Abstract
In the southeast of the Qinghai-Tibetan Plateau of China, Mono Maple is a common species in reforestation processes. The paper mainly investigated the changes in morphological, photosynthetic and physiological responses of Mono Maple seedlings to UV-B radiation, nitrogen supply and their combination. The experimental design included two levels of UV-B treatments (ambient UV-B, 11.02 KJ m−2 day−1; enhanced UV-B, 14.33 KJ m−2 day−1) and two nitrogen levels (0; 20 g N m−2 a−1)—to determine whether the adverse effects of UV-B on plants are eased by nitrogen supply. Enhanced UV-B caused a marked decline in growth parameters, net photosynthetic rate, and photosynthetic pigments, whereas it induced an increase in reaction oxygen species (hydrogen peroxide accumulation and the rate of superoxide radical production) and malondialdehyde content. Enhance UV-B also induced an increase in antioxidant compounds of Mono Maple, such as UV-B absorbing compounds, proline content, and activities of antioxidant enzymes (peroxidase, superoxide dimutase and catalase). On the other hand, nitrogen supply caused an increase in some growth parameters, net photosynthetic rate, photosynthetic pigments and antioxidant compounds (peroxidase, proline content and UV-B absorbing compounds), and reduced the content of reaction oxygen species (H2O2 accumulation, the rate of O − 2 production) and malondialdehyde content under ambient UV-B. However, under enhanced UV-B, nitrogen supply inhibited some growth parameters, and increased H2O2 accumulation, the rate of O − 2 production and MDA content, though proline content, UV-B absorbing compounds and activities of POD and SOD increased. These results implied that enhanced UV-B brought harmful effects on Mono Maple seedlings and nitrogen supply made plants more sensitive to enhanced UV-B, though increased some antioxidant activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In spite of the current efforts to restrict the production of ozone-depleting substances, thinning of the stratospheric ozone layer and increased penetration of ultraviolet-B radiation to the earth’s surface will continue for decades (De La Rose et al. 2001). Atmospheric ozone remains depleted and the annual average ozone loss is approximately 3% globally (Executive summary 2003). Enhanced UV-B radiation could induce over production of free radicals and result in oxidative stress eventually (Yu et al. 2004). The harmful effects of UV-B radiation on plants are often a consequence of reactive oxygen species (ROS) production (Strid et al. 1994).
In addition to UV-B radiation, Human activities have significantly altered the global nitrogen cycle, with the development of industry and agriculture. Ever increasing amount of nitrogen are imported into the terrestrial ecosystems through nitrogen deposition. In the European livestock and industrialized areas, nitrogen deposition is more than 25 kg N hm−2 a−1 (Binkley et al. 2000). In the north-eastern United States, the current nitrogen deposition is more than 10–20 times background nitrogen (Magill et al. 1997). At present, China is one of the three greatest nitrogen deposition regions (Li et al. 2003).
Nitrogen is the mineral nutrient needed in the largest amounts by plants and it is usually also the limiting factor for plant growth in terrestrial ecosystems (Vitousek and Howarth 1991), particularly in tundra, boreal as well as alpine ecosystems (Xu et al. 2003). In contrast to UV-B radiation, nitrogen addition improves plant growth, net photosynthesis and changes nutrient cycle in Crytomeria japonica and Pinus densiflora seedlings (Nakaji et al. 2001), and reduces production of free radicals in Coffea Arabica (Ramalho et al. 1998).
Both UV-B radiation and nitrogen supply are expected to increase simultaneously with changes of global climate in future. Nitrogen can affect UV-B response in plants (Pinto et al. 1999; Correia et al. 2005). Levizou and Manetas (2001) reported that supplemental UV-B radiation improved growth in Phlomis fruticosa at high nutrient level, whereas greater growth inhibition by UV-B has been reported in nitrate-replete than nitrate-deficient crop plants (Hunt and McNeil 1998). Tosserams et al. (2001) reported that photosynthetic rate of Plantaago lanceolata with high UV-B was not influenced by differential quantities of multiple mineral supply. Previous studies have mainly focused on crop and herb plants. However, only limited papers have been reported the effects of nitrogen and UV-B radiation on woody plants (De La Rose et al. 2001, 2003; Lavola et al. 2003).
Mono Maple (Acer Mono Maxim) is a common species in reforestation processes in the southeast of the Qinghai-Tibetan Plateau of China. This paper studies the short-term effects of UV-B and nitrogen supply on growth, photosynthetic and antioxidant responses of Mono Maple under open semi-field conditions—to know the relation between growth, photosynthesis and antioxidant compounds of Mono Maple under enhanced UV-B, nitrogen supply and their combination. This will be helpful for understanding of the combined effects on tree species, and the reforestation processes of forest under future changes in global climate. We hypothesize that nitrogen supply will ease the adverse effects of UV-B radiation on Mono Maple seedlings.
