy metal removal by native IOB from polluted soil. Bacteria such as Acidithiobacillus ferrooxidans can be used to reduce the soil solution pH lower than 2.5 and to increase the oxidation–reduction potential at room temperature when adjusted to a pH of 4. Iron oxidation through such bacteria was accelerated by the addition of ammonium sulfate [(NH4)2SO4] and potassium phosphate (K2HPO4). Zinc and manganese had higher percent removal rates than copper. Metal speciation, together with the soil types studied, affected metal removal by IOB. A potential technique for the efficient removal of arsenic from groundwater was discovered to be the biotic oxidation of iron by the bacteria Gallionella ferruginea and Leptothrix ochracea, which offered an economical and environmentally beneficial alternative. The microorganisms and iron oxides that were formed in the upflow filtration columns, during this process, provide an ideal environment for the absorption and arsenic removal from the aqueous streams (Katsoyiannis and Zouboulis 2004). Blais et al. (1993) described their research on the function of IOB in the sequestration of toxic metals from sewage sludge. Iron-oxidizing microorganisms lower the pH of sewage sludge and increase its oxidation–reduction potential. These were found to be the metals removed from sludge by A. ferrooxidans (previously known as Thiobacillus ferrooxidans) in the following ascending order: manganese, zinc, nickel, cadmium, copper, lead, and chromium, with the sludge form used during the experiment having a little effect. Xiang et al. (2000) conducted another batch of investigation aimed at the heavy metal removal from sewage sludge utilizing indigenous IOB. When iron was supplemented to inoculated sets as the ferrous sulfate form, the sludge pH fell from 2.0 to 2.5. Following 16 days of treatment with iron oxidizers, there was a significant decline in the metal contents (chromium, copper, lead, nickel, and zinc) available in sewage sludge, attaining agriculturally acceptable levels, indicating that biological agents can be used to remediate metal contaminants. Recently, the contribution of microaerophilic IOB in arsenic (As) removal was reported by Tong et al. (2019) through the adsorbed/immobilized of arsenite and arsenate that resulted in ferric oxyhydroxide formation following the prominent activity of iron oxidizers with a significant arsenic adsorption affinity. The As content was reduced from 600 to 4.8 g/L following iron oxidizer treatment. The presence of an arsenite oxidase gene, which is accountable for the formation of arsenite from arsenate in microbes, was also discovered in the study. The generation of biogenic iron oxides as a result of the iron oxidation by the bacteria Gallionella and Leptothrix has a tremendous potential for phosphate elimination from the solution phase (Buliauskaite et al. 2020). Moreover, Gallionella sp. has been shown to have an overall phosphate removal capacity than Leptothrix sp. When compared to chemically manufactured iron oxides, biologically derived iron oxides had a greater phosphate sequestration performance, implying that they could be used in phosphate recovery treatment systems. Adsorption and precipitation play a substantial role in phosphate elimination by biogenic iron oxides, according to their findings. Another recent study showed that IOB can fortunately bioremediate groundwater contaminated with prominent levels of iron and manganese (Aziz et al. 2020). Not long ago, the ability of bacteria A. ferrooxidans to extract heavy metals (chromium, copper, nickel, and zinc) from electroplating sludge under low-voltage stimulation paves the door for industrial-scale metal contamination treatment (Wu et al. 2020). Under laboratory conditions with a pH of 2.0–3.0 and Fe concentrations near 11 g/L, A. ferrooxidans catalyzes the synthesis of crystalline biogenic tooeleite, which is an iron-arsenic-based mineral and is thought to remove roughly 95% of As(III) (Li et al. 2020a, b). The research revealed key principles driving biogenic mineral formation, which could lead to the advancement of a treatment system that uses rapidly proliferating IOB to decontaminate As-damaged environments (Li et al. 2020a, b; Singh et al. 2021).
17.8 Future Direction and Conclusion
In the future, nitrate-reducing bacteria may be a valuable microbe for the Biofloc Technology of fish culture to reduce nitrate, which is a severe concern in the system. However, more research into this topic is required. Besides, the pathogenic nature of these bacteria needs to be thoroughly studied to avoid any disease outbreak in the future. Moreover, bioremediation with iron-degrading bacteria can minimize iron toxicity, which is both economical and ecologically sound. Furthermore, this technology will be a boon. However, it is critical to investigate all of the mechanisms involved in the process. Furthermore, the microorganisms must be completely defined before they can be deployed in the bioremediation of iron in an environment where in situ bioremediation has limited. In addition, the remediation of environments contaminated by metalloids, metals, and hazardous organics may benefit from the use of IOB that are resilient to greater metal concentrations and can swiftly adapt to changing natural environmental circumstances.
