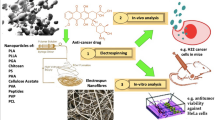
Abstract
Nanofibers fabricated by electrospinning as drug-delivery systems provide high therapeutic efficiency at a lower dose. The literature discussed in this chapter sheds light on the research innovations for developing multifunctional polyurethane nanofibers for the triggered delivery of drugs to treat various cancers. Being a hydrophobic polymer, it is easy to electrospun, and these nanofibers exhibit high tensile strength and elastic properties. Moreover, incorporating anticancer drugs into polyurethane and its derivatives may boost the anticancer agents’ accessibility to tumor sites. This may further reduce cancer cells that the first dose of the medicines does not manage to kill and lessen the adverse side effects. This chapter will highlight general nanotechnological tools that can help to diagnose cancers, e.g., quantum dots, nanoshells, and gold nanoparticles. Particular emphasis is placed on using polyurethane nanofibers for targeted drug delivery and controlled release. Finally, we will learn how polyurethane nanofibers can be incorporated with various compounds to induce hyperthermia effectively for cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Cancer is defined by uncontrolled multiplication and the absence of cell death, resulting in an abnormal mass or tumor (Bertolaso 2016). The main difference between cancer and a tumor is that the latter is localized; these cells grow in a specific location and can be removed surgically. In contrast, cancerous cells spread from one place to another and kill the host. Cancer is caused by a variety of factors, including genetic factors (mutations, translocations, and hereditary) and environmental factors (UV, chemicals, viral infections) (Parsa 2012). All cells have protooncogenes; if these genes are transformed into oncogenes, the normal cell will be changed into a cancer cell (Bishop 1987). Furthermore, mutations in the tumor suppressor gene increase the risk of evolving a tumor or cancer. As a result, the growth-promoting protooncogene and the growth-restricting tumor suppressor genes must be appropriately balanced for cell growth control.
Various treatments are available for curing cancer, such as surgery, radiation, chemotherapy, hormone therapy, immune therapy, etc. (Abbas et al. 2018). All the treatments aim to remove cancerous tissues altogether without harming healthy tissues, which is difficult to achieve. Among existing cancer treatments, chemotherapy is the most employed form of intervention. However, this is associated with detrimental side effects caused by chemotherapeutic agents, such as drug resistance and high cost (Mitra et al. 2015). These treatments may cause kidney failure, nerve injury, nausea, cell toxicity, and death if high doses are administrated (Baudino 2015). To address the limitations of current therapy, researchers are looking for new promising anticancer agents with higher efficacy and fewer side effects.
Nanotechnology has improved drug-delivery routes in recent years by reducing the adverse effects of delivering a minute concentration of drugs (Patra et al. 2018). In general, a nano-drug-delivery system allows for more controlled delivery (release rate and time) and the maintenance of drug concentration at a certain level within the valid therapeutic window. It is regarded that nanotechnology-based targeted drug-delivery systems such as liposomes, hydrogels, nanoparticles (NPs), and nanofibers could provide anticancer drugs with sustained release (Kumar et al. 2012). However, in recent years, there has been a surge in interest in using electrospun nanofibers for drug delivery (Contreras-Cáceres et al. 2019). These nanofibers’ popularity can be attributed to their high porosity, large surface area, tiny size, and interconnecting channels, which offer a valuable strategy for high loading and the release of drugs. These ID nanostructures, e.g., the nanofibers in current times, are mainly produced by the electrospinning technique. Electrospinning offers greater flexibility in selecting biodegradable or nonbiodegradable materials to form nanofibers resulting in remarkable properties such as more advanced control over drug release kinetics (Elsadek et al. 2022). Moreover, the site-specific delivery of drugs using a patch system in a particular area is the main benefit of the fibrous carriers. Additionally, many drugs may be directly contained within the fibers and then released sustainably. Because of their large surface area, these nanofibers can cause a water-insoluble drug to decompose slowly and then release and protect it from corrosion by stomach acid and enzymes, improving drug stability (Castillo-Henríquez et al. 2020).
Several anticancer drugs have been loaded into various electrospun nanofibers for cancer treatment, including paclitaxel (PTX) (Ma et al. 2011; Faraji Dizaji et al. 2020), doxorubicin (DOX) (Abasian et al. 2019), 5-fluorouracil (5-FU) (Grant et al. 2021), and camptothecin (Amna et al. 2020). The nanofibers’ high surface-to-volume ratio is a unique structural feature; however, it causes undesirable initial burst release of drugs. The drug loading may require a different approach depending on the type of drug incorporated, such as small molecule drugs, proteins, and genes, to achieve a successful release profile. Medications can be simply mixed with the polymer solution (Hu et al. 2015), physically or chemically surface immobilized (Yoo et al. 2009), or indirectly loaded onto the nanofiber (Thakkar and Misra 2017). The simplest way to incorporate drugs into nanofibers is to dissolve the drugs and polymer in a single solvent and then co-electrospin them. Nanofibers have high drug-loading efficiency due to a high surface-to-volume ratio, which can further increase when combined with self-assembled NPs or microspheres that are evenly distributed within the nanofiber mats (Forouharshad and Ajalloueian 2022; Fan et al. 2016).
Drug-loaded nanofibers can be fabricated from both natural and synthetic polymers; particularly, biodegradable polymers are preferred. Biodegradable polymers are particularly interested in electrospinning because they eliminate the need for a second surgery to remove the implanted carrier (Tian et al. 2012). Various synthetic polymers such as polylactic acid (PLA) (Abasian et al. 2019; Dai et al. 2017), polycaprolactone (PCL) (Yan et al. 2020), and polyethylene oxide (PEO) (Darbasizadeh et al. 2021; Rengifo et al. 2019) have been extensively studied to fabricate nanofibers with desired properties for drug-delivery applications. However, hydrophobic polymers are accessible to electrospin because of the highly volatile solvents available, and the nanofibers exhibit high tensile strength and elastic properties (Son et al. 2014). One of the most well-liked polymers is biodegradable polyurethane, in which each molecule comprises a hard segment, a soft segment, and a chain extender. It is inexpensive, highly biocompatible, and thus proper for drug administration systems (Bahadur et al. 2017). For instance, incorporating anticancer drugs into polyurethane and its derivatives may boost the anticancer agents’ accessibility to tumor areas, reduce cancer cells that the first dose of the drugs did not manage to kill, and lessen the adverse side effects (Krukiewicz and Zak 2016). However, polyurethane nanofibers for drug-delivery applications have received less focus. Therefore, this chapter aims to examine the uses of electrospun polyurethane nanofibers for cancer drug delivery.