Materials and methods
Plant material and experimental design
The experiment was conducted in open semi-field conditions for one growing season in Maoxian Ecological Station of Chinese Academy of Sciences, Sichuan province, China (31°41′ N, 103°53′ E, 1820 m a.s.l.). Uniform 1-year-old Mono Maple (Acer Mono Maxim) seedlings from a local nursery were selected based on plant height, basal diameter and fresh mass. The average plant height, basal diameter and fresh mass were separately 50.4 ± 1.5 cm, 5.3 ± 0.5 mm, 13.5 ± 1.0 g. Seedlings were transplanted into plastic pots (25 × 35 cm), one seedling per pot. The substrate used for growing the seedlings was sieved topsoil from under a forest canopy. In a preliminary experiment, the plastic pots did not affect growth of seedlings root during a 2-year growth period.
The experiment consisted of four treatments: (1) ambient UV-B without additional nitrogen supply (control, C); (2) ambient UV-B with additional nitrogen supply (N); (3) enhanced UV-B without additional nitrogen supply (UV-B); (4) enhanced UV-B with additional nitrogen supply (UV-B + N). Each treatment had 3 blocks and each block had 10 pots. The pots within blocks were rotated approximately every 20 days.
UV-B treatments and nitrogen treatments
Supplementary UV-B was supplied by UV-B fluorescent lamps (Beijing Electronic Resource Institute, Beijing, China) mounted in metal frames with minimum shading. The distance from the lamps to the top of plant apex was 100 cm and kept constant throughout the experiment. In ambient UV-B frames, UV-B from the lamps was excluded by wrapping the tubes with 0.125 mm polyester film, which transmits UV-A. In enhanced UV-B frames, lamps were wrapped with 0.10 mm cellulose diacetate film, which transmits both UV-B and UV-A. Vertical polyester curtains were placed between the frames in order to prevent the UV-B radiation from reaching the control seedlings (De La Rose et al. 2003). Films were replaced every week and the lamps were replaced monthly. The spectral irradiance from the lamps was determined with an Optronics Model 742 (Optronics Laboratory Inc., Orlando, FL) spectroradiometer. The spectral irradiance was weighted according to the generalized plant action spectrum (Caldwell 1971) and normalized at 300 nm to obtain effective radiation (UV-BBE). The supplemental UV-BBE dose was 3.31 KJ m−2day−1 (a 30% difference in ambient UV-BBE) in addition to the effective 11.02 KJ m−2day−1 UV-BBE (ambient UV-BBE) from sky. All pots also received natural solar radiation. Seedlings were irradiated for 8 h daily centered on the solar noon.
Nitrogen was added as a 9.5 mM NH4NO3 solution (300 ml) to the potted soil surface ever 3 days. The treatment without additional nitrogen supply was watered with 300 ml water at the same interval. The nitrogen amount added to the soil was equivalent to 20 g N m−2 a−1 on the basis of soil surface area. Nitrogen amount was based on the similar studies (Bowden et al. 2004; Nakaji et al. 2001).
Growth parameters
Six randomly selected seedlings from each treatment were harvested, and then divided into leaf, stem and root. Roots were divided into coarse roots and fine roots, and fine roots were defined as those less than 2 mm in diameter. Roots were rinsed free of soil. Biomass samples were dried to constant mass. Leaf area for each seedling was determined by Portable Laser Area Meter (CI-203, CID Inc., USA). Fine root/coarse root ratio and specific leaf area (SLA, leaf area per unit leaf mass) were then calculated. Leaf thickness was measured with a photomicroscope fitted with a micrometer employing 25 semi-thin transverse sections cut from leaf material prepared for electron microscopy studies pertaining to the leaf from six different plants
Gas change and photosynthetic pigments
After 60 days of UV-B irradiation, on a cloudless day, CO2 exchange rates were measured with a portable photosynthesis system (LI-6400, Lincoln, NE, USA) in the open circuit mode. Atmospheric conditions consisted of photosynthetic photon flux density of 1,000 ± 50 μmol m−2 s−1 and leaf temperature was maintained at 25 ± 1.5°C. Measurements were made around midday. Net photosynthetic rate (A, μmol CO2 m−2 s−1), stomatal conductance (Gs, mmol m−2 s−1) and intercellular CO2 concentration (Ci, μmol mol−1) were measured.
The young fully expanded leaves were taken and ground in 80% acetone for determination of chlorophyll and carotenoid content. Total chlorophyll (Chl (a + b)), chlorophyll a (Chl a), chlorophyll b (Chl b) and total carotenoid (Car) content were determined according to Lichtenthaler (1987).