References
Aziz HA, Tajarudin HA, Wei THL, Alazaiza MYD (2020) Iron and manganese removal from groundwater using limestone filter with iron-oxidized bacteria. Int J Environ Sci Technol 17(5):2667–2680. https://doi.org/10.1007/s13762-020-02681-5
Beller HR, Zhou P, Legler TC, Chakicherla A, Kane S, Letain TE, O’Day PA (2013) Genome-enabled studies of anaerobic, nitrate-dependent iron oxidation in the chemolithoautotrophic bacterium Thiobacillus denitrificans. Front Microbiol 4:249. https://doi.org/10.3389/fmicb.2013.00249
Bird LJ, Coleman ML, Newman DK (2013) Iron and copper act synergistically to delay anaerobic growth of bacteria. Appl Environ Microbiol 79(12):3619–3627. https://doi.org/10.1128/AEM.03944-12
Bird LJ, Saraiva IH, Park S, Calçada EO, Salgueiro CA, Nitschke W, Louro RO, Newman DK (2014) Nonredundant roles for cytochrome c 2 and two high-potential iron-sulfur proteins in the photoferrotroph Rhodopseudomonas palustris TIE-1. J Bacteriol 196(4):850–858. https://doi.org/10.1128/JB.00843-13
Blais JF, Tyagi RD, Auclair JC (1993) Metals removal from sewage sludge by indigenous iron-oxidizing bacteria. J Environ Sci Health A 28(2):443–467. https://doi.org/10.1080/10934529309375888
Bose A, Newman DK (2011) Regulation of the phototrophic iron oxidation (pio) genes in Rhodopseudomonas palustris TIE-1 is mediated by the global regulator, FixK. Mol Microbiol 79(1):63–75. https://doi.org/10.1111/j.1365-2958.2010.07430.x
Bose A, Gardel EJ, Vidoudez C, Parra EA, Girguis PR (2014) Electron uptake by iron-oxidizing phototrophic bacteria. Nat Commun 5(1):1–7. https://doi.org/10.1038/ncomms4391
Brons HJ, Hagen WR, Zehnder AJ (1991) Ferrous iron dependent nitric oxide production in nitrate reducing cultures of Escherichia coli. Arch Microbiol 155(4):341–347. https://doi.org/10.1007/BF00243453
Bryce C, Blackwell N, Schmidt C, Otte J, Huang YM, Kleindienst S, Tomaszewski E, Schad M, Warter V, Peng C, Byrne JM (2018) Microbial anaerobic Fe (II) oxidation–ecology, mechanisms and environmental implications. Environ Microbiol 20(10):3462–3483. https://doi.org/10.1111/1462-2920.14328
Buchwald C, Grabb K, Hansel CM, Wankel SD (2016) Constraining the role of iron in environmental nitrogen transformations: dual stable isotope systematics of abiotic NO2− reduction by Fe (II) and its production of N2O. Geochim Cosmochim Acta 186:1–12. https://doi.org/10.1016/j.gca.2016.04.041
Buliauskaite R, Wilfert P, Suresh Kumar P, de Vet WW, Witkamp GJ, Korving L, van Loosdrecht MC (2020) Biogenic iron oxides for phosphate removal. Environ Technol 41(2):260–266. https://doi.org/10.1080/09593330.2018.1496147
Bury N, Grosell M (2003) Iron acquisition by teleost fish. Comp Biochem Physiol C Toxicol Pharmacol 135(2):97–105. https://doi.org/10.1016/S1532-0456(03)00021-8
Bury NR, Grosell M, Wood CM, Hogstrand C, Wilson RW, Rankin JC, Busk M, Lecklin T, Jensen FB (2001) Intestinal iron uptake in the European flounder (Platichthys flesus). J Exp Biol 204(21):3779–3787. https://doi.org/10.1242/jeb.204.21.3779
Byrne JM, Klueglein N, Pearce C, Rosso KM, Appel E, Kappler A (2015) Redox cycling of Fe (II) and Fe (III) in magnetite by Fe-metabolizing bacteria. Science 347(6229):1473–1476. https://doi.org/10.1126/science.aaa4834
Byrne JM, Van Der Laan G, Figueroa AI, Qafoku O, Wang C, Pearce CI, Jackson M, Feinberg J, Rosso KM, Kappler A (2016) Size dependent microbial oxidation and reduction of magnetite nano-and micro-particles. Sci Rep 6(1):1–13. https://doi.org/10.1038/srep30969
