Abstract
Static magnetic field (SMF) exists in nature widely and plays an essential role in the biological evolution. Due to the rapid development of superconducting technology, the intensities of SMFs used for medical and academic research purposes have steadily increased in recent years. This chapter presents an overview on the biological effects induced by SMFs with intensities ranging from mT to several Teslas (T). The effects of SMFs on microorganisms are divided into six sections, including cellular growth and viability, morphological and biochemical modifications, genotoxicity, gene and protein expression, magnetosome formation sensing magnetic field, and application of SMFs on antibiotic resistance, fermentation, and wastewater treatment. The effects of SMFs on plants are divided into six sections, including germination, growth, gravitropism, photosynthesis, redox status, and cryptochromes (CRYs) sensing magnetic field. The effects of SMFs on animals are divided into seven sections, including Caenorhabditis elegans, insects, Helix pomatia, aquatic animals, Xenopus laevis, mice and rats, and magnetic sensing protein in animals. This chapter will be very helpful for better understanding the biological responses to SMFs in different species and their underlying mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Static magnetic field (SMF) is a ubiquitous environmental factor for all living organisms during the evolution process. A variety of organisms including bacteria, algae, snails, planaria, honeybee, salmon, lobsters, salamanders, homing pigeons, robins, mice, and possibly humans have been demonstrated the ability to sense magnetic fields (MFs) for orientation in navigation, migration, homing, escaping, and nest building (Qin et al. 2016). Although the biophysical mechanisms of magnetoreception are poorly understood, three main hypothesis for magnetosensing have been proposed: (1) magnetic induction, which can only be applied to marine creatures, owing to the high conductivity of salt water; (2) the magnetite hypothesis that proposes a process mediated by crystals of permanently magnetic material (magnetite) with an evolutionary genetics hypothesis for magnetite formation; (3) the radical pair mechanism (RPM) which relies on a chemical reaction involving specialized photoreceptors (Fedele et al. 2014; Bellinger et al. 2022). However, the recently proposed MagR/Cry-based biocompass model combines the concepts of ferrimagnetism and the involvement of Cry in magnetoreception, which have also attracted a lot of attention (Qin et al. 2016).
Since the Industrial Revolution in the 1850s, human-made sources of SMFs have become inevitable environmental factors for organisms on Earth. In particular, the development of electromagnets in the nineteenth century and superconducting magnets in the mid-twentieth century has greatly increased the risk of the exposure of organisms to higher magnetic fields. Acute and chronic exposure of organisms to SMFs, which are often ten and more times greater than geomagnetic fields, have been investigated for decades. However, the exact mechanisms underlying the influence of SMFs on living systems are still largely unknown, and until now there is no unique theory about magnetic field–organism interaction. In this review, we limit our discussion on the evidence of the biological response of SMFs with the intensities ranging from a few mT to several Teslas (T) on microorganisms, plants, and animals and explore recent results on the investigation of magnetoreception in these organisms.
7.2 SMFs on Microorganisms
7.2.1 SMFs on Cellular Growth and Viability
The influence of magnetic fields with various flux densities on the growth rate and viability of microbes has been investigated in bacteria, yeast, and plant pathogenic fungi. Under low and moderate magnetic fields, the inhibition on the growth of microorganisms has been reported in various bacteria species. Bajpai et al. (2012) showed that a SMF of 100 mT suppressed the growth of both gram-positive (S. epidermidis) and gram-negative bacteria (Escherichia coli, E. coli), which was related to the cellular membrane damage. Fan et al. (2018) discussed the effect of long-term exposure to a moderate SMF on Enterococcus faecalis and showed that the cellular proliferation of Enterococcus faecalis (E. faecalis) was inhibited by a SMF of 170 mT with 120 h exposure. The moderate SMFs in the range from 50 to 500 mT on the growth of Streptococcus pyogenes (S. pyogenes) was investigated by Morrow et al. (2007) and was found that the growth inhibition was observed up to 300 mT, but an increase in growth rate when cells were exposed to 500 mT. Although a SMF of 300 mT had no influence on the growth of E. coli in nutrient rich Luria Bertani (LB) medium, it increased bacterial cell culture density during late growth in diluted LB (Potenza et al. 2004). El May et al. (2009) also reported that a SMF of 200 mT failed to alter cellular growth but induced a decrease of colony-forming units (CFU) between 3 and 6 h followed by an increase from 6 to 9 h. Ben Mouhoub et al. (2018) showed that 57 mT SMF improved the viability of Salmonella Hadar compared with the control group. Under high magnetic fields, Kazuhiro et al. (1997) reported that the cellular growth of Bacillus subtilis MI113 and genetically transformed B. subtilis MI113 (pC112) was significantly increased by exposure to homogeneous 7 T and inhomogeneous 5.2–6.1 T magnetic fields. Moreover, a SMF of 5.2–6.1 T promoted survival rate of E. coli B cells of stationary phase, which the CFU number and the amount of S factor encoded by the rpoS gene were much higher than that under a geomagnetic field (Horiuchi et al. 2001). These observations suggest that SMFs are not always negative to the growth of microorganism, which are closely related to the intensities of SMFs, types of bacteria, and exposure manners.
The combined effects of SMF and other environmental factors on the growth of microorganisms are largely unknown. Ji et al. (2009) showed that a SMF of 450 mT inhibited the growth and even killed E. coli, in which the inhibitory effect was increased with temperature. Masahiro et al. (2000) compared the effect of SMF exposure up to 100 mT on the culture of Streptococcus mutans (S. aureus) and E. coli grown in aerobic and anaerobic conditions. They found that the bacterial growth was inhibited by the SMF in anaerobic conditions, but remained unaffected when the SMF was applied in aerobic conditions, indicating that oxygen played an inhibitory effect for the magnetic field. Letuta and Berdinskiy (2019) found that the concurrent treatment of isotope 67Zn and 25–35 mT SMF increased the colony formation ability and growth rate constant of E. coli by 2–4 times compared with non-magnetic zinc isotopes 64,66Zn.
There are few studies on the growth and sporulation of phytopathogenic microscopic fungi under the static magnetic fields. Nagy and Fischl (2004) showed that the applied magnetic fields with flux intensities ranging from 0.1 to 1 mT decreased the growth of phytopathogenic fungi colonies and the number of Fusarium oxysporum conidia, while the number of the developed conidia of Alternaria alternata and Curvularia inaequalis was increased. Maria Cristina et al. (2003) and Jan et al. (2007) provided further evidence on the growth depression of fungi exposed to SMF. A 1.5–2 times faster growth rate was found in Aspergillus niger exposed to a static B-field varying from 40 to 80 T than in sham controls and the B-field exposure could have an effect on the biodegradability of materials by enhancing the growth rate and the aggressiveness of the fungus. However, Ruiz-Gómez et al. (2004) reported that magnetic fields had no effect on fungal growth.
In yeast, Lucielen Oliveira et al. (2010) showed that a SMF of 25 mT resulted in an increase of glutathione content and biomass in Saccharomyces cerevisiae (S. cerevisiae). Muniz et al. (2007) reported that the biomass (g/L) increment of S. cerevisiae DAUFPE-1012 was 2.5 times greater in cultures exposed to 220 mT SMF as compared with non-exposed cultures. Kthiri et al. (2019) furtherly reported that under the treatment of 250 mT SMF, the growth and viability of Saccharomyces cerevisiae and colony formation decreased significantly after 6 h, but increased from 6 to 9 h. In contrast, Malko et al. (1994) reported that yeast cells subjected to a static MF of 1.5 T over the course of seven cell divisions displayed growth rates similar to unexposed cells, indicating that moderate SMF had minimal effect on the growth of yeast. With the intensity increase of SMFs, Masakazu et al. (2004) found that gradient magnetic fields of 14 T exhibited the decelerated growth in a liquid–gas mixture system.
7.2.2 SMF on Morphological and Biochemical Modifications
The morphological study of SMF treated cells using transmission electron microscope (TEM) revealed that bacterial cell wall was ruptured by SMF exposure (Ji et al. 2009). Quiñones-Peña et al. (2017) reported that the prototype of E. coli: strain-EPEC E2348/69 exposed to 107 mT SMF reduced its aggregation and altered the adhesion pattern, which was related to the expression of its BFP cilia. A SMF of 200 mT significantly altered the phospholipid proportions in Salmonella typhimurium (S. typhimurium) wild type and dam mutant strain, which the most affected were those of the acidic phospholipids, cardiolipins (CL) (Mouadh et al. 2012). Egami et al. (2010) investigated the effect of SMFs on the budding of S. typhimurium and found that the size of budding yeast cells and the budding angle were affected by a SMF of 2.93 T. In homogeneous magnetic field, the budding direction of daughter yeast cells was mainly oriented in the direction of magnetic field B; in contrast, in inhomogeneous magnetic field, the daughter yeast cells tended to bud along the axis of capillary flow in regions where the magnetic gradient was high.
Microorganisms as models for analyzing fundamental metabolic responses to magnetic fields have great advantages, as they represent simple unicellular organisms. Letuta (2020) found that the maximum concentration of ATP was generated under the action of the magnetic isotope 25Mg and an electro SMF of 70–90 mT. The composition of membrane lipids in S. typhimurium was disturbed by 200 mT SMF, which the bacteria tried to change SFA, UFA, and CFA, and hydroxyl FA levels to maintain membrane fluidity, while the UFAs/SFAs ratio of Salmonella reached equilibrium after 9 h of exposure (Ramla et al. 2017). Similarly, Mihoub et al. (2012) showed that a SMF of 200 mT significantly affected the lipid proportions in membrane, leading to an unusual accumulation of the acidic phospholipids cardiolipins, with a significant increase of membrane cyclic fatty acids and a meaningful increase of the total unsaturated fatty acids to total saturated fatty acids ratios of the exposed cells. Tang et al. (2019) exposed Flavobacterium m1–14 to 100 mT SMF for 0, 24, 48, 72, or 120 h, respectively, and found that the length of the cells increased significantly by SMF treatment. Compared with the control group, after 24 h, 48 h, 72 h, and 120 h of 100 mT SMF treatment, the length of the cell increased by 123%, 258%, 70.1%, and 31.2%, respectively; among them, the cells treated by the magnetic field for 48 h were more elongated (Fig. 7.1). The inhibition of mycelia growth by a SMF of 300 mT was accompanied by morphological and biochemical changes, and Ca2+-dependent signal transduction pathways were involved in conidia germination (Maria Cristina et al. 2003). The patterns of metabolites released from S. pyogenes exposed to different magnetic flux intensities ranging from 50 to 500 mT were significantly altered (Morrow et al. 2007). A SMF of 250–300 mT elicited the maximal release of the majority of metabolites. Hu et al. (2009) reported that the composition and conformation of nucleic acid, protein, and fatty acid of E. coli were altered by 10 T SMF, which were reflected by the changes of spectral region of Fourier-transform infrared (FTIR) spectroscopy combined with cluster analysis. She et al. (2009) further found that 3.46–9.92% of the disorder coils in the secondary structures of protein were altered into α-helices by 10 T SMF; in contrast, 10 T SMF had little influence on Staphylococcus aureus (S. aureus).
Scanning electron micrographs of the cellular morphology following different treatments. (a) Untreated bacteria; (b) exposed to 100 mT SMF for 24 h; (c) exposed to 100 mT SMF for 48 h; (d) exposed to 100 mT SMF for 72 h; (e) exposed to 100 mT SMF for 120 h; (f) length of bacteria in different exposure times. [Reprinted with permission from (Tang et al. 2019)]
7.2.3 SMF on Genotoxicity
In living organisms, the production of free radicals has the potential to interact with DNA and plays an important role both in the aging process and environmental stress related adverse effects. Exposure of cells to 300 mT SMF significantly reduced the yield of 8-hydroxyguanine in extracted DNA compared to controls, suggesting some possible antioxidant protection to S. pyogenes at this field strength (Morrow et al. 2007). Carlioz and Touati (1986) showed an induction of the expression of a soxS::lacZ fusion gene following strong SMF exposure. Fan et al. (2018) confirmed that Enterococcus faecalis (E. faecalis) could induce a stress response by upregulating the expression of dnaK gene and the expression of virulence genes efaA and ace under the treatment of SMF. Righi et al. (2020) exposed irradiated Deinococcus radiodurans (D. radiodurans) cells to SMF and found that their cell viability was improved, which might be due to the improvement of the efficiency of DNA fragment recombination by SMF exposure.
The direct evidence on the genotoxicity of SMFs is limited and controversial. Mahdi et al. (1994) exposed various mutant strains of E. coli to a homogeneous SMF of either 500 mT or 3 T. No evidence of increased DNA damage was detected in SMF-sexposed E. coli, even with bacterial strains disabled for DNA repair. Masateru et al. (1999) performed a bacterial mutation assay to determine the mutagenic potential of SMF. No mutagenic effects were detected in four uvrB strains of S. typhimurium (TA98, TA100, TA1535 and TA1537) and E. coli WP2uvrA. Schreiber et al. (2001) also reported that exposures to a SMF of 7.2 T did not show any alteration in the number of His+ revertants in Salmonella mutagenicity test. Yoshie et al. (2012) reported that no statistically significant differences in the mutation frequency in thymine synthesis genes were observed between SMF-exposed cells and unexposed cells at any of the applied magnetic flux intensities. SMFs up to 13 T caused neither mutagenicity nor co-mutagenicity in the superoxide dismutase (SOD)-deficient E. coli strain QC774 or in its parental strain GC4468, suggesting that exposure to high SMFs did not affect the behavior of superoxide in these microorganisms. However, the modification of chromatin conformation was reported in E. coli cells by Belyaev et al. (1994). Zhang et al. (2003) showed a dose–response relationship between the magnetic flux intensity (5 and 9 T SMF) and an increase in mutation frequency in the SOD-deficient E. coli strain QC774.
7.2.4 SMF on Gene and Protein Expression
Differential gene expression is a critical event, common to all biological systems, allowing the accurate response under normal conditions and adaptation to various environmental stresses including magnetic fields. Tsuchiya et al. (1999) reported that inhomogeneous magnetic fields ranging from 5.2 to 6.1 T enhanced the transcription of the rpoS gene in E. coli. Three cDNAs were found to be expressed only in E. coli exposed to 300 mT SMF, whereas one cDNA was more expressed in the controls (Potenza et al. 2004). El May et al. (2009) found that the expression level of the 16S rRNA mRNA in Salmonella Hadar (S. Hadar) remained stable during the exposure of 200 mT SMF, while mRNAs of rpoA, katN, and dnaK genes were over-expressed following 10 h of SMF exposure. Ikehata et al. (2003) reported that a slight decrease in the expression of genes related to respiration was observed in the budding yeast, Saccharomyces cerevisiae (S. cerevisiae), exposed to 14 T SMF, whereas no changes were observed with field strengths <5 T. Although 14.1 T SMF caused little effects on cell growth of Shewanella oneidensis (S. onedensis) MR-1, apparent changes at transcriptional levels were detected in exposed cells, in which 21 genes were upregulated while other 44 genes were downregulated (Gao et al. 2005). In contrast, Potenza et al. (2012) reported that no differences were observed in gene expression in Tuber borchii mycelium after exposure to SMF, and only the activities of glucose 6-phosphate dehydrogenase and hexokinase were increased. These results indicated that the effects of the magnetic fields on the expression of genes are variable and dependent on parameters applied as well as the cell type.
Protein is the essential unit for biological activities in cells and their functions are determined by the sequence of amino acid including primary and tertiary structures of protein. Snoussi et al. (2012) investigated the effect of 200 mT SMF on the outer membrane protein pattern in S. Hadar. They found that a total of 11 proteins displaying more than a twofold change were differentially expressed in exposed cells, among which 7 were upregulated and 4 downregulated. The proteomic analysis provided a further overview of potentially important cytosolic proteins, in which a total of 35 proteins displaying more than a twofold change were differentially expressed in exposed cells, among which 25 were upregulated and 10 were downregulated. The stress response to a SMF of 200 mT was essentially set up to avoid oxidative damages, with the overexpression of proteins directly involved in oxidative stress response and metabolic switches to counteract oxidative stress (Snoussi et al. 2016).
