Abstract
The use of plants and their products as sources of antioxidants to enhance health and food preservation is currently of great interest. Many plant species, especially those belonging to the Lamiaceae family have exhibited strong antioxidant activity. Salvia macrosiphon is an endemic medicinal plant in Iran, belonging to Lamiaceae family and has many pharmaceutical properties. This study was carried to evaluate the effects of different concentrations of sodium chloride [0.4 (control), 2.3, 4.5 and 6.8 dSm−1] on growth, total phenolic content and antioxidant activity of S. macrosiphon plants. Results showed that salinity stress affects plant growth by changing plants’ both fresh and dry weights. In addition, although total phenolic content in S. macrosiphon plants decreased with salinity, there was an increase in leaf antioxidant content. The absence of correlation between phenolic contents present in S. macrosiphon plants and antioxidant activity could be explained by the fact that other antioxidant molecules are also synthesized under stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of plants and their products as sources of antioxidants to enhance health and food preservation is of great current interest (Rice-Evans et al. 1997). Epidemiological studies have suggested positive associations between the diet rich in fruits and vegetables and disease prevention (Scalbert and Williamson 2000). These health promoting effects have been attributed to the antioxidant-active components present in such diet (Kaur and Kapoor 2001). Antioxidants are known to act as free radical scavengers and their decomposition resulting in the suppression of some disorders (Halliwell 2000). However, there are concerns about the use of synthetic antioxidants, because of their instability and their possible carcinogenic effects (Namiki 1990). Consequently, in recent years, there has been a growing interest in the studies of natural health substances and additives (Tomaino et al. 2005) as the potential antioxidant sources. The synthesis of natural antioxidants and their accumulation in plants are generally stimulated in response to abiotic stresses such as salinity (Shannon et al. 1994). Indeed, when plants are subjected to environmental stresses such as salinity, reactive oxygen species (ROS) are generated in response to stress conditions (Dat et al. 2000). The negative effect at the various environmental stresses is at least partially due to the generation of reactive oxygen species and/or inhibition of the system which defends against them (Asada 1997). Salinity stress also limits plant growth by adversely affecting various physiological and biochemical processes like photosynthesis, antioxidant activity, nitrogen metabolism and ion homeostasis (Misra et al. 2006). To control the levels of reactive oxygen species and to protect the cells under stress conditions, plant tissues contain several reactive oxygen species scavenging enzymes and a network of low molecular weight antioxidants such as ascorbate, glutathione, phenolic compounds and tocopherols (Blokhina et al. 2003).
Because of the difficulty in detecting the activity of each antioxidant component separately, several methods have been developed for determining the total antioxidant activity (TAA) of fruits, vegetables and foods (Wang et al. 1997; Benzie and Strain 1997). Moreover, due to interferences by the processes of synergisms and antagonisms among different antioxidants, the sum of antioxidant activities might not reflect the total antioxidant potential of the tissues under study. For these reasons, the concept of total antioxidant activity has been introduced (Pellegrini et al. 1999).
Many plant species, especially those belonging to the Lamiaceae family have been shown to possess strong antioxidant activities (Hirasa and Takemasa 1998). Salvia macrosiphon Boiss is one of the most important members of the Lamiaceae family which belongs to genus Salvia. It is a perennial, herbaceous and strongly aromatic (lemon-scented) plant which is endemic to Iran. The GC-mass analyses of S. macrosiphon essential oils have been shown to contain: linalool (26.3 %), hexylhexanoate (9.6 %), hexyl isovalerate (9.3 %), hexyl-2-methyl-butanoate (8.9 %), sclareol (7.2 %) and hexyl octanoate (6.1 %) as the major compounds in this plant (Javidnia et al. 2005). S. macrosiphon is generally known for its multiple pharmacological effects including its analgesic and anti-inflammatory activities (Karami et al. 2012). Moreover, some studies have reported a powerful antimicrobial property (Vahdani et al. 2011), inhibitory effects on the tumor cell lines (Amirghofran et al. 2010) and strong antioxidant activity (Gohari et al. 2011) of the essential oils and extracts of this plant. Several genus of Lamiaceae family such as salvia species are used in folk medicine as antiseptics, astringents and spasmolytics. Many studies have indicated the antioxidant, antimicrobial and antiviral activities of some Salvia species extract (Yamini et al. 2007). Although there are reports on the medicinal properties of S. macrosiphon, to the best of our knowledge, no studies have been done on the effects of salt stress on antioxidant activities of this plant under environmental stresses.
