Abstract
Background: 1,4-Dioxane, also known as dioxane, is a water-miscible synthetic industrial chemical used as a stabilizer for chlorinated solvents and feedstock chemical for a wide range of industrial consumer products. It is a heterocyclic organic ether that, through consumer products and industrial, municipal, and domestic effluents, can get into the environment. Due to its volatility and miscibility, short-term exposure results in irritation of the nose, eyes, and throat, while excessive amounts damage the liver and kidney. Long-term exposure results in carcinogenicity to humans that may associate with death. Statement of problem: 1,4-Dioxane is nonbiodegradable in nature and hence persists in the environmental compartments; some methods such as UV peroxide oxidation, direct UV photolysis, and activated carbon adsorption were reported to be effective in the removal of dioxane in the environment. Yet, their adaptation challenges such as complex matrices, running costs, mass balance, and stoichiometry limitations hinder their efficiency. Finding: Mimicking natural or integrated techniques such as bacteriological transformation of dioxane via aerobic, anaerobic, microcosm, integrated microbial community, and co-metabolic techniques is among the robust eco-friendly technologies against these limitations. Soil matrix offers enormous microbial consortium for nature-based remediation of dioxane with high turnup than single microbial strains. Since bacteriological remediation offers adoptable, flexible, and quick implementation strategies that minimizes the use of synthetic chemicals, its fundamental understanding will be inevitable. Conclusion: Nature-based remediation of dioxane is an undoubtable future since apart from the natural occurrence of soil bacteria responsible for degradation, their natural adaptation flexibility, energy conservation, and release of harmless by-products without formation of secondary synergic harmful contaminants present a relatively affordable technique.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
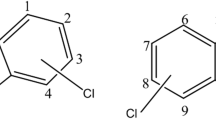
1,4-Dioxane (C4H8O2) is an industrial solvent produced for diverse direct applications including raw material and as a solvent. Its chemical abstract service number (CAS no.) assigned by the American Chemical Society is 123-91-1 with synonyms including dioxan, diethylene dioxide, dioxane, p-dioxane, diethylene oxide, glycol ethylene ether, or diethylene ether (McFee et al. 1994). At laboratory scale, this colorless and flammable liquid is used as a stable reaction media and as an extraction liquid for vegetable and animal oils (ATSDR 2006). Dioxane is useful as a growth substrate in organisms (Barajas-Rodriguez et al. 2019) and solvent in paints, ink, cosmetics, detergent, varnishes, and cleaning and shining fluids. With its nonpolar and aprotic solvent as it is presented in Fig. 4.1, dioxane is in addition utilized in the processing of petrochemicals, pesticide, pharmaceuticals, plastics and rubber, explosives, polishing, and pulp and paper as a solvent (DES 2018).
Among the chemical structures presented in Fig. 4.1, structure B containing two planes of symmetry is the most stable due to minimum atomic constraints. The laboratory-scale production is shown in Fig. 4.2, while the commercial production of dioxane follows reaction root shown in Fig. 4.3.
Laboratory production of 1,4-dioxane (van Buskirk 2014)
Industrial production of 1,4-dioxane (Wilbur and Jones 2012)
Through literature survey, there are no industries in the world that directly produce 1,4-dioxane, implying that its production might be sporadic. The produced dioxane above undergoes heating with acids, distillation, salting out with CaCl2, and distillation again in order to attain high level of purity. More than 90% of the produced dioxane is used for stabilization of chlorinated solvents (US EPA 2014a) such as trichloroethene and 1,1,1-trichloroethane (Milavec et al. 2020).
4.1.1 Properties of 1,4-Dioxane
Dioxane forms an adduct with 1,1,1-trichloroethane (TCE) that inhibits through poisoning the catalytic reaction between TCE and aluminum containers used for transportation of chlorinated solvents (Adamson et al. 2015). In most reactions and solvent requirements, dioxane has replaced tetrahydrofuran, which has high toxicity level than dioxane (Madhu 2018), high boiling point, hygroscopicity, diether chelating ligand (Tusher et al. 2021), and high water miscibility. According to the hard and soft Lewis acids and bases (HSAB) theory (Pinter et al. 2013), dioxane is a hard base implying that it is weakly polarizable, has high charge states, is less volatile, is nonpolar, and has small molecule (Davarani et al. 2012). Dioxane can react as a monodentate with uranium to produce useful materials (Fig. 4.4) for nuclear chemistry (Monreal et al. 2011).
Stable compounds of uranium-containing dioxane. [Image adopted with permission from Monreal et al. (2011)]
However, the bidentate ligand is complex due to bond constraints. The possible reaction reported in Fig. 4.5 indicates the reaction between dioxane and Grignard reagent to form a polymer (Che-revision Administrator 2014).
4.1.2 Sources and Occurrence of 1,4-Dioxane
Reports on the direct production of 1,4-dioxane are rare; however, it occurs in most consumer products such as in cosmetics as a trace contaminant (Hossein et al. 2022) that can penetrate human and animal skin (U.S. FDA 2016). Industrial manufacturing of chlorinated solvents, papers and pulp, agricultural pesticides, organic chemical, dyes, and rubber and textile processing are potential sources of dioxane (Sun et al. 2016). It is a by-product during the manufacturing of aircraft deicing fluids, dyes, antifreeze, greases, paint strippers, and other consumer products (Hossein et al. 2022). Its high water miscibility and use in domestic activities including cleansing and bathing, recreational swimming, industrial production, and automobile activities release substantial amount of dioxane in terrestrial and marine ecosystem (Karges et al. 2018). Through its environmental partitioning, dioxane is rarely available in the atmosphere due to low volatility (Adamson et al. 2021), surface water (Karges et al. 2020), soil (Hinchee et al. 2018), and groundwater (Yamamoto et al. 2018a). The oceanic occurrence of dioxane is also reported by Scaratti et al. (2020). The occurrence of dioxane in the body is rare since it is transformed to β-hydroxyethoxyacetic acid and eliminated through urination (ITRC 2020a). Its occurrence in the drinking water (Adamson et al. 2021), food (Broughton et al. 2019), and particulate matters (Lee and Choo 2013) has proved the occurrence of dioxane in all ecosystem compartments. Thus, mostly use and disposal of chlorinated compounds and consumer products, water and wastewater treatment plants, accidental releases, landfills, and pipe leakages are the major sources of dioxane to the environment.
The LD50 of dioxane in rats is 5170 mg/kg, while the no-observed adverse effect to human being is 400 mg/m3 (Supprenant 2012). Dioxane falls among EPA’s unregulated contaminant monitoring rule (UCMR) that qualifies it as an emerging contaminant (Suthersan et al. 2016; Sarma 2022), and in addition dioxins are classified as persistent organic pollutants (Miraji et al. 2021).
4.1.3 Effects and Fate of 1,4-Dioxane
Compared to other solvents such as ethanol whereby the USA alone produced 84% (6.3 million m3) of the world in the year 2020 (AFDC 2022), recent data of 1,4-dioxane production are limited. For example, in the year 1985, global production of dioxane was 14,000 tons (13.6 million liters) (Wiki 2022); in the year 1990, about 18 million pounds was produced (7.9 million liters); and in the year 2002, the USA alone produced ten million pounds (4.4 million liters) (Wilbur and Jones 2012) indicating a gradual decrease. The absence of recent data on the direct global production of dioxane is not clear, yet most consumer products such as automotive coolants, chemical manufacturing, and textile contain dioxane. Dioxane is volatile (EPA 2010) with atmospheric half-life of 1–3 days (US EPA 2014b). There are several federal standard guidelines for dioxane exposure. Some of them are shown in Table 4.1 (US EPA 2014a).
