Abstract
Heavy metals (HMs) are natural assets on the planet, but they have become a serious threat to environmental pollution due to fast industrial enterprise, metropolitanism, and recent technological breakthroughs. The principal causes of HMs pollution in soil, water, and air are industrial waste, organic compounds, pesticides, paints (including small-medium industries), and mining activities. The land, water, and air utilized in farming are of major significance and may have an impact on the health of living organisms. Novel and robust ecotechnologies are needed to avoid HM contamination in the environment. Microbial bioremediation has long been recognized as the most well-understood biotechnological process for environmental restoration. For the treatment of HM-contaminated environmental sites, microbial bioremediation is a cost-effective option. Researchers worldwide are making strides in discovering new bacterial strains with plasmid-linked degradation/reduction ability. Genetic engineering and molecular biology aided in the development of microbes that would produce the desired results in the environment. Recent advances in microbial bioremediation techniques include biostimulation, bioaugmentation, bioaccumulation, biosorption, and the use of biofilms. This chapter assembles data on recent developments and applications of microbe-mediated bioremediation of HM-contaminated soils.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
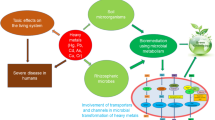
The environment is made up of several complicated variables such as land, air, and water. The existence of humans and other living entities such as animals, plants, and bacteria is based on their positive correlation (Arora et al. 2018). Heavy metal (HM) pollution has become a serious risk to the environment and food production as a result of the massive expansion in the global population and quick progress in modern agriculture (Selvi et al. 2015). Many studies have reported it as a global problem in countries like India, Bangladesh, Italy, Germany, Greece, Hong Kong, China, Turkey, Iran, etc. (Chikumbusko et al. 2017). The lack of understanding about safe effluent disposal and the failure to impose strong regulatory standards have contributed to environmental degradation (Khalid et al. 2017). Vast amounts of solid waste in various harmful forms have been generated as a consequence of these circumstances leading to contamination of the entire ecosystem. The wastewaters, which may exceed authorized limits specified by international regulatory bodies, will have an impact on the quality of surface water and land (EPA 1992, 2002).
Because of their nonbiodegradability, bioaccumulation, environmental stability, persistence, and biotoxicity, HMs make a major environmental risk to living creatures and environments (Khan et al. 2019). They can directly affect the physical and chemical properties of the soils, air, and water, which cause environmental pollution (Fig. 11.1) (Omwene et al. 2018). They can also disrupt the natural ecosystem and have a direct and continual impact on human health through food chains, leading to various diseases (Weber et al. 2020; Suhani et al. 2021),such as paralysis agitans, Alzheimer’s, Sclerosis, cancer, hardening of the arteries, etc. (Muszyńska and Hanus-Fajerska 2015).
Many treatment approaches, such as physical, chemical, and biological, have recently been proposed to clean up HM pollution in the air, water, and soil. HM treatment processes include adsorption, heat treatment, chlorination, ion exchange, chemical extraction, bioleaching, and electrokinetics. According to reports, the majority of the aforementioned techniques are only intended to be used as single remedial methods. Despite their success, these methods have downsides such as inefficiency, cost, and failure during large-scale adoption, among other things (Volesky and Holan 1995; Selvi et al. 2019).
In this chapter, we have discussed the HM sources, their harmful effects, issues associated with the disposal and recycling of HM-containing products, and different microbial-based methods for abatement and opportunities.
11.2 Toxicity of Heavy Metals and the Environment
The biosphere is the most significant part of the environment where biotic entities, i.e., animals and plants, interact with abiotic surroundings, viz., soil, water, and air (Mahmoudi 2003). Pollution is defined as any human action that reduces the quality of the natural environment. Pollution of the environment is nothing new, yet it is still the world’s most serious problem and the primary cause of diseases and loss of life. Environmental pollution is generally worse in middle- and low-income countries as compared to the developed ones, partly due to poverty, ineffective legislation, and a lack of awareness about pollution. Environmental pollution is caused by HMs through a variety of factors, including industrial growth, urbanization, population increase, exploration, mining, deforestation, bush burning, dumping of agricultural and residential wastes in water bodies, use of pesticides in aquatic animal harvesting, inappropriate disposal of technological wastes, etc. (Landrigan et al. 2018) The repercussions affect not just people but also other land and aquatic species, including microbes, which support biogeochemical cycles required for a healthy ecosystem (Ukaogo et al. 2020).
11.3 Heavy Metals
HMs are metallic chemical elements with a relatively high density, which are harmful or lethal even at low doses. Almost all the HMs are hazardous to human health above a certain concentration and pose a risk to the environment. HMs include cadmium (Cd), zinc (Zn), molybdenum (Mo), mercury (Hg), nickel (Ni), chromium (Cr), strontium (Sr), arsenic (As), vanadium (V), boron (B), cobalt (Co), copper (Cu), molybdenum (Mo), tin (Sn), lead (Pb), etc. HMs including Cu, Ni, Fe, Zn, B, and Mo are necessary for plant growth, but when their concentrations exceed the permissible limits, they can harm animals and plants. Among all the HMs, Pb, Hg, Cd, and As are not required for the growth and development of plants and animals.
11.3.1 Sources of Heavy Metal Pollution
HMs in the soil aggregate due to a variety of causes that may be natural and anthropogenic sources (Fig. 11.2) (He et al. 2012).