7.2.5 Magnetosome Formation Sensing Magnetic Fields
Microbial magnetosomes represent a special category of intracellular organelles that are synthesized by magnetotactic bacteria (MTB). As a group of Gram-negative aquatic prokaryotes, MTB had a broad range of morphological types, including vibrioid, coccoid, rod, and spirillum. They used the magnetosomes to sense and modify their orientation according to the magnetic field (Moisescu et al. 2014). Magnetosomes comprised magnetic iron-bearing inorganic crystals enveloped by an organic membrane (Staniland et al. 2007). The membrane of magnetosomes contained a unique set of proteins that were thought to direct the biomineralization of magnetite crystals and magnetosome chain formation and regulation (Komeili et al. 2004). Forty-eight proteins were identified as magnetosome-specific proteins in Magnetospirillum magneticum (M. magneticum) AMB-1, and at least 13 proteins were potentially involved in the formation of magnetosomes, which were encoded by the mam and mms genes (Matsunaga et al. 2005). Among the genes known to be essential for magnetosome formation, magA, mms6, mamA, and mms13 were involved in iron uptake (Chikashi et al. 1995; Grünberg et al. 2001), synthesis of magnetite crystals of a uniform size and narrow size distribution with a cubo-octahedral morphology (Amemiya et al. 2007), magnetosome assembly (Komeili et al. 2004), and formation of magnetosomes, respectively. The superior crystalline and magnetic properties of magnetosomes have been attracting much interest in studying biomineralization and medical applications such as drug delivery, magnetic resonance imaging, and array-based assaying (Yoshino and Matsunaga 2006; Matsunaga et al. 2007; Barber-Zucker et al. 2016).
Wang et al. (2008) found that exposure to hypomagnetic field less than 500 nT restrained the growth of M. magneticum strain AMB-1 during the stationary phase, but increased the percentage of bacteria that contained mature SD magnetosomes in their exponential growth phase. The average size of magnetic particles in cells exposed to hypomagnetic field was larger (>50 nm) and they contained a larger proportion (57%) of SD particles compared to those grown in the geomagnetic field only. 200 mT SMF could impair the cellular growth and raise Cmag values of the cultures (Wang et al. 2009). The number of magnetic particles per cell and the linearity of magnetosome chain were affected by SMF exposure. Moreover, the expression of mamA, mms13, magA genes was upregulated by SMF. Blondeau et al. (2018) explored the effect of magnetotactic bacterium AMB-1 magnetosome chain alignment under conditions of limited external magnetic field. The bacteria were under a silica matrix, and some bacteria exposed to a field of 80 mT exhibited several magnetic lines. The chains of magnetosomes were arranged parallel to each other but offset relative to the longitudinal axis of the bacteria as shown in Fig. 7.2.
Electron microscopy observations of magnetic and crystallographic orientations in magnetosomes. (a) TEM image of an AMB-1 cell in suspension observed after 7 days of incubation in absence of a magnetic field (black arrow shows the selected chain for off-axis image), (b) corresponding magnetic phase contours of magnetosome chains determined by off-axis EH (d, g) TEM images of encapsulated AMB-1 bacteria observed after 7 days of incubation in presence of a magnetic field (white arrows show the selected chains for off-axis images), (e, h) corresponding magnetic phase contours of magnetosomes chains determined by off-axis EH, and (c, f, i) corresponding HRTEM images with 〈111〉 directions determined by using Selected Area Electron Diffraction (SAED) and materialized by yellow bars. [Reprinted from (Blondeau et al. 2018), open access]
7.2.6 Application of Static Magnetic Fields on Antibiotic Resistance, Fermentation, and Wastewater Treatment
The application of SMF of 0.5 ± 2 mT significantly enhanced the activity of the antibiotic gentamicin against Pseudomonas aeruginosa (Benson et al. 1994). Stansell et al. (2001) found that exposure of E. coli to SMF of 4.5 mT significantly increased its antibiotic resistance. Tagourti et al. (2010) showed that exposure to a 200 mT SMF increased the efficiency of gentamicin against S. Hadar, but did not affect the diameter of the inhibition zone of some other antibiotics actives on Enterobacteria: penicillin, oxacillin, cephalotin, neomycin, amikacin, tetracycline, erythromycin, spiramycin, chloramphenicol, nalidixic acid, and vancomycin. However, Grosman et al. (1992) reported that a SMF of 0.5 ± 4.0 T had no significant influence on the growth of two strains of E. coli or S. aureus after exposure time of 30 ± 120 min, nor were there any effects on sensitivity to several antibiotics.
The influence of SMF on fermentation process has been investigated in biomass, and enzyme activity (Motta et al. 2001). da Motta et al. (2004) showed that exposure to 220 mT SMF significantly increased the biomass (g/L) of S. cerevisiae strain by 2.5-fold and the concentration of ethanol by 3.4-fold as compared with SMF non-exposed cultures. Glucose consumption was higher in magnetized cultures, which was correlated to the ethanol yield. Invertase is an enzyme (b-fructofuranosidase, EC 3.2.1.26) used to produce noncrystallizable sugar syrup from sucrose. Taskin et al. (2013) showed that the maximum invertase activity and biomass concentration were achieved with the spores exposed to 5 mT SMF.
Enhancement of biochemical processes by SMF has been applied in biological wastewater treatment. SMF had a positive effect on activated sludge biomass growth and dehydrogenase activity, which was similar to the observation in p-nitroaniline removal with activated sludge (Niu et al. 2014). Low and moderate SFMs could enhance the activities and growth of nitrite-oxidizing bacteria, increasing the removal of organic pollutants from wastewater (Jia et al. 2018). The effect of SMF exposure on the biodegradation rate of a mixture of pollutants was investigated by three strains including Pseudomonas stutzeri LBR (KC157911), Cupriavidus metallidurans LBJ (KU659610), and Rhodococcus equi LBB (KU743870) isolated and identified near Bizerte, Tunisia. Mansouri et al. (2019) applied 200 mT to these three strains and found there was an increase by 20% in the growth of the exposed bacterial population compared to controls, and 98% of biodegradation of DDT and 90% for BaP after 30 days of follow-up. The efficiency of phenol biodegradation was greatly increased by 30% under moderate SMFs (Kriklavova et al. 2014). Krzemieniewski et al. (2003) reported that a SMF of 400–600 mT stimulated the conditioning of wastewater sludge. A significant 30% increase in maximum nitrogen removal rate and an approximate 1/4 saving in cultivation time were achieved by using a SMF of 60 mT, indicating that the magnetic field was useful and reliable for fast start-up of anammox process (Liu et al. 2008). In algal-bacterial symbiotic system, Tu et al. (2015) reported that SMF stimulated both algal growth and oxygen production, suggesting that magnetic field could reduce the energy consumption required for aeration during the degradation of organic matter in municipal wastewater. Although SMFs have shown interesting potential in biodegradation of wastewater, there are some negative results. Mateescu et al. (2011) showed that SMFs of 500 and 620 mT produced an atypical growth in the fungus that was characterized by less and swollen, bombastic colonies which did not spread on the entire surface of the culture medium. Jasmina et al. (2012) reported that SMF (B = 17 mT) negatively influenced the growth of E. coli and Pseudomonas putida that were commonly found in wastewater treatment plants, but positively influenced enzymatic activity.
In addition to the application in wastewater biological treatment, SMFs also have broad application prospects for decolorization and de-oiling. In terms of decolorization reactions, Shao et al. (2019) studied the decolorization effect of marine microbial communities on azo dyes under SMF and found that the decolorization, chemical oxygen demand (COD) removal, and detoxification efficiency were higher at 45.3 mT SMF. Tan et al. (2020) found that the SMF and the salt-tolerant yeast Candida tropicalis SYF-1 co-enhanced SBR (named MSF-SBR) had higher and more stable ARB (acidity) under high salt and continuous operating conditions Red B processing efficiency (Shao et al. 2019). Ren et al. (2018) studied the effect of SMF on the high-efficiency oil-removing bacteria Acinetobacter B11, and the results showed that under a low-intensity magnetic field of 15–35 mT, the permeability of the cell membrane was increased and superoxide disproportionation was improved. Enzyme (SOD) activity effectively enhanced the lipid degradation performance of bacteria.
7.3 SMF on Plants
7.3.1 SMF on Germination
Magnetic seed treatment is one of the physical presowing seed treatments that have been reported to enhance the germination of crop plants. The rate and percentage of germination were increased by low and moderate SMFs in barley seed, rice (Oryza sativa L.) seeds, chickpea (Cicer arietinum L.) seeds, sunflower seeds, bean seeds, wheat seeds, okra (Abelmoschus esculentus cv. Sapz pari), garden pea (Pisum sativum L. cv. climax), mung beans seeds, onion seeds (c.v. Giza Red), and cumin seeds. However, there are few reports on negative results of germination stimulated by moderate SMFs. The effects of SMFs at various intensities and exposure periods on the germination of different plants were summarized in Table 7.1.
The coeffects of SMFs with other factors on germination have been investigated to obtain higher germination. Poinapen et al. (2013) investigated the magnetic flux intensity, together with exposure time, seed orientation (North and South polarity), and relative humidity (RH) in tomato (Solanum lycopersicum L.) var. MST/32 seeds. They found that higher germination (∼11.0%) was observed in magnetically exposed seeds than in non-exposed ones, suggesting a significant effect of non-uniform SMFs on seed performance with respect to RH, and more pronounced effects were observed during seed imbibition rather than during later developmental stages. Jovicic-Petrovic et al. (2021) found that the synergistic effect of B. amyloliquefaciens D5 ARV and 90 mT exposure increased the germination rate of white mustard (Sinapis alba L.) by 53.20%.
The mechanism of SMF on germination is not very clear. Bahadir et al. (2018) reported that 125 mT SMF treatment improved the germination of Lathyrus chrysanthus Boiss by breaking dormancy. Raipuria et al. (2021) showed that 200 mT SMF promoted nitric oxide via nitric oxide synthase to ameliorate the UV-B stress during germination of soybean seedlings. Kataria et al. (2020) reported the role of nitric oxide (NO) at 200 mT SMF induced seed germination and early growth characteristics of soybean (Glycine max) seedlings under salt stress and found that pretreatment of seeds with 200 mT SMF positively stimulated the germination and then promoted the seedling growth.
7.3.2 SMF on Growth
The effects of SMFs on growth have been well studied in various seeds of crop, vegetable, and fruit. Extremely low magnetic field at 47 ± 5 μT promoted the maize seedling growth (Hajnorouzi et al. 2011). Besides, Vashisth and Nagarajan (2010) found that under the same conditions, seedlings of sunflower showed higher seedling dry weight, root length, root surface area, and root volume; moreover, in germinating seeds, enzyme activities of amylase, dehydrogenase, and protease were significantly higher in treated seeds than controls as shown in Fig. 7.3. The beneficial effects of low SMFs on the growth have been well investigated in potato plantlets, barley seeds, soybean, corn, Zea mays, pea, and radish seedlings as shown in Table 7.2.
Effect of pre-germination exposure of sunflower seeds on (a) speed of germination and (b) seedling vigor. [Reprinted with permission from (Vashisth and Nagarajan 2010)]
7.3.3 SMF on Gravitropism
Gravitropism is the most conspicuous response to the gravitational force in plants, which plays an essential role in maintaining the spatial orientation of seedlings and stable balance of massive plants. The ability of plants to sense gravity is largely attributed to starch-filled amyloplasts, which is a long-lived response throughout the entire life. Kuznetsov and Hasenstein (1996) reported that high-gradient magnetic fields (HGMFs) induced intracellular magnetophoresis of amyloplasts. The shoots of lazy-2 mutant of tomato (Lycopersicon esculentum Mill., cv. Ailsa Craig) exhibited negative gravitropism in the dark, but responded positively gravitropically in red light. The induced magnetophoretic curvature showed that lazy-2 mutants perceived the displacement of amyloplasts in a similar manner than wild type and the high MF did not affect the graviresponse mechanism (Hasenstein and Kuznetsov 1999). Weise et al. (2000) reported that Arabidopsis stems positioned in a high-gradient magnetic field (HGMF) on a rotating clinostat showed the lack of apical curvature after basal amyloplast displacement, indicating that gravity perception in the base was not transmitted to the apex. Jin et al. (2019) reported that root growth was significantly enhanced by SMFs in an intensity and magnetic direction dependent way, which was mediated by CRY and auxin signaling pathways in Arabidopsis. Hasenstein et al. (2013) examined the movement of starch grains of corn, wheat, and potato (Solanum tuberosum) in suspension during parabolic flights and found that magnetic gradients were able to move diamagnetic compounds under weightless or microgravity conditions and serve as directional stimulus during seed germination in low-gravity environments. Yano et al. (2001) reported that the primary roots of radish (Raphanus sativus L.) seedlings responded tropically to the 13–68 mT SMF with the tropism appearing to be negative and the roots responded significantly to the south pole of the magnet.
7.3.4 SMF on Photosynthesis
The effects of SMF on the photosynthesis have been investigated in various plants including soybean, corn, Lemna minor, and lettuce. Shine et al. (2011) reported that presowing magnetic treatment could improve biomass accumulation in soybean. Polyphasic chlorophyll a fluorescence transient from magnetically treated soybean plants gave a higher fluorescence yield. Baghel et al. (2016) provided further evidence that polyphasic chlorophyll a fluorescence (OJIP) transient from magnetically treated plants gave a higher fluorescence yield at J–I–P phase. Moreover, nitrate reductase activity, PIABS, photosynthetic pigments, and net rate of photosynthesis were also higher in plants that emerged from soybean seeds exposed to 200 mT SMF. In corn plants, Anand et al. (2012) reported that SMFs of 100 and 200 mT increased the photosynthesis, stomatal conductance, and chlorophyll content. The pretreatment of seeds of two corn cultivars with different magnetic treatments significantly alleviated the drought-induced adverse effects on growth by improving chlorophyll, photochemical quenching, and non-photochemical quenching (Javed et al. 2011). Jan et al. (2015) found that the reduced geomagnetic field (GMF) significantly stimulated growth rate of the total frond area in the magnetically treated Lemna minor plants, while the enhanced GMF pointed toward inhibition of growth rate in exposed plants in comparison to control, but the difference was not statistically significant. All photosynthetic pigments in lettuce seeds (Lactuca sativa var. capitata L.) were induced markedly under 0.44 T, 0.77 T, and 1 T SMF, especially chlorophyll a (Latef et al. 2020).
There are few studies on the coeffects of SMFs and other environmental factors on photosynthesis. Kataria et al. (2021) reported that 200 mT SMF pretreatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Fatima et al. (2021a) found that 200 mT SMF pretreatment caused enhancement of leaf growth along with photosynthesis even under the presence of ambient UV-B stress. Moreover, pretreatment with 50–300 mT SMF increased water uptake by the midrib of soybean (Glycine max, variety JS-335), which in turn led to an increase in photosynthesis and stomatal conductance (Fatima et al. 2017). In addition, Jovanić and Sarvan (2004) reported that SMF induced significant changes in bean leaf fluorescence spectra and temperature, which the fluorescence intensity ratio (FIR) and change of leaf temperature βT were increased with the increase of MF intensity.
7.3.5 SMF on Redox Status
The uncoupling of free radicals including reactive oxygen/nitrogen species (ROS/RNS) is involved in the underlying mechanism of SMF induced oxidative stress in plants. The activities of free radical scavenging enzymes, including catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione transferase (GT), peroxidase (POD), ascorbate peroxidase (APX), and polyphenol oxidase (POP), have been well documented to be altered by SMF exposure in various plants, including pea, radish (Raphanus sativus), Leymus chinensis, soybean, cucumber (Cucumis sativus), broad bean, corn, parsley (Petroselinum crispum), and wheat (Regoli et al. 2005; Baby et al. 2011; Jouni et al. 2012). Mohammadi et al. (2018) found that 0.2 mT SMF increased the contents of nitric oxide (NO), hydrogen peroxide (HO), and salicylic acid (SA) in tobacco cells (Nicotiana tabacum cv. Barley 21), and suggested that a signaling pathway activated by SMF starting from accumulation of NO and HO, then increased the cyclic nucleotides and subsequent decreased the cyclin-dependent kinases A (CDKA) and D-type cyclin (CycD). Cakmak et al. (2012) reported that SMF of 7 mT increased lipid peroxidation and H2O2 levels in shallot (Allium ascalonicum) leaves. Jouni et al. (2012) found that treatment of plants with 15 mT SMF caused accumulation of reactive oxygen species (ROS), lowered the antioxidant defense system, and increased the peroxidation of membrane lipids in broad bean (Vicia faba L.). Shokrollahi et al. (2018) found that 20 mT SMF decreased ferrous and HO contents, content and activity of ferritin and catalase in soybean plants, but the opposite responses were observed under 30 mT treatments. Shine et al. (2012) showed that SMFs of 150 and 200 mT enhanced production of ROS mediated by cell wall peroxidase, while the increase in the cytosolic peroxidase activity indicated that this antioxidant enzyme had a vital role in scavenging the increased H2O2 produced in seedlings from the magnetically treated soybean seeds. In mung bean seedlings treated with 600 mT SMF followed by cadmium stress, Chen et al. (2011) found that the concentration of malondialdehyde, H2O2, and O− were decreased, while the NO concentration and NOS activity were increased compared to cadmium stress alone, indicating that MF compensates for the toxicological effects of cadmium exposure were related to NO signal.