11.2 Nanotechnology in Cancer Diagnosis
Currently, we can diagnose cancer using imaging tools and the morphological examination of tissues or cells. Moreover, imaging procedures like endoscopy, ultrasonography, computed tomography, and magnetic resonance imaging are often employed (Frangioni 2008). However, these traditional approaches to cancer diagnosis have numerous drawbacks because they cannot identify cancer at a very early stage, lack specificity, and have severe side effects. By that time, innumerable tumors may have increased and even metastasized. The current monsterlike situation of cancer has forced researchers to create several methods for accurate diagnosis. As a result, researchers turn to a different technique: a nanotechnology-based diagnostic technique that is being developed as a potentially helpful tool for speedy, practical, and affordable cancer detection (Zhang et al. 2019).
Nanotechnology involves creating and assembling NPs, which are submicroscopic objects with diameters between 1 and 100 nm. A subfield of nanotechnology known as nanomedicine has evolved as a cutting-edge technique for using NPs to detect and treat human disease (Lee et al. 2012). Nanomedicine enables early diagnosis, treatment with few side effects, and noninvasive evaluation of treatment effectiveness. Cancer nanotechnology aims for cellular and molecular elements to interact with nanoscale devices, specifically concerning cancer diagnosis and treatment (Cuenca et al. 2006). The potential of cancer nanotechnology resides in the ability to create therapeutic vehicles with distinct features that, due to their small size, can profoundly and specifically enter tumors. One of the most critical steps in any diagnosing process is screening. Effective screening at the beginning of the disease can help to decrease its adverse effects. Nanotechnology makes it possible to swiftly and precisely detect cancer-related substances, allowing for observing molecular changes even when they are only present in a small percentage of cells. NPs can bind cancer biomarkers, such as proteins, carbohydrates, and nucleic acids, which are expressly released by the cancer cells and thus help diagnose and target drug delivery to the tumor site (Wu and Qu 2015). This would make it possible to diagnose cancer early, which is essential for enhancing cancer treatment. Nanotechnology will also reduce screening tools, meaning many tests can be run on a single device. As a result, cancer screening is quicker and more economical. Quantum dots, nanoshells, and gold nanoparticles (Au NPs) are among the significant nanomaterials involved in cancer diagnosis that are covered here.
11.2.1 Quantum Dots
Quantum dots are highly fluorescent nanometer-size crystals of semiconductor materials that exhibit strong fluorescence emitting different colors concerning their size (Zhang et al. 2008). QDs are resistant to photobleaching, have a high detection intensity, and are thus frequently used for diagnostic applications (Smith et al. 2014). Depending on their size and composition, they have an intrinsic fluorescence emission spectrum with a wavelength ranging from 400 to 2000 nm and are made up of an inorganic elemental core (such as cadmium or mercury) and an outer layer of metal (Nikalaje and Shende 2018). The optical characteristics of NPs are strongly influenced by their structure, particularly the color (wavelength) and diameter of quantum dots. The quantum dots can be injected into a subject and then detected by exciting them to emit light. For instance, cadmium selenide NPs (also known as quantum dots) illuminate when exposed to UV light. They seep into cancerous tumors when injected. The glowing tumor is visible to the surgeon, who can utilize it as a reference point for more precise tumor excision. Nida et al. investigated the use of quantum dots conjugated to anti-epidermal growth factor receptor antibodies to detect precancerous biomarkers in vitro. They found illumination of SiHa cervical cancer cells due to epidermal growth factor receptor overexpression (Nida et al. 2005).
11.2.2 Nanoshells
Nanoshells may be spherical in shape with a diameter of 10–300 nm, have a dielectric core often made of silica, and are encased in a thin metal shell made of gold (Zhao et al. 2014). Nanoshells have the ability to either absorb or scatter light. While scattering nanoshells are utilized as contrast agents, absorbing nanoshells are primarily used to induce hyperthermia (increase in body temperature) (Lal et al. 2008). Hyperthermia, commonly referred to as thermal therapy, is one of the main techniques to eradicate tumors where tumor cells are killed by subjecting them to high temperatures using nanoshells. Hyperthermia involves heating tumor cells to a temperature of 40–45 °C to inhibit tumor cells by initiating a sequence of thermally induced metabolic processes, such as apoptosis (Beik et al. 2016). Compared to normal cells, malignant cells are more sensitive to high temperatures and, therefore, can be killed explicitly by hyperthermia using nanoshells.
11.2.3 Gold (Au) NPs
With their distinctive unusual optical and electrical properties and minimal toxicity, Au NPs are one of the most alluring families used in cancer diagnostics (Fan et al. 2020). This is because Au can be easily synthesized and has been approved for use in the treatment of human disease due to its high biocompatibility. These NPs have been investigated as a delivery system for therapeutic agents, in photodynamic therapy for cancer treatment, and as diagnostic tools to find disease-related biomarkers (Singh et al. 2018). These Au NPs act as contrast agents by scattering visible light in vitro. Au NPs can also be used in conjugation with antibodies for biopsies and identification of cervical and pancreatic cancers (Jain et al. 2007). Smilowitz et al. enhanced radiation and X-ray imaging in mice by using Au NPs (Hainfeld et al. 2004). They discovered that because the tumor’s vascular permeability was higher, Au NPs aggregated there, and a higher contrast was visible. These findings demonstrate the potential of Au NPs to increase tumor diagnostic rates. Thus, Au NPs are expected to play a more critical role in X-ray scattering imaging. A different work by Lu et al. showed multifunctional oval-shaped Au NPs for the targeted detection of breast cancer using a straightforward colorimetric and highly sensitive two-photon scattering assay (Lu et al. 2010). Oval-shaped Au NPs caused a noticeable color change and a 13-fold increase in two-photon scattering intensity compared to the breast cancer SK-BR-3 cell line. The technique successfully identified cancerous and noncancerous cells and set them apart from other breast cancer cell lines that exhibit low levels of human epidermal growth factor receptor 2. These discoveries have significant implications for early detection because the technology can detect malignancies inside the body that are only a few millimeters in diameter. As a result, Au NPs are a priority-based diagnostic tool for many malignancies.
