Abstract
Iron-oxidizing bacteria (FeOB) refers to a group of bacteria with the ability to exchange and accumulate divalent iron dissolved in water as trivalent iron inside and outside the bacterial cell. Most FeOB belong the largest bacterial phylum, Proteobacteria. Within this phylum, FeOB with varying physiology with regards to their response to oxygen (obligate aerobes, facultative and obligate anaerobes) and pH optimum for proliferation (neutrophiles, moderate and extreme acidophiles) can be found. Although FeOB have been reported from a wide variety of environments, most of them have not been isolated and their biochemical characteristics remain largely unknown. This is especially true for those living in the marine realm, where the properties of FeOB was not known until the isolation of the Zetaproteobacteria Mariprofundus ferrooxydans, first reported in 2007. Since the proposal of Zetaproteobacteria by Emerson et al., the detection and isolation of those microorganisms from the marine environment has greatly escalated. Furthermore, FeOB have also recently been reported from works on ocean drilling and metal corrosion. This review aims to summarize the current state of phylogenetic and physiological diversity in marine FeOB, the significance of their roles in their environments (on both global and local scales), as well as their growing importance and applications in the industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is one of the most common elements on Earth and the fourth most abundant element in the Earth’s crust (Wedepohl 1995). Iron has a wide range of oxidation states but exists mostly in + 2 or + 3 states in the natural environment. The valence of iron depends on the prevailing environmental physicochemical conditions, such as pH, O2 concentration, and redox potential. Iron occurs in many mineral phases, including (hydr)oxides, carbonates, silicates and sulfides. The oxidation of ferrous to ferric iron releases energy, which is harnessed by some iron-oxidizing prokaryotes. Ferrous iron is stable under anoxic conditions but can autoxidize in air. Some microbial communities that are unable to use solar energy via photosynthesis use energy from iron oxidation for CO2 assimilation (Schwertmann and Cornell 2000).
Iron-oxidizing chemolithoautotrophs were first identified in the nineteenth century (Ehrenberg 1836; Winogradsky 1888). These microorganisms became important for understanding the global iron cycle (Bach and Edwards 2003) and also for industrial applications in biomining (Bacelar-Nicolau and Johnson 1999; Rohwerder et al. 2003; Rawlings and Johnson 2007). Recently, interest has grown in iron oxidation and its impacts on biogeochemical elemental cycles in acidic and circumneutral environments under micro-aerobic and anaerobic conditions (Straub et al. 1996; Emerson and Moyer 1997; Baker and Banfield 2003; Edwards et al. 2000, 2003, 2004; Hegler et al. 2012; Klueglein and Kappler 2013). There are many kinds of microorganisms that utilize iron in the marine environment which contribute to this. Among those that inhabit the ocean, most known lineages fall into one class: Zetaproteobacteria.
Distribution of Zetaproteobacteria
All Zetaproteobacteria strains isolated so far originate from the marine environment (Table 1). Recent culture-dependent and -independent microbiological characterizations have revealed that a species of Zetaproteobacteria, Mariprofundus ferrooxydans (Emerson et al. 2007) and its relatives commonly occur in oceanic environments around the world (Table 2; Fig. 1). Mariprofundus ferrooxydans strains PV-1T and JV-1 were first isolated from the Loihi seamount vent field (Emerson and Moyer 2002; Emerson et al. 2007) and classified in Gammaproteobacteria, but detailed phylogenetic analysis subsequently showed that they were in fact strains of the novel class now generally known as Zetaproteobacteria (Emerson et al. 2007). Singer et al. conducted a genomic analysis of M. ferrooxydans PV-1 and confirmed the presence of potential genes contributing to iron oxidation (Singer et al. 2011). The biogeographic distribution of deep-sea Zetaproteobacterial populations has been investigated in several Pacific submarine hydrothermal systems (McAllister et al. 2011) which identified 28 OTUs, some of which were endemic to a single locality, suggesting Zetaproteobacteria is much more diverse than expected.

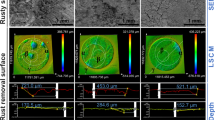
a and b are reproduces with permission from American Society for Microbiology: [Appl Environ Microbiol] (Makita et al. 2016). Panel d is reproduced with permission from Springer International Publishing AG: [Arch Microbiol] (Makita et al. 2017)
Environments where Zetaproteobacteria have been detected. Deep-sea hydrothermal sediment from the NW Eifuku seamount in the North Mariana Volcanic Arc (Latitude/Longitude (L/L) = 21.4850°/144.0430°, Depth:1545 m) (a), the Urashima site in the Mariana Trough (L/L = 13.51861°/144.0833°, Depth:2930 m) (b), the Tarama Knoll hydrothermal field in the Okinawa Trough (L/L = 25.0916°/124.5416°, Depth:1532 m) (c), and the Bayonnaise knoll on the Izu-Ogasawara Arc (L/L = 31.95112°/139.73529°, Depth:772 m) (d). An overview of the Nagahama Bay at the Satsuma IWO-jima in the Kagoshima, Japan (L/L = 30.7930°/130.2960°, Depth: ca.4 m) (e). Brown discoloration of seawater indicates the presence of iron oxide. Shallow hydrothermal iron-oxide deposited in Nagahama Bay (f).
Indeed, although deep-sea hydrothermal fields, particularly microbial mats and Fe-rich hydrothermally influenced sediments in relatively low-temperature areas (Laufer et al. 2017), was initially considered to be the main habitat of Zetaproteobacteria; recently they have been recovered from a wide range of other marine environments such as surface of shallow sediments, beach aquifer, and surface water (Table 2; Fig. 1). In addition, some Zetaproteobacteria members have been also found in association with metallic corrosion moiety (McBeth et al. 2011). Nevertheless, the true diversity, physiology, and ecology of Zetaproteobacteria (Fig. 2) still remains much unknown due to the fact that only very few strains have been isolated for further studies.
