Abstract
Cellulose has been considered as the most emerging biodegradable polymer. This is one of the most abundant biopolymers found in plants, fungi, and the cell wall of some bacteria. The main advantage of this renewable polymer is the presence of tunable hydroxyl functionalities in addition to biocompatibility and biodegradability. However, the presence of strong H-bonding between the chains of cellulose renders the polymer to be water insoluble limiting its processability. Hence, researchers found easy ways to solubilize the cellulose chains by functionalizing the hydroxyl groups through etherification, esterification, nucleophilic substitution, oxidation, and copolymerization. The degree of substitution plays a crucial role in determining the chemical, mechanical, and physical properties of cellulose derivatives which are critically discussed in the chapter. This chapter also focuses on the types of sources and corresponding properties of cellulose. A list of several cellulose derivatives is provided along with a discussion on how they can be used in several areas such as biomedical field, agriculture, pharmaceutical industries, biofuel generation, superabsorbents, electronics, etc.
Mouli Sarkar, Ashank Upadhyay, Dharmendra Pandey are equaly contributed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
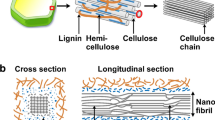
Biopolymers have significantly drawn attention for creating numerous biodegradable products. These biopolymers are synthesized within the biological cells through enzymatic pathways. Among several polysaccharides such as starch, cellulose, xanthan, pullulan, hyaluronic acid, chitosan, etc., cellulose gains popularity as it contains large numbers of OH groups that can be modified according to the requirements. It shields plants, and fungi from external chemical, biological, and mechanical disturbances. The large supramolecular structure containing large amounts of OH moieties makes cellulose hydrophilic as they are susceptible to water attack. About 40–50 weight percent of the woody biomass in plants is made up of cellulose, the most common polymer, and a considerable amount of this biomass is found in crystalline forms [1]. Chemically, cellulose is a long linear chain of anhydro-D-glucopyranosyl units joined by β-(1,4)-glycosidic linkages (Fig. 1a) [2]. The cyclic hemiacetal groups contain equatorial OH groups (in the C1 position) which defines that the cellulose structure exists in β-form (or β isomer), whereas, in the case of α isomer, the OH group remains in the axial position. The different isomers differentiate cellulose from other polysaccharides in terms of its biodegradation through hydrolysis reaction [3].
a Structure of cellulose; b Cellulose structure featuring inter and intramolecular H-bonding in the repeating unit and reducing (right) and non-reducing (left) end groups. Redrawn from the Ref. [4]
X-ray diffraction, 13C CP/MAS, and solid-state NMR reveal two crystalline forms of cellulose structure, viz. Iα and Iβ. These varieties completely depend upon sources. Valonia, bacterial cellulose, and the cell wall of Glaucocystis are rich in Iα, whereas tunicate (Halocynthia roretzi) or animal cellulose are rich in Iβ [5, 6]. The OH groups in the cellulose network control the chemical and physical characteristics of the cellulose chains. The intramolecular H-bonds between OH-groups of glucose units of the same cellulose units provide rigidity and thermostability of the chains, whereas, intermolecular H-bonds between two different cellulose chains are responsible for the development of the supramolecular structures as shown in Fig. 1b [4].
In addition to controlling their chemical reactivity, these OH groups can be modified by a variety of chemical reactions like esterification, acetylation, and nitration. Although cellulose is recognized to be the most plentiful and cost-effective biopolymer, its poor water solubility limits its commercial applications [7].
To address this problem, researchers have developed a variety of derivatives, including methylcellulose (MC), hydroxyethyl cellulose (HEC), carboxymethylcellulose (CMC), as well as cellulose acetate (CA) and hydroxypropyl methylcellulose (HPMC). The advantage of functionalization is to make a significant impact on the properties of cellulose by breaking H-bonding [2, 8]. Due to their simplicity of processing, these cellulose derivatives are also employed extensively in the cosmetic and pharmaceutical industries. Mostly, industries employ them commercially in a variety of forms, including tablet and capsule coating materials, stabilizers, thickening agents, bioadhesives, mucoadhesives, pressure-sensitive adhesives, binders, gelling agents, flavor maskers, fillers, free-flowing agents, and hemostatic compounds [9, 10].
2 Sources
French scientist Anselme Payen first extracted cellulose from the cell walls of the plant in 1938 and, later, the chemical structure was determined by Hermann Staudinger in 1920. Finally, synthetic cellulose was first chemically synthesized in the laboratory by Kobayashi and Shoda in 1992 [11].
Wood is the primary resource of cellulose. Others are bacterial cellulose, algal cellulose, and tunicate cellulose [2, 12, 13]. Bacterial cellulose is very pure, highly crystalline, and contains a high DP (degree of polymerization). It has been investigated that, annually a tree can produce 1011–1012 tonnes of cellulose during photosynthesis [14]. Figure 2a illustrates the different sources of cellulose.
2.1 Wood and Plant Cellulose
The primary source of cellulose is wood and plants. The sources vary as corn, cotton, jute, pineapple leaves, flax, hemp, potato peel waste, cereal straws, oil palm biomass, cotton, etc. [2, 16]. The cellulose content in the plant varies with the location. In the soft woody part, it ranges from 30 to 75%, while, in the case of the hard woody part, it is around 40 to 50%. Fibers extracted from both these parts exhibit similarities in composition and structure. They are made of carbohydrates, (such as cellulose, hemicellulose, and lignin) having a complex structure. Hemicelluloses are found in terrestrial plants and algae. Xylan-type polysaccharides are mostly characterized by hemicellulose, whereas lignin is the highly branched 3D complex network having several functional groups. The nature of cellulose highly varies with the amount of lignin, hemicellulose, and fibers.
2.2 Bacterial Cellulose (BC)
Bacterial cellulose (BC) is often very pure (no lignin or hemicelluloses). It is highly crystalline having a high degree of polymerization. It becomes more significant that numerous bacteria belonging to the genera Acetobacter, Agrobacterium, Sarcina, and Rhizobium produce cellulose [17]. Agro-industrial wastes are commonly used as carbon sources to manufacture BC, and the yield of bacterial culture can reach 40%. Most often, BC is acquired in pure form having a purity of more than 90%. BC is capable of being transformed into a variety of morphological forms, including spheres, films, fleeces, and hollow particles. Pure cellulose face masks also frequently have a higher biodegradability than commercial counterparts, which is an additional benefit. A fibrous network of cellulose chains gives them a porous structure, and they are typically wide ribbon-shaped fibrils (diameter ~100 nm), which contain numerous nanofibrils (diameter ~8 nm). As a result, distinctive nanomorphology has a wide surface area, high water holding capacity (~99% water), high wet strength, strong elasticity, and conformability.
