Abstract
Climate change fluctuations, specifically CO2 concentration, temperature, rainfall patterns, droughts, and soil salinity, are increasing due to anthropogenic activities. These variations are identified as major constraints to plant survival and therefore limit plant growth and productivity. Photosynthesis inhibition, excessive ROS (reactive oxygen species) production, biomass reduction, increased pathogen infestation, and ultimately lower yields are the major limiting attributes that have attracted a lot of attention from researchers worldwide. Since climate change predictions indicate that ecological damage will be more frequent and severe in the upcoming futuristic scenarios, the question of fulfilling the food requirement of the ever-growing population becomes imperative. Plants are sensitive to the effects of climate change. Alterations in photosynthesis and carbon assimilation mechanisms are attributed to reduced productivity. To cope with these stresses, secondary metabolite production elicits defensive responses in plants. These natural by-products are synthesized from primary metabolites and protect against various abiotic and biotic stresses. Synthesis and accumulation of secondary metabolites differ among plant species growing in different environmental conditions. Phenolics, flavonoids, alkaloids, terpenoids, tannins, glucosinolates, and so on are a useful array of natural products that increase plant resistance against various stresses. Although these are synthesized in minimal concentrations, they display a crucial role in the scavenging of ROS molecules.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Anthropogenic activities including the burning of fossil fuels, urbanization, and a rise in the concentration of greenhouse gases (GHGs) are the major factors responsible for global climate change (Dutta et al. 2020). Elevated concentration of GHGs in the atmosphere since the industrial revolution has increased the concentration of CO2 from 280 ppm to >410 ppm and is expected to rise further to 730–1000 ppm by 2100 (IPCC 2014). Recently, IPCC (2021) revealed that this enhancement in atmospheric GHGs further raises the global temperature approximately by 0.84–1.10 °C, and consequently disturbing the rainfall patterns and prevailing drought conditions in arid regions of the world (IPCC 2014). Such variability in climate is influencing crop production with each successive year and somewhere evokes an uncertainty in terms of food production (Reddy and Hodges 2000). It is predicted that agricultural outputs will be declined (10–20%) by the end of 2080 in developing countries (Thompson and Cohen 2012). Consequently, the subject of achieving food security worldwide becomes a daunting task with an ever-growing population (Barnett 2011; Funk and Brown 2009; Rice and Garcia 2011). In the current scenario, about 1 billion people are food-deprived, 150 million children are chronically undernourished, 50 million children are acutely malnourished with a higher mortality rate, and another 38 million children are overweight (Misselhorn et al. 2012; Fanzo 2018). The situation in India is similar to the global scenario: whether the increasing population and demand for food supply will continue to rise with climate change. Temperature increases of 1–2 °C have a negative influence on the productivity of major cereal crops, which in turn affects the nutritional status of the population (Easterling et al. 2007; Rao et al. 2016).
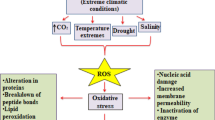
Climate change unavoidably disturbs plants by hampering the physiological and biochemical processes such as altered photosynthesis, plant–water interactions, and CO2 assimilations, which severely affects their growth and yield (Fig. 6.1) (Anjum et al. 2011). These variations induced oxidative stress in plants via increased generation of reactive oxygen species (ROS), leading to lipid peroxidation, DNA damage, and inactivation of important enzymes (Akula and Ravishankar 2011). In addition to this, overproduction of ROS also inhibits CO2 fixation in chloroplasts, as they are the primary source of ROS generation (Asada 2006). In response to such constraints, plants have acquired alternative strategies such as increased antioxidative response, phytohormones, osmotic adjustment, and enhanced production of secondary metabolites (Yadav et al. 2021; Jogawat et al. 2021; Zandalinas et al. 2022). Secondary metabolites play a vital role in plant defense against herbivory, insect attack, and environmental stress (Chomel et al. 2016). Several biotic and abiotic stresses act as an elicitor for the stimulation of secondary metabolites (Radman et al. 2003; Ghorbanpour et al. 2014). Their synthesis and accumulation differ among plant species grown under different environmental conditions (Radušienė et al. 2012). Shikimate pathway, acetate–malonate pathway, and side reactions involving glycolysis and TCA cycle are different metabolic routes through which biosynthesis of secondary metabolites takes place in plants (Geilfus 2019; Nabavi et al. 2020). Phenolics, flavonoids, and terpenes synthesized in very low concentrations facilitate antioxidative defense mechanisms, thus increasing their acclimatization to oxidative stress in plants (Edreva et al. 2008). Phytohormones, particularly ABA and jasmonic acid (JA), are positively correlated with the production of secondary metabolites, as they work in a synergistic manner. For example, ABA and JA were responsible for the increase in phenolics and flavonoid contents in Castanea sativa (Camisón et al. 2019). This could protect the plants against increased oxidative stress through the activation of NAC transcription factors (Choudhary et al. 2021).
6.2 Elevated CO2 and Temperature Stress
Increased anthropogenic activities have accelerated the level of CO2 concentrations in the atmosphere. At the time of pre-industrialization, the CO2 levels were 280 ppm initially, but with increasing trends, it has been reported to be nearly 410 ppm (September 2019) (IPCC 2019). In view of this, a two-fold increase in CO2 concentration has been expected (IPCC 2013). These elevated levels of CO2 not only causes global warming but also reduces ecosystem productivity. According to NOAA (2020), the surface temperature of land and oceans is 0.98 °C warmer than the twentieth-century average (13.9 °C). Kimball (2016) found that increasing CO2 concentration by 200 ppm will increase canopy temperature (ET) by 0.7 °C. Increased ET becomes a problem, particularly for developing countries, as it has reduced crop productivity and grain yield (Chaturvedi et al. 2017; Wang et al. 2017). CO2 levels play a significant role in plant metabolic processes. Short-term exposure to elevated CO2 (~400 ppm) reported enhanced photosynthesis, biomass, and decreased oxidative stress. Various plants, such as Solanum lycopersicum L., Stevia rebaudiana L., and Parthenium hysterophorus L., have demonstrated the beneficial impact of elevated CO2 (Hussin et al. 2017; Bajwa et al. 2019; Pan et al. 2020). However, prolonged exposure to higher CO2 levels (~800 ppm), promoted negative effects on plant growth, i.e., reduced photosynthesis, and altered biomass that ultimately affected crop yield and its quality (Wang et al. 2013). For instance, decreased photosynthesis and fruit yield has been observed under high CO2 concentrations in strawberry (Balasooriya et al. 2018). This significant reduction is due to the low availability of RuBisCO content and nitrogen concentration (Gamage et al. 2018; Rosa et al. 2019). Many crops, including Lactuca sativa and Spinacia oleracea, had lower nutritional quality (Mg, N, Fe, Zn, and S) under increased CO2 concentrations (Giri et al. 2016; Dong et al. 2018).
Variable environmental factors influence secondary metabolite biosynthesis in plants. Phenolics such as flavonoids, condensed tannins, and alkaloids in response to elevated CO2 concentration have significantly modulated secondary metabolism in plants (Levine et al. 2008; Jia et al. 2014). CO2 enrichment in the atmosphere increases the susceptibility of plants to insect attack by boosting photosynthesis and higher production of carbohydrates (Ainsworth and Rogers 2007; Bernacchi et al. 2007). To avoid insect damage, plants allocate the primary metabolites to secondary metabolites grown under high CO2 levels. In woody plants, phenolic compounds and terpenoids provide defense against herbivory at higher CO2 concentrations (Feeny 1976; Rhoades and Cates 1976). Robinson et al. (2012) reported increased total phenolics (19%), flavonoids (27%), and tannins (22%) in plants grown under elevated CO2. On the other hand, flavonoids such as quercetin, fisetin, and kaempferol were enhanced in the leaves and rhizomes of ginger, hence exhibiting higher antioxidative defense responses (Ghasemzadeh et al. 2010). Similarly, soybean plants mediate anti-herbivory by increasing the ratios of quercetin and kaempferol while decreasing the genistein concentration (Piubelli et al. 2005). Higher phenylalanine ammonia-lyase (PAL) enzyme activity is linked with the upregulation of secondary metabolites in elevated CO2. For example, a significant increase in phenolics and flavonoid concentration was observed in Eleais guneensis L. due to increased PAL enzyme activity (Ibrahim and Jaafar 2012). Similarly, Triticum aestivum L. exhibited higher PAL activity along with an accumulation of phenolic compounds (Mishra et al. 2013). Generally, warming conditions are associated with phenolic contents in leaves and increased terpenoid concentrations in foliage (Peñuelas and Staudt 2010; Zvereva and Kozlov 2006). However, under elevated CO2, phenolic concentrations were increased in the foliage while decreased in woody tissues (Zvereva and Kozlov 2006). On the other hand, terpenoid concentration was significantly lowered as CO2 concentration increased in conifers. Similarly, the emission of phenolics and flavonoid content was significantly intensified by elevated temperature in Zingiber officinale L. (Ghasemzadeh et al. 2011). Several plant species, such as Thymus hyemalis L., Thymus vulgaris L., Valeriana jatamansi L., and Camellia sinensis L., have been reported with an increased concentration of secondary metabolites in plants (Biel et al. 2005; Vurro et al. 2009; Li et al. 2017; Kaundal et al. 2018). Sobuj et al. (2018) observed the differential response of flavonoid concentration in male and female plants. Under elevated CO2 conditions, female plants had a significantly higher concentration of flavonoids as compared to male plants. The anti-carcinogenic and anti-inflammatory activities of glucoraphanin and sulforaphane have been linked to increased hydrolysis of glucosinolates (GSs) in response to elevated CO2 (Table 6.1) (Almuhayawi et al. 2020). Jasmonic acid (JA) plays an integral role in plant defense mechanisms through the elicitation of different secondary metabolites such as alkaloids, flavonoids, phenylpropanoids, and terpenoids (Tamogami et al. 1997). For example, higher ascorbic acid and carotenoid content have been observed in Origanum majorana L. (Złotek 2017). Similarly, various plants have been reported to produce differential secondary metabolites being elicited by JA (Thakur et al. 2019).
