Abstract
Until recently, gastric cancer was the most common cause of cancer deaths. Despite the rapid fall in incidence, gastric cancer is still the fifth most common cancer and third leading cause of cancer-related mortality worldwide. The lifetime risk of gastric cancer to age 74 remains between 1 and 3% in most European countries and as high as 5–20% in some parts of Asia. The discovery of Helicobacter pylori as the cause of atrophic gastritis, the lesion that predisposes to gastric cancer, has resulted in increasing attempts to eliminate gastric cancer by eradicating H. pylori. Here we review the worldwide changes in gastric cancer incidence and the current lifetime risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Until the mid- to late twentieth century, gastric cancer was the most common cause of cancer-related mortality in most countries [1]. In the United States (USA), gastric cancer retained that title until 1952 when the age-adjusted cancer mortality rate of lung cancer surpassed gastric cancer in men [2]. Gastric cancer’s prominence in the nineteenth century is evidenced by the large body of literature from that era dedicated to it. For example, the 1903 English translation of Riegel’s book Diseases of the Stomach cites 158 references from 1878 to 1896 and refers to Leube in Ziemssen’s Handbook of Special Pathology and Therapy for the older literature up to the late 1870s [3]. Examples include Marc d’Espine who reported that between 1838 and 1855, 45% of fatal cancers in Geneva were gastric [4]. Virchow, using autopsy material, calculated that between 1852 and 1855 in Wurzburg, Germany, 34.6% of all fatal cancers were gastric [3]. In 1889, Haberelin in Switzerland found 11,422 gastric cancers (41.5%) among 27,500 cancers that ended fatally [5]. The UK Registrar General’s annual report from 1897 to 1900 found that gastric cancer was the leading cause of death (20.6%) among 12 different malignant diseases [4].
Worldwide statistics remained rare until 1915 when Frederick L. Hoffman, statistician for the Prudential Insurance Company of America and chairman of the Committee of Statistics, American Society for the Control of Cancer, published a book entitled The Mortality from Cancer Throughout the World [1]. Hoffman’s book was one of the first that attempted to pull together all available data and to detail plans for subsequent collection of cancer statistics. He provided data from 23 countries and innumerable subpopulations pointing out the difficulties in obtaining reliable data. His book confirmed that in most countries, including Western countries and Japan, gastric cancer was the most common cause of cancer-related death [1].
2 Gastric Cancer and Gastritis
The late nineteenth century was also a time of intense interest in gastric physiology and diseases, including gastric cancer and peptic ulcer disease. One focus was on histologic damage to the stomach, gastritis, and its relation to disease [6]. The association of gastric cancer with chronic gastritis and gastric atrophy was well established in the late 1800s and early 1900s [7, 8]. Both Charles Mayo and Arthur Hurst are credited with the statement that gastric carcinoma never involves a healthy stomach [9, 10]. In 1879, von den Vender made the association of gastric cancer with achlorhydria [8, 11], which was confirmed and greatly expanded (e.g., by Comfort in 1934) [12]. von den Vender’s observation stimulated extensive research examining acid secretion and gastritis [8, 11]. In the Schorstein Lecture of 1929, Sir Arthur Hurst reported that gastrectomy specimens of patients with chronic ulcer and carcinoma had consistently shown gastritis to be present throughout the stomach [9]. He further noted that “chronic gastritis, which precedes the onset of carcinoma, is in fact the most common predisposing condition” [9] and that “the ideal form of prophylaxis [for gastric cancer] would be not merely to recognize and treat the common precancerous gastric conditions, chronic gastritis and chronic gastric ulcer, but to prevent their development” [9].