2.3 Algal Cellulose
Another source of cellulose is algae. It is highly crystalline. Algae cellulose is specifically produced from brown species, red species (Gelidium elegans), and green species (Cladophora). Algal cellulose grows more quickly than plant cellulose, giving it a competitive edge in industrial applications. Oceans, lakes, ponds, and wastewaters are just a few of the environments where algae can develop, and they can produce cellulose [15]. Algal cellulose is not produced in its purest form because hemicellulose, protein, and lignin are highly associated with them. Red algae and green algae are good sources of cellulose. The main types of carbohydrates found in red algae, or Gelidium elegans, are cellulose and agar. Green algal cellulosic cellulose exhibits outstanding qualities such as large specific surface area, high porosity (mesopores), and high crystallinity (~70%) among others.
2.4 Tunicate Cellulose
The only known animal source of cellulose is tunicates. They are marine invertebrate animals, and cellulose is extracted from the outer tissue of these animals. It contains a large amount of cellulose (~60%) and a small amount of nitrogenous compound (~27%). In tunicates, cellulose is also present as a nano fibrillar form arranged in a multi-layered structure at the surface of its epidermis. The shape and size of these nanofibril bundles vary with different species. Commonly, the length and width of nanofibrils are in the range of 100 nm–2 μm and 10 nm–30 nm, respectively. It also has a high specific surface area (150–170 m2 g−1). The extraction of cellulose from various sources is one of the important processes and most of the commercially used cellulose is primarily extracted from plant-based sources. In the following section, we are going to discuss the extraction process of cellulose from plant-based sources (Table 1).
3 Extraction of Cellulose
Different types of structural polymers like polysaccharides and polyphenolic compounds make up the constituent of a typical plant. The cell wall of a plant comprises of cellulose, pectin, and hemicellulose. Cellulose is present in the form of fibrillar and is the major part of the biomass [16]. Conventionally, cellulose is isolated from plants starting with the pulping process. This process removes the extractable materials like lignin, and hemicellulose without degrading the fibrillar structure of cellulose. Bleaching is carried out as the next process which utilizes oxygen, ozone, and hydrogen peroxide. The final product after bleaching mainly contains alpha-cellulose and hemicelluloses in residual amounts. This process extracts 40% cellulose, 10–11% as secondary products like furfural, xylose, and acetic acid, and the remaining as waste material [26]. Salimi et al. elaborate on the stepwise extraction of cellulose [27]. In addition to fibrillated cellulose, cellulose nanoparticles can also be produced and a scheme to obtain the same is shown in Fig. 3, a detailed discussion of which is provided in the next section [28,29]

Modified from Gopakumar et al. [28]
Scheme of extraction process of cellulose.
3.1 Cellulose Particles
Based on the size and morphology, the cellulose particles have been broadly characterized into cellulose crystals and cellulose fibers. These differ in size, shape, crystallinity, aspect ratio, and physiochemical properties [2, 15, 20].
3.2 Cellulose Fibers
Naturally found cellulose is present in the form of microfibrils which assemble and organize themselves in the form of cellulosic fibers. Three types of cellulose fibers are known viz. pulp fibers whose length is found in the range of 1–10 mm, staple fibers with a length of approximately 60 mm, and strand fibers with a length range of 20–100 cm. Strand fibers contain multiple cells, whereas staple fibers contain a single cell. Cotton fibers are an example of staple fibers with a length range of 25–45 mm. These two types of fibers are obtained from wild plants and crops via a pulping process. The fibers obtained are further disintegrated into micro and nanofibrils by a mechanical process that includes homogenization and microfluidization. The length of cellulose nanofibrils obtained is generally 1 µm and the width ranges from 2 to 100 nm. The fiber size is mainly depending on the pretreatment, fibrillation process, and source. Cellulose nanofibrils have superior mechanical strength with a tensile strength of ~1 GPa and an elastic modulus range of ~14–36 GPa [30].
3.3 Cellulose Crystals
Enzymatic, mechanical, and chemical treatments of cellulose microfibrils lead to the formation of cellulose crystals. During treatment, the cellulose microfibrils get fragmented into microcrystalline cellulose and cellulose nanocrystals. Microcrystals have a high degree of crystallinity and form rod-like stiff particles. Microcrystalline cellulose (MCC) was first introduced as an ingredient for direct tableting. The common source for pharmaceutical MCC is wood cellulose. Cellulose nanocrystals (CNCs) particles are harnessed from the cellulose microfibrils’ crystalline regions. CNCs are synthesized via sulfuric hydrolysis of cellulose, which leads to the disintegration of highly crystalline CNCs particles which are eventually extracted. The final characteristic of CNCs depends on the cellulose source and extraction parameters like temperature of hydrolysis, controlled time, and further modification like dialysis and neutralization. CNCs display exceptional mechanical and thermal properties like high TS, modulus, low density, and high thermal properties [31]. Due to the superior properties of CNCs, various potential applications have been explored [32].
4 Properties of Cellulose
4.1 Solubility
As we have seen that plants contain hemicellulose and cellulose known as holocellulose, lignin, and inorganic materials (such as ash). Cellulose can be simply hydrolyzed by acids to form water-soluble sugars and is resistant to strong alkalis. Cellulose is proportionally resistant to oxidizing agents. Hemicellulose forms a supportive matrix for the cellulose microfibrils which are hydrophilic in nature. This hydrophilicity helps its easy dissolution in alkali and easy hydrolysis in acids. The harder part (lignin) does not get hydrolyzed in acids but is soluble in hot alkali, gets condensed in the presence of phenol, and quickly oxidized. A mixture of dimethyl sulfoxide (DMSO) and 10–20% (w/v) tetra butyl ammonium fluoride trihydrate can dissolve cellulose without any pretreatment at room temperature. Cellulose is insoluble in water and other organic solvents which limits its applications. The OH groups present in the cellulose form H bonding restricting the entry of solvent molecules [33]. This is the main reason for the insolubility of cellulose. It can only be dissolved when this interaction is broken. This can be induced by the addition of functional groups in the cellulose backbone which break the intermolecular H-bonding and in turn solvate the chains. Due to the compact structure of cellulose, a complex solvent system that required minimum energy of dissolution is used in dissolving cellulose, Initially, Copper complexes (Cuoxam) were used to dissolve the cellulose [34]. However, other metal complexes based on Co, Ni, Cd, and Zn are also being used for dissolving cellulose. Thus cellulose can be easily dissolved in phosphoric acid and trifluoroacetic acids by disrupting the Hydrogen bonds. Various ionic liquids are found to be suitable agents for dissolving cellulose since the solvents are eco-friendly, less toxic, having good thermal stability and recyclability. However, the high cost and energy-intensive nature of ionic liquids limit their usage.
4.2 Mechanical Property
Cellulose shows high crystallinity and high intermolecular interaction which manifests itself in the form of superior mechanical properties, viz. tensile strength and modulus. The rigid cellulose nanocomposites have a modulus similar to that of Kevlar and Steel. Bacterial cellulose (BC) is softer and finds application in the replacement of collagen networks. Looking at the impressive mechanical properties of cellulose leads to potential applications in wound healing, tissue engineering, and drug delivery where a strong and stable building block is required [35].