Breathing of contaminated air (ATSDR 2006); skin adsorption via use of shampoo, toothpaste, and other cosmetics (Alsohaimi et al. 2020); ingestion via packaging material (Gi et al. 2018); food and food supplements/additives (Mo et al. 2022); pesticide remnants in agricultural products (Begum et al. 2016); groundwater (Chu et al. 2018); occupational exposure (US EPA 2014a); industrial effluents (Stepien et al. 2014); and use of spermicidal sponge (ATSDR 2012) are the potential roots of exposure to dioxane.
Experimental research on dioxane has established LD50 of about 2 g/kg for cats and rabbits, 5.7–5.9 g/kg in mice, and 3.15–4 g/kg in guinea pigs. In this study, kidney and liver were the chronic affected organs (OEHHA 1998). The United States Environmental Protection Agency (US EPA) has reported 35 μg/L of dioxane as a cancer risk level (US EPA 2012) and classified it as group B2 chemical that probably causes human carcinogenic effect (McElroy et al. 2019). The Office of Environmental Health Hazard Assessment (OEHHA) has recommended 3 ppb of dioxane as a maximum level in the drinking water (OEHHA 1998). Evidence of dioxane carcinogenicity is reported and summarized by the Scientific Committee on Consumer Safety in its report number SCCS/1570/15 indicating that while the EU classifies it as a carcinogen category 2, the IARC classifies it as a group 2B carcinogen chemical. The SCCS recommends 55 μg/day; Australia has the highest recommendation of 420 μg/day, while Japan has the lowest recommendation of 4.3 μg/day (US EPA 2012).
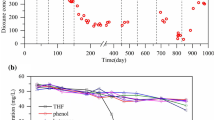
Short-term or acute exposure to 1,4-dioxane may result in various effects, but the common ones are drowsiness; nausea; irritation of the eyes, throat, and nose; and headache (ATSDR 2012; US EPA 2014a). Long-term chronic exposure is associated with drying and cracking of skin and liver, dermatitis, eczema, and kidney damage (ATSDR 2012; US EPA 2014a). Recently, there has been an exponential growth in the global reports regarding 1,4-dioxane as indicated in Fig. 4.6.
Exponential growth is an indication of global awareness on the environmental impact of 1,4-dioxane, with the leading research area being chemistry as indicated in Fig. 4.7.
Its occurrence as an impurity in most consumer products (Fuh et al. 2005) presents a challenge in its roots and mitigation approaches. Thus, apart from dioxane that will enter living organisms and causes adverse effects, the remaining will be contaminant in the environmental ecosystem that requires natural remediation for the ecosystem safety.
4.2 Remediation of 1,4-Dioxane
Chemical and physical properties of 1,4-dioxane lead to ineffective traditional remediation especially through chemical treatment methods (ITRC 2020b). Furthermore, these properties render conventional unit processes involved in drinking and wastewater treatment ineffective too (ITRC 2020b). Although under aerobic conditions 1,4-dioxane is biodegradable via direct metabolism or co-metabolism, the relative roles of these processes are dependent on both the concentration of 1,4-dioxane and the presence of co-contaminants (Zhang et al. 2017; He et al. 2018; Broughton et al. 2019; Polasko et al. 2019; ITRC 2020b; Johnson et al. 2020; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021). Inhibitory co-contaminants frequently complicate the biological treatment of 1,4-dioxane (Johnson et al. 2020). Because of its uses as a solvent, wetting agent, and stabilizer for chlorinated solvents, used in metal vapor degreasing, 1,4-dioxane is frequently found with a variety of co-contaminants, including heavy metals like hexavalent chromium [Cr(VI)] (Zhang et al. 2017; He et al. 2018; Broughton et al. 2019; Polasko et al. 2019; ITRC 2020b; Johnson et al. 2020). Cr(VI) occurs naturally in groundwater due to geological formations, but it also has anthropogenic sources that can overlap with 1,4-dioxane.
Microbes that use 1,4-dioxane as a carbon or energy source, as well as those that co-metabolize it after growth on other organic substrates, can biodegrade it (Zhang et al. 2017; He et al. 2018; Broughton et al. 2019; Polasko et al. 2019; ITRC 2020b; Johnson et al. 2020). In this case, using microbes such as bacterial strains for biodegradation of 1,4-dioxane is of interest for environmental and public health safety. These techniques may be used to remediate 1,4-dioxane in contaminated water, wastewater, and soil. The composition of soil microbial community in contaminated soils is potential for the effectiveness of the remediation processes.
4.3 Soil Microbial Constituents
Soil comprises organic matters, minerals, gases, liquids, and living things, which contribute to supporting life (Haumaier et al. 2001; Pettit 2004; Amundson et al. 1998). In addition to a porous phase containing water and gases, soil consists of a solid phase comprised of minerals and organic matter (Haumaier et al. 2001; Amundson et al. 1998) as presented in Fig. 4.8. The solid, liquid, and gaseous states may coexist in the soil depending on the climatic conditions (Haumaier et al. 2001; Amundson et al. 1998). Climate; relief such as elevation, orientation, and slope of the terrain; organisms; and soil’s parent materials interact over time to produce soil. The produced soil continuously changes due to various physical, chemical, and biological processes, including weathering and related erosion (Velde 2013; Okon and Antia 2022). Soil ecologists view soil as an ecosystem having deep internal connections and complexity (Hopp and McDonnell 2009; Häring et al. 2012; Velde 2013; Reinhold-Hurek et al. 2015; Okon and Antia 2022).
Soil microbial constituents are the main factor that determines the functions of soil microorganisms and how they impact soil characteristics and nutrient constituents (Haumaier et al. 2001; Amundson et al. 1998; Reinhold-Hurek et al. 2015). The first known bacteria and microbes on earth are thought to have originated in the waters between two and four billion years ago (Margulis and Sagan 1997; Nisbet and Sleep 2001; Altermann et al. 2006).
These bacteria could fix nitrogen, and as they grew over time, they released oxygen into the environment (Margulis and Sagan 1997; Nisbet and Sleep 2001; Altermann et al. 2006). This process is crucial because it impacts soil fertility and structure. There are several soil microorganisms including bacteria, actinomycetes, fungus, algae, and protozoa (Edwards and Fletcher 1988; Belnap 2001; Bhattarai et al. 2015; Balasubramanian 2017). Each group has traits that characterize and define their roles in the soil (Lopez de Ceballos et al. 1983; Shormanov et al. 2012; Sei et al. 2013; Li et al. 2018; Yamamoto et al. 2018b; Tusher et al. 2021; Wang et al. 2021b). Each gram of soil around and surrounding plant roots, or the rhizosphere, contains up to ten billion bacteria (Liu et al. 2007; Sei et al. 2013; Miao et al. 2019; Polasko et al. 2019), which can be utilized for remediation purposes and restoration of natural environments.