Carlson HK, Clark IC, Blazewicz SJ, Iavarone AT, Coates JD (2013) Fe (II) oxidation is an innate capability of nitrate-reducing bacteria that involves abiotic and biotic reactions. J Bacteriol 195(14):3260–3268. https://doi.org/10.1128/JB.00058-13
Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ (2011) Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J 5(4):717–727. https://doi.org/10.1038/ismej.2010.173
Chaudhuri SK, Lack JG, Coates JD (2001) Biogenic magnetite formation through anaerobic biooxidation of Fe (II). Appl Environ Microbiol 67(6):2844–2848. https://doi.org/10.1128/AEM.67.6.2844-2848.2001
Chen XP, Zhu YG, Hong MN, Kappler A, Xu YX (2008) Effects of different forms of nitrogen fertilizers on arsenic uptake by rice plants. Environ Toxicol Chem 27(4):881–887. https://doi.org/10.1897/07-368.1
Clement JC, Shrestha J, Ehrenfeld JG, Jaffé PR (2005) Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol Biochem 37(12):2323–2328. https://doi.org/10.1016/j.soilbio.2005.03.027
Colman BP, Fierer N, Schimel JP (2008) Abiotic nitrate incorporation, anaerobic microsites, and the ferrous wheel. Biogeochemistry 91(2):223–227. https://doi.org/10.1007/s10533-008-9281-9
Colmer AR, Temple KL, Hinkle ME (1950) An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. J Bacteriol 59(3):317–328. https://journals.asm.org/doi/pdf/10.1128/jb.59.3.317-328.1950
Croal LR, Johnson CM, Beard BL, Newman DK (2004) Iron isotope fractionation by Fe (II)-oxidizing photoautotrophic bacteria. Geochim Cosmochim Acta 68(6):1227–1242. https://doi.org/10.1016/j.gca.2003.09.011
Croal LR, Jiao Y, Newman DK (2007) The fox operon from Rhodobacter strain SW2 promotes phototrophic Fe (II) oxidation in Rhodobacter capsulatus SB1003. J Bacteriol 189(5):1774–1782. https://doi.org/10.1128/JB.01395-06
Crowe SA, Jones C, Katsev S, Magen C, O’Neill AH, Sturm A, Canfield DE, Haffner GD, Mucci A, Sundby B, Fowle DA (2008) Photoferrotrophs thrive in an Archean Ocean analogue. Proc Natl Acad Sci 105(41):15938–15943. https://doi.org/10.1073/pnas.0805313105
Datta S, Singh S, Kumar V, Dhanjal DS, Sidhu GK, Amin DS, Kumar S, Singh J, Singh J (2020) Endophytic bacteria in xenobiotic degradation. In: Microbial endophytes. Woodhead Publishing, Sawston, pp 125–156. https://doi.org/10.1016/B978-0-12-818734-0.00006-1
Davidson EA, Chorover J, Dail DB (2003) A mechanism of abiotic immobilization of nitrate in forest ecosystems: the ferrous wheel hypothesis. Glob Chang Biol 9(2):228–236. https://doi.org/10.1046/j.1365-2486.2003.00592.x
Ducluzeau AL, Van Lis R, Duval S, Schoepp-Cothenet B, Russell MJ, Nitschke W (2009) Was nitric oxide the first deep electron sink? Trends Biochem Sci 34(1):9–15. https://doi.org/10.1016/j.tibs.2008.10.005
Ebrahiminezhad A, Manafi Z, Berenjian A, Kianpour S, Ghasemi Y (2017) Iron-reducing bacteria and iron nanostructures. J Adv Med Sci Appl Technol 3(1):9–16. https://doi.org/10.18869/nrip.jamsat.3.1.9
Eggerichs T, Wiegand M, Neumann K, Opel O, Thronicker O, Szewzyk U (2020) Growth of iron-oxidizing bacteria Gallionella ferruginea and Leptothrix cholodnii in oligotrophic environments: Ca, Mg, and C as limiting factors and G. ferruginea necromass as C-source. Geomicrobiol J 37(2):190–199. https://doi.org/10.1080/01490451.2019.1686667
Ehrenreich A, Widdel F (1994) Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Environ Microbiol 60(12):4517–4526. https://doi.org/10.1128/aem.60.12.4517-4526.1994
Emerson D, Moyer C (1997) Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol 63(12):4784–4792. https://doi.org/10.1128/aem.63.12.4784-4792.1997