4.3 Hygroscopic Property
Cellulose is highly hygroscopic and attracts water, this is by the virtue of hydrogen bonds present in the structure. Cellulose absorbs water almost 30% of its biomass and gets swollen, the water permeates into the structure via the amorphous region (disordered domains) [36, 37]. This swelling of cellulose assists in the aqueous processing of cellulose and its composites. The hygroscopic nature of cellulose leads to lower wet strength and which might pose limiting parameters for various applications.
4.4 Structure and Degradability
The backbone of cellulose is structured on β-1,4-glycoside-linked glucose units. The rigid structure of cellulose can be attributed to the intermolecular hydrogen bonds leading it to good mechanical properties making it useful as a building material in plants. These superstructures produced are stable till significantly a higher temperature. The degradation of cellulose happens below the theoretical melting point of 260–270 °C. The melting point can be reduced by breaking the H-bonding in the structure, this can be effectively done by derivatizing the hydroxyl group of cellulose. Some of the most commonly used cellulose derivatives which are used industrially are cellulose acetate, cellulose acetobutyrate, ethyl cellulose, and benzyl cellulose. Thermoplasticity in these derivatives is achieved by complete derivatization of hydroxyl group. The average degree of substitution (DS) value is the number of substituted OH groups per anhydroglucose unit. The substitution level is inversely proportional to the biodegradability of cellulose derivatives as enzyme attack requires a free glucose unit [38].
4.5 Mechanism of Degradation
One of the well-recognized degradation routes for cellulose is by the brown rot fungi which produce H2O2, which in turn assists in the production of hydroxyl radical (·OH). The attack by ·OH leads to the cleavage of the cellulose chains generating lactone as shown in Fig. 4. The ·OH radical is produced via the Fenton reaction (H2O2 + Fe2+ → H2O + Fe3+ + OH) in which iron is used from the wood itself. The hydrogen abstraction by ·OH is a fast reaction that leaves behind a carbon-centered radical which rapidly reacts with oxygen from the environment, which subsequently eliminates ·OOH [39].
Plausible mechanism of degradation of cellulose by Brown rot fungi, reproduced with permission from Hammel et al. [39]
5 Functional Derivatives of Cellulose—Synthesis, and Biodegradability
As we have depicted in the previous section that crystallinity and hydrogen bonding in the cellulose structure leads to difficulty in the processability of cellulose; hence, it is essential to functionalize the cellulose. Esterification, etherification, nucleophilic substitution, oxidation, and copolymerization are the most common routes to achieve functionalization in cellulose. Functionalization has a two-fold advantage, viz. feasible processibility, and better plastic properties. The functionalization helps in breaking the crystalline structure and assists in solubilization which further assists in processing. The amount of substitution has an impact on biodegradability. As the size and degree of substituent groups increase, the biodegradability of the cellulose decreases [40].
The OH groups in cellulose being susceptible to reactions, is utilized for the functionalization to undergo various kinds of reactions. Figure 5 shows the chemical structure of cellulose after functionalization. The degree of substitution affects the final properties of the polymer obtained. To design a new functionalized cellulose-based polymer, it is essential to understand the changes in properties achieved with the substitution. The various cellulose derivatives are discussed in detail below (Table 2).
Cellulose derivatives, redrawn from [2]
5.1 Cellulose Ethers
When OH in the anhydroglucose unit of cellulose is substituted with the alkyl group, cellulose ethers are obtained. Cellulose ethers are synthesized by the reaction of cellulose with NaOH and subsequently reacting with alkyl chloride as shown in the scheme below (Fig. 6). Cellulose ethers consist of methylcellulose, ethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, and carboxymethylcellulose and their derivatives.
The above scheme represents the summary of the synthesis of cellulose derivatives viz. esters and ether, redrawn from [2]
5.1.1 Methylcellulose (MC)
Methylcellulose is the simplest functionalized biodegradable cellulose ether. It has a vast application in the food industry, tissue engineering, coating, and preparation of mulch films. It comes with the advantage of easy processability, large availability, and low cost. The biodegradability however limits its application in high-end products. Hence, two methods which helps to modulate the biodegradability of the polymers with required properties are the incorporation of fillers with a high aspect ratio to increase the tortuous path, and secondly by incorporation of crosslinks such as glutaraldehyde (Glu) between the polymer chains. This will delay the hydrolysis and provide an enhanced property with the loss in biodegradability [41].
Methylcellulose is synthesized in an alkaline medium in the presence of methylating agents like methyl chloride or dimethyl sulfate [42]. Degree of substitution depends on the synthesis conditions such as reaction time and methylating agent. As the degree of substitution is increased from the range 1.4–2.0 to 2.4–2.8, the solubility of methylcellulose is enhanced in water as well as in some organic solvents [43].
5.1.2 Carboxymethylcellulose (CMC)
CMC is one of the most popular and commercially available cellulose ethers. It is biodegradable, non-toxic, water-soluble, and has better chemical stability. CMC has myriad applications by the virtue of its properties in the food industry, textile industry, paper industry, drugs and cosmetics, leather, films, filaments, paints, and lacquers [44]. CMC is an efficacious thickening agent, binder, and emulsion stabilizer, and has film formability. CMC is synthesized by a reaction of cellulose with monochloroacetic acid in which the hydroxyl group (predominantly C2) is substituted by carboxymethyl groups (–CH2–COOH).
The degree of substitution is typically between 0.6 and 1.25. The properties are dependent on the large molecular structure, degree of substitution, and molecular weight [45]. The sodium salt is soluble in water and hence CMC is usually used as its sodium salt.
5.1.3 Ethyl Cellulose (EC)
Ethyl cellulose is another commercial biodegradable cellulose ether that is formed when the hydroxyl group is substituted by the ethyl group. Ethyl cellulose is synthesized by the reaction of cellulose alkali with ethyl chloride at 60 °C for several hours [46]. For commercial products, normally the degree of substitution lies in the range of 2–2.6 [47]. The properties of ethyl cellulose depend on the molecular weight, degree of etherification, and molecular uniformity.
EC has an excellent film-forming capacity with superior barrier properties and hydrophobicity making it a suitable candidate for biomedical applications, majorly in drug delivery. Ethyl cellulose is generally brittle and hence plasticizers are added in order to enhance its flexibility, thermal stability, and processability [48].
5.1.4 Hydroxyethyl Cellulose (HEC)
Hydroxyethyl cellulose is synthesized by reacting alkali cellulose with ethylene dioxide to achieve hydroxy ethyl group (–CH2–CH2–OH) at 2, 4 and 6 positions of glycosyl unit of cellulose. This OH group acts as a reactive center which can be utilized for further modification. Hydroxyethyl cellulose is highly soluble in water and other organic solvents. Mixing cellulose with HEC allows easy processing of it, and diversifies its biomedical application [49].
