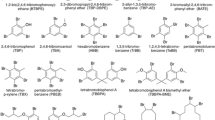
Abstract
Novel brominated flame retardants (NBFRs) and legacy BFRs have been used in industrial and home applications to reduce the risk of ignition. However, the use of flame retardants is of particular concern due to the likelihood of being found in high concentrations, persistence in the environment, and for bioaccumulation in the environment. BFRs are of interest due to the potential toxicity to humans and endocrine-disrupting properties. To adress toxicity and persistence of BFRs, new or novel BFRs (NFBRs) have been introduced as a replacement. However, NBFRs have similar chemical properties and environmental fates as legacy BFRs. This chapter discusses various methods of abiotic and biotic degradation of BFRs, culturing conditions, potential microorganisms, and enzymes that can biodegrade BFRs from various environmental sources. We include the proposed mechanisms of biodegradation and persistence in the environment for several congeners. Water matrices are also discussed as an environmental source since BFRs in sedimentation are not well known and pose an essential factor in assessing the amount of BFRs present in the environment. The presence of BFRs in our environment have been concerning as they have been linked by various studies to the decline of sperm counts and fertility issues of both genders as well as contribute to cognitive and developmental problems in children.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brominated flame retardants
- Bioremediation
- Halogenated
- Endocrine disruptor
- Polybrominated diphenyl ethers (PBDEs)
- Neurotoxicity
- Decabrominated diphenyl ether (BDE-209)
- Decabromodiphenyl ethane (DBDPE)
- New brominated flame retardants
- Tetrabromobisphenol A (TBBPA)
- Minimal salts media (MSM)
- Hexabromocyclododecanes (HBDC)
5.1 Introduction
Epoxy resins are widely used for many products but are highly combustible. Furthermore, the amount of heat created and the toxic gases released once ignited are significant causes of deaths and property damage. For this reason, flame retardants (FRs) have been developed to mitigate this problem (Waaijers and Parsons 2016). BFRs represent various chemicals applied to many products, including plastics, polymers, textiles, wood, or other ignitable objects, to prevent combustion. During the process of combustion, free radicals are formed. Halogens, namely bromine, are good at detaining free radicals and lowering the decomposition temperature (Kodavanti et al. 2017). Approximately one-quarter of the world’s flame retardants are brominated (Andersson et al. 2006). There are three main groups of BFRs used. These are decabrominated diphenyl ether (BDE-209), tetrabromobisphenol A (TBBPA), and hexabromocyclododecane (HBCD) (Andersson et al. 2006).
BFRs, also known as organohalogens, comprise a group of chemicals that use flame retardants, which is concerning due to their toxicity, bioaccumulation, and environmental accumulation. NBFRs have been cited as being neurotoxic in children who are still developing (Roze et al. 2009). Due to bioaccumulation and toxicity, BFRs have been banned in various countries, and novel BFRs are being used as an alternative to BFRs. However, novel BFRs have been shown to accumulate in aquatic matrices, air, sediment, and sludge and bioaccumulate in animals (Xiong et al. 2019). In this review, we will also focus on water matrices as sedimentation is a part of land management that is often overlooked and often harbors pollutants that are harmful to aquatic ecosystems (Bakker et al. 2008).
5.2 Toxicity of BFRS
The toxicity of BFRs has been cited in many publications. BFRs are lipophilic and are stored in adipose tissue, and have the ability to biomagnify in the food chain. This is a potential risk considering that the animals humans consume have been shown to harbor BFRs. Evidence shows that DBDPE was found to induce oxidative stress, changes in morphology, and transcriptomics in white rot fungus P. ostreatus (Wang et al. 2022a). This study observed a decrease in fungal biomass in concentrations from 1 to 50 mg L−1 DBDPE. A decrease in superoxide dismutase, catalase, and glutathione was also observed. This is significant since all three are crucial in the detoxification process. Most significant was the role in the downregulation of genes involved in metabolisms such as oxidative phosphorylation, TCA cycle, and carbon metabolism. This study highlights transcriptomics as an essential tool in molecular biology for understanding the mechanisms of toxicity after exposure to pollutants. Moreover, a multi-omics approach can accurately map the pathways in response to pollutant exposure, thus revealing the mechanisms of toxicity for organisms (Li et al. 2022).
Kidney miRNAs from grass carp were found to be significantly changed after exposure to BFR and DBDPE (Gan et al. 2016). The study found that five kidney miRNAs were significantly downregulated, while 36 kidney miRNAs were significantly upregulated. Interestingly, miR-155, miR-205, and most miR-10 family members were upregulated. miR-155 is known to regulate immune response, miR-205 is linked to nephropathy, and dysregulation of miR-10 family members is associated with various cancers (Gan et al. 2016). The study proposes using miRNAs as biomarkers for evidence of environmental toxicity. This could be a useful tool to determine the extent of toxicity in organisms.
Sun et al. (2020) found that exposure to DBDPE and BDE-209 was linked to hepatoxicity, liver pathology, and changes in the morphology of the liver. These changes also included an increase in liver weight, with an abnormal liver/body ratio. The study also found that BDE-209 and DBDPE could induce inflammation and oxidative stress, increase serum glucose levels, and interfere with metabolic pathways through the downregulation of enzymes in rats. A recent publication found similar results with DBDPE and BDE-209. Jing et al. (2019) linked BDE-209 to morphological and structural changes in the heart. The study on male rats also found that decabromodiphenyl ethane (DBDPE) and BDE-209 increased inflammatory markers, interleukin-1 beta (IL-1 b), as well as IL6 and IL10. The results of the study also indicated that BDE-209 had stronger toxic effects and could cause oxidative stress, inflammation, and heart damage. DBDPE also caused oxidative stress, lipid peroxidation, genetic toxicity, and DNA damage in the earthworm, Eisenia fetida (Zhao et al. 2020). Lipid peroxidation and enzyme inhibition were also found in a study by Feng et al. (2013) in Carassius auratus. These findings present severe implications for public health as chronic exposure and high concentrations of DBDPE lead to heart and liver disease, lipid peroxidation, and possibly DNA damage.
Ji et al. (2014) discovered gender-specific responses to TBBPA. The physiological effects on the model organism, Mytilus galloprovincialis, were determined using iTRAQ-based proteome analysis. TBBPA exposure may cause a variety of physiological responses, including apoptosis, signal transduction, immunological and oxidative stress, and energy disturbance. More importantly, the study revealed gender-specific responses and encouraged inclusion of both genders when investigating the effects of environmental toxicity of BFRs. Several articles highlight a cocktail of pollutants, including BFRs that may be responsible for the declining sperm counts and semen quality within the past few decades (Ingle et al. 2018; Yu et al. 2018). In addition, BFRs were cited as causing fertility problems in both genders as well as developmental problems in children (Kodavanti et al. 2022).
5.3 Persistence of BFRs in the Environment
Polybrominated diphenyl ethers (PBDEs) are ubiquitous in the environment as they are present in air, soil, and water. PBDEs, penta-PBDE, octa-PBDE, and deca-PBDEs, have been on the persistent organic pollutants (POPs) list since 2017 (Jing et al. 2019; Ezechiáš et al. 2014). Altogether, there are 209 different congeners. Since PBDEs are not bound to other chemicals, they can be easily added to furniture or textiles. In addition, they are volatile and quickly released into the air (Webster et al. 2009). For this reason, household dust and indoor air have a higher concentration of PBDEs than outdoors. Novel PBDEs have been used as a replacement for legacy PBDEs however, they are also persistent in the environment and are toxic and biomagnified in the food chain (Ezechiáš et al. 2014).