UV-B absorbing compounds, malondialdehyde and proline content
UV-B absorbing compounds in fully developed leaf were extracted with acidified methanol solution (methanol: water: HCl = 79: 20: 1, v/v/v). Samples were heated in a water bath (90°C) for one hour. The absorbance at 285 nm was recorded using a scanning spectrophotometer (Unicam UV-330, Thermo spectronic, England, UK).
The free proline concentration was determined according to the method described by Bates et al. (1973). The young fully expanded leaves (1 g) were homogenized in 5 ml of 3% sulfosalicylic acid and homogenate was centrifuged at 20,000 × g for 5 min. About 0.5 ml of the supernatant was incubated at 100°C for 60 min with 0.5 ml of glacial acetic acid and 0.5 ml of ninhydrin reagent. After termination of the reaction in an ice bath, the reaction mixture was extracted with 3 ml of toluene, and the absorbance was read at 520 nm.
The degree of lipid peroxidation was assessed by the amount of malondialdehyde (MDA). MDA content was determined by the thiobarbituric acid (TBA) reaction. The young fully expanded leaves (0.5 g) were homogenized with 5 ml of 20% trichloroacetic acid (TCA) and homogenate was centrifuged at 3,500 × g for 20 min. 2 ml of 20% TCA containing 0.5% TBA and 100 μl 4% butylated hydroxytoluene in ethanol were added to 2 ml of the supernatant. The mixture was heated at 95°C for 30 min and then quickly cooled on ice. The contents were centrifuged at 10,000 × g for 15 min and the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. MDA content was calculated using an extinction coefficient of 155 mM−1 cm−1. Results were expressed as μmol g FW−1.
The rate of superoxide radical production and hydrogen peroxide content
The rate of superoxide radical production (O −2 ) was measured as described by Ke et al. (2002), by monitoring the nitrite formation from hydroxyiamine in the presence of O −2 . The young fully expanded leaves (0.5 g) was homogenized with 1.5 ml of 65 mM potassium phosphate (pH 7.8) and centrifuged at 5,000 × g for 10 min. The incubation mixture contained 0.45 ml of 65 mM phosphate buffer (pH 7.8), 0.5 ml of 10 mM hydroxulamine hydrochloride, and 0.5 ml of the supernatant. After incubation at 25°C for 20 min, 8.5 mM sulfanilamide and 3.5 mM α-naphthylamine were added to the incubation mixture. After reaction at 25°C for 20 min, the absorbance in the aqueous solution was read at 530 nm. A standard curve with NO −2 was used to calculate the production rate of O −2 from the chemical reaction of O −2 and hydroxylamine.
Hydrogen peroxide (H2O2) content was determined according to Prochazkova et al. (2001). The youngest fully expanded leaves (0.5 g) were ground with 5 ml cooled acetone in a cold room (10°C). Mixture was filtered with filter paper followed by the addition of 2 ml titanium reagent and 5 ml ammonium solution to precipitate the titanium–hydrogen peroxide complex. The reaction mixture was centrifuged at 10,000 × g for 10 min. The precipitate was dissolved in 5 ml 2 M H2SO4 and than recentrifuged. Supernatants were read at 415 nm.
Antioxidant enzymes activity
Extracts for the determination of antioxidant enzymes activities were prepared from 1.0 g of fully development new leaves homogenized under ice-cold conditions in 3 ml of extraction buffer, containing 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP and 0.5 % (v/v) Triton X-100. The homogenates were centrifuged at 10,000 × g for 30 min and the supernatant was used for the assays.
Superoxide dimutase activity (SOD, EC 1.15.1.1) was assayed by the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT), as described by Becana et al. (1986). The reaction mixture consisted of 50 μl of enzyme extract and 3 ml O −2 generating mixture solution containing 50 mM potassium-phosphate (pH 7.8), 0.1 mM Na2 EDTA, 13 mM methionine, 75 μM NBT, and 16.7 μM riboflavin. Test tubes were shaken and placed 30 cm from light bank consisting of six 15-W fluorescent lamps. The reaction was allowed to run for 10 min and stopped by switching the light off. The reduction in NBT was followed by reading absorbance at 560 nm. Blanks and controls were run the same way but without illumination and enzyme, respectively. One unit of SOD was defined as the amount of enzyme, which produced a 50% inhibition of NBT reduction under the assay conditions (Costa et al. 2002).
Catalase activity (CAT, EC 1.11.1.6) activity was determined in the homogenates by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2), 10 mM H2O2 and 50 μl enzyme extract (Kato and Shimizu 1987). The activity was calculated using the extinction coefficient (40 mM−1 cm−1) for H2O2.