5.1.5 Hydroxypropyl Cellulose (HPC)
Hydroxypropyl cellulose is a biodegradable polymer in which the OH group is substituted by 2-hydroxypropyl. Like HEC, HPC also has a secondary alcohol group that can be utilized for further functionalization of the cellulose chains for biomedical applications such as tissue engineering. HPC forms a liquid crystal when dissolved in water depicting an interesting property of mechanochromism meaning it shows a change in color on the application of pressure. This property makes it a suitable candidate for application in sensing applications [50].
5.2 Cellulose Esters
Cellulose esters offer the advantage of solubility in a common solvent and easy melt processability over cellulose. It also affords cellulose to be molded into 3D shapes, drawn into thin wires, and solution cast into films, sheets, or coating applications [51]. The modification can also be done over the surface of the cellulose substrate keeping the crystallinity intact to avail high mechanical properties [52]. Common cellulose esters are discussed below.
5.2.1 Cellulose Acetate (CA)
Cellulose acetate is synthesized from the acetylation of cellulose. The DS defines the properties of the CA. DS of 2.5 is the most common level that provides optimum molecular weight and rheological/solution properties. These properties help CA to be used in various applications like textiles, thermoplastic, films, and cigarette filters [53].
The key mechanism of biodegradation of CA is chemical hydrolysis and acetyl esterases as the first step which subsequently leads to the degradation of backbone which mainly contains cellulose. Various studies have confirmed that CA is indeed biodegradable in natural environment. One interesting study is done by Komarek et al. in which acetyl carbon was labeled with 14C and CO2 evolution was monitored. The study compared CA with DS of 1.85, 2.07, and 2.57, and found a reduction in degradation rate by increasing the level of acetylation [54].
5.2.2 Cellulose Nitrate (CN)
Cellulose nitrate also known as nitrocellulose, is synthesized by the substitution of an OH group with a nitrate group by treating cellulose with concentrated nitric acid. The typical DS is 2.2–2.8 which eventually decides its properties and application. A major application of CN is in explosives, plastics, coating, and ink industries [55]. CN is found to be 40% biodegradable only due to inhibition by various products formed during the degradation of main degrading enzymes [56].
5.2.3 Cellulose Sulfate (CS)
Sulfonation of cellulose is carried out using sulfuric acid, sulfur trioxide, and chlorosulfonic acid. It is water soluble, antiviral, antibacterial, and has anticoagulant properties due to the presence of a sulfate group. CS shows excellent biocompatibility, film-forming ability, and biodegradability. These properties make it highly viable for application in tissue engineering and drug delivery [57]. In vitro studies showed favorable biodegradable properties of sodium cellulose sulfate (NaCS) and chitosan-based films. The DS also has an inverse effect on the biodegradability of CS [58].
6 Applications
6.1 Packaging Industry
Plastics are commercial materials derived mainly from synthetic polymers. These materials are cheap and remain intact throughout their packaging life. But synthetic polymer leads to some side effects on human beings, animals, and the environment. The usage of plastic bags cannot be reduced as they are easy to handle. That’s why to get rid of the sustainability issues, it is important to introduce biodegradable polymers to reduce the solid waste in the environment. Among the well-known biopolymers, cellulose has been extensively used in the food packaging industry [59].
In order to convert cellulose into a polymeric substance, first cellulose has to be extracted from natural resources. The best methods to extract cellulose were described by Han and Rowell and later by Borella et al. [52, 53, 60]. Recently, partially biodegradable biocomposites and green biocomposites gained much attention in synthesizing biobased products, which can be degraded easily by microorganisms [3, 52, 53]. Biocomposites consist of biofiber and matrix polymer. Partially biodegradable biocomposites contain biodegradable biofiber and non-biodegradable polymer matrix whereas, biodegradable biocomposites consist of both biodegradable fiber and polymer. Also, there exist hybrid biocomposites which contain two or more biofiber in combination with a polymer [61]. In recent days, biodegradable polymers blended with inert polymer matrices are becoming more attractive. In this case, upon disposal of the plastic in the environment, the biodegradable part can be degraded by microorganisms and the residual polymer component loses its integrity and fades away. A cellulose ester known as cellulose acetate butyrate (CAB) can be added to other cellulose ester-based film formers as well as employed as a reactive polyol during curing of the coating. The regenerated cellulose fiber, i.e., cellophane is used as breathable packaging material for baked food. Apart from that, cellulose esters and cellulose ethers are being used in the processing of film coatings, edible films, etc. [62].
6.2 Pharmaceutical Industry
Cellulose-based polymers are gaining much importance in the field of pharmaceuticals. Especially, cellulose ethers and cellulose esters are having unique physicochemical and mechanochemical properties by which they can be used broadly as healthcare products in the form of tablets, gelling agents, bioadhesives, mucoadhesives, etc. Another well-known derivative of cellulose MCC is a multifunctional excipient such as silicified MCC (SMCC) which is being extensively used instead of only MCC. Recently, cellulose is converted to nanocomposites which are also used as drug-delivery agents and as ion-sensing materials [63,64,65].
6.3 Electronic Industry
In order to meet the sustainability issues, it is necessary to utilize renewable energy sources. Thus biodegradable materials such as cellulose derivatives are highly used in the field of the electro-technological field for their lightweight, low cost, transparency, portability, and flexibility in electronic and solar energy conversion [66, 67]. Cellulose fibers having a diameter of ~20 µm, would have been suitable for usage in solar cells, but their high surface roughness and porous structure render their effectiveness in coatings. Also, the fiber diameter is much larger than the wavelength of visible light. So, traditional papers made of cellulose are opaque which is difficult for using the materials for transparent devices. Chang et al. showed that O-(2,3-dihydroxypropyl) cellulose (DHPC) produces a smooth surface with excellent ductility and transparency and can be effectively used in a flexible solar cell. However, due to poor mechanical strength, this derivative of cellulose is further reinforced by rigid tunicate cellulose nanocrystals (TCNCs) [68].
6.4 Metal Industry
Water bodies are playing a major role as a carrier of heavy metals, non-metals, and organic dyes. Cellulose backbone contains a huge amount of OH groups which can interact with metal ions through electrostatic force and hydrogen bonding. Besides, these OH groups can be converted into several other functional groups (phosphonate, sulfonate, amine, carboxyl, ether, ester, etc.) which change the surface charge over the cellulose matrix. These modifications provide excellent interaction with metal ions. Hajeeth et al. employed cellulose extracted from sisal fiber in the form of a cellulose-g-acrylic acid copolymer to adsorb Ni(II) and Cu(II) from aqueous solutions [69]. Cellulose was also successfully modified to produce cellulose-amine and cellulose-thio adsorbents. The obtained sample showed strong adsorption potential towards Hg(II) in aqueous solutions [59]. Cellulose can also be used as an adsorbent when combined with montmorillonite (NaMMT). It was applied to eliminate Cr(VI) from the aqueous solutions. Other modified cellulose were listed in Table 3.