Due to various physiological properties such as low vapor pressure, low Henry’s law constant, low solubility, and low octanol-water partition, legacy BFRs such as TBBPA can quickly be deposited onto the soil, sediments, and particles in the atmosphere (Dong et al. 2022; Sunday et al. 2022). The majority of BFRs are additives. They are added and mixed as the polymer is being made but are not usually bound to the polymer covalently (Yu et al. 2016). Therefore, PBDEs are considered additives, allowing them to easily volatilize away from the original product they were added to and subsequently enter the environment (Yang et al. 2018). More concerning is that little is known about the toxicity of partially degraded BFRs or lower brominated BFRs as a result of natural environmental processes. Moreover, assessing the amount of BFRs trapped in sedimentation is difficult. Figure 5.1 illustrates the broad distribution of BFRs in soil and sedimentation in Europe, Asia, and the USA. Lao et al. (2023) found that the amounts of BFRs, especially PBDEs, present in sediment were significantly correlated with the amount of industrialization and output of the surrounding geographical areas along the Pearl River Delta (Lao et al. 2023). Figure 5.1 shows the distribution of various BFRs in Asia, Europe, the USA, and Japan in soil and sediment.
BFR consumption and distribution in soil and sediment. [Retrieved from Yu et al. (2016)]
5.3.1 Potential Exposure to Legacy BFRs and NBFRs in Indoor and Outdoor Settings
The persistence of legacy BFRs in indoor settings poses a health risk for humans, and the same may be expected of NBFRs. Four main routes of toxicity were identified. These are ingestion of indoor dust, absorption through the skin, inhalation of BFRs in indoor dust, and ingestion of BFRs in food (Zuiderveen et al. 2020). Reche et al. (2019) collected samples of outdoor ambient air, indoor workplace ambient air, and indoor dust across Spain to determine concentrations and trends for each. The study found that high concentrations of outdoor PBDE ranging from 1.18 to 28.6 pg m−3 were correlated to outdoor landfills and recycling centers. In addition, high dechlorane plus (DP) concentrations in indoor air at concentrations of 2.90–42.6 pg m−3 were strongly correlated to new electronic devices.
A similar study by McGrath et al. (2018) conducted in Melbourne, Australia, tested 51 dust samples from homes, offices, and vehicles to identify prominent BFRs in each setting. The BFRs tested were eight PBDEs (−28, −47, −99, −100, −153, −154, −183, and −209) and seven NBFRs (PBT, PBEB, HBB, EH-TBB, BEH-TEBP, BTBPE, and DBDP) identified using selective pressurized liquid extraction (S-PLE) and gas chromatography coupled to tandem mass spectrometry (GC-MSMS). The study also found that legacy and NBFRs were linked to specific areas, such as offices with the highest concentrations of penta-BDE. At the same time, homes and vehicles contained higher levels of EH-TBB and BDE 209. In addition, toddlers were more at risk by up to 2 orders of magnitude than adults for exposure to PBDEs and NBFRs. This is especially concerning since evidence suggests that BFRs are neurotoxic to children (Roze et al. 2009).
Ingestion of BFRs has been found to vary with different foods and locations close to e-waste recycling areas. Various studies have found the presence of PBDEs in fish and meat products in China (Shi et al. 2018). Sun et al. (2014) found that daily consumption of PBDEs in the coastal regions of South China ranged from 1.42 to 5.91 ng d−1. HBCDs were ubiquitous in human milk and food products of animal origin in China despite that HBCD has been restricted since 2016 (Shi et al. 2018). The implications of this are that HBCD and relevant congeners are likely airborne, and contaminated dust is being ingested and tends to persist in the environment and biomagnify in the food chain.
5.4 Biodegradation of BFRs in Soils and Sedimentation Under Anaerobic and Aerobic Conditions
The kinetic rates for the biodegradation of BFRs in both anaerobic and aerobic soils differ. Generally, degradation kinetics are faster in aerobic soils (Nyholm et al. 2010). However, biodegradation is much more substantial and efficient under anaerobic conditions (Gerecke et al. 2006). During the anaerobic digestion of organic micropollutants, four main stages occur. These consist of methanogenesis, hydrolysis, acidogenesis, and acetogenesis (Carneiro et al. 2020). These processes are also influenced by microorganisms and cometabolite biotransformation (Arias et al. 2018).
Biodegradation of other BFRs, such as tetrabromobisphenol A (TBBPA), was found to be influenced by the complexity of the carbon source. Complex sources such as wastewater, as opposed to glucose, were biodegraded slower (Macêdo et al. 2022). This was further corroborated by Balaban et al. (2021) demonstrating that the addition of a vitamin source delayed bacterial growth and, as a result, reduced TBNPA and DBNPG biodegradation. Moreover, the study concluded that concentrations higher than 0.5 mg L−1 inhibited biodegradation in Clostridium spp. This is in agreement with Wang et al. (2022b), who stated that higher concentrations of DBDPE inhibit biodegradation. Concentrations higher than 50 mg L−1 were too toxic for the organisms.
Clostridium spp. has also proven to be effective in the biodegradation of HBCD (Li et al. 2020). In this study, both Bacillus spp. and Clostridium spp. were capable of biodegradation of up to 70% and 77% from cell suspensions taken from Chiang Chung soil and riverbank soil, respectively. The biodegradation was conducted under aerobic conditions. The biodegradation kinetics was slower in soil than in soil suspension for this study.
Huang et al. utilized a system of maize plants and P. aeruginosa strain HS9 to remove HBCD from the soil. The optimal temperature and pH reported were 30 °C at pH8, respectively, for the increased degradation rate of hexabromocyclododecane (HBCD). The study reported that the HS9 strain could remove 69% of the 1.7 mg L−1 of HBCDs (α-, β-, and γ-HBCD) in 14 days. The addition of HS9 was also found to stimulate plant growth by removing HBCDs from the soil. Further, adding HS9 enriched the number of microbes in rhizospheric soil. This included fungal microbes, an essential part of soil microbial ecosystems. Peng et al. (2015) reported degradation of HBCD and a-HBCD to 90% under similar culturing conditions of 30 °C and pH 7 using the bacterial strain Achromobacter sp. Stepwise increasing additions of HBCD were added to culturing conditions to optimize the ability to biodegrade α-, β-, and γ-HBCD in a study by Geng et al. (2019). The biodegradation of HBCD was under aerobic conditions using a Pseudomonas sp. strain GJY at 30 °C at pH 7. Soil samples were collected from Ziya e-waste recycling centers in Tianjin, China. The strains were derived from the soil samples using extinction-dilution techniques. The degradation of each isomer was conducted in MSM with each diastereoisomer. After 8 days, the results showed degradation efficiencies of 85.38%, 82.64%, and 75.5% for α-, β-, and γ-HBCD, respectively.