By 1950, the gastritis-carcinoma sequence had been firmly established, with gastritis recognized as the soil from which cancer arose [8, 13]. Research on gastritis continued with many studies worldwide seeking to discover the cause of gastritis, which would likely also be the cause of gastritis-associated diseases, including peptic ulcer disease and gastric cancer. Unfortunately, the discovery of Helicobacter pylori and proof that it was the major cause of gastritis was not immediately integrated into this plethora of data regarding gastritis. Instead, this new finding attracted many new investigators who seemed ignorant of prior research. Additionally, most early epidemiology studies regarding gastric cancer and H. pylori were based on H. pylori serology, which, in retrospect, provided misleading data as serology often becomes negative following development of gastric atrophy [14,15,16]. Thus, early studies significantly underestimated the attributable risk of H. pylori on gastric cancer. Proof that gastric cancer was strongly linked to gastric atrophy, which was previously established by early researchers, had to be rediscovered before it caused investigators to relate H. pylori to gastric cancer. Rather than confirm that the long sought-after cause of gastritis, gastric cancer, and peptic ulcer had been found, observations (e.g., such as the association of H. pylori with gastric cancer) were treated as unique, deserving to be published in the most prestigious journals. This delay in recognizing that the discovery of H. pylori closed the loop in the long search for the cause of gastric cancer and peptic ulcer disease resulted in the delayed realization that elimination of H. pylori could eliminate both diseases.
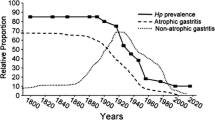
It is now well accepted that the most common cause of gastric cancer is infection with H. pylori and that the major risk factor is the development of atrophic gastritis [8, 17,18,19]. Factors that affect H. pylori infection and/or atrophic gastritis can influence gastric cancer incidence. The impact of environmental factors, such as diet or H. pylori infection, on the incidence and pattern of gastric disease is best appreciated when analyzing birth cohorts [20]. Increased access to clean water and improved sanitation over time have resulted in reduced H. pylori infection rates among younger generations compared to older generations [18]. Additionally, the anatomic pattern of H. pylori gastritis (pangastritis vs. antral predominant) leads to different disease presentations. Pangastritis with atrophy presents as gastric ulcer and cancer, whereas antral predominant gastritis presents as duodenal ulcer disease. Thus, a change in the pattern of disease from predominantly gastric ulcer and gastric cancer to duodenal ulcer among Western countries in the late nineteenth and early twentieth century signified a change in the pattern of gastritis [18]. In contrast, the overall decline in gastric cancer and both gastric and duodenal ulcers in the latter part of the twentieth century reflected the progressive fall in H. pylori prevalence [18].
3 The Gastric Cancer Cascade
In 1975, Correa described a cascade of superficial gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and finally gastric cancer [21, 22]. He termed the basic pattern of gastritis “multifocal atrophic gastritis.” It is now recognized that his description of a multifocal process was related to his method of determining the pattern of intestinal metaplasia (e.g., staining for sucrase activity) (Fig. 2.1). While multifocal staining identified intestinal metaplasia, it could not recognize the lawn of underlying atrophy then called pyloric or pseudopyloric metaplasia that represented the primary atrophic event [23, 24]. Pyloric metaplasia is now easily recognized histologically by immunohistochemical staining of corpus mucosa for spasmolytic polypeptide (SPEM) [25]. Islands of intestinal metaplasia develop within this lawn of SPEM within which gastric cancer arises (Fig. 2.2) [23, 24]. The cascade is now best described as H. pylori infection, superficial gastritis, atrophic gastritis, metaplastic epithelia, intramucosal neoplasia, and finally invasive cancer. Most no longer believe that intestinal metaplasia evolves into gastric cancer. However, the stem cell for gastric cancer remains unknown [26]. Nonetheless, mucosal atrophy and loss of parietal cells in the corpus results in hypochlorhydria or achlorhydria which forms the milieu in which gastric cancer develops [13, 27]. Extensive atrophy of the gastric corpus also leads to decreased numbers of chief cells, which primarily produce pepsinogen (PG) I. Serum PGI levels < 70 μg/L or PGI/PGII ratio < 3 is widely used as a biomarker for severe atrophic gastritis involving the corpus [28, 29].