Temperature stress also affects plant ontology and metabolic processes, i.e., physiological and biochemical changes such as chlorophyll pigment breakdown, leaf senescence, membrane damage, and protein denaturation (Waraich et al. 2012). Other effects of higher temperature (heat stress) can be identified by decreased quantum efficiency of Photosystem II (PSII), stomatal conductance, CO2 fixation, altered secondary metabolites, and ROS generation (Hasanuzzaman et al. 2013; Verma and Shukla 2015). However, low temperature is responsible for disturbing the plant–water interactions and metabolic activities, ultimately hampering the plant growth and productivity (Chinnusamy et al. 2007). Plant growing under low temperature synthesizes cryoprotective substances such as soluble sugars (trehalose, raffinose, stachyose, and saccharose), sugar alcohols (inositol, ribitol, and sorbitol), and nitrogen-containing compounds (glycine betaine, proline) to maximize cold stress tolerance (Janská et al. 2010). In Arnica montana, enhanced ratios of quercetin–kaempferol have been reported under low temperature (Albert et al. 2009). Increased artemisinin content has been observed after exposure of Artemisia annua to cold stress (Yin et al. 2008; Vashisth et al. 2018).
Variations in temperature influence the biosynthesis and accumulation of alkaloids in plants. For instance, in Papaver somniferum L., the accumulation of morphinane, benzylisoquinoline, and phthalisoquinoline becomes restricted at low temperature (Bernáth and Tetenyi 1979). Contrary to this, the concentration of isoflavonoids (genistein, genistin, and daidzein) is significantly enhanced in the roots of Glycine max L. at low temperature (Janas et al. 2002). Similarly, several studies have been reported with increased alkaloid contents in plants incubated at a higher temperature. For example, Lupinus angustifolius has been reported with higher alkaloids concentration when grown under elevated temperature (Jansen et al. 2009). In Catharanthus roseus L., increased concentrations of catharanthine, vindoline, and vinblastine were observed at a higher temperature, while incubation at low temperature resulted in a two- to four-fold reduction of catharanthine and vindoline contents (Dutta et al. 2007). These findings suggest that higher temperature enhanced the concentration of alkaloids in plants and low temperature significantly hinders their biosynthetic pathway genes (Dutta et al. 2007). The antioxidative properties of terpenes provide stability to the thylakoid membrane of the chloroplast. In Chamomilla recutita, the combination of photoperiod (21-3h) and temperature (20 ± 2 °C) resulted in the highest concentration of α-bisabolol (Fahlén et al. 1997). Temperature dependency is correlated with the yield of terpenoids. For example, pine species have been reported with increased emissions of sesquiterpene compounds (α-bergamotene, α-farnesene, β-caryophyllene, and β-farnesene) at elevated temperature (Table 6.1) (Helmig et al. 2007). Differential response of temperature on volatile organic compounds (VOCs) has been studied in Betula pendula and Populus tremula, resulting in an exponential increase in DMNT (4,8-dimethyl-nona-1,3,7-triene) concentration (Ibrahim et al. 2010). In the roots of Panax ginseng and Panax quinquefolius, ginsenoside content was significantly enhanced under elevated temperature, while photosynthesis and biomass were considerably reduced (Yu et al. 2005; Jochum et al. 2007).
6.3 Drought Stress
High temperature and solar radiations are accompanied by water deficit conditions that induce drought (Xu et al. 2010). Among abiotic stressors, drought hampers agricultural productivity by upto 50–70% (Verma and Deepti 2016). It has been estimated that drought affects 40% of the global population and now has been predicted to pose a risk of displacement to 700 million populations by 2030 (WHO 2020). Drought stress severely alters plant growth through photosynthesis inhibition, decreased stomatal conductance, CO2 assimilation, and leaf senescence (Nezhadahmadi et al. 2013; Wang et al. 2018; Zargar et al. 2017). Plant defense responses, including secondary metabolites production, are triggered by decreased water potential and turgor pressure caused by increased transpiration rate (Ashraf et al. 2018). Drought stress induces ROS production through oxidative stress, resulting in enhanced production of flavonoids and phenolic acids (Larson and Weber 2018). Through transcriptomics, Morales et al. (2017) identified pathways as well as genes involved in drought-tolerant quinoa. Upregulation of drought-tolerant genes such as GmbZIP44, GmbZIP46, GmbZIP62, and GmbZIP78 has been known to provide tolerance against drought (Xie et al. 2009). In addition, enhanced expression of the GmbZIP1 gene in wheat has been reported as an excellent resource for overcoming drought stress (Gao et al. 2011). Activation of the PAL gene resulted in enhanced phenolic and flavonoid contents in Lactuca sativa L. (Rajabbeigi et al. 2013). Various plants such as Artemisia, Hypericum brasiliense, Hypericum perforatum, and Trachyspermum ammi have been reported with increased secondary metabolites such as artemisinin, betulinic, ruetin, hyperforin, and quercitin (Azhar et al. 2011; Zobayed et al. 2007; Verma and Shukla 2015). Similarly, water-deficit conditions (80–85% field capacity) decreased the number of total flavonoids in Glechoma longituba (Zhang et al. 2012).
The major enzymes responsible for the biosynthesis of flavonoids include chalcone synthase (CHS), chalcone isomerase (CHI), flavone synthase (FNS), flavanone 3-hydroxylase (F3H), flavonol synthase (FLS), dihydroflavonol 4--reductase (DFR), and anthocyanidin synthase (ANS) (Shih et al. 2008). The antioxidant property of flavonoids lies in the position of hydroxyl groups and carbon modifications such as glycosylation, methylation, and prenylation (Rice-Evans et al. 1997). Flavonoids under drought stress act as an antioxidant and protect plants from severe damage induced under water-deficit conditions (Nichols et al. 2015). For instance, in Pisum sativum L., flavonoid concentration was significantly increased by 45% in response to drought stress (Nogués et al. 1998). Similarly, roots of Scutellaria baicalensis were reported with elevated concentrations of flavonoids (Yuan et al. 2012). This increased accumulation of flavonoids represents effective detoxification of H2O2 molecules induced via drought stress (Hernández et al. 2009). In addition, drought stress also influenced phenolic concentration in plants, which was mediated via alteration in the phenylpropanoid pathway (Table 6.2) (Gharibi et al. 2019; Rezayian et al. 2018; Li et al. 2018). Salvia dolomitica and Salvia officinalis showed an increase in flavonoids (101%) and phenolics (139%) content under drought conditions (Caser et al. 2018, 2019). Drought stress reduced oil, sesamin, and quercetin concentration, however, a significant increment was noticed in flavonoids and phenolics contents in Sesamum indicum L " (Kermani et al. 2019). Biosynthesis of glycine betaine via enhanced expressions of glycine betaine hydrogenase was responsible for the alleviation of drought stress in C. roseus (Jaleel et al. 2007). Similarly, the artificial introduction of mannitol in seedlings elevated the concentrations of carbohydrates, proline, thymol, and γ-terpinene (Razavizadeh and Komatsu 2018). Water stress altered essential oil content (geraniol and citral) in Cymbopogon citratus L. (Singh-Sangwan et al. 1994). However, moderate drought conditions exhibited a higher concentration of β-thujone and camphor in Salvia officinalis (Bettaieb et al. 2009). Additionally, Nowak et al. (2010) reported higher concentrations of monoterpenes (33%) in the same plant. Essential oil contents do not always increase; however, it depends on the plant species and the severity of the stress. Paulsen and Selmar (2016) reported a considerable increase in terpene content, whereas the total amount of terpene was markedly reduced due to biomass reduction.
6.4 Salinity Stress
Salinity stress is one of the major limiting factors in plant growth and development. Due to increased anthropogenic activities and global climate change, it is projected to worsen in the near future (Rengasamy 2010). For instance, salinity stress significantly decreased crop yield by 10–50% in most salt-sensitive plant species (Panta et al. 2014). Globally, salinization has recorded an estimated economic loss of US$ 27.3 billion/year (Qadir et al. 2014). This significant increasing trend in salinity becomes a subject of great concern for national as well as global food security. Keeping this in view, the Indian government has planned to restore 26 million ha of salt-affected lands by 2030 (Kumar and Sharma 2020). Photosynthesis inhibition, ROS production, and reduced germination are some of the negative impacts commonly observed under salt stress. The generation of ROS mediated via salt stress alters plant metabolic activities such as the disruption of membrane and ion toxicity (Ashraf et al. 2015; Chaudhary and Choudhary 2021). Secondary metabolites can scavenge ROS through the enhanced accumulation of phenolic compounds. Polyphenol concentrations significantly increased in Cakile maritime after exposure to different concentrations of NaCl (0, 100, 400 mM), indicating a protective role against salt stress (Ksouri et al. 2007). Similarly, Cynara cardunculus were reported to have increased phenolic contents on exposure to moderate levels of NaCl (>75 mM) (Hanen et al. 2008). Fagopyrum esculentum L. under variable salt concentrations (10–200 mM) showed a remarkable increase in phenolic contents (isoorientin, rutin, orientin, and vitexin) compared to control (Lim et al. 2012). Exposure to increased salinity levels (0–200 mM) significantly enhanced the total non-flavonoids (30%), total phenolics (135%), and total tannins (72%) content in Brassica napus L. (Falcinelli et al. 2017). In contrast, Brassica oleracea L. showed a decrease in phenolic compounds (chlorogenic and derivatives of sinapic acid), indicating the accumulation of phenolic acids in a plant-specific manner (Lopez-Berenguer et al. 2009). Similarly, Salvia macrosiphon L. has been reported with a remarkable decrease in total phenolics (2.6 times) after exposure to 8 dS ms−1 salinity level (Valifard et al. 2017). Furthermore, this lack of correlation, however, depends on the synergistic interactions of different antioxidant molecules (Tarchoune et al. 2012a, b). The effects of different salt concentrations on various plant species are shown in Table 6.3.