Ascorbate peroxidase activity (APX, EC 1.11.1.11) was assayed as described by Nakano and Asada (1981) using a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM H2O2, 0.5 mM ascorbate and 0.1 mM EDTA. The hydrogen peroxide-dependent oxidation of ascorbate was followed monitoring the absorbance decrease at 290 nm (extinction coefficient 2.8 mM−1 cm−1).
Peroxidase activity (POD, EC 1.11.1.7) was based on the determination of guaiacol oxidation (extinction coefficient 26.6 mM−1 cm−1) at 470 nm by H2O2. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 20.1 mM guaiacol, 12.3 mM H2O2, and enzyme extract in a 3 ml volume (Ekmekci et al. 2005).
Glutathione reductase activity (GR, EC 1.6.4.2) was measured by monitoring the decrease in absorbance at 340 nm (extinction coefficient 6.2 mM−1 cm−1) due to NADPH oxidation. The reaction mixture contained 50 μl enzyme extract, 1 mM EDTA, 0.5 mM GSSG, 0.15 mM NADPH and 50 mM Tris–HCl buffer (pH 7.5) (Costa et al. 2002).
Soluble protein contents were also determined as described by Bradford (1976), using bovine serum albumin as a calibration standard.
Statistical analysis
All data were subjected to an analysis of variance that tested the UV-B radiation, nitrogen and UV-B × nitrogen interaction effects, and the significance of the single factors calculated as well as the interaction between the factors calculated. All statistical analyses were performed using the Software Statistical Package for the Social Science (SPSS) version 11.0.
Results
The effects of enhanced UV-B and nitrogen addition on growth parameters
Increased UV-B significantly decreased plant height, total biomass, coarse roots mass, fine roots mass and fine root/coarse root ratio in Mono Maple seedlings (Table 1). The reduced ratio of fine root/coarse root indicated that the effects of enhanced UV-B on fine roots were more than those on coarse roots. On the other hand, nitrogen supply significantly increased plant height, total biomass, coarse roots mass, fine roots mass only under ambient UV-B, whereas it reduced coarse roots mass under enhanced UV-B. The ratio of fine root/coarse root was not affected by nitrogen supply. Moreover, the interactive effects of UV-B and nitrogen were detected on total biomass, coarse roots mass and fine roots mass.
Leaf morphology was significantly affected by enhanced UV-B (Table 2, Plate 1). Enhanced UV-B caused a reduction in total number of leaves, leaf mass and total leaf area. Nitrogen supply induced an increase in leaf mass and total leaf area under ambient UV-B, and reduced total number of leaves and total leaf area under enhanced UV-B. Specific leaf area (SLA) and leaf thickness was not affected by nitrogen supply and enhanced UV-B. A prominent UV-B × nitrogen interaction was observed on total number of leaves, leaf mass and total leaf area. Compared to enhanced UV-B alone, leaf morphological responses of plants were more sensitive to enhanced UV-B under nitrogen supply.
The effects of enhanced UV-B and nitrogen addition on photosynthetic parameters
Enhanced UV-B markedly reduced net photosynthetic rate (A) and stomatal conductance to water vapour (Gs), and increased intercellular CO2 concentration (Ci) of Mono Maple (Fig. 1A, B, C). Net photosynthetic rate and stomatal conductance to water vapour of plants grown at ambient UV-B were increased by nitrogen supply. A prominent UV-B × nitrogen interaction was observed on stomatal conductance to water vapour and intercellular CO2 concentration. The reduction in net photosynthetic rate and stomatal conductance to water vapour by enhanced UV-B was more marked under nitrogen supply than that under without additional nitrogen supply.
The effects of enhanced UV-B and nitrogen addition on leaf morphology of Mono Maple seedlings. C, ambient UV-B without additional nitrogen supply; N, ambient UV-B with additional nitrogen supply; UV-B, enhanced UV-B without additional nitrogen supply; UV-B+N, enhanced UV-B with additional nitrogen supply
Net photosynthetic rate (A), stomatal conductance to water vapour (B) and intercellular CO2 concentration (C) of Mono Maple seedlings affected by enhanced UV-B and nitrogen addition. C, ambient UV-B without additional nitrogen supply; N, ambient UV-B with additional nitrogen supply; UV-B, enhanced UV-B without additional nitrogen supply; UV-B + N, enhanced UV-B with additional nitrogen supply. The bars with different letters are significantly different from each other (P < 0.05). Values are means of six replicates ± SE. F N, nitrogen effect; F UV-B, UV-B radiation effect; F UV-B × N, interaction effect of UV-B and nitrogen
Enhanced UV-B markedly reduced Chl a, Chl b, Chl (a + b) and carotenoid content. A parallel decrease in Chl a and Chl b resulted in no significant change in Chl a/b ratio under enhanced UV-B (Table 3). On the other hand, Chl a, Chl b and Chl (a + b) content of plants grown at ambient UV-B were enhanced by nitrogen supply, but they were not influenced by nitrogen supply under enhanced UV-B (Table 3). The ratio of Chl a/b was reduced by nitrogen supply, suggesting that the effects of nitrogen on Chl b were more than those on Chl a. Nitrogen supply did not induce the changes of carotenoid content. Significant interactive effects of UV-B and nitrogen were also detected on Chl a, Chl b, total Chl (a + b) content and carotenoid content.