Another synergistic application for degradation was applied to degrade BDE-209. Chang et al. (2021) utilized a novel bio-slurry bioreactor (NBB), which consisted of UVA LED irradiation coupled with biodegradation by microbes in a Ca-montmorillonite clay slurry. The NBB coupled UV-resistant bacteria, Stenotrophomonas sp., Pseudomonas sp., and Microbacterium sp., and UV photolysis was carried out in a clay slurry under anaerobic conditions. Quantification of debromination was done by measuring Br− using ion chromatography. Biodegradation was done by using BDE-209 as the sole source of carbon. The degradation kinetics were most efficient with the coupled system than either alone.
Yan et al. (2018) reported using soil columns under anaerobic conditions to degrade pentabromodiphenyl ether (BDE-91). The study replaced oxygen with sulfate as the final electron acceptor in columns filled with soil to remediate contaminated reclaimed water to recharge groundwater. Interestingly, this study used sulfate-reducing bacteria and archaea for the biodegradation of BDE-91. Elevated levels of sulfate were found to enhance the biodegradation of BDE-91.
Anaerobic conditions were utilized in a study by Ramaswamy et al. (2021). Dehalococcoides mccartyi strain CG1 debrominated tetrabromobisphenol A (TBBPA) ultimately into BPA in 10 days. Dehalococcoides mccartyi strain CG1 was able to utilize TBBPA as a sole source of carbon. Furthermore, a proteomic analysis revealed that the reductive dehalogenase, PcbA1, was responsible for debromination of TBBPA. Therefore, the acceleration of biodegradation of TBBPA was interpreted as metabolic utilization of TBBPA. Furthermore, a 92-fold increase in cell density of D. mccartyi strain CG1 demonstrated further evidence of this.
BDE-209 and other polybrominated diphenyl ethers can be broken down by coupling a Fenton system with persulfate (Wu et al. 2020). This abiotic approach can remove BDE-209 from soils or other hard surfaces. The study reported degradation efficiency ranging from 73.4 to 95.8%. The addition of persulfate resulted in the generation of SO4. The SO4 was believed to make a nucleophilic attack on BDE-209, resulting in debromination to lower brominated constituents and pentabromophenol (Wu et al. 2020). The mechanism was achieved through the cleavage of C–O bonds and subsequent replacement with OH groups (Wu et al. 2020). The resulting products, nona-BDEs and pentabromophenols, were vulnerable to further transformation. The study presents this data as a cost-effective solution for removing BDE-209 from soils in an aerobic environment.
Larger eukaryote organisms are potential solutions to removing BFRs from the soil. Earthworms were used in a study by Qiao et al. (2022) for the potential removal of BFRs: BDE 209, PBT, DBDPE, BTBPE, and HBB. The removal of these BFRs varied with each BFR. BFRs with similar molecular weights and chemical structures had similar enrichment and removal results. The study also demonstrated the potential for secondary pollution from worm castings that were not retained in the earthworm. The use of earthworms could remove BDE-209 and DBDPE from the soil. The removal of the other BFRs could have been more efficient.
5.5 Biodegradation of Contaminated Water and Sediment Matrices
Systematic surveillance of BFRs in wastewater treatment plants (WWTPs) is crucial for understanding efficient methods of removal and contamination. Moreover, little is known about certain BFRs, such as 1,2-dibromo-4-(1,2-bromomethyl) cyclohexane (TBECH), in aqueous environments. Anaerobic digestion is a well-studied aspect of wastewater treatment. Hydrolysis is essential in this process and facilitates the enzymatic breakdown of larger molecules into monomers (Macêdo et al. 2022). Carneiro et al. (2020) found that hydrolysis and acidogenesis were crucial steps in removing organic micropollutants from wastewater. Wastewater treatment plants could detoxify wastewater by coupling bioremediation methods with regular treatments. Ruan et al. (2019) discovered that BFRs were prevalent in influent water sampled from various WWTPs in Hong Kong. This work emphasized the need to monitor the enantiomer-specific behavior of chiral BFRs in the various treatments used in WWTPs. The study also discovered that biological treatment resulted in more efficient BFR elimination and enantiomer-specific degradation of chiral BFRs.
A novel approach of combining an upflow anaerobic sludge blanket bioreactor (UASB) and integrated fixed film/activated sludge (IFAS) system can increase the efficiency of organic micropollutant removal as was done in a study by Arias et al. (2018). This innovative approach reduced nitrogen by using methane as an electron donor. The study reported that the system consisting of methanotrophs and heterotrophic denitrifiers removed 93% of chemical oxygen demand and dissolved methane in the UASB effluent. The purpose of the system was to increase microbial diversity to achieve more efficient removal of organic micropollutants. However, different culturing conditions, which will be discussed further, are essential for achieving this goal.
In a study by Balaban et al. (2021), a four-strain consortium was used to degrade both TBNPA and DBNPG. Table 5.1 lists the genera, GenBank accession number, and species in the consortium that biodegraded both TBNPA and DBNPG. Interestingly, the study reported that both compounds were degraded at similar rates. The authors hypothesized that this might be due to similar enzymes and metabolic pathways of degradation for both. The study proposed a monooxygenase pathway for degradation. When both TBNPA and DBNPG were added, the degradation kinetics were almost twice as long (from 3–4 days to 7 days). Bacterial growth was determined to be the limiting factor, and a carbon source was needed for biodegradation. Yeast extract and glucose significantly enhanced biodegradation (3–7 days), while the vitamin mix slowed degradation kinetics (1–2 months).
Liang et al. (2019a) found that efficient removal of typical BFR, 2,4,6-tribromophenol (TBP), was possible using Bacillus sp. GZT. This study also identified genes and enzymes corresponding to the bioremediation of TBP and proposed an enzymatic pathway (pictured below in Fig. 5.2) for biodegradation. Interestingly, the study proposed the possibility of biodegradation enhancement and tolerance with recombinant strains containing the genes: tbpA, tbpB, tbpC, tbpD, and tbpE.
Lin et al. (2021) used a microbial fuel cell to bioremediate and detoxify wastewater. Microbial fuel cells (MFCs) have been used more recently to biodegrade various organic compounds and recover energy by increasing the electron transfer rate and biodegradation efficiency (Hassan et al. 2018). The efficiency of using MFCs is evident with a less toxic final product of bioremediation and energy recovery through bioelectrochemical processes (Hassan et al. 2018). Lin et al. used different genera of bacteria for dehalogenation (Pseudomonas), electroactive bacteria (Desulfovibrio), and aromatic ring-cleaving bacteria (Geobacter) in the MFC for further biodegradation than bisphenol A.
5.6 Catalytic Methods of Degradation and Reduction of BFRs in Wastewater, Aquatic Matrices, and Sediment
The presence of BFRs in sedimentation from a temporospatial aspect needs to be better studied and understood. In a study by Vauclin et al. (2021), sediment cores were taken along the backwater areas along the Rhône River. An age-depth model was established to find how the concentrations of BFRs and other pollutants were prevalent for each period. The findings were consistent with phasing out certain pollutants, such as polychlorinated biphenyls, which showed lower concentrations after phasing out. The study also found that novel and legacy BFRs reached peak concentrations in the early 2000s and have remained stable since the 2010s. The study highlighted the importance of sediment cores for determining spatiotemporal trends in both legacy and novel BFRs. Due to the hydrophobicity of BFRs, sedimentation often becomes a sink, and high concentrations of BFRs can be found in river sedimentation near e-waste sights (Yu et al. 2016). Xiong et al. (2017) proposed bioaugmentation with Bacillus sp. GZT for TBP removal from river water/sediment. This is in addition to the phyla isolated from sediments. These were Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes. In this study, the biodegradation of TBP was enhanced by supplementation with NaCl, glucose, yeast extract, sodium propionate, and humic acids.