Salt stress stimulates the production of tropane alkaloids in Datura innoxia L. (Brachet and Cosson 1986). In C. roseus, vincristine content was significantly enhanced in response to 150 mM NaCl but gradually declined with increasing salinity levels (Osman et al. 2007). Ali et al. (2008) reported altered ricinine content in Ricinus communis L. Similarly, reserpine and vincristine (alkaloids) contents significantly increased in C. roseus and R. tetraphylla, respectively (Ahl and Omer 2011). Rosmarinus officinalis governs increased concentrations of camphor and cineole on account of salt stress, whereas borneol, camphene, nopol, and α-terpineol concentrations were decreased significantly (Tounekti et al. 2011). Furthermore, roots of Zea mays L. drastically improved the zealexins levels by five-fold at higher levels of NaCl (500 mM); however, kauralexins contents increased upto two-fold at lower levels (100 mM) (Vaughan et al. 2015). Different concentrations of salt (0–150 mM) significantly enhanced the expression of flavonoid biosynthetic genes (CHS, FS, and PAL) and resulted in increased production of lutein and quercetin in Solanum nigrum L. (Ben Abdallah et al. 2016).
6.5 UV-B Stress
Depletion of the ozone layer raises its concern over increased exposure to UV-B radiation on plants and animals. UV-B radiation, which comprises 0.5% of total solar radiation, possesses a significant impact on terrestrial life forms (Rozema et al. 2009; Verdaguer et al. 2012; Correia et al. 2012). Equatorial regions receive about 12 kJ m−2 d−1 of solar UV-B radiation (Forster 2011). During the pre-1980s, about 6–14% of increment was detected. However, current scenarios reflect this percentage remaining elevated for the next decades (WMO 2010). UV-B influenced plants by reducing photosynthesis, biomass, deformities in chloroplast structure, and increased ROS generation (Pandey and Chaplot 2007; Yang et al. 2007; Kataria et al. 2014; Yao and Liu 2006; Kakani et al. 2003; Choudhary et al. 2017). Elevated UV-B levels significantly altered the concentrations of secondary metabolites such as alkaloids, anthocyanins, cyanogenic glycosides, flavonoids, and tannins in plants (Table 6.4) (Hirata et al. 1993; Morales et al. 2010; Gouvea et al. 2012). For instance, in C. roseus, the amount of catharanthine and vindoline production was significantly enhanced after supplemental UV-B radiation (Ramani and Jayabaskaran 2008). In another study, increased kaempferol and quercetin contents have been reported in Populus trichocarpa (Warren et al. 2003). Similarly, different rice cultivars have been observed with increased C-glycosyl flavones content under high UV-B intensity. Enhanced UV-B radiation leads to a more pronounced effect on flavanols accumulation in Trifolium repens, resulting in increased quercetin levels by 200% (Hofmann et al. 2000). These flavanols protect by acting as UV-B filters and further help to scavenge ROS (Agati et al. 2009, 2011).
UV-B-absorbing compounds such as flavonoids and hydroxycinnamic acids (derivatives of phenolic acids) confer protection at elevated UV-B levels (Agati and Tattini 2010; Jansen et al. 2008; Qian et al. 2020). For example, an increased concentration of flavonoids was reported in Lactuca sativa and Gynura bicolor, which were grown under ambient and high UV-B radiation (García-Macías et al. 2007; Schirrmacher et al. 2004). Accumulation of flavonoids in leaf epidermis confers resistance to the detrimental effects of UV-B radiation. Higher flavonoid contents illustrate increased PAL activity, a key enzyme involved in the phenylpropanoid pathway (Liu et al. 2002). Quercetin and kaempferol levels are certainly beneficial for plants to quench free radicals generated at the initial stage of UV-B exposure (Harborne and Williams 2000). Important crop plants, mainly Vigna radiata L., Pisum sativum L., and Glycine max L., demonstrated enhanced concentrations of quercetin and kaempferol contents induced via elevated UV-B exposure (Choudhary and Agrawal 2014a, b, 2016). In Fagopyrum tataricum, UV-B treatment resulted in a dramatic increase in concentrations of rutin (4.82 mg/g) DW and quercetin (0.04 mg/g) DW, respectively (Huang et al. 2016). Similarly, Mao et al. (2017) reported enhanced concentrations of rutin and quertein (flavonoids) in soyabean. Prolonged exposure to UV-B resulted in upregulation of flavonoid synthetic genes, i.e., FLS and F3′H in Gingko biloba (Zhao et al. 2020). The highest estimated flavonoid concentration was recorded in Alternanthera sessilis (Klein et al. 2018). In Olea europaea L. leaves, abundant concentrations of luteolin-7-O-glucoside account for the species’ high tolerance to UV-B stress (Dias et al. 2020).
UV-B elicitation greatly influences the biosynthesis of phenolic compounds in plants. Increased ROS production initially triggered by UV-B resulted in enhanced phenolic contents that acts direct scavenger of ROS (Solovchenko and Merzlyak 2008). Ambient UV-B doses significantly enhanced flavonoid concentration in root and leaves of Tropaeolum majus L. and Brassica oleracea, suggesting UV-B as a systemic inducer of phenolic compounds in plants (Schreiner et al. 2009; Neugart et al. 2012). Phenolic compounds under UV-B exposure become elevated in postharvested fruits and crops, including apples, peaches, onions, and strawberries (Marais et al. 2001; Kataoka and Beppu 2004; Higashio et al. 2004). The upregulation of phenylpropanoid enzymes by UV-B causes an increase in phenolic concentration (Tomás-Barberán and Espín 2001; Treutter 2005). Moderate UV-B exposure increased catharanthine concentration in C. roseus (Ramani and Chelliah 2007). Various plants such as Acorus calamus, Cyambopogon citratus, Mentha piperata, and Ocimum basilicum have been reported with important pharmacological compounds, induced via UV-B (Kumari et al. 2009a, b; Dolzhenko et al. 2010; Maffei and Scannerini 2000). Sesquiterpenes such as artemisinin and Germacrene-D concentrations were found to be elevated by 11.6% and 10.5% under UV-B exposure (Kumari and Agrawal 2011; Rai et al. 2011). Another plant, Glycyrrhiza uralensis, exhibited a 1.5-fold increase in Glycyrrhizin content on exposure to UV-B dose (0.43 W m−2) (Afreen et al. 2005). One of the major pharmacological important diterpenes, carnosic acid, present in R. officinalis, becomes elevated with UV-B dose (31 kJ m−2 d−1) (Luis et al. 2007).
Exposure to UV-B radiation induced the production of terpenoids in various medicinal plants such as Artemisia annua, Curcuma caesia, Cuminum cyminum L., and Vitis vignifera L. (Li et al. 2021; Jaiswal et al. 2020; Ghasemi et al. 2019; Gil et al. 2012). These terpenoids protect the plant leaves from heat stress induced via UV-B (Liu et al. 2017). Withaferin A and withanolide A contents are increased by 1.38- and 3.42-fold in Withania coagulans L. (Tripathi et al. 2021). Thus, it can be concluded that UV-B can be used as a potential elicitor in increasing the contents of pharmacologically important compounds (Tripathi et al. 2021; Takshak and Agrawal 2014, 2015; Choudhary et al. 2021).
6.6 Tropospheric Ozone Stress
Ozone (O3) is a potent air pollutant and greenhouse gas that may influence vegetation and human health directly or indirectly (DeLang et al. 2021; Wedow et al. 2021). Consumption of fossil fuel increases the concentration of precursor gases such as nitrogen oxide, carbon monoxide, and volatile organic compounds (VOCs), including methane and CO2 that drive increased O3 concentrations (Bhatia et al. 2012). Currently, tropospheric O3 has reached 35–40 ppm globally and is expected to rise further to 70 ppm or more by 2050 (Frei 2015; Sicard et al. 2017; Pfister et al. 2014). Being a strong antioxidant, O3 incorporates into plant tissues through stomata and induces ROS production that ultimately causes lipid peroxidation, DNA and RNA degradation, and programmed cell death (Mishra and Agrawal 2015; Picchi et al. 2017; Choudhury et al. 2017). Likewise, a variety of responses marked by elevated O3, i.e., foliar injury, reduced chlorophyll and RuBisCO content, stomatal conductance, photosynthesis inhibition, and alteration in carbon allocation, cause a reduction in biomass, yield, and its quality (Emberson 2020). The activation of the PAL enzyme corresponds to increased production of flavonoids, phenolic acids, and monolignols, which improves the tolerance ability of plants by acting as scavengers against O3 stress (Iriti and Faoro 2009). Long-term exposure to elevated O3 concentrations concerning accumulation of phenolic compounds has been extensively studied (Richet et al. 2012). In Linum usitatissimum L., various secondary metabolites (flavonoids, anthocyanins, lignin, and wax) were enhanced under elevated O3 (27.7–59.0 ppb) (Tripathi and Agrawal 2013). This enhancement reflects more utilization of assimilate in the production of secondary metabolites and less availability for reproductive organs that ultimately contribute to less yield (Singh et al. 2014). Fatima et al. (2018) investigated the effects of treatment of higher O3 concentration (ambient + 30 ppb) on different wheat cultivars. These findings state that higher induction of flavonoids and total phenols subsequently declined reproductive structures and final yield. Differential responses in the accumulation of total phenolic contents in early and late sown cultivars of wheat indicated a correlation with higher ascorbic acid involved in the production of polyphenols (Yadav et al. 2019). Furthermore, various plants such as wheat, caster, groundnut, and cotton elucidate the sensitivity to ozone pollution (Chaudhary et al. 2021; Rathore and Chaudhary 2019; Ghosh et al. 2020a, b; Chaudhary and Rathore 2021a, b).
