Abstract
Gastric cancer is the fifth most common cancer accounting for close to 7 % of all human cancers. Despite the decrease in incidence, gastric cancer remains the most common cause of gastrointestinal cancer-related death, with more than 800,000 fatalities annually. The disease is more common in men than in women, and non-cardia gastric cancer is twice as common as cardia cancer. More than two-thirds of gastric cancer occur in East Asia, in particular, in China, Japan, and Korea. There are large regional and racial differences in the incidence of gastric cancer. These differences are related to prevalence of Helicobacter pylori (H. pylori), diet, and other risk factors. The mortality of gastric cancer closely matches the regional differences in incidence. The age-standardized incidence and mortality rates of gastric cancer are expected to further decrease due to improvement in socioeconomic conditions and decreasing prevalence of H. pylori. Population screening and intervention, as well as general health measures such as antismoking campaigns, can accelerate the changing epidemiology of gastric cancer. In the absence of such measures, gastric cancer will for long remain a very common and lethal disease. This chapter reviews the epidemiology of gastric cancer, with focus on regional differences in incidence and mortality, risk factors for gastric cancer. It further summarizes the changing epidemiology of gastric cancer in recent decades and the expected future trends.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The first cancer registries started in the late nineteenth century among others in Germany. At that time, 30–40 % of all cancers were of gastric origin [1]. That vast predominance of gastric cancer was strongly related to the ubiquitous colonization of Helicobacter pylori (H. pylori) from early childhood onwards. Similar findings were observed elsewhere. For instance in the 1930s, gastric cancer was almost twice as common as cause of cancer-related death than any other individual malignancy [2]. Since then, we have seen significant changes that are still ongoing. Between 1990 and 2014, the average life expectancy of the world population increased by 6.3 years [3]. This marked change was most marked in Southeast Asia where life expectancy increased by more than 8 years. These improvements were firstly due to prevention and improved outcome of infectious diseases, among others, as a result of further implementation of vaccination programs and clean water supply. Other marked improvements were noted in the outcome of treatment of patients with cancer. Both these factors have changed the epidemiology of gastric cancer.
2 Incidence and Mortality
2.1 Incidence
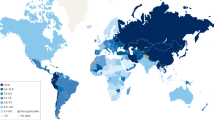
The incidence of non-cardia gastric cancer has in Western countries continuously decreased over the past 80 years; this decrease was more than 80 % in the United States [2]. In Asia, these changes occurred later and were so far less pronounced [4, 5]. Gastric cancer remained the most common malignancy worldwide until 1975. Nowadays, gastric cancer is the fifth most common cancer after cancers of the lung, breast, colorectum, and prostate, making up for 6.7 % of all human cancers [6]. The annual incidence worldwide approximates 951,000 cases [7]. This includes 260,000 patients with cardia gastric cancer, and 691,000 with non-cardia gastric cancer. These match age-standardized incidence rates of, respectively, 3.3 and 8.8 per 100,000 worldwide. More than two-thirds of gastric cancer occur in East Asia, in particular, in China, Japan, and Korea [4] (Fig. 21.1). In 167 (91 %) of 184 countries for which there are data, non-cardia is more common than cardia gastric cancer with an average ratio of 2:1. The few countries in which the incidence of cardia gastric cancer is higher than or equal to non-cardia cancer can, in particular, be found in Northwestern Europe and include Denmark, Finland, and Sweden. These countries are characterized by high socioeconomic status, low H. pylori prevalence, and relatively low proportion of the population consisting of first- and second-generation immigrants. In other Western countries such as the United States and Canada, the incidence of non-cardia gastric cancer is only marginally higher than that of cardia gastric cancer. This pattern markedly differs from that in most Asian and South American countries where non-cardia gastric cancer tends to be considerably more common than cardia gastric cancer. In Eastern and Southeastern Asia, for instance, the age-standardized incidence rates for cardia and non-cardia gastric cancer approximate 9 and 22 cases per 100,000 per year [7]. A similar pattern existed in most Western countries until recently. Within individual countries, marked differences in the incidence of gastric cancer are often observed. One consistent observation is the considerably higher incidence in various indigenous populations [8]. These differences are observed in many different countries with indigenous populations often suffering from an at least fourfold increased standardized incidence ratio of gastric cancer compared with the national average. Other groups also differ in the incidence of gastric cancer. In the United States, for instance, the incidence of non-cardia gastric cancer is considerably higher among African-Americans and Hispanics than among Caucasians [9]. In other countries, these differences among population subgroups can even be larger. In Malaysia, for instance, the incidence is more than fivefold higher among Chinese and Indian men and women than among Malay men and women [10].