4.4 Bacteriological Transformation of 1,4-Dioxane in the Soil
In most environments, 1,4-dioxane does not biodegrade easily possibly due to their chemical stability and high miscibility to water (Klečka and Gonsior 1986; Suh and Mohseni 2004; Ghosh et al. 2010; Stepien et al. 2014). However, several microorganisms have been identified that can biodegrade 1,4-dioxane, either directly or through co-metabolism (Altermann et al. 2006; Inoue et al. 2016, 2018; Zhang et al. 2017; Li et al. 2020; Johnson et al. 2020; Ramalingam and Cupples 2020a; Zippilli et al. 2021; Murnane et al. 2021b; Kikani et al. 2021; Wang et al. 2021a). Microorganisms use 1,4-dioxane as a growth substrate during metabolic biodegradation, but growth is slow unless 1,4-dioxane concentrations are high to levels greater than 100 mg/L (Ramalingam and Cupples 2020a; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021). An additional growth substrate such as methane must be supplied during co-metabolic biodegradation to support biomass growth and induce the appropriate 1,4-dioxane-degrading enzymes (Gedalanga et al. 2014; US EPA 2017; Zhang et al. 2017; Aoyagi et al. 2018; He et al. 2018; Hamid et al. 2020; Ramalingam and Cupples 2020a, b; Murnane et al. 2021b). Unlike metabolic degradation, co-metabolic processes can reduce 1,4-dioxane to very low concentrations, which is important for greener ecosystems. These processes may be used separately or in combination based on needs. Details of 1,4-dioxane degradation conditions are presented in Table 4.2.
Some bacterial strains such as Amycolata sp. CB1190 and P. carboxydivorans RM-31 are capable of degradation of 1,4-dioxane in various reaction conditions. The utility of these microbes for environmental remediation of contaminants is undeniable. For example, Mahendra and colleagues investigated 1,4-dioxane, a probable human carcinogen and an important emerging contaminant (Mahendra and Alvarez-Cohen 2006). Their results revealed that among 20 bacteria strains, 13 were capable of biodegrading dioxane (Mahendra and Alvarez-Cohen 2006), indicating potential utility in remediation purposes.
The application of bacteria strains in the biodegradation of contaminants, including 1,4-dioxane, was also reported by several researchers (Metsä-Ketelä et al. 2013; Hamid et al. 2020; Murnane et al. 2021b).
4.4.1 Aerobic Biotransformation
Although they are uncommon, several microorganisms that can metabolize 1,4-dioxane in aerobic conditions have been isolated. Among the microbes that can biotransform 1,4-dioxane in aerobic conditions, Pseudonocardia dioxanivorans CB1190 is the best studied strain (Arve 2015; Isaka et al. 2016; Zhang et al. 2017; Aoyagi et al. 2018; Guan et al. 2018; He et al. 2018; Miao et al. 2018; Barajas-Rodriguez et al. 2019; Ramalingam and Cupples 2020b; Johnson et al. 2020; Dang and Cupples 2021; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021). Industrial activated sludge was used to enrich the bacterium and then fed with tetrahydrofuran and finally 1,4-dioxane. Studies indicate that high levels of chlorinated solvents, their by-products, and some metals may inhibit 1,4-dioxane CB1190 strain degradation (Arve 2015; Isaka et al. 2016; Zhang et al. 2017; Aoyagi et al. 2018; Guan et al. 2018; He et al. 2018; Miao et al. 2018; Barajas-Rodriguez et al. 2019; Ramalingam and Cupples 2020b; Johnson et al. 2020; Dang and Cupples 2021; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021). The most effective inhibitors of 1,4-dioxane degradation are presented in Fig. 4.9.
It is reported that about 5 mg/L of 1,1-dichloroethene is an inhibitor of 1,4-dioxane biodegradation (Arve 2015; Isaka et al. 2016; Zhang et al. 2017; Aoyagi et al. 2018; Guan et al. 2018; He et al. 2018; Miao et al. 2018; Barajas-Rodriguez et al. 2019; Ramalingam and Cupples 2020b; Johnson et al. 2020; Dang and Cupples 2021; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021). The most effective metal inhibitor of 1,4-dioxane degradation by CB1190 was Cu(II), which lengthened the lag time at 1 mg/L and significantly decreased 1,4-dioxane degradation rates at 10 and 20 mg/L Cu(II). While Zn(II) did not impact biodegradation of 1,4-dioxane at the highest test concentration (20 mg/L Zn), Cd(II), Ni(II), and Ni(II) were less sensitive to 1,4-dioxane degradation (Arve 2015; Isaka et al. 2016; Zhang et al. 2017; Aoyagi et al. 2018; Guan et al. 2018; He et al. 2018; Miao et al. 2018; Barajas-Rodriguez et al. 2019; Ramalingam and Cupples 2020b; Johnson et al. 2020; Dang and Cupples 2021; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021). This result indicates that 1,4-dioxane occurs as a mixture of several contaminants; therefore, the more effective approach will be to propose and improve techniques for co-biotransformation of 1,4-dioxane and its co-contaminants in the environments. For the treatment of industrial wastewater contaminated with 1,4-dioxane, biodegradation is a viable, economical, and environmentally benign solution. A schematic and proposed chemical equation for aerobic biotransformation is presented in Fig. 4.10.
In a study, Tusher and colleagues explored metabolic bacteria from a stable microbial community that degrades 1,4-dioxane (Tusher et al. 2021). Pseudonocardia sp. (TS28), Dokdonella sp. (TS32), and Afipia sp. (TS43) were three bacterial strains that were discovered to be capable to use 1,4-dioxane as their only source of carbon and energy to break down organic compounds (Tusher et al. 2021). This was a pioneer study that detailed the participation of the genus Dokdonella in the biodegradation of 1,4-dioxane as presented in Fig. 4.11. Genus Dokdonella possesses inducible 1,4-dioxane-degrading enzymes’ potential for remediation purposes (Tusher et al. 2021).
The findings of this study also contributed to our understanding of how various 1,4-dioxane degraders interact and cohabit in a consortium while utilizing a single carbon supply to create an effective biological 1,4-dioxane treatment system.
4.4.2 Aerobic Co-metabolic Biotransformation of 1,4-Dioxane
A simultaneous degradation method of two compounds is known as co-metabolism, whereby the degradation of a second compound always depends on the presence of the first compound. Organisms involved in co-metabolizing 1,4-dioxane, in contrast to 1,4-dioxane-metabolizing microorganisms, can break down 1,4-dioxane after growing on a main growth-supporting substrate (Kashimoto et al. 1989; Mahendra et al. 2013; US EPA 2014a, 2017; Arve 2015; Zhang et al. 2017, b; Johnson et al. 2020; Miao et al. 2020; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021; Tusher et al. 2021). Figure 4.12 shows some main processes involved in co-metabolism.