The effects of enhanced UV-B and nitrogen addition on ROS and MDA content
Figure 2 showed that enhanced UV-B induced an increase in H2O2 accumulation, the rate of O −2 production and MDA content. H2O2 content, the rate of O −2 production and MDA content were reduced by nitrogen supply under ambient UV-B, whereas they were significantly increased under enhanced UV-B (Fig. 2A, B and C). Significant interaction between UV-B × nitrogen was also found on H2O2 content, the rate of O −2 production and MDA content. The responses of H2O2 content, the rate of O −2 production and MDA content to enhanced UV-B were greater under nitrogen supply.
H2O2 content (A), the rate of O −2 production (B) and MDA content (C) of Mono Maple seedlings affected by enhanced UV-B and nitrogen addition. C, ambient UV-B without additional nitrogen supply; N, ambient UV-B with additional nitrogen supply; UV-B, enhanced UV-B without additional nitrogen supply; UV-B + N, enhanced UV-B with additional nitrogen supply. The bars with different letters are significantly different from each other (P < 0.05). Values are means of six replicates ± SE. F N, nitrogen effect; F UV-B, UV-B radiation effect; F UV-B × N, interaction effect of UV-B and nitrogen. H2O2, hydrogen peroxide; O −2 , superoxide radical; MDA, malondialdehyde
The effects of enhanced UV-B and nitrogen addition on UV-B absorbing compounds and proline content
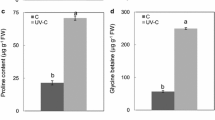
Both enhanced UV-B and nitrogen supply significantly increased UV-B absorbing compounds and proline content respectively (Fig. 3A and B). The effects of UV-B radiation × nitrogen interaction were not found on UV-B absorbing compounds and proline content.
UV-B absorbing compounds (A), proline content (B) of Mono Maple seedlings affected by enhanced UV-B and nitrogen addition. C, ambient UV-B without additional nitrogen supply; N, ambient UV-B with additional nitrogen supply; UV-B, enhanced UV-B without additional nitrogen supply; UV-B + N, enhanced UV-B with additional nitrogen supply. The bars with different letters are significantly different from each other (P < 0.05). Values are means of six replicates ± SE. F N, nitrogen effect; F UV-B, UV-B radiation effect; F UV-B × N, interaction effect of UV-B and nitrogen
The effects of enhanced UV-B and nitrogen addition on antioxidant enzymes
Enhanced UV-B significantly increased activities of POD, SOD and CAT (Table 4). On the other hand, nitrogen supply increased POD activity under ambient UV-B, and increased activities of POD and SOD under enhanced UV-B. Moreover, activities of APX and GR did not significantly affected by enhanced UV-B or nitrogen supply. Significant interaction between nitrogen and UV-B radiation was not found on activities of five antioxidant enzymes studied in the paper.
Discussion
The effects of enhanced UV-B on Mono Maple
The effects of enhanced UV-B on plants were generally detrimental, e.g., reducing growth and biomass (Sullivan and Teramura 1992; De La Rose et al. 2003), and affecting biomass partition and root/shoot ratio (Yang et al. 2005). In our study, enhanced UV-B significantly reduced total biomass by 55% (without nitrogen supply) and 59% (with nitrogen supply) separately (Table 1). This may be due to the decrease of total leaf area that limited the photosynthesis as shown here and in previous studies (Antonelli et al. 1997). The reduced rate of fine root/coarse root showed that fine roots were more sensitive to enhanced UV-B than coarse roots.
The reduced growth was closely related to a strong reduction in photosynthesis under enhanced UV-B (Kulandaivelu et al. 1989). The negative effects of UV-B on photosynthetic processes have been demonstrated in earlier publication (Allen et al. 1998; Correia et al. 2005). As demonstrated here, Correia et al. (2005) reported that intense UV-B reduced net photosynthetic rate, stomata conductance to water vapour and increased the intercellular CO2 concentration of maize. Intercellular CO2 concentration is a reliable indication to estimate the changes of net photosynthesis rate from stomatal limitation or non-stomatal limitation (Farquhar and Sharkey 1982). Our results further confirm that the reduction in photosynthesis of high UV-B treated plants was more closely related to non-stomata than to stomata limitations.