Antral mucosa (yellow) (a) or pseudopyloric metaplasia (light yellow) (b). Atrophy advances as proximally into the corpus as a lawn of pseudopyloric metaplasia that can also be recognized by immunohistochemical staining for spasmolytic polypeptide (SPEM). Islands of intestinal metaplasia (blue hatch) subsequently develop within the lawn and over time may expand (c)
The severity and extent of atrophy is now often classified using a five-point scale (0–4), the Operative Link for Gastritis Assessment (OLGA) or Gastric Intestinal Metaplasia Assessment (OLGIM) staging systems, used for cancer risk stratification [30, 31]. The OLGIM system, which stages the severity of intestinal metaplasia in the corpus and antrum, has been reported to show better interobserver agreement compared to the OLGA [30]. Scores of 3 or 4 are associated with a markedly increased risk of gastric cancer [31]. Higher OLGA staging has also been shown to correlate with the results of PGI/PGII ratio testing. Intestinal metaplasia is an easily recognizable manifestation of atrophy and, in the right circumstance, identifies the presence of severe atrophy that may warrant surveillance in high-risk populations [28]. Conversely, focal gastric intestinal metaplasia without background atrophy can also develop after mucosal injury and is not associated with heightened gastric cancer risk [29].
4 Atrophic Gastritis, Intestinal Metaplasia, and Gastric Cancer Risk
Worldwide, gastric cancer risk reflects the prevalence and age of onset of atrophic gastritis [17]. For example, in a Swedish cohort, the annual incidence of gastric cancer was 20/100,000 person-years in those with non-atrophic mucosa, 100/100,000 person-years in atrophic gastritis, and highest in those with intestinal metaplasia (129/100,000 person-years) and intramucosal neoplasia (263/100,000 person-years) [32]. In a US cohort of 4146 patients in northern California with atrophic gastritis, as evidenced by the presence of intestinal metaplasia, the incidence of gastric carcinoma was 72/100,000 person-years, which was 2.6 times the risk seen in the general population [33]. The incidence of gastric carcinoma rose to 767/100,000 person-years in those with low-grade intramucosal neoplasia. In a similar study in southern California, the age-adjusted incidence of gastric carcinoma arising in a cohort with atrophic gastritis, evidence by the presence of intestinal metaplasia, was 172/100,000 person-years compared to 9.67/100,000 person-years in the reference population [34]. The risk of carcinoma with gastric atrophy is especially heightened in non-Whites. In the Northern California study, Hispanics independently predicted the development of gastric carcinoma in the context of atrophic gastritis after adjusting for age and H. pylori status [33].
5 Worldwide Epidemiology of Gastric Cancer
In 1975, gastric cancer was the most common cause of cancer worldwide [35]. Since then, gastric cancer incidence has declined. Now, gastric cancer is the fifth most common cancer and third leading cause of cancer-related mortality worldwide [as reported by the World Health Organization (WHO) International Agency for Research on Cancer (IARC) 2012 data] [35]. The incidence appears to be decreasing due to improved diet and food storage (less use of salt and more fresh fruits, vegetables, and vitamin C) [36, 37], decreased smoking [37], improved sanitation, and reduced transmission of H. pylori infection [38].
As early as 1915, it was clear that the risk of gastric cancer was not uniform and was reduced among populations with predominantly vegetarian diets [1]. For example, among 1000 autopsies performed in India, there was a single case of gastric cancer, and among 396 cases of carcinoma seen at the Mayo Hospital in Lahore, Pakistan between 1882 and 1903, there was no case of gastric cancer [1].
Figures 2.3, 2.4, 2.5, 2.6, and 2.7 illustrate the cumulative lifetime risk of gastric cancer, the risk to age 74, in males from 2012 by country. The cancer incidence shown was reported from the WHO IARC GLOBOCAN project, which is the highest quality data on cancer incidence for 184 countries [35]. The 2012 GLOBOCAN estimates of cancer incidence were reported based on Cancer Incidence in Five Continents volumes IX (1998–2002) and X (2003–2007) [39, 40]. Additionally, Fig. 2.7 shows the reported gastric cancer incidence for subpopulations at highest risk based on Cancer Incidence in Five Continents volumes VIII (1993–1997) and X (2003–2007) [40, 41].