Some main processes involved in co-metabolism (Mahendra et al. 2013)
Co-metabolic active bacteria such as R. rhodochrous ATCC 21198 can often break down co-substrates at lower concentrations below those attained by organisms that metabolize and thrive on them since co-substrate is not utilized as either primary carbon or energy sources (Kashimoto et al. 1989; Mahendra et al. 2013; US EPA 2014a, 2017; Arve 2015; Zhang et al. 2017, b; Johnson et al. 2020; Miao et al. 2020; Metabolism A, Cometabolism A, Biodegradation A, Tools MB 2021; Tusher et al. 2021). Numerous bacteria such as Mycobacterium austroafricanum JOB5 (Arve 2015; Zhang et al. 2017; Johnson et al. 2020; Tusher et al. 2021) can co-metabolize 1,4-dioxane in various conditions. These include model organisms that utilize well-characterized enzymes like soluble methane monooxygenase (sMMO) and grow on primary substrates like toluene or methane, and other 1,4-dioxane co-metabolizing strains reported to thrive on THF, ethane, and isobutane. Numerous bacterial monooxygenases have the capacity to oxidize numerous co-substrates at once; if fully studied and utilized, this may have a promising future in the remediation of environmental contaminants.
Hatzinger and colleagues assessed methane and ethane capacity to promote the aerobic co-metabolism that breaks down 1,4-dioxane in groundwater aquifers (Hatzinger et al. 2017). Ethane encouraged M. sphagni ENV482, which was isolated from a separate aquifer, to aerobically break down 1,4-dioxane (Hatzinger et al. 2017). According to this study, ethane, a common by-product of the biotic or abiotic reductive dechlorination of chlorinated ethanes and ethenes, may act as a substrate to speed up the breakdown of 1,4-dioxane in aquifers, especially in areas where these by-products combine with aerobic groundwater (Hatzinger et al. 2017). A similar study was conducted by Murnane and colleagues on co-metabolic transformation of 1,1,1-trichloroethane and 1,4-dioxane by pure cultures of R. rhodochrous (ATCC strain 21198) (Murnane et al. 2021a). Results indicated that the transformation of dioxane occurred without a lag phase for cells grown on 2-butanol, while an induction period of several hours was required for 1-butanol-grown cells (Murnane et al. 2021a). A similar observation was archived with activity-based labelling patterns for monooxygenase hydroxylase components and specific rates of tetrahydrofuran degradation (Murnane et al. 2021a). The study further reported lower rates of oxygen gas consumption in the reactors containing tetra-s-butylorthosilicate, which has benefits for in situ bioremediation. Indicating the structure of SRC is important when developing passive aerobic co-metabolic treatment systems.
4.4.3 Anaerobic Biotransformation of 1,4-Dioxane
Little metabolic or co-metabolic anaerobic 1,4-dioxane biodegradation has been observed thus far (Skinner et al. 2009; Göen et al. 2016; Guan et al. 2018). There was no evidence of 1,4-dioxane biodegradation in microcosm research employing samples of aquifer material from several 1,4-dioxane-impacted sites. On 1,4-dioxane, an iron-reducing bacterium did grow anaerobically with chemical processes (Arve 2015; Zhang et al. 2017; Hamid et al. 2020; Ramalingam and Cupples 2020a; Dang and Cupples 2021; Sengupta and Dhal 2021). Fe(III)-reducing facultative anaerobe S. oneidensis can produce hydroxyl radicals, which degrade 1,4-dioxane as indicated in Fig. 4.13 (Sekar and DiChristina 2014; Sekar et al. 2016).
S. oneidensis produces Fe(II) in anaerobic circumstances, which interacts chemically with H2O2 to yield hydroxyl radicals that can result in oxidative 1,4-dioxane breakdown (Sekar and DiChristina 2014; Sekar et al. 2016).
4.5 Roles of Soil Bacteria in the Biotransformation of 1,4-Dioxane
A cyclic molecule such as 1,4-dioxane with two ether moieties was thought to be relatively unmanageable to biodegradation, until a recent study that demonstrated that it may be efficiently biotransformed (Zhang et al. 2016, 2017; He et al. 2018). Dioxane-degrading bacteria are promising in situ bioremediation agents for cleaning up 1,4-dioxane-contaminated soils because it is less expensive than using advanced technologies. Several strains that may co-metabolically degrade dioxane have been isolated from wastewater treatment facilities or dioxane-affected areas (He et al. 2018). Numerous research emphasized the significance of monooxygenase enzymes in dioxane breakdown, and a gene cluster and sequence identified in P. dioxanivorans CB1190 that can aid in this process (i.e., thmADBC) (Mahendra and Alvarez-Cohen 2006; Kim et al. 2009; Sales et al. 2011). The gene cluster encodes dioxane monooxygenases, the dissolving di-iron (SDIMO) that initiates the breakdown of dioxane. The di-iron monooxygenase contains multiple bacterial enzyme components that can catalyze the oxidation of a variety of priority pollutants such as chlorinated solvents and aromatic hydrocarbons, among other contaminants, implying that they have inherent bioremediation potential (Notomista et al. 2003; Grostern et al. 2012).
A garden soil with unknown history of exposure to dioxane has shown a capability to degrade 500 mg/L dioxane as the sole source of carbon and energy to non-detectable levels within a week (He et al. 2018). Filtering through the aquifer material hinders the subsurface dispersion of inoculated bacteria, which is particularly problematic when the cultures aggregate. The archetypal dioxane degraders, which aggregate in suspension and include P. dioxanivorans (CB1190) and M. dioxanotrophicus (PH-06), are severely constrained by this (Grostern et al. 2012). Remarkably, the SDIMO consortia (A and B) enriched by He et al. (2018) from garden soil did not clump as much as the archetype, making them better candidates for in situ bioaugmentation at areas with inadequate indigenous dioxane biodegradation ability. Figure 4.14 shows findings indicating that there is a substantial growth of bacteria when exposed in the dioxane, while simultaneous decrease in dioxane concurrently occurs.
Degradation of dioxane-enriched consortia and two archetypes (left) and concurrent growth of consortia (right). [Source: He et al. (2018)]
Tenfold serial dilution was adopted during conduction of experiments in Fig. 4.14 where ammonium mineral salts were used as growth medium with an initial dioxane concentration of 500 mg/L, while autoclaved bacteria were used as control.
4.6 Fate of 1,4-Dioxane Biotransformation Products
Numerous intermediates produced by the biotransformation of 1,4-dioxane in environmental matrices have the potential to interact with the environment and result in secondary pollution issues. Therefore, it is crucial to comprehend their fate and the effects of these items. Spectrometry, chromatography, nuclear magnetic resonance spectroscopy (NMR), gas chromatography-mass spectroscopy (GC-MS), high-pressure liquid chromatography (HPLC), and gas chromatography-flame ionization detection (GC-FID) are some techniques that can be used to quantify and qualitatively estimate the extent of changes in 1,4-dioxane (Kikani et al. 2022). The production of metabolites with are structurally distinct via redox mechanisms is the consequence of 1,4-dioxane transformation by biomaterials. There have been papers on microbial systems’ qualitative evaluation of 1,4-dioxane intermediates and metabolites. The intermediates produced by Cordyceps sinensis during the biodegradation of 1,4-dioxane are shown in Fig. 4.15 (Nakamiya et al. 2005; Mahendra et al. 2007; Kang and Doty 2014).