The reduction in photosynthetic pigments may play an important role in reduced photosynthetic rate under enhanced UV-B. A decrease of total chlorophyll and carotenoid content of plants grown at high UV-B was observed (Table 3). Similar results were also reported in previous publications (Casati et al. 2001; Correia et al. 2005). The decrease of total chlorophyll content may be due to the decrease of carotenoid content, since carotenoid protects chlorophyll from photooxidative destruction (Singh 1996). Carotenoid is the quenching agent of short wave radiation with high energy and could exert their protective function as antioxidants to inactivate UV-B-induced radicals in the photosynthetic membrane (Götz et al. 1999). The decrease in carotenoid content suggested that enhanced UV-B caused considerable oxidative stress by accumulation of ROS (Fig. 2A and B).
Proline metabolism is a typical mechanism of biochemical adaptation in living organisms subjected to stress conditions (Delauney and Verma 1993). Though mainly involved in the water-stress syndrome, proline reportedly accumulated in the shoots of rice, mustard and mung bean seedlings exposed to UV radiation (Saradhi et al. 1995). Stress-related alterations in proline metabolism may impinge on systems of redox control of plant gene expression (Hare and Cress 1997), which may protect plant cells against peroxidative processes (Saradhi et al. 1995). On the other hand, UV-B absorbing compounds could protect plants from the harm of UV-B radiation by reductions in the transmittance of UV photons through leaf tissue (Day and Neale 2002). In our study on Mono Maple, enhanced UV-B significantly increased proline content and UV-B absorbing compounds. This may provide an ecological adaptation for young seedlings by enhanced defense substance content under stress conditions.
It is generally accepted that the mechanism of UV-B toxicity involves oxidative damage (Pruvot et al. 1996; Mackerness et al. 1999; Du and Jin 2000; Santos et al. 2004). In our study, enhanced UV-B significantly increased ROS and MDA content in leaves of Mono Maple (Fig. 2). In order to balance and control the oxygen toxicity, plants have developed antioxidative systems. SOD, APX, POD, GR and CAT are important enzymes in plants that protect plants against oxidative damage. Enhanced UV-B induced the activities of POD, CAT and SOD as the increasing requirement of screening ROS (Table 4). ROS induced antioxidant gene expression to confer plants tolerance to survive stressful conditions.
The effects of nitrogen addition on Mono Maple
Nitrogen supply accelerates some growth parameters of Mono Maple seedlings under ambient UV-B. This agrees well with the results of earlier studies (Deckmyn and Impens 1997), however, some growth parameters were inhibited by nitrogen supply under enhanced UV-B. This indicated that the effects of high UV-B on growth completely overshadowed effects of nitrogen supply, whereas nitrogen supply increased for growth, morphological and physiological responses of Mono Maple to ambient UV-B.
Nitrogen is closely related to photosynthesis in plants. Being on an important constituent of photosynthetic apparatus (Correia et al. 2005), net photosynthetic rate and chlorophyll content were stimulated by nitrogen supply under ambient UV-B. In the contrast, nitrogen supply did not affect the sensitivity of net photosynthesis rate and chlorophyll content to high UV-B in our study, suggesting that enhanced UV-B shield the influence of nitrogen supply on photosynthetic parameters.
Nitrogen supply evidently increased proline content of Mono Maple leaves (Fig. 3). Proline accumulation induced by nitrogen supply may be because it is a nitrogen-storage compound (Ahmad and Hellebust 1988) and that synthesis and accumulation of proline are simulated by nitrogen supply (Sánchez et al. 2002). Nitrogen supply also increased UV-B absorbing compounds, this response offered supplemental protection of plants to UV-B radiation.
Nitrogen supply reduced H2O2 content, the rate of O −2 production and MDA content under ambient UV-B. This may be related to the increase of antioxidant enzymes activities under nitrogen supply (Table 4), since nitrogen nutrition could improve synthesis and physiological activities of antioxidant enzymes. In contrast, nitrogen nutrition could reduce deoxidization capacity, which would lead to the reduced rate of ROS production and the less ROS accumulation (Xiao et al. 1998). However, H2O2 content, the rate of O −2 production and MDA content of plants grown at enhanced UV-B were increase by nitrogen supply (Fig. 2). This demonstrates that nitrogen supply made plants more sensitive to enhanced UV-B, though increased some antioxidant activity.