6.5 Biomedical Industry
Cellulose contains abundant OH groups over its backbone. Hence, cellulose hydrogel can be easily produced by physical crosslinking [91]. There are some water-soluble cellulose derivatives, such as Methylcellulose, Hydroxypropyl Cellulose, Hydroxypropyl Methylcellulose, and Carboxymethylcellulose that are often used for the fabrication of cellulose-hydrogels. It was shown that, when OH moieties are partially substituted by methyl or hydroxypropyl groups, both hydrogen bonding and hydrophobic interaction play a key role in determining the gelation ability of the hydrogels [92]. In the case of chemically crosslinking structures, some di-functional reagents are employed to provide a three-dimensional shape to the cellulose hydrogel structure. Sannino et al. prepared super-hydro porous hydrogels based on crosslinking of CMC and HEC with a crosslinker such as DVS (divinyl sulfone) (Fig. 7). The main feature of these hydrogels is that they can change their sorption capability depending on the ionic strength and pH of the excipient. The super-adsorbent hydrogel has been efficiently applied for the treatment of edemas [93,94,95]. Apart from medication, cellulose-based hydrogels are often used in tissue engineering, sensors, agriculture, water purification, and chromatographic support which are discussed below [96,97,98,99].
Scheme of formation of HEC and CMC crosslinked network, redrawn with permission from Ayouch et al. [100]
In the biomedical field, hydrogels have never-ending applications. It has the ability to remove excess water from the body and is highly effective in the treatment of edema. Polyelectrolyte-based cellulosic hydrogels, such as sodium salt of carboxymethylcellulose and hydroxyethyl Cellulose are highly pH-dependent and get removed from the body with feces without affecting the function of other body parts. The water uptake capability varies with the changing ratio of NaCMC/HEC [101]. Besides, superabsorbent cellulose-based hydrogels are being extensively used in personal care products like diapers as these hydrogels absorb liquid and keep that place dry, and also prevent diaper rash and any other fungal attack. These products are also cheap and safe to use. Furthermore, these superabsorbents prevent leakage and reduce the risk of fecal contamination, and gastrointestinal problems [102, 103]. In 1966, Harmon and Herper separately patented superabsorbent materials [104].
The superabsorbent cellulosic system contains three main parts (1) an envelope of non-oven tissue, (2) a plastic cover material, and (3) an absorbent made of wood pulp cellulose mixed with some hydroporous superabsorbent polymer (SAP). The latter two components were usually non-biodegradable. Hence, for the recovery of cellulose materials and recycling of the absorbent, usage of cellulose-based hydrogels was suggested. Among them, NaCMC and HEC crosslinked with DVS show similar properties to SAP and produce higher retention ability and swelling ratio [105].
6.6 Agricultural Industry
In order to optimize the water resources in the agricultural field, especially for the cultivation in the desert area, a constant water supply is highly needed. So, the superabsorbent provides the required quantity of water throughout the time by releasing the water slowly through a diffusion-drive mechanism [106]. Cellulose-based hydrogels perform as an alternative to acrylate-based hydrogel which is not biodegradable. Some cellulose-based SAP hydrogels were suggested which have the absorption ability of almost one liter of water per gram of hydrogel [107, 108].
6.7 Biofuel Industry
In recent days biofuel supplies 86% of the energy of the world. With the increment in population, energy usage is also expected to surge in the coming days. High usage of petroleum-based fuels leads to an impact on environmental changes like global warming and the reduction of biomass. In that case, cellulose is the most active renewable resource used in the production of energy in the world (chemical energy stored in biomass is approximately 6–7 times that of total human energy consumption per year) [109]. Moreover, a human can not digest cellulose, so it is advantageous to use this polymer as it does not compete with food resources. Production of biofuel from cellulosic materials is generally carried out by the processes of gasification, pyrolysis, and hydrolysis. First, the woody biomass is disintegrated to produce isolated cellulose from other constituents of lignocellulosic materials. Next, cellulose is depolymerized (basically converted to glucose units), followed by conversion of glucose into biofuels via biological treatment, and finally purification of the biofuel. In the meantime, it is necessary to deoxygenate the biomass as it reduces the heat content thereby lessening the chance of blending with other fossil fuels [110]. The detailed procedure for the formation of biofuel was studied by Fatehi [87].
6.8 Paper Industry
The major ingredient of paper is cellulose fibers. The quality of paper highly depends on the size and quality of cellulose fiber. Higher the cellulose content in the paper pulp, the better the quality of the paper. In earlier times, the paper used to be made up of bamboo fiber, and silk. Nowadays, it has been replaced by cellulose-based, plant fiber, which has excellent writable properties and is also cheap. Cellulose-based papers are highly porous, rough, and hydrophilic. Three kinds of pulp were studied such as chemical pulp, semi-chemical pulp, and mechanical pulp during paper production. Industrially, the chemical pulp is being extensively used as paper produced from this becomes strong, stable, and easy to bleach, as compared to other pulps [111]. Recently, nano-fibrillated cellulose has become an industrially promising material for the formation of papers. It acts as a reinforcing material on the fiber surface. Also, the nano-fibrillated cellulose plays the role of filler and is incorporated in the voids and pores between the fibers [112].
7 Conclusion
Cellulose being a ubiquitous and easily biodegradable polymer has an immense potential to replace petroleum-based non-degradable polymers. Cellulose possesses fascinating mechanical and thermal properties making it comparable with that of conventional polymers. Cellulose is majorly extracted from the plant via multiple stages of chemical treatment. Other sources include bacteria and marine invertebrate animals called tunicates. Hydrogen bonding present in the Cellulose makes them crystalline, thereby enhancing their mechanical property, but reducing solubility in various solvents, thus affects its solution processability. To enhance the processability of cellulose and to provide better plastic properties, it has been functionalized via various routes viz. etherification and esterification. The free OH group in cellulose assists in their functionalization to produce cellulose derivatives. For example, CMC and EC are some of the commercially available Cellulose ether derivatives used in myriads of application. Cellulose acetate, cellulose nitrate and cellulose sulfate are some of the common cellulose esters discussed in this chapter. By a virtue of their property, cellulose has a great potential to be used in various areas, viz. packaging, pharmaceutical, electronic, biomedical, biofuel, and paper industry. With the development of nanotechnology, cellulose–inorganic hybrid hydrogels are found to have multifunctional applications. Besides, microbial cellulose has become popular in the generation of biomedical devices such as in wound healing, organ replacement etc. Cellulose has become the most studied biopolymer that can be easily processed and industrialized . Therefore, the development of cellulose derivatives along with other polymers as well as inorganic fillers has the potential to show extensive application in our daily life. Nonetheless, they suffer from challenges such as source variation, availability, lack of economically attractive extraction procedures, etc., which require to be overcome in the near future for their widespread applications.