7.3.6 Cryptochromes Sensing Magnetic Field
Cryptochromes (CRYs) are flavoproteins that direct a diverse array of developmental processes in response to blue light in plants (Yu et al. 2010). CRY has been suggested to be a potential magnetoreceptor for light-initiated electron transfer chemistry which might be magnetically sensitive to virtue of the radical pair mechanism (Evans and Davidson 2013; Hore and Mouritsen 2016). Geomagnetic field (GMF) has been hypothesized to affect the redox balance of cryptochromes and the related signaling state (Vanderstraeten et al. 2015); however, the influence of strong SMF on the function of CRYs is still largely unexplored.
Three CRYs, CRY1, CRY2, and CRY3 are encoded in Arabidopsis genome (Lin and Todo 2005). CRY1 and CRY2 function as major blue light receptors regulating blue light induced de-etiolation, photoperiodic flowering, and circadian clock (Liu et al. 2016). Xu et al. (2014) found SMF of 500 μT modified the function of CRYs. The blue light-dependent phosphorylations of CRY1 and CRY2 were enhanced in Arabidopsis seedlings grown in a 500 μT MF, whereas the near-null MF weakened the blue light-dependent phosphorylation of CRY2 but not CRY1; in the darkness, dephosphorylations of CRY1 and CRY2 were slowed down in the 500 μT MF, whereas dephosphorylations of CRY1 and CRY2 were accelerated in the near-null MF. According to the calculation of radical pair mechanism in a relatively realistic model of the radical pair system in Arabidopsis CRY1, Solov’yov et al. (2007) showed that 500 μT MF could increase the signaling activity of cryptochrome by up to 10%, suggesting that the function of CRYs was affected by magnetic field. Pooam et al. (2019) investigated the response of Arabidopsis CRY1 in vivo to 500 μT SMF using both plant growth and light-dependent phosphorylation as an assay, then they found that the magnetically sensitive reaction step in the cryptochrome photocycle must occur during flavin reoxidation, and likely involved the formation of ROS. Ahmad et al. (2007) reported that 500 μT MF enhanced the blue light-dependent inhibition of hypocotyl growth of Arabidopsis. Hypocotyl growth of Arabidopsis mutants lacking CRYs was unaffected by the increase of magnetic intensity, while cryptochrome-dependent responses, such as blue light-dependent anthocyanin accumulation and blue light-dependent degradation of CRY2 protein, were enhanced at the higher magnetic intensity. However, with experimental conditions chosen to match Ahmad’s study, Harris et al. (2009) found that in no case consistent, statistically significant MF responses were detected.
CRYs evolved from photolyases are conserved across many different species. In addition to plants, the expression of CRYs has been detected in migratory birds and the eyes of mammals, which were putative sites for magnetoreceptors in vertebrates, and there was no evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening (Möller et al. 2004; Nießner et al. 2013; Edelman et al. 2015). In animals, CRYs also functioned as circadian photoreceptors in the Drosophila brain, mediating the light resetting of the 24 h clock; but in vertebrates, the CRYs acted as the main negative regulators for the circadian feedback loop, due to the difference in light sensing (Yoshii et al. 2009; Fedele et al. 2014). Non-Drosophila insects can also encode CRY1 and CRY2, but CRY1 retain their light-sensing properties, whereas the CRY2s act as vertebrate-like negative regulators. Marley et al. (2014) reported that MF exposure coupled with CRY photoactivation during embryogenesis was sufficient to produce heightened seizure susceptibility in resultant Drosophila third instar (L3) larvae. Giachello et al. (2016) provided evidence that exposure to a MF of 100 mT was sufficient to potentiate the ability of light-activated cryptochrome to increase neuronal action potential firing, indicating that the activity of cryptochrome was sensitive to an external MF that was capable of modifying animal behavior.
7.4 SMF on Animals
7.4.1 SMF on Caenorhabditis elegans
Caenorhabditis elegans (C. elegans) is a small free-living nematode that has been widely utilized to address fundamental questions of developmental biology, neurobiology, and behavioral biology. C. elegans is similar to higher eukaryotes in many molecular and cellular pathways (Kaletta and Hengartner 2006) and offers unique advantages, including the ease of maintenance, small size, short life cycle, genetic manipulability, stereotypical development, and high-throughput capability. As about 50% of its genes have human homologs, C. elegans based assays are increasingly used to evaluate potential toxicity of different stressors in humans and mechanisms of toxicity by physical and chemical exposures (Kazazian Jr. 2004; Dengg and van Meel 2004; Rajini et al. 2008; Sprando et al. 2009; Boyd et al. 2010).
Recent evidence has shown that the C. elegans oriented to the earth’s magnetic field during vertical burrowing migrations neuron pair (Vidal-Gadea et al. 2015). A pair of neurons called the AFD neurons, which carry information about temperature and chemical stimuli from the environment, were critical for magnetic navigation in C. elegans. The further investigation showed the unique spatiotemporal trajectories of magnetotactic processes in C. elegans under different external conditions including temporal, spatial, and environmental factors. They found that the magnetic orientation of these “small worm” might be stronger under dry conditions (<50% RH) (Bainbridge et al. 2020). Using worms with mutations at some of the genes expressed in the AFD neurons and a calcium sensitive protein, it was found that the tax-4 gene, which encoded an ion channel protein similar to a photoreceptor found in the retina of human eyes, was required for magnetotaxis (Rankin and Lin 2015). These data represented a significant advance in our understanding of the neurobiology underlying how organisms navigate using the Earth’s magnetic field. Recently, Cheng et al. (2022) found that exposure C. elegans to 0.5 T and 1 T SMFs greatly decreased the avoidance behavior of the pathogenic Pseudomonas aeruginosa. The total serotonin level was significantly increased by exposure to 0.5 T and 1 T SMF; in contrast, SMFs had few effects on other three neurotransmitters including choline, γ-aminobutyric acid (GABA), dopamine as shown in Fig. 7.4. These data indicated that moderate-intensity SMFs induced neurobehavioral disorder might be modulated by serotonin in C. elegans.
Effects of 0.5 T and 1 T SMFs exposure for 48 h on neurons and neurotransmitters of C. elegans. (a) Fluorescence imaging of each neurotransmitter neurons; from top to bottom are cholinergic neurons, GABAergic neurons, dopaminergic neurons, and serotonergic neurons. (b) Analysis of fluorescence intensity of four neurotransmitter systems (n ≥ 30 nematodes/group). (c) Serotonin concentrations after long-term exposure to SMF. [Reprinted with permission from (Cheng et al. 2022)]
The biological effects of SMFs on C. elegans have been focused on the development, aging process, behavior, and global gene expression. Hung et al. (2010) reported that treatment with 200 mT SMF reduced the development time from the L2 to the L3 stage by 20%, from L3 to L4 by 23%, and from L4 to young adult by 31%. With SMF treatment, the average lifespan was reduced from 31 to 24 days in wild-type nematodes. The upregulation of lim-7, clk-1, daf-2, unc-3, and age-1 by SMF treatment was verified by quantitative real-time PCR; in contrast, lifespan analyses showed that SMF treatment had no effect on let-7, unc-3, and age-1 mutants, indicating that the induction of gene expression by SMFs was selective and dose-dependent. Lee et al. (2012) showed that long-term and low-dosage exposure to 200 mT SMF was capable of inducing an apoptosis-mediated behavioral decline in nematodes. 26 differentially expressed genes including apoptosis, oxidative stress, and cancer-related genes were identified, indicating that a global molecular response to SMF exposure occurred. Mutations in genes involved in major apoptotic pathways, that is, ced-3, ced-4, and ced-9, abolished this SMF-induced behavioral decline. Kimura et al. (2008) reported that genes involved in motor activity, actin binding, cell adhesion, and cuticles were transiently and specifically induced by 3 or 5 T SMF exposure in C. elegans. Several genes encoding apoptotic cell-death activators and secreted surface proteins were upregulated by ionizing radiation, instead of SMFs. Exposure to 3 or 5 T SMFs did not induce DNA double-strand breaks or germline cell apoptosis during meiosis. However, we found that 8.5 T SMFs resulted in a time-dependent lifespan decrease and alteration of development rate and stages in C. elegans. Germ cell apoptosis dramatically increased upon exposure to 8.5 T SMF in worms via core apoptotic machinery, which could be prevented by concurrent treatment with a free radical scavenger, dimethyl sulfoxide (Wang et al. 2015). Yang et al. (2022) further explored the biological effects of 10 T SMF on sperms and their offspring in him-5 male mutants of C. elegans and found that sperms were sensitive targets of high SMFs as shown in Figs. 7.5 and 7.6. Although 10 T SMF had little effect on the morphology of sperms, the size of unactivated sperms and the function of sperms were modified by SMF exposure, leading to diminish the reproductive capacity of him-5 male worms. These observations provided interesting information regarding the adverse effects of high SMFs on the reproductive function of C. elegans and their offspring, which could improve our understanding of the fundamental aspects of high SMFs on biological system.
10 T SMF accelerated the activation of sperms. (a) The male him-5 mutants were exposed to 10 T SMF, and the premature activation of sperm was measured. White arrows represent activated sperm (pseudopodia). (b) The percentage of premature activation of sperm with 10 T SMF exposure. (c) Relative mRNA expression of swm-1and try-5 genes in male him-5 mutants with 10 T SMF exposure. Data were pooled from three independent experiments. Error bars indicate ± SEM; *p < 0.05, compared with the control group. Scale bars, 50 μm. [Reprinted with permission from (Yang et al. 2022)]
Graphical abstract. Effects of 10 T static magnetic field on the function of sperms and their offspring in Caenorhabditis elegans. [Reprinted with permission from (Yang et al. 2022)]
7.4.2 SMF on Insects
Magnetic fields have been shown to affect the orientation, oviposition development, fecundity, and behaviors for a wide variety of insects. The insect eggs have advantages in magnetic exposure for a large number of eggs which can be placed into the magnet at the same time. The SMF at 4.5 mT had no effect on egg lying, but increased mortality of eggs, larvae, and pupa, and diminished adult viability in Drosophila (Ramirez et al. 1983). Decreased hatching rate after exposure to a weak SMF during early embryogenesis was also obtained in D. melanogaster and Heliothis virescens (tobacco budworm) (Ho et al. 1992; Pan 1996). A significant increase of Hylotrupes bajulus viability and larval mass was reported after exposure to a SMF of 98 mT (Rauš Balind et al. 2009). The SMF of 60 mT reduced the embryonic and post-embryonic development and induced weaker viability in two different species, Drosophila melanogaster and Drosophila hydei (Savić et al. 2011). Todorovic et al. (2019) found that chronic exposure to 110 mT SMF significantly decreased the gut mass and the activity of glutathione reductase (GR) and glutathione S-transferase (GST) as compared to the control in Blaptica dubia (B. dubia). They further reported that 110 mT SMF decreased nymph body mass and glycogen content in the fat body but increased all examined parameters of locomotion, indicating that B. dubia nymphs were sensitive to SMF exposure (Todorovic et al. 2020). Oak and beech populations of Drosophila subobscura had longer development time, and lower viability was observed in N and S groups of 2.4 T SMF, which was mediated by oxidative stress (Todorović et al. 2015). Apparent hatching delay of strong magnetic fields was observed in mosquito eggs in the center of 9.4 and 14.1 T magnets (Pan and Liu 2004).
In insects, the neuroendocrine system is a main regulator of all aspects of life processes, such as development and behavior, and the detection and activity of an external magnetic field may be transmitted by the neuroendocrine system (Blanchard and Blackman 1994; Gilbert et al. 1996). A SMF of 375 mT caused the disturbance of development and survival of pupae of the honeybee and Tenebrio molitor, yellow mealworm (Prolic and Jovanovic 1986; Prolić and Nenadović 1995). The morphometric parameters of the A1 and A2′ neurosecretory neurons of the protocerebrum as well as the morphometric parameters of the corpora allata were changed by a SMF of 320 mT (Perić-Mataruga et al. 2008). However, SMF of 50 mT did not effect on pupa-adult development dynamic of two examine Tenebrio species, but modulated their motor behavior (Todorović et al. 2013).
The antennal lobe of Drosophila provides an ideal intact neural network model to investigate neural circuit function (Ng et al. 2002). Yang et al. (2011) found that a SMF of 3.0 T modilated the rhythmic spontaneous activities of large LNs and correlated activity of ipsilateral pairs of large LN/LN in Drosophila antennal lobe, indicating that Drosophila could be an ideal intact neural circuit model to evaluate the effects of magnetic field stimulations.
Mutagenic effects of a static magnetic field were investigated by increasing mutation rate in population of Drosophila exposed to magnetic field 10–12 times greater than geomagnetic one (Giorgi et al. 1992). Exposure to 2, 5, or 14 T fields caused a statistically significant enhancement in somatic recombination frequency in the postreplication repair-deficient flies, whereas the frequency of somatic recombination remained unchanged in the nucleotide excision repair-deficient flies and in DNA repair-proficient flies after exposure (Takashima et al. 2004).
7.4.3 SMF on Helix pomatia
Helix pomatia possesses simple nerve system and displays simple behavioral repertoire. Single identified neurons have been documented as a good experimental model for the relatively large size, easy manipulation, consistent position on the surface of the ganglia, and consistent type of synaptic connections. Nikolić et al. (2008) reported that the magnetic field of 2.7 mT intensity caused changes in the amplitude and duration of action potential of the Br neuron in subesophageal ganglia of the garden snail Helix pomatia, whereas the 10 mT magnetic field changed the resting potential, amplitude spike, firing frequency, and duration of action potential of the Br neuron. Moreover, significant increase of the activity of Na+/K+-ATPase and the expression of its α-subunit in nervous system were observed in Helix pomatia exposed to 10 mT SMF (Nikolić et al. 2013). With single, 30-min long, and whole body exposed to 147 mT, Hernádi and László (2014) reported that SMF exposure mediated peripheral thermal nociceptive threshold by affecting the serotonerg as well as the opioiderg system.
7.4.4 SMF on Aquatic Animals
Sea urchins are the only invertebrates with the same development patterns as mammals. Moreover, the gametes of sea urchins can be obtained easily, the eggs and early embryos are transparent, and the early development of embryos is highly synchronous. A SMF of 30 mT delayed the onset of mitosis in two species of sea urchins, Lytechinus pictus and Strongylocentrotus purpuratus. There was an eightfold increase in the incidence of exogastrulation in Lytechinus pictus embryos exposed to SMFs, while magnetic fields had no effects on species Strongylocentrotus purpuratus embryos (Levin and Ernst 1997). Exposure of fertilized eggs of Echinometra mathaei to 30, 40, and 50 mT of magnetic fields delayed the onset of early cleavage division and significantly decreased the cleaved cells for exposed embryos. As the increase of intensity of the magnetic fields, earlier appearances of abnormalities were observed (Sakhnini and Dairi 2004).
The interaction among neurons in escape circuit of crayfish has been well studied. As the lateral giant (LG) neuron was easy to access for electrophysiological study, Ye et al. (2004) found that exposure to SMF at 4.74–43.45 mT increased the amplitude of action potential (AP) in LG depending upon both the intensity of field and duration of field exposure, which was mediated by the increasing level of intracellular Ca2+ in the LG. The excitatory postsynaptic potential (EPSP) produced via electrical and chemical synapses in the lateral giant neuron was enhanced after 30 min of SMF exposure (8.08 mT). Perfusion of field-exposed crayfish bath solution or preloading of Ca2+ chelator and intracellular Ca2+ release blocker failed to observe the SMF-induced enhancement on EPSP (Yeh et al. 2008).