The present study was undertaken for the first time to investigate the effects of salinity stress on growth, total phenolic content and antioxidant activity of this plant. It is hoped that results obtained may be useful to both plant breeders and growers of this very important medicinal plant.
2 Materials and Methods
2.1 Experimental
Experiments were carried out in the research greenhouse of Biology Department, College of Sciences, Shiraz University, Shiraz, Iran. Experiments lasted for 7 months from December 2012 to June 2013. Seeds of S. macrosiphon were kindly provided by Research Center for Agriculture and Natural Resources, Shiraz, Iran. To break the seed dormancy, they were soaked in boiling water for 10 min and then placed in Petridishes moistened with distilled water and kept in refrigerator (4 °C) 7 days Seeds were then sown in plastic pots containing silt and powdered leaves (1:2) and were allowed to grow in the greenhouse with the mean day/night temperature and relative humidity of 29 ± 4/17 ± 2 °C, and 38 ± 5/50 ± 5 % respectively. Sixty days after seed germination, uniform seedlings with two nodes and four opposite leaves were transplanted into big plastic pots (30 × 50 cm). Each pot was filled with 10 kg of air-dried soil and two seedlings were used per pot. Physical and chemical properties of the soil used are presented in Table 1.
2.2 Irrigation
Eight weeks after transplanting, plants were subjected to different levels of salinity supplied with irrigated water. To prevent osmotic shock to seedlings, salt solutions were added gradually at several stages lasting 3 weeks. To keep the levels of soil salt concentration constant, distilled water was used in subsequent irrigations. At the end of salt treatments, total soil salinities, including control, were determined by EC meter (0.40, 2.3, 4.5, 6.8 dSm−1). Salt stress symptoms (leaf tip chlorosis and necrosis) in plants treated with high salt concentrations appeared after 3 weeks. At this time, the seedlings were harvested.
2.3 Growth Parameter Measurements
Plants were weighed individually for their fresh weight and then placed in Whatman paper bags. The bags were kept for 72 h in an oven set at 70 °C and shoot and root dry weights were determined by analytical balance.
2.4 Total Phenolic Extraction
Harvested leaves were dried at room temperature for 1 week. The leaves were extracted by stirring 200 mg of dry leaf powder in 2 mL methanol–acetic acid (85:15 v/v). The extracts were kept at −18 °C in the dark. After 24 h, extracts were sonicated for 15 min and centrifuged at 10,000×g in refrigerated centrifuge for 20 min. The supernatants were removed; n-Hexane was added (1:1 v/v) and mixed thoroughly. The mixture was centrifuged at 10,000×g in a refrigerated centrifuge for 20 min. The polyphenol fraction (lower phase) was removed, filtered through 0.2 μm membrane filter and stored at −18 °C for future analysis (Justesen et al. 1998).
2.5 Total Phenolics Measurement
Total phenolic content was determined by the Folin–Ciocalteu method (Singleton and Rossi 1965). Two hundred microliters of diluted samples was added to 1 mL of 1:10 diluted Folin–Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min and then 800 µL of 7 % Na2CO3 solution was added. After incubation for 90 min at 23 °C, the solutions absorbance was read at 760 nm by microplate reader, model BioTek ELx808. Solutions of gallic acid (0–500 mg/L) were used to prepare the standard curves. Results were expressed as milligram gallic acid equivalent (mg AGE)/g leaf dry weight.