11.3 Electrospinning
A number of advanced techniques have been developed for the generation of 1D nanostructures, including electron beam or focused ion beam (Tseng 2005), hydrothermal synthesis (Hu et al. 2007), chemical vapor deposition (Zang et al. 2009), electrospinning (Shafi et al. 2023), etc. Among these methods, electrospinning is the simplest method to produce nanofibers with unique properties such as small diameter, high surface area, adjustable porosity, and small fiber-to-fiber distance (Jeckson et al. 2021). This technique was demonstrated more than 90 years ago and was first patented in the 1930s (Subbiah et al. 2005). However, it did not receive much attention till the early 1990s. It has a low start-up cost and is compatible with other pre-/posttreatment methods. To date, the electrospinning technique has evolved as the ideal technique used to make nanofibers. Therefore, in this chapter’s coming text, we will elaborate the electrospinning technique that has been used to deliver anticancer drugs. Unlike other methods for forming nanostructures, electrospinning is based on the electric field force acting on the polymer solution (this solution can inherit anticancer drugs). In electrospinning, solidified fiber can be achieved by stretching the electrified jet for the electrostatic repulsions between the surface charges and the eventual evaporation of solvent (Li and Wang 2013). A basic setup of electrospinning is shown in Fig. 11.1. This mainly consists of syringe pump, a spinneret, a high-voltage power supply, and a collector (Long et al. 2019). During the process, the polymer solution is placed into a syringe, and then it is pushed to the tip of the spinneret by external pumping (desired flow rate) to provide a continuous flow of solution. When a droplet is formed at the spinneret, and electrical voltage (typically 1–30 kV) is applied between the tip of the spinneret and the collector placed in front of it, the solvent quickly evaporates from the jets, and solid nanofibers are finely deposited on the collector. Typically, the diameters of the electrospun fibers can be controlled in the range of tens of nanometers to micrometers, and the fibers can be deposited as nonwoven mats or aligned into uniaxial arrays and further stacked into multilayered architectures. Here collector plate should be of good electric conductivity to neutralize the charge carried by the polymer nanofiber. This technique could be applied to synthetic and natural polymers, polymer alloys, and polymer-incorporated functional nanomaterials. These unique advantages of electrospinning impart multifunctional properties to nanofibers for diverse applications. To achieve the desired fiber diameter, bead-free fibers, as well as a porous and uniform fibrous mat, optimization of the electrospinning parameters is necessary (Ibrahim and Klingner 2020). These electrospinning parameters include three main components—solution, process, and ambient—as shown in Fig. 11.2. All of these parameters are extremely important, and they have to be adjusted carefully for successful electrospinning.
11.4 Polyurethane as a Polymer for Electrospinning
Polyurethane is a synthetic polymer formed by the polycondensation of isocyanates and polyols (alcohol that has two or more hydroxyl groups within a molecule), with a third component acting as an extender in some cross-linked products, as shown in Fig. 11.3 (Akindoyo et al. 2016). Dr. Otto Bayer invented and studied polyurethane for the first time in 1937 (Joseph et al. 2018). It is one of the most adaptable macromolecular compounds, with soft and hard segments. The former is created through the isocyanate-polyol reaction, while the latter is created through the isocyanate-chain extender reaction (Das and Mahanwar 2020). Due to the versatility of this polymer, it has practically been used in every field of material engineering (e.g., coatings for aircraft to all types of shoes) (Sikdar et al. 2022). The main benefits of polyurethane are its durability, toughness, and chemical resistance. The urethane group is repeating unit in polyurethane and is formed by the reaction of alcohol and isocyanate; however, this polymer also contains ethers, esters, urea, and some aromatic compounds. The properties of a polyurethane are typically determined by the polyols and isocyanates from which they are derived. Generally, stretchy polymers can be obtained from long segments of polyols with low cross-linking, whereas rigid polymers can be obtained from shorter chains with high cross-linking. Moreover, another group of compounds that often play essential roles are chain extenders and cross-linkers (Bin Ying et al. 2020). These are highly beneficial in improving the morphology of polyurethane adhesives, elastomers, fibers, and a variety of other significant microcellular and skin foams. The hard segments, which are made of isocyanate and chain extenders, are immobile and stiff, whereas the soft segments, which are made of polyols, can move freely and frequently appear in foil forms (Mohamad Sadeghi and Sayaf 2012). Covalent bonding between the hard and soft segments inhibits plastic flow within the polymer chains, resulting in elastomeric resilience (Prisacariu 2011).
11.5 Electrospun Polyurethane Nanofibers for Drug Delivery Against Cancer
Compared to conventional drug-delivery strategies, such as using films and gels, the nanofibers have a large surface area to volume ratio and enough porosity, making it feasible to deliver homogeneous, significant, and regulated dosages of pharmacological preparation at the target site. Nanofibrous drug-delivery systems also provide high therapeutic efficiency at the lower dose, reduced toxicity (since the drug is delivered to the precise action site), accessibility to a broad application area, and a low incidence of side effects (Joshi et al. 2014). Polyurethane has been chosen as the preferred polymer for creating nanofibers due to several benefits, including good strength, chemical resistance, biocompatibility, and a high elastic memory for sustaining tension (Vaithylingam et al. 2017). Pertinently, it can also support patients in the sense of comfort due to its high moisture transmission rate, which is important for breathability and softening at body temperature, both of which are required to retain their qualities if used as a patch system delivery.
To load and regulate the release of doxorubicin, Kilic et al. fabricated polyurethane nanofibers (Kiliç et al. 2018). When the kinetics of the release of 3 and 6 mg of doxorubicin was investigated at pH 4.5 and 7.5, it was found that the drug loaded to drug released was inverse. At pH 4.5, polyurethane nanofibers loaded with doxorubicin exhibited immediate release behavior and more than 70% in the initial 10 min. In contrast, at pH 7.5, doxorubicin-loaded nanofibers exhibited slower release, and 80% of the drug was released in 50 min. Most drugs are said to accumulate on the fiber surface during the electrospinning process, which is why the literature claims that the electrospun fibers typically exhibit an initial surge release (Yu et al. 2014). Drug release at pH 4.5 was seen to be in this situation, whereas pH 7.5 saw a more regulated release. However, the initial burst release of drugs from the nanofiber surfaces is the primary problem with drug-loaded nanofibrous systems. An easy, unique solution to these issues is the combination of drug-loaded NPs inside the nanofibers. The drug nanocarriers then incorporated into electrospun nanofibers can result in prolonged and sustained release.