Maximum-likelihood phylogenetic tree of isolated marine iron-oxidizing bacterial constructed based on the 16S rRNA gene (1349-bp). Those FeOB with limited physiological data available are excluded. Sequence similarity analysis of the 16S rRNA gene was conducted using BLAST (Altschul et al. 1997; Benson et al. 1998). The phylogenetic trees were reconstructed by the maximum-likelihood (ML) method in the MEGA 5.0 package (Tamura et al. 2011), using Jukes-Cantor model distance. Bootstrap values were calculated using 1000 replications for the ML tree. Numbers in parentheses indicate GenBank/EMBL/DDBJ database accession numbers
Features of Zetaproteobacteria
As Zetaproteobacteria became recognized as a cosmopolitan group, their potential significant roles in biogeochemical processes in iron-rich redox-cline environments have begun to be investigated (Emerson and Moyer 2010).
Mariprofundus ferrooxydans, the best known Zetaproteobacteria, is an iron-oxidizing neutrophilic chemolithoautotroph that produces helical Fe-(oxy)hydroxide “stalks” (Emerson and Moyer 2002; Emerson et al. 2007; Chan et al. 2011; Figs. 3, 4). These stalks morphologically resemble those produced by the Betaproteobacteria genus Gallionella (Ehrenberg 1836; Pringsheim 1949; Kucera and Wolfe 1957; Ghiorse 1984; Hallbeck and Pedersen 1990, 1991; Hallbeck et al. 1993; Hallberg and Ferris 2004; Hanert 2006), and as such, helical Fe-(oxy)hydroxide stalks in deep-sea hydrothermal environments were long regarded as potential products of deep-sea Gallionella populations (Halbach et al. 2001), until the first isolation of M. ferrooxydans (Emerson et al. 2007; Emerson and Moyer 2002).

Panel a is reproduced with permission from PLOS: [PLoS One] (Singer et al. 2007), panels c and e are reproduced by permission from Frontiers Media S. A.: [Front Microbiol] (Chiu et al. 2017), panels b and d are reproduced by permission from Springer International Publishing AG.: [Arch Microbiol] (Makita et al. 2017), panel f is reproduced by permission from PLOS: [PLoS One] (Emerson et al. 2007), and panel g is reprinted by permission from Macmillan Publishers Ltd: [ISME Journal] (Mori et al. 2017)
Mariprofundus sp. culture using the gradient tube method. The state of M. ferrooxydans PV-1 (a) and M. micogutta ET2 (b) in culture with gradient medium. State of M. aestuarium CP-5 and M. ferrinatatus CP-8 on day 8 of culture in gradient medium (c). The orange-brown band is a colony band. Light micrographs showing cells with filamentous iron oxides produced by M. micogutta ET2 (d). Cells are DAPI stained and fluorescence images show the cells in blue. Phase contrast and fluorescence micrograph (overlay) of strain CP-5 cells (green), stained with SYBR Green I, and iron oxide dreads (e). Light micrographs showing M. ferrooxydans PV-1 cells (green), stained with Syto, attached at the end of filaments (f). Microscopic images of Ghiorsea bivora TAG-1, cells (green) were stained with SYTO13 (g). Scale bars indicate 10 µm (d), 2 µm (e), 5 µm (f), and 10 µm (g), respectively.

Panel a is reproduced with permission from Macmillan Publishers Ltd: [ISME Journal] (Chan et al. 2011), panel b is reproduced with permission from PLOS: [PLoS One] (Singer et al. 2007), panels c and d are reproduced with permission from Frontiers Media S. A.: [Front Microbiol] (Chiu et al. 2017), and panels e and f are reproduced with permission from Springer International Publishing AG.: [Arch Microbiol] (Makita et al. 2017)
Transmission electron microscope (TEM) images of M. ferrooxydans’s cell with stalk (a), (b). Inset: smaller cell and stalk, displayed at the same scale showing that smaller cells produce narrower stalks with fewer filaments (a). Scanning electron micrographs (SEM) of dreads produced by the strain CP-8, with the likely location of a missing cell denoted by a yellow oval (c). Dreads surrounding a freshwater betaproteobacterial FeOB Ferriphaselus sp. strain R-1 cell, highlighted in yellow (d). SEM micrograph showing the cell and extracellular materials of M. micogutta ET2 (e), and filamentous iron oxides (f). Scale bars: 500 nm (a), (b), 1 µm (c), (d), 100 nm (e) and 1 µm (f), respectively.
Mariprofundus micogutta ET2 was recently isolated from deep-sea hydrothermal environments (Makita et al. 2017; Fig. 2), and M. aestuarium CP-5 and M. ferrinatatus CP-8 from near shore environments (Chiu et al. 2017). These three strains produce iron oxides products that are distinct in morphology from the usual stalks, with M. micogutta ET2 forming filamentous structures and M. aestuarium CP-5 and M. ferrinatatus CP-8 forming dread-like structures (Fig. 3e). Furthermore, the iron oxides produced by M. aestuarium CP-5 and M. ferrinatatus CP-8 are very similar to those produced by the freshwater iron oxidizing Betaproteobacteria Ferriphaselus sp. strain R-1 in morphology (Kato et al. 2015; Fig. 4c, d). As previously mentioned, stalks made by M. ferrooxydans PV-1 is also similar in morphology to those produced by Gallionella ferruginea (Hallbeck and Pedersen 1990). It is notable that in both cases, the laboratory culturing conditions of the relevant Zeta- and Betaproteobacteria strains were very similar. As it is difficult to precisely determine the morphology of iron oxides produced by each strain in their natural environment (due to multiple strains being present together), it is possible that they produce different iron oxides structures in nature. Taken together, these cases potentially indicate that the condition of laboratory culturing can have an determining influence on the morphological structure of iron-oxides produced.