Age-standardized incidence rates of gastric cancer (Adapted from http://globocan.iarc.fr/Default.aspx)
The highest incidences of both cardia gastric cancer are found in China, Central Asia, parts of Eastern Europe and Baltic countries, Central America, and parts of South America [7] (Fig. 21.1). The lowest incidences are observed in the Sahel region and sub-Saharan Africa. The pattern for non-cardia gastric cancer shows significant similarity, with minor differences. The highest incidences are observed in the countries mentioned with inclusion of Russia, the lowest in central and some parts of southern Africa, Northwestern Europe, and North America [7]. The variation in age-standardized incidence rates per 100,000 person-years is marked. It ranges in men from approximately 1 in Botswana and Mozambique to more than 40 in parts of Central Asia such as Mongolia. In women, this range reaches from 0.5 in Botswana and Mozambique to 20 in Guatemala. The latter together with Peru is one of the few countries where the incidence of non-cardia cancer is slightly higher in women than in men. Globally, approximately 631,000 gastric cancer cases diagnosed in 2012 were male and 320,000 were female. The global male-female ratio is most marked for cardia cancer ranging from 1.5 in Southern Africa to 4 in North America with an average of 3 [7]. For non-cardia cancer, the global male-female ratio was approximately 2 with the highest difference in Southeast Asia.
2.2 Mortality
The developments in the incidence of gastric cancer are closely matched by geographic differences and changes over time in mortality due to the disease [6] (Fig. 21.2). Between 1990 and 2014, the global age-standardized death rate due to gastric cancer declined by 36 % from 21.7 to 13.8 per 100,000 population [3]. Although this decline was more marked than for any other gastrointestinal malignancy, the age-standardized death rate for gastric cancer remained the highest of any of gastrointestinal cancer. Over the same time period, the number of people dying of gastric cancer actually increased from 763,000 to 841,000 per year [3]. This rise was due to the increase in numbers and life expectancy of the world population, in particular, in areas with a high gastric cancer incidence. The comparison between annual numbers of newly diagnosed patients (see below) and those dying of the disease shows that even today the global disease fatality rate may amount to 85 %. This fatality rate is significantly lower in countries with population screening programs aiming for early detection of gastric cancer [11]. In Japan, the 5-year survival rate is now approximately 69 % [12], while even better results are achieved in high-volume centers in Korea [13]. In Western countries, the majority of cases are detected at a more advanced stage. Survival rates for non-cardia cancer reach 30 %, while survival rates for cardia cancer remain as low as 20 % [14], with multimodality treatment contributing to this effect [15]. Observations in the Surveillance Epidemiology and End Results (SEER) database suggest that the difference in gastric cancer survival may also be related to host and disease factors, since patients of Asian descent had a 12 % better survival than Caucasian patients [16]. This effect persisted after correction for tumor grade and number of lymph nodes investigated. Depending on stage, Asian patients had a 13–72 months longer median survival than corresponding Caucasian patients.