Shahrousvand et al. used a technique to create the poly (ε-caprolactone) (PCL)-diol based (b)-polyurethane (Shahrousvand et al. 2016). In short, hexamethylene diisocyanate and 12 g PCL-diol were combined in a glass vial reactor and heated to 85 °C for 3 h. The reaction was then continued for another 20 min after 1.08 g of 1,4-butanediol was added to the reactor’s contents. The reactor’s contents were then dried for 24 h at 70 °C in a vacuum oven. A three-time deionized water wash was performed on the synthesized PCL-diol-b-polyurethane. Then, PCL-diol-b-polyurethane/Au composite nanofibers were made by electrospinning PCL-diol-b-polyurethane solutions, which were combined with Au NPs (Irani et al. 2017). The Au NPs were used to increase the anticancer activity of nanofibers against glioblastoma cells because of their small size, low toxicity, and high potential to pass the blood-brain barrier. Successful encapsulation of TMZ is one of the most effective treatments for glioblastoma because of its capacity to pass through the blood-brain barrier. A sustained delivery method of TMZ from nanofibers was observed, allowing for a continuous release for the treatment of glioblastoma (Fig. 11.4). Because TMZ has a short plasma half-life of 1.8 h, polymeric micro/NPs have been used to encapsulate it for controlled release. The cytotoxicity of the produced nanofibers against U-87 human glioblastoma cells showed that, in contrast to free TMZ, which essentially does not affect the percentage of cell multiplication, the Au coating on the nanofibers’ surface improved the cytotoxicity of PCL-diol-b-polyurethane/Au@TMZ nanofibers (Fig. 11.5).
TMZ release from (a) PCL-diol-b-polyurethane/TMZ, (b) PCL-diol-b-polyurethane/Au@TMZ, (c) Au-coated PCL-diol-b-polyurethane/Au@TMZ nanofibers, and fitting data with Korsmeyer-Peppas kinetic model. During the first 24 h, Au-coated PCL-diol-b-polyurethane/Au@TMZ nanofibrous formulations showed lower burst release (24%) in comparison with PCL-diol-b-polyurethane/Au@TMZ (32%) and PCL-diol-b-polyurethane/TMZ (30%). PU polyurethane. (Reproduced from Irani et al. 2017 with copyright permission from Elsevier’s)
In vitro cytotoxicity of TMZ and TMZ-loaded synthesized nanofibers. PCL-diol-b polyurethane/Au@TMZ and gold-coated PCL-diol-b-polyurethane/Au@TMZ nanofibers exhibited more cytotoxicity against U-87 cells than pure TMZ after 3, 5, and 7 days, which could be attributed to the slower release of TMZ from nanofibers. PU polyurethane. (Reproduced from Irani et al. 2017 with copyrights permission of Elsevier’s)
In another study, Seyyedi et al. used acrylated polyurethane/nanohydroxyapatite (n-Hap) nanocomposites to incorporate the anticancer drug PTX (Seyyedi and Molajou 2021). The results showed that the synthetic fibers are excellent candidates for drug loading as the loading efficiency was more than 90% for all manufactured nanofibers. However, the average fiber diameter increased from 290 to 360 nm by loading PTX into the nanofibers. Although the hydrophilic nature of n-Hap NPs could lead to a faster release of drug molecules, the filling of some nanofiber pores with n-HAp NPs prevented PTX molecules from being released from the nanofibers, which neutralized the effect of hydrophilicity on the release rate of PTX and led to a gradually increasing drug release percentage.
For the prolonged release of DOX and folic acid (FA) against breast cancer, UiO-66 metal-organic framework (MOF) NPs loaded with carboxymethyl chitosan (CMC)/poly(ethylene oxide) (PEO)/polyurethane core-shell nanofibers were fabricated by Farboudi et al. (2020a). However, due to their hydrophilicity, CMC/PEO nanofibers could not release anticancer medications over an extended period. The hydrophobic polymer polyurethane was applied to the chitosan nanofibrous layer to increase the hydrophobicity of the nanofibers. FA and DOX had better than 95% drug-loading efficiency for the fabricated MOF-loaded nanofibers. Neither the DOX nor the FA drugs were released in a burst from the FA-DOX/UiO-66 loaded CMC/PEO nanofibers throughout 72 h. The co-delivery of DOX and FA from DOX-FA/UiO-66 nanofiber led to the cell death rise from 41 ± 1% to 54 ± 2%, as shown in Fig. 11.6. The DAPI staining also revealed nuclear fragmentation in the chromatin and apoptosis in MCF-7 cells as indicated by the reduction in the intensity of the dye. As a result, simultaneous administration of DOX and FA into the core-shell fibers may considerably improve the apoptotic nuclei (Fig. 11.7).