Catalytic methods of removing BFRs from wastewater have been explored in various studies. These methods include photocatalytic, electrocatalytic, and plasma catalytic degradation and reduction. Sedimentation can reduce and debrominate BFRs through microorganisms or reductive catalysis. Green rust (GR), which consists of layers of sedimentation with Fe(II) and high amounts of Fe2+–O–Fe3+ in the iron hydroxide layer, is capable of reductive catalysis (O’Loughlin and Burris 2004). Figure 5.2 illustrates the proposed mechanism of reduction by GR-CuNPs, which primarily occurs on the surface of the GR. O’Loughlin and Burris (2004) demonstrated that the addition of Cu and Ag significantly enhanced the reducing capabilities of GR on halogenated organic compounds. Fang et al. (2019) also confirmed that adding Cu nanoparticles (Cu NP) to GR enhanced the reduction of TBBPA. The GR was interlayered with Cl−, SO42−, and CO32−. The GR(Cl)-Cu NP obtained the greatest degradation efficiency at 92.11%.
Proposed mechanism of reduction by GR-Cu NPs. The reduction of TBBPA occurs on the surface of Cu NPs, and electrons from GR are transferred to the active sites of Cu NPs. GR(Cl) is transformed into two byproducts, goethite and magnetite, after TBBPA is reduced. [Retrieved from Fang et al. (2019)]
Enhanced heterogeneous photo-Fenton catalytic photodegradation was utilized by Huang et al. (2020). An efficient degradation rate of 97.4% was achieved by coupling bio-template synthesized ceria with natural ferrihydrites in a novel heterogeneous photo-Fenton system. Furthermore, adding bio-template synthesized ceria with natural ferrihydrites resulted in the regeneration of Fe2+ and the production of photoelectrons, which is often a limiting factor.
Natural organic matter is an environmentally friendly alternative for use in photocatalytic degradation. Soluble organic matter can form active free radicals such as OH when hit with visible light that, in turn, can oxidize BFRs (Dong et al. 2022). Natural organic matter such as humic acids and carboxylate ions in the environment has proven to be a promising solution to BFRs. Humic acids can form reactive oxygen species or photochemically produced reactive intermediates capable of degrading persistent organic pollutants (Dong et al. 2022). For example, Son et al. (2019) used Aldrich humic acid to photodegrade HBCD and its three diastereoisomers in simulated solar light. Likewise, Zhang et al. (2018) found that dissolved organic matter could photodegrade novel BFR, 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE), in simulated light with the addition of chlorine.
5.7 Mechanisms of Biodegradation
The debromination of BFRs is the most important step in the biodegradation of BFRs since it allows for complete mineralization (Segev et al. 2009a). In general, the more bromines in a BFR, the slower the biodegradation rate. Typically, the arrangement and amount of bromines or halogens are also inversely proportional to biodegradation kinetics. Under anaerobic conditions, biodegradation of PBDEs favors reductive bromination or reduces the number of bromines (Zhao et al. 2018). In aerobic degradation, cleavage of the aromatic ring occurred first, followed by debromination and hydroxylation (Zhao et al. 2018). It is important to note that reductive removal of the halogen or debromination forming a halide anion is crucial in reducing the toxicity of the compound in question (Hug et al. 2013). This outlines the importance of organohalide respiration, which refers to the respiration process where anaerobic bacteria use halogenated hydrocarbons as a final electron acceptor (Hug et al. 2013).
The fate and intermediate lower brominated BFRs, such as in PBDEs, can often be even more environmentally toxic than the parental species (Pan et al. 2016). Therefore, it is important to understand the fate, mechanisms, and degradation kinetics of both novel and legacy BFRs undergoing natural photodegradation (Zhang et al. 2018). Pan et al. (2016) reviewed the fate of PBDEs in various environmental matrices, including aqueous, organic, solid, gas, and Ti–O2-mediated phases, to understand the natural photodegradation of PBDE congeners.
In some instances, the method of degradation influenced the resulting congeners. For example, Wang et al. (2019) investigated the debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), which had preferential debromination at the para-bromine in the H-transfer system to generate BDE-17, while the preference was ortho-bromine in an electron transfer system to generate BDE-28. Both methods were part of a nanoscale zerovalent iron (n-ZVI) system and six n-ZVI-based bimetallic systems (Fe/Cu, Fe/Ni, Fe/Pd, Fe/Ag, Fe/Pt, and Fe/Au). Interestingly, the metals Pd, Pt, Ni, Cu, and Au use hydrogen gas to debrominated PBDEs. The study determined that bimetallic and NaBH4, Fe/Pt, Fe/Ni, and Fe/Pd preferred H-transfer mechanisms, while e-transfer mechanisms preferred Fe/Ag. Conversely, Fe/Cu and Fe/Au preferentially debrominate equally under e-transfer and H-transfer mechanisms. The mechanism for debromination of halogenated compounds has been divided into two hypotheses. The first hypothesis has been to attribute dehalogenation to electron transfer, where the difference in corrosion potential between Fe and the additive metal allowed for more current and, thus, more electron transfer (Yan et al. 2010; Wang et al. 2019). The other hypothesis is that the additive’s ability to absorb hydrogen would dictate the speed of hydrogen transfer and, thus, dehalogenation (Chun et al. 2010; Wang et al. 2019). Chun et al. (2010) also concluded that the size and distribution of the metal additives on the surface of Fe were the most important variable. Hydrogen transfer in a palladized zerovalent zinc (Pd/ZVZ) system is also pH dependent, as illustrated in a study by Xu et al. (2020).
As previously mentioned, Geng et al. (2019) conducted biodegradation studies using Pseudomonas strain GJY. The study also proposed a mechanism of biodegradation of the three diastereoisomers of HBCD. In addition, they tracked subsequent metabolites using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MSMS). The study proposed that the pathway for HBCD biodegradation consisted of ring opening, hydroxyl substitution, and debromination. This pathway differs from other biotransformation studies of HBCD and highlights that microorganisms influence how HBCD isomers are distributed in environmental settings. Figure 5.3 highlights a proposed pathway of debromination of HBCD-contaminated soil from Chiang Chun (Li et al. 2020).