Emerson D, Fleming EJ, McBeth JM (2010) Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol 64:561–583. https://doi.org/10.1146/annurev.micro.112408.134208
Finneran KT, Anderson RT, Nevin KP, Lovley DR (2002) Potential for bioremediation of uranium-contaminated aquifers with microbial U (VI) reduction. Soil Sediment Contam 11(3):339–357. https://doi.org/10.1080/20025891106781
Gemaque T, Costa DPD, Pereira LV (2019) Evaluation of iron toxicity in the tropical fish Leporinus friderici. Biomed J Sci Tech Res 18(2):13436–13441. https://doi.org/10.26717/BJSTR.2019.18.003127
Hallbeck L, Pedersen K (1991) Autotrophic and mixotrophic growth of Gallionella ferruginea. Microbiology 137(11):2657–2661. https://doi.org/10.1099/00221287-137-11-2657
Hallberg KB, González-Toril E, Johnson DB (2010) Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 14(1):9–19. https://doi.org/10.1007/s00792-009-0282-y
He S, Tominski C, Kappler A, Behrens S, Roden EE (2016) Metagenomic analyses of the autotrophic Fe (II)-oxidizing, nitrate-reducing enrichment culture KS. Appl Environ Microbiol 82(9):2656–2668. https://doi.org/10.1128/AEM.03493-15
He S, Barco RA, Emerson D, Roden EE (2017) Comparative genomic analysis of neutrophilic iron (II) oxidizer genomes for candidate genes in extracellular electron transfer. Front Microbiol 8:1584. https://doi.org/10.3389/fmicb.2017.01584
Hedrich S, Schlömann M, Johnson DB (2011) The iron-oxidizing proteobacteria. Microbiology 157(6):1551–1564. https://doi.org/10.1099/mic.0.045344-0
Hegler F, Posth NR, Jiang J, Kappler A (2008) Physiology of phototrophic iron (II)-oxidizing bacteria: implications for modern and ancient environments. FEMS Microbiol Ecol 66(2):250–260. https://doi.org/10.1111/j.1574-6941.2008.00592.x
Heising S, Schink B (1998) Phototrophic oxidation of ferrous iron by a Rhodomicrobium vannielii strain. Microbiology 144(8):2263–2269. https://doi.org/10.1099/00221287-144-8-2263
Hohmann C, Winkler E, Morin G, Kappler A (2010) Anaerobic Fe (II)-oxidizing bacteria show As resistance and immobilize As during Fe (III) mineral precipitation. Environ Sci Technol 44(1):94–101. https://doi.org/10.1021/es900708s
Höhn R, Isenbeck-Schröter M, Kent DB, Davis JA, Jakobsen R, Jann S, Niedan V, Scholz C, Stadler S, Tretner A (2006) Tracer test with As (V) under variable redox conditions controlling arsenic transport in the presence of elevated ferrous iron concentrations. J Contam Hydrol 88(1–2):36–54. https://doi.org/10.1016/j.jconhyd.2006.06.001
Holmes DE, Risso C, Smith JA, Lovley DR (2011) Anaerobic oxidation of benzene by the hyperthermophilic archaeon Ferroglobus placidus. Appl Environ Microbiol 77(17):5926–5933. https://doi.org/10.1128/AEM.05452-11
Hundley GC, Navarro FKSP, Ribeiro Filho OP, Navarro RD (2018) Integration of Nile tilapia (Oreochromis niloticus L.) production Origanum majorana L. and Ocimum basilicum L. using aquaponics technology. Acta Sci Technol 40:e35460–e35460. https://doi.org/10.4025/actascitechnol.v40i1.35460
Hutchins DA, Hare CE, Weaver RS, Zhang Y, Firme GF, DiTullio GR, Alm MB, Riseman SF, Maucher JM, Geesey ME, Trick CG (2002) Phytoplankton iron limitation in the Humboldt Current and Peru Upwelling. Limnol Oceanogr 47(4):997–1011. https://doi.org/10.4319/lo.2002.47.4.0997
Imhoff J (2005) Rhodomicrobium Duchow and Douglas 1949, 415 AL emend. Imhoff, Trüper and Pfennig 1984, 341. Bergey’s Manual® of Systematic Bacteriology, pp 543–545
Jiao Y, Newman DK (2007) The pio operon is essential for phototrophic Fe (II) oxidation in Rhodopseudomonas palustris TIE-1. J Bacteriol 189(5):1765–1773. https://doi.org/10.1128/JB.00776-06