References
Solala I, Iglesias MC, Peresin MS (2019) On the potential of lignin-containing cellulose nanofibrils (LCNFs): a review on properties and applications. Cellulose 27:1853–1877
Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, Bacabac RG, Klein-Nulend J (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28:1893–1931
Chandra R, Rustgi R (1998) Biodegradable polymers. Prog Polym Sci 23:1273–1335
Shen T, Gnanakaran S (2009) The stability of cellulose: a statistical perspective from a coarse-grained model of hydrogen-bond networks. Biophys J 96:3032
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose iα from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125:14300–14306
Bochek AM (2003) Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ J Appl Chem 76:1711–1719
Kamel S, Ali N, Jahangir K, Shah SM, El-Gendy AA (2008) Pharmaceutical significance of cellulose: a review. Express Polym Lett 2:758–778
Tkacheva NI, Morozov SV, Grigor’Ev IA, Mognonov DM, Kolchanov NA (2013) Modification of cellulose as a promising direction in the design of new materials. Polym Sci Ser B 55:409–429
Abeer MM, Mohd Amin MCI, Martin C (2014) A review of bacterial cellulose-based drug delivery systems: their biochemistry, current approaches and future prospects. J Pharm Pharmacol 66:1047–1061
Hon DNS (1994) Cellulose: a random walk along its historical path. Cellul 11(1):1–25
Si X, Lu F, Chen J, Lu R, Huang Q, Jiang H, Taarning E, Xu J (2017) A strategy for generating high-quality cellulose and lignin simultaneously from woody biomass. Green Chem 19:4849–4857
Courtenay JC, Sharma RI, Scott JL (2018) Recent advances in modified cellulose for tissue culture applications. Mol 23:654
Heinze T, El Seoud OA, Koschella A (2018) Production and characteristics of cellulose from different sources. 1–38
Hardouin K, Bedoux G, Burlot AS, Nyvall-Collén P, Bourgougnon N (2014) Enzymatic recovery of metabolites from seaweeds: potential applications. Adv Bot Res 71:279–320
Abdul Khalil HPS, Bhat AH, Ireana Yusra AF (2012) Green composites from sustainable cellulose nanofibrils: a review. Carbohydr Polym 87:963–979
Ullah H, Santos HA, Khan T (2016) Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 23:2291–2314
Campano C, Balea A, Blanco A, Negro C (2016) Enhancement of the fermentation process and properties of bacterial cellulose: a review. Cellulose 23:57–91
Gorgieva S, Trček J (2019) Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials 9:1–20
Müller A, Zink M, Hessler N, Wesarg F, Müller FA, Kralisch D, Fischer D (2014) Bacterial nanocellulose with a shape-memory effect as potential drug delivery system. RSC Adv 4:57173–57184
Zhao Y, Zhang Y, Lindström ME, Li J (2015) Tunicate cellulose nanocrystals: Preparation, neat films and nanocomposite films with glucomannans. Carbohydr Polym 117:286–296
Zhao Y, Li J (2014) Excellent chemical and material cellulose from tunicates: Diversity in cellulose production yield and chemical and morphological structures from different tunicate species. Cellulose 21:3427–3441
Samiee S, Ahmadzadeh H, Hosseini M, Lyon S (2019) Algae as a source of microcrystalline cellulose. Adv Bioprocess Altern Fuels, Biobased Chem Bioprod Technol Approaches Scale-Up Commer. https://doi.org/10.1016/B978-0-12-817941-3.00017-6
Chen YW, Lee HV, Juan JC, Phang SM (2016) Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr Polym 151:1210–1219
Hua K, Rocha I, Zhang P, Gustafsson S, Ning Y, Strømme M, Mihranyan A, Ferraz N (2016) Transition from bioinert to bioactive material by tailoring the biological cell response to carboxylated nanocellulose. Biomacromolecules 17:1224–1233
Bhat AH, Dasan YK, Khan I, Soleimani H, Usmani A (2017) Application of nanocrystalline cellulose: processing and biomedical applications. Cellul Nanofibre Compos Prod Prop Appl 215–240
Salimi S, Sotudeh-Gharebagh R, Zarghami R, Chan SY, Yuen KH (2019) Production of nanocellulose and its applications in drug delivery: a critical review. ACS Sustain Chem Eng 7:15800–15827
Gopakumar DA, Arumughan V, Pasquini D, Leu SY, Abdul Khalil HPS, Thomas S (2019) Nanocellulose-based membranes for water purification. Nanoscale Mater Water Purif 59–85
Hasan N, Rahman L, Kim SH, Cao J, Arjuna A, Lallo S, Jhun BH, Yoo JW (2020) Recent advances of nanocellulose in drug delivery systems. J Pharm Investig 50:553–572
Han JS, Rowell JS (2008) Chemical composition of fibers. Cellulose 283:83–134
Ng HM, Sin LT, Tee TT, Bee ST, Hui D, Low CY, Rahmat AR (2015) Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos Part B Eng 75:176–200
Pachuau L (2017) Application of nanocellulose for controlled drug delivery. Nanocellul Nanohydrogel Matrices Biotechnol Biomed Appl 1–19
Lindman B, Medronho B, Alves L, Costa C, Edlund H, Norgren M (2017) The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys Chem Chem Phys 19:23704–23718
Saalwächter K, Burchard W, Klüfers P, Kettenbach G, Mayer P, Klemm D, Dugarmaa S (2000) Cellulose solutions in water containing metal complexes. Macromolecules 33:4094–4107
Xiong R, Hu K, Grant AM, Ma R, Xu W, Lu C, Zhang X, Tsukruk VV (2016) Ultrarobust transparent cellulose nanocrystal-graphene membranes with high electrical conductivity. Adv Mater 28:1501–1509
Benítez AJ, Torres-Rendon J, Poutanen M, Walther A (2013) Humidity and multiscale structure govern mechanical properties and deformation modes in films of native cellulose nanofibrils. Biomacromolecules 14:4497–4506
Yan G, Chen B, Zeng X, Sun Y, Tang X, Lin L (2020) Recent advances on sustainable cellulosic materials for pharmaceutical carrier applications. Carbohydr Polym 244:116492
Brodman B, Devine MP (1981) Microbial attack of nitrocellulose
Hammel KE, Kapich AN, Jensen KA, Ryan ZC (2002) Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb Technol 30:445–453
Nawrath C, Poirier Y, Somerville C (1995) Plant polymers for biodegradable plastics: cellulose, starch and polyhydroxyalkanoates. Mol Breed 1:105–122
Rimdusit S, Jingjid S, Damrongsakkul S, Tiptipakorn S, Takeichi T (2008) Biodegradability and property characterizations of methyl cellulose: effect of nanocompositing and chemical crosslinking. Carbohydr Polym 72:444–455