The proposed pathway of HBCD biodegradation through the process of debromination from soils collected from Chiang Chun. The metabolites were identified via gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). [Retrieved from Li et al. (2020)]
5.8 Genes and Enzymes Involved in the Biodegradation of BFRs
Genome annotations and identification of enzymes involved in biodegradation pathways are crucial for successful biodegradation of BFRs and other pollutants. Liu et al. (2015) overexpressed tbbpa A for biodegradation studies of TBBPA. This gene was identified due to upregulation when exposed to TBBPA. Whole-genome sequencing of Ochrobactrum sp. strain T was compared to the NCBI database for identification of potential TBBPA-degrading genes. Gene tbbpa A was identified and cloned into an expression vector. The constructed strain was able to degrade TBBPA and removed bromine with 78% efficiency and demineralization at 37.8% efficiency in 96 h. This was observed to be very similar to the parental strain. These results demonstrated the possibility of creating constructs for the purpose of biodegradation of POPs. Culturing conditions were aerobic in mineral medium at 37 °C at pH 7. Table 5.2 lists the genes and enzymes that have been identified as capable of degradation and mineralization of BFRs. Some enzymes may be capable of biodegradation of multiple BFRs with similar structures.
Whole-genome sequencing of microorganisms capable of debromination as well as biodegradation is another important step in deducing the molecular mechanisms involved in bioremediation of BFRs (Shah et al. 2018; Liang et al. 2019a). Genes encoding enzymes that play an important role in the biodegradation of BFRs are ideal targets for genomic analysis when considering potential candidates for biodegradation. Wang et al. (2022b) isolated extracellular enzymes, MnP, Lip, Lac, and cytochrome P450, which aided in the biodegradation of DBDPE. The most important extracellular enzyme for degradation was Lac. The study also noted that antioxidant enzymes CAT and SOD were important for reducing the toxicity of P. ostreatus.
Enzymes have a potential to degrade BFRs more efficiently and rapidly than the organism itself. This was illustrated in a study by Liu et al. (2015). Crude enzyme extract was isolated from P. aeruginosa LY11. This strain is known for biodegradation of BDE-209. The crude enzyme was extracted through sonication, centrifugation, and finally filtration through a 0.22 μm filter. The resulting filtrate is what was considered crude enzyme extract. In this study, the crude enzyme was able to degrade BDE-209 in a shorter time period of 5 h and more efficiently at 92.77%. This study provides insight into the possibility of using crude enzyme extract from other microorganisms previously known to biodegrade specific BFRs. The potential to mass produce crude enzyme extract through over-expression systems may be a more efficient means of bioremediation of BFRs.
5.9 Culturing Conditions in Bioremediation Strategies
The culturing conditions for many degradation systems varied with each type of BFR. Some were cultured in aerobic or anaerobic conditions where temperature, pH, and culture supplementation varied with each organism. For some, supplementation was necessary for bioremediation, while other studies used the BFR as the sole carbon source. Table 5.3 summarizes the culturing conditions for various BFRs from optimized protocols for degradation and mineralization.
Tokarz et al. (2008) reported that adding vitamin B12 enhanced the debromination kinetics. Other studies showed enhancements with the addition of glucose or other carbohydrates (Wang et al. 2022b). However, some additions for one strain may enhance degradation and inhibit others. This was evident when adding a vitamin mix slowed the degradation rate of BFRs (Balaban et al. 2021). Some bacterial strains are capable of biodegradation when the BFR is the sole source of carbon. This was observed for the Bacillus cereus JP12 strain (Lu et al. 2013), where BDE-209 was added to MSM at 30 °C, pH 6. Degradation efficiency was enhanced by adding other carbon sources, surfactants, and metals Cu2+ and Zn2+. Similarly, zerovalent iron enhanced the biodegradation of BDE-209 and BDE-28 (Yang et al. 2017). Shah et al. (2018) also used Bacillus cereus for successful biodegradation of HBCD with another strain called HBCD-sjtu at a higher pH of 7 at 30 °C. This demonstrates that physiological conditions were as important as culturing and media for the biodegradation of certain BFRs. It also demonstrated that despite using the same genus and species of bacteria, the BFR degradation influenced the culturing conditions. Optimal temperatures ranged from 30 °C at pH 7 (Peng et al. 2015) to 35 °C at pH 9 (Chang et al. 2021), depending on the bacterial strain. Changes in pH and temperature resulted in less biodegradation efficiency and was dependent on the BFR.
5.10 Conclusion and Future Direction
The possibility of phasing out NBFRs and using bio-sustainable organobromine BFRs is a promising solution to the problem of BFR environmental contamination. Sequencing genomes of microorganisms capable of biodegradation of BFRs is essential for understanding the molecular mechanisms of biodegradation. The potential for isolating genes and enzymes that biodegrade BFRs is high when microorganisms are collected from e-waste sites or near industrial facilities where BFRs are present. There have been many different genera and species of bacteria that have demonstrated the ability to biodegrade various BFRs. However, culturing conditions vary with each species of microbes used.
Another important consideration is the possibility that microorganisms that can remove chloride from hexachlorocyclohexanes may also debrominate BFRs, especially when the chemical structures are similar. Various studies, as previously mentioned, demonstrated that bacterial strains with dehalogenases may also remove halogens from different compounds, thus implying that microorganisms can bioremediate a variety of POPs.
The strategies presented here include various methods to address emerging BFR pollutants from different environmental sources. Catalysis is a proven method for efficient mineralization and biodegradation in abiotic methods. Using natural organic matter is an environmentally friendly way to oxidize BFRs and a promising way to address environmental pollution. One example discussed is humic acids, which form free radicals when exposed to light. In biotic methods, several organisms and systems have been presented. The supplementation and culturing conditions varied with each BFR and organism. The addition of enzymes proved essential for biodegradation and highlights the possibility of engineering enzymes for the degradation of BFRs or in synergy with microorganisms.
The urgent need to remove POPs has never been more evident. Several publications have identified various pollutants including BFRs as being responsible for declining sperm counts over the past few decades. The exposure to BFRs through ingestion, inhalation, and absorption through the skin has taken their toll on the fertility of both genders as well as developmental consequences for children. This exposure to BFRs is most prevalent in indoor settings, e-waste recycling centers, and other industrial locations where BFRs are manufactured. Therefore, the best strategy moving forward is to bioremediate BFRs from wastewater treatment plants before they are released into the surrounding environment.