11.3.1.1 Natural Sources
Under different environmental conditions, HMs are naturally emitted through volatile organic compounds, forest fire, sea-salt sprays, volcanic ash, rock depletion, and dirt particles, which are the causes of HM pollution. HMs can be found in the form of oxides, silicates, sulfides, phosphates, sulfates, hydroxides, and organic molecules. Pb, Ni, Cr, Cd, As, Hg, Se, Zn, and Cu are some of the most regularly used HMs. While these HMs are found in smaller quantities in humans and other animals, they may cause severe health concerns (Ali et al. 2021).
11.3.1.2 Anthropogenic Sources
There are a lot of anthropogenic factors of HM concentrations in the environment, but the most significant are rising industrialization and urbanization in recent days. Fertilization, pesticide application, air deposition, sewage irrigation, mining, and sludge application are responsible for HM accumulation in the environment; in addition to these factors, melting activities for metallic ores, industrial wastes, combustion of fossil fuel refinement, and refinishing contribute to HM accumulation (Srivastava et al. 2017). The primary cause of metal pollution in the atmosphere is assumed to be coal burning (Antoniadis et al. 2017).
11.3.2 Environmental Impacts of Heavy Metals
Heavy metals have a lot of negative consequences when they are present in the environment. The hydrosphere, lithosphere, and biosphere are all affected due to these HMs (Masindi and Muedi 2018) (Fig. 11.2). They can directly impact the physical and chemical properties of the sediment, soils, and water to hinder microbial activities. They can also destabilize the natural ecosystem and have a direct and long-term impact on the human body, leading to a variety of diseases and issues in mankind (Ali et al. 2021).
Population increase is one of the major issues in the world. Due to the increasing population, the use of HMs also increased, and with this contamination, major environmental components cause serious issues worldwide (Masindi and Muedi 2018).
11.3.2.1 Effect on Soil
HMs affect the agroecosystem through both natural and artificial sources. Several studies have found that natural HM pollution sources are usually high when compared to anthropogenic activity. The parent material from which HMs are derived is the major source of HMs in soil. Human activities disrupt the nature’s slow-moving geochemical cycle of HMs, resulting in accumulation in the soil (Dixit et al. 2015). Different anthropogenic activities like the refinement of fossil fuels through combustion (Muradoglu et al. 2015), smelting and extracting metals (Chen et al. 2015), municipal trash disposal (Khan et al. 2016), fertilizer application (Atafar et al. 2010), pesticide usage (Ogunlade and Agbeniyi 2011), sewage application (Sun et al. 2013; Srivastava et al. 2016), etc. cause HM pollution.
HMs in soil cause a severe problem because they accumulate in food chains, damaging the entire ecosystem. Organic pollutants are biodegradable, but their biodegradation rate is slowed by the presence of HMs in nature, which doubles the organic and HM pollution. HMs can harm animals, humans, plants, and ecosystems in a variety of ways. Direct ingestion, absorption by plants, transfer through food chains, drinking polluted water, and changes in soil color, pH, porosity, and natural chemistry all impact soil quality (Musilova et al. 2016).
11.3.2.2 Effects on Water
The kind of soil, rock, and water movement, all influence the metal composition of surface water such as rivers, lakes, and ponds. Metals on the soil’s surface are carried away by the wind and end up in sewage and reservoirs (Salem et al. 2000). Rainwater becomes polluted as it travels through the atmosphere. The passage of numerous industrial wastewaters into water sources contaminates them, which contain a large number of HM leachates from landfills and liquid disposal in deep wells, and contaminates the groundwater (Oyeku and Eludoyin 2010). The metal level of water is affected by a variety of elements, including pH, life forms, ion exchange, temperature, vaporization, absorption, and others.
11.3.2.3 Effects on Air
Surface degradation and loss of colloids release HMs into the atmosphere as vapors. Mineral dust, particles of sea salt, volcanoes, and forest fires are all atmospheric sources of HMs (Colbeck 1995). HM air pollution can come from a variety of industrial activities that produce dust particles, such as metal smelters and cement factories, in addition to these natural sources. In the atmosphere, unstable metals such as gaseous pollutants particles of Sb, Se, Hg, and As are transmitted. Metals like Zn, Pb, and Cu are carried as particulates which pollutes the air (Selvi et al. 2019).
11.3.2.4 Human Exposure to Heavy Metals
Poison HMs get into the human body through a variety of mechanisms, including ingestion, inhalation, and skin absorption. People in underdeveloped countries are more exposed to hazardous metals (Eqani et al. 2016) because many people are unaware of the dangers of HM exposure and the ramifications for human health (Afrin et al. 2015). HMs may be present in the workplace and the environment. HMs are ingested by mining and industrial workers through metal particles containing dust and particulate matter. Welders exposed to welding fumes for an extended period had considerably greater blood levels of the HMs such as Cr, Ni, Cd, and Pb than the control group, as well as elevated oxidative stress (Mahmood et al. 2015). Cigarette smoking is a major source of human exposure to harmful HMs, i.e., Cd (Järup 2003) and other HMs found in tobacco leaves. Among these HMs, As is one of the most hazardous metalloids on the earth. According to the World Health Organization (WHO), the limit of As in the drinking water is 5–10 μg/L, but in many countries like Bangladesh and some parts of India, As concentration is more than 50 μg/L, causing many diseases such as skin cancers, kidney cancer, lung cancer, and bladder cancer apart from long-term exposure of inorganic arsenic hypertension, diabetes, reproductive disorders, and cardiovascular diseases (Santra et al. 2013).