Very recently, two strains (TAG-1 and SV-108) of a novel Zetaproteobacteria named Ghiorsea bivora were isolated (Mori et al. 2017), both strains were unique in being able to utilize either Fe(II) or molecular hydrogen (H2) as the sole electron donor and oxygen as the terminal electron acceptor. Other known iron oxidizing bacteria thus far did not have the ability to use molecular hydrogen as the sole electron donor. Both strains precipitated iron oxides as amorphous particulates, but did not produce stalk structures which is consistent with the fact that they do not possess known putative genes for stalk-formation (xag operon) which are conserved in the genomes of several Mariprofundus species that do produce stalks (Kato et al. 2015). Presumably, these two strains of G. bivora produce a different type of exopolymer that prevents them from becoming encrusted in iron oxides, as has been proposed for several non-stalk forming freshwater FeOB (Emerson et al. 2007).
The iron oxides produced by Zetaproteobacteria can provide excellent habitational space and substrates for the microbial community, as the iron oxides structures serve as stable, porous and complex skeletons containing a variety of inorganic and organic substrates suitable for microbial growth and survival. It has been indicated that the distinctive iron oxides, such as helical twists and filaments, formation is based on association with exopolysaccarides (EPS) of cell surfaces, stalks and biofilms (Chan et al. 2009, 2011; Toner et al. 2009, 2012; Kikuchi et al. 2011, 2014; Wu et al. 2014). Although the composition and structure of EPS from Zetaproteobacteria is not fully understood, Chan et al. (2009) and Mitsunobu et al. (2012) suggested that the EPS was composed of acidic polysaccharide. These EPS and other organic compounds associated with the iron oxide production would serve as energy and carbon sources for mixotrophic and heterotrophic populations in iron mat microbial communities. It is likely that the chemical composition and structure of stalks produced by Zetaproteobacteria such as the genus Mariprofundus may be highly novel. A summary of genome and physiological characteristics of each Zetaproteobacteria strains isolated thus far is shown in Table 1.
Enrichment and isolation procedures for Zetaproteobacteria
Most Zetaproteobacteria isolated so far have been isolated by the gradient tube culture method (Emerson and Moyer 2002; Emerson and Floyd 2005), which supplies oxygen in gas phase from the top and iron from the bottom to generate a concentration gradient of the two across the medium to form a oxidation/reduction boundary region. In gradient culture, FeS or zerovalent iron is used as the iron source. The existence of a gradient allows iron oxidizing bacteria to localize in their optimum environment in the medium and grow abundantly. Therefore, it is an effective method for culturing iron oxidizing bacteria whose optimal conditions of oxygen concentration and iron concentration are difficult to predict and prepare a priori. Mariprofundus aestuarium and M. ferrinatatus were isolated by five transplantations in gradient tubes, for example (Chiu et al. 2017). Photographs of an actual ongoing gradient culture is shown in Fig. 3.
However, an issue exists with the gradient culture method, in that agar is used to make the concentration gradient which renders counting the number of cells or examining the culture conditions difficult, when compared to using a liquid medium. Therefore, even when the initial examination of culturing conditions, accumulation of culture, and purification is carried out with a gradient medium, subsequent isolation work and experiments investigating the properties of the isolated strain is often carried out in liquid medium instead. For example, Mariprofundus micogutta was isolated by a serial dilution method in a liquid medium after enrichment in gradient tubes (Makita et al. 2017). Ghiorsea bivora, which is able to utilize Fe(II) or H2, was also isolated in a liquid medium with zero valent iron powder by serial dilutions method in petri plates (Mori et al. 2017). Since zerovalent iron reacts with water to generate hydrogen, this method suited G. bivora well.
In another different method, Laufer et al. (2017) isolated a new Zetaproteobacteria close to Mariprofundus sp. M34 (98% sequence similarity) using zero-valent iron (ZVI) plates (McBeth et al. 2011; Laufer et al. 2017) with artificial seawater (ASW) medium and CO2 as a carbon source.
Other noteworthy marine FeOB
Not all marine FeOBs are Zetaproteobacteria. For example, Sudek et al. (2009) reported a heterotrophic Gammaproteobacteria (e.g., Pseudoalteromonas sp. and Pseudomonas sp.), isolated from a volcanic seamount, with the ability to catalyze ferrous iron oxidation under micro-aerobic conditions. In addition, Alphaproteobacteria (e.g., Hyphomonas sp. from hydrothermal fields on the Juan de Fuca ridge) have been shown to oxidize Fe(II) (Edwards et al. 2003).
Recently, interesting results were obtained from culture experiments using subsurface sediment core samples obtained by International Ocean Drilling Program expeditions, for example the Gammaproteobacterial Marinobacter sp. (strain NP-6) with neutrophilic iron-oxidizing capabilities was isolated from aphyric, cryptocrystalline basalt over 300 m deep below the seafloor in the North Pond site of the Mid-Atlantic Ridge (Zhang et al. 2016). In vitro nitrogen stimulated cultivation of the same core sediments revealed, for the first time, the existence of microorganisms that grow by using basalt as the iron source. Such results indicate that iron oxidation is a key energetic process in maintaining microbial communities in subsurface basalts.
In addition, a CFB group-bacteria Prolixibacter sp. that oxidize zerovalent iron from crude oil was sampled from an oil well in Akita Prefecture, Japan (Iino et al. 2014, 2015). A noteworthy characteristic of this bacteria is that it can grow by oxidizing zerovalent iron in an anaerobic environment with nitrate.
Industrial applications
Iron-oxidizing bacteria (FeOB) have rich potential applications in many fields. On the seafloor, organic matter (e.g., polysaccharide) produced by FeOB are coated with iron oxides and over time these accumulate to form thick iron mats. These iron oxide structures are able to serve as a medium for the absorption of toxic trace metals, such as Mn, As, Pb, Cd, Cs and Sr (Dyer et al. 2000; Katsoyiannis and Zouboulis 2006; Pokhrel and Viraraghavan 2009; Langley et al. 2009; Sahabi et al. 2010). In fact, in municipalities that utilize groundwater extensively for daily use, such as Nara, Kyoto, Osaka and Kita-Kyushu in Japan and Haiphong City, Vietnam (Tamura et al. 1999; Thapa Chhetri et al. 2013; Yayam 2014), iron oxide-based biofiltration systems are already in use for the removal of metals such as Fe, Mn, and Al from ground water. Installation of iron oxide-based biofiltration systems by municipal water treatment facilities can lead to significant cost savings. In Jyoyo City’s (Kyoto, Japan) water treatment facility, construction costs are cut by 30% compared to traditional treatment systems, chemical costs are reduced by more than 40%, and power consumption is reduced by 5% (Suzuki 2012).