References
Andersson PL, Öberg K, Örn U (2006) Chemical characterization of brominated flame retardants and identification of structurally representative compounds. Environ Toxicol Chem 25(5):1275–1282. https://doi.org/10.1897/05-342R.1
Arias A, Alvarino T, Allegue T, Suárez S, Garrido JM, Omil F (2018) An innovative wastewater treatment technology based on UASB and IFAS for cost-efficient macro and micropollutant removal. J Hazard Mater 359:113–120. https://doi.org/10.1016/j.jhazmat.2018.07.042
Bakker MM, Govers G, van Doorn A, Quetier F, Chouvardas D, Rounsevell M (2008) The response of soil erosion and sediment export to land-use change in four areas of Europe: the importance of landscape pattern. Geomorphology 98:213–226. https://doi.org/10.1016/j.geomorph.2006.12.027
Balaban N, Gelman F, Taylor AA, Walker SL, Bernstein A, Ronen Z (2021) Degradation of brominated organic compounds (flame retardants) by a four-strain consortium isolated from contaminated groundwater. Appl Sci (Switzerland) 11(14):6263. https://doi.org/10.3390/app11146263
Carneiro RB, Gonzalez-Gil L, Londoño YA, Zaiat M, Carballa M, Lema JM (2020) Acidogenesis is a key step in the anaerobic biotransformation of organic micropollutants. J Hazard Mater 389:121888. https://doi.org/10.1016/j.jhazmat.2019.121888
Chang YT, Chen HC, Chou HL, Li H, Boyd SA (2021) A coupled UV photolysis-biodegradation process for the treatment of decabrominated diphenyl ethers in an aerobic novel bioslurry reactor. Environ Sci Pollut Res 28(5):6078–6089. https://doi.org/10.1007/s11356-020-10753-9
Chen J, Wang C, Pan Y, Farzana SS, Tam NFY (2018) Biochar accelerates microbial reductive debromination of 2,2″,4,4″-tetrabromodiphenyl ether (BDE-47) in anaerobic mangrove sediments. J Hazard Mater 341:177–186. https://doi.org/10.1016/j.jhazmat.2017.07.063
Chou HL, Hwa MY, Lee YC, Chang YJ, Chang YT (2016) Microbial degradation of decabromodiphenyl ether (DBDE) in soil slurry microcosms. Environ Sci Pollut Res 23(6):5255–5267. https://doi.org/10.1007/s11356-015-5767-x
Chun CL, Baer DR, Matson DW, Amonette JE, Penn RL (2010) Characterization and reactivity of iron nanoparticles prepared with added Cu, Pd, and Ni. Environ Sci Technol 44:5079–5085. https://doi.org/10.1021/es903278e
Dong J, Li G, Gao J, Zhang H, Bi S, Liu S (2022) Science of the total environment catalytic degradation of brominated flame retardants in the environment: new techniques and research highlights. Sci Total Environ 848:157695. https://doi.org/10.1016/j.scitotenv.2022.157695
Ezechiáš M, Covino S, Cajthaml T (2014) Ecotoxicity and biodegradability of new brominated flame retardants: a review. Ecotoxicol Environ Saf 110:153–167. https://doi.org/10.1016/j.ecoenv.2014.08.030
Fang L, Liu R, Xu L, Li J, Huang LZ, Li F (2019) Enhanced debromination of tetrabromobisphenol A by zero-valent copper-nanoparticle-modified green rusts. Environ Sci Nano 6:970–980. https://doi.org/10.1039/c8en01289j
Feng M, Li Y, Qu R, Wang L, Wang Z (2013) Oxidative stress biomarkers in freshwater fish Carassius auratus exposed to decabromodiphenyl ether and ethane, or their mixture. Ecotoxicology 22:1101–1110. https://doi.org/10.1007/s10646-013-1097-2
Gan L, Xiong Y, Dong F, Yu Y, Zhang L, Shunmei E, Hu G (2016) Profiling kidney microRNAs from juvenile grass carp (Ctenopharyngodon idella) after 56 days of oral exposure to decabromodiphenyl ethane. J Environ Sci (China) 44:69–75. https://doi.org/10.1016/j.jes.2015.09.022
Geng J, Han M, Yang X, Li Y, Bartlam M, Wang Y (2019) Different biotransformation of three hexabromocyclododecane diastereoisomers by Pseudomonas sp. under aerobic conditions. Chem Eng J 374:870–879. https://doi.org/10.1016/j.cej.2019.05.232
Gerecke AC, Giger W, Hartmann PC, Heeb NV, Kohler HPE, Schmid P, Kohler M (2006) Anaerobic degradation of brominated flame retardants in sewage sludge. Chemosphere 64(2):311–317. https://doi.org/10.1016/j.chemosphere.2005.12.016
Hassan H, Jin B, Donner E, Vasileiadis S, Saint C, Dai S (2018) Microbial community and bioelectrochemical activities in MFC for degrading phenol and producing electricity: microbial consortia could make differences. Chem Eng J 332:647–657. https://doi.org/10.1016/j.cej.2017.09.114
Heeb NV, Wyss SA, Geueke B, Fleischmann T, Kohler HPE, Lienemann P (2014) LinA2, a HCH-converting bacterial enzyme that dehydrohalogenates HBCDs. Chemosphere 107:194–202. https://doi.org/10.1016/j.chemosphere.2013.12.035
Huang L, Wang W, Shah SB, Hu H, Xu P, Tang H (2019) The HBCDs biodegradation using a Pseudomonas strain and its application in soil phytoremediation. J Hazard Mater 380:120833. https://doi.org/10.1016/j.jhazmat.2019.120833
Huang X, Zhu N, Mao F, Ding Y, Zhang S, Liu H, Li F, Wu P, Dang Z, Ke Y (2020) Enhanced heterogeneous photo-Fenton catalytic degradation of tetracycline over yCeO2/Fh composites: performance, degradation pathways, Fe2+ regeneration and mechanism. Chem Eng J 392:123636. https://doi.org/10.1016/j.cej.2019.123636
Hug LA, Maphosa F, Leys D, Löffler FE, Smidt H, Edwards EA, Adrian L (2013) Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Philos Trans R Soc B Biol Sci 368:20120322. https://doi.org/10.1098/rstb.2012.0322
Ingle ME, Mínguez-Alarcón L, Carignan CC, Butt CM, Stapleton HM, Williams PL, Ford JB, Hauser R, Meeker JD (2018) The association between urinary concentrations of phosphorous-containing flame retardant metabolites and semen parameters among men from a fertility clinic. Int J Hyg Environ Health 221:809–815. https://doi.org/10.1016/j.ijheh.2018.05.001
Ji C, Wu H, Wei L, Zhao J (2014) iTRAQ-based quantitative proteomic analyses on the gender-specific responses in mussel Mytilus galloprovincialis to tetrabromobisphenol A. Aquat Toxicol 157:30–40. https://doi.org/10.1016/j.aquatox.2014.09.008
Jing L, Sun Y, Wang Y, Liang B, Chen T, Zheng D, Shi Z (2019) Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats. Chemosphere 223:675–685. https://doi.org/10.1016/j.chemosphere.2019.02.115
Kim YM, Nam IH, Murugesan K, Schmidt S, Crowley DE, Chang YS (2007) Biodegradation of diphenyl ether and transformation of selected brominated congeners by Sphingomonas sp. PH-07. Appl Microbiol Biotechnol 77:187–194. https://doi.org/10.1007/s00253-007-1129-z
Kodavanti PRS, Stoker TE, Fenton SE (2017) Brominated flame retardants. In: Reproductive and developmental toxicology. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-804239-7.00038-X
Kodavanti PRS, Stoker TE, Fenton SE, Curras-Collazo M (2022) Brominated flame retardants. In: Reproductive and developmental toxicology. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-323-89773-0.00036-9