As an increasingly important model species in genetic and neurobehavioral studies, zebrafish (Danio rerio) is an excellent organism for better understanding the biological mechanism of SMFs. Using a fast, fully automated assay system relying on negative reinforcement, Shcherbakov et al. (2005) recorded statistically highly significant reactions to weak magnetic field changes in Mozambique tilapia, a fish migrating regularly between freshwater and the sea, and non-migratory zebrafish. Takebe et al. (2012) found that zebrafish responded to a magnetic field as weak as the geomagnetic field by bidirectional orientation with group-specific preferences regardless of close kinships. SMFs with density from 4.7 to 11.7 T profoundly disturbed the orientation and locomotion behaviors of adult zebrafish, and the independence of these effects from other sensory modalities suggested that they were mediated by the vestibular system as shown in Fig. 7.7 (Ward et al. 2014). In addition, the SMFs could be disrupting metabolism and immunity of the Caspian kutum fry during acute and subacute exposures (Loghmannia et al. 2015). Ge et al. (2019) showed that 9.0 T SMF exposure had no effect on the survival and overall development of zebrafish embryos, but slowed down the development speed of the whole animal. They surmised that microtubule and spindle positioning were perturbed under such high SMF.
Adult zebrafish behavior outside and inside of an 11.7 T vertical magnetic field. Tracing of adult zebrafish path in visible green light during 1 min prior to magnetic field entry (a) and during 1 min inside the magnet (b). X- and Y-position coordinates are displayed as a function of time. Upon entry into the magnet, fish swimming becomes erratic, with frequent rolling, tight circling and increased swimming velocity. [Reprinted from (Ward et al. 2014), open access]
7.4.5 SMF on Xenopus laevis
Xenopus embryos are thought to be a useful tool for studying vertebrate development, and gene expression for their embryogenesis is rapid and completed outside of the female. The hatching rate of embryos of the frog Rana pipiens subjected to the field of a 1 T permanent magnet was found to be reduced (Neurath 1968). Ueno et al. (1984) investigated embryos of African clawed toads exposed to 1 T magnetic field and found that the magnetic field exerted no harmful or modifying effects on gastrulation and neurulation; however, exposed embryos occasionally developed into tadpoles with reduced pigmentation, axial anomalies, or microcephaly. Compared to the first and the second cleavage, the third cleavage was the most susceptible to reorientation in strong SMFs. Exposure to SMF at 16.7 T altered the direction of the third cleavage furrow from its normal horizontal type to the perpendicular type, which was confirmed by embryos exposed to 8 T (Denegre et al. 1998; Eguchi et al. 2006). These results indicated that SMFs might act directly on the microtubules of the mitotic apparatus to cause distortion of the third cleavage furrow. Kawakami et al. (2006) found that a SMF of 11–15 T significantly retarded normal development and induced microcephaly, two heads, abnormal cement glands, and multiple malformations. Moreover, the gene expression of Xotx2 (an important regulator of fore and midbrain morphogenesis) and Xag1 (essential for cement gland formation) was greatly suppressed by strong SMF. Mietchen et al. (2005) investigated the morphology of fertilizable Xenopus laevis eggs with and without jelly coat that were subjected to a SMF of up to 9.4 T and found that no effect was observed when the jelly layers of the eggs were left intact, indicating the action of magnetic fields might involve cortical pigments or associated cytoskeletal structures normally held in place by the jelly layers.
The effects of SMF exposure on nerve conduction were investigated in frog sciatic nerves. A significant increase in the nerve conduction velocity (NCV) of compound action potentials (CAP) in sciatic nerves was observed by exposure to a uniform SMF of 1.16 T. Edelman et al. (1979) observed a significant increase in the amplitude of CAP in frog sciatic nerves when a uniform SMF of 385 or 600 mT was applied perpendicular to the axis of the nerve fibers. Although NCV of CAP was not affected by the 8 T SMF, Eguchi et al. (2003) reported that under SMF exposure an optimal time interval existed in the relative refractory period (1.0–1.1 ms) during which some ions move dynamically through specific ion channels. Satow et al. (2001) found that 0.65 T SMF increased excitability in bullfrog sartorius muscle during the recovery period in a conditioning-test stimulation paradigm. With the exposure of in vitro frog sciatic nerve fibers to moderate-intensity gradient SMF up to 0.7 T, Okano et al. (2012) found the values of the nerve conduction velocity of C fibers were significantly reduced by Bmax of 0.7 T SMF but not by 0.21 T SMF, relative to the unexposed control. Although the mechanistic reasons for this decrease have yet to be clarified, SMF could affect the behavior of some types of ion channels associated with C fibers.
7.4.6 SMF on Mice and Rats
7.4.6.1 SMF on Bone Growth, Healing, and Loss
SMF has been considered as a physical therapy on bone health maintenance and bone disorders treatment for it can enhance bone fracture healing and bone formation by osteoblast both in vivo and in vitro (Trock 2000; Miyakoshi 2005; Saunders 2005; Wang et al. 2011). Zhang et al. (2018a) found that 4 mT SMF could inhibit the structural deterioration of trabecular and cortical bone and reduce mechanical strength in T1DM rats. They compared the microstructure and mechanical properties of mouse bone under either hypomagnetic field (HyMF, 500 nT) or moderate SMF (MMF, 0.2 T) and found that exposure to MMF for 4 weeks had a significant effect on bone biology mechanical properties but bone microarchitecture was not affected, whereas HyMF significantly inhibited mouse growth and bone elasticity (Zhang et al. 2018b). Shan et al. (2021) reported that the tooth movement speed was significantly faster and the periodontal ligament (PDL) width was significantly increased under a SMF of 20–204 mT. 2–4 T SMFs improved bone microstructure and strength by stimulating bone formation and inhibiting bone resorption (Yang et al. 2021).
With implantation of magnetized rods into the middle diaphysis of rat femurs to generate SMF, Yan et al. (1998) found that the femurs adjacent to magnetized specimens had significantly higher bone mineral density (BMD) and calcium content than those adjacent to the unmagnetized specimen. The significantly reduced BMD in this ischemic bone model could be prevented by long-term SMF exposure of 3 weeks (Xu et al. 2001). The SMF accelerated not only the bone neoformation but also the integration of the bone grafts (Puricelli et al. 2009; Leesungbok et al. 2013). Kotani et al. (2002) showed that high SMF of 8 T stimulated ectopic bone formation in and around subcutaneously implanted bone morphogenetic protein (BMP) 2-containing pellets in mice, in which the orientation of bone formation was parallel to the magnetic field. Using ovariectomized (OVX) rat model to represent the clinical features of bone loss, Xu et al. (2010) observed that SMF significantly increased the BMD of osteoporotic lumbar vertebrate without affecting the E2 (17-b-estradiol) levels of serum compared with sham control. Taniguchi et al. (2004) examined the effect of the whole-body exposure to SMF on bone formation and found that SMF could contribute to the relief of pain induced by adjuvant arthritis and BMD was also accelerated significantly. However, with the same SMF exposure device, Taniguchi and Kanai (2007) reported that SMF did inhibit the bone loss of tibia in OVX rats to some extent, but its BMD was still much lower than normal rats, which might be due to the enhanced locomotor activity.
7.4.6.2 SMF on Cardiovascular System
7.4.6.2.1 Blood Pressure and Blood Flow
SMF in the mT range has been reported to modulate circulatory hemodynamics and/or arterial blood pressure (BP) and baroreflex sensitivity (BRS). Okano and Ohkubo (2003, 2005, 2006) found that whole-body exposure to SMF suppressed spontaneously hypertensive rats (SHR), which was mediated by nitric oxide (NO) pathway, Ca2+ channel, and hormonal regulatory systems. With the calculation of the hematological characteristics, Tasic et al. (2021) found that SMFs with different orientations had adverse effects on the hematological indicators of spontaneously hypertensive rats, but their cardiac and renal morphological features were not affected. Li et al. (2020) found that 20–150 mT SMF had antithrombotic effects in constructed rat and mouse thrombosis models, indicating a non-invasive prevention and treatment way for clot-related diseases.
It is well known that surface temperature and cutaneous blood flow are closely parallel to each other. Ichioka et al. (2003) reported that the whole body of anesthetized rats exposed to 8 T SMF was associated with reduced skin blood flow and temperature, which could be recovered after removal of the animal from the magnet. Both increases and decreases in skin and rectal temperatures were observed in mice exposed to SMFs with intensities ranging from 0.4 to 8 T. In contrast to these observations, no evidence was found for a change in body temperature of rodents exposed to strong homogeneous or gradient magnetic fields (Tenforde 1986).
7.4.6.2.2 Cardiac Function
Blood flow in an applied magnetic field gives rise to induce voltages in the aorta and other major arteries of the central circulatory system that can be observed as superimposed electrical signals in the electrocardiogram (ECG). The largest magnetically induced voltage occurs during pulsatile blood flow into the aorta and results in an increased signal at the location of the T-wave in the ECG. A marked increased T-wave in the ECG records was observed in squirrel monkeys during the exposure to stationary fields of 2–7 T and rabbits exposed to 1 T SMF (Beischer and Knepton Jr. 1964; Togawa et al. 1967). Similar observation was reported by Gaffey and Tenforde (1981) that a field strength dependent increase in the amplitude of the T-wave signal in the rat ECG was revealed during exposure to homogeneous stationary magnetic fields of 2 T, which might be due to a superimposed electrical potential generated by aortic blood flow in the presence of a stationary magnetic field. The exposure of rats to a SMF of 128 mT decreased the activities of glutathione peroxidase (GPx) and the CuZn superoxide dismutase (CuZn-SOD) in rat cardiac muscle (Amara et al. 2009).
7.4.6.2.3 Hematological Parameters
The effects of SMFs on hematological parameters have been studied in rats at the intensity of 128 mT. Amara et al. (2006b) reported that a SMF of 128 mT significantly decreased the growth rates, but increased the plasmatic total protein levels, hemoglobin, red blood cells, white blood cells, platelet number, and the activities of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) in male Wistar rats; in contrast, the glucose concentration was unaffected. Milovanovich et al. (2016) showed that both upward- and downward-oriented SMF of 128 mT caused a reduction in the amount of total white blood cells (WBC). Chater et al. (2006) found that subacute exposure to a SMF of 128 mT stimulated biosynthesis of plasma corticosterone and metallothionein activities in female rats, while increased blood glucose and decreased insulin release, leading to a diabetic-like state in pregnant rats. Elferchichi et al. (2016) showed an impaired glucose homeostasis and a deregulated lipid metabolism after SMF exposure in adult rats. But, they noticed that a SMF of 128 mT induced a pseudoanemia status with increased monocarboxylate transporters (MCT4) and glucose transporter 4 (Glut4). Atef et al. (1995) investigated changes of hemoglobin (Hb)’s characteristics in Swiss mice using hundreds of mT for 10 min and found that the rate of Hb oxidative reaction was declined by 350–400 mT. However, Djordjevich et al. (2012) found that differently oriented SMF of 16 mT did not alter hemoglobin and hematocrit, although the upward and downward fields caused statistically significant higher levels of serum transferrin.
In addition, the supplementation with vitamin D corrected and restored glycemia and insulinemia in SMF-exposed rats (Lahbib et al. 2015). Selenium (Se) improved adverse oxidative stress in blood induced by SMF, whereas zinc supplementation could prevent toxic effects of SMFs probably by its antioxidant proprieties (Ghodbane et al. 2011).
7.4.6.3 SMF on Digestive System
The effects of SMFs on digestive system are largely unknown and most of studies mainly focus on the intensity of 128 mT. A SMF of 128 mT increased total GSH levels and the activity of superoxide dismutase (SOD) and catalase (CAT) in rat liver and hepatocyte apoptosis through a caspase-independent pathway involving mitochondrial apoptosis-inducing factor (AIF), which was restored by Se and vitamin E supplementations (Ghodbane et al. 2015). Amara et al. (2009) found that exposure of rats to a SMF of 128 mT increased the 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) concentration in kidney.
7.4.6.4 SMF on Endocrine System
The influence of SMFs on endocrine system has been linked to their function, such as insulin, pineal gland, and testis. Jing et al. (2010) found that 180 mT SMF exposure could significantly accelerate the diabetic wound (DW) closure process and enhance the wound tensile strength (TS); however, 180 mT local SMF exposure had slight effect on insulin secretion or pancreatic cells of diabetic rats (Rosmalen et al. 2002). Under the neodymium permanent magnets, Feng et al. (2022) found that SMFs promoted diabetic mice wound healing by suppressing oxidative stress. Elferchichi et al. (2011) showed that the metabolic alterations following exposure to a SMF of moderate intensity could trigger the development of a pre-diabetic state. Exposure to a SMF of 128 mT induced an increase in plasma glucose level and a decrease in plasma insulin concentration in rats, which could be corrected by vitamin D supplementation (Lahbib et al. 2010, 2015). Moreover, β cell insulin content, the expression of glucose transporter GLUT2 and islet area were lower in SMF-exposed group compared to control. Tang et al. (2021) found that moderate-intensity SMFs could cause the abnormalities of glucose metabolism in rats’ brain in an intensity-dependent way, which was closely related to anxiety behavior as shown in Fig. 7.8. However, László et al. (2011) showed that daily SMF exposure repeated for several weeks was protective against the development of high blood glucose level in diabetic mice. Li et al. (2020) also reported that moderate intensity of SMFs, 400 mT and 600 mT, had the protective effects on diabetic mice. Yu et al. (2021) further found that downward 100 mT SMF could reduce the occurrence of hyperglycemia, fatty liver, weight gain, and tissue damage effectively, while upward SMF cannot. Both weak static fields (800 G) for periods between 12 h and 8 days and a 7-Tesla MRI magnet for 45 min had slight effect on nighttime pineal, serum melatonin levels, 5-hydroxytryptamine (5-HT), and 5-hydroxyindole acetic acid (5-HIAA) in exposed rats (Kroeker et al. 1996). Abdelmelek et al. (2006) reported that a SMF of 128 mT induced an increase in norepinephrine content in rat gastrocnemius muscle.
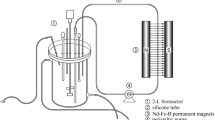
Schematic diagram of static magnetic field exposure. (a) Whole body of the rats was exposed using the superconducting magnet exposure source. (b) Organic squirrel cages developed by our laboratory according to the MF distribution map of the superconducting magnet. (c) Overall device of organic squirrel cage. (d) Single device of organic squirrel cage. (e) Cabinet of organic squirrel cage. (f) Lid of organic squirrel cage. [Reprinted with permission from (Tang et al. 2021)]
7.4.6.5 SMF on Lymphatic System
Bellossi (1986) showed that the lifetime was prolonged significantly by uniform SMFs of 600 or 800 mT in female AKR mice, which developed spontaneous lymphoblastic leukemia. Yang et al. (2009) observed that SMFs of 200–400 mT prolonged the average lifetime of mice bearing L1210 leukemia cells and increased the spleen and thymus index in normal mice. Milovanovich et al. (2016) reported that a SMF of 128 mT caused a reduction in the amount of lymphocytes in serum and a decrease of granulocytes in the spleen, kidney inflammation, a specific redistribution of pro-inflammatory cells in blood and various organs. De Luka et al. (2016) showed that a SMF of 1 mT reduced the content of zinc in mouse spleen, while copper amount remained unchanged.
7.4.6.6 SMF on Nervous System
The nervous system, including brain, spiral cord, and neurons, is important target of magnetic fields. SMF exposure had a strong modulation effect on cellular hydration in different tissues of rats including brain tissue. Krištofiková et al. (2005) showed functional teratogenic risks of the alterations in the orientation of 140 mT SMF for postnatal brain development and functional specialization of both hippocampi in rats. The whole-body SMF exposure and local SMF exposure on the spine resulted in practically identical ear thicknesses and significant effects of the SMF might involve a lower spinal response to the SMF exposure, and showed that local SMF exposure on the spine affected ear thickness, indicating that the place of local SMF action may be in the lower spinal region (Kiss et al. 2015). Dincic et al. (2018) reported increased synaptosome ATPase activities in rat synaptosomes exposed to 1 mT SMF. Veliks et al. (2004) investigated the influence of 100 mT SMF on autonomic nervous system in rat brain by evaluating heart rate and rhythmicity and found that the effectiveness of SMF in large measure depended on both functional peculiarities and functional activities of brain autonomic centers. Yakir-Blumkin et al. (2020) implanted a small magnetic sheet into the rat skull, which an average magnetic field intensity of 4.3 mT in the subventricular zone (SVZ) and 12.9 mT in the endothelial layer, and found that low-intensity SMF exposure enhanced the proliferation of SVZ cells in young adult rats and DCX-expressing new cells in the neocortical area.