Cumulative lifetime risk of gastric cancer up to age 74 in males by country in Africa for 2012 calculated from [35]
Cumulative lifetime risk of gastric cancer up to age 74 in males by country in North and Central/South Americas for 2012 calculated from [35]
Cumulative lifetime risk of gastric cancer up to age 74 in males by country in Asia for 2012 calculated from [35]
Cumulative lifetime risk of gastric cancer up to age 74 in males by country in Europe and Oceania for 2012 calculated from [35]
For all countries with data available in 2012 and 1997, gastric cancer risk has decreased over time with the exception of Uganda, Algeria, Cuba, China, Vietnam, India, and Denmark. The highest cumulative lifetime risk of gastric cancer is in La Reunion, Mauritius, and Kenya in Africa (Fig. 2.3); Chile, Guatemala, and Costa Rica in Central/South America (Fig. 2.4); South Korea, Mongolia, and Japan in Asia (Fig. 2.5); and Belarus, Russia, and Albania in Europe (Fig. 2.6). The lifetime risk to age 74 remains less than 1% in most African countries and between 0.5 and 3% in most European and American countries. Some subpopulations with highest cumulative risk of gastric cancer are shown in Fig. 2.7. The highest rates of gastric cancer are in specific counties of China, Japan, and South Korea, with the highest cumulative risk (22%) in Yangcheng County, China.
6 Quality in Reporting of Cancer Incidence and Mortality
Gastric cancer epidemiologic data quality has varied over time and still varies across countries. Early on, in the nineteenth and much of the twentieth century, gastric cancer reporting and data collection were not standardized. During that era, gastric cancer statistics relied on autopsy-derived collectible statistics. The reporting was likely biased based on who underwent autopsies (which varied by socioeconomic or other factors), which autopsies were recorded (e.g., population deaths vs. hospital-specific deaths), and which autopsies may not have reflected disease patterns on a population level. Additionally, cited cause of death in the early literature may have inaccurately classified cancer-related death (e.g., cause of death recorded as cachexia rather than gastric cancer).
When interpreting gastric cancer incidence, the age structure of the background population must be accounted for. Since the majority of gastric cancers occur after age 60, populations with shorter life expectancies are expected to have a lower gastric cancer incidence. This phenomenon may partly explain the low incidence observed in African countries. Also, failure to age-adjust in a population with a shorter life expectancy can markedly underestimate the gross incidence of disease, predominantly in the elderly.
The World Health Organization (WHO)-reported cancer incidence is age-standardized to allow for comparisons between groups and time intervals. However, the quality of cancer incidence data varies greatly between countries. Some countries provide high-quality data from >50% of the population (e.g., USA), whereas other countries have high-quality data from <10% of the population (e.g., China). Some developing countries are classified as having only lower-quality regional data available (e.g., Kenya). Therefore, since standardization in reporting of cancer varies widely across countries, variability in estimation of cancer incidence may exist.
7 Conclusions
Variability in gastric cancer statistical reporting has posed some limitations in interpreting temporal trends in gastric cancer patterns. Over the past three decades, the WHO IARC has provided high-quality gastric cancer data, but there continues to be variability in the quality of data reported by each country. Gastric cancer was the leading cause of cancer-related death until 1975. Since then, gastric cancer incidence has been declining worldwide, likely due to improved diet and food storage, decreased smoking, and decreased H. pylori transmission. Currently, South Korea, Mongolia, and Japan have the highest lifetime risk of gastric cancer. Eradication of H. pylori will eventually make gastric cancer a rare disease [42].
References
Hoffman FL. The mortality from cancer throughout the world. Newark: Prudential Press; 1915.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
Rielgel F. Carcinoma of the stomach. In: Stockton CG, editor. Diseases of the stomach in Nothnagel’s encyclopedia of practice of medicine. Nothnagel’s practice. Philadelphia: W.B. Saunders & Company; 1903. p. 644.
Williams WR. The increase of cancer, and its concomitants. In: Williams WR, editor. The natural history of cancer. New York: William Wood and Company; 1908. p. 55.
Haberlin H. Uber verbreitung und aetiologie des magenkrebses. Ueber neue diagnostische Humsmittel beim magenhkrebs. Arch F Klin, Med; Xlv. 1889.
Faber K. Chronic gastritis: its relation to achylia and ulcer. Lancet. 1927;2:902–7.
Faber K. Gastritis and its consequences. Paris: Oxford University Press; 1935.
Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45:1–8.
Hurst AF. Schorstein lecture on the precursors of carcinoma of the stomach. Lancet. 1929;214:1023–8.