The overall public is exposed to HMs through food and water. Intensification of industrial and agricultural activity has resulted from globalization, urbanization, and rapid economic development. Toxic HMs could be released into the water, air, and soil as a result of these actions. HMs bioaccumulate in human food systems, eventually reaching the human body and causing different diseases (Ali et al. 2019) (Fig. 11.3).
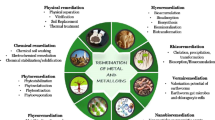
11.4 Environmental Heavy Metal Remediation
HMs emitted from several sites are released into the environment, either directly or indirectly, affecting humans, animals, and plants. Increased human exposure to HMs has serious health consequences and causes environmental degradation (Rzymski et al. 2014). The severity of negative health impacts varies depending on the duration of exposure, concentration, chemical form, and type of HMs. HM pollution in soil has led to ecosystem degradation, reduced food quality, and decreased soil health. HM concentrations in India’s industrial zones are far greater than the WHO’s allowed limit, putting humans at risk (Manivasagam 1987). The failure of respective government environmental safety agencies in developing countries to impose strict regulations and the unreliability of current individual treatment technologies in situ and a wide range of applications are all contributing to the health problems associated with metal pollution.
11.4.1 Biological Remediation
Biological remediation is the utilization of living organisms to clean up HMs in the soil. Here we mainly discussed microbial remediation and phytoremediation.
11.4.1.1 Microbial Remediation
Environmental HM contamination has seriously threatened all ecosystems (Okolo et al. 2016). According to Environmental Protection Agency, parameterization is a natural activity in which microbial processes are utilized to break down or convert dangerous substances into less harmful ones, ultimately eliminating toxins from the environment. During the microbial process, microorganisms utilize chemical pollutants as an energy source in their metabolic processes. Synthetic nutrients limit microbial development in the soil (Ahirwar et al. 2016). Microorganisms can degrade, detoxify, and decontaminate substances and even accumulate toxic organic and inorganic substances from a variety of places in the environment; some bacteria will be described biochemically, and it will be determined whether they can tolerate heavy metals like copper and zinc. In the last 20 years, bioremediation strategies have made significant advances, with the ultimate goal of efficiently restoring damaged regions in an eco-friendly and low-cost manner (Ambaye et al. 2022).
11.4.1.1.1 Bacterial Remediation Capacity of Heavy Metals
Microbial biomass contains a variety of biosorption properties that range greatly among microorganisms. However, each microbial cell’s biosorption ability is determined by its pretreatment and experimental settings. Bacteria are essential bioabsorbents because of their widespread distribution, size, capacity to thrive in controlled environments, and resistance to environmental conditions (Srivastava et al. 2015). Their remarkable biosorption abilities are due to their high surface-to-volume ratios and probable active chemisorption sites on the cell wall (Mosa et al. 2016). Bacteria thrive in mixed cultures because they are more stable and survive longer (Sannasi et al. 2006). As a result, consortia are metabolically efficient for metal biosorption and more suitable for field application (Kader et al. 2007). De et al. (2008) used an Acinetobacter sp. bacterial consortium to decrease Cr by 78%. B. megaterium, B. niger, and Penicillium sp. had the greatest ability to reduce Pb (2.13–0.03 mg/L), Cr (1.38–0.08 mg/L), and Cd (0.4–0.03 mg/L), respectively.
11.4.1.1.2 Plant Growth-Promoting Endophyte-Mediated Phytoremediation
Endophytic bacteria have shown to assist host plants in adjusting to harsh soil conditions and improve phytoremediation capacity by modifying metal bioavailability in soil, increasing plant growth, decreasing metal phytotoxicity, lowering mental stress, and changing metal translocation in plants (Ma et al. 2011). These bacterial endophytes contribute to the detoxification of metal-polluted soils by improving plant metal tolerance capacity and growth and increasing uptake capacity in plants as discussed (Table 11.1).
11.4.1.1.3 Fungi Remediation Capacity of Heavy Metal
Because of their strong metal uptake and restoration capabilities, fungi are often used as biosorbents to eliminate toxic metals (Fu et al. 2012). Dead fungal biomass from Penicillium chrysogenum, Saccharomyces cerevisiae, Aspergillus niger, and Rhizopus oryzae can convert hazardous Cr (VI) to less dangerous or nontoxic Cr (Park et al. 2005). Luna et al. (2016) also reported that Candida sphaerica creates biosurfactants that remove Pb (79%), Zn (90%), and Fe (95%). Likewise surfactin, rhamnolipid, and sophorolipid were also tested for HM (Cu and Zn) removal by Mulligan et al. (2001).