Shaily and colleagues (Mahendra et al. 2007) described a process for dioxane oxidation by monooxygenase-expressing cells that does not result in the buildup of hazardous intermediate compounds in the environment. In this mechanism, dioxane is first transformed to 2-hydroxy-1,4-dioxane, which is then instantaneously oxidized to 2-hydroxyethoxyacetic acid. During a second monooxygenation step, 2-hydroxy-1,4-dioxane is further hydroxylated, yielding a mixture of dihydroxy-ethoxyacetic acids with a hydroxyl group in the ortho or para position. After the second ether bond is broken, small organic molecules such as ethylene glycol, glycolate, glyoxylate, and oxalate are progressively formed and mineralized to CO2 via common cellular metabolic mechanism.
4.7 Conclusion
The occurrence of 1,4-dioxane in the water, wastewater, soil, drinking water, and groundwater is globally reported. This chemical is classified as a carcinogenic compound, posing a threat to ecosystems upon its chronic exposure. Its occurrence in many consumer products as either an impurity or a solvent and its high miscibility to water lead to its widespread contamination, complicating its remediation methods. Bacteria such as Pseudonocardia dioxanivorans CB1190 through aerobic biotransformation, Rhodococcus rhodochrous ATCC 21198 through co-metabolic transformation, and Shewanella oneidensis through anaerobic transformation have shown a significant bioreduction of 1,4-dioxane concentration in the contaminated environments. The role of these bacteria is through either consumption of 1,4-dioxane as a source of energy, thus reducing its concentration, or transformation to different products that are harmless compared to parent molecule. In the future, relying of microbial remediation of 1,4-dioxane is expected to take a pace since nature does not leave behind unattended waste resulting in an ecological safety.
References
Adamson DT, Anderson RH, Mahendra S, Newell CJ (2015) Evidence of 1,4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1,4-dioxane. Environ Sci Technol 49:6510–6518. https://doi.org/10.1021/acs.est.5b00964
Adamson DT, Uhlir G, Rauch SR et al (2021) Trends in 1,4-dioxane analyses: implications for identification and characterization of contaminated groundwater sites. Groundw Monit Remediat 41:29–40. https://doi.org/10.1111/gwmr.12427
AFDC (2022) Alternative Fuels Data Center. In: Generic. https://afdc.energy.gov/data/. Accessed 2 Aug 2022
Alsohaimi IH, Khan MR, Ali HM et al (2020) Solvent extraction and gas chromatography–mass spectrometric determination of probable carcinogen 1,4-dioxane in cosmetic products. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-62149-x
Altermann W, Kazmierczak J, Oren A, Wright DT (2006) Cyanobacterial calcification and its rock-building potential during 3.5 billion years of earth history. Geobiology 4:147–166
Amundson R, Stern L, Baisden T, Wang Y (1998) The isotopic composition of soil and soil-respired CO2. Geoderma 82:83–114
Aoyagi T, Morishita F, Sugiyama Y et al (2018) Identification of active and taxonomically diverse 1,4-dioxane degraders in a full-scale activated sludge system by high-sensitivity stable isotope probing. ISME J 12:2376–2388. https://doi.org/10.1038/s41396-018-0201-2
Arve P (2015) Microcosm study of 1,4-dioxane biotransformation. Clemson University, Clemson
ATSDR (2006) 1,4-Dioxane (C4H8O2) CAS 123-9191; UN 1165
ATSDR (2012) Toxicological profile for 1,4-dioxane. Agency for Atlanta, Atlanta
Balasubramanian A (2017) Soil microorganisms. Univeristy of Mysore, Mysore
Barajas-Rodriguez FJ, Murdoch LC, Falta RW, Freedman DL (2019) Simulation of in situ biodegradation of 1,4-dioxane under metabolic and cometabolic conditions. J Contam Hydrol 223:103464. https://doi.org/10.1016/j.jconhyd.2019.02.006
Begum G et al (2016) A study of potential cytotoxic effect of 1,4-dioxane oh human hepatic cell line (HEP10). Int J Recent Sci Res 7:14320–14325
Belnap J (2001) Microbes and microfauna associated with biological soil crusts. In: Biological soil crusts: structure, function, and management. Springer, Berlin, pp 167–174
Bhattarai A, Bhattarai B, Pandey S (2015) Variation of soil microbial population in different soil horizons. J Microbiol Exp 2:44
Broughton A, Sepulveda A, Foster K et al (2019) 1,4-Dioxane: emerging technologies for an emerging contaminant. Remediation 29:49–63. https://doi.org/10.1002/rem.21613
Che-revision Administrator (2014) Main group organometallics. In: Generic. http://ueache.weebly.com/main-group-organometallics.html. Accessed 2 Aug 2022
Chu MYJ, Bennett PJ, Dolan ME et al (2018) Concurrent treatment of 1,4-dioxane and chlorinated aliphatics in a groundwater recirculation system via aerobic cometabolism. Groundw Monit Remediat 38:53–64. https://doi.org/10.1111/gwmr.12293
Dang H, Cupples AM (2021) Identification of the phylotypes involved in cis-dichloroethene and 1,4-dioxane biodegradation in soil microcosms. Sci Total Environ 794:148690. https://doi.org/10.1016/j.scitotenv.2021.148690
Davarani SSH, Masoomi L, Banitaba MH et al (2012) A new aluminium hydroxide coating on fused silica fiber for the determination of 1,4-dioxane in surfactants and detergents using HS-SPME-GC. Chromatographia 75:371–377. https://doi.org/10.1007/s10337-012-2213-9
DeRosa CT, Wilbur S, Holler J et al (1996) Health evaluation of 1,4-dioxane. Toxicol Ind Health 12:1–43. https://doi.org/10.1177/074823379601200101
DES (2018) 1,4-Dioxane: Health Information Summary. 1–2
Edwards CA, Fletcher KE (1988) Interactions between earthworms and microorganisms in organic-matter breakdown. Agric Ecosyst Environ 24:235–247
EPA (2010) 1,4-Dioxane
Fuh CB, Lai M, Tsai HY, Chang CM (2005) Impurity analysis of 1,4-dioxane in nonionic surfactants and cosmetics using headspace solid-phase microextraction coupled with gas chromatography and gas chromatography-mass spectrometry. J Chromatogr A 1071:141–145. https://doi.org/10.1016/j.chroma.2004.09.012
Gedalanga PB, Pornwongthong P, Mora R et al (2014) Identification of biomarker genes to predict biodegradation of 1,4-dioxane. Appl Environ Microbiol 80:3209–3218. https://doi.org/10.1128/AEM.04162-13
Ghosh P, Samanta AN, Ray S (2010) Oxidation kinetics of degradation of 1,4-dioxane in aqueous solution by H2O2/Fe(II) system. J Environ Sci Health Part A 45:395–399