Bockus HL. Carcinoma of the stomach. In: Bockus HL, editor. Gastroenterology, vol. 1. Philadelphia: W.B. Saunders Co.; 1963. p. 748.
von den Velden R. Ueber vorkommen und mandgel der freien salzsaure in magensaft bei gastrektasie. Deutsches Arch F Klin Med. 1879;23:369–99.
Comfort MW, Vanzant FR. Gastric acidity in carcinoma of the stomach. Am J Surg. 1934;26:447–56.
Comfort MW. Gastric acidity before and after development of gastric cancer: its etiologic, diagnostic and prognostic significance. Ann Intern Med. 1951;36:1331–48.
Kikuchi S, Nakajima T, Kobayashi O, et al. Effect of age on the relationship between gastric cancer and Helicobacter pylori. Tokyo Research Group of Prevention for Gastric Cancer. Jpn J Cancer Res. 2000;91:774–9.
Masci E, Viale E, Freschi M, et al. Precancerous gastric lesions and Helicobacter pylori. Hepatogastroenterology. 1996;43:854–8.
Urita Y, Hike K, Torii N, et al. Serum pepsinogens as a predicator of the topography of intestinal metaplasia in patients with atrophic gastritis. Dig Dis Sci. 2004;49:795–801.
Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35(Suppl 12):90–7.
Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–204.
Graham DY, Shiotani A. The time to eradicate gastric cancer is now. Gut. 2005;54:735–8.
Wang C, Weber A, Graham DY. Age, period, and cohort effects on gastric cancer mortality. Dig Dis Sci. 2015;60:514–23.
Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60.
Correa P, Cuello C, Duque E, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027–35.
El-Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–36.
Graham DY, Kato M, Asaka M. Gastric endoscopy in the 21st century: appropriate use of an invasive procedure in the era of non-invasive testing. Dig Liver Dis. 2008;40:497–503.
Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46.
Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–31.
Miftahussurur M, Yamaoka Y, Graham DY. Helicobacter pylori as an oncogenic pathogen, revisited. Expert Rev Mol Med. 2017;19:e4.
Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–8.
El-Zimaity HMT, Gutierrez O, Kim JG, et al. Geographic differences in the distribution of intestinal metaplasia in duodenal ulcer patients. Am J Gastroenterol. 2001;96:666–72.
Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–8.
Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–6.
Song H, Ekheden IG, Zheng Z, et al. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867.
Li D, Bautista MC, Jiang SF, et al. Risks and predictors of gastric adenocarcinoma in patients with gastric intestinal metaplasia and dysplasia: a population-based study. Am J Gastroenterol. 2016;111:1104–13.
Reddy KM, Chang JI, Shi JM, et al. Risk of gastric cancer among patients with intestinal metaplasia of the stomach in a US Integrated Health Care System. Clin Gastroenterol Hepatol. 2016;14:1420–5.
Ferlay JSI, Soerjomatarm I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide; IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013.
Tsugane S, Akabane M, Inami T, et al. Urinary salt excretion and stomach cancer mortality among four Japanese populations. Cancer Causes Control. 1991;2:165–8.
Kamada T, Haruma K, Ito M, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter. 2015;20:192–8.
Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150:1113–24.
Curado MP, Edwards B, Shin HR, et al. Cancer incidence in five continents, Vol. IX. IARC Sci Publ. 2007;160:1–837.
Forman D, Bray F, Brewster DH, et al. Cancer incidence in five continents, Vol. X. IARC Sci Publ. 2014;164:1–1365.
Parkin DM, Whelan SL, Ferlay J, et al. Cancer incidence in five continents, Vol. VIII. IARC Sci Publ. 2002;155:1–781.
Lee YC, Chiang TH, Liou JM, et al. Mass Eradication of Helicobacter pylori to prevent gastric cancer: theoretical and practical considerations. Gut Liver. 2016;10:12–26.
Acknowledgments
Support: Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center.
Disclosures: Dr. Graham is a consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies. Drs. Tan and Balakrishnan do not have any relevant disclosures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tan, M.C., Balakrishnan, M., Graham, D.Y. (2019). Gastric Cancer Worldwide Except Japan. In: Shiotani, A. (eds) Gastric Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-13-1120-8_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-1120-8_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1119-2
Online ISBN: 978-981-13-1120-8
eBook Packages: MedicineMedicine (R0)