11.4.1.1.4 Algae Remediation Capacity of Heavy Metal
In comparison to other microbial bioabsorbents, algae are autotrophic, meaning they consume few nutrients and produce a large amount of biomass. These bioabsorbents have also been utilized to remove HMs from the environment due to their high sorption ability (Abbas et al. 2014). Adsorption or integration of algae biomass into cells is employed for bioremediation of HM-polluted wastewater. Phycoremediation is employing several forms of algae and cyanobacteria to remove or degrade toxicants to remediate HMs (Chabukdhara et al. 2017). Algae include chemical moieties on their surface that act as metal-binding sites, including hydroxyl, carboxyl, phosphate, and amide (Abbas et al. 2014). Dead Chlorella vulgaris cells were utilized by Hussian and Napiórkowska-Krzebietke et al. to remove Cd2+, Cu2+, and Pb2+ ions from aqueous solutions under various pH, bioabsorbent dosage, and contact time conditions. These findings demonstrate that the biomass of C. vulgaris is an exceptionally efficient bioabsorbent for the removal of Cd2+, Cu2+, and Pb2+ at 95.5%, 97.7%, and 99.4%, respectively, from a mixed solution containing 50 mg/dm3 of each metal ion (Goher et al. 2016).
11.4.1.2 Heavy Metal Removal Using Biofilm
In many experiments, biofilms were utilized to remove HMs. Biofilm is a type of bioremediation that also serves as a biological stabilizer. Biofilms have an extremely high tolerance for hazardous inorganic elements, even at deadly doses. According to research on Rhodotorula mucilaginosa, metal elimination efficacy varied from 4.79 to 10.25% for planktonic cells and from 91.71 to 95.39% for biofilm cells (Goher et al. 2016). Biosorbents or exopolymeric substances present in biofilms that contain molecules with a surfactant or emulsifying qualities might be used in biofilm bioremediation approaches (El-Masry et al. 2004).
11.4.1.2.1 Metal-Microbe Interaction
The bacterial cell takes up the heavy metal by different methods, either active transport, ion exchange, electrostatic interaction, complexation, or the production of extracellular polysaccharides (Srivastava et al. 2017) (Fig. 11.4).
When microbes interact with heavy metals, they accumulate in the microbial cell and can be detoxified by mechanisms such as bioadsorption, biomineralization, biodegradation, bioleaching, biotransformation, and bioaccumulation.
11.4.1.3 Methods for Heavy Metal Remediation Using Microorganisms
11.4.1.3.1 Biosorption
Although the terms bioaccumulation and biosorption are often used interchangeably, they differ in how pollutants are sequestered. Biosorption, according to Volesky, is the adsorption of chemicals from solution by biological materials via physiochemical absorption pathways such as electrostatic forces and ion/proton displacement (Volesky and Holan 1995). Biosorption has been proven to remove a wide range of HMs from aqueous solutions, including very hazardous metal ions such as Cd, Cr, Pb, Hg, and As (Saba et al. 2019). As a result, the diversity of cell wall architectures is critical to biosorption success. By using bioabsorbents like microorganisms (both live and dead), agricultural waste, and other industrial wastes, biosorption can remove pollutants and build the framework for long-term metal removal and recovery (Inoue et al. 2017). Numerous parameters such as pH, temperature, shaking speed, initial pollutant concentration, and bioabsorbent amount are considered to improve biosorption effectiveness. The chemical composition of each contaminant, biomass size, interaction between distinct metallic ions, and ionic strength influence the binding mechanism. Biosorption is also appealing because of a variety of benefits, including low operating costs due to its reversible process, no increase in chemical oxygen demand (COD), ease of desorption, and high adsorption rate. However, other factors must be considered, such as the potential toxicity of pollutants to bacterial cells if living cells are used in this procedure.
11.4.1.3.2 Bioaccumulation Process
Bioaccumulation, on the other hand, is a natural active metabolic process in which HMs accumulate and are taken up by proteins into intracellular living bacterial cells. The initial stage is the adsorption of HMs onto cells, and the metal species are then carried inside the cells, where the HMs can be sequestered by proteins, the lipid bilayer as an import system, and peptide ligands as a storage system (Mishra and Malik 2013). Metal ions were uptake by numerous substances inside the cell cytoplasm to create big ions in intracellular sequestration. Gram-negative bacteria enhanced absorption from the periplasm into the cytoplasm is dependent on the expression of inner membrane importers in extracellular sequestration (Saier 2016; Diep et al. 2018).
11.4.1.3.3 Biomineralization
Biomineralization is the process by which microorganisms mediate and catalyze inorganic reactions to form new mineral assemblages. Therefore, some microorganisms produce certain extracellular polymeric substances (EPSs) or biosurfactant in the extracellular environment, or sometimes they will also produce it in the intracellularly environment which will help to convert the heavy metal into the minerals, a process called biomineralization. Various bacteria like Bacillus, Streptococcus, etc. help in the biomineralization process (Arnold et al. 2021).
11.4.1.3.4 Bioleaching for Bioremediation
“The dissolving of metals from their mineral source using specific naturally existing microbes” or “the use of microbes to alter metal elements so that the elements can be recovered when water is filtered through it” are two definitions of bioleaching (Mishra et al. 2005). Ni, Cu, Zn, Co, Au, Pb, and As have all been dissolved with it. This process is useful for extracting valuable metal compounds from solid substrates and detoxifying HM-contaminated wastes such as ores, energy, or landfill space as a technology (Singh and Li 2015). However, an investigation has demonstrated that chemolithotrophic techniques cannot handle industrial waste materials containing substantial levels of important metals (Sajjad et al. 2019). Additionally, this is dependent on the metal compounds in the waste as vanadium, chromium, copper, and zinc may all be fully recovered (Blaise et al. 2010). Thiobacilli have also been able to detoxify HM-contaminated sewage sludge, soil, sediment, and water (Blaise et al. 2010). From bacteria to fungi and algae, many microorganisms have been isolated from mining and environmental bioleaching settings. Rhodotorula sp., Trichosporon sp. (yeasts), Acidithiobacillus sp. (Bacteria), Eutrepia sp. (flagellates), protozoa, and amoebas have all been isolated from a copper mine. Some thermophilic bacteria (particularly Sulfolobus sp.) have also been isolated and enhanced from bioleaching environments.