Gi M, Fujioka M, Kakehashi A et al (2018) In vivo positive mutagenicity of 1,4-dioxane and quantitative analysis of its mutagenicity and carcinogenicity in rats. Arch Toxicol 92:3207–3221. https://doi.org/10.1007/s00204-018-2282-0
Göen T, von Helden F, Eckert E et al (2016) Metabolism and toxicokinetics of 1,4-dioxane in humans after inhalational exposure at rest and under physical stress. Arch Toxicol 90:1315–1324. https://doi.org/10.1007/s00204-015-1567-9
Grostern A, Sales CM, Zhuang W-Q et al (2012) Glyoxylate metabolism is a key feature of the metabolic degradation of 1,4-dioxane by Pseudonocardia dioxanivorans strain CB1190. Appl Environ Microbiol 78:3298–3308. https://doi.org/10.1128/AEM.00067-12
Guan X, Liu F, Wang J et al (2018) Mechanism of 1,4-dioxane microbial degradation revealed by 16S rRNA and metatranscriptomic analyses. Water Sci Technol 77:123–133. https://doi.org/10.2166/wst.2017.498
Hamid H, Li LY, Grace JR (2020) Aerobic biotransformation of fluorotelomer compounds in landfill leachate-sediment. Sci Total Environ 713:136547. https://doi.org/10.1016/j.scitotenv.2020.136547
Hand S, Wang B, Chu K-H (2015) Biodegradation of 1,4-dioxane: effects of enzyme inducers and trichloroethylene. Sci Total Environ 520:154–159. https://doi.org/10.1016/j.scitotenv.2015.03.031
Häring T, Dietz E, Osenstetter S et al (2012) Spatial disaggregation of complex soil map units: a decision-tree based approach in Bavarian forest soils. Geoderma 185:37–47
Hatzinger PB, Banerjee R, Rezes R et al (2017) Potential for cometabolic biodegradation of 1,4-dioxane in aquifers with methane or ethane as primary substrates. Biodegradation 28:453–468. https://doi.org/10.1007/s10532-017-9808-7
Haumaier L, Six J, Simpson R (2001) Sources and composition of soil organic matter fractions between and within soil aggregates. Eur J Soil Sci 52:607–618
He Y, Mathieu J, da Silva MLB et al (2018) 1,4-Dioxane-degrading consortia can be enriched from uncontaminated soils: prevalence of mycobacterium and soluble di-iron monooxygenase genes. Microb Biotechnol 11:189–198. https://doi.org/10.1111/1751-7915.12850
Hinchee RE, Dahlen PR, Johnson PC, Burris DR (2018) 1,4-Dioxane soil remediation using enhanced soil vapor extraction: I. Field demonstration. Groundw Monit Remediat 38:40–48. https://doi.org/10.1111/gwmr.12264
Hopp L, McDonnell JJ (2009) Connectivity at the hillslope scale: identifying interactions between storm size, bedrock permeability, slope angle and soil depth. J Hydrol 376:378–391
Hossein M, Chande O, Eunice FNM (2022) Exposure to 1,4-dioxane and disinfection by-products due to the reuse of wastewater. In: Sarma H, Dominguez DC, Lee W-Y (eds) . Elsevier, Emerging contaminants in the environment, pp 87–109
House AJ, Hyman MR (2010) Effects of gasoline components on MTBE and TBA cometabolism by Mycobacterium austroafricanum JOB5. Biodegradation 21:525–541
Inoue D, Tsunoda T, Sawada K et al (2016) 1,4-Dioxane degradation potential of members of the genera Pseudonocardia and Rhodococcus. Biodegradation 27:277–286. https://doi.org/10.1007/s10532-016-9772-7
Inoue D, Tsunoda T, Yamamoto N et al (2018) 1,4-Dioxane degradation characteristics of Rhodococcus aetherivorans JCM 14343. Biodegradation 29:301–310. https://doi.org/10.1007/s10532-018-9832-2
Isaka K, Udagawa M, Kimura Y et al (2016) Biological wastewater treatment of 1,4-dioxane using polyethylene glycol gel carriers entrapping Afipia sp. D1. J Biosci Bioeng 121:203–208. https://doi.org/10.1016/j.jbiosc.2015.06.006
ITRC (2020a) Toxicity and risk assessment: 1,4-dioxane, pp 2–5
ITRC (2020b) Remediation and treatment technologies. Fact Sheets, pp 1–7
Johnson NW, Gedalanga PB, Zhao L et al (2020) Cometabolic biotransformation of 1,4-dioxane in mixtures with hexavalent chromium using attached and planktonic bacteria. Sci Total Environ 706:135734. https://doi.org/10.1016/j.scitotenv.2019.135734
Kang JW, Doty SL (2014) Cometabolic degradation of trichloroethylene by Burkholderia cepacia G4 with poplar leaf homogenate. Can J Microbiol 60:487–490. https://doi.org/10.1139/cjm-2014-0095
Karges U, Becker J, Püttmann W (2018) 1,4-Dioxane pollution at contaminated groundwater sites in western Germany and its distribution within a TCE plume. Sci Total Environ 619:712–720. https://doi.org/10.1016/j.scitotenv.2017.11.043
Karges U, Ott D, De Boer S, Püttmann W (2020) 1,4-Dioxane contamination of German drinking water obtained by managed aquifer recharge systems: distribution and main influencing factors. Sci Total Environ 711:134783. https://doi.org/10.1016/j.scitotenv.2019.134783
Kashimoto T, Takayama K, Mimura M et al (1989) Evaluation of toxic effects on yusho causal substances by chick embryo hepatic microsomal enzymes activities. Fukuoka Igaku Zasshi 80:210–220
Kelley SL, Aitchison EW, Deshpande M et al (2001) Biodegradation of 1,4-dioxane in planted and unplanted soil: effect of bioaugmentation with Amycolata sp. CB1190. Water Res 35:3791–3800. https://doi.org/10.1016/s0043-1354(01)00129-4
Kikani M, Bhojani G, Amit C, Kumar Madhava A (2021) Chemo-metrically formulated consortium with selectively screened bacterial strains for ameliorated biotransformation and detoxification of 1,4-dioxane. J Hazard Mater 413:125456. https://doi.org/10.1016/j.jhazmat.2021.125456
Kikani M, Vijaybhai G, Prasad T et al (2022) Remedial strategies for abating 1,4-dioxane pollution-special emphasis on diverse biotechnological interventions. Environ Res 214:113939. https://doi.org/10.1016/j.envres.2022.113939
Kim Y-M, Jeon J-R, Murugesan K et al (2009) Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation 20:511–519. https://doi.org/10.1007/s10532-008-9240-0
Klečka GM, Gonsior SJ (1986) Removal of 1,4-dioxane from wastewater. J Hazard Mater 13:161–168
Lee KC, Choo KH (2013) Hybridization of TiO2 photocatalysis with coagulation and flocculation for 1,4-dioxane removal in drinking water treatment. Chem Eng J 231:227–235. https://doi.org/10.1016/j.cej.2013.07.023
Li M, Yang Y, He Y et al (2018) Detection and cell sorting of Pseudonocardia species by fluorescence in situ hybridization and flow cytometry using 16S rRNA-targeted oligonucleotide probes. Appl Microbiol Biotechnol 102:3375–3386. https://doi.org/10.1007/s00253-018-8801-3
Li F, Deng D, Li M (2020) Distinct catalytic behaviors between two 1,4-dioxane-degrading monooxygenases: kinetics, inhibition, and substrate range. Environ Sci Technol 54:1898–1908. https://doi.org/10.1021/acs.est.9b05671