Weed invasion delineates the struggle of crop plants for their healthy survival under progressive climate change (Clements et al. 2014). It has been reported that weed interference causes an annual yield loss of 34% in some agronomically important crop species (Oerke 2006). The reason behind the aggressiveness of weeds lies in their higher content of phenolics and alkaloids, which alters the nutrient uptake in the soil (Majeed et al. 2012). A recent study was performed on Chenopodium album L. and Triticum aestivum L. plants to investigate the allelopathic interaction with a concomitant elevation in the concentration of O3. The study revealed that O3 raised the concentrations of ferulic acid (FA) and p-coumaric acid (CA) in the roots of the former and attributed a negative change in the root length of the latter (Ghosh et al. 2020a, b). Greater tolerance to O3 stress is determined by increased PAL activity (Di Baccio et al. 2008). Elevated O3 concentrations significantly increased total phenolic contents and PAL activity in Vigna radiata L. (Mishra and Agrawal 2015). Accumulated phenolic compounds triggered by higher O3 levels during the initial days of exposure were later observed with a slight decrement in Salvia officinalis L. This suggested that higher doses of O3 displayed a priming effect, and later, these plants failed to invest in their response strategy, indicating a slow production of secondary metabolites (Marchica et al. 2021). Brassica campestris L., a rich source of glucosinolate (GLS), exhibited an alteration in the amount of indole, aliphatic, and aromatic GLS (Han et al. 2021).
Exposure to higher levels of O3 also affects isoprene emissions. Isoprene biosynthesis in plants maintains photochemical efficiency and ROS levels induced via excess O3 (Pollastri et al. 2019; Loreto and Velikova 2001). O3-induced emission of isoprene has been documented in several studies (Hewitt et al. 2009; Arab et al. 2016). Taking this into account, date palm has a high potential to resist photochemical changes induced by short-term exposure to O3 (Du et al. 2018). A more realistic Free-air CO2 enrichment (FACE) study demonstrated that emission of isoprene declined significantly with higher O3 concentrations, but the number of total monoterpenes stimulated in date palm leaves was attributed to increased emission of aldehyde volatiles (Table 6.5) (Paoletti et al. 2021).
6.7 Conclusion
Climate change caused by increased anthropogenic activities has significantly altered CO2 concentrations, temperature fluctuations, water-deficit conditions, salinity stress, UV-B intensity, and tropospheric ozone concentrations on Earth’s surface. This is accompanied by a parallel decrease in physiological processes in plants. Elevated CO2 concentrations induce photosynthetic processes, but plants become more susceptible to insect attack at the same time. To avoid insect damage, plants allocate photo-assimilates to secondary metabolite production. Phenolics and terpenoids decreased as CO2 concentrations increased; however, these were significantly intensified by elevated temperature. Higher temperature prevails drought conditions and, with a concomitant increase in salt levels, severely impacts plant growth and yield via ROS production and reduced osmotic potential, which mediates biochemical changes. To confer resistance, plants facilitate antioxidative defense mechanisms through enhanced production of phenolics and flavonoids. Similarly, increased UV-B exposure and ozone stress induce morphological, physiological, and biochemical alterations in plants. Despite this, it needs to further investigate the synergistic role of different abiotic stresses responsible for actual synthesis and modulation at the same time. More importantly, scientists mimic the climate change perspectives through experimental studies, which raises concern about achieving food security and nutritional status worldwide.
References
Afreen F, Zobayed SMA, Kozai T (2005) Spectral quality and UV-B stress stimulate glycyrrhizin concentration of Glycyrrhiza uralensis in hydroponic and pot system. Plant Physiol Biochem 43(12):1074–1081
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photoprotection. New Phytol 186(4):786–793
Agati G, Stefano G, Biricolti S, Tattini M (2009) Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann Bot 104(5):853–861
Agati G, Biricolti S, Guidi L, Ferrini F, Fini A, Tattini M (2011) The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J Plant Physiol 168(3):204–212
Ahl SA, Omer EA (2011) Medicinal and aromatic plants production under salt stress. A review. Herba Polonica 57(2):72–87
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30(3):258–270
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731
Albert A, Sareedenchai V, Heller W, Seidlitz HK, Zidorn C (2009) Temperature is the key to altitudinal variation of phenolics in Arnica montana L. cv. ARBO. Oecologia 160(1):1–8
Ali RM, Elfeky SS, Abbas H (2008) Response of salt stressed Ricinus communis L. to exogenous application of glycerol and/or aspartic acid. J Biol Sci 8(1):171–175
Almuhayawi MS, AbdElgawad H, Al Jaouni SK, Selim S, Hassan AH, Khamis G (2020) Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem 328:127102
Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6(9):2026–2032
Arab L, Kreuzwieser J, Kruse J, Zimmer I, Ache P, Alfarraj S, Al-Rasheid KA, Schnitzler JP, Hedrich R, Rennenberg H (2016) Acclimation to heat and drought—lessons to learn from the date palm (Phoenix dactylifera). Environ Exp Bot 125:20–30
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Ashraf M, Iqbal M, Hussain I, Rasheed R (2015) Physiological and biochemical approaches for salinity tolerance. In: Managing salt tolerance in plants: molecular and genomic perspectives, p 79
Ashraf MA, Iqbal M, Rasheed R, Hussain I, Riaz M, Arif MS (2018) Environmental stress and secondary metabolites in plants: an overview. In: Plant metabolites and regulation under environmental stress, pp 153–167
Azhar N, Hussain B, Ashraf MY, Abbasi KY (2011) Water stress mediated changes in growth, physiology and secondary metabolites of desi ajwain (Trachyspermum ammi L.). Pak J Bot 43(1):15–19
Bajwa AA, Wang H, Chauhan BS, Adkins SW (2019) Effect of elevated carbon dioxide concentration on growth, productivity and glyphosate response of parthenium weed (Parthenium hysterophorus L.). Pest Manag Sci 75(11):2934–2941
Balasooriya HN, Dassanayake KB, Seneweera S, Ajlouni S (2018) Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria× ananassa) growth and fruit yield. Int J Biol Biomol Agric Food Biotechnol Eng 12:279–287
Barnett J (2011) Dangerous climate change in the Pacific Islands: food production and food security. Reg Environ Chang 11(1):229–237
Ben Abdallah S, Aung B, Amyot L, Lalin I, Lachâal M, Karray-Bouraoui N, Hannoufa A (2016) Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosynthesis in Solanum nigrum. Acta Physiol Plant 38(3):1–13
Bernacchi CJ, Kimball BA, Quarles DR, Long SP, Ort DR (2007) Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol 143(1):134–144
Bernáth J, Tetenyi P (1979) The Effect of environmental factors on growth. Development and alkaloid production of Poppy (Papaver somniferum L.): I. Responses to day-length and light intensity. Biochem Physiol Pflanz 174(5–6):468–478
Bettaieb I, Zakhama N, Wannes WA, Kchouk ME, Marzouk B (2009) Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci Hortic 120(2):271–275
Bhatia A, Tomer R, Kumar V, Singh SD, Pathak H (2012) Impact of tropospheric ozone on crop growth and productivity—a review. J Sci Ind Res 71(2):97–112
Biel C, Save R, Cristobal R, Cases MA, del Medio Natural ADC (2005) Effects of atmospheric carbon dioxide concentrations on Thymus vulgaris, Thymus zygis and Thymus hyemalis. Acta Hortic 676:61–65
Brachet J, Cosson L (1986) Changes in the total alkaloid content of Datura innoxia Mill. subjected to salt stress. J Exp Bot 37(5):650–656
Camisón Á, Martín MÁ, Sánchez-Bel P, Flors V, Alcaide F, Morcuende D, Pinto G, Solla A (2019) Hormone and secondary metabolite profiling in chestnut during susceptible and resistant interactions with Phytophthora cinnamomi. J Plant Physiol 241:153030
Caser M, D’Angiolillo F, Chitarra W, Lovisolo C, Ruffoni B, Pistelli L, Pistelli L, Scariot V (2018) Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant Growth Regul 84(2):383–394
Caser M, Chitarra W, D’Angiolillo F, Perrone I, Demasi S, Lovisolo C, Pistelli L, Scariot V (2019) Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind Crop Prod 129:85–96
Chaturvedi AK, Bahuguna RN, Pal M, Shah D, Maurya S, Jagadish KS (2017) Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crop Res 206:149–157
Chaudhary N, Choudhary KK (2021) Photosynthetic responses and tolerance mechanism of crop plants against salt stress. In: Srivastava PK, Kumar J, Prasad SM (eds) Salt stress responses in plants: perception, signaling, omics and tolerance mechanisms. Nova Science Publications, pp 65–84