Researchers can examine the particular processes that sustain microbial successions and the impact of community structure on the environment in bioleaching heaps used for copper removal (Wang et al. 2020). Advances in DNA sequencing technology have made it possible to gather unprecedented amounts of information on the genomes of bioleaching bacteria, allowing for the development of metabolic potential estimation techniques and environmental level interactions.
11.4.1.4 Phytoremediation
The use of plants to clean up HMs in the soil is defined as phytoremediation. These include phytoaccumulation, phytostabilization, and phytodegradation (Fig.11.5). The hyperaccumulating plant will take up the heavy metal that will get accumulated within the plant (Marques et al. 2009; Muthusaravanan et al. 2018; Chaney and Baklanov 2017) (Table 11.2).
In this case, plants will take it up, and sometimes, phytodegradation or the breakdown, phytostabilization, and rhizosphere degradation, that is, degrading of metal in the rhizosphere will happen.
11.5 Conclusion
HM pollution is a serious environmental problem that occurs due to a variety of human activities and has a significant impact on humans and the environment. Because of the biotechnological potential of microbes in removing or recovering metals, our focus has shifted to eco-friendly treatments such as phytoremediation and microbial remediation, which entail HM absorption by microorganisms. Apart from their contributions, biosorbents are potentially beneficial and readily available for removing HMs and for protecting nature and the environment using a bioremediation process. Although just a few studies have been done on this subject, bacteria are one of the most significant microbiological approaches for bioremediation. As heavy-metal pollution alleviators, more research is needed to get the most out of bacterial systems and to determine the specific and unambiguous mechanisms involved in HMs removal by bacteria, fungus, and algae. For the betterment of our environment, we need environmentally friendly remediation solutions based on plants and microorganisms that are viable alternatives to physical and chemical removal methods.
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals. A review. J Chem Sci Technol 3:74–102
Achal V, Kumari D, Pan X (2011) Bioremediation of chromium contaminated soil by a brown-rot fungus Gloeophyllum sepiarium. Res J Microbiol 6:166
Afrin R, Mia M, Ahsan M, Akbor A (2015) Concentration of heavy metals in available fish species (Bain, Mastacembelus armatus; Taki, Channa punctatus and Bele, Glossogobius giuris) in the Turag River, Bangladesh. Biol Sci - PJSIR 58:104–110
Ahirwar NK, Gupta G, Singh R, Singh V (2016) Isolation, identification and characterization of heavy metal resistant bacteria from industrial affected soil in Central India. Int J Pure Appl Biosci 4:88–93
Alford ÉR, Pilon Smits EA, Fakra SC, Paschke MW (2012) Selenium hyperaccumulation by Astragalus (Fabaceae) does not inhibit root nodule symbiosis. Am J Bot 99:1930–1941
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals. Environmental Persistence, Toxicity, and Bioaccumulation. J Chem:1–14
Ali MM, Hossain D, Khan MS, Begum M, Hasan Osman MH, Nazal MK, Zhao H (2021) Environmental pollution with heavy metals: a public health concern. IntechOpen
Ambaye TG, Chebbi A, Formicola F, Prasad S, Gomez FH, Franzetti A, Vaccari M (2022) Remediation of soil polluted with petroleum hydrocarbons, and their reuse for agriculture. Recent progress, challenges, and perspectives. Chemosphere:133572
Antoniadis V, Levizou E, Shaheen S, Ok Y, Sebastian A, Baum C, Prasad M, Wenzel W, Rinklebe J (2017) Trace elements in the soil-plant interface. Phytoavailability, translocation, and phytoremediation. A review Earth Sci Rev 171:621–645
Arnold A, Dennison E, Kovacs CS, Mannstadt M, Rizzoli R, Brandi ML, Clarke B, Thakke RV (2021) Hormonal regulation of biomineralization. Nat Rev Endocrinol 17:261–275
Arora NK, Fatima T, Mishra I, Verma M, Mishra J, Mishra V (2018) Environmental sustainability, challenges and viable solutions. Environ Sustain 1:309–340
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi A (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Balamurugan D, Udayasooriyan C, Kamaladev B (2014) Chromium (VI) reduction by pseudomonas putida and Bacillus subtilis isolated from contaminated soils. Int J Environ Sci 5:522
Blaise JF, Tyagi RD, Auclai JC (2010) Metals removal from sewage sludge by indigenous iron oxidizing bacteria. J Environ Sci 28:443–467
Bothe H (2011) Plants in heavy metal soils. In: Detoxification of heavy metals. Springer, Berlin. Heidelberg, pp 35–57
Broadhurst CL, Chaney RL (2016) Growth and metal accumulation of an Alyssum murale nickel hyperaccumulator ecotype co-cropped with Alyssum montanum and perennial ryegrass in serpentine soil. Front Plant Sci 7:451