Flow cytometry analyses of MCF-7 cells treated with the (a) control group, (b) core-shell nanofibers, (c) DOX/UiO-66 loaded core-shell fibers, and (d) DOX-FA/UiO-66 loaded core-shell fibers after 24 h. PU polyurethane. (Reproduced from Farboudi et al. 2020a with copyright permission of Elsevier)
DAPI stained images of (a) untreated MCF-7 cells, (b) MCF-7 cells treated with core-shell nanofiber, (c) MCF-7 cells treated with DOX/UiO-66 loaded core-shell fibers, and (d) MCF-7 cells treated with DOX-FA/UiO-66 loaded core-shell fibers after 24 h. (Reproduced from Farboudi et al. 2020a with copyright permission of Elsevier’s)
Moreover, the antitumor effectiveness of anticancer drug-delivery systems can be increased by including mixtures of cytotoxic drugs within the drug carriers. For instance, Farboudi et al. synthesized magnetic Au-coated PCL-b-polyurethane/poly(N-isopropylacrylamide)-grafted-chitosan core-shell nanofibers for controlled release of PTX and 5-FU toward 4T1 breast cancer cells (Farboudi et al. 2020b). Coating core-shell nanofibers with magnetic Au NPs caused a progressive rise in surface roughness. Therefore, the drug-loaded nanofibers coated with magnetic Au NPs were a good substrate for 4T1 cancer cell proliferation and treatment of breast cancer cells (Fig. 11.8). Tumor growth was continuously inhibited by the prolonged release of anticancer drugs from drug-loaded nanofibers and magnetic gold-coated nanofibers, respectively. After 20 days, the presence of drug-loaded nanofibers coated with magnetic Au NPs resulted in the least amount of tumor growth, as shown in Fig. 11.9. The outcomes demonstrated that the simultaneous insertion of PTX and 5-FU into the core-shell fibers and coating of nanofibers with magnetic Au NPs were beneficial for boosting the anticancer activity of produced nanofibrous carrier toward 4T1 breast cancer cells (Fig. 11.10). During the G2-M phases, PTX encourages microtubule assembly and hinders the formation of mitotic spindles. These activities reduce the growth of tumor cells. 5-FU, on the other hand, can stop the growth of tumor cells by interfering with nucleic acid metabolism during the G1-S phase of the cell cycle. In order to prevent the dispersion of drug-loaded nanofibrous carriers onto normal cells, the external magnetic field can control the residence time of drug-loaded nanofibers coated with magnetic Au NPs on the surface of cancer cells. This could result in the presence of a high concentration of drugs in the target area. Therefore, in the presence of a magnetic Au NPs coated-core-shell nanofibrous carrier, the external magnetic field causes the apoptosis of breast cancer cells. Thus, the localized delivery of anticancer drugs through the nanofibrous drug-delivery systems has more significant benefits in treating breast cancer.
AFM analysis of (a) pure nanofibers and (b) Au NP-coated nanofibers. The surface roughness of the core-shell nanofibers was less rough, as shown in figure; however, as the nanofibers were coated with magnetic Au NPs, the surface roughness increased. (Reproduced from Farboudi et al. 2020b with copyrights permission of Elsevier)
The figure showed that in the presence of an external magnetic field, the PTX and 5-FU loaded nanofibers coated with magnetic Au NPs resulted in the most nominal tumor growth after 20 days, whereas the pure nanofibers did not have obvious cytotoxicity toward 4T1 breast cancer cells and the tumor volume increased rapidly. (Reproduced from Farboudi et al. 2020b with copyright permission of the Elsevier’s)
Cell viability testing revealed that incorporating PTX and 5-FU into the core-shell fibers and coating nanofibers with magnetic Au NPs resulted in the most remarkable cytotoxicity toward 4T1 breast cancer cells. (Reproduced from Farboudi et al. 2020b with copyright permission of the Elsevier’s)
11.6 Thermo-Responsive Nanofibers
The cancer cells are sensitive to body temperatures above normal (hyperthermia), making them susceptible to cell death. Given this, one of the main techniques for treating a cancer cell is hyperthermia. In order to provide simultaneous release of PTX and 5-FU, Erik et al. created poly-(N-isopropylacrylamide-co-N-(hydroxymethyl)acrylamide) (p-NP-HM)/polyurethane core-shell nanofibers (Aguilar et al. 2017). Figure 11.11 demonstrates that the drug release profile of the composites has a temperature-sensitive response. The drug release was observed to be zero at 25 °C (room temperature) and 36.5 °C, corresponding to normal body temperature. However, the release of 28% and 29.5% PTX and 2.3% and 23.7% 5-FU drugs occurred when the medium temperature reached 40 and 50 °C, respectively. This suggests that drug release increases as the temperature exceeds normal body temperature.
In vitro drug release profiles of both PTX and 5-FU from the core-shell nanofiber composite mat at different temperatures, time points, and cycles. Each temperature point was maintained for 10 min. PU polyurethane. (Reproduced from Aguilar et al. 2017 with copyright permission of Elsevier’s)
The primary mechanism for releasing the drugs was the component of p-NP-HM switching from a hydrophilic to a hydrophobic phase. The in vitro drug release investigation for PTX and 5-FU showed good controlled release profiles when the alternating magnetic field was applied. Cell staining experiments have been used to visually explore the live/dead ratio utilizing (Resazurin red, live cells, and Sytox green, dead/injured cells) for ESO26 (adenocarcinoma). The morphological studies for OE21 (squamous cell carcinoma) cancer cell lines using DAPI and rhodamine dyes are shown in Fig. 11.12. In the case of ESO26 cells, all treatment groups have a visibly larger distribution of dead cells than the control group. By using DAPI and rhodamine labeling, the morphological investigation of OE21 revealed nucleus blebbing, restricted cell spreading, and stunted actin expansion. This suggests cytotoxicity across all treatment groups. Favorable results can be achieved especially with HHRx-hyperthermia (45 °C) with dual drug release and LHRx-hyperthermia (40 °C) with single drug release treatment groups. Thus, it was shown that the drug-loaded nanofibers were highly effective against the cancer cell lines ESO26 and OE21, and they might be used as a promising material for cancer therapy by combining the effects of hyperthermia and chemotherapy.
Confocal images of (a) ESO26 cell line live/dead staining and (b) OE21 cell line DAPI and rhodamine stains. Treatment group abbreviations: HHRx = hyperthermia (45 °C) with dual drug release, LHRx = hyperthermia (40 °C) with single drug release, H = hyperthermia (45 °C), HfreeRx = hyperthermia (45 °C) with free combination drugs, freeRx = free combination drugs. (Reproduced from Aguilar et al. 2017 with copyrights permission of Elsevier’s)
In a different study, superparamagnetic iron NPs (γ-Fe2O3) were coupled with polyurethane nanofibers to treat cancer by heating the body to a high temperature (Song et al. 2018). The composite fibrous membrane created by in situ electrospinning demonstrated effective heating ability and well-maintained cycle heating performance in the presence of an alternating magnetic field when using the auxiliary electrode. The magnetic composite fibrous membrane created in situ by the auxiliary electrode is a remarkable option for magnetic hyperthermia in cancer treatment. This encouraging result came about because of the inclusion of a conical auxiliary electrode made of aluminum. Superparamagnetic iron oxide NPs were enclosed in polyurethane nanofiber by Amarjargal et al. (2013). The amount of magnetic Fe3O4 NPs in/on the membranes increased along with a steady increase in the heating rate. This electrospun nanofiber may be a possible candidate for a novel heat-generating substrate for localized hyperthermia cancer therapy.