Behavioral effects are essential response of nervous system function. Exposure to 128 mT SMF not only altered emotional behavior of rats in the plus maze and long-term spatial memory, but also led to cognitive impairments or at least to substantial attention disorders in the Morris water maze (Ammari et al. 2008). Saeedi Goraghani et al. (2019) found that simultaneous exposure to 5 mT SMF increased the neurobehavioral effects of MK-801, N-methyl d-aspartate (NMDA) receptor blocker, in male Wistar rats. Maaroufi et al. (2013) showed that SMF exposure had no massive effect but affected long-term spatial memory. Weiss et al. (1992) confirmed that acute behavioral and neural effects on rats became apparent at 4 T in a simple T-maze study. A 30 min exposure of rats to a 9.4 T superconducting magnet induced tight circling locomotor activity, conditioned taste aversion (CTA), and the express of c-Fos in specific vestibular and visceral nuclei within the brainstem (Nolte et al. 1998; Snyder et al. 2000). They extended the studies on the relationship of rat behavior and SMF of 7 or 14 T and found that depressed drinking, more circling, and less rearing actions were observed in SMF-exposed group, while CTA was acquired in a short time, and the direction of circling was dependent on the orientation of SMF to rats as shown in Fig. 7.9 (Houpt et al. 2007, 2012). The behavior response of magnetic field exposure was abolished by chemical labyrinthectomy, suggesting that the vestibular apparatus of the intact inner ear is the locus of magnetic field interaction (Houpt et al. 2007; Cason et al. 2009). Tkac et al. (2021) found that 16.4 T SMF induced long-term impairment of the vestibular system in mice, while 10.5 T SMF exposure had no effect.
Examples of rats during (a) sham exposure, (b) 14.1 T magnetic field with head up, and (c) 14.1 T magnetic field with head down. Panels on the left are frames from the video recording. Panels on the right demonstrate the quantification of head tilt calculated as the angle from the nose (N) to the midpoint (M) between the position of the left eye (L) and right eye (R). A deviation from the perpendicular toward the rat’s right was assigned a negative angle (a), while a deviation toward the rat’s left was assigned a positive angle (c). [Reprinted with permission from (Houpt et al. 2012)]
Magnetic therapy as a non-contact, non-invasive, and cheap physiotherapeutic method has been used for analgesic modulation. Gyires et al. (2008) reported that acute exposure of mice to 2–754 mT SMF resulted in an opioid-mediated analgesic action in the writhing test in the mouse. Exposure of mice to both inhomogeneous (3–477 mT) and homogeneous (145 mT) SMF generated an analgesic effect toward visceral pain elicited by chemically induced pain (Kiss et al. 2013). Zhu et al. (2017) found that the orofacial pain levels of mice in the environment of 20–204 mT SMF could be reduced and significantly downregulated P2X3 receptors of trigeminal ganglion (TG) in mice during experimental tooth movement.
Using EEG detection, Rivadulla et al. (2018) found that 0.5 T SMF treatment for 1–2 h could reduce epileptiform activity in anesthetized rats and monkeys. Antal and László (2009) found that inhomogeneous subchronic SMF could prohibit the increased sensitivity of mice to mechanical stimuli in neuralgia in mice, which was in consistent with the pain suppression by SMF of clinical magnetic resonance order. With rat model of Huntington disease, the static magnetic field north and south promoted a distinct behavioral profile and morphological preservation after 7 days of lesion with quinolinic acid associated with apomorphine (APO) (Giorgetto et al. 2015). Lv et al. (2022) found that 7 T SMF exposure for 8 h attenuated the depressive state of depressed mice, including reducing the immobility time of the tail suspension test and increasing sucrose preference. Brain tissue analysis showed that 11.1–33.0 T and 7 T SMF can increase oxytocin by 164.65% and 36.03%, respectively, promoting the increase of c-Fos level in the hippocampus by 14.79%. However, Sekino et al. (2006) reported that a SMF of 8 T upregulated the action potentials of nerve C fiber, which enhanced pain feeling in rats for the C fiber is functioned as pain transmitter.
7.4.6.7 SMF on Reproduction and Development
The adverse effects of SMF on aspects of spermatogenesis, organogenesis, or even ontogenesis in humans have cause great concern in recent years. Embryonic development is a highly sensitive process to SMFs. Many researchers have explored the biological effects of SMF exposure with different magnetic field intensities and different exposure methods on mice and their embryos. The exposure modes were mainly intermittent short-term and continuous long-term exposure, and studies found that different steady-state magnetic field parameters and exposure methods have different effects on the organism as shown in Table 7.3.
7.4.7 Magnetic Sensing Protein in Animals
Many animals have evolved to sense the direction of the geomagnetic field for orientation, navigation, and migration over long distances. The blue light receptor CRYs that could form radical pairs after exposure to blue light were suggested to be a magnetoreceptor based on the proposition that radical pairs were involved in the magnetoreception. CRYs are expressed not only in plant, but also in newts, fruit flies, birds, and the eyes of mammals (Möller et al. 2004; Nießner et al. 2013). Gegear et al. (2008) reported that cry mutants of Drosophila melanogaster showed neither naive nor a magnetic field, while the wild-type flies showed significant naive and trained responses to the magnetic field. Expression of monarch butterfly (Danaus plexippus) cryptochrome gene in Drosophila cry mutants rescued the responses to the magnetic field (Gegear et al. 2010). Marley et al. (2014) reported that MF exposure coupled with CRY photoactivation during embryogenesis was sufficient to produce heightened seizure susceptibility in resultant Drosophila third instar (L3) larvae. Giachello et al. (2016) provided new evidence that exposure to MF of 100 mT was sufficient to potentiate the ability of light-activated CRY to increase neuronal action potential firing, indicating that the activity of CRY was sensitive to an external MF that was capable of modifying animal behavior. CRYs also function as circadian photoreceptors in the Drosophila brain, mediating the light resetting of the 24 h clock, but in vertebrates, the CRYs act as the main negative regulators for the circadian feedback loop, due to the difference in light sensing (Yoshii et al. 2009; Fedele et al. 2014). Non-Drosophila insects encode CRY1 and CRY2, but CRY1 retains their light-sensing properties, whereas the CRY2s act as vertebrate-like negative regulators.
In order to investigate a possible interaction between CRY4 and the iron-sulfur-containing assembly protein (ISCA1) from European robin (Erithacus rubecula), CRY4 has recently been proposed to be relevant for magnetic field sensing. Kimø et al. (2018) reported that the ISCA1 complex and CRY4 were capable of binding; however, the peculiarities of this binding argue strongly against ISCA1 as relevant for magnetoreception. In the fruit fly, CRY plays a light-independent role as “assembling” protein in the rhabdomeres of the compound eyes (Schlichting et al. 2018). Schleicher et al. (2017) demonstrated that photo-induced electron transfer reactions in Drosophila melanogaster cryptochrome were indeed influenced by magnetic fields of a few millitesla. Günther et al. (2018) sequenced night-migratory European robin (Erithacus rubecula) Cry4 from the retina and predicted the currently unresolved structure of the erCry4 protein, which suggested that erCry4 should bind Flavin. They also found that Cry1a, Cry1b, and Cry2 mRNA displayed robust circadian oscillation patterns, whereas Cry4 showed only a weak circadian oscillation. CRYs are sensing magnetic fields in insects as well as in humans. Nohr et al. (2017) presented compelling evidence for an extended electron transfer cascade in the Drosophila cryptochrome and identified W394 as a key residue for flavin photoreduction and formation of a spin-correlated radical pair with a sufficient lifetime for high-sensitivity magnetic field sensing. Xu et al. (2021) found that the photochemistry of cryptochrome 4 (CRY4) from the night-migratory European robin (Erithacus rubecula) was magnetically sensitive in vitro, and more so than CRY4 from two non-migratory bird species, chicken (Gallus gallus) and pigeon (Columba livia). Site-specific mutations of ErCRY4 revealed the roles of four successive flavin-tryptophan radical pairs in generating magnetic field effects and in stabilizing potential signaling states in a way that could enable sensing and signaling functions to be independently optimized in night-migratory birds. Wan et al. (2021) reported that monarchs responded to a reversal of the inclination of the Earth’s magnetic field in an UV-A/blue light and CRY1, but not CRY2, dependent manner, and further demonstrated that both antennae and eyes, which expressed CRY1, were magnetosensory organs.
7.5 Conclusion and Perspectives
SMFs are constant fields, which do not change in intensity or direction over time. There are four SMF parameters relevant for the interaction with a biological system: target tissue(s), magnet characteristics, magnet support device, and dosing regimen. Although the interaction of SMFs with living organisms is a rapidly growing field of investigation, many inconsistencies and seemingly contradictory observations exist. These inconsistencies in the literature are linked to the lack of appropriate systematic approaches to isolate the bioeffects of the treatment relative to other factors including geomagnetic field, the use of different exposure systems, different biological model systems, and the lack of uniformity in culture conditions.
With rapid development of superconducting technology, the magnetic flux density of SMFs used for medical and academic research purposes has steadily increased. Exposure to several Tesla (T) or higher from magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) instruments has become common in pursuit of higher resolution and sensitivity, and human and animal studies have been performed at up to 9.4 and 21.1 T, respectively. In the meanwhile, strong SMFs may also be generated by thermonuclear reactors, magnetohydrodynamic systems, and superconducting generators. The facilities equipped with bubble chambers, particle accelerators, superconducting spectrometers, and isotope devices with high magnetic flux density separation units may have areas around these. However, data on living organisms from exposure to strong SMFs have not been sufficient to evaluate these potential ecosystem risks and explore the function of magnetoreception.
References
Abdelmelek H, Molnar A, Servais S, Cottet-Emard J, Pequignot J, Favier R, Sakly M (2006) Skeletal muscle HSP72 and norepinephrine response to static magnetic field in rat. J Neural Transm 113(7):821–827
Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W (2007) Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 225(3):615–624
Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, Favier A, Sakly M, Ben Rhouma K (2006a) Effects of subchronic exposure to static magnetic field on testicular function in rats. Arch Med Res 37(8):947–952
Amara S, Abdelmelek H, Salem MB, Abidi R, Sakly M (2006b) Effects of static magnetic field exposure on hematological and biochemical parameters in rats. Braz Arch Biol Technol 49:889–895
Amara S, Douki T, Garel C, Favier A, Sakly M, Rhouma KB, Abdelmelek H (2009) Effects of static magnetic field exposure on antioxidative enzymes activity and DNA in rat brain. Gen Physiol Biophys 28(3):260–265
Amemiya Y, Atsushi A, Sarah SS, Tsuyoshi T, Tadashi M (2007) Controlled formation of magnetite crystal by partial oxidation of ferrous hydroxide in the presence of recombinant magnetotactic bacterial protein Mms6. Biomaterials 28(35):5381–5389
Ammari M, Jeljeli M, Maaroufi K, Sakly M, Abdelmelek H, Roy V (2008) Static magnetic field exposure affects behavior and learning in rats. Electromagn Biol Med 27(2):185–196
Anand A, Nagarajan S, Verma A, Joshi D, Pathak P, Bhardwaj J (2012) Pre-treatment of seeds with static magnetic field ameliorates soil water stress in seedlings of maize (Zea mays L.). Indian J Biochem Biophys 49(1):63–70
Ananta V, Shantha N (2010) Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J Plant Physiol 167(2):149–156
Antal M, László J (2009) Exposure to inhomogeneous static magnetic field ceases mechanical allodynia in neuropathic pain in mice. Bioelectromagnetics 30(6):438–445
Atef M, Abd Ei-Baset M, Ell-Kareem A, Aida S, Fadel M (1995) Effects of a static magnetic field on haemoglobin structure and function. Int J Biol Macromol 17(2):105–111
Baby SM, Narayanaswamy GK, Anand A (2011) Superoxide radical production and performance index of photosystem II in leaves from magnetoprimed soybean seeds. Plant Signal Behav 6(11):1635–1637
Baghel L, Kataria S, Guruprasad KN (2016) Static magnetic field treatment of seeds improves carbon and nitrogen metabolism under salinity stress in soybean. Bioelectromagnetics 37(7):455–470
Bahadir A, Beyaz R, Yildiz M (2018) Effect of magnetic field on in vitro seedling growth and shoot regeneration from cotyledon node explants of Lathyrus chrysanthus boiss. Bioelectromagnetics 39(7):547–555
Bainbridge C, Clites BL, Caldart CS, Palacios B, Rollins K, Golombek DA, Pierce JT, Vidal-Gadea AG (2020) Factors that influence magnetic orientation in Caenorhabditis elegans. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 206(3):343–352
Bajpai I, Saha N, Basu B (2012) Moderate intensity static magnetic field has bactericidal effect on E. coli and S. epidermidis on sintered hydroxyapatite. J Biomed Mater Res Part B Appl Biomater 100(5):1206–1217
Baniasadi F, Hajiaghalou S, Shahverdi A, Pirhajati V, Fathi R (2021) Static magnetic field halves cryoinjuries of vitrified mouse COCs, improves their functions and modulates pluripotency of derived blastocysts. Theriogenology 163:31–42
Barber-Zucker S, Keren-Khadmy N, Zarivach R (2016) From invagination to navigation: the story of magnetosome-associated proteins in magnetotactic bacteria. Protein Sci 25(2):338–351
Beischer D, Knepton J Jr (1964) Influence of strong magnetic fields on the electrocardiogram of squirrel monkeys (Saimiri sciureus). Aerosp Med 35:939–944
Bellinger MR, Wei J, Hartmann U, Cadiou H, Winklhofer M, Banks MA (2022) Conservation of magnetite biomineralization genes in all domains of life and implications for magnetic sensing. Proc Natl Acad Sci U S A 119(3):e2108655119
Bellossi A (1986) Effect of static magnetic fields on survival of leukaemia-prone AKR mice. Radiat Environ Biophys 25(1):75–80