2.6 Antioxidant Activities
Harvested leaves were dried at room temperature for 1 week. Leaf extracts were obtained by stirring 1 g of dried-leaf powder with 10 mL pure methanol for 30 min. The extracts were then kept for 24 h at 4 °C, filtered through Whatman No. 4 filter papers, the filtrates were dried using rotary evaporator at 45 °C and stored at 4 °C for later analysis.
2.6.1 1, 1-Diphenyl-2-picrylhydrazyl (DPPH˚) Scavenging Activity
The effect of methanolic extracts on DPPH˚ degradation was estimated according to (Hanato et al. 1988; Bruits et al. 2001). The dried plant extracts were dissolved in pure methanol at different rates (1, 3.2, 6.25, 12.5, 25, 50, 100, 200, 400, 800, 1600, 3200 µg mL−1) and then 20 µL of these solutions was added to 200 µL of a 100 mmol L−1 DPPH˚ methanolic solution. The mixture was shaken vigorously and left standing at room temperature for 30 min. The absorbance of the resulting solutions was then read at 517 nm after 30 min by microplate reader model BioTek ELx808. The free radical scavenging activities of the solutions were expressed as IC50 (mg mL−1), that is the antiradical dose required to cause a 50 % inhibition. A lower IC50 value corresponds to a higher antioxidant activity of the plant extract (Patro et al. 2005). The potential of extracts to scavenge DPPH˚ radicals was calculated using the following equation:
DPPH radical scavenging activity (%) = 100 − [(Absorbance of sample − Absorbance of blank) × 100/Absorbance of control]. Methanol (200 µl) plus extracting solution (20 µl) was used as blank, while DPPH solutions plus methanol were used as negative control. The positive control was DPPH solution plus different concentrations of gallic acid. The free radical scavenging activities of the solutions were expressed as IC50 (mg mL−1) which was calculated graphically using different concentrations of samples versus DPPH inhibition percentage.
2.7 Statistical Analysis
Three replications were used for each treatment. Data were expressed as means. The means were compared using the one-way and multivariate analysis of variances (ANOVA) followed by Duncan’s multiple range tests. The differences between individual means were deemed to be significant at P < 0.05.
3 Results
3.1 Effect of Salt Stress on Plant Growth
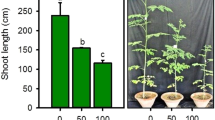
The rates of plant growth were adversely affected by the increase in external NaCl concentration (Table 2). However, the root fresh weights were not affected by external salt concentrations significantly. At external NaCl of 6.8 dSm−1, the root dry weight decreased by 40 % as compared to control plants. The shoot fresh weight was decreased by 17 and 58 % at the external NaCl salinity of 4.5 and 6.8 dSm−1 respectively. However, salinity did not affect shoot dry weights significantly as compared with control plants and those grown under moderate salinity (2.5 dSm−1).
3.2 Effect of Salt Stress on Total Phenolic Contents
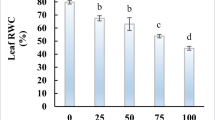
The absorbance values of the plant extract solutions reacted with Folin–Ciocalteu reagent in comparison with those of gallic acid standard solutions are shown in Fig. 1. Salt treatments decreased leaf total phenolic compounds significantly. At 6.8 dSm−1 NaCl, total phenolics were 3.76 mg AGE g−1 DW which was reduced by 2.6 times as compared to control leaves (Fig. 1).
3.3 Effect of Salt Stress on Leaves Antioxidant Activity
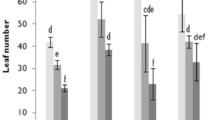
The effects of salt stress on leaf extracts antioxidant activity were determined by DPPH free radical scavenging activity. The leaves of S. macrosiphon plants exhibited a significant increase in antioxidant activity under salt stress. At 4.5 dSm−1 NaCl, leaf extracts with the IC50 value of 7.20 mg mL−1 displayed the highest free radicals quenching activity as compared to other treatments (Fig. 2).