Chaudhary IJ, Rathore D (2021a) Assessment of dose–response relationship between ozone dose and groundnut (Arachis hypogaea L.) cultivars using Open Top Chamber (OTC) and Ethylenediurea (EDU). Environ Technol Innov 22:101494
Chaudhary IJ, Rathore D (2021b) Assessment of ozone toxicity on cotton (Gossypium hirsutum L.) cultivars: its defensive system and intraspecific sensitivity. Plant Physiol Biochem 166:912–927
Chaudhary N, Bonfil DJ, Tas E (2021) Physiological and yield responses of spring wheat cultivars under realistic and acute levels of ozone. Atmosphere 12(11):1392
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12(10):444–451
Chomel M, Guittonny-Larchevêque M, Fernandez C, Gallet C, DesRochers A, Paré D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104(6):1527–1541
Choudhary KK, Agrawal SB (2014a) Cultivar specificity of tropical mung bean (Vigna radiata L.) to elevated ultraviolet-B: changes in antioxidative defense system, nitrogen metabolism and accumulation of jasmonic and salicylic acids. Environ Exp Bot 99:122–132
Choudhary KK, Agrawal SB (2014b) Ultraviolet-B induced changes in morphological, physiological, and biochemical parameters of two cultivars of pea (Pisum sativum L.). Ecotoxicol Environ Saf 100:178–187
Choudhary KK, Agrawal SB (2016) Assessment of fatty acid profile and seed mineral nutrients of two soybean (Glycine max L.) cultivars under elevated UV-B: role of ROS pigments and antioxidants. Photochem Photobiol 92:134–143
Choudhary KK, Chaudhary N, Agrawal SB, Agrawal M (2017) Reactive oxygen species: generation, damage, and quenching in plants during stress. In: Singh VP, Singh S, Tripathi DK, Prasad SM, Chauhan DK (eds) Reactive oxygen species in plants: boon or bane—revisiting the role of ROS. Wiley, pp 89–115
Choudhary KK, Singh S, Agrawal M, Agrawal SB (2021) Role of jasmonic and salicylic acid signaling in plants under UV-B stress. In: Aftab T, Yusuf M (eds) Jasmonates and salicylates signaling in plants. Signaling and communication in plants. Springer, Cham, pp 45–63
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90(5):856–867
Clements DR, DiTommaso A, Hyvönen T (2014) Ecology and management of weeds in a changing climate. In: Chauhan B, Mahajan G (eds) Recent advances in weed management. Springer, New York, pp 13–37
Correia CM, Coutinho JF, Bacelar EA, Gonçalves BM, Björn LO, Moutinho Pereira J (2012) Ultraviolet-B radiation and nitrogen affect nutrient concentrations and the amount of nutrients acquired by above-ground organs of maize. Sci World J 2012:11
DeLang MN, Becker JS, Chang KL, Serre ML, Cooper OR, Schultz MG, Schröder S, Lu X, Zhang L, Deushi M, Josse B (2021) Mapping yearly fine resolution global surface ozone through the Bayesian maximum entropy data fusion of observations and model output for 1990–2017. Environ Sci Technol 55(8):4389–4398
Di Baccio D, Castagna A, Paoletti E, Sebastiani L, Ranieri A (2008) Could the differences in O3 sensitivity between two poplar clones be related to a difference in antioxidant defense and secondary metabolic response to O3 influx? Tree Physiol 28(12):1761–1772
Dias MC, Pinto DC, Freitas H, Santos C, Silva AM (2020) The antioxidant system in Olea europaea to enhanced UV-B radiation also depends on flavonoids and secoiridoids. Phytochemistry 170:112199
Dolzhenko Y, Bertea CM, Occhipinti A, Bossi S, Maffei ME (2010) UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha× piperita L.). J Photochem Photobiol B Biol 100(2):67–75
Dong J, Gruda N, Lam SK, Li X, Duan Z (2018) Effects of elevated CO2 on nutritional quality of vegetables: a review. Front Plant Sci 9:924
Du B, Kreuzwieser J, Winkler JB, Ghirardo A, Schnitzler JP, Ache P, Alfarraj S, Hedrich R, White P, Rennenberg H (2018) Physiological responses of date palm (Phoenix dactylifera) seedlings to acute ozone exposure at high temperature. Environ Pollut 242:905–913
Dutta A, Sen J, Deswal R (2007) Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G Don. Plant Cell Rep 26(10):1869–1878
Dutta P, Chakraborti S, Chaudhuri KM, Mondal S (2020) Physiological responses and resilience of plants to climate change. In: Rakshit A, Singh H, Singh A, Singh U, Fraceto L (eds) New frontiers in stress management for durable agriculture. Springer, Singapore, pp 3–20
Easterling WE, Aggarwal PK, Batima P, Brander KM, Erda L, Howden SM, Kirilenko A, Morton J, Soussana JF, Schmidhuber J, Tubiello FN (2007) Food, fibre and forest products. Climate Change 2007:273–313
Edreva A, Velikova V, Tsonev T, Dagnon S, Gürel A, Aktaş L, Gesheva E (2008) Stress-protective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol 34(1-2):67–78
Emberson L (2020) Effects of ozone on agriculture, forests and grasslands. Phil Trans R Soc A 378(2183):20190327
Fahlén A, Welander M, Wennersten R (1997) Effects of light–temperature regimes on plant growth and essential oil yield of selected aromatic plants. J Sci Food Agric 73(1):111–119
Falcinelli B, Sileoni V, Marconi O, Perretti G, Quinet M, Lutts S, Benincasa P (2017) Germination under moderate salinity increases phenolic content and antioxidant activity in rapeseed (Brassica napus var oleifera Del.) sprouts. Molecules 22(8):1377
Fanzo J (2018) Challenges and impacts of poor nutrition (No. 2175-2019-152)
Fatima A, Singh AA, Mukherjee A, Agrawal M, Agrawal SB (2018) Variability in defence mechanism operating in three wheat cultivars having different levels of sensitivity against elevated ozone. Environ Exp Bot 155:66–78
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interaction between plants and insects. Recent advances in phytochemistry. Springer, Boston, MA, pp 1–40
Forster PM (2011) Stratospheric changes and climate. Scientific assessment of ozone depletion: 2010. Global Ozone research and monitoring project—report no. 52. World Meteorological Organization, Geneva, Switzerland, pp 1–60
Frei M (2015) Breeding of ozone resistant rice: relevance, approaches and challenges. Environ Pollut 197:144–155
Funk CC, Brown ME (2009) Declining global per capita agricultural production and warming oceans threaten food security. Food Secur 1(3):271–289
Gamage D, Thompson M, Sutherland M, Hirotsu N, Makino A, Seneweera S (2018) New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ 41(6):1233–1246
Gao SQ, Chen M, Xu ZS, Zhao CP, Li L, Xu HJ, Tang YM, Zhao X, Ma YZ (2011) The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol Biol 75(6):537–553
García-Macías P, Ordidge M, Vysini E, Waroonphan S, Battey NH, Gordon MH, Hadley P, John P, Lovegrove JA, Wagstaffe A (2007) Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J Agric Food Chem 55(25):10168–10172
Geilfus CM (2019) Plant secondary compounds. In: Controlled environment horticulture. Springer, Cham, pp 19–33
Gharibi S, Tabatabaei BES, Saeidi G, Talebi M, Matkowski A (2019) The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochemistry 162:90–98
Ghasemi S, Kumleh HH, Kordrostami M (2019) Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma 256(1):279–290
Ghasemzadeh A, Jaafar HZ, Rahmat A (2010) Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 15(11):7907–7922
Ghasemzadeh A, Jaafar HZ, Rahmat A (2011) Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts. J Med Plants Res 5(7):1147–1154
Ghorbanpour M, Khavazi K, Hatami M (2014) Chemical compositions and antimicrobial activity of Salvia officinalis L. essential oil under rhizobacteria (Pseudomonas fluorescens and Putida) inoculation. Eur J Soil Biol
Ghosh A, Agrawal M, Agrawal SB (2020a) Effect of water deficit stress on an Indian wheat cultivar (Triticum aestivum L. HD 2967) under ambient and elevated level of ozone. Sci Total Environ 714:136837
Ghosh A, Pandey B, Agrawal M, Agrawal SB (2020b) Interactive effects and competitive shift between Triticum aestivum L.(wheat) and Chenopodium album L.(fat-hen) under ambient and elevated ozone. Environ Pollut 265:114764
Gil M, Pontin M, Berli F, Bottini R, Piccoli P (2012) Metabolism of terpenes in the response of grape (Vitis vinifera L.) leaf tissues to UV-B radiation. Phytochemistry 77:89–98
Giri A, Armstrong B, Rajashekar CB (2016) Elevated carbon dioxide level suppresses nutritional quality of lettuce and spinach. Am J Plant Sci 7(01):246
Gouvea DR, Gobbo-Neto L, Lopes NP (2012) The influence of biotic and abiotic factors on the production of secondary metabolites in medicinal plants. In: Plant bioactives and drug discovery: principles, practice, and perspectives, vol 17, p 419
Han YJ, Gharibeshghi A, Mewis I, Förster N, Beck W, Ulrichs C (2021) Effect of different durations of moderate ozone exposure on secondary metabolites of Brassica campestris L. ssp. chinensis. J Hortic Sci Biotechnol 96(1):110–120
Hanen F, Ksouri R, Megdiche W, Trabelsi N, Boulaaba M, Abdelly C (2008) Effect of salinity on growth, leaf-phenolic content and antioxidant scavenging activity in Cynara cardunculus L. In: Öztürk M, Ashraf M, Grignon C (eds) Abdelly C. Biosaline agriculture and high salinity tolerance, Birkhäuser, Basel, pp 335–343
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55(6):481–504
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684