Jiao Y, Kappler A, Croal LR, Newman DK (2005) Isolation and characterization of a genetically tractable photoautotrophic Fe (II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl Environ Microbiol 71(8):4487–4496. https://doi.org/10.1128/AEM.71.8.4487-4496.2005
Kappler A, Newman DK (2004) Formation of Fe (III)-minerals by Fe (II)-oxidizing photoautotrophic bacteria. Geochim Cosmochim Acta 68(6):1217–1226. https://doi.org/10.1016/j.gca.2003.09.006
Kashefi K, Lovley DR (2003) Extending the upper temperature limit for life. Science 301(5635):934–934. https://doi.org/10.1126/science.1086823
Katsoyiannis IA, Zouboulis AI (2004) Biological treatment of Mn (II) and Fe (II) containing groundwater: kinetic considerations and product characterization. Water Res 38(7):1922–1932. https://doi.org/10.1016/j.watres.2004.01.014
Klueglein N, Zeitvogel F, Stierhof YD, Floetenmeyer M, Konhauser KO, Kappler A, Obst M (2014) Potential role of nitrite for abiotic Fe (II) oxidation and cell encrustation during nitrate reduction by denitrifying bacteria. Appl Environ Microbiol 80(3):1051–1061. https://doi.org/10.1128/AEM.03277-13
Kostka JE, Wu J, Nealson KH, Stucki JW (1999) The impact of structural Fe (III) reduction by bacteria on the surface chemistry of smectite clay minerals. Geochim Cosmochim Acta 63(22):3705–3713. https://doi.org/10.1016/S0016-7037(99)00199-4
Li B, Tian C, Zhang D, Pan X (2014) Anaerobic nitrate-dependent iron (II) oxidation by a novel autotrophic bacterium, Citrobacter freundii strain PXL1. Geomicrobiol J 31(2):138–144. https://doi.org/10.1080/01490451.2013.816393
Li B, Pan X, Zhang D, Lee DJ, Al-Misned FA, Mortuza MG (2015) Anaerobic nitrate reduction with oxidation of Fe (II) by Citrobacter Freundii strain PXL1–a potential candidate for simultaneous removal of As and nitrate from groundwater. Ecol Eng 77:196–201. https://doi.org/10.1016/j.ecoleng.2015.01.027
Li Q, Zhang M, Yang J, Liu Q, Zhang G, Liao Q et al (2020a) Formation and stability of biogenic tooeleite during Fe (II) oxidation by Acidithiobacillus ferrooxidans. Mater Sci Eng C 111:110755. https://doi.org/10.1016/j.msec.2020.110755
Li X, Qiao J, Li S, Häggblom MM, Li F, Hu M (2020b) Bacterial communities and functional genes stimulated during anaerobic arsenite oxidation and nitrate reduction in a paddy soil. Environ Sci Tech 54(4):2172–2181. https://doi.org/10.1021/acs.est.9b04308
Liu T, Chen D, Li X, Li F (2019) Microbially mediated coupling of nitrate reduction and Fe (II) oxidation under anoxic conditions. FEMS Microbiol Ecol 95(4):fiz030. https://doi.org/10.1093/femsec/fiz030
Lonergan DJ, Jenter HL, Coates JD, Phillips EJ, Schmidt TM, Lovley DR (1996) Phylogenetic analysis of dissimilatory Fe (III)-reducing bacteria. J Bacteriol 178(8):2402–2408. https://doi.org/10.1128/jb.178.8.2402-2408.1996
Lovley DR (2003) Cleaning up with genomics: applying molecular biology to bioremediation. Nat Rev Microbiol 1(1):35–44. https://doi.org/10.1038/nrmicro731
Lovley DR, Phillips EJ (1986) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51(4):683–689. https://doi.org/10.1128/aem.51.4.683-689.1986
Magot M (2005) Indigenous microbial communities in oil fields. In: Petroleum microbiology. ASM Press, Washington, DC, pp 21–33. https://doi.org/10.1128/9781555817589.ch2
Means JL, Crerar DA, Duguid JO (1978a) Migration of radioactive wastes: radionuclide mobilization by complexing agents. Science 200(4349):1477–1481. https://doi.org/10.1126/science.200.4349.1477