Chabukdhara M, Gupta SK, Gogoi M (2017) Phycoremediation of heavy metals coupled with generation of bioenergy. In: Algal biofuels. Springer, Cham, pp 163–188
Chaney RL, Baklanov IA (2017) Phytoremediation and phytomining status and promise. Adv Bot Res 83:189–221
Chen X, Lu X, Yang G (2012) Sources identification of heavy metals in urban topsoil from inside the Xi’an Second Ringroad NW China using multivariate statistical methods. Catena 98:73–78. https://doi.org/10.1016/j.catena.2012.06.007
Chen ZF, Zhao Y, Fan LD, Xing L, Yan YJ (2015) Cadmium (Cd) localization in tissues of cotton (Gossypium hirsutum L.) and its phytoremediation potential for Cd-contaminated soils. Bull Environ Conta Toxico 95:784–789
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:752708
Chikumbusko C, Ishmael B, Deliwe D, Rex M, Benard T, Morris M, Russel C, Stephen K, Samson M (2017) A review of heavy metals in soil and aquatic systems of urban and semi-urban areas in Malawi with comparisons to other selected countries. Afr J Environ Sci Technol 11:448–460
Claire-Lise M, Nathalie V (2012) The use of the model species Arabidopsis halleri towards phytoextraction of cadmium polluted soils. Nat Biotechnol 30:9–14
Coelho LM, Rezende HC, Coelho LM, De Sousa PA, Melo DF, Coelho NM (2015) Bioremediation of polluted waters using microorganisms. Adv Bioremediat Waste Water Pollut Soil 10:5772–60770
Colbeck I (1995) Particle emission from outdoor and indoor sources in airborne particulate matter. In: Kouimtzis T, Samara C (eds) The handbook of environ Chem, vol 4. Springer, Berlin, Heidelberg, pp 1–33
De J, Ramaia N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10:471–477
Diep P, Mahadevan R, Yakunin AF (2018) Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front Bioeng Biotechnol 6:157. https://doi.org/10.3389/fbioe.2018.00157
Dixit R, Wasiullah MD, Pandiyan K, Singh U, Sahu A, Shukla R, Singh B, Rai SP, Lade H, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment, An overview of principles and criteria of fundamental processes. Sustainability 7:2189–2212
Dong G, Wang Y, Gong L, Wang M, Wang H, He N, Zheng Y, Li Q (2013) Formation of soluble Cr (III) end-products and nano-particles during Cr (VI) reduction by Bacillus cereus strain XMCr-6. Biochem Eng J 70:166–172
El-Masry MH, El-Bestawy E, El-Adl NI (2004) Bioremediation of vegetable oil and grease from polluted wastewater using a sand biofilm system. World J Microbiol Biotechnol 20:551–557
EPA (1992) Ground water issue- behaviour of metals in soil. U.S. Environmental Protection Agency, EPA/540/S-92/018
EPA (2002) Supplemental guidance for developing soil screening levels for superfund sites. US EPA, Office of Solid Waste and Emergency Response, Washington, DC
Eqani S, Khalid R, Bostan N, Saqib Z, Mohmand J, Rehan M, Ali N, Katsoyiannis I, Shen H (2016) Human lead (Pb) exposure via dust from different land use settings of Pakistan a case study from two urban mountainous cities. Chemosphere 155:259
Fu YQ, Li S, Zhu HY, Jiang R, Yin LF (2012) Biosorption of copper (II) from aqueous solution by mycelial pellets of Rhizopus oryzae. Afr J Biotechnol 11:1403–1411
Fulekar MH (2016) Phytoremediation of heavy metals by Helianthus annuus in aquatic and soil environment. Int J Curr Microbiol App Sci 5:392–404
Goher ME, AM AE, Abdel-Satar AM, Ali MH, Hussian AE, Napiórkowska-Krzebietke A (2016) Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J Elem 21:3
He B, Yun Z, Sh J, Jiang G (2012) Research progress of heavy metal pollution in China sources, analytical methods, status, and toxicity. Chin Sci Bull 58:134–140
Inoue K, Parajuli D, Ghimire KN, Biswas BK, Kawakita H, Oshima T, Ohto K (2017) Biosorbents for removing hazardous metals and metalloids. Materials 10:8–857
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Kader J, Sannasi P, Othman O, Ismai BS, Salmijah S (2007) Removal of Cr (VI) from aqueous solutions by growing and non-growing populations of environmental bacterial consortia. Glob J Environ Res 1:12–17
Khalid S, Shahid M, Niazi N, Murtaza B, Bib I, Dumat C (2017) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268
Khan I, Ghani A, Rehman AU, Awan SA, Noreen A, Khalid I (2016) Comparative analysis of heavy metal profile of Brassica campestris (L.) and Raphanus sativus (L.) irrigated with municipal waste water of Sargodha city. J Clin Toxicol 6:1–4
Khan M, Dai X, Ni Q, Zhang C, Cui X, Lu M, Deng M, Yang X, He Z (2019) Toxic metal pollution and ecological risk assessment in sediments of water reservoirs in Southeast China. Soil Sediment Contaminat Int J 28:695–715
Kumar Ramasamy R, Congeevaram S, Thamaraiselvi K (2011) Evaluation of isolated fungal strain from e-waste recycling facility for effective sorption of toxic heavy metal Pb (II) ions and fungal protein molecular characterization- a mycoremediation approach. Asian J Exp Biol Sci 2:342–347
Landrigan PJ, Fuller R, Acosta NJ, Adeyi O, Arnold R, Baldé AB, Zhong M (2018) The lancet commission on pollution and health. Lancet 391:462–512