Helmig D, Ortega J, Duhl T, Tanner D, Guenther A, Harley P, Wiedinmyer C, Milford J, Sakulyanontvittaya T (2007) Sesquiterpene emissions from pine trees—identifications, emission rates and flux estimates for the contiguous United States. Environ Sci Technol 41(5):1545–1553
Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14(3):125–132
Hewitt CN, MacKenzie AR, Di Carlo P, Di Marco CF, Dorsey JR, Evans M, Fowler D, Gallagher MW, Hopkins JR, Jones CE, Langford B (2009) Nitrogen management is essential to prevent tropical oil palm plantations from causing ground-level ozone pollution. Proc Natl Acad Sci U SA 106(44):18447–18451
Higashio H, Hirokane H, Sato F, Tokuda S, Uragami A (2004) Effect of UV irradiation after the harvest on the content of flavonoid in vegetables. In: V International postharvest symposium, vol 682, pp 1007–1012
Hirata K, Asada M, Yatani E, Miyamoto K, Miura Y (1993) Effects of near-ultraviolet light on alkaloid production in Catharanthus roseus plants. Planta Med 59(01):46–50
Hofmann RW, Swinny EE, Bloor SJ, Markham KR, Ryan KG, Campbell BD, Jordan BR, Fountain DW (2000) Responses of nine Trifolium repens L. populations to ultraviolet-B radiation: differential flavonol glycoside accumulation and biomass production. Ann Bot 86(3):527–537
Huang X, Yao J, Zhao Y, Xie D, Jiang X, Xu Z (2016) Efficient rutin and quercetin biosynthesis through flavonoids-related gene expression in Fagopyrum tataricum Gaertn. hairy root cultures with UV-B irradiation. Front Plant Sci 7:63
Hussin S, Geissler N, El-Far MM, Koyro HW (2017) Effects of salinity and short-term elevated atmospheric CO2 on the chemical equilibrium between CO2 fixation and photosynthetic electron transport of Stevia rebaudiana Bertoni. Plant Physiol Biochem 118:178–186
Ibrahim MH, Jaafar HZ (2012) Impact of elevated carbon dioxide on primary, secondary metabolites and antioxidant responses of Eleais guineensis Jacq. (Oil Palm). Molecules 17(5):5195–5211
Ibrahim MA, Mäenpää M, Hassinen V, Kontunen-Soppela S, Malec L, Rousi M, Pietikäinen L, Tervahauta A, Kärenlampi S, Holopainen JK, Oksanen EJ (2010) Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J Exp Bot 61(6):1583–1595
IPCC (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor MMHL, Allen SK, Boschung J, Nauels A, Xia Y, Bex B, Midgley PM (eds) Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, vol 2. Cambridge University Press, pp 1–27
IPCC (2014) Climate change 2014: synthesis report. In: Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, Church JA, Clarke L, Dahe Q, Dasgupta P, Dubash NK et al (eds) Proceedings of the contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, p 151
IPCC (2019) In: Shukla PR, Skea J, Calvo Buendia E, Masson-Delmotte V, Pörtner HO, Roberts DC, Zhai P, Slade R, Connors S, Van Diemen R, Ferrat M et al (eds) Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. IPCC, Geneva, p 865
IPCC (2021) Summary for policymakers. In: Masson Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N et al (eds) Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press
Iriti M, Faoro F (2009) Chemical diversity and defense metabolism: how plants cope with pathogens and ozone pollution. Int J Mol Sci 10(8):3371–3399
Jaiswal D, Pandey A, Mukherjee A, Agrawal M, Agrawal SB (2020) Alterations in growth, antioxidative defense and medicinally important compounds of Curcuma caesia Roxb. under elevated ultraviolet-B radiation. Environ Exp Bot 177:104152
Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Gopi R, Somasundaram R, Panneerselvam R (2007) Water deficit stress mitigation by calcium chloride in Catharanthus roseus: Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf B: Biointerfaces 60(1):110–116
Janas KM, Cvikrová M, Pałagiewicz A, Szafranska K, Posmyk MM (2002) Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci 163(2):369–373
Jansen MA, Hectors K, O’Brien NM, Guisez Y, Potters G (2008) Plant stress and human health: do human consumers benefit from UV-B acclimated crops? Plant Sci 175(4):449–458
Jansen G, Jürgens HU, Ordon F (2009) Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius cultivars. J Agron Crop Sci 195(3):172–177
Janská A, Maršík P, Zelenková S, Ovesná J (2010) Cold stress and acclimation–what is important for metabolic adjustment? Plant Biol 12(3):395–405
Jia X, Wang W, Chen Z, He Y, Liu J (2014) Concentrations of secondary metabolites in tissues and root exudates of wheat seedlings changed under elevated atmospheric CO2 and cadmium-contaminated soils. Environ Exp Bot 107:134–143
Jochum GM, Mudge KW, Thomas RB (2007) Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae). Am J Bot 94(5):819–826
Jogawat A, Yadav B, Lakra N, Singh AK, Narayan OP (2021) Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: a review. Physiol Plant 172(2):1106–1132
Kakani VG, Reddy KR, Zhao D, Sailaja K (2003) Field crop responses to ultraviolet-B radiation: a review. Agric For Meteorol 120(1–4):191–218
Kataoka I, Beppu K (2004) UV irradiance increases development of red skin color and anthocyanins in Hakuho’Peach. HortScience 39(6):1234–1237
Kataria S, Jajoo A, Guruprasad KN (2014) Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. J Photochem Photobiol B Biol 137:55–66
Kaundal M, Bhatt V, Kumar R (2018) Elevated CO2 and temperature effect on essential oil content and composition of Valeriana jatamansi Jones. with organic manure application in a Western Himalayan region. J Essent Oil-Bear Plants 21(4):1041–1050
Kermani SG, Saeidi G, Sabzalian MR, Gianinetti A (2019) Drought stress influenced sesamin and sesamolin content and polyphenolic components in sesame (Sesamum indicum L.) populations with contrasting seed coat colors. Food Chem 289:360–368
Kimball BA (2016) Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr Opin Plant Biol 31:36–43
Klein FRS, Reis A, Kleinowski AM, Telles RT, Amarante LD, Peters JA, Braga EJB (2018) UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Bot Bras 32:615–623
Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem 45(3–4):244–249
Kumar P, Sharma PK (2020) Soil salinity and food security in India. Front Sustain Food Syst 4:174
Kumari R, Agrawal SB (2011) Comparative analysis of essential oil composition and oil containing glands in Ocimum sanctum L. (Holy basil) under ambient and supplemental level of UV-B through gas chromatography–mass spectrometry and scanning electron microscopy. Acta Physiol Plant 33(4):1093–1101
Kumari R, Agrawal SB, Sarkar A (2009a) Evaluation of changes in oil cells and composition of essential oil in lemongrass (Cymbopogon citratus (DC) Stapf.) due to supplemental ultraviolet-B irradiation. Curr Sci 97:1137–1142
Kumari R, Singh S, Agrawal SB (2009b) Effects of supplemental ultraviolet-B radiation on growth and physiology of Acorus calamus L. (sweet flag). Acta Biol Cracov Ser Bot 51:19–27
Larson RA, Weber EJ (2018) Reaction mechanisms in environmental organic chemistry. Routledge
Levine LH, Kasahara H, Kopka J, Erban A, Fehrl I, Kaplan F, Zhao W, Littell RC, Guy C, Wheeler R, Sager J (2008) Physiologic and metabolic responses of wheat seedlings to elevated and super-elevated carbon dioxide. Adv Space Res 42(12):1917–1928
Li X, Zhang L, Ahammed GJ, Li ZX, Wei JP, Shen C, Yan P, Zhang LP, Han WY (2017) Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camellia sinensis L. Sci Rep 7(1):1–12
Li M, Li Y, Zhang W, Li S, Gao Y, Ai X, Zhang D, Liu B, Li Q (2018) Metabolomics analysis reveals that elevated atmospheric CO2 alleviates drought stress in cucumber seedling leaves. Anal Biochem 559:71–85
Li Y, Qin W, Fu X, Zhang Y, Hassani D, Kayani SI, Xie L, Liu H, Chen T, Yan X, Peng B (2021) Transcriptomic analysis reveals the parallel transcriptional regulation of UV-B-induced artemisinin and flavonoid accumulation in Artemisia annua L. Plant Physiol Biochem 163:189–200
Lim JH, Park KJ, Kim BK, Jeong JW, Kim HJ (2012) Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem 135(3):1065–1070
Liu C, Blount JW, Steele CL, Dixon RA (2002) Bottlenecks for metabolic engineering of isoflavone glycoconjugates in Arabidopsis. Proc Natl Acad Sci U S A 99:14578–14583
Liu H, Cao X, Liu X, Xin R, Wang J, Gao J, Wu B, Gao L, Xu C, Zhang B, Grierson D (2017) UV-B irradiation differentially regulates terpene synthases and terpene content of peach. Plant Cell Environ 40(10):2261–2275
Lopez-Berenguer C, Martínez-Ballesta MDC, Moreno DA, Carvajal M, Garcia-Viguera C (2009) Growing hardier crops for better health: salinity tolerance and the nutritional value of broccoli. J Agric Food Chem 57(2):572–578
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127(4):1781–1787
Luis JC, Pérez RM, González FV (2007) UV-B radiation effects on foliar concentrations of rosmarinic and carnosic acids in rosemary plants. Food Chem 101(3):1211–1215
Maffei M, Scannerini S (2000) UV-B effect on photomorphogenesis and essential oil composition in peppermint (Mentha piperita L.). J Essent Oil Res 12(5):523–529