Liu J, Lee LS, Nies LF et al (2007) Biotransformation of 8:2 fluorotelomer alcohol in soil and by soil bacteria isolates. Environ Sci Technol 41:8024–8030. https://doi.org/10.1021/es0708722
Lopez de Ceballos M, Guisado E, Sanchez-Blazquez P et al (1983) Long-term social isolation in the rat induces opposite changes in binding to alpha 1- and alpha 2-adrenoceptors in the brain and vas deferens. Neurosci Lett 39:217–222. https://doi.org/10.1016/0304-3940(83)90080-0
Madhu (2018) Difference between tetrahydrofuran and dioxane. In: Generic. https://www.differencebetween.com/difference-between-thf-and-dioxane/. Accessed 3 Aug 2022
Mahendra S, Alvarez-Cohen L (2005) Pseudonocardia dioxanivorans sp. nov., a novel actinomycete that grows on 1,4-dioxane. Int J Syst Evol Microbiol 55:593–598. https://doi.org/10.1099/ijs.0.63085-0
Mahendra S, Alvarez-Cohen L (2006) Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ Sci Technol 40:5435–5442. https://doi.org/10.1021/es060714v
Mahendra S, Petzold CJ, Baidoo EE et al (2007) Identification of the intermediates of in vivo oxidation of 1,4-dioxane by monooxygenase-containing bacteria. Environ Sci Technol 41:7330–7336. https://doi.org/10.1021/es0705745
Mahendra S, Grostern A, Alvarez-Cohen L (2013) The impact of chlorinated solvent co-contaminants on the biodegradation kinetics of 1,4-dioxane. Chemosphere 91:88–92. https://doi.org/10.1016/j.chemosphere.2012.10.104
Margulis L, Sagan D (1997) Microcosmos: four billion years of microbial evolution. University of California Press, Oakland
McElroy AC, Hyman MR, Knappe DRU (2019) 1,4-Dioxane in drinking water: emerging for 40 years and still unregulated. Curr Opin Environ Sci Health 7:117–125. https://doi.org/10.1016/j.coesh.2019.01.003
McFee AF, Abbott MG, Gulati DK, Shelby MD (1994) Results of bone marrow micronucleus studies on 1,4-dioxane. Mutat Res 322:141–150
Metabolism A, Cometabolism A, Biodegradation A, Tools MB (2021) Biodegradation—1,4-dioxane, pp 20–25
Metsä-Ketelä M, Oja T, Taguchi T et al (2013) Biosynthesis of pyranonaphthoquinone polyketides reveals diverse strategies for enzymatic carbon–carbon bond formation. Curr Opin Chem Biol 17:562–570. https://doi.org/10.1016/j.cbpa.2013.06.032
Miao Y, Johnson NW, Heck K et al (2018) Microbial responses to combined oxidation and catalysis treatment of 1,4-dioxane and co-contaminants in groundwater and soil. Front Environ Sci Eng 12:1–13. https://doi.org/10.1007/s11783-018-1071-6
Miao Y, Johnson NW, Gedalanga PB et al (2019) Response and recovery of microbial communities subjected to oxidative and biological treatments of 1,4-dioxane and co-contaminants. Water Res 149:74–85. https://doi.org/10.1016/j.watres.2018.10.070
Miao Y, Johnson NW, Phan T et al (2020) Monitoring, assessment, and prediction of microbial shifts in coupled catalysis and biodegradation of 1,4-dioxane and co-contaminants. Water Res 173:115540. https://doi.org/10.1016/j.watres.2020.115540
Milavec J, Tick GR, Brusseau ML, Carroll KC (2020) 1,4-Dioxane cosolvency impacts on trichloroethene dissolution and sorption. Environ Pollut 252:777–783. https://doi.org/10.1016/j.envpol.2019.05.156.1
Miraji H, Ripanda A, Moto E (2021) A review on the occurrences of persistent organic pollutants in corals, sediments, fish and waters of the Western Indian Ocean. Egypt J Aquat Res 47:373–379. https://doi.org/10.1016/j.ejar.2021.08.003
Mo X, Liu Q, Gao L et al (2022) The industrial solvent 1,4-dioxane causes hyperalgesia by targeting capsaicin receptor TRPV1. BMC Biol 20:1–14. https://doi.org/10.1186/s12915-021-01211-0
Monreal MJ, Thomson RK, Cantat T et al (2011) UI4(1,4-dioxane)2, [UCl4(1,4-dioxane)]2, and UI3(1,4-dioxane)1.5: stable and versatile starting materials for low- and high-valent uranium chemistry. Organometallics 30:2031–2038. https://doi.org/10.1021/om200093q
Murnane RA, Chen W, Hyman M, Semprini L (2021a) Long-term cometabolic transformation of 1,1,1-trichloroethane and 1,4-dioxane by Rhodococcus rhodochrous ATCC 21198 grown on alcohols slowly produced by orthosilicates. J Contam Hydrol 240:103796. https://doi.org/10.1016/j.jconhyd.2021.103796
Murnane RA, Chen W, Semprini L (2021b) Rhodococcus rhodochrous ATCC 21198 grown on alcohols slowly produced by orthosilicates. Oregon State University, Corvallis
Nakamiya K, Hashimoto S, Ito H et al (2005) Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl Environ Microbiol 71:1254–1258. https://doi.org/10.1128/AEM.71.3.1254-1258.2005
Nam JH, Ventura JRS, Yeom IT et al (2016) Structural and kinetic characteristics of 1,4-dioxane-degrading bacterial consortia containing the phylum TM7. J Microbiol Biotechnol 26:1951–1964. https://doi.org/10.4014/jmb.1601.01095
Nisbet EG, Sleep NH (2001) The habitat and nature of early life. Nature 409:1083–1091
Notomista E, Lahm A, Di Donato A, Tramontano A (2003) Evolution of bacterial and archaeal multicomponent monooxygenases. J Mol Evol 56:435–445. https://doi.org/10.1007/s00239-002-2414-1
OEHHA (1998) 1,4-Dioxane action level
Okon OG, Antia UE (2022) Pedogenesis and soil biota interactions in the pedosphere. In: Structure and functions of pedosphere. Springer, pp 1–21
Parales RE, Adamus JE, White N, May HD (1994) Degradation of 1,4-dioxane by an actinomycete in pure culture. Appl Environ Microbiol 60:4527–4530. https://doi.org/10.1128/aem.60.12.4527-4530.1994
Patt TE, Abebe HM (1995) United States patent 5399495: microbial degradation of chemical pollutants. The Upjohn Company, Kalamazoo
Pettit RE (2004) Humates General Info
Pinter B, Nagels N, Herrebout WA, De Proft F (2013) Halogen bonding from a hard and soft acids and bases perspective: investigation by using density functional theory reactivity indices. Chem A Eur J 19:519–530. https://doi.org/10.1002/chem.201202567
Polasko AL, Zulli A, Gedalanga PB et al (2019) A mixed microbial community for the biodegradation of chlorinated ethenes and 1,4-dioxane. Environ Sci Technol Lett 6:49–54. https://doi.org/10.1021/acs.estlett.8b00591
Ramalingam V, Cupples AM (2020a) Anaerobic 1,4-dioxane biodegradation and microbial community analysis in microcosms inoculated with soils or sediments and different electron acceptors. Appl Microbiol Biotechnol 104:4155–4170. https://doi.org/10.1007/s00253-020-10512-3
Ramalingam V, Cupples AM (2020b) Enrichment of novel actinomycetales and the detection of monooxygenases during aerobic 1,4-dioxane biodegradation with uncontaminated and contaminated inocula. Appl Microbiol Biotechnol 104:2255–2269. https://doi.org/10.1007/s00253-020-10376-7