Age-standardized mortality rates of gastric cancer (Adapted from http://globocan.iarc.fr/Default.aspx)
2.3 Incidence and Mortality in Lynch Syndrome Carriers
The previous observations were also reflected in the patterns of gastric cancer among carriers of a hereditary cancer syndrome. For instance, in the first description of Lynch syndrome in 1913 in the United States, gastric cancer was the predominant cancer affecting family members [17]. This usually already occurred around the age of 40 years. This is thought to have resulted from early exposure to H. pylori, most likely in combination with a diet rich in salt and nitrosamines. With changes in diet and a decrease in exposure to H. pylori, the predominant cancer in Lynch carriers became colorectal cancer, usually at an age above 40 years. In a nationwide cohort of Lynch carriers in the Netherlands, we observed a 50 % decline in gastric cancer incidence between 1970 and 2000 [18].
3 Risk Factors
3.1 Helicobacter pylori
The most important risk factor for non-cardia gastric cancer is H. pylori gastritis. The International Agency for Research on Cancer (IARC) thus classified H. pylori as a class I carcinogen [19]. Based on nested case-control in longitudinal cohorts [20] as well as retrospective case-control studies, H. pylori gastritis has been estimated to be associated with a 17-fold increased risk for development of non-cardia gastric cancer [21]. Based on this relative risk and the high global prevalence of H. pylori, 89 % of all non-cardia gastric cancers are thought to be attributable to H. pylori colonization and the resulting gastritis in all H. pylori-positive subjects [22]. This risk depends on the severity of gastritis and is thus higher in those who have more severe chronic active mucosal inflammation as a result of colonization with a more pathogenic H. pylori strain [21, 23, 24]. Likewise, gastric cancer in H. pylori-positives occurs also more frequently in hosts with a more pro-inflammatory genotype [25, 26]. These observations form the basis for the recommendation to consider H. pylori eradication either as a population approach in regions with high gastric cancer incidence [27, 28] or as targeted approach, for instance, in subjects with a positive family history for gastric cancer [29, 30]. When applied prior to the stage of development of intestinal metaplasia, this intervention can strongly reduce the risk of cancer development [31], but it appears to have little preventive effect in those with intestinal metaplasia [31]. In those subjects, cancer can occur even more than a decade after H. pylori eradication [32] (see also Chap. 53).
3.2 Other Risk Factors
There are a range of other factors that either increase or decrease the risk of gastric cancer, in particular factors related to diet [33]. A high intake of salt is associated with an estimated up to threefold increased risk of gastric cancer [34]. In a Japanese study that followed 2476 subjects for 14 years, those in the highest quartile of salt intake had a 2.98-fold increased risk of gastric cancer compared with those in the lowest quartile [35]. This relative risk remained after correction for confounders such as age, sex, and presence of atrophic gastritis. In a retrospective study from Portugal comparing salt intake in 422 patients with gastric cancer versus 649 community controls, the highest tertile of salt intake was associated with a 2.6-fold increased risk for gastric cancer [36]. Very similar data were observed elsewhere, such as in China and Latin America [37, 38].
A diet that is short on fresh fruits and vegetables also increases the risk of gastric cancer. In a retrospective case-control study from Portugal, the highest tertile of fruit intake in the population was associated with an odds ratio (OR) of 0.47 and 0.53 for, respectively, cardia and non-cardia gastric cancer [39]. For the highest tertile of vegetable intake, OR were 0.59 and 0.60, these results were in line with a meta-analysis of literature findings [39]. A meta-analysis of a range of studies from South America reported that high intake of fruits and vegetables was associated with a respective 32 and 42 % decrease in risk of gastric cancer, without specification of tumor subsite [38]. The same meta-analysis also reported a 2.3-fold increased risk for gastric cancer in those with high intake of chili peppers, but also noted that the data were limited. An increase of risk for gastric cancer has also been reported with high intake of processed and red meat, with relative risks ranging from 1.5 to 1.6 in different studies and a meta-analysis from South America [38], while another meta-analysis reported a 15–38 % increased risk with every 30 g/day increase in meat consumption [40]. A large European population study following 521,000 men and women for a median 6.5 years concluded that the increased risk with high meat intake was most pronounced in H. pylori-positives, with an OR of 5.3 with every 100 g/day increase in meat intake [41].