Belyaev I, Alipov Y, Shcheglov V, Polunin V, Aizenberg O (1994) Cooperative response of Escherichia coli cells to the resonance effect of millimeter waves at super low intensity. Electro Magnetobiol 13:53–66
Ben Mouhoub R, El May A, Boujezza I, Sethom MM, Feki M, Landoulsi A (2018) Viability and membrane lipid composition under a 57 mT static magnetic field in Salmonella hadar. Bioelectrochemistry 122:134–141
Benson DE, Grissom CB, Burns GL, Mohammad SF (1994) Magnetic field enhancement of antibiotic activity in biofilm forming Pseudomonas aeruginosa. ASAIO J 40(3):M371–M376
Blanchard J, Blackman C (1994) Clarification and application of an ion parametric resonance model for magnetic field interactions with biological systems. Bioelectromagnetics 15(3):217–238
Blondeau M, Guyodo Y, Guyot F, Gatel C, Menguy N, Chebbi I, Haye B, Durand-Dubief M, Alphandery E, Brayner R, Coradin T (2018) Magnetic-field induced rotation of magnetosome chains in silicified magnetotactic bacteria. Sci Rep 8(1):7699
Boyd WA, McBride SJ, Rice JR, Snyder DW, Freedman JH (2010) A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol Appl Pharmacol 245(2):153–159
Cakmak T, Dumlupinar R, Erdal S (2010) Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 31(2):120–129
Cakmak T, Cakmak ZE, Dumlupinar R, Tekinay T (2012) Analysis of apoplastic and symplastic antioxidant system in shallot leaves: impacts of weak static electric and magnetic field. J Plant Physiol 169(11):1066–1073
Carbonell MV, Martinez E, Amaya JM (2000) Stimulation of germination in rice (Oryza sativa L.) by a static magnetic field. Electro Magnetobiol 19(1):121–128
Carbonell M, Florez M, Martínez E, Maqueda R, Amaya J (2011) Study of stationary magnetic fields on initial growth of pea (Pisum sativum L.) seeds. Seed Sci Technol 39(3):673–679
Carlioz A, Touati D (1986) Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J 5(3):623–630
Cason AM, Kwon B, Smith JC, Houpt TA (2009) Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol Behav 97(1):36–43
Chater S, Abdelmelek H, Pequignot JM, Sakly M, Rhouma KB (2006) Effects of sub-acute exposure to static magnetic field on hematologic and biochemical parameters in pregnant rats. Electromagn Biol Med 25(3):135–144
Chen YP, Li R, He JM (2011) Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 20(4):760–769
Cheng L, Yang B, Du H, Zhou T, Li Y, Wu J, Cao Z, Xu A (2022) Moderate intensity of static magnetic fields can alter the avoidance behavior and fat storage of Caenorhabditis elegans via serotonin. Environ Sci Pollut Res Int 29(28):43102–43113
Chikashi N, James Grant B, Koji S, Tadashi M (1995) An iron-regulated gene, magA, encoding an iron transport protein of Magnetospirillum sp. strain AMB-1. J Biol Chem 270(47):28392–28396
da Motta MA, Muniz JBF, Schuler A, Da Motta M (2004) Static magnetic fields enhancement of Saccharomyces cerevisae ethanolic fermentation. Biotechnol Prog 20(1):393–396
De Luka SR, Ilić AŽ, Janković S, Djordjevich DM, Ćirković S, Milovanovich ID, Stefanović S, Vesković-Moračanin S, Ristić-Djurović JL, Trbovich AM (2016) Subchronic exposure to static magnetic field differently affects zinc and copper content in murine organs. Int J Radiat Biol 92(3):140–147
De Souza A, Garcí D, Sueiro L, Gilart F, Porras E, Licea L (2006) Pre-sowing magnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics 27(4):247–257
Denegre JM, Valles JM Jr, Lin K, Jordan W, Mowry KL (1998) Cleavage planes in frog eggs are altered by strong magnetic fields. Proc Natl Acad Sci U S A 95(25):14729–14732
Dengg M, van Meel JC (2004) Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J Pharmacol Toxicol Methods 50(3):209–214
Dincic M, Krstic DZ, Colovic MB, Nesovic Ostojic J, Kovacevic S, De Luka SR, Djordjevic DM, Cirkovic S, Brkic P, Todorovic J (2018) Modulation of rat synaptosomal ATPases and acetylcholinesterase activities induced by chronic exposure to the static magnetic field. Int J Radiat Biol 94(11):1062–1071
Djordjevich DM, De Luka SR, Milovanovich ID, Janković S, Stefanović S, Vesković-Moračanin S, Ćirković S, Ilić AŽ, Ristić-Djurović JL, Trbovich AM (2012) Hematological parameters’ changes in mice subchronically exposed to static magnetic fields of different orientations. Ecotoxicol Environ Saf 81:98–105
Edelman A, Teulon J, Puchalska I (1979) Influence of the magnetic fields on frog sciatic nerve. Biochem Biophys Res Commun 91(1):118–122
Edelman NB, Fritz T, Nimpf S, Pichler P, Lauwers M, Hickman RW, Papadaki-Anastasopoulou A, Ushakova L, Heuser T, Resch GP, Saunders M, Shaw JA, Keays DA (2015) No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening. Proc Natl Acad Sci U S A 112(1):262–267
Egami S, Naruse Y, Watarai H (2010) Effect of static magnetic fields on the budding of yeast cells. Bioelectromagnetics 31(8):622–629
Eguchi Y, Ogiue-Ikeda M, Ueno S (2003) Control of orientation of rat Schwann cells using an 8-T static magnetic field. Neurosci Lett 351(2):130–132
Eguchi Y, Ueno S, Kaito C, Sekimizu K, Shiokawa K (2006) Cleavage and survival of Xenopus embryos exposed to 8 T static magnetic fields in a rotating clinostat. Bioelectromagnetics 27(4):307–313
El May A, Snoussi S, Ben Miloud N, Maatouk I, Abdelmelek H, Ben Aïssa R, Landoulsi A (2009) Effects of static magnetic field on cell growth, viability, and differential gene expression in Salmonella. Foodborne Pathog Dis 6(5):547–552
Elferchichi M, Mercier J, Bourret A, Gross R, Lajoix AD, Belguith H, Abdelmelek H, Sakly M, Lambert K (2011) Is static magnetic field exposure a new model of metabolic alteration? Comparison with Zucker rats. Int J Radiat Biol 87(5):483–490
Elferchichi M, Mercier J, Ammari M, Belguith H, Abdelmelek H, Sakly M, Lambert K (2016) Subacute static magnetic field exposure in rat induces a Pseudoanemia status with increase in MCT4 and Glut4 proteins in glycolytic muscle. Environ Sci Pollut Res Int 23(2):1265–1273
Evans JA, Davidson AJ (2013) Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci 119:283–323
Fan W, Huang Z, Fan B (2018) Effects of prolonged exposure to moderate static magnetic field and its synergistic effects with alkaline pH on Enterococcus faecalis. Microb Pathog 115:117–122
Fatima A, Kataria S, Baghel L, Guruprasad KN, Agrawal AK, Singh B, Sarkar PS, Shripathi T, Kashyap Y (2017) Synchrotron-based phase-sensitive imaging of leaves grown from magneto-primed seeds of soybean. J Synchrotron Radiat 24:232–239
Fatima A, Kataria S, Agrawal A, Singh B, Kashyap Y, Jain M, Brestic M, Allakhverdiev S, Rastogi A (2021a) Use of synchrotron phase-sensitive imaging for the investigation of magnetopriming and solar UV-exclusion impact on soybean (Glycine max) leaves. Cell 10(7):1725
Fatima A, Kataria S, Prajapati R, Jain M, Agrawal A, Singh B, Kashyap Y, Tripathi D, Singh V, Gadre R (2021b) Magnetopriming effects on arsenic stress-induced morphological and physiological variations in soybean involving synchrotron imaging. Physiol Plant 173(1):88–99
Fedele G, Green EW, Rosato E, Kyriacou CP (2014) An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat Commun 5:4391
Feng C, Yu B, Song C, Wang J, Zhang L, Ji X, Wang Y, Fang Y, Liao Z, Wei M, Zhang X (2022) Static magnetic fields reduce oxidative stress to improve wound healing and alleviate diabetic complications. Cell 11(3):443
Florez M, Carbonell Padrino M, Martinez E (2004) Early sprouting and first stages of growth of rice seeds exposed to a magnetic field. Electromagn Biol Med 23:167–176
Florez M, Carbonell MV, Martínez E (2007) Exposure of maize seeds to stationary magnetic fields: effects on germination and early growth. Environ Exp Bot 59(1):68–75
Gaffey C, Tenforde T (1981) Alterations in the rat electrocardiogram induced by stationary magnetic fields. Bioelectromagn Environ Sci Pollut Res 2(4):357–370
Gao W, Liu Y, Zhou J, Pan H (2005) Effects of a strong static magnetic field on bacterium Shewanella oneidensis: an assessment by using whole genome microarray. Bioelectromagnetics 26:558–563
Ge S, Li J, Huang D, Cai Y, Fang J, Jiang H, Hu B (2019) Strong static magnetic field delayed the early development of zebrafish. Open Biol 9(10):190137
Gegear RJ, Casselman A, Waddell S, Reppert SM (2008) Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 454(7207):1014–1018
Gegear RJ, Foley LE, Casselman A, Reppert SM (2010) Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 463(7282):804–807
Ghodbane S, Amara S, Arnaud J, Garrel C, Faure H, Favier A, Sakly M, Abdelmelek H (2011) Effect of selenium pre-treatment on plasma antioxidant vitamins A (retinol) and E (α-tocopherol) in static magnetic field-exposed rats. Toxicol Ind Health 27(10):949–955
Ghodbane S, Ammari M, Lahbib A, Sakly M, Abdelmelek H (2015) Static magnetic field exposure-induced oxidative response and caspase-independent apoptosis in rat liver: effect of selenium and vitamin E supplementations. Environ Sci Pollut Res Int 22(20):16060–16066
Giachello CN, Scrutton NS, Jones AR, Baines RA (2016) Magnetic fields modulate blue-light-dependent regulation of neuronal firing by cryptochrome. J Neurosci 36(42):10742–10749
Gilbert SF, Opitz JM, Raff RA (1996) Resynthesizing evolutionary and developmental biology. Dev Biol 173(2):357–372
Giorgetto C, Silva ECM, Kitabatake TT, Bertolino G, de Araujo JE (2015) Behavioural profile of wistar rats with unilateral striatal lesion by quinolinic acid (animal model of Huntington disease) post-injection of apomorphine and exposure to static magnetic field. Exp Brain Res 233(5):1455–1462
Giorgi G, Guerra D, Pezzoli C, Cavicchi S, Bersani F (1992) Genetic effects of static magnetic fields. Body size increase and lethal mutations induced in populations of Drosophila melanogaster after chronic exposure. Genet Sel Evol 24(5):393–413
Grosman Z, Kolár M, Tesaríková E (1992) Effects of static magnetic field on some pathogenic microorganisms. Acta Univ Palacki Olomuc Fac Med 134:7–9
Grünberg K, Wawer C, Tebo BM, Schüler D (2001) A large gene cluster encoding several magnetosome proteins is conserved in different species of magnetotactic bacteria. Appl Environ Microbiol 67(10):4573–4582
Günther A, Einwich A, Sjulstok E, Feederle R, Bolte P, Koch K, Solov'yov I, Mouritsen H (2018) Double-cone localization and seasonal expression pattern suggest a role in magnetoreception for European robin cryptochrome 4. Curr Biol 28(2):211–223
Gyires K, Zádori ZS, Rácz B, László J (2008) Pharmacological analysis of inhomogeneous static magnetic field-induced antinociceptive action in the mouse. Bioelectromagnetics 29(6):456–462
Hajnorouzi A, Vaezzadeh M, Ghanati F, Jamnezhad H, Nahidian B (2011) Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J Plant Physiol 168(10):1123–1128
Harris SR, Henbest KB, Maeda K, Pannell JR, Timmel CR, Hore PJ, Okamoto H (2009) Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J R Soc Interface 6(41):1193–1205
Hasenstein KH, Kuznetsov OA (1999) The response of lazy-2 tomato seedlings to curvature-inducing magnetic gradients is modulated by light. Planta 208(1):59–65
Hasenstein KH, John S, Scherp P, Povinelli D, Mopper S (2013) Analysis of magnetic gradients to study gravitropism. Am J Bot 100(1):249–255
Hernádi L, László JF (2014) Pharmacological analysis of response latency in the hot plate test following whole-body static magnetic field-exposure in the snail Helix pomatia. Int J Radiat Biol 90(7):547–553
High WB, Sikora J, Ugurbil K, Garwood M (2000) Subchronic in vivo effects of a high static magnetic field (9.4 T) in rats. J Magn Reson Imaging 12(1):122–139
Ho MW, Stone TA, Jerman I, Bolton J, Bolton H, Goodwin BC, Saunders PT, Robertson F (1992) Brief exposures to weak static magnetic field during early embryogenesis cause cuticular pattern abnormalities in Drosophila larvae. Phys Med Biol 37(5):1171
Hore PJ, Mouritsen H (2016) The radical-pair mechanism of magnetoreception. Annu Rev Biophys 45:299–344
Horiuchi S, Ishizaki Y, Okuno K, Ano T, Shoda M (2001) Drastic high magnetic field effect on suppression of Escherichia coli death. Bioelectrochemistry 53(2):149–153
Houpt TA, Cassell JA, Riccardi C, DenBleyker MD, Hood A, Smith JC (2007) Rats avoid high magnetic fields: dependence on an intact vestibular system. Physiol Behav 92(4):741–747
Houpt TA, Cassell J, Carella L, Neth B, Smith JC (2012) Head tilt in rats during exposure to a high magnetic field. Physiol Behav 105(2):388–393
Hoyer C, Vogt MA, Richter SH, Zaun G, Zahedi Y, Maderwald S, Ladd ME, Winterhager E, Grümmer R, Gass P (2012) Repetitive exposure to a 7 Tesla static magnetic field of mice in utero does not cause alterations in basal emotional and cognitive behavior in adulthood. Reprod Toxicol 34(1):86–92
Hozayn M, Amal A, Abdel-Rahman H (2015) Effect of magnetic field on germination, seedling growth and cytogenetic of onion (Allium cepa L.). Afr J Agric Res 10:849–857
Hu X, Qiu Z, Wang Y, She Z, Qian G, Ren Z (2009) Effect of ultra-strong static magnetic field on bacteria: application of Fourier-transform infrared spectroscopy combined with cluster analysis and deconvolution. Bioelectromagnetics 30(6):500–507
Hung YC, Lee JH, Chen HM, Huang GS (2010) Effects of static magnetic fields on the development and aging of Caenorhabditis elegans. J Exp Biol 213(12):2079–2085
Ichioka S, Minegishi M, Iwasaka M, Shibata M, Nakatsuka T, Ando J, Ueno S (2003) Skin temperature changes induced by strong static magnetic field exposure. Bioelectromagnetics 24(6):380–386
Iimoto M, Watanabe KN, Fujiwara K (1996) Effects of magnetic flux density and direction of the magnetic field on growth and CO2 exchange rate of potato plantlets in vitro. Acta Hortic 440:606–610
Ikehata M, Iwasaka M, Miyakoshi J, Ueno S, Koana T (2003) Effects of intense magnetic fields on sedimentation and gene expression profile in budding yeast. J Appl Phys 93:6724–6726
Jan N, Ludĕk S, Lukáš F, Iva S, Vladimír V (2007) Effects of low-frequency magnetic fields on the viability of yeast Saccharomyces cerevisiae. Bioelectrochemistry 70(1):115–121
Jan L, Fefer D, Košmelj K, Gaberščik A, Jerman I (2015) Geomagnetic and strong static magnetic field effects on growth and chlorophyll a fluorescence in Lemna minor. Bioelectromagnetics 36(3):190–203
Jasmina F, Barbara K, Brigita T, Vanja K, Ines M-M (2012) Effects of low-density static magnetic fields on the growth and activities of wastewater bacteria Escherichia coli and Pseudomonas putida. Bioresour Technol 120:225–232
Javed N, Ashraf M, Akram NA, Al-Qurainy F (2011) Alleviation of adverse effects of drought stress on growth and some potential physiological attributes in maize (Zea mays L.) by seed electromagnetic treatment. Photochem Photobiol 87(6):1354–1362
Ji W, Huang H, Deng A, Pan C (2009) Effects of static magnetic fields on Escherichia coli. Micron 40(8):894–898
Jia W, Zhang J, Lu Y, Li G, Yang W, Wang Q (2018) Response of nitrite accumulation and microbial characteristics to low-intensity static magnetic field during partial nitrification. Bioresour Technol 259:214–220
Jin Y, Guo W, Hu X, Liu M, Xu X, Hu F, Lan Y, Lv C, Fang Y, Liu M, Shi T, Ma S, Fang Z, Huang J (2019) Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci Rep 9:14384