Viera RGP, Filho GR, de Assunção RMN, da Carla C, Vieira JG, de Oliveira GS (2007) Synthesis and characterization of methylcellulose from sugar cane bagasse cellulose. Carbohydr Polym 67:182–189
Vieira JG, Filho GR, Meireles CDS, Faria FAC, Gomide DD, Pasquini D, Cruz SFD, De Assunção RMN, Motta LADC (2012) Synthesis and characterization of methylcellulose from cellulose extracted from mango seeds for use as a mortar additive. Polímeros 22:80–87
Hollabaugh CB, Burt LH, Walsh AP (2002) Carboxymethylcellulose. Uses and applications. Ind Eng Chem 37:943–947
Turaev AS (1996) Dependence of the biodegradability of carboxymethylcellulose on its supermolecular structure and molecular parameters. Chem Nat Compd 312(31):254–259
Rekhi GS, Jambhekar SS (2008) Ethylcellulose—a polymer review. https://doi.org/10.3109/03639049509048096 21:61–77
Koch W (1937) Properties and uses of ethylcellulose. Ind Eng Chem 29:687–690
Yang D, Peng X, Zhong L, Cao X, Chen W, Zhang X, Liu S, Sun R (2014) “Green” films from renewable resources: properties of epoxidized soybean oil plasticized ethyl cellulose films. Carbohydr Polym 103:198–206
Wang W, Zhang P, Zhang S, Li F, Yu J, Lin J (2013) Structure and properties of novel regenerated cellulose fibers prepared in NaOH complex solution. Carbohydr Polym 98:1031–1038
Kamita G, Frka-Petesic B, Allard A, Dargaud M, King K, Dumanli AG, Vignolini S (2016) Biocompatible and sustainable optical strain sensors for large-area applications. Adv Opt Mater 4:1950–1954
Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC, Tindall D (2001) Advances in cellulose ester performance and application. Prog Polym Sci 26:1605–1688
Berlioz S, Molina-Boisseau S, Nishiyama Y, Heux L (2009) Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 10:2144–2151
Puls J, Wilson SA, Hölter D (2011) Degradation of cellulose acetate-based materials: a review. J Polym Environ 19:152–165
Komarek RJ, Gardner RM, Buchanan CM, Gedon S (1993) Biodegradation of radiolabeled cellulose acetate and cellulose propionate. J Appl Polym Sci 50:1739–1746
Neves A, Angelin EM, Roldão É, Melo MJ (2019) New insights into the degradation mechanism of cellulose nitrate in cinematographic films by Raman microscopy. J Raman Spectrosc 50:202–212
Sundaram ST, Sharma A, Zhang YZ, Brodman BW (2006) Biodegradation of nitrocellulose. 13:283–298. https://doi.org/10.1080/07370659508019389
Palaninathan V, Raveendran S, Rochani AK, Chauhan N, Sakamoto Y, Ukai T, Maekawa T, Kumar DS (2018) Bioactive bacterial cellulose sulfate electrospun nanofibers for tissue engineering applications. J Tissue Eng Regen Med 12:1634–1645
Wu QX, Guan YX, Yao SJ (2018) Sodium cellulose sulfate: a promising biomaterial used for microcarriers’ designing. Front Chem Sci Eng 13:46–58
Weber CJ, Haugaard V, Festersen R, Bertelsen G (2002) Production and applications of biobased packaging materials for the food industry. Food Addit Contam 19(Suppl):172–177
Borella S, Ménot G, Leuenberger M (2004) Sample homogeneity and cellulose extraction from plant tissue for stable isotope analyses. Handb Stable Isot Anal Tech 507–522
Mohanty AK, Misra M, Drzal LT (eds) (2005) Natural fibers, biopolymers, and biocomposites, 1st edn. CRC Press
Debeaufort F, Voilley A (1995) Methyl cellulose-based edible films and coatings I. Effect of plasticizer content on water and 1-octen-3-ol sorption and transport. Cellulose 2:205–213
Sarkar C, Chowdhuri AR, Garai S, Chakraborty J, Sahu SK (2019) Three-dimensional cellulose-hydroxyapatite nanocomposite enriched with dexamethasone loaded metal–organic framework: a local drug delivery system for bone tissue engineering. Cellulose 26:7253–7269
Sarkar C, Kumari P, Anuvrat K, Sahu SK, Chakraborty J, Garai S (2018) Synthesis and characterization of mechanically strong carboxymethyl cellulose–gelatin–hydroxyapatite nanocomposite for load-bearing orthopedic application. J Mater Sci 53:230–246
Sarkar C, Chowdhuri AR, Kumar A, Laha D, Garai S, Chakraborty J, Sahu SK (2018) One pot synthesis of carbon dots decorated carboxymethyl cellulose-hydroxyapatite nanocomposite for drug delivery, tissue engineering and Fe3+ ion sensing. Carbohydr Polym 181:710–718
Harper CA (2000) Modern plastics handbook. McGraw-Hill Education
Liu Y, Pharr M, Salvatore GA (2017) Lab-on-skin: a review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 11:9614–9635
Cheng Q, Ye D, Yang W, Zhang S, Chen H, Chang C, Zhang L (2018) Construction of transparent cellulose-based nanocomposite papers and potential application in flexible solar cells. ACS Sustain Chem Eng 6:8040–8047
Hajeeth T, Vijayalakshmi K, Gomathi T, Sudha PN (2013) Removal of Cu(II) and Ni(II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int J Biol Macromol 62:59–65
Mautner A, Maples HA, Kobkeatthawin T, Kokol V, Karim Z, Li K, Bismarck A (2016) Phosphorylated nanocellulose papers for copper adsorption from aqueous solutions. Int J Environ Sci Technol 13:1861–1872
Taha AA, Wu Y, Wang H, Li F (2012) Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. J Environ Manag 112:10–16
Boisa N (2013) Adsorption of chlorendic acid onto hydrophilic fumed titanium dioxide (P25). 2:183. http://www.sciencepublishinggroup.com
Suopajärvi T, Liimatainen H, Karjalainen M, Upola H, Niinimäki J (2015) Lead adsorption with sulfonated wheat pulp nanocelluloses. J Water Process Eng C:136–142
Hokkanen S, Repo E, Suopajärvi T, Liimatainen H, Niinimaa J, Sillanpää M (2014) Adsorption of Ni(II), Cu(II) and Cd(II) from aqueous solutions by amino modified nanostructured microfibrillated cellulose. Cellulose 21:1471–1487
Gurgel LVA, Perin de Melo JC, de Lena JC, Gil LF (2009) Adsorption of chromium (VI) ion from aqueous solution by succinylated mercerized cellulose functionalized with quaternary ammonium groups. Bioresour Technol 100:3214–3220
Liu P, Sehaqui H, Tingaut P, Wichser A, Oksman K, Mathew AP (2014) Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 21:449–461
Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC, Tindall D (2001) Advances in cellulose ester performance and application. Prog Polym Sci 9:1605–1688
Mohanty AK, Misra M, Drzal LT (eds) (2005) Natural fibers, biopolymers, and biocomposites. https://doi.org/10.1201/9780203508206