Lao Z, Li H, Liao Z, Liu Y, Ying G, Song A, Liu M, Liu H, Hu L (2023) Spatiotemporal transitions of organophosphate esters (OPEs) and brominated flame retardants (BFRs) in sediments from the Pearl River Delta, China. Sci Total Environ 855:158807. https://doi.org/10.1016/j.scitotenv.2022.158807
Li Y-J, Li M-H, Shih Y (2020) Aerobic degradation and the effect of hexabromocyclododecane by soil microbial communities in Taiwan. Environ Int 145:106128. https://doi.org/10.1016/j.envint.2020.106128
Li R, Luo C, Qiu J, Li Y, Zhang H, Tan H (2022) Metabolomic and transcriptomic investigation of the mechanism involved in enantioselective toxicity of imazamox in Lemna minor. J Hazard Mater 425:127818. https://doi.org/10.1016/j.jhazmat.2021.127818
Liang Z, Li G, Mai B, An T (2019a) Biodegradation of typical BFRs 2,4,6-tribromophenol by an indigenous strain Bacillus sp. GZT isolated from e-waste dismantling area through functional heterologous expression. Sci Total Environ 697:134159. https://doi.org/10.1016/j.scitotenv.2019.134159
Liang Z, Li G, Mai B, Ma H, An T (2019b) Application of a novel gene encoding bromophenol dehalogenase from Ochrobactrum sp. T in TBBPA degradation. Chemosphere 217:507–515. https://doi.org/10.1016/j.chemosphere.2018.11.004
Lin XQ, Li ZL, Nan J, Su JH, Liang B, Li CJ, Wang AJ (2021) Biodegradation and metabolism of tetrabromobisphenol A in microbial fuel cell: behaviors, dynamic pathway and the molecular ecological mechanism. J Hazard Mater 417:126104. https://doi.org/10.1016/j.jhazmat.2021.126104
Liu Y, Gong AJ, Qiu LN, Li JR, Li FK (2015) Biodegradation of decabromodiphenyl ether (BDE-209) by crude enzyme extract from Pseudomonas aeruginosa. Int J Environ Res Public Health 12:11829–11847. https://doi.org/10.3390/ijerph120911829
Lu M, Zhang ZZ, Wu XJ, Xu YX, Su XL, Zhang M, Wang JX (2013) Biodegradation of decabromodiphenyl ether (BDE-209) by a metal resistant strain, Bacillus cereus JP12. Bioresour Technol 149:8–15. https://doi.org/10.1016/j.biortech.2013.09.040
Macêdo WV, Poulsen JS, Oliveira GHD, Nielsen JL, Zaiat M (2022) Tetrabromobisphenol A (TBBPA) biodegradation in acidogenic systems: one step further on where and who. Sci Total Environ 808:152016. https://doi.org/10.1016/j.scitotenv.2021.152016
McGrath TJ, Morrison PD, Ball AS, Clarke BO (2018) Concentrations of legacy and novel brominated flame retardants in indoor dust in Melbourne, Australia: an assessment of human exposure. Environ Int 113:191–201. https://doi.org/10.1016/j.envint.2018.01.026
Nyholm JR, Lundberg C, Andersson PL (2010) Biodegradation kinetics of selected brominated flame retardants in aerobic and anaerobic soil. Environ Pollut 158(6):2235–2240. https://doi.org/10.1016/j.envpol.2010.02.010
O’Loughlin EJ, Burris DR (2004) Reduction of halogenated ethanes by green rust. Environ Toxicol Chem 23:41–48. https://doi.org/10.1897/03-45
Pan Y, Tsang DCW, Wang Y, Li Y, Yang X (2016) The photodegradation of polybrominated diphenyl ethers (PBDEs) in various environmental matrices: kinetics and mechanisms. Chem Eng J 297:74–96. https://doi.org/10.1016/j.cej.2016.03.122
Peng X, Huang X, Jing F, Zhang Z, Wei D, Jia X (2015) Study of novel pure culture HBCD-1, effectively degrading hexabromocyclododecane, isolated from an anaerobic reactor. Bioresour Technol 185:218–224. https://doi.org/10.1016/j.biortech.2015.02.093
Qiao Z, Lu C, Han Y, Luo K, Fu M, Zhou S, Peng C, Zhang W (2022) Enrichment and removal of five brominated flame retardants in the presence of co-exposure in a soil-earthworm system. SSRN Electron J 2022:9154. https://doi.org/10.2139/ssrn.4089154
Ramaswamy R, Zhao S, Bae S, He J (2021) Debromination of TetraBromoBisphenol-A (TBBPA) depicting the metabolic versatility of Dehalococcoides. J Hazard Mater 419:126408. https://doi.org/10.1016/j.jhazmat.2021.126408
Reche C, Viana M, Querol X, Corcellas C, Barceló D, Eljarrat E (2019) Particle-phase concentrations and sources of legacy and novel flame retardants in outdoor and indoor environments across Spain. Sci Total Environ 649:1541–1552. https://doi.org/10.1016/j.scitotenv.2018.08.408
Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF (2009) Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect 117:1953–1958. https://doi.org/10.1289/ehp.0901015
Ruan Y, Zhang K, Lam JCW, Wu R, Lam PKS (2019) Stereoisomer-specific occurrence, distribution, and fate of chiral brominated flame retardants in different wastewater treatment systems in Hong Kong. J Hazard Mater 374:211–218. https://doi.org/10.1016/j.jhazmat.2019.04.041
Segev O, Kushmaro A, Brenner A (2009a) Environmental impact of flame retardants (persistence and biodegradability). Int J Environ Res Public Health 6(2):478–491. https://doi.org/10.3390/ijerph6020478
Segev O, Meusel W, Friedenberger M, Brenner A, Kushmaro A (2009b) Aerobic biodegradation of the brominated flame retardants, dibromoneopentyl glycol and tribromoneopentyl alcohol. Biodegradation 20(5):621–627. https://doi.org/10.1007/s10532-009-9249-z
Shah SB, Ali F, Huang L, Wang W, Xu P, Tang H (2018) Complete genome sequence of Bacillus sp. HBCD-sjtu, an efficient HBCD-degrading bacterium. 3 Biotech 8:291. https://doi.org/10.1007/s13205-018-1326-8
Shi Z, Zhang L, Li J, Wu Y (2018) Legacy and emerging brominated flame retardants in China: a review on food and human milk contamination, human dietary exposure, and risk assessment. Chemosphere 198:522–536. https://doi.org/10.1016/j.chemosphere.2018.01.161
Son MH, Gong J, Seo S, Yoon H, Chang YS (2019) Photosensitized diastereoisomer-specific degradation of hexabromocyclododecane (HBCD) in the presence of humic acid in aquatic systems. J Hazard Mater 369:171–179. https://doi.org/10.1016/j.jhazmat.2019.02.035
Sun YX, Hao Q, Xu XR, Luo XJ, Wang SL, Zhang ZW, Mai BX (2014) Persistent organic pollutants in marine fish from Yongxing Island, South China Sea: levels, composition profiles and human dietary exposure assessment. Chemosphere 98:84–90. https://doi.org/10.1016/j.chemosphere.2013.10.008
Sun Y, Wang Y, Liang B, Chen T, Zheng D, Zhao X, Shi Z (2020) Hepatotoxicity of decabromodiphenyl ethane (DBDPE) and decabromodiphenyl ether (BDE-209) in 28-day exposed Sprague-Dawley rats. Sci Total Environ 705:135783. https://doi.org/10.1016/j.scitotenv.2019.135783
Sunday OE, Bin H, Guanghua M, Yao C, Zhengjia Z, Xian Q, Xiangyang W, Weiwei F (2022) Review of the environmental occurrence, analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environ Res 206:112594. https://doi.org/10.1016/j.envres.2021.112594