Lee YC, Chang SP (2011) The biosorption of heavy metals from aqueous solution by spirogyra and Cladophora filamentous macroalgae. Bioresour Technol 102:5297–5304
Lívia de CFMH, Benedito C (2015) Potential application of modified Saccharomyces cerevisiae for removing lead and cadmium. J Bioremed Biodegr 6:2
Luna JM, Rufin RD, Sarubbo LA (2016) Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties. Process Saf Environ Prot 102:558–566
Ma Y, Guo L, Wang H, Bai B, Shi D (2011) Accumulation, distribution, and physiological contribution of oxalic acid and other solutes in an alkali-resistant forage plant, Kochia sieversiana, during adaptation to saline and alkaline conditions. J Plant Nutr Soil Sci 174:655–663
Mahmood Q, Wang J, Pervez A, Meryem S, Wasee M, Ullah Z (2015) Health risk assessment and oxidative stress in workers exposed to welding fumes. Toxicol Environ Chem 97:634–639
Mahmoudi S (2003) Alexandre Timoshenko, environmental negotiator handbook (London, the Hague and Boston Kluwer law international 2003) xvi 541 pages. Yearb Int Environ Law 14:844–848
Mane PC, Bhosle AB (2012) Bioremoval of some metals by living algae spirogyra sp. and Spirulina sp. from aqueous solution
Manivasagam N (1987) Industrial effluents origin characteristics effects. In: Analysis and Treatment. Coimbatore Shakti Publications, pp 79–92
Marques AP, Rangel AO, Castro PM (2009) Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Crit Rev Environ Sci Technol 39:622–654
Masindi V, Muedi K (2018) Environmental contamination by heavy metals. Heavy Metals 10:115–132
Mengoni A, Cecchi L, Gonnelli C (2012) Nickel hyperaccumulating plants and Alyssum bertolonii model systems for studying biogeochemical interactions in serpentine soils. In: Bio-Geo Interactions in Metal-Contaminated Soils. Springer, Berlin, Heidelberg, pp 279–296
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43:1162–1222
Mishra D, Dong-Jin K, Jong-Gwan A, Young-Ha R (2005) Bioleaching, a microbial process of metal recovery. A Review. Met Mater Int 11(3):249–256
Mosa KA, Saadoun I, Kumar K, Helm M, Dhankher OP (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci 7:303
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater an evaluation. Eng Geol 60:193–207
Muradoglu F, Gundogdu M, Ercisli S, Encu T, Balta F, Jaafar HZ, Zia-Ul-Haq M (2015) Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res 48(1):7
Musilova J, Arvay J, Vollmannova A, Tot T, Tomas J (2016) Environmental contamination by heavy metals in region with previous mining activity. Bull Environ Contam Toxicol 97:569–575
Muszyńska E, Hanus-Fajerska E (2015) Why are heavy metal hyperaccumulating plants so amazing. Biotechnol Acta 4:265–271
Muthusaravanan S, Sivarajasekar N, Vivek J, Paramasivan T, Naushad M, Prakashmaran J, Al-Duaij OK (2018) Phytoremediation of heavy metals, mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359
Nalla S, Hardaway CJ, Sneddon (2012) Phytoextraction of selected metals by the first and second growth seasons of Spartina alterniflora. Instrum Sci Technol 40(1):17–28
Nematian MA, Kazemeini F (2013) Accumulation of Pb, Zn, Cu and Fe in plants and hyperaccumulator choice in Galali iron mine area, Iran. Int j Agric Crop Sci 5:4–426
Ogunlade MO, Agbeniyi SO (2011) Impact of pesticides use on heavy metals pollution in cocoa soils of Cross-River state Nigeria. Afr J Agric Res 6:3725–3728
Okolo NV, Olowolafe EA, Akawu I, Okoduwa SIR (2016) Effects of industrial effluents on soil resources in Challawa industrial area Kano Nigeria. Glob Ecol Conserv 5(1):1–10
Omwene P, Öncel M, Çelen M, Kobya M (2018) Heavy metal pollution and spatial distribution in surface sediments of Mustafakemalpaşa stream located in the world's largest borate basin (Turkey). Chemosphere 208:782–792
Oyeku OT, Eludoyin AO (2010) Heavy metal contamination of groundwater resources in a Nigerian urban settlement. Afr J Environ Sci Technol 4:201–214
Park D, Yun YS, Jo JH, Park JM (2005) Mechanism of hexavalent chromium removal by dead fungal biomass of aspergillus Niger. Water Res 39:533–540
Rahman A, Nahar N, Nawani NN, Jass J, Hossain K, Saud ZA, Saha AK, Ghosh S, Olsson B, Mandal A (2015) Bioremediation of hexavalent chromium (VI) by a soil-borne bacterium Enterobacter cloacae B2-DHA. J Environ Sci Health Part A 50:1136–1147
Rathinasabapathi B (2011) Arsenic hyperaccumulator fern pteris vittata utilities for arsenic phytoremediation and plant biotechnology in working with ferns. Springer, New York, pp 261–269
Rzymski P, Niedzielski P, Poniedziałek B, Klimaszyk P (2014) Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ Monitor Asses 186:3199–3212
Saba RY, Ahmed M, Sabri AN (2019) Potential role of bacterial extracellular polymeric substances as biosorbent material for arsenic bioremediation. J bioremediat 23(2):72–81