Moreover, iron oxides produced by FeOB during the course of metabolism are often in the form and structure of amorphous nano-sized particles which have numerous potential industrial applications. One example is the tubular iron oxide nanoparticles produced during the growth of the freshwater iron-oxidizing betaproteobacterium Leptothrix ochracea. They have a unique charge–discharge property, making them a suitable material for negative electrodes in lithium ion secondary batteries (Hashimoto et al. 2014). Another example is the iron oxide produced by M. ferrooxydans which has applications as novel multifunctional drug carriers for triggered therapeutics release as well as cancer hyperthermia applications (Kumeria et al. 2016). In addition, iron oxides of Mariprofundus sp. processed at 800 °C were the most optimal for photocatalytic applications the degradation rhodamine B (Wang et al. 2016).
Extracellular polysaccharide (EPS) of FeOB, which are supports of iron oxide, also have potential industrial applications. Although the chemical composition, structure, and mechanism of production of EPS from Zetaproteobacteria are not entirely known, the chemical composition and structure of the extra-cellular sheath produced by the iron-oxidizing Betaproteobacteria Leptothrix cholodnii has been elucidated (Emerson and Ghiorse 1993; Takeda et al. 2005; Makita et al. 2006). The 2-(cysteinyl)amido-2-deoxy-D-galacturonic acid residue connecting the main hetero-polysaccharide chain to the peptide side chain exhibits a novel structure (Makita et al. 2006). While the details of EPS remain largely unknown for most FeOB, these potentially novel structures may be useful for the pharmaceutical and textile industries. For instance, moisturizing agent derived from a polysaccharide produced by Alcaligenes latus absorbs 1000 times its own weight in water, which can be used as medicament and cosmetics (Kurane et al. 1994). Recently, M. ferrooxydans has been successfully cultivated using electric energy (Mogi et al. 2013; Summers et al. 2013). This indicates M. ferrooxydans exhibits inter-membrane electron transfer mechanisms from outside its cell. This electrochemical culture method may allow a continuous production of organic macromolecules from iron oxidizing bacteria for use in industry. In this way, FeOB have attractive potential applications in producing organic matter from carbon dioxide.
Furthermore, the study of the interaction between FeOB and solid-phase iron (e.g., iron in reinforced concrete) is industrially important. FeOB that grow by oxidizing zerovalent iron, such as the aforementioned CFB group-bacteria, Prolixibacter denitrificans and P. bellariivorans (Iino et al. 2014, 2015), are the source of microbial induced corrosion (MIC) of industrial products which a major issue limiting the lifespan of such products (Javaherdashti 2008). Although zerovalent iron does not exist in the natural environment, it is widely used in industrial products. Future researches on such bacteria that play a role in MIC is expected to yield effective counter-measures for this issue.
Conclusion
Until the last decade, marine FeOB were not recognized as significant players in in-situ ecosystems in marine environments. Although their existence and importance in the carbon cycle were predicted, few species could be isolated. However, in recent years, studies on the isolation and identification of FeOB inhabiting marine environments have been rapidly increasing (Emerson et al. 2007; Chiu et al. 2017; Laufer et al. 2017; Makita et al. 2017; Mori et al. 2017), with the number of successful isolations likely to increase with time. It is thought that FeOB play an important role in iron and carbon circulation in the ocean as it utilizes iron and CO2 in the seawater. Although the biogeochemical iron cycle is unable to function independently from the carbon cycle, a relatively small amount of carbon is sufficient to drive and maintain it. Further investigation into the role of FeOB in CO2 fixation in the marine environment will contribute to the understanding of the overall global carbon cycle. Furthermore, knowledge of the mechanism and total amount of CO2 fixation by FeOB can also lead to industrially applicable methods for immobilizing CO2 to help mitigate the effects of climate change. For that, the identification of enzymes involved in iron oxidation and using such enzymes as biomarkers to reveal the true global distribution of FeOB will be key.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Bacelar-Nicolau P, Johnson DB (1999) Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures. Appl Environ Microbiol 65:585–590
Bach W, Edwards KJ (2003) Iron and sulfide oxidation within the basaltic ocean crust: Implications for chemolithoautotrophic microbial biomass production. Geochim Cosmochim Acta 67:3871–3887
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152
Benson DA, Boguski MS, Lipman DJ, Ostell J, Ouellette BF (1998) GenBank Nucl Acids Res 26:1–7
Cao H, Wang Y, Lee OO, Zeng X, Shao Z, Qian P-Y (2014) Microbial sulfur cycle in two hydrothermal chimneys on the Southwest Indian Ridge. mBio 5(1):e00980-13. https://doi.org/10.1128/mBio.00980-13