Kim Y, McCoy LT, Feit C, Mubarak SA, Sharma S, Minko S (2021) Carboxymethyl cellulose enhanced production of cellulose nanofibrils. Fibers 9:57
Ahmadzadeh S, Khaneghah AM (2020) Role of green polymers in food packaging. Encycl Renew Sustain Mater 305–319
Fotie G, Limbo S, Piergiovanni L (2020) Manufacturing of food packaging based on nanocellulose: current advances and challenges. Nanomater 10:1726
Bourges X, Weiss P, Daculsi G, Legeay G (2002) Synthesis and general properties of silated-hydroxypropyl methylcellulose in prospect of biomedical use. Adv Colloid Interface Sci 99:215–228
Tang Y, Wang X, Li Y, Lei M, Du Y, Kennedy JF, Knill CJ (2010) Production and characterisation of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr Polym 3:833–841
Williamson SL, Armentrout RS, Porter RS, McCormick CL (1998) Microstructural examination of semi-interpenetrating networks of poly(N, N-dimethylacrylamide) with cellulose or chitin synthesized in lithium chloride/N, N-dimethylacetamide. Macromolecules 31:8134–8141
Kim J, Yun S, Ounaies Z (2006) Discovery of cellulose as a smart material. Macromolecules 39:4202–4206
Mattar H, Baz Z, Saleh A, Shalaby AS, Elsayed Azzazy A, Salah H, Ismail I (2020) Nitrocellulose: structure, synthesis, characterization, and applications. Water, Energy, Food Environ J An Int J 1:1–15
Fatehi P, Fatehi P (2013) Production of biofuels from cellulose of woody biomass. Cellul Biomass Convers. https://doi.org/10.5772/50740
US5414079A—Oxidized cellulose—Google Patents. https://patents.google.com/patent/US5414079A/en. Accessed 6 Nov 2022
Gloor WE, Mahlman BH, Ullrich RD (2002) Hydroxyethylcellulose and Its Uses. Ind Eng Chem 42:2150–2153
De Silva DJ, Olver JM (2005) Hydroxypropyl methylcellulose (HPMC) lubricant facilitates insertion of porous spherical orbital implants. Ophthal Plast Reconstr Surg 21:301–302
Chang C, Zhang L (2011) Cellulose-based hydrogels: present status and application prospects. Carbohydr Polym 84:40–53
Li L, Thangamathesvaran PM, Yue CY, Tam KC, Hu X, Lam YC (2001) Gel network structure of methylcellulose in water. Langmuir 17:8062–8068
Sannino A, Esposito A, De Rosa A, Cozzolino A, Ambrosio L, Nicolais L (2003) Biomedical application of a superabsorbent hydrogel for body water elimination in the treatment of edemas. J Biomed Mater Res A 67:1016–1024
Sannino A, Madaghiele M, Conversano F, Mele G, Maffezzoli A, Netti PA, Ambrosio L, Nicolais L (2004) Cellulose derivative-hyaluronic acid-based microporous hydrogels cross-linked through divinyl sulfone (DVS) to modulate equilibrium sorption capacity and network stability. Biomacromolecules 5:92–96
Sannino A, Maffezzoli A, Nicolais L (2003) Introduction of molecular spacers between the crosslinks of a cellulose-based superabsorbent hydrogel: effects on the equilibrium sorption properties. J Appl Polym Sci 90:168–174
Ibrahim SM, El Salmawi KM, Zahran AH (2007) Synthesis of crosslinked superabsorbent carboxymethyl cellulose/acrylamide hydrogels through electron-beam irradiation. J Appl Polym Sci 104:2003–2008
Zhou J, Chang C, Zhang R, Zhang L (2007) Hydrogels prepared from unsubstituted cellulose in NaOH/urea aqueous solution. Macromol Biosci 7:804–809
Zhou D, Zhang L, Guo S (2005) Mechanisms of lead biosorption on cellulose/chitin beads. Water Res 39:3755–3762
Sannino A, Pappadà S, Giotta L, Valli L, Maffezzoli A (2007) Spin coating cellulose derivatives on quartz crystal microbalance plates to obtain hydrogel-based fast sensors and actuators. J Appl Polym Sci 106:3040–3050
Ayouch I, Kassem I, Kassab Z, Barrak I, Barhoun A, Jacquemin J, Draoui K, Achaby ME (2021) Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf Interfaces 24:101124
Sannino A, Pappadà S, Madaghiele M, Maffezzoli A, Ambrosio L, Nicolais L (2005) Crosslinking of cellulose derivatives and hyaluronic acid with water-soluble carbodiimide. Polymer (Guildf) 46:11206–11212
Noonan C, Quigley S, Curley MAQ (2006) Skin integrity in hospitalized infants and children: a prevalence survey. J Pediatr Nurs 21:445–453
Holaday B, Waugh G, Moukaddem VE, West J, Harshman S (1995) Diaper type and fecal contamination in child day care. J Pediatr Health Care 9:67–74
Gene Harper B, Niles Bashaw R, Leroy Atkins B (1966) Absorbent product containing a hydrocelloidal composition
Sannino A, Mensitieri G, Nicolais L (2004) Water and synthetic urine sorption capacity of cellulose-based hydrogels under a compressive stress field. J Appl Polym Sci 91:3791–3796
Dortdivanlioglu B, Linder C (2019) Diffusion-driven swelling-induced instabilities of hydrogels. J Mech Phys Solids 125:38–52
Lenzi F, Sannino A, Borriello A, Porro F, Capitani D, Mensitieri G (2003) Probing the degree of crosslinking of a cellulose based superabsorbing hydrogel through traditional and NMR techniques. Polymer (Guildf) 44:1577–1588
Sannino A, Esposito A, Nicolais L, Del Nobile MA, Giovane A, Balestrieri C, Esposito R, Agresti M (2000) Cellulose-based hydrogels as body water retainers. J Mater Sci Mater Med 11:247–253
Zhang YHP (2009) A sweet out-of-the-box solution to the hydrogen economy: is the sugar-powered car science fiction? Energy Environ Sci 2:272–282
Cherubini F, Strómman AH (2010) Production of biofuels and biochemicals from lignocellulosic biomass: estimation of maximum theoretical yields and efficiencies using matrix algebra. Energy Fuels 24:2657–2666
Rimadhanti Ningtyas K, Nugraha Agassi T, Gina Putri P (2022) Utilization of waste cellulose raw material for making paper pulp. IOP Conf Ser Earth Environ Sci 1012:012091
Li A, Xu D, Luo L et al (2021) Overview of nanocellulose as additives in paper processing and paper products. Nanotechnol Rev 10:264–281
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sarkar, M., Upadhyay, A., Pandey, D., Sarkar, C., Saha, S. (2023). Cellulose-Based Biodegradable Polymers: Synthesis, Properties, and Their Applications. In: Saha, S., Sarkar, C. (eds) Biodegradable Polymers and Their Emerging Applications. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-99-3307-5_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-3307-5_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-3306-8
Online ISBN: 978-981-99-3307-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)