The risk of gastric cancer is increased in smokers. In a European prospective cohort, smoking increased the risk of gastric cancer with approximately 80 % after adjusting for other factors such as intake of fruit and vegetables [42]. Other studies confirm this correlation [38, 43, 44]. A US population microsimulation model showed that reduction in smoking significantly contributed to the decline in the incidence of non-cardia gastric cancer and can further do so with in the coming period on top of changes in H. pylori prevalence [45].
Other factors that have been associated with gastric cancer risk, but less consistent, include alcohol and green tea consumption. In a systematic review of six cohort studies on green tea intake, a limited protective effect was noted on non-cardia gastric cancer but only in women who drank at least five cups of tea per day [46]. There is no convincing evidence for a modulation of gastric cancer risk by coffee consumption [33, 47]. The evidence for a link between both cardia and non-cardia gastric cancer has not been consistently reported, with a recent meta-analysis concluding that the relative risks were not significantly increased [48].
Together, the abovementioned risk factors have been adequately used for implementation in a prediction model for gastric cancer in Korea as a high-incidence country [49]. In Western Europe, as a low-incidence region, it has been estimated that 19 % of all gastric cancers and even 62 % of all cardia cancers could be prevented by universal adoption of a healthy lifestyle including use of a healthy diet and refraining from smoking and alcohol consumption [50].
4 Future Trends
Continuation of the trends of recent decades will likely imply a significant decrease in the number of newly diagnosed patients as well as mortality rates due to gastric cancer despite an expanding and aging world population. The largest changes are likely to occur in current high-incidence countries, in particular, with changing epidemiology of H. pylori. This effect may in some areas such as Japan and Korea be augmented by gastric cancer screening programs [51, 52], and further among others in Japan, and parts of China, and Taiwan by population screening programs aiming to screen and treat for H. pylori and/or atrophic gastritis [27, 31, 53].
A similar effect may occur in Western countries, even in those that already have low incidences of gastric cancer. It has, for instance, been estimated that the decrease would be 66 % for mortality and 50 % for incidence within Europe when comparing 2030 with 2005 [54]. This estimate is supported by the observation of a marked decrease in the prevalence of atrophic gastritis and intestinal metaplasia in the general population [55]. Since these lesions are predominant risk factors for later development of gastric cancer, their prevalence is a looking glass for the incidence of gastric cancer in a population in the following two decades [56]. However, despite the decrease, atrophic gastritis and intestinal metaplasia remain common conditions in those above the age of 50 years even in Western Europe [55, 57].
Some effects may however interfere with this trend. These include the observation that cardia cancer is becoming predominant in Western countries [7]. The incidence of this tumor shows much less of a change over the past decades. For instance, in the United States, the incidence of cardia cancer actually tended to increase between 1976 and 2007 in all racial groups [9]. Over the same time period, the incidence of non-cardia gastric cancer stabilized or even slightly increased in men and women below the age of 50 years [58]. A similar stabilization of non-cardia gastric cancer has recently been observed in the Netherlands [59]. Furthermore, there are some observations that the decreasing prevalence of H. pylori in subsequent birth cohorts may have slowed or stopped in a Western country such as the Netherlands for reasons which are not yet fully understood, but may include a marked increase in the use daycare at very young age [60, 61].