Chan CS, Fakra SC, Edwards DC, Emerson D, Banfield JF (2009) Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim Cosmochim Acta 73:3807–3818
Chan CS, Fakra SC, Emerson D, Fleming EJ, Edwards KJ (2011) Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: implications for biosignature formation. ISME J 5:717–727. https://doi.org/10.1038/ismej.2010.173
Chiu BK, Kato S, McAllister SM, Field EK, Chan CS (2017) Novel pelagic iron-oxidizing Zetaproteobacteria from the Chesapeake bay oxic-anoxic transition zone. Front Microbiol 18(8):1280. https://doi.org/10.3389/fmicb.2017.01280
Dang H, Chen R, Wang L, Shao S, Dai L, Ye Y, Guo L, Huang G, Klotz MG (2011) Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environ Microbiol 13:3059–3074. https://doi.org/10.1111/j.1462-2920.2011.02583.x
Davis RE, Stakes DS, Wheat CG, Moyer CL (2009) Bacterial variability within an iron-silica-manganese-rich hydrothermal mound located off-axis at the Cleft Segment, Juan de Fuca Ridge. Geomicrobiol J 26:570–580
Davis RE, Moyer C, McAllister S, Rassa A, Tebo B (2010) Spatial and temporal variability of microbial communities from pre- and post-eruption microbial mats collected from Loihi Seamount, Hawaii. Abstr. In: 13th International Symposium on Microbial Ecology, abstr. PS.01.015
Dhillon A, Teske A, Dillon J, Stahl DA, Sogin ML (2003) Molecular characterization of sulfate-reducing bacteria in the Guaymas Basin. Appl Environ Microbiol 69:2765–2772. https://doi.org/10.1128/AEM.69.5.2765-2772.2003
Dickinson I, Goodall-Copestake W, Thorne MA, Schlitt T, Ávila-Jiménez ML, Pearce DA (2016) Extremophiles in an Antarctic marine ecosystem. Microorganisms 4:8. https://doi.org/10.3390/microorganisms4010008
Dyer A, Pillinger M, Harjulab R, Aminc S (2000) Sorption characteristics of radionuclides on synthetic birnessite-type layered manganese oxides. J Mater Chem 10:1867–1874. https://doi.org/10.1039/B002435J
Eder W, Jahnke LL, Schmidt M, Huber R (2001) Microbial diversity of the brine-seawater interface of the Kebrit Deep, Red Sea, studied via 16S rRNA gene sequences and cultivation methods. Appl Environ Microbiol 67:3077–3085. https://doi.org/10.1128/AEM.67.7.3077-3085.2001
Edwards KJ, Bond PL, Gihring TM, Banfield JF (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796–1799
Edwards KJ, Rogers DR, Wirsen CO, McCollom TM (2003) Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic α- and γ-Proteobacteria from the deep sea. Appl Environ Microbiol 69:2906–2913
Edwards KJ, Bach W, McCollom TM, Rogers DR (2004) Neutrophilic iron-oxidizing bacteria in the ocean: their habitats, diversity, and roles in mineral deposition rock alteration, and biomass production in the deep-sea. Geomicrobiol J 21:393–404
Edwards KJ, Glazer BT, Rouxel OJ, Bach W, Emerson D, Davis RE, Toner BM, Chan CS, Tebo BM, Staudigel H, Moyer CL (2011) Ultra-diffuse hydrothermal venting supports Fe-oxidizing bacteria and massive umber deposition at 5000 m off Hawaii. ISME J. https://doi.org/10.1038/ismej.2011.48
Ehrenberg CG (1836) Vorläufige mitteilungen über das wirkliche vorkommen fossiler infusorien und ihre große verbreitung. Poggendorff’s Ann Phys Chem 38:213–227
Emerson D, Ghiorse WC (1993) Ultrastructure and chemical composition of the sheath of Leptothrix discophora SP-6. J Bacteriol 175(24):7808–7818
Emerson D, Moyer CL (1997) Isolation and characterization of novel iron-oxidizingbacteria that grow at circumneutral pH. Appl Environ Microbiol 63:4784–4792
Emerson D, Moyer CL (2002) Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi Seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl Environ Microbiol 68:3085–3093
Emerson D, Floyd MM (2005) Enrichment and isolation of iron-oxidizing bacteria at neutral pH. In: Methods in enzymology, vol. 397. Academic Press, Cambridge MA, pp 112–123
Emerson D, Moyer CL (2010) Microbiology of seamounts: common patterns observed in community structure. Oceanography 23:148–163
Emerson D, Rentz JA, Lilburn TG, Davis RE, Aldrich H, Chan C, Moyer CL (2007) A novel lineage of Proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2:e667. https://doi.org/10.1371/journal.pone.0000667
Field EK, Kato S, Findlay AJ, MacDonald DJ, Chiu BK, Luther GW, Chan CS (2016) Planktonic marine iron oxidizers drive iron mineralization under low-oxygen conditions. Geobiology 14:499–508. https://doi.org/10.1111/gbi.12189
Fleming EJ, Davis RE, McAllister SM, Chan CS, Moyer CL, Tebo BM, Emerson D (2013) Hidden in plain sight: discovery of sheath-forming, iron-oxidizing Zetaproteobacteria at Loihi Seamount, Hawaii, USA. FEMS Microbiol Ecol 85:116–127. https://doi.org/10.1111/1574-6941.12104
Forget NL, Murdock SA, Juniper SK (2010) Bacterial diversity in Fe-rich hydrothermal sediments at two South Tonga Arc submarine volcanoes. Geobiology 8:417–432. https://doi.org/10.1111/j.1472-4669.2010.00247.x
Fullerton H, Hager KW, McAllister SM, Moyer CL (2017) Hidden diversity revealed by genome-resolved metagenomics of iron-oxidizing microbial mats from Lō’ihi Seamount, Hawai’i. ISME J 11(8):1900–1914. https://doi.org/10.1038/ismej.2017.40
Ghiorse WC (1984) Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol 38:515–550
Glazer BT, Rouxel OJ (2009) Redox speciation and distribution within diverse iron-dominated microbial habitats at Loihi Seamount. Geomicrobiol J 26:606–622