Majeed A, Chaudhry Z, Muhammad Z (2012) Allelopathic assessment of fresh aqueous extracts of Chenopodium album L. for growth and yield of wheat (Triticum aestivum L.). Pak J Bot 44(1):165–167
Mao B, Wang Y, Zhao TH, Tian RR, Wang W, Ye JS (2017) Combined effects of elevated O3 concentrations and enhanced UV-B radiation of the biometric and biochemical properties of soybean roots. Front Plant Sci 8:1568
Marais E, Jacobs G, Holcroft DM (2001) Postharvest irradiation enhances anthocyanin synthesis in apples but not in pears. HortScience 36(4):738–740
Marchica A, Ascrizzi R, Flamini G, Cotrozzi L, Tonelli M, Lorenzini G, Nali C, Pellegrini E (2021) Ozone as eustress for enhancing secondary metabolites and bioactive properties in Salvia officinalis. Ind Crop Prod 170:113730
Mishra AK, Agrawal SB (2015) Biochemical and physiological characteristics of tropical mung bean (Vigna radiata L.) cultivars against chronic ozone stress: an insight to cultivar-specific response. Protoplasma 252(3):797–811
Mishra AK, Rai R, Agrawal SB (2013) Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum L.) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian J Biochem Biophys 50:139–149
Misselhorn A, Aggarwal P, Ericksen P, Gregory P, Horn-Phathanothai L, Ingram J, Wiebe K (2012) A vision for attaining food security. Curr Opin Environ Sustain 4(1):7–17
Morales LO, Tegelberg R, Brosche M, Keinänen M, Lindfors A, Aphalo PJ (2010) Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol 30(7):923–934
Morales A, Zurita-Silva A, Maldonado J, Silva H (2017) Transcriptional responses of Chilean quinoa (Chenopodium quinoa Wild.) under water deficit conditions uncovers ABA-independent expression patterns. Front Plant Sci 8:216
Nabavi SM, Šamec D, Tomczyk M, Milella L, Russo D, Habtemariam S, Suntar I, Rastrelli L, Daglia M, Xiao J, Giampieri F (2020) Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol Adv 38:107316
Neugart S, Zietz M, Schreiner M, Rohn S, Kroh LW, Krumbein A (2012) Structurally different flavonol glycosides and hydroxycinnamic acid derivatives respond differently to moderate UV-B radiation exposure. Physiol Plant 145(4):582–593
Nezhadahmadi A, Prodhan ZH, Faruq G (2013) Drought tolerance in wheat. Sci World J 2013
Nichols SN, Hofmann RW, Williams WM (2015) Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ Exp Bot 119:40–47
NOAA (National Centers for Environmental Information) (2020). State of the climate: global climate report for annual 2020. https://www.ncdc.noaa.gov/sotc/global/202013. Accessed 15 Mar 2021
Nogués S, Allen DJ, Morison JI, Baker NR (1998) Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol 117(1):173–181
Nowak M, Kleinwaechter M, Manderscheid R, Weigel HJ, Selmar D (2010) Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J Appl Bot Food Qual 83(2):133–136
Oerke EC (2006) Crop losses to pests. J Agric Sci 144(1):31–43
Osman ME, Elfeky SS, El-Soud KA, Hasan AM (2007) Response of Catharanthus roseus shoots to salinity and drought in relation to vincristine alkaloid content. Asian J Plant Sci
Pan T, Wang Y, Wang L, Ding J, Cao Y, Qin G, Yan L, Xi L, Zhang J, Zou Z (2020) Increased CO2 and light intensity regulate growth and leaf gas exchange in tomato. Physiol Plant 168(3):694–708
Pandey J, Chaplot K (2007) Effects of enhanced UV-B radiation on physiological and biochemical characteristics of wheat [Triticum aestivum (L.) var. Raj 3077]. Res Crops 8(2):401
Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S (2014) Halophyte agriculture: success stories. Environ Exp Bot 107:71–83
Paoletti E, Hoshika Y, Arab L, Martini S, Cotrozzi L, Weber D, Ache P, Neri L, Baraldi R, Pellegrini E, Müller HM (2021) Date palm responses to a chronic, realistic ozone exposure in a FACE experiment. Environ Res 195:110868
Paulsen J, Selmar D (2016) Case study: the difficulty of correct reference values when evaluating the effects of drought stress: a case study with Thymus vulgaris. J Appl Bot Food Qual 89:287–289
Peñuelas J, Staudt M (2010) BVOCs and global change. Trends Plant Sci 15(3):133–144
Pfister GG, Walters S, Lamarque JF, Fast J, Barth MC, Wong J, Done J, Holland G, Bruyère CL (2014) Projections of future summertime ozone over the US. J Geophys Res Atmos 119(9):5559–5582
Picchi V, Monga R, Marzuoli R, Gerosa G, Faoro F (2017) The ozone-like syndrome in durum wheat (Triticum durum Desf.): mechanisms underlying the different symptomatic responses of two sensitive cultivars. Plant Physiol Biochem 112:261–269
Piubelli GC, Hoffmann-Campo CB, Moscardi F, Miyakubo SH, Neves De Oliveira MC (2005) Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? J Chem Ecol 31(7):1509–1525
Pollastri S, Jorba I, Hawkins TJ, Llusià J, Michelozzi M, Navajas D, Peñuelas J, Hussey PJ, Knight MR, Loreto F (2019) Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol 223(3):1307–1318
Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Drechsel P, Noble AD (2014) Economics of salt-induced land degradation and restoration. Nat Resour Forum 38(4):282–295
Qian C, Chen Z, Liu Q, Mao W, Chen Y, Tian W, Liu Y, Han J, Ouyang X, Huang X (2020) Coordinated transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol Plant 13(5):777–792
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37(1):91–102
Radušienė J, Karpavičienė B, Stanius Ž (2012) Effect of external and internal factors on secondary metabolites accumulation in St. John’s worth. Bot Lith 18(2):101–108
Rai R, Meena RP, Smita SS, Shukla A, Rai SK, Pandey-Rai S (2011) UV-B and UV-C pre-treatments induce physiological changes and artemisinin biosynthesis in Artemisia annua L.—an antimalarial plant. J Photochem Photobiol B Biol 105(3):216–225
Rajabbeigi E, Eichholz I, Beesk N, Ulrichs C, Kroh LW, Rohn S, Huyskens-Keil S (2013) Interaction of drought stress and UV-B radiation-impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). J Appl Bot Food Qual 86(1):190–197
Ramani S, Chelliah J (2007) UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant Biol 7(1):1–17
Ramani S, Jayabaskaran C (2008) Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J Mol Signal 3(1):1–6
Rao CR, Raju BMK, Rao AS, Rao KV, Rao VUM, Ramachandran K, Venkateswarlu B, Sikka AK, Rao MS, Maheswari M, Rao CS (2016) A district level assessment of vulnerability of Indian agriculture to climate change. Curr Sci 110:1939–1946
Rathore D, Chaudhary IJ (2019) Ozone risk assessment of castor (Ricinus communis L.) cultivars using open top chamber and ethylenediurea (EDU). Environ Pollut 244:257–269
Razavizadeh R, Komatsu S (2018) Changes in essential oil and physiological parameters of callus and seedlings of Carum copticum L. under in vitro drought stress. J Food Meas Charact 12(3):1581–1592
Reddy KR, Hodges HF (eds) (2000) Climate change and global crop productivity. CABI
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Funct Plant Biol 37(7):613–620
Rezayian M, Niknam V, Ebrahimzadeh H (2018) Differential responses of phenolic compounds of Brassica napus under drought stress. Iran J f Plant Physiol 8(3):2417–2425
Rhoades DF, Cates RG (1976) Toward a general theory of plant anti-herbivore chemistry. In: Wallace JW, Mansell RL (eds) Biochemical interaction between plants and insects. Recent advances in phytochemistry. Springer, Boston, MA, pp 168–213
Rice JC, Garcia SM (2011) Fisheries, food security, climate change, and biodiversity: characteristics of the sector and perspectives on emerging issues. ICES J Mar Sci 68(6):1343–1353
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2(4):152–159
Richet N, Tozo K, Afif D, Banvoy J, Legay S, Dizengremel P, Cabané M (2012) The response to daylight or continuous ozone of phenylpropanoid and lignin biosynthesis pathways in poplar differs between leaves and wood. Planta 236(2):727–737
Robinson EA, Ryan GD, Newman JA (2012) A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol 194(2):321–336
Rosa BL, Souza JP, Pereira EG (2019) Increased atmospheric CO2 changes the photosynthetic responses of Acrocomia aculeata (Arecaceae) to drought. Acta Bot Bras 33:486–497
Rozema J, Blokker P, Fuertes M, Broekman R (2009) UV-B absorbing compounds in present-day and fossil pollen, spores, cuticles, seed coats and wood: evaluation of a proxy for solar UV radiation. Photochem Photobiol Sci 8(9):1233–1243
Schirrmacher G, Schnitzler WH, Grassmann J (2004) Determination of secondary plant metabolites and antioxidative capacity as new parameter for quality evaluation: Indicated by the new Asia salad Gynura bicolor. J Appl Bot 78(2):133–134
Schreiner M, Krumbein A, Mewis I, Ulrichs C, Huyskens-Keil S (2009) Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innovative Food Sci Emerg Technol 10(1):93–96
Shih CH, Chu H, Tang LK, Sakamoto W, Maekawa M, Chu IK, Wang M, Lo C (2008) Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 228(6):1043–1054
Sicard P, Anav A, De Marco A, Paoletti E (2017) Projected global ground-level ozone impacts on vegetation under different emission and climate scenarios. Atmos Chem Phys 17(19):12177–12196
Singh AA, Agrawal SB, Shahi JP, Agrawal M (2014) Assessment of growth and yield losses in two Zea mays L. cultivars (quality protein maize and nonquality protein maize) under projected levels of ozone. Environ Sci Pollut Res 21(4):2628–2641