4 Discussion
Salinity stress limits plant growth and development by adversely affecting various physiological and biochemical processes like photosynthesis, antioxidant activity, nitrogen metabolism and ion homeostasis (Misra et al. 2006). Our results revealed that plants treated with low salt concentrations (2.3 dSm−1) did not exhibit significant changes in their growth parameters as compared to control. When exposed to moderate salinity (4.5 dSm−1), they showed a decrease in biomass production. However, high salinity stress (6.8 dSm−1) limited the growth drastically by affecting both plant dry and fresh weights. Nevertheless, this Salvia species was able to survive at this NaCl concentration. Our results are similar to those reported by Ben Taarit et al. (2009) who reported a reduction in biomass production in Salvia officinalis when exposed to 50 and 75 mM NaCl.
Since the important roles of antioxidant molecules in the detoxification of free radicals are well recognized, nutritionist, clinical researchers and various segments of food and pharmaceutical industries have an increasing interest in determining the antioxidant potential of physiological fluids, foods and natural products (Halliwell 2000). During salinity stress, plants protect themselves against oxidative damages caused by ROS using both enzymatic and non-enzymatic antioxidant mechanisms (Ardic et al. 2009). Among non-enzymatic antioxidants, plant tissues contain a network of low-molecular mass antioxidants such as ascorbate, glutathione, phenolic compounds and tocopherols whose production could be induced by environmental stresses such as salinity (Blokhina et al. 2003). In the present study, our results showed that NaCl salinity increased total antioxidant activity in leaf methanolic extract (Fig. 2). However, salinity caused a marked reduction in the total phenolic content in all NaCl treatments. The major cause of total phenolic oxidation in S. macrosiphon plants can be due to increased activity of peroxidase (POD) under salt stress conditions (Tarchoune et al. 2010; Tarchoune et al. 2012a, b). On the other hand, the lack of correlation between total phenolic contents in S. macrosiphon plants and antioxidant activity could be explained by the fact that the levels of single antioxidant molecules do not necessarily reflect their total antioxidant activity, which depends on the synergistic and redox interactions among the different antioxidant molecules present in S. macrosiphon (Tarchoune et al. 2012a, b). The present results obtained for S. macrosiphon were in line with those findings by Tarchoune et al. (2012a, b) who reported for Ocimum basillcum L. plants under salinity treatments. These authors found that although the major phenolic acids in O. basillcum decreased with salinity, an increase in total antioxidant activity caused by the increase in glutathione and ascorbic acid content under the same conditions was observed. In conclusion, the use of moderate salinity levels can be an effective method in making S. macrosiphon a superior pharmaceutical asset. These facts should be taken into consideration in the economic cultivation of this valuable medicinal plant. Further study will be needed to determine the effects of salinity on the physiological and biochemical processes of the S. macrosiphon plants at the reproductive and vegetative stages.
Abbreviations
- DPPH:
-
1, 1-Diphenyl-2-picrylhydrazyl
- AGE:
-
Gallic acid equivalent
References
Amirghofran ZFZ, Javidnia K, Miri R (2010) The cytotoxic activity of various herbals against different tumor cells: an in vitro study. Iran Red Crescent Med J 12(3):260–265
Ardic M, Sekmen AH, Tokur S (2009) Antioxidant responses of chickpea plants subjected to boron toxicity. Plant Biol 11:228–328
Asada A (1997) The role of ascorbate peroxidase and mono dehydroascorbate reductase in H2O2 scavenging in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, New York, pp 715–735
Ben MT, Msaada K, Hosni K, Marzouk B (2009) Changes in fatty acid and essential oil composition of sage (Salvia officinalis L.) leaves under NaCl stress. Food Chem 119:951–956