Halbach M, Koschinsky A, Halbach P (2001) Report of the discovery of Gallionella ferruginea from an active hydrothermal field in the deep sea. InterRidge News 10:18–20
Hallbeck L, Pedersen K (1990) Culture parameters regulating stalk formation and growth rate of Gallionella ferruginea. J Gen Microbiol 136:1675–1680
Hallbeck L, Pedersen K (1991) Autotrophic and mixotrophic growth of Gallionella ferruginea. J Gen Microbiol 137:2657–2661
Hallbeck L, Ståhl F, Pedersen K (1993) Phylogeny and phenotypic characterization of the stalk-forming and iron-oxidizing bacterium Gallionella ferruginea. Microbiology 139(7):1531–1535
Hallberg R, Ferris FG (2004) Biomineralization by Gallionella. Geomicrobiol J 21:325–330
Handley KM, Boothman C, Mills RA, Pancost RD, Lloyd JR (2010) Functional diversity of bacteria in a ferruginous hydrothermal sediment. ISME J 4:1193–1205. https://doi.org/10.1038/ismej.2010.38
Hanert HH (2006) The genus Gallionella. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH (eds) The prokaryotes, vol 7. Springer, New York, pp 990–995
Hashimoto H, Kobayashi G, Sakuma R, Fujii T, Hayashi N, Suzuki T, Kanno R, Takano M, Takada J (2014) Bacterial nanometric amorphous Fe-based oxide: a potential lithium-ion battery anode material. ACS Appl Mater Interfaces 2014 6:5374–5378
Hegler F, Lösekann-Behrens T, Hanselmann K, Behrens S, Kappler A (2012) Influence of seasonal and geochemical changes on the geomicrobiology of an iron carbonate mineral water spring. Appl Environ Microbiol 78(20):7185–7196
Hodges TW, Olson JB (2009) Molecular comparison of bacterial communities within iron-containing flocculent mats associated with submarine volcanoes along the Kermadec Arc. Appl Environ Microbiol 75:1650–1657
Iino T, Ito K, Wakai S, Tsurumaru H, Ohkuma M, Harayama S (2014) Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1-1. Appl Environ Microbiol 81(5):1839–1846. https://doi.org/10.1128/AEM.03741-14
Iino T, Sakamoto M, Ohkuma M (2015) Prolixibacter denitrificans sp. nov., an iron-corroding, facultatively aerobic, nitrate-reducing bacterium isolated from crude oil, and emended descriptions of the genus Prolixibacter and Prolixibacter bellariivorans. Int J Syst Evol Microbiol 65(9):2865–2869. https://doi.org/10.1099/ijs.0.000343
Javaherdashti R (2008) Microbiologically influenced corrosion: an engineering insight. Springer, New York
Juniper SK, Fouquet Y (1988) Filamentous iron-silica deposits from modern and ancient hydrothermal sites. Can Miner 26:859–869
Kato S, Kobayashi C, Kakegawa T, Yamagishi A (2009a) Microbial communities in iron-silica-rich microbial mats at deep-sea hydrothermal fields of the Southern Mariana Trough. Environ Microbiol 11:2094–2111
Kato S, Yanagawa K, Sunamura M, Takano Y, Ishibashi J, Kakegawa T, Utsumi M, Yamanaka T, Toki T, Noguchi T, Kobayashi K, Moroi A, Kimura H, Kawarabayasi Y, Marumo K, Urabe T, Yamagishi A (2009b) Abundance of Zetaproteobacteria within crustal fluids in back-arc hydrothermal fields of the Southern Mariana Trough. Environ Microbiol 11:210–3222
Kato S, Ohkuma M, Powell DH, Krepski ST, Oshima K, Hattori M, Shapiro N, Woyke T, Chan CS (2015) Comparative genomic insights into ecophysiology of neutrophilic, microaerophilic iron oxidizing bacteria. Front Microbiol 6:1265. https://doi.org/10.3389/fmicb.2015.01265
Katsoyiannis IA, Zouboulis AI (2006) Use of iron- and manganese-oxidizing bacteria for the combined removal of iron, manganese and arsenic from contaminated groundwater. Water Qual Res J Can 41(2):117–129
Kennedy CB, Scott SD, Ferris FG (2003a) Characterization of bacteriogenic iron oxide deposits from Axial Volcano, Juan de Fuca Ridge, Northeast Pacific Ocean. Geomicrobiol J 20:199–214
Kennedy CB, Martinez RE, Scott SD, Ferris FG (2003b) Surface chemistry and reactivity of bacteriogenic iron oxides from Axial Volcano, Juan de Fuca Ridge, north-east Pacific Ocean. Geobiology 1:59–69
Kennedy CB, Scott SD, Ferris FG (2003c) Ultrastructure and potential sub- seafloor evidence of bacteriogenic iron oxides from Axial Volcano, Juan de Fuca Ridge, north-east Pacific Ocean. FEMS Microbiol Ecol 43:247–254
Kikuchi S, Makita H, Mitsunobu S, Terada Y, Yamaguchi N, Takai K, Takahashi Y (2011) Application of synchrotron based µ-XRF-XAFS to the speciation of Fe on single stalk in bacteriogenic iron oxides (BIOS). Chem Lett 40:680–681
Kikuchi S, Makita H, Takai K, Yamaguchi N, Takahashi Y (2014) Characterization of biogenic iron oxides collected by the newly designed liquid culture method using diffusion chambers. Geobiology. https://doi.org/10.1111/gbi.12073
Klueglein N, Kappler A (2013) Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1—questioning the existence of enzymatic Fe(II) oxidation. Geobiology 11(2):180–190. https://doi.org/10.1111/gbi.12019
Kucera S, Wolfe RS (1957) A selective enrichment method for Gallionella ferruginea. J Bacteriol 74:344–349
Kumeria T, Maher S, Wang Y, Kaur G, Wang L, Erkelens M, Forward P, Lambert MF, Evdokiou A, Losic D (2016) Naturally derived iron oxide nanowires from bacteria for magnetically triggered drug release and cancer hyperthermia in 2D and 3D culture environments: bacteria bio film to potent cancer therapeutic. Biomacromolecules 17(8):2726–2736. https://doi.org/10.1021/acs.biomac.6b00786