In conclusion, enhanced UV-B led to a marked decline in growth, net photosynthetic rate, and photosynthetic pigments, whereas it induced an increase in reaction oxygen species and MDA content, although enhance UV-B caused an increase in antioxidant compounds of Mono Maple seedlings. Nitrogen supply improved for the growth of plants under ambient UV-B, as could be seen by the increase of growth parameters, net photosynthetic rate, photosynthetic pigments and antioxidant compounds, and reduction in the content of reaction oxygen species and malondialdehyde content. However, nitrogen supply makes Mono Maple seedlings more sensitive to enhanced UV-B, though some antioxidant compounds increased. Obviously, nitrogen supply could not ease the harmful effects of high UV-B on plants, but aggravated the harm on plants.
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione redutase
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- O −2 :
-
Superoxide radical
- POD:
-
Peroxidase
- ROS:
-
Reaction oxygen species
- SOD:
-
Superoxide dimutase
References
Ahmad I, Hellebust JA (1988) The relationship between inorganic nitrogen metabolism and proline accumulation in osmoregulatory responses of two euryhaline microalgae. Plant Physiol 88:348–354
Allen DJ, Nogués S, Baker NR (1998) Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot 49:1775–1788
Antonelli F, Grifoni D, Sabatini F, Zipoli G (1997) Morphological and physiological responses of bean plants to supplemental UV radiation in a Mediterranean climate. Plant Ecol 128:127–136
Bates LS, Waldren RP, Teare IK (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Becana M, Aparicio-Tejo P, Irigoyen JJ, Sánchez-Díaz M (1986) Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 82:1169–1171
Binkley D, Son Y, Valentine WD (2000) Do forests receive occult inputs of nitrogen? Ecosystems 3:321–331
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manage 196:43–56
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caldwell MM (1971) Solar ultraviolet radiation and the growth and development of higher plants In: Giese AC (ed) Phytophysiology Academic Press, New York, pp 131–177
Casati P, Lara MV, Andreo CS (2001) Regulation of enzymes involved in C4 photosynthesis and the antioxidant metabolism by UV-B radiation in Egeria densa, a submersed aquatic species. Photosynthesis Res 71:251–264
Correia CM, Moutinho-Pereira JM, Coutinho JF, Björn LO, Torres-Pereira JMG (2005) Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: a Mediterranean field study. Eur J Agron 22:337–347
Costa H, Gallego SM, Tomaro ML (2002) Effects of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci 162:939–945
Day TA, Neale PJ (2002) Effects of UV-B radiation on terrestrial and aquatic primary producers. Ann Rev Ecol Syst 33:371–396
De La Rose TM, Aphalo PJ, Lehto T (2003) Effects of ultraviolet-B radiation on growth, mycorrhizas and mineral nutrition of silver birch (Betula pendula Roth) seedlings grown in low-nutrient conditions. Global Change Biol 9:65–73
De La Rose TM, Julkunen-Tiitto R, Lehto T, Aphalo PJ (2001) Secondary metabolites and nutrient concentrations in silver birch seedlings under five levels of daily UV-B exposure and two relative nutrient addition rates. New Phytologist 150:121–131
Deckmyn G, Impens I (1997) Combined effects of enhanced UV-B radiation and nitrogen deficiency on the growth, composition and photosynthesis of rye (Secale cereale). Plant Ecol 128:235–240
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmo-regulation in plants. Plant J 4:215–223
Du YJ, Jin YH (2000) Effect of far ultraviolet radiation on lipid peroxidation and inherent protection system in seedlings of Taxus cuspidata, Chin. J Appl Ecol 11:660–664 (in Chinese)
Ekmekci Y, Terzioglu S (2005) Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic Biochem Physiol 83:69–81
Executive summary (2003) Environmental effects of ozone depletion and its interactions with climate change: 2002 assessment. Photochem Photobiol Sci 2:1–4
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. J Anne Rev Plant Physiol 33:317–345
Götz T, Windhövel U, Böger P, Sandmann G (1999) Protection of photosynthesis against ultraviolet-B radiation by carotenoids in transformants of the Cyanobacterium Synechococcus PCC7942. Plant Physiol 120:599–604
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plant. Plant Growth Regul 21:79–102
Hunt JE, McNeil DL (1998) Nitrogen status affects UV-B sensitivity of cucumber. Aust J Plant Physiol 25:79–86
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Ke D, Wang A, Sun G, Dong L (2002) The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot Sin 44:551–556