11.7 Conclusion
Nanotechnology enables early cancer diagnosis and prevention in the fight against the disease’s pain, suffering, and mortality. We may be able to prevent cancer even before it begins with nanomedicine. With such technology, nanomedicine has the potential to increase the life span of human beings. The literature included in this chapter emphasizes the importance of electrospun polyurethane nanofibers as a drug-delivery mechanism for cancer treatment. Different delivery carriers can be created by mixing and matching various polymers. The electrospun polyurethane nanofibers have a variety of benefits as drug-delivery vehicles. These polyurethane drug products can provide patients with a sensation of comfort because of their high moisture vapor transmission rate, which is crucial for breathability and softening at body temperature, both of which are necessary for maintaining their properties. Additionally, polyurethane has drawn attention among the numerous polymeric architectures for electrospinning because of its higher strength, chemical resistance, and biocompatibility. Drugs can be incorporated into the electrospun nanofibers by simply mixing with the polymer solution, physically or chemically (surface immobilized), or indirectly loaded onto the nanofiber. Although nanofibers possess high drug incorporation efficiency, it further increases when combined with self-assembled NPs or microspheres that are evenly distributed within the nanofibers. Thus, polyurethane based drug-delivery devices in nanofibers are promising systems that can provide vast benefits and drug therapy for cancer.
References
Abasian P et al (2019) Incorporation of magnetic NaX zeolite/DOX into the PLA/chitosan nanofibers for sustained release of doxorubicin against carcinoma cells death in vitro. Int J Biol Macromol 121:398–406. https://doi.org/10.1016/J.IJBIOMAC.2018.09.215
Abbas Z, Rehman S, Abbas Z, Rehman S (2018) An overview of cancer treatment modalities. In: Neoplasm. InTech. https://doi.org/10.5772/INTECHOPEN.76558
Aguilar LE, GhavamiNejad A, Park CH, Kim CS (2017) On-demand drug release and hyperthermia therapy applications of thermoresponsive poly-(NIPAAm-co-HMAAm)/polyurethane core-shell nanofiber mat on non-vascular nitinol stents. Nanomed Nanotechnol Biol Med 13(2):527–538. https://doi.org/10.1016/J.NANO.2016.12.012
Akindoyo JO, Beg MDH, Ghazali S, Islam MR, Jeyaratnam N, Yuvaraj AR (2016) Polyurethane types, synthesis and applications—a review. RSC Adv 6(115):114453–114482. https://doi.org/10.1039/C6RA14525F
Amarjargal A, Tijing LD, Park CH, Im IT, Kim CS (2013) Controlled assembly of superparamagnetic iron oxide nanoparticles on electrospun PU nanofibrous membrane: a novel heat-generating substrate for magnetic hyperthermia application. Eur Polym J 49(12):3796–3805. https://doi.org/10.1016/J.EURPOLYMJ.2013.08.026
Amna T, Hassan MS, Sheikh FA (2020) Nanocamptothecins as new generation pharmaceuticals for the treatment of diverse cancers: overview on a natural product to nanomedicine. Appl Nanotechnol Biomed Sci:39–49. https://doi.org/10.1007/978-981-15-5622-7_3
Bahadur A et al (2017) Biocompatible waterborne polyurethane-urea elastomer as intelligent anticancer drug release matrix: a sustained drug release study. React Funct Polym 119:57–63. https://doi.org/10.1016/J.REACTFUNCTPOLYM.2017.08.001
Baudino TA (2015) Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol 12:3–20
Beik J et al (2016) Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release 235:205–221. https://doi.org/10.1016/J.JCONREL.2016.05.062
Bertolaso M (2016) Cancer biology. Hist Philos Theory Life Sci 18:1–16. https://doi.org/10.1007/978-94-024-0865-2_1/COVER
Bin Ying W et al (2020) Waterproof, highly tough, and fast self-healing polyurethane for durable electronic skin. ACS Appl Mater Interfaces 12(9):11072–11083. https://doi.org/10.1021/ACSAMI.0C00443
Bishop JM (1987) The molecular genetics of cancer. Science 235(4786):305–311. https://doi.org/10.1126/SCIENCE.3541204
Castillo-Henríquez L, Vargas-Zúñiga R, Pacheco-Molina J, Vega-Baudrit J (2020) Electrospun nanofibers: a nanotechnological approach for drug delivery and dissolution optimization in poorly water-soluble drugs. ADMET DMPK 8:325–353. https://doi.org/10.5599/admet.844
Contreras-Cáceres R et al (2019) Electrospun nanofibers: recent applications in drug delivery and cancer therapy. Nanomaterials (Basel) 9:656. https://doi.org/10.3390/nano9040656
Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR (2006) Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 107(3):459–466. https://doi.org/10.1002/CNCR.22035
Dai J, Jin J, Yang S, Li G (2017) Doxorubicin-loaded PLA/pearl electrospun nanofibrous scaffold for drug delivery and tumor cell treatment. Mater Res Express 4(7):075403. https://doi.org/10.1088/2053-1591/AA7479
Darbasizadeh B et al (2021) Electrospun doxorubicin-loaded PEO/PCL core/sheath nanofibers for chemopreventive action against breast cancer cells. J Drug Deliv Sci Technol 64:102576. https://doi.org/10.1016/J.JDDST.2021.102576
Das A, Mahanwar P (2020) A brief discussion on advances in polyurethane applications. Adv Ind Eng Polym Res 3(3):93–101. https://doi.org/10.1016/J.AIEPR.2020.07.002
Elsadek NE et al (2022) Electrospun nanofibers revisited: an update on the emerging applications in nanomedicine. Materials (Basel) 15(5):1934. https://doi.org/10.3390/MA15051934
Fan R et al (2016) Dual drug loaded biodegradable nanofibrous microsphere for improving anti-colon cancer activity. Sci Rep 6(1):1–13. https://doi.org/10.1038/srep28373