Means JL, Crerar DA, Borcsik MP, Duguid JO (1978b) Adsorption of Co and selected actinides by Mn and Fe oxides in soils and sediments. Geochim Cosmochim Acta 42(12):1763–1773. https://doi.org/10.1016/0016-7037(78)90233-8
Mishra M, Singh SK, Kumar A (2021) Role of omics approaches in microbial bioremediation. In: Microbe mediated remediation of environmental contaminants. Woodhead Publishing, Sawston, pp 435–445. https://doi.org/10.1016/B978-0-12-821199-1.00036-5
Noffke A, Hensen C, Sommer S, Scholz F, Bohlen L, Mosch T, Graco M, Wallmann K (2012) Benthic iron and phosphorus fluxes across the Peruvian oxygen minimum zone. Limnol Oceanogr 57(3):851–867. https://doi.org/10.4319/lo.2012.57.3.0851
Pettijohn FJ (1963) Chemical composition of sandstones, excluding carbonate and volcanic sands: representative analyses. US Government Printing Office. https://books.google.co.in/books?hl=en&lr=&id=NCQlAQAAIAAJ&oi=fnd&pg=PA1&dq=Pettijohn,+F.+J.,+1963.+Chemical+composition+of+sandstones%3B+excluding+carbonate+and+volcanic++sands.+United+States+Geological+Survey+Professional+Paper+440-S,+21.&ots=g7AhbkE-w8&sig=JdO9iFuF7rtc-gFTwlkv0zd5fes#v=onepage&q&f=false
Picardal F (2012) Abiotic and microbial interactions during anaerobic transformations of Fe (II) and NOx. Front Microbiol 3:112. https://doi.org/10.3389/fmicb.2012.00112
Prince RC, Walters CC (2016) Biodegradation of oil hydrocarbons and its implications for source identification. In: Standard handbook oil spill environmental forensics. Academic Press, London, pp 869–916. https://doi.org/10.1016/B978-0-12-803832-1.00019-2
Raiswell R, Canfield DE (2012) The iron biogeochemical cycle past and present. Geochem Perspect 1(1):1–2. https://pubs.geoscienceworld.org/perspectives/article-abstract/1/1/1/251624/The-Iron-Biogeochemical-Cycle-Past-and-Present?redirectedFrom=fulltext
Rickard D (2012) Sedimentary iron biogeochemistry. In: Developments in sedimentology, vol 65. Elsevier, Amsterdam, pp 85–119. https://doi.org/10.1016/B978-0-444-52989-3.00003-9
Scholz F, Severmann S, McManus J, Hensen C (2014) Beyond the Black Sea paradigm: the sedimentary fingerprint of an open-marine iron shuttle. Geochim Cosmochim Acta 127:368–380. https://doi.org/10.1016/j.gca.2013.11.041
Scholz F, Löscher CR, Fiskal A, Sommer S, Hensen C, Lomnitz U, Wuttig K, Göttlicher J, Kossel E, Steininger R, Canfield DE (2016) Nitrate-dependent iron oxidation limits iron transport in anoxic ocean regions. Earth Planet Sci Lett 454:272–281. https://doi.org/10.1016/j.epsl.2016.09.025
Senn DB, Hemond HF (2002) Nitrate controls on iron and arsenic in an urban lake. Science 296(5577):2373–2376. https://doi.org/10.1126/science.1072402
Singh VK, Chaudhari AK, Notarte KIR, Kumar A, Singh R, Bhadouria R (2021) Metal-oxidizing microbes and potential application in bioremediation. In: Microbe mediated remediation of environmental contaminants. Woodhead Publishing, Sawston, pp 107–114. https://doi.org/10.1016/B978-0-12-821199-1.00011-0
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62(4):1458–1460. https://doi.org/10.1128/aem.62.4.1458-1460.1996
Su J, Luo X, Huang T, Ma F, Zheng S, Lu J (2017) Effect of nitrite on autotrophic denitrification efficiency by Pseudomonas sp. SZF15. Environ Eng Sci 34(8):577–584. https://doi.org/10.1089/ees.2016.0173
Sudek LA, Templeton AS, Tebo BM, Staudigel H (2009) Microbial ecology of Fe (hydr)oxide mats and basaltic rock from Vailulu’u Seamount, American Samoa. Geomicrobiol J 26(8):581–596. https://doi.org/10.1080/01490450903263400