5 Conclusions
Gastric cancer has for long been the most common human malignancy. Despite a marked decline in incidence during the past decades, it still is the fifth most common cancer accounting for close to 7 % of all human cancers. The disease is more common in men than in women, and more often affects the body and antrum of the stomach than the cardia. Despite the decrease in incidence, gastric cancer remains the most common cause of gastrointestinal cancer-related death, with more than 800,000 fatalities annually. There are large regional and racial differences in the incidence of gastric cancer. These differences are related to the prevalence of H. pylori, diet, and other risk factors. The mortality of gastric cancer closely matches the regional differences in incidence. Globally, the majority of patients affected by gastric cancer still die as a result of the disease. The high incidences and fatality rates are remarkable in view of the fact that gastric cancer is largely a preventable disease and can also be detected and treated with excellent outcome at a precursor or early cancer stage. The age-standardized incidence and mortality rates of gastric cancer are expected to further decrease due to improvement in socioeconomic conditions and decreasing prevalence of H. pylori. Population screening and intervention, as well as general health measures such as antismoking campaigns, can accelerate the changing epidemiology of gastric cancer. In the absence of such measures, gastric cancer will for long remain a very common and lethal disease.
References
Boas I. Diagnostik und Therapie der Magenkrankheiten. Leipzig: Georg Thieme; 1925.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71.
Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–60.
Tanaka M, Ma E, Tanaka H, Ioka A, Nakahara T, Takahashi H. Trends of stomach cancer mortality in Eastern Asia in 1950-2004: comparative study of Japan, Hong Kong and Singapore using age, period and cohort analysis. Int J Cancer. 2012;130:930–6.
Forman D, Bray F, Brewster DH, Gombe Mbalawa C. Kohler B, Piñeros M, et al. Cancer incidence in five continents, vol. X (electronic version). Lyon: International Agency for Research on Cancer; 2013. http://ci5.iarc.fr/Default.aspx. Accessed 11 Jan 2015.
Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881–8.
Arnold M, Moore SP, Hassler S, Ellison-Loschmann L, Forman D, Bray F. The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut. 2014;63:64–71.
Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–9.
Lim KG. Malays in peninsular Malaysia may have the lowest incidence of stomach cancer in the world. Med J Malaysia. 2009;64:91–2.
Kimman M, Norman R, Jan S, Kingston D, Woodward M. The burden of cancer in member countries of the Association of Southeast Asian Nations (ASEAN). Asian Pac J Cancer Prev. 2012;13:411–20.
Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–16.
Shen ZL, Song KY, Ye YJ, Xie QW, Liang B, Jiang K, et al. Significant differences in the clinicopathological characteristics and survival of gastric cancer patients from two cancer centers in China and Korea. J Gastric Cancer. 2015;15:19–28.
Dassen AE, Dikken JL, Bosscha K, Wouters MW, Cats A, van de Velde CJ, et al. Gastric cancer: decreasing incidence but stable survival in the Netherlands. Acta Oncol. 2014;53:138–42.
Seyedin S, Wang PC, Zhang Q, Lee P. Benefit of adjuvant chemoradiotherapy for gastric adenocarcinoma: a SEER population analysis. Gastrointest Cancer Res. 2014;7:82–90.
Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965–71.
Warthin A. Heredity with reference to carcinoma: as shown by the study of the cases examined in the pathological laboratory of the University of Michigan. Arch Intern Med. 1913;12:546–55.
Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487–92.
Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the evaluation of carcinogenic risks to humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241.
Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53.
Simán JH, Engstrand L, Berglund G, Forsgren A, Florén CH. Helicobacter pylori and CagA seropositivity and its association with gastric and oesophageal carcinoma. Scand J Gastroenterol. 2007;42:933–40.
Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–90.
Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–80.
Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91–9.
Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: a HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–70.
Hunt RH, Camilleri M, Crowe SE, El-Omar EM, Fox JG, Kuipers EJ, et al. The stomach in health and disease. Gut. 2015;64:1650–68.
Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–82.
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on management of Helicobacter pylori gastritis. Gut. 2015;64:1353–67.
Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection – the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–64.
Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa. Endoscopy. 2012;44:74–94.
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–94.
de Vries AC, Kuipers EJ, Rauws EA. Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol. 2009;104:1342–5.
Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820–32.
Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–13.
Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201.
Peleteiro B, Lopes C, Figueiredo C, Lunet N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104:198–207.
Zhong C, Li KN, Bi JW, Wang BC. Sodium intake, salt taste and gastric cancer risk according to Helicobacter pylori infection, smoking, histological type and tumor site in China. Asian Pac J Cancer Prev. 2012;13:2481–4.