Tokarz JA, Ahn MY, Leng J, Filley TR, Nies L (2008) Reductive debromination of polybrominated diphenyl ethers in anaerobic sediment and a biomimetic system. Environ Sci Technol 42:1157–1164. https://doi.org/10.1021/es071989t
Uhnáková B, Petříčková A, Biedermann D, Homolka L, Vejvoda V, Bednář P, Papoušková B, Šulc M, Martínková L (2009) Biodegradation of brominated aromatics by cultures and laccase of Trametes versicolor. Chemosphere 76:826–832. https://doi.org/10.1016/j.chemosphere.2009.04.016
Van der Merwe JJ (2002) Production of laccase by the white-rot fungus Pycnoporus sanguineus. University of the Free State, Bloemfontein
Vauclin S, Mourier B, Dendievel AM, Marchand P, Vénisseau A, Morereau A, Lepage H, Eyrolle F, Winiarski T (2021) Temporal trends of legacy and novel brominated flame retardants in sediments along the Rhône River corridor in France. Chemosphere 271:129889. https://doi.org/10.1016/j.chemosphere.2021.129889
Waaijers SL, Parsons JR (2016) Biodegradation of brominated and organophosphorus flame retardants. Curr Opin Biotechnol 38:14–23. https://doi.org/10.1016/j.copbio.2015.12.005
Wang R, Tang T, Lu G, Zheng Z, Huang K, Li H, Tao X, Yin H, Shi Z, Lin Z, Wu F, Dang Z (2019) Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems. Sci Total Environ 661:18–26. https://doi.org/10.1016/j.scitotenv.2019.01.166
Wang S, Li W, Chen Y, Liu L, Hou S, Qu J, You H (2022a) Toxicity evaluation of decabromodiphenyl ethane (DBDPE) to Pleurotus ostreatus: oxidative stress, morphology and transcriptomics. J Hazard Mater 431(2021):128625. https://doi.org/10.1016/j.jhazmat.2022.128625
Wang S, Li W, Liu L, Qi H, You H (2022b) Biodegradation of decabromodiphenyl ethane (DBDPE) by white-rot fungus Pleurotus ostreatus: characteristics, mechanisms, and toxicological response. J Hazard Mater 424:127716. https://doi.org/10.1016/j.jhazmat.2021.127716
Webster TF, Harrad S, Millette JR, Holbrook RD, Davis JM, Stapleton HM, Covaci A (2009) Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ Sci Technol 43(9):3067–3072. https://doi.org/10.1021/es803139w
Wu N, Qu R, Li C, Bin-Jumah M, Allam AA, Cao W, Wang Z (2020) Enhanced oxidative degradation of decabromodiphenyl ether in soil by coupling Fenton-persulfate processes: insights into degradation products and reaction mechanisms. Sci Total Environ 737:139777. https://doi.org/10.1016/j.scitotenv.2020.139777
Xiong J, Li G, An T (2017) The microbial degradation of 2,4,6-tribromophenol (TBP) in water/sediments interface: investigating bioaugmentation using Bacillus sp. GZT Sci Total Environ 575:573–580. https://doi.org/10.1016/j.scitotenv.2016.09.017
Xiong P, Yan X, Zhu Q, Qu G, Shi J, Liao C, Jiang G (2019) A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ Sci Technol 53:13551–13569. https://doi.org/10.1021/acs.est.9b03159
Xu Y, Liang C, Zhang T, Tao X, Wang R, Huang K, Pan Z, Dang Z, Yin H, Lu G (2020) Debromination of polybrominated diphenyl ethers (PBDEs) by palladized zerovalent zinc particles: influence factors, pathways and mechanism. Chemosphere 253:126726. https://doi.org/10.1016/j.chemosphere.2020.126726
Yan W, Herzing AA, Li XQ, Kiely CJ, Zhang WX (2010) Structural evolution of Pd-doped nanoscale zero-valent iron (nZVI) in aqueous media and implications for particle aging and reactivity. Environ Sci Technol 44:4288–4294. https://doi.org/10.1021/es100051q
Yan Y, Rene ER, Ma M, Ma W, Li X, Lun X (2018) Role of sulfate on the potential biodegradation of pentabromodiphenyl ether (BDE-99) in soil columns with reclaimed water and microbial community. Int Biodeterior Biodegrad 132:1–9. https://doi.org/10.1016/j.ibiod.2018.05.001
Yang CW, Huang HW, Chang BV (2017) Microbial communities associated with anaerobic degradation of polybrominated diphenyl ethers in river sediment. J Microbiol Immunol Infect 50:32–39. https://doi.org/10.1016/j.jmii.2014.12.009
Yang J, Huang D, Zhang L, Xue W, Wei X, Qin J, Ou S, Wang J, Peng X, Zhang Z, Zou Y (2018) Multiple-life-stage probabilistic risk assessment for the exposure of Chinese population to PBDEs and risk managements. Sci Total Environ 643:1178–1190. https://doi.org/10.1016/j.scitotenv.2018.06.200
Yu G, Bu Q, Cao Z, Du X, Xia J, Wu M, Huang J (2016) Brominated flame retardants (BFRs): a review on environmental contamination in China. Chemosphere 150:479–490. https://doi.org/10.1016/j.chemosphere.2015.12.034
Yu YJ, Lin BG, Liang WB, Li LZ, Chen XC, Xu XY, Xiang MD, Huang S (2018) Associations between PBDEs exposure from house dust and human semen quality at an e-waste areas in South China—a pilot study. Chemosphere 198:266–273. https://doi.org/10.1016/j.chemosphere.2018.01.150
Zhang YN, Wang J, Chen J, Zhou C, Xie Q (2018) Phototransformation of 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) in natural waters: important roles of dissolved organic matter and chloride ion. Environ Sci Technol 52:10490–10499. https://doi.org/10.1021/acs.est.8b03258
Zhao C, Yan M, Zhong H, Liu Z, Shi L, Chen M, Feng H (2018) Biodegradation of polybrominated diphenyl ethers and strategies for acceleration: a review. Int Biodeterior Biodegrad 129:23–32. https://doi.org/10.1016/j.ibiod.2017.12.010
Zhao Y, Sun L, Li Q, Yan X, Li Z, Liu B, Li G (2020) Use of integrated biomarker response for evaluating antioxidant stress and DNA damage of earthworms (Eisenia fetida) in decabromodiphenyl ethane-contaminated soil. Environ Pollut 264:114706. https://doi.org/10.1016/j.envpol.2020.114706
Zuiderveen EAR, Slootweg JC, de Boer J (2020) Novel brominated flame retardants—a review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere 255:126816. https://doi.org/10.1016/j.chemosphere.2020.126816
Acknowledgments
The authors would like to thank the Department of Molecular and Cellular Physiology at Stanford University, Stanford, CA 94305, and Bodoland University in India for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nava, A., Sarma, H. (2023). Persistence, Toxicity, and Strategies for Remediation of Brominated Flame Retardants in Soil and Sedimentation in Aquatic Matrices Under Aerobic and Anaerobic Conditions. In: Sarma, H., Joshi, S. (eds) Land Remediation and Management: Bioengineering Strategies. Springer, Singapore. https://doi.org/10.1007/978-981-99-4221-3_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-4221-3_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4220-6
Online ISBN: 978-981-99-4221-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)