Reinhold-Hurek B, Bünger W, Burbano CS et al (2015) Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol 53:403–424
Sales CM, Mahendra S, Grostern A et al (2011) Genome sequence of the 1,4-dioxane-degrading Pseudonocardia dioxanivorans strain CB1190. J Bacteriol 193:4549–4550. https://doi.org/10.1128/JB.00415-11
Sarma H (2022) Understanding emerging contaminants in soil and water: current perspectives on integrated remediation approaches. In: Emerging contaminants in the environment: challenges and sustainable practices. Elsevier, Amsterdam, pp 1–38
Scaratti G, De Noni JA, José HJ, de Fatima Peralta Muniz Moreira R (2020) 1,4-Dioxane removal from water and membrane fouling elimination using CuO-coated ceramic membrane coupled with ozone. Environ Sci Pollut Res 27:22144–22154. https://doi.org/10.1007/s11356-019-07497-6
Sei K, Miyagaki K, Kakinoki T et al (2013) Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 24:665–674. https://doi.org/10.1007/s10532-012-9614-1
Sekar R, DiChristina TJ (2014) Microbially driven Fenton reaction for degradation of the widespread environmental contaminant 1,4-dioxane. Environ Sci Technol 48:12858–12867. https://doi.org/10.1021/es503454a
Sekar R, Taillefert M, DiChristina TJ (2016) Simultaneous transformation of commingled trichloroethylene, tetrachloroethylene, and 1,4-dioxane by a microbially driven Fenton reaction in batch liquid cultures. Appl Environ Microbiol 82:6335–6343. https://doi.org/10.1128/AEM.02325-16
Sengupta I, Dhal PK (2021) Impact of elevated phosphogypsum on soil fertility and its aerobic biotransformation through indigenous microorganisms (IMO’s) based technology. J Environ Manag 297:113195. https://doi.org/10.1016/j.jenvman.2021.113195
Shormanov VK, Chigareva EN, Vladimirenko EN (2012) Chemical toxicological identification of esfenvalerate. Sud Med Ekspert 55:37–41
Skinner K, Cuiffetti L, Hyman M (2009) Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp. Appl Environ Microbiol 75:5514–5522. https://doi.org/10.1128/AEM.00078-09
Stepien DK, Diehl P, Helm J et al (2014) Fate of 1,4-dioxane in the aquatic environment: from sewage to drinking water. Water Res 48:406–419
Suh JH, Mohseni M (2004) A study on the relationship between biodegradability enhancement and oxidation of 1,4-dioxane using ozone and hydrogen peroxide. Water Res 38:2596–2604
Sun B, Ko K, Ramsay JA (2011) Biodegradation of 1,4-dioxane by a Flavobacterium. Biodegradation 22:651–659. https://doi.org/10.1007/s10532-010-9438-9
Sun M, Lopez-Velandia C, Knappe DRU (2016) Determination of 1,4-dioxane in the cape fear river watershed by heated purge-and-trap preconcentration and gas chromatography-mass spectrometry. Environ Sci Technol 50:2246–2254. https://doi.org/10.1021/acs.est.5b05875
Supprenant KS (2012) Dioxane. Ullmann’s Encycl. Ind, Chem, p 6
Suthersan S, Quinnan J, Horst J et al (2016) Making strides in the management of “emerging contaminants”. Groundw Monit Remediat 36:15–25. https://doi.org/10.1111/gwmr.12143
Tusher TR, Shimizu T, Inoue C, Chien MF (2021) Isolation and characterization of novel bacteria capable of degrading 1,4-dioxane in the presence of diverse co-occurring compounds. Microorganisms 9:1–14. https://doi.org/10.3390/microorganisms9050887
U.S. FDA (2016) 1,4-Dioxane: a manufacturing byproduct. In: Generic. https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/14-dioxane-cosmetics-manufacturing-byproduct. Accessed 2 Aug 2022
US EPA (2012) 2012 Edition of the drinking water standards and health advisories. EPA, Washington, DC
US EPA (2014a) Technical fact sheet—1,4-dioxane
US EPA (2014b) Technical fact sheet—1,2,3-trichloropropane
US EPA (2017) Technical fact sheet: 1.4-dioxane
van Buskirk G (2014) Method and process for the degradation of cyclic ethers in ethoxylate-containing actives 1:33
Velde B (2013) Origin and mineralogy of clays: clays and the environment. Springer Science & Business Media
Wang P, Li F, Wang W et al (2021a) Cometabolic degradation of 1,4-dioxane by a tetrahydrofuran-growing Arthrobacter sp. WN18. Ecotoxicol Environ Saf 217:112206. https://doi.org/10.1016/j.ecoenv.2021.112206
Wang Y, Ma F, Yang J et al (2021b) Xanthobacter dioxanivorans sp. nov., a 1,4-dioxane-degrading bacterium. Int J Syst Evol Microbiol 71:005139. https://doi.org/10.1099/ijsem.0.005139
Wiki (2022) 1,4-Dioxane. In: Generic. https://en.wikipedia.org/wiki/1,4-Dioxane#:~:text=4.4-Cosmetics-,Synthesis,between,11%2C000-and-14%2C000-tons. Accessed 2 Aug 2022
Wilbur S, Jones DRJ (2012) Toxicological profile for 1,4-dioxane. In: ATSDR (ed) Production, import/export, use, and disposal. Agency for Toxic Substances and Disease Registry, Atlanta
Yamamoto N, Inoue D, Sei K et al (2018a) Field test of on-site treatment of 1,4-dioxane-contaminated groundwater using Pseudonocardiasp. D17. J Water Environ Technol 16:256–268. https://doi.org/10.2965/jwet.18-033
Yamamoto N, Saito Y, Inoue D et al (2018b) Characterization of newly isolated Pseudonocardia sp. N23 with high 1,4-dioxane-degrading ability. J Biosci Bioeng 125:552–558. https://doi.org/10.1016/j.jbiosc.2017.12.005
Zhang S, Gedalanga PB, Mahendra S (2016) Biodegradation kinetics of 1,4-dioxane in chlorinated solvent mixtures. Environ Sci Technol 50:9599–9607. https://doi.org/10.1021/acs.est.6b02797
Zhang S, Gedalanga PB, Mahendra S (2017) Advances in bioremediation of 1,4-dioxane-contaminated waters. J Environ Manag 204:765–774. https://doi.org/10.1016/j.jenvman.2017.05.033
Zhou Y, Huang H, Shen D (2016) Multi-substrate biodegradation interaction of 1,4-dioxane and BTEX mixtures by Acinetobacter baumannii DD1. Biodegradation 27:37–46. https://doi.org/10.1007/s10532-015-9753-2
Zippilli C, Botta L, Bizzarri BM et al (2021) Laccase-catalyzed 1,4-dioxane-mediated synthesis of Belladine N-oxides with anti-influenza a virus activity. Int J Mol Sci 22:1337. https://doi.org/10.3390/ijms22031337
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Miraji, H., Ripanda, A., Bakari, R., Sarma, H. (2023). Biotransformation of 1,4-Dioxane by the Use of Bacteria in the Soil. In: Sarma, H., Joshi, S. (eds) Land Remediation and Management: Bioengineering Strategies. Springer, Singapore. https://doi.org/10.1007/978-981-99-4221-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-4221-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4220-6
Online ISBN: 978-981-99-4221-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)