Jing D, Shen G, Cai J, Li F, Huang J, Wang Y, Xu Q, Tang C, Luo E (2010) Effects of 180 mT static magnetic fields on diabetic wound healing in rats. Bioelectromagnetics 31(8):640–648
Jouni FJ, Abdolmaleki P, Ghanati F (2012) Oxidative stress in broad bean (Vicia faba L.) induced by static magnetic field under natural radioactivity. Mutat Res 741(1–2):116–121
Jovanić B, Sarvan M (2004) Permanent magnetic field and plant leaf temperature. Electromagn Biol Med 23(1):1–5
Jovicic-Petrovic J, Karlicic V, Petrovic I, Cirkovic S, Ristic-Djurovic JL, Raicevic V (2021) Biomagnetic priming-possible strategy to revitalize old mustard seeds. Bioelectromagnetics 42(3):238–249
Kaletta T, Hengartner MO (2006) Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5(5):387–399
Kataria S, Jain M, Tripathi DK, Singh VP (2020) Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol Plant 168(2):422–436
Kataria S, Jain M, Rastogi A, Brestic M (2021) Static magnetic field treatment enhanced photosynthetic performance in soybean under supplemental ultraviolet-B radiation. Photosynth Res 150:263–278
Kawakami S, Kashiwagi K, Furuno N, Yamashita M, Kashiwagi A, Tanimoto Y (2006) Effects of strong static magnetic fields on amphibian development and gene expression. Jpn J Appl Phys 45(7):6055
Kazazian HH Jr (2004) Mobile elements: drivers of genome evolution. Science 303(5664):1626–1632
Kazuhiro N, Kazumasa O, Takashi A, Makoto S (1997) Effect of high magnetic field on the growth of Bacillus subtilis measured in a newly developed superconducting magnet biosystem. Bioelectrochem Bioenerg 43(1):123–128
Kimø S, Friis I, Solov'yov I (2018) Atomistic insights into cryptochrome interprotein interactions. Biophys J 115(4):616–628
Kimura T, Takahashi K, Suzuki Y, Konishi Y, Ota Y, Mori C, Ikenaga T, Takanami T, Saito R, Ichiishi E (2008) The effect of high strength static magnetic fields and ionizing radiation on gene expression and DNA damage in Caenorhabditis elegans. Bioelectromagnetics 29(8):605–614
Kiss B, Gyires K, Kellermayer M, László JF (2013) Lateral gradients significantly enhance static magnetic field-induced inhibition of pain responses in mice—a double blind experimental study. Bioelectromagnetics 34(5):385–396
Kiss B, Laszlo JF, Szalai A, Porszasz R (2015) Analysis of the effect of locally applied inhomogeneous static magnetic field-exposure on mouse ear edema—a double blind study. PLoS One 10(2):e0118089
Komeili A, Vali H, Beveridge TJ, Newman DK (2004) Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc Natl Acad Sci U S A 101(11):3839–3844
Kotani H, Kawaguchi H, Shimoaka T, Iwasaka M, Ueno S, Ozawa H, Nakamura K, Hoshi K (2002) Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J Bone Miner Res 17(10):1814–1821
Kriklavova L, Truhlar M, Skodovaa P, Lederer T, Jirku V (2014) Effects of a static magnetic field on phenol degradation effectiveness and Rhodococcus erythropolis growth and respiration in a fed-batch reactor. Bioresour Technol 167:510–513
Krištofiková Z, Čermák M, Benešová O, Klaschka J, Zach P (2005) Exposure of postnatal rats to a static magnetic field of 0.14 T influences functional laterality of the hippocampal high-affinity choline uptake system in adulthood; in vitro test with magnetic nanoparticles. Neurochem Res 30(2):253–262
Kroeker G, Parkinson D, Vriend J, Peeling J (1996) Neurochemical effects of static magnetic field exposure. Surg Neurol 45(1):62–66
Krzemieniewski M, Dębowski M, Janczukowicz W, Pesta J (2003) Effect of sludge conditioning by chemical methods with magnetic field application. Pol J Environ Stud 12:595–605
Kthiri A, Hidouri S, Wiem T, Jeridi R, Sheehan D, Landouls A (2019) Biochemical and biomolecular effects induced by a static magnetic field in Saccharomyces cerevisiae: evidence for oxidative stress. PLoS One 14(1):e0209843
Kuznetsov OA, Hasenstein KH (1996) Intracellular magnetophoresis of amyloplasts and induction of root curvature. Planta 198(1):87–94
Lahbib A, Elferchichi M, Ghodbane S, Belguith H, Chater S, Sakly M, Abdelmelek H (2010) Time-dependent effects of exposure to static magnetic field on glucose and lipid metabolism in rat. Gen Physiol Biophys 29(4):390
Lahbib A, Ghodbane S, Louchami K, Sener A, Sakly M, Abdelmelek H (2015) Effects of vitamin D on insulin secretion and glucose transporter GLUT2 under static magnetic field in rat. Environ Sci Pollut Res Int 22(22):18011–18016
László JF, Pórszász R (2011) Exposure to static magnetic field delays induced preterm birth occurrence in mice. Am J Obstet Gynecol 205(4):362–326
László JF, Szilvási J, Fényi A, Szalai A, Gyires K, Pórszász R (2011) Daily exposure to inhomogeneous static magnetic field significantly reduces blood glucose level in diabetic mice. Int J Radiat Biol 87(1):36–45
Latef AAAH, Dawood MFA, Hassanpour H, Rezayian M, Younes NA (2020) Impact of the static magnetic field on growth, pigments, osmolytes, nitric oxide, hydrogen sulfide, phenylalanine ammonia-lyase activity, antioxidant defense system, and yield in lettuce. Biology 9(7):112
Lee CH, Chen HM, Yeh LK, Hong MY, Huang GS (2012) Dosage-dependent induction of behavioral decline in Caenorhabditis elegans by long-term treatment of static magnetic fields. J Radiat Res 53(1):24–32
Leesungbok R, Ahn S-J, Lee S-W, Park G-H, Kang J-S, Choi J-J (2013) The effects of a static magnetic field on bone formation around a sandblasted, large-grit, acid-etched–treated titanium implant. J Oral Implantol 39(S1):248–255
Letuta UG (2020) Magnetic isotopes of 25 Mg and 67 Zn and magnetic fields influence on adenosine triphosphate content in Escherichia coli. J Phys Conf Ser 1443:012015
Letuta UG, Berdinskiy VL (2019) Biological effects of static magnetic fields and zinc isotopes on E. coli bacteria. Bioelectromagnetics 40(1):62–73
Levin M, Ernst SG (1997) Applied DC magnetic fields cause alterations in the time of cell divisions and developmental abnormalities in early sea urchin embryos. Bioelectromagnetics 18(3):255–263
Li Q, Fang Y, Wu N, Gu L, Li H, Liao Z, Liu M, Fang Z, Zhang X (2020) Protective effects of moderate intensity static magnetic fields on diabetic mice. Bioelectromagnetics 41(8):598–610
Lin C, Todo T (2005) The cryptochromes. Genome Biol 6(5):1–9
Liu H, Gu F, Dong S, Liu W, Wang H, Chen Z, Wang J (2016) CONSTANS-like 9 (COL9) delays the flowering time in Oryza sativa by repressing the Ehd1 pathway. Biochem Biophys Res Commun 479(2):173–178
Liu S, Yang F, Meng F, Chen H, Gong Z (2008) Enhanced anammox consortium activity for nitrogen removal: impacts of static magnetic field. J Biotechnol 138(3):96–102
Loghmannia J, Heidari B, Rozati SA, Kazemi S (2015) The physiological responses of the Caspian kutum (Rutilus frisii kutum) fry to the static magnetic fields with different intensities during acute and subacute exposures. Ecotoxicol Environ Saf 111:215–219
Lucielen Oliveira S, Ranulfo Monte A, Cristina G-D, Jorge C (2010) Effects of magnetic fields on biomass and glutathione production by the yeast Saccharomyces cerevisiae. Process Biochem 45(8):1362–1367
Lv Y, Fan Y, Tian X, Yu B, Song C, Feng C, Zhang L, Ji X, Zablotskii V, Zhang X (2022) The anti-depressive effects of ultra-high static magnetic field. J Magn Reson Imaging 56:354–365
Maaroufi K, Ammari M, Elferchichi M, Poucet B, Sakly M, Save E, Abdelmelek H (2013) Effects of combined ferrous sulphate administration and exposure to static magnetic field on spatial learning and motor abilities in rats. Brain Inj 27(4):492–499
Mahdi A, Gowland P, Mansfield P, Coupland R, Lloyd R (1994) The effects of static 3.0 T and 0.5 T magnetic fields and the echo-planar imaging experiment at 0.5 T on E. coli. Br J Radiol 67:983–987
Malko J, Constantinidis I, Dillehay D, Fajman W (1994) Search for influence of 1.5 Tesla magnetic field on growth of yeast cells. Bioelectromagnetics 15(6):495–501
Mansouri A, Abbes C, Ben Mouhoub R, Ben Hassine S, Landoulsi A (2019) Enhancement of mixture pollutant biodegradation efficiency using a bacterial consortium under static magnetic field. PLoS One 14(1):e0208431
Maria Cristina A, Augusto A, Barbara C, Sabrina B, Maria Piera P, Francesco U, Elena P (2003) Morphological and biochemical modifications induced by a static magnetic field on Fusarium culmorum. Biochimie 85(10):963–970
Marley R, Giachello CN, Scrutton NS, Baines RA, Jones AR (2014) Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci Rep 4(1):1–4
Martinez E, Carbonell Padrino M, Amaya J (2000) A static magnetic field of 125 mT stimulates the initial growth stages of barley (Hordeum vulgare L.). Electro Magnetobiol 19:271–277
Masahiro K, Muneyo Y, Isao K, Moriyasu W (2000) Effect of static magnetic fields on bacteria: Streptococcus mutans, Staphylococcus aureus, and Escherichia coli. Pathophysiology 7(2):143–148
Masakazu I, Masateru I, Junji M, Shoogo U (2004) Strong static magnetic field effects on yeast proliferation and distribution. Bioelectrochemistry 65(1):59–68
Masateru I, Takao K, Yuji S, Hidesuke S, Masayoshi N (1999) Mutagenicity and co-mutagenicity of static magnetic fields detected by bacterial mutation assay. Mutat Res 427(2):147–156
Mateescu C, Burunţea N, Stancu N (2011) Investigation of Aspergillus niger growth and activity in a static magnetic flux density field. Rom Biotechnol Lett 16:6364–6368
Matsunaga T, Okamura Y, Fukuda Y, Wahyudi AT, Murase Y, Takeyama H (2005) Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res 12(3):157–166
Matsunaga T, Takeyuki S, Masayoshi T, Atsushi A (2007) Molecular analysis of magnetotactic bacteria and development of functional bacterial magnetic particles for nano-biotechnology. Trends Biotechnol 25(4):182–188
Mevissen M, Buntenkötter S, Löscher W (1994) Effects of static and time-varying (50-Hz) magnetic fields on reproduction and fetal development in rats. Teratology 50(3):229–237
Mietchen D, Keupp H, Manz B, Volke F (2005) Non-invasive diagnostics in fossils-magnetic resonance imaging of pathological belemnites. Biogeosciences 2(2):133–140
Mihoub M, El May A, Aloui A, Chatti A, Landoulsi A (2012) Effects of static magnetic fields on growth and membrane lipid composition of Salmonella typhimurium wild-type and dam mutant strains. Int J Food Microbiol 157(2):259–266
Milovanovich ID, Ćirković S, De Luka SR, Djordjevich DM, Ilić AŽ, Popović T, Arsić A, Obradović DD, Oprić D, Ristić-Djurović JL (2016) Homogeneous static magnetic field of different orientation induces biological changes in subacutely exposed mice. Environ Sci Pollut Res Int 23(2):1584–1597
Miyakoshi J (2005) Effects of static magnetic fields at the cellular level. Prog Biophys Mol Biol 87(2–3):213–223
Mohammadi F, Ghanati F, Sharifi M, Chashmi N (2018) On the mechanism of the cell cycle control of suspension-cultured tobacco cells after exposure to static magnetic field. Plant Sci 277:139–144
Moisescu C, Ardelean I, Benning L (2014) The effect and role of environmental conditions on magnetosome synthesis. Front Microbiol 5:49
Möller A, Sagasser S, Wiltschko W, Schierwater B (2004) Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91(12):585–588
Morrow A, Dunstan R, King B, Roberts T (2007) Metabolic effects of static magnetic fields on Streptococcus pyogenes. Bioelectromagnetics 28(6):439–445
Motta MA, Montenegro EJN, Stamford T, Silva AR, Silva FR (2001) Changes in Saccharomyces cerevisiae development induced by magnetic fields. Biotechnol Prog 17:970–973
Mouadh M, El May A, Aloui A, Chatti A, Landoulsi A (2012) Effects of static magnetic fields on growth and membrane lipid composition of Salmonella typhimurium wild-type and dam mutant strains. Int J Food Microbiol 157(2):259–266
Muhammad D, Zia ul H, Jamil Y (2012) Effect of pre-sowing magnetic field treatment to garden pea (Pisum sativum L.) seed on germination and seedling growth. Pak J Bot 44:1851–1856
Muniz JBF, Marcelino M, Motta MD, Schuler ARP, da Motta MA (2007) Influence of static magnetic fields on S. cerevisiae biomass growth. Braz Arch Biol Technol 50:515–520
Nagy P, Fischl G (2004) Effect of static magnetic field on growth and sporulation of some plant pathogenic fungi. Bioelectromagnetics 25(4):316–318
Narra VR, Howell RW, Goddu SM, Rao DV (1996) Effects of a 1.5-Tesla static magnetic field on spermatogenesis and embryogenesis in mice. Invest Radiol 31(9):586–590
Naz A, Jamil Y, Ul Haq Z, Ahmad M, Ashraf I, Khera RA (2012) Enhancement in the germination, growth and yield of okra (Abelmoschus esculentus) using pre-sowing magnetic treatment of seeds. Indian J Biochem Biophys 49:211–214
Neurath PW (1968) High gradient magnetic field inhibits embryonic development of frogs. Nature 219(5161):1358–1359
Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenböck G (2002) Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36(3):463–474
Nießner C, Denzau S, Stapput K, Ahmad M, Peichl L, Wiltschko W, Wiltschko R (2013) Magnetoreception: activated cryptochrome 1a concurs with magnetic orientation in birds. J R Soc Interface 10(88):20130638
Nikolić L, Kartelija G, Nedeljković M (2008) Effect of static magnetic fields on bioelectric properties of the Br and N1 neurons of snail Helix pomatia. Comp Biochem Physiol A Mol Integr Physiol 151(4):657–663
Nikolić L, Bataveljić D, Andjus PR, Nedeljković M, Todorović D, Janać B (2013) Changes in the expression and current of the Na+/K+ pump in the snail nervous system after exposure to a static magnetic field. J Exp Biol 216(18):3531–3541
Niu C, Liang W, Ren H, Geng J, Ding L, Xu K (2014) Enhancement of activated sludge activity by 10–50 mT static magnetic field intensity at low temperature. Bioresour Technol 159:48–54
Nohr D, Paulus B, Rodriguez R, Okafuji A, Bittl R, Schleicher E, Weber S (2017) Determination of radical–radical distances in light-active proteins and their implication for biological magnetoreception. Angew Chem Int Ed Engl 56(29):8550–8554
Nolte CM, Pittman DW, Kalevitch B, Henderson R, Smith JC (1998) Magnetic field conditioned taste aversion in rats. Physiol Behav 63(4):683–688
Okano H, Ohkubo C (2003) Effects of static magnetic fields on plasma levels of angiotensin II and aldosterone associated with arterial blood pressure in genetically hypertensive rats. Bioelectromagnetics 24(6):403–412
Okano H, Ohkubo C (2005) Exposure to a moderate intensity static magnetic field enhances the hypotensive effect of a calcium channel blocker in spontaneously hypertensive rats. Bioelectromagnetics 26(8):611–623
Okano H, Ohkubo C (2006) Elevated plasma nitric oxide metabolites in hypertension: synergistic vasodepressor effects of a static magnetic field and nicardipine in spontaneously hypertensive rats. Clin Hemorheol Microcirc 34(1–2):303–308
Okano H, Ino H, Osawa Y, Osuga T, Tatsuoka H (2012) The effects of moderate-intensity gradient static magnetic fields on nerve conduction. Bioelectromagnetics 33(6):518–526
Okazaki R, Ootsuyama A, Uchida S, Norimura T (2001) Effects of a 4.7 T static magnetic field on fetal development in ICR mice. J Radiat Res 42(3):273–283