Kurane R, Hatamochi K, Kakuno T, Kiyohara M, Kawaguchi K, Mizuno Y, Hirano M, Taniguchi Y (1994) Purification and characterization of lipid bioflocculant produced by Rhodococcus erythropolis. Biosci Biotechnol Biochem 58:1977–1982. https://doi.org/10.1271/bbb.58.1977
Langley S, Gault AG, Ibrahim A, Takahashi Y, Renaud R, Fortin D, Clark ID, Ferris FG (2009) Sorption of strontium onto bacteriogenic iron oxides. Environ Sci Technol 43:1008–1014. https://doi.org/10.1021/es802027f
Laufer K, Nordhoff M, Halama M, Martinez RE, Obst M, Nowak M, Stryhanyuk H, Richnow HH, Kappler A (2017) Microaerophilic Fe(II)-oxidizing Zetaproteobacteria isolated from low-Fe marine coastal sediments: physiology and composition of their twisted stalks. Appl Environ Microbiol 83(8):e03118–e03116. https://doi.org/10.1128/AEM.03118-16
Makita H, Nakahara Y, Fukui H, Miyanoiri Y, Katahira M. Takeda M, Koizumi J (2006) Identification of 2-(Cysteinyl)amido-2-deoxy-D-galacturonic acid residue from the sheath of Leptothrix cholodnii. Biosci Biotechnol Biochem 70:1265–1268. https://doi.org/10.1271/bbb.70.1265
Makita H, Kikuchi S, Mitsunobu S, Takaki Y, Yamanaka T, Toki T, Noguchi T, Nakamura K, Abe M, Hirai M, Yamamoto M, Uematsu K, Miyazaki J, Nunoura T, Takahashi Y, Takai K (2016) Comparative analysis of microbial communities in iron-dominated flocculent mats in deep sea hydrothermal environments. Appl Environ Microbiol 82(19):5741–5755. https://doi.org/10.1128/AEM.01151-16
Makita H, Tanaka E, Mitsunobu S, Miyazaki M, Nunoura T, Uematsu K, Takaki Y, Nishi S, Shimamura S, Takai K (2017) Mariprofundus micogutta sp. nov., a novel iron-oxidizing zetaproteobacterium isolated from a deep-sea hydrothermal field at the Bayonnaise knoll of the Izu-Ogasawara arc, and a description of Mariprofundales ord. nov. and Zetaproteobacteria classis nov. Arch Microbiol 199(2):335–346. https://doi.org/10.1007/s00203-016-1307-4
Makita H, Nishi S, Takaki Y, Tanaka E, Nunoura T, Mitsunobu S, Takai K (2018) Draft genome sequence of Mariprofundus micogutta strain ET2. Genome Announ. 6(20):e00342-18
McAllister SM, Davis RE, McBeth JM, Tebo BM, Emerson D, Moyer CL (2011) Biodiversity and emerging biogeography of the neutrophilic iron-oxidizing Zetaproteobacteria. Appl Environ Microbiol 77(15):5445–5457. https://doi.org/10.1128/AEM.00533-11
McAllister SM, Barnett JM, Heiss JW, Findlay AJ, MacDonald DJ, Dow CL, Luther GW, Michael HM, Chan CS (2015) Dynamic hydrologic and biogeochemical processes drive microbially enhanced iron and sulfur cycling within the intertidal mixing zone of a beach aquifer. Limnol Oceanogr 60:329–345. https://doi.org/10.1111/lno.10029
McBeth JM, Little BJ, Ray RI, Farrar KM, Emerson D (2011) Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl Environ Microbiol 77:1405–1412
Mitsunobu S, Shiraishi F, Makita H, Orchtt B, Kikuchi S, Jorgensen B, Takahashi Y (2012) Bacteriogenic Fe(III)(oxyhydr)oxides characterized by synchrotron microprobe coupled with spatially-resolved phylogenetic analysis. Environ Sci Technol 46:3304–3311
Mogi T, Ishii T, Hashimoto K, Nakamura R (2013) Low-voltage electrochemical CO2 reduction by bacterial voltage-multiplier circuits. Chem Commun 49:3967–3969
Mori JF, Scott JJ, Hager KW, Moyer CL, Küsel K, Emerson D (2017) Physiological and ecological implications of an iron- or hydrogen-oxidizing member of the Zetaproteobacteria, Ghiorsea bivora, gen. nov., sp. nov. ISME J 11(11):2624–2636. https://doi.org/10.1038/ismej.2017.132
Moyer CL, Dobbs FC, Karl DM (1994) Estimation of diversity and community structure through restriction fragment length polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol 60:871–879
Moyer CL, Dobbs FC. Karl DM (1995) Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl Environ Microbiol 61:1555–1562
Mumford AC, Adaktylou IJ, Emerson D (2016) Peeking under the iron curtain: development of a microcosm for imaging the colonization of steel surfaces by Mariprofundus sp. strain DIS-1, an oxygen-tolerant Fe-oxidizing bacterium. Appl Environ Microbiol 82(22):6799–6807
Orcutt BN, Bach W, Becker K, Fisher AT, Hentscher M, Toner BM, Wheat CG, Edwards KJ (2011) Colonization of subsurface microbial observatories deployed in young ocean crust. ISME J 5:692–703. https://doi.org/10.1038/ismej.2010.157
Peng X, Ta K, Chen S, Zhang L, Xu H (2015) Coexistence of Fe(II)- and Mn(II)-oxidizing bacteria govern the formation of deep sea umber deposits. Geochim Cosmochim Acta 169:200–216. https://doi.org/10.1016/j.gca.2015.09.011
Pokhrel D, Viraraghavan T (2009) Biological filtration for removal of arsenic from drinking water. J Environ Manag 90(5):1956–1961. https://doi.org/10.1016/j.jenvman.2009.01.004
Pringsheim EG (1949) Iron bacteria. Biol Rev Camb Philos Soc 24:200–245
Rassa AC, McAllister SM, Safran SA, Moyer CL (2009) Zeta-Proteobacteria dominate the colonization and formation of microbial mats in low-temperature hydrothermal vents at Loihi Seamount, Hawaii. Geomicrobiol J 26:623–638
Rawlings DE, Johnson DB (2007) The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315–324
Reyes C, Dellwig O, Dähnke K, Gehre M, Noriega-Ortega B, Böttcher ME, Meister P, Friedrich MW (2016) Bacterial communities potentially involved in iron-cycling in Baltic Sea and North Sea sediments revealed by pyrosequencing. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiw054