Kulandaivelu G, Margatham S, Nedunchezhian N (1989) On the possible control of ultraviolet-B induced response in growth and photosynthetic activities in higher plants. Physiol Plant 76:398–404
Lavola A, Aphalo PJ, Lahti M, Julkunen-Tiitto R (2003) Nutrient availability and the effect of increasing UV-B radiation on secondary plant compounds in Scots pine. Environ Exp Bot 49:49–60
Levizou E, Manetas Y (2001) Combined effects of enhanced UV-B radiation and additional nutrients on growth of two Mediterranean plant species. Plant Ecol 154:181–186
Li DJ, Mo JM, Fang YT, Pen SL, Gundersen P (2003) Impact of nitrogen deposition on forest plants. Acta Ecologica Sinica 23:1891–1900 (in Chinese)
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mackerness SAH, Jordan BRand Thomas B (1999) Reactive oxygen species in the regulation of photosynthetic genes by ultraviolet-B radiation (UV-B: 280–320 nm) in green and etiolated buds of pea (Pisum sativum L.). J Photochem Photobiol B: Biol 48:180–188
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7:402–415
Nakaji T, Fukami M, Dokiya Y, Izuta T (2001) Effects of high nitrogen load on growth, photosynthesis and nutrient status of Cryptomeria japonica and Pinus desiflora seedlings. Trees 15:453–461
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Pinto ME, Casati P, Hsu TP, Ku MS, Edwards GE (1999) Effects of UV-B radiation on growth, photosynthesis, UV-B absorbing compounds and NADP-malic enzyme in bean (Phaseolus vulgaris L.) grown under different nitrogen conditions. J Photochem Photobiol B 48:200–209
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Pruvot G, Masimino J, Peltier G, Rey P (1996) Effects of low temperature, high salinity and exogenous ABA on the synthesis of two chloroplastic drought-induced proteins in Solanum tuberosum. Plant Physiol 97:123–131
Ramalho JC, Campos PS, Teixeria M, Nunes MA (1998) Nitrogen dependent changes in antioxidant system and in fatty acid composition of chloroplast membranes from Coffea Arabica L. plants submitted to high irradiance. Plant Sci 135:115–124
Sánchez E, Ruiz JM, Romero L (2002) Proline metabolism in response to nitrogen toxicity in fruit of French bean plants. Sci Hortic 93:225–233
Santos I, Fidalgo F, Almeida JM (2004) Biochemical and ultrastructural changes in leaves of potato plants grown under supplementary UV-B radiation. Plant Sci 167:925–935
Saradhi PP, Alia SA, Prasad KV (1995) Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Commun 209:1–5
Singh A (1996) Growth, physiological, and biochemical responses of three tropical legumes to enhanced UV-B radiation. Can J Bot 74:135–139
Strid A, Chow WS, Anderson JM (1994) UV-B damage and protection at the molecular level in plants. Photosynth Res 39:475–489
Sullivan JH, Teramura AH, Ziska LH (1992) Variation in UV-B sensitivity in plants from a 3000 m elevational gradient in Hawaii. Am J Bot 79:737–743
Tosserams M, Smet J, Magendans E, Rozema J (2001) Nutrient availability influences UV-B sensitivity of Plantago lanceolata. Plant Ecol 154:159–168
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in sea. How can it occur? Biogeochemistry 13:87–115
Xiao K, Zhang RX, Qian WP (1998) The physiological mechanism of senescence and photosynthetic function decline of flag leaf in wheat regulated by nitrogen nutrition. Plant Nutr Fertil Sci 4: 371–378 (in Chinese)
Xu XL, Ou YH, Pei ZY, Zhou CP (2003) Fate of N15 labeled nitrate and ammonium salts added to an alpine meadow in the Qinghai-Xizang Plateau. Acta Bot Sin 45:276–281 (in Chinese)
Yang YQ, Yao YA, Xu G, Li CY (2005) Growth and physiological responses to drought and elevated ultraviolet-B in two contrasting populations of Hippophae Rhamnoides. Physiol Plant 124:431–440
Yu J, Tang XX, Zhang PY, Tian JY, Cai HJ (2004) Effects of CO2 enrichment on photosynthesis, lipid peroxidation and activities of antioxidative enzymes of platymonas subcordiformis subjected to UV-B radiation stress. Acta Botanica Sinica 46:682–690 (in Chinese)
Acknowledgements
During this work the senior author was supported by the National Natural Science Foundation of China (No. 30530630), the Talent Plan of the Chinese Academy of Sciences and “Knowledge Innovation Engineering” of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, X., Liu, Q. Changes in morphological, photosynthetic and physiological responses of Mono Maple seedlings to enhanced UV-B and to nitrogen addition. Plant Growth Regul 50, 165–177 (2006). https://doi.org/10.1007/s10725-006-9116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-006-9116-4