Pan H (1996) The effect of a 7 T magnetic field on the egg hatching of Heliothis virescens. Magn Reson Imaging 14(6):673–677
Pan H, Liu X (2004) Apparent biological effect of strong magnetic field on mosquito egg hatching. Bioelectromagnetics 25(2):84–91
Payez A, Ghanati F, Behmanesh M, Abdolmaleki P, Hajnorouzi A, Rajabbeigi E (2013) Increase of seed germination, growth and membrane integrity of wheat seedlings by exposure to static and a 10-kHz electromagnetic field. Electromagn Biol Med 32:417–429
Perić-Mataruga V, Prolić Z, Nenadović V, Vlahović M, Mrdaković M (2008) The effect of a static magnetic field on the morphometric characteristics of neurosecretory neurons and corpora allata in the pupae of yellow mealworm Tenebrio molitor (Tenebrionidae). Int J Radiat Biol 84(2):91–98
Poinapen D, Brown DC, Beeharry GK (2013) Seed orientation and magnetic field strength have more influence on tomato seed performance than relative humidity and duration of exposure to non-uniform static magnetic fields. J Plant Physiol 170(14):1251–1258
Pooam M, Arthaut LD, Burdick D, Link J, Martino CF, Ahmad M (2019) Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 249(2):319–332
Potenza L, Ubaldi L, De Sanctis R, De Bellis R, Cucchiarini L, Dachá M (2004) Effects of a static magnetic field on cell growth and gene expression in Escherichia coli. Mutat Res 561(1–2):53–62
Potenza L, Saltarelli R, Polidori E, Ceccaroli P, Amicucci A, Zeppa S, Zambonelli A, Stocchi V (2012) Effect of 300 mT static and 50 Hz 0.1 mT extremely low frequency magnetic fields on Tuber borchii mycelium. Can J Microbiol 58:1174–1182
Prolic Z, Jovanovic Z (1986) Influence of magnetic-field on the rate of development of honeybee preadult stage. Period Biol 88(2):187–188
Prolić Z, Nenadović V (1995) The influence of a permanent magnetic field on the process of adult emergence in Tenebrio molitor. J Insect Physiol 41(12):1113–1118
Puricelli E, Dutra NB, Ponzoni D (2009) Histological evaluation of the influence of magnetic field application in autogenous bone grafts in rats. Head Face Med 5(1):1–6
Qin S, Yin H, Yang C, Dou Y, Liu Z, Zhang P, Yu H, Huang Y, Feng J, Hao J, Hao J, Deng L, Yan X, Dong X, Zhao Z, Jiang T, Wang HW, Luo SJ, Xie C (2016) A magnetic protein biocompass. Nat Mater 15(2):217–226
Quiñones-Peña MA, Tavizon G, Puente JL, Martínez-Anaya C, Hernández-Chiñas U, Eslava CA (2017) Effects of static magnetic fields on the enteropathogenic Escherichia coli. Bioelectromagnetics 38(7):570–578
Raipuria RK, Kataria S, Watts A, Jain M (2021) Magneto-priming promotes nitric oxide via nitric oxide synthase to ameliorate the UV-B stress during germination of soybean seedlings. J Photochem Photobiol B Biol 220:112211
Rajini P, Melstrom P, Williams PL (2008) A comparative study on the relationship between various toxicological endpoints in Caenorhabditis elegans exposed to organophosphorus insecticides. J Toxicol Environ Health A 71(15):1043–1050
Ramadan LA, Abd-Allah AR, Aly HA, Saad-El-Din AA (2002) Testicular toxicity effects of magnetic field exposure and prophylactic role of coenzyme Q10 and l-carnitine in mice. Pharmacol Res 46(4):363–370
Ramirez E, Monteagudo JL, Garcia-Gracia M, Delgado JM (1983) Oviposition and development of Drosophila modified by magnetic fields. Bioelectromagnetics 4(4):315–326
Ramla A, Imed C, Ahmed L (2017) Influence of static magnetic field exposure on fatty acid composition in Salmonella Hadar. Microb Pathog 108:13–20
Rankin CH, Lin CH (2015) Magnetosensation: finding a worm’s internal compass. Elife 4:e09666
Rauš Balind S, Todorović D, Prolić Z (2009) Viability of old house borer (Hylotrupes bajulus) larvae exposed to a constant magnetic field of 98 mT under laboratory conditions. Arch Biol Sci 61(1):129–134
Regoli F, Gorbi S, Machella N, Tedesco S, Benedetti M, Bocchetti R, Notti A, Fattorini D, Piva F, Principato G (2005) Pro-oxidant effects of extremely low frequency electromagnetic fields in the land snail Helix aspersa. Free Radic Biol Med 39(12):1620–1628
Ren Z, Leng X, Liu Q (2018) Effect of a static magnetic field on the microscopic characteristics of highly efficient oil-removing bacteria. Water Sci Technol 77(2):296–303
Righi H, Arruda-Neto JDT, Gomez JGC, da Silva LF, Somessari ESR, Lemos ACC (2020) Exposure of Deinococcus radiodurans to both static magnetic fields and gamma radiation: observation of cell recuperation effects. J Biol Phys 46(3):309–324
Rivadulla C, Aguilar J, Coletti M, Aguila J, Prieto S, Cudeiro J (2018) Static magnetic fields reduce epileptiform activity in anesthetized rat and monkey. Sci Rep 8(1):15985
Rosmalen JG, Leenen PJ, Pelegri C, Drexhage HA, Homo-Delarche F (2002) Islet abnormalities in the pathogenesis of autoimmune diabetes. Trends Endocrinol Metab 13(5):209–214
Ruiz-Gómez MJ, Prieto-Barcia MI, Ristori-Bogajo E, Martı́nez-Morillo, M. (2004) Static and 50 Hz magnetic fields of 0.35 and 2.45 mT have no effect on the growth of Saccharomyces cerevisiae. Bioelectrochemistry 64(2):151–155
Saeedi Goraghani M, Ahmadi-Zeidabadi M, Bakhshaei S, Shabani M, Ghotbi Ravandi S, Rezaei Zarchi S, Nozari M (2019) Behavioral consequences of simultaneous postnatal exposure to MK-801 and static magnetic field in male Wistar rats. Neurosci Lett 701:77–83
Saito K, Suzuki H, Suzuki K (2006) Teratogenic effects of static magnetic field on mouse fetuses. Reprod Toxicol 22(1):118–124
Sakhnini L, Dairi M (2004) Effects of static magnetic fields on early embryonic development of the sea urchin Echinometra mathaei. IEEE Trans Magn 40(4):2979–2981
Satow Y, Matsunami K, Kawashima T, Satake H, Huda K (2001) A strong constant magnetic field affects muscle tension development in bullfrog neuromuscular preparations. Bioelectromagnetics 22(1):53–59
Saunders R (2005) Static magnetic fields: animal studies. Prog Biophys Mol Biol 87(2–3):225–239
Savić T, Janać B, Todorović D, Prolić Z (2011) The embryonic and post-embryonic development in two Drosophila species exposed to the static magnetic field of 60 mT. Electromagn Biol Med 30(2):108–114
Schleicher E, Biskup T, Rodriguez R, Weber S, Hore PJ, Timmel CR, Mackenzie SR (2017) Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci Rep 7:42228
Schlichting M, Rieger D, Cusumano P, Grebler R, Costa R, Mazzotta G, Helfrich-Förster C (2018) Cryptochrome interacts with actin and enhances eye-mediated light sensitivity of the circadian clock in Drosophila melanogaster. Front Mol Neurol 11:238
Schreiber WG, Teichmann EM, Schiffer I, Hast J, Akbari W, Georgi H, Graf R, Hehn M, Spiebeta HW, Thelen M, Oesch F, Hengstler JG (2001) Lack of mutagenic and co-mutagenic effects of magnetic fields during magnetic resonance imaging. J Magn Reson Imaging 14:779–788
Sekino M, Tatsuoka H, Yamaguchi S, Eguchi Y, Ueno S (2006) Effects of strong static magnetic fields on nerve excitation. IEEE Trans Magn 42(10):3584–3586
Shan Y, Han H, Zhu J, Yan X, Zhang X, Long H, Jian F, Li X, Wang Y, Lai W (2021) The effects of static magnetic field on orthodontic tooth movement in mice. Bioelectromagnetics 42(5):398–406
Shao Y, Mu G, Song L, Yan S, Tan L (2019) Enhanced biodecolorization performance of azo dyes under high-salt conditions by a marine microbial community exposed to moderate-intensity static magnetic field. Environ Eng Sci 36(2):186–196
Shcherbakov D, Winklhofer M, Petersen N, Steidle J, Hilbig R, Blum M (2005) Magnetosensation in zebrafish. Curr Biol 15(5):R161–R162
She Z, Xing H, Zhao X, Ren Z, Ding G (2009) FTIR investigation of the effects of ultra-strong static magnetic field on the secondary structures of protein in bacteria. Infrared Phys Technol 52(4):138–142
Shine M, Guruprasad K, Anand A (2011) Enhancement of germination, growth, and photosynthesis in soybean by pretreatment of seeds with magnetic field. Bioelectromagnetics 32(6):474–484
Shine M, Guruprasad K, Anand A (2012) Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 33(5):428–437
Shokrollahi S, Ghanati F, Sajedi R, Sharifi M (2018) Possible role of iron containing proteins in physiological responses of soybean to static magnetic field. J Plant Physiol 226:163–171
Snoussi S, May AE, Coquet L, Chan P, Jouenne T, Landoulsi A, Dé E (2012) Adaptation of salmonella Enterica hadar under static magnetic field: effects on outer membrane protein pattern. Proteome Sci 10(1):6
Snoussi S, El May A, Coquet L, Chan P, Jouenne T, Dé E, Landoulsi A (2016) Unraveling the effects of static magnetic field stress on cytosolic proteins of Salmonella by using a proteomic approach. Can J Microbiol 62(4):338–348
Snyder DJ, Jahng JW, Smith JC, Houpt TA (2000) c-Fos induction in visceral and vestibular nuclei of the rat brain stem by a 9.4 T magnetic field. Neuroreport 11(12):2681–2685
Solov’yov IA, Chandler DE, Schulten K (2007) Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys J 92(8):2711–2726
Sprando RL, Olejnik N, Cinar HN, Ferguson M (2009) A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a complex object parametric analyzer and sorter, and axenic liquid media. Food Chem Toxicol 47(4):722–728
Staniland S, Ward B, Harrison A, van der Laan G, Telling N (2007) Rapid magnetosome formation shown by real-time X-ray magnetic circular dichroism. Proc Natl Acad Sci U S A 104(49):19524–19528
Stansell MJ, Winters WD, Doe RH, Dart BK (2001) Increased antibiotic resistance of E. coli exposed to static magnetic fields. Bioelectromagnetics 22(2):129–137
Subber AR, Hail RCA, Jabail WA, Hussein HF (2012) Effects of magnetic field on the growth development of Zea mays seeds. Seeds 2:7
Tablado L, Soler C, Núñez M, Núñez J, Pérez-Sánchez F (2000) Development of mouse testis and epididymis following intrauterine exposure to a static magnetic field. Bioelectromagnetics 21(1):19–24
Tagourti J, El May A, Aloui A, Chatti A, Ben Aissa R, Landoulsi A (2010) Static magnetic field increases the sensitivity of Salmonella to gentamicin. Ann Microbiol 60(3):519–522
Takashima Y, Miyakoshi J, Ikehata M, Iwasaka M, Ueno S, Koana T (2004) Genotoxic effects of strong static magnetic fields in DNA-repair defective mutants of Drosophila melanogaster. J Radiat Res 45(3):393–397
Takebe A, Furutani T, Wada T, Koinuma M, Kubo Y, Okano K, Okano T (2012) Zebrafish respond to the geomagnetic field by bimodal and group-dependent orientation. Sci Rep 2:727
Tan L, Mu G, Shao Y, Ning S, Shi S (2020) Combined enhancement effects of static magnetic field (SMF) and a yeast Candida tropicalis SYF-1 on continuous treatment of Acid Red B by activated sludge under hypersaline conditions. J Chem Technol Biotechnol 95(3):840–849
Tang H, Wang P, Wang H, Fang Z, Yang Q, Ni W, Sun X, Liu H, Wang L, Zhao G, Zheng Z (2019) Effect of static magnetic field on morphology and growth metabolism of Flavobacterium sp. m1–14. Bioprocess Biosyst Eng 42(12):1923–1933
Tang S, Ye Y, Yang L, Hao Y, Yu C, Yan H, Xing Y, Jia Z, Hu C, Zuo H, Li Y (2021) Static magnetic field induces abnormality of glucose metabolism in rats’ brain and results in anxiety-like behavior. J Chem Neuroanat 113:101923
Taniguchi N, Kanai S (2007) Efficacy of static magnetic field for locomotor activity of experimental osteopenia. Evid Based Complement Alternat Med 4(1):99–105
Taniguchi N, Kanai S, Kawamoto M, Endo H, Higashino H (2004) Study on application of static magnetic field for adjuvant arthritis rats. Evid Based Complement Alternat Med 1(2):187–191
Tarlochan S, Pandey O (2015) Effect of electric and magnetic treatments on germination of bitter gourd (Momordica charantia) seed. Int J Agric Biol 17:351–356
Tasic T, Lozic M, Glumac S, Stankovic M, Milovanovich I, Djordjevich DM, Trbovich AM, Japundzic-Zigon N, De Luka SR (2021) Static magnetic field on behavior, hematological parameters and organ damage in spontaneously hypertensive rats. Ecotoxicol Environ Saf 207:111085
Taskin M, Esim N, Genisel M, Örtücü S, Hasenekoglu I, Canli O, Erdal S (2013) Enhancement of invertase production by Aspergillus niger OZ-3 using low-intensity static magnetic fields. Prep Biochem Biotechnol 43:177–188
Tenforde T (1986) Thermoregulation in rodents exposed to high-intensity stationary magnetic fields. Bioelectromagnetics 7(3):341–346
Tkac I, Benneyworth MA, Nichols-Meade T, Steuer EL, Larson SN, Metzger GJ, Ugurbil K (2021) Long-term behavioral effects observed in mice chronically exposed to static ultra-high magnetic fields. Magn Reson Med 86(3):1544–1559
Todorović D, Marković T, Prolić Z, Mihajlović S, Rauš S, Nikolić L, Janać B (2013) The influence of static magnetic field (50 mT) on development and motor behaviour of Tenebrio (Insecta, Coleoptera). Int J Radiat Biol 89(1):44–50
Todorović D, Perić-Mataruga V, Mirčić D, Ristić-Djurović J, Prolić Z, Petković B, Savić T (2015) Estimation of changes in fitness components and antioxidant defense of Drosophila subobscura (Insecta, Diptera) after exposure to 2.4 T strong static magnetic field. Environ Sci Pollut Res Int 22(7):5305–5314
Todorovic D, Ilijin L, Mrdakovic M, Vlahovic M, Filipovic A, Grcic A, Peric-Mataruga V (2019) Long-term exposure of cockroach Blaptica dubia (Insecta: Blaberidae) nymphs to magnetic fields of different characteristics: effects on antioxidant biomarkers and nymphal gut mass. Int J Radiat Biol 95(8):1185–1193
Todorovic D, Ilijin L, Mrdakovic M, Vlahovic M, Grcic A, Petkovic B, Peric-Mataruga V (2020) The impact of chronic exposure to a magnetic field on energy metabolism and locomotion of Blaptica dubia. Int J Radiat Biol 96(8):1076–1083
Togawa T, Okai O, Oshima M (1967) Observation of blood flow EMF in externally applied strong magnetic field by surface electrodes. Med Biol Eng 5(2):169–170
Trock DH (2000) Electromagnetic fields and magnets: investigational treatment for musculoskeletal disorders. Rheum Dis Clin North Am 26(1):51–62
Tsuchiya K, Kazumasa O, Takashi A, Kan T, Hideo T, Makoto S (1999) High magnetic field enhances stationary phase-specific transcription activity of Escherichia coli. Bioelectrochem Bioenerg 48(2):383–387
Tu R, Jin W, Xi T, Yang Q, Han SF, Abomohra AE-F (2015) Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res 86:132–138
Ueno S, Harada K, Shiokawa K (1984) The embryonic development of frogs under strong DC magnetic fields. IEEE Trans Magn 20(5):1663–1665
Vanderstraeten J, Burda H, Verschaeve L, De Brouwer C (2015) Could magnetic fields affect the circadian clock function of cryptochromes? Testing the basic premise of the cryptochrome hypothesis (ELF magnetic fields). Health Phys 109(1):84–89
Vashisth A, Joshi DK (2017) Growth characteristics of maize seeds exposed to magnetic field. Bioelectromagnetics 38(2):151–157
Vashisth A, Nagarajan S (2008) Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.). Bioelectromagnetics 29(7):571–578
Vashisth A, Nagarajan S (2010) Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J Plant Physiol 167(2):149–156
Vashisth A, Meena N, Krishnan P (2021) Magnetic field affects growth and yield of sunflower under different moisture stress conditions. Bioelectromagnetics 42(6):473–483
Veliks V, Ceihnere E, Svikis I