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A: Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl Microbiol Biotechnol 63:239–248
Rubin-Blum M, Antler G, Tsadok R, Shemesh E, Austin JA Jr, Coleman DF, Goodman-Tchernov BN, Ben-Avraham Z, Tchernov D (2014) First evidence for the presence of iron-oxidizing Zetaproteobacteria at the Levantine continental margins. PLoS ONE 9:e91456. https://doi.org/10.1371/journal.pone.0091456
Sahabi DM, Takeda M, Suzuki I, Koizumi J (2010) Comparison of arsenate, lead, and cadmium adsorption onto aged biofilter media. J Environ Eng 136(5):493–500
Schwertmann U, Cornell RM (2000) The iron oxides in the laboratory: preparation and characterization. Wiley-VCH, New York
Singer E, Emerson D, Webb EA, Barco RA, Kuenen JG, Nelson WC, Chan CS, Comolli LR, Ferriera S, Johnson J, Heidelberg JF, Edwards KJ (2011) Mariprofundus ferrooxydans PV-1 the first genome of a marine Fe(II) oxidizing Zetaproteobacterium. PLoS ONE 6(9):e25386. https://doi.org/10.1371/journal.pone.0025386
Staudigel H, Hart SR, Pile A, Bailey BE, Baker ET, Brooke S, Connelly DP, Haucke L, German CR, Hudson I, Jones D, Koppers AA, Konter J, Lee R, Pietsch TW, Tebo BM, Templeton AS, Zierenberg R, Young CM (2006) Vailulu’u Seamount, Samoa: life and death on an active submarine volcano. Proc Natl Acad Sci USA 103(17):6448–6453. https://doi.org/10.1073/pnas.0600830103
Stauffert M, Cravo-Laureau C, Jezequel R, Barantal S, Cuny P, Gilbert F, Cagnon C, Militon C, Amouroux D, Mahdaoui F, Bouyssiere B, Stora G, Merlin F-X, Duran R (2013) Impact of oil on bacterial community structure in bioturbated sediments. PLoS ONE 8:e65347. https://doi.org/10.1371/journal.pone.0065347
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62:1458–1460
Sudek LA, Templeton AS, Tebo BM, Staudigel H (2009) Microbial ecology of Fe (hydr)oxide mats and basaltic rock from Vailulu’u Seamount, American Samoa. Geomicrobiol J 26:581–596
Summers ZM, Gralnick JA, Bond DR (2013) Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes. MBio 4(1):e00420–e00412. https://doi.org/10.1128/mBio.00420-12
Suzuki I (2012) Seibutsu-Kogaku Kaishi. Soc Biosci Bioeng 90(4):170–173
Sylvan JB, Pyenson BC, Rouxel O, German CR, Edwards KJ (2012) Time-series analysis of two hydrothermal plumes at 9°50′N East Pacific Rise reveals distinct, heterogeneous bacterial populations. Geobiology 10:178–192. https://doi.org/10.1111/j.1472-4669.2011.00315.x
Takeda M, Makita H, Ohno K, Nakahara Y, Koizumi J (2005) Structural analysis of the sheath of a sheathed bacterium, Leptothrix cholodnii. Int J Biol Macromol 37:92–98
Tamura T, Tsunai K, Ishimaru Y, Nakata A (1999) Iron and manganese removal by iron bacteria in ground water. Suidou-Kyokai-Zasshi 68(6):2–13 (1999)
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thapa Chhetri R, Suzuki I, Fujita T, Takeda M, Koizumi J, Fujikawa Y, Minami A, Hamasaki T, Sugahara M (2013) Bacterial diversity in biological filtration system for the simultaneous removal of arsenic, iron and manganese from groundwater. J Water Environ Technol 12(2):135–149. https://doi.org/10.2965/jwet.2014.135
Toner MB, Santelli CM, Marcus MA, Wirth R, Chan CS, McCollom T, Bach W, Edwards KJ (2009) Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochim Cosmochim Acta 73:388–403
Toner MB, Berquo T, Michel FM, Sorensen JV, Templeton AS, Edwards KJ (2012) Mineralogy of iron microbial mats from Loihi Seamount. Front Microbiol 3:118. https://doi.org/10.3389/fmicb.2012.00118
Wang L, Kumeria T, Santos A, Forward P, Lambert MF, Losic D (2016) Iron oxide nanowires from bacteria biofilm as an efficient visible-light magnetic photocatalyst. ACS Appl Mater Interfaces 8(31):20110–20119. https://doi.org/10.1021/acsami.6b06486
Wedepohl HK (1995) The composition of the continental crust. Geochimicaet Cosmochimica Acta 59(7):1217–1232
Winogradsky S (1888) Ueber Eisenbacterien. Bot Zeit 17:262–269
Wu W, Swanner ED, Hao L, Zeitvogel F, Obst M, Pan Y, Kappler A (2014) Characterization of the physiology and cell-mieneral interactions of the marine anoxygenic phototrophic Fe(II) oxidizer Rhodovulum iodosum-implications for Precambrian Fe(II) oxidation. FEMS Microbiol Ecol 88:503–515
Yayam M (2014) Upward biological contact filtration. In: Nakamoto N, Graham N, Collins R, Gimbel R (eds) Progress in slow sand and alternative biofiltration processes, Chap. 66. London: IWA Publishing
Zhang X, Fang J, Bach W, Edwards KJ, Orcutt BN, Wang F (2016) Nitrogen stimulates the growth of subsurface basalt-associated microorganisms at the Western flank of the mid-Atlantic Ridge. Front Microbiol 3(7):633. https://doi.org/10.3389/fmicb.2016.00633
Acknowledgements
Dr. Chong Chen and Dr. Donald Pan (JAMSTEC) are gratefully acknowledged for their help in improving an earlier version of the manuscript. This research was partially supported by KAKENHI JP26820389 and JP18K04595 to HM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makita, H. Iron-oxidizing bacteria in marine environments: recent progresses and future directions. World J Microbiol Biotechnol 34, 110 (2018). https://doi.org/10.1007/s11274-018-2491-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-018-2491-y