Fan M et al (2020) Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 10(11):4944. https://doi.org/10.7150/THNO.42471
Faraji Dizaji B et al (2020) Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of paclitaxel toward prostate cancer cells death. Int J Biol Macromol 164:1461–1474. https://doi.org/10.1016/J.IJBIOMAC.2020.07.228
Farboudi A et al (2020a) UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int J Biol Macromol 150:178–188. https://doi.org/10.1016/J.IJBIOMAC.2020.02.067
Farboudi A et al (2020b) Synthesis of magnetic gold coated poly (ε-caprolactonediol) based polyurethane/poly(N-isopropylacrylamide)-grafted-chitosan core-shell nanofibers for controlled release of paclitaxel and 5-FU. Int J Biol Macromol 150:1130–1140. https://doi.org/10.1016/J.IJBIOMAC.2019.10.120
Forouharshad M, Ajalloueian F (2022) Tunable self-assembled stereocomplexed-polylactic acid nanoparticles as a drug carrier. Polym Adv Technol 33(1):246–253. https://doi.org/10.1002/PAT.5510
Frangioni JV (2008) New technologies for human cancer imaging. J Clin Oncol 26(24):4012. https://doi.org/10.1200/JCO.2007.14.3065
Grant JJ et al (2021) Electrospun fibres of chitosan/PVP for the effective chemotherapeutic drug delivery of 5-fluorouracil. Chemosensors 9(4):70. https://doi.org/10.3390/CHEMOSENSORS9040070
Hainfeld JF, Slatkin DN, Smilowitz HM (2004) The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 49(18):N309. https://doi.org/10.1088/0031-9155/49/18/N03
Hu H, Huang X, Deng C, Chen X, Qian Y (2007) Hydrothermal synthesis of ZnO nanowires and nanobelts on a large scale. Mater Chem Phys 106(1):58–62. https://doi.org/10.1016/J.MATCHEMPHYS.2007.05.016
Hu J, Prabhakaran MP, Tian L, Ding X, Ramakrishna S (2015) Drug-loaded emulsion electrospun nanofibers: characterization, drug release and in vitro biocompatibility. RSC Adv 5(121):100256–100267. https://doi.org/10.1039/C5RA18535A
Ibrahim HM, Klingner A (2020) A review on electrospun polymeric nanofibers: production parameters and potential applications. Polym Test 90:106647. https://doi.org/10.1016/J.POLYMERTESTING.2020.106647
Irani M, Sadeghi GMM, Haririan I (2017) The sustained delivery of temozolomide from electrospun PCL-diol-b-PU/gold nanocompsite nanofibers to treat glioblastoma tumors. Mater Sci Eng C 75:165–174. https://doi.org/10.1016/J.MSEC.2017.02.029
Jain PK, ElSayed IH, El-Sayed MA (2007) Au nanoparticles target cancer. Nano Today 2(1):18–29. https://doi.org/10.1016/S1748-0132(07)70016-6
Jeckson TA, Neo YP, Sisinthy SP, Gorain B (2021) Delivery of therapeutics from layer-by-layer electrospun nanofiber matrix for wound healing: an update. J Pharm Sci 110(2):635–653. https://doi.org/10.1016/J.XPHS.2020.10.003
Joseph J, Patel RM, Wenham A, Smith JR (2018) Biomedical applications of polyurethane materials and coatings. Int J Surf Eng Coat 96(3):121–129. https://doi.org/10.1080/00202967.2018.1450209
Joshi M, Butola BS, Saha K (2014) Advances in topical drug delivery system: micro to nanofibrous structures. J Nanosci Nanotechnol 14(1):853–867. https://doi.org/10.1166/JNN.2014.9083
Kiliç E, Yakar A, Pekel Bayramgil N (2018) Preparation of electrospun polyurethane nanofiber mats for the release of doxorubicine. J Mater Sci Mater Med 29(1):1–9. https://doi.org/10.1007/S10856-017-6013-5/FIGURES/7
Krukiewicz K, Zak JK (2016) Biomaterial-based regional chemotherapy: local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater Sci Eng C 62:927–942. https://doi.org/10.1016/J.MSEC.2016.01.063
Kumar S, Kushwaha S, Awani R (2012) Novel drug delivery system for anticancer drug: a review. Int J PharmTech Res 4:542–553. Available: https://www.researchgate.net/publication/287478414. Accessed 10 Oct 2022
Lal S, Clare SE, Halas NJ (2008) Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res 41(12):1842–1851. https://doi.org/10.1021/AR800150G
Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC (2012) Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev 41(7):2656–2672. https://doi.org/10.1039/C2CS15261D
Li Z, Wang C (2013) Introduction of electrospinning. In: Springer briefs in materials. Springer, pp 1–13. https://doi.org/10.1007/978-3-642-36427-3_1/COVER
Long YZ, Yan X, Wang XX, Zhang J, Yu M (2019) Electrospinning: the setup and procedure. In: Electrospinning: nanofabrication and applications, pp 21–52. https://doi.org/10.1016/B978-0-323-51270-1.00002-9
Lu W et al (2010) Multifunctional oval-shaped gold-nanoparticle-based selective detection of breast cancer cells using simple colorimetric and highly sensitive two-photon scattering assay. ACS Nano 4(3):1739–1749. https://doi.org/10.1021/NN901742Q
Ma G, Liu Y, Peng C, Fang D, He B, Nie J (2011) Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohydr Polym 86(2):505–512. https://doi.org/10.1016/J.CARBPOL.2011.04.082
Mitra AK et al (2015) Novel delivery approaches for cancer therapeutics. J Control Release 219:248. https://doi.org/10.1016/J.JCONREL.2015.09.067
Mohamad Sadeghi GM, Sayaf M (2012) From PET waste to novel polyurethanes. In: Material recycling—trends and perspectives. InTech. https://doi.org/10.5772/31642
Nida DL, Rahman MS, Carlson KD, Richards-Kortum R, Follen M (2005) Fluorescent nanocrystals for use in early cervical cancer detection. Gynecol Oncol 99(3):S89–S94. https://doi.org/10.1016/J.YGYNO.2005.07.050
Nikalaje Y, Shende V (2018) Recent development applications of nanotechnology in cancer: a literature review of imaging and treatment. World J Pharm Pharm Sci. https://doi.org/10.20959/wjpps20182-10868