Singh-Sangwan N, Abad Farooqi AH, Singh Sangwan R (1994) Effect of drought stress on growth and essential oil metabolism in lemongrasses. New Phytol 128(1):173–179
Sobuj N, Virjamo V, Zhang Y, Nybakken L, Julkunen-Tiitto R (2018) Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L.). Environ Exp Bot 146:34–44
Solovchenko AE, Merzlyak MN (2008) Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ J Plant Physiol 55(6):719–737
Takshak S, Agrawal SB (2014) Effect of ultraviolet-B radiation on biomass production, lipid peroxidation, reactive oxygen species, and antioxidants in Withania somnifera. Biol Plant 58(2):328–334
Takshak S, Agrawal SB (2015) Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: augmentation of secondary metabolites and antioxidants. Plant Physiol Biochem 97:124–138
Tamogami S, Rakwal R, Kodama O (1997) Phytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid. FEBS Lett 412(1):61–64
Tarchoune I, Sgherri C, Baatour O, Izzo R, Lachaal M, Navari-Izzo F (2012a) Phenolic acids and total antioxidant activity in Ocimum basilicum L. grown under Na2SO4 medium. J Med Plants Res 6(48):5868–5875
Tarchoune I, Sgherri C, Izzo R, Lachaâl M, Navari-Izzo F, Ouerghi Z (2012b) Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) under different sodium salts. Acta Physiol Plant 10:738–847
Thakur M, Bhattacharya S, Khosla PK, Puri S (2019) Improving production of plant secondary metabolites through biotic and abiotic elicitation. J Appl Res Med Aromat Plants 12:1–12
Thompson B, Cohen MJ (eds) (2012) The impact of climate change and bioenergy on nutrition. Springer
Tomás-Barberán FA, Espín JC (2001) Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric 81(9):853–876
Tounekti T, Vadel AM, Ennajeh M, Khemira H, Munné-Bosch S (2011) Ionic interactions and salinity affect monoterpene and phenolic diterpene composition in rosemary (Rosmarinus officinalis). J Plant Nutr Soil Sci 174(3):504–514
Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7(06):581–591
Tripathi R, Agrawal SB (2013) Interactive effect of supplemental ultraviolet B and elevated ozone on seed yield and oil quality of two cultivars of linseed (Linum usitatissimum L.) carried out in open top chambers. J Sci Food Agric 93(5):1016–1025
Tripathi D, Meena RP, Pandey-Rai S (2021) Short term UV-B radiation mediated modulation of physiological traits and withanolides production in Withania coagulans (L.) Dunal under in-vitro condition. Physiol Mol Biol Plants 27(8):1823–1835
Valifard M, Mohsenzadeh S, Kholdebarin B (2017) Salinity effects on phenolic content and antioxidant activity of Salvia macrosiphon. Iran J Sci Technol Trans A Sci 41(2):295–300
Vashisth D, Kumar R, Rastogi S, Patel VK, Kalra A, Gupta MM, Gupta AK, Shasany AK (2018) Transcriptome changes induced by abiotic stresses in Artemisia annua. Sci Rep 8(1):1–14
Vaughan MM, Christensen S, Schmelz EA, Huffaker A, Mcauslane HJ, Alborn HT, Romero M, Allen LH, Teal PE (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ 38(11):2195–2207
Verdaguer D, Llorens L, Bernal M, Badosa J (2012) Photomorphogenic effects of UVB and UVA radiation on leaves of six Mediterranean sclerophyllous woody species subjected to two different watering regimes at the seedling stage. Environ Exp Bot 79:66–75
Verma AK, Deepti S (2016) Abiotic stress and crop improvement: current scenario. Adv Plants Agric Res 4(4):00149
Verma N, Shukla S (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med Aromat Plants 2(4):105–113
Vurro E, Bruni R, Bianchi A, di Toppi LS (2009) Elevated atmospheric CO2 decreases oxidative stress and increases essential oil yield in leaves of Thymus vulgaris grown in a mini-FACE system. Environ Exp Bot 65(1):99–106
Wang L, Feng Z, Schjoerring JK (2013) Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): a meta-analytic test of current hypotheses. Agric Ecosyst Environ 178:57–63
Wang P, Marsh EL, Ainsworth EA, Leakey AD, Sheflin AM, Schachtman DP (2017) Shifts in microbial communities in soil, rhizosphere and roots of two major crop systems under elevated CO2 and O3. Sci Rep 7(1):1–12
Wang Z, Li G, Sun H, Ma L, Guo Y, Zhao Z, Gao H, Mei L (2018) Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol Open 7(11):bio035279
Waraich EA, Ahmad R, Halim A, Aziz T (2012) Alleviation of temperature stress by nutrient management in crop plants: a review. J Soil Sci Plant Nutr 12(2):221–244
Warren JM, Bassman JH, Fellman JK, Mattinson DS, Eigenbrode S (2003) Ultraviolet-B radiation alters phenolic salicylate and flavonoid composition of Populus trichocarpa leaves. Tree Physiol 23(8):527–535
Wedow JM, Ainsworth EA, Li S (2021) Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response. Trends Biochem Sci 46(12):992–1002
WHO (World Health Organization) (2020) The state of food security and nutrition in the world 2020: transforming food systems for affordable healthy diets, vol 2020. Food and Agriculture Organization
WMO (World Meteorological Organization) (2010) Scientific assessment of ozone depletion: 2010. Global Ozone Research and Monitoring Project-Report No 53. 2010
Xie ZM, Zou HF, Lei G, Wei W, Zhou QY, Niu CF, Liao Y, Tian AG, Ma B, Zhang WK, Zhang JS (2009) Soybean Trihelix transcription factors GmGT-2A and GmGT-2B improve plant tolerance to abiotic stresses in transgenic Arabidopsis. PLoS One 4(9):e6898
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5(6):649–654
Yadav DS, Rai R, Mishra AK, Chaudhary N, Mukherjee A, Agrawal SB, Agrawal M (2019) ROS production and its detoxification in early and late sown cultivars of wheat under future O3 concentration. Sci Total Environ 659:200–210
Yadav B, Jogawat A, Rahman MS, Narayan OP (2021) Secondary metabolites in the drought stress tolerance of crop plants: a review. Gene Rep 23:101040
Yang SH, Wang LJ, Li SH, Duan W, Loescher W, Liang ZC (2007) The effects of UV-B radiation on photosynthesis in relation to Photosystem II photochemistry, thermal dissipation and antioxidant defenses in winter wheat (Triticum aestivum L.) seedlings at different growth temperatures. Funct Plant Biol 34(10):907–917
Yao X, Liu Q (2006) Changes in morphological, photosynthetic and physiological responses of Mono Maple seedlings to enhanced UV-B and to nitrogen addition. Plant Growth Regul 50(2):165–177
Yin LL, Zhao C, Huang Y, Yang RY, Zeng QP (2008) Abiotic stress-induced expression of artemisinin biosynthesis genes in Artemisia annua L. Chin J Appl Environ Biol 14(1):1–5
Yu KW, Murthy HN, Hahn EJ, Paek KY (2005) Ginsenoside production by hairy root cultures of Panax ginseng: influence of temperature and light quality. Biochem Eng J 23(1):53–56
Yuan Y, Liu Y, Wu C, Chen S, Wang Z, Yang Z, Qin S, Huang L (2012) Water deficit affected flavonoid accumulation by regulating hormone metabolism in Scutellaria baicalensis Georgi roots. PLoS One 7(10):e42946
Zandalinas SI, Balfagón D, Gómez-Cadenas A, Mittler R (2022) Plant responses to climate change: metabolic changes under combined abiotic stresses. J Exp Bot 73(11):3339–3354
Zargar SM, Gupta N, Nazir M, Mahajan R, Malik FA, Sofi NR, Shikari AB, Salgotra RK (2017) Impact of drought on photosynthesis: Molecular perspective. Plant Gene 11:154–159
Zhang L, Wang Q, Guo Q, Chang Q, Zhu Z, Liu L, Xu H (2012) Growth, physiological characteristics and total flavonoid content of Glechoma longituba in response to water stress. J Med Plants Res 6(6):1015–1024
Zhao B, Wang L, Pang S, Jia Z, Wang L, Li W, Jin B (2020) UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind Crop Prod 151:112483
Złotek U (2017) Effect of jasmonic acid and yeast extract elicitation on low-molecular antioxidants and antioxidant activity of marjoram (Origanum majorana L.). Acta Scientiarum Polonorum Technologia. Alimentaria 16(4):371–377
Zobayed SMA, Afreen F, Kozai T (2007) Phytochemical and physiological changes in the leaves of St. John’s wort plants under a water stress condition. Environ Exp Bot 59(2):109–116
Zvereva EL, Kozlov MV (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a meta-analysis. Glob Chang Biol 12(1):27–41
Acknowledgments
P.S. is thankful to CSIR, New Delhi, India, for the Junior Research Fellowship (F. No.: 09/0013(12956)/2021-EMR-I). K.K.C. is grateful to UGC-BSR Start-up Grant (No. F. 30-432/2018(BSR)), New Delhi, India, and to the seed grant, Institute of Eminence (IoE—Dev. Scheme no. 6031), Banaras Hindu University, Varanasi, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, P., Choudhary, K.K. (2023). Impacts of Climate Alterations on the Biosynthesis of Defensive Natural Products. In: Kannaujiya, V.K., Sinha, R.P., Rahman, M.A., Sundaram, S. (eds) Photoprotective Green Pharmacology: Challenges, Sources and Future Applications. Springer, Singapore. https://doi.org/10.1007/978-981-99-0749-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-99-0749-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0748-9
Online ISBN: 978-981-99-0749-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)