Sun W, Sierra-Alvarez R, Milner L, Oremland R, Field JA (2009) Arsenite and ferrous iron oxidation linked to chemolithotrophic denitrification for the immobilization of arsenic in anoxic environments. Environ Sci Technol 43(17):6585–6591. https://doi.org/10.1021/es900978h
Tong H, Liu C, Hao L, Swanner ED, Chen M, Li F, Xia Y, Liu Y, Liu Y (2019) Biological Fe (II) and As (III) oxidation immobilizes arsenic in micro-oxic environments. Geochim Cosmochim Acta 265:96–108. https://doi.org/10.1016/j.gca.2019.09.002
Waikhom D, Ngasotter S, Soniya Devi L, Devi S, Singh AS (2020) Role of microbes in petroleum hydrocarbon degradation in the aquatic environment: a review. Int J Curr Microbiol Appl Sci 9:2990–2903. https://doi.org/10.20546/ijcmas.2020.905.342
Wang X, Liu T, Li F, Li B, Liu C (2018) Effects of simultaneous application of ferrous iron and nitrate on arsenic accumulation in rice grown in contaminated paddy soil. ACS Earth Space Chem 2(2):103–111. https://doi.org/10.1021/acsearthspacechem.7b00115
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4(10):752–764. https://doi.org/10.1038/nrmicro1490
Weber KA, Hedrick DB, Peacock AD, Thrash JC, White DC, Achenbach LA, Coates JD (2009) Physiological and taxonomic description of the novel autotrophic, metal oxidizing bacterium, Pseudogulbenkiania sp. strain 2002. Appl Microbiol Biotechnol 83(3):555–565. https://doi.org/10.1007/s00253-009-1934-7
Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B (1993) Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362(6423):834–836. https://doi.org/10.1038/362834a0
Wu P, Zhang LJ, Lin CB, Xie XX, Yong XY, Wu XY, Zhou J, Jia HH, Wei P (2020) Extracting heavy metals from electroplating sludge by acid and bioelectrical leaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 191:105225. https://doi.org/10.1016/j.hydromet.2019.105225
Xiang L, Chan LC, Wong JWC (2000) Removal of heavy metals from anaerobically digested sewage sludge by isolated indigenous iron-oxidizing bacteria. Chemosphere 41(1-2):283–287. https://doi.org/10.1016/S0045-6535(99)00422-1
Zagury GJ, Narasiah KS, Tyagi RD (1994) Adaptation of indigenous iron-oxidizing bacteria for bioleaching of heavy metals in contaminated soils. Environ Technol 15(6):517–530. https://doi.org/10.1080/09593339409385458
Zahedi S, Vaezzade H, Rafati M, Zarei Dangesaraki M (2014) Acute toxicity and accumulation of iron, manganese and, aluminum in Caspian kutum Fish (Rutilus kutum). Iran J Toxicol 8(24):1028–1033. http://ijt.arakmu.ac.ir/article-1-299-en.html
Zhang H, Wang H, Yang K, Chang Q, Sun Y, Tian J, Long C (2015) Autotrophic denitrification with anaerobic Fe2+ oxidation by a novel Pseudomonas sp. W1. Water Sci Technol 71(7):1081–1087. https://doi.org/10.2166/wst.2015.071
Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB (2015) The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126(3):251–267. https://doi.org/10.1007/s10533-015-0166-4
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Waikhom, D., Ngasotter, S., Devi, L.S., Singh, S.K., Munilkumar, S. (2023). Iron-Degrading Bacteria in the Aquatic Environment: Current Trends and Future Directions. In: Soni, R., Suyal, D.C., Morales-Oyervides, L., Sungh Chauhan, J. (eds) Current Status of Fresh Water Microbiology. Springer, Singapore. https://doi.org/10.1007/978-981-99-5018-8_17
Download citation
DOI: https://doi.org/10.1007/978-981-99-5018-8_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5017-1
Online ISBN: 978-981-99-5018-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)