Bonequi P, Meneses-González F, Correa P, Rabkin CS, Camargo MC. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control. 2013;24:217–31.
Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16:312–27.
Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: a meta-analysis. J Natl Cancer Inst. 2006;98:1078–87.
González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:345–54.
González CA, Pera G, Agudo A, Palli D, Krogh V, Vineis P, et al. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer. 2003;107:629–34.
Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut. 2010;59:39–48.
Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701.
Yeh JM, Hur C, Schrag D, Kuntz KM, Ezzati M, Stout N, et al. Contribution of H. pylori and smoking trends to US incidence of intestinal-type noncardia gastric adenocarcinoma: a microsimulation model. PLoS Med. 2013;10:e1001451.
Inoue M, Sasazuki S, Wakai K, Suzuki T, Matsuo K, Shimazu T, et al. Green tea consumption and gastric cancer in Japanese: a pooled analysis of six cohort studies. Gut. 2009;58:1323–32.
Liu H, Hua Y, Zheng X, Shen Z, Luo H, Tao X, et al. Effect of coffee consumption on the risk of gastric cancer: a systematic review and meta-analysis of prospective cohort studies. PLoS One. 2015;10:e0128501.
Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23:28–36.
Eom BW, Joo J, Kim S, Shin A, Yang HR, Park J, et al. Prediction model for gastric cancer incidence in Korean population. PLoS One. 2015;10:e0132613.
Buckland G, Travier N, Huerta JM, Bueno-de-Mesquita HB, Siersema PD, Skeie G, et al. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int J Cancer. 2015;137:598–606.
Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895–905.
Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the national cancer screening programme in Korea. Br J Cancer. 2015;112:608–12.
Yoshida T, Kato J, Inoue I, Yoshimura N, Deguchi H, Mukoubayashi C, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134:1445–57.
Amiri M, Janssen F, Kunst AE. The decline in stomach cancer mortality: exploration of future trends in seven European countries. Eur J Epidemiol. 2011;26:23–8.
de Vries AC, Meijer GA, Looman CW, Casparie MK, Hansen BE, van Grieken NC, et al. Epidemiological trends of pre-malignant gastric lesions: a long-term nationwide study in the Netherlands. Gut. 2007;56:1665–70.
de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–52.
den Hoed CM, van Eijck BC, Capelle LG, van Dekken H, Biermann K, Siersema PD, et al. The prevalence of premalignant gastric lesions in asymptomatic patients: predicting the future incidence of gastric cancer. Eur J Cancer. 2011;47:1211–8.
Anderson WF, Camargo MC, Fraumeni Jr JF, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–8.
Holster IL, Aarts MJ, Tjwa ET, Lemmens VE, Kuipers EJ. Trend breaks in incidence of non-cardia gastric cancer in the Netherlands. Cancer Epidemiol. 2014;38:9–15.
Roosendaal R, Kuipers EJ, Buitenwerf J, van Uffelen C, Meuwissen SG, van Kamp GJ, et al. Helicobacter pylori and the birth cohort effect: evidence of a continuous decrease of infection rates in childhood. Am J Gastroenterol. 1997;92:1480–2.
den Hoed CM, Vila AJ, Holster IL, Perez-Perez GI, Blaser MJ, de Jongste JC, et al. Helicobacter pylori and the birth cohort effect: evidence for stabilized colonization rates in childhood. Helicobacter. 2011;16:405–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Kuipers, E.J. (2016). Gastric Cancer: Synopsis and Epidemiology of Gastric Cancer. In: Kim, N. (eds) Helicobacter pylori. Springer, Singapore. https://doi.org/10.1007/978-981-287-706-2_21
Download citation
DOI: https://doi.org/10.1007/978-981-287-706-2_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-705-5
Online ISBN: 978-981-287-706-2
eBook Packages: MedicineMedicine (R0)