Parsa N (2012) Environmental factors inducing human cancers. Iran J Public Health 41(11):1. Available: /pmc/articles/PMC3521879/. Accessed 10 Oct 2022
Patra JK et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):1–33. https://doi.org/10.1186/S12951-018-0392-8
Prisacariu C (2011) Polyurethane elastomers. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0514-6
Rengifo AFC et al (2019) PEO-chitosan nanofibers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline for skin cancer treatment. Eur Polym J 119:335–343. https://doi.org/10.1016/J.EURPOLYMJ.2019.08.001
Seyyedi M, Molajou A (2021) Nanohydroxyapatite loaded-acrylated polyurethane nanofibrous scaffolds for controlled release of paclitaxel anticancer drug. J Res Sci Eng Technol 9(1):50–61. https://doi.org/10.24200/JRSET.VOL9ISS01PP50-61
Shafi H et al (2023) Super disintegrating oromucosal nanofiber patch of zolmitriptan for rapid delivery and efficient brain targeting. Chem Eng J 463:142481. https://doi.org/10.1016/j.cej.2023.142481
Shahrousvand M, Sadeghi GMM, Salimi A (2016) Artificial extracellular matrix for biomedical applications: biocompatible and biodegradable poly (tetramethylene ether) glycol/poly (ε-caprolactone diol)-based polyurethanes. J Biomed Sci Polym Ed 27(17):1712–1728. https://doi.org/10.1080/09205063.2016.1231436
Sikdar P, Dip TM, Dhar AK, Bhattacharjee M, Hoque MS, Bin Ali S (2022) Polyurethane (PU) based multifunctional materials: emerging paradigm for functional textiles, smart, and biomedical applications. J Appl Polym Sci 139(38):e52832. https://doi.org/10.1002/APP.52832
Singh P, Pandit S, Mokkapati VRSS, Garg A, Ravikumar V, Mijakovic I (2018) Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci 19(7):1979. https://doi.org/10.3390/IJMS19071979
Smith AM, Dave S, Nie S, True L, Gao X (2014) Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev Mol Diagn 6(2):231–244. https://doi.org/10.1586/14737159.6.2.231
Son YJ, Kim WJ, Yoo HS (2014) Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch Pharm Res 37(1):69–78. https://doi.org/10.1007/S12272-013-0284-2/FIGURES/3
Song C et al (2018) Electric field-assisted in situ precise deposition of electrospun γ-Fe2O3/polyurethane nanofibers for magnetic hyperthermia. Nanoscale Res Lett 13(1):1–11. https://doi.org/10.1186/S11671-018-2707-Y/FIGURES/9
Subbiah T, Bhat GS, Tock RW, Parameswaran S, Ramkumar SS (2005) Electrospinning of nanofibers. J Appl Polym Sci 96(2):557–569. https://doi.org/10.1002/APP.21481
Thakkar S, Misra M (2017) Electrospun polymeric nanofibers: new horizons in drug delivery. Eur J Pharm Sci 107:148–167. https://doi.org/10.1016/J.EJPS.2017.07.001
Tian H, Tang Z, Zhuang X, Chen X, Jing X (2012) Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci 37(2):237–280. https://doi.org/10.1016/J.PROGPOLYMSCI.2011.06.004
Tseng AA (2005) Recent developments in nanofabrication using focused ion beams. Small 1(10):924–939. https://doi.org/10.1002/SMLL.200500113
Vaithylingam R, Ansari MNM, Shanks RA (2017) Recent advances in polyurethane-based nanocomposites: a review. Polym Plast Technol Eng 56(14):1528–1541. https://doi.org/10.1080/03602559.2017.1280683
Wu L, Qu X (2015) Cancer biomarker detection: recent achievements and challenges. Chem Soc Rev 44(10):2963–2997. https://doi.org/10.1039/C4CS00370E
Yan E et al (2020) pH-sensitive core-shell electrospun nanofibers based on polyvinyl alcohol/polycaprolactone as a potential drug delivery system for the chemotherapy against cervical cancer. J Drug Deliv Sci Technol 55:101455. https://doi.org/10.1016/J.JDDST.2019.101455
Yoo HS, Kim TG, Park TG (2009) Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev 61(12):1033–1042. https://doi.org/10.1016/J.ADDR.2009.07.007
Yu H, Jia Y, Yao C, Lu Y (2014) PCL/PEG core/sheath fibers with controlled drug release rate fabricated on the basis of a novel combined technique. Int J Pharm 469(1):17–22. https://doi.org/10.1016/J.IJPHARM.2014.04.045
Zang CH et al (2009) Optical properties of a ZnO/P nanostructure fabricated by a chemical vapor deposition method. J Phys Chem C 113(43):18527–18530. https://doi.org/10.1021/JP905648M
Zhang H, Yee D, Wang C (2008) Quantum dots for cancer diagnosis and therapy: biological and clinical perspectives. Nanomedicine (London) 3(1):83–91. https://doi.org/10.2217/17435889.3.1.83
Zhang Y, Li M, Gao X, Chen Y, Liu T (2019) Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol 12(1):1–13. https://doi.org/10.1186/S13045-019-0833-3/FIGURES/2
Zhao J, Wallace M, Melancon MP (2014) Cancer theranostics with gold nanoshells. Nanomedicine 9(13):2041–2057. https://doi.org/10.2217/NNM.14.136
Acknowledgments
This work is supported by the research grants received by Dr. Faheem A. Sheikh from the Science and Engineering Research Board (SERB), Grant/Award Number: CRG/220/000113, and a Council of Scientific and Industrial Research (CSIR) sponsored project (22(0846)/20/EMR-II). Anjum Hamid is grateful for the fellowship she received from the Maulana Azad National Fellowship (MANF).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rather, A.H. et al. (2023). Polyurethane Nanofibers Fabricated by Electrospinning as Drug Carrier Systems for the Treatment of Cancer. In: Sheikh, F.A., Majeed, S., Beigh, M.A. (eds) Interaction of Nanomaterials With Living Cells. Springer, Singapore. https://doi.org/10.1007/978-981-99-2119-5_11
Download citation
DOI: https://doi.org/10.1007/978-981-99-2119-5_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2118-8
Online ISBN: 978-981-99-2119-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)