Benzie IFF, Strain JJ (1997) Ferric reducing antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modify ed version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Blokhina O, Virolainen E, Fagerstedt K (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bruits M, Asres K, Bucar F (2001) The antioxidant activity of the essential oils of Artemisia Afra, Artemisia byssinica and juniperusprocera. Phytother Res 15:103–108
Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Gohari AR, Ebrahimi H, Saeidnia S, Foruzani M, Ebrahimi P, Ajani Y (2011) Flavones and flavone glycosides from Salvia macrosiphon Boiss. Iran J Pharm Res 10(2):247–251
Halliwell B (2000) The antioxidant paradox. The Lancet 355:1179–1180
Hanato H, Kagawa T, Yasuhara T (1988) Two new flavonoids and other constituents in licorice root their relative astringency and radical scavenging effect. Chem Pharma Bull 36:1090–1097
Hirasa K, Takemasa M (1998) Spice science and technology. Marcel Dekker, New York, pp 163–200
Javidnia K, Miri R, Jamalia A (2005) Composition of the essential oil of Salvia macrosiphon Boiss. from Iran. Flavour Fragr J 20:542–543
Justesen U, Knuthsen P, Leth T (1998) Quantitative analysis of flavonols, flavones and flavanons in fruits, vegetables and beverages by HPLC with photodiode array and mass spectrometric detection. J Chromatogr 799:101–110
Karami M, Gohari AR, Naghshvar F, Salarnia A, Alemy SH (2012) Comparison effects of methanolic extracts of Salvia Macrosiphon and Withania Coagulans on Withdrawal syndrome in mice. J Pharm Sci 18(3):183–186
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables—the millennium’s health. Int J Food Sci 36:703–725
Misra N, Gupta AK, Dwivedi UN (2006) Changes in free amino acids and stress protein synthesis in two genotypes of green gram under salt stress. J Plant Sci 1:56–66
Namiki M (1990) Antioxidant/antimutagenesin food. Crit Rev Food Sci Nutr 29:273–300
Patro BS, Bauri AK, Mishra S (2005) Chattopadhyay, antioxidant activity of Myristicama labarica extracts and their constituents. J Agric Food Chem 53:6912–6918
Pellegrini N, Re Y, Yang M, Rice-Evans CA (1999) Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,20-azino bis-3-ethylenebenzothiazoline-6-sulphonic acid radical decolorization assay. Methods Enzymol 299:379–389
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2(4):152–159
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073–2085
Shannon MC, Grieve CM, Francois LE (1994) Whole-plant response to salinity. In: Wilkinson RE (ed) Plant-environment interactions. Marcel Dekker, New York, pp 199–244
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–153
Tarchoune I, Sgherri C, Izzo R, Lachaal M, Ouerghi Z, Navari-Izzo F (2010) Antioxidant responses of Ocimumbasillcum to sodium chloride or sodium sulphate salinization. J Plant Biochem Physiol 48:772–777
Tarchoune I, Sgherri C, Baatour O, Izzo R, Lachaal M, Navari-Izzo F, Ouerghi Z (2012a) Phenolic acids and total antioxidant activity in Ocimum basillcum L. grown under Na2SO4 medium. J Med Plants Res 6(48):5868–5875
Tarchoune I, Sgherri C, Izzo R, Lachaal M, Ouerghi Z, Navari-Izzo F (2012b) Changes in the antioxidative system of Ocimum basillcum L. (cv Fine) under different sodium salts. Acta Physiol Plant 10:738–847
Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pasqualem A (2005) Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 89:549–554
Vahdani M, Faridi P, Zarshenas MM, Javadpour S, Abolhassanzadeh Z, Moradi N, Bakzadeh Z, Karmostaji A, Mohagheghzadeh A, Ghasemi Y (2011) Major compounds and antimicrobial activity of essential oils from five Iranian endemic medicinal plants. J Pharmacol 3:48–53
Wang H, Cao G, Prior RL (1997) Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem 45:304–309
Yamini Y, Khajeh M, Ghasemi E, Mirza M, Javidnia K (2007) Comparison of essential oil compositions of Salvia mirzayanii obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem 108:341–346
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valifard, M., Mohsenzadeh, S. & Kholdebarin, B. Salinity Effects on Phenolic Content and Antioxidant Activity of Salvia macrosiphon . Iran J Sci Technol Trans Sci 41, 295–300 (2017). https://doi.org/10.1007/s40995-016-0022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-016-0022-y