Saier MH (2016) Transport protein evolution deduced from analysis of sequence topology and structure. Curr Opin Struct Biol 38:9–17
Sajjad W, Zheng G, Din G, Ma X, Rafiq M, Xu W (2019) Metals extraction from sulfide ores with microorganisms: the bioleaching technology and recent developments. Trans Indian Inst Metals 72(3):559–579. https://doi.org/10.1007/s12666-018-1516-4
Salem HM, Eweida EA, Farag A (2000) Heavy metals in drinking water and their environmental impact on human health. ICEHM Cairo Uni Egypt 542:556
Sannasi P, Kader J, Othman O, Salmijah S (2006) Single and multi-metal removal by an environmental mixed bacterial isolate. Modern Multidiscip Appl Microbiol Exploit Microb Interact 1:36–141
Santra SC, Samal AC, Bhattacharya P, Banerjee S, Biswas A, Majumdar J (2013) Arsenic in foodchain and community health risk: a study in Gangetic West Bengal. Procedia Environ 18:2–13
Selvi A, Das D, Das N (2015) Potentiality of yeast Candida sp. SMN04 for degradation of cefdinir, a cephalosporin antibiotic, kinetics, enzyme analysis and biodegradation pathway. Environ Technol 36:3112–3124
Selvi A, Rajasekar A, Theerthagiri J, Ananthaselvam A, Sathishkumar K, Madhavan J, Rahman PK (2019) Integrated remediation processes toward heavy metal removal/recovery from various environments. A Review Front Environ Sci:7
Singh N, Li JH (2015) Bio-extraction of metals as secondary resources from E-waste. Appl Mech Mater 768:602
Slatter K (1998) Nickel accumulation and tolerance in Berkheya coddii and its application in phytoremediation (Doctoral dissertation)
Srivastava S, Dwivedi AK (2015) Biological wastes the tool for biosorption of arsenic. J Bioremed Biodegr 7:323
Srivastava V, De Araujo ASF, Vaish B, Bartelt-Hunt S, Singh P, Singh R (2016) Biological response of using municipal solid waste compost in agriculture as fertilizer supplement. Rev Environ Sci and Bio/Technol 677:69
Srivastava V, Sarkar A, Singh S, Singh P, De Araujo AS, Sing RP (2017) Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front Environ Sci 5:64
Srivastava V, Ismail SA, Singh P, Singh RP (2015) Urban solid waste management in the developing world with emphasis on India challenges and opportunities. Rev Environ Sci and Bio/Technol 317:337
Su Y, Han FX, Chen J, Sridhar BM, Monts DL (2008) Phytoextraction and accumulation of mercury in three plant species Indian mustard (Brassica juncea) beard grass (Polypogon monospeliensis) and Chinese brake fern (Pteris vittata). Int J Phytoremediation 10:547–560
Suhani I, Sahab S, Srivastava V, Singh RP (2021) Impact of cadmium pollution on food safety and human health. Curr Opin Toxicol 27(1):7
Sukumar M (2010) Reduction of hexavalent chromium by Rhizopus oryzae. Afr J Environ Sci Technol 4:412–418
Sun WH, Jiang YX, Li X (2013) Research of the evaluation on heavy-metal pollution in rice by sewage irrigation. Appl Mech Mater 295:1594–1599
Taştan BE, Ertuğrul S, Dönmez G (2010) Effective bioremoval of reactive dye and heavy metals by aspergillus versicolor. Bioresour Technol 101:870–876
Ukaogo P, Ewuzi U, Onwuk C (2020) Environmental pollution, causes, effects, and the remedies. Microbes Environ:419–429
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Wang Y et al (2020) Stabilization of heavy metal-contaminated soils by biochar: challenges and recommendations. Sci Total Environ 729:139060. https://doi.org/10.1016/j.scitotenv.2020.139060
Weber A, Sales C, de Souza FF, Melo R, Bazzoli N, Rizzo E (2020) Effects of metal contamination on liver in two fish species from a highly impacted neotropical river: a case study of the Fundão dam, Brazil. Ecotoxicol Environ Safety 190:110165
Zhang Z, Wen X, Huang Y, Inoue C, Liang Y (2017) Higher accumulation capacity of cadmium than zinc by Arabidopsis halleri ssp. Germmifera in the field using different sowing strategies. Plant Soil 418:165–176
Zhuang P, Ye ZH, Lan CY, Xi ZW, Shu WS (2005) Chemically assisted phytoextraction of heavy metal contaminated soils using three plant species. Plant Soil 276:153–162
Acknowledgments
The authors acknowledge the Director, CSIR National Botanical Research Institute, for providing facilities and support during the study. This work is supported by the CSIR-Network (MLP0048 and MLP0049) and an in-house project (OLP109) funded by the Council of Scientific and Industrial Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Majhi, B., Semwal, P., Mishra, S.K., Srivastava, V., Singh, R.P., Chauhan, P.S. (2023). Metalliferous Soil Remediation Through Heavy Metal-Resistant Plant Growth-Promoting Bacteria: Prospects and Paradigms. In: Singh, R.P., Singh, P., Srivastava, A. (eds) Heavy Metal Toxicity: Environmental Concerns, Remediation and Opportunities. Springer, Singapore. https://doi.org/10.1007/978-981-99-0397-9_11
Download citation
DOI: https://doi.org/10.1007/978-981-99-0397-9_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0396-2
Online ISBN: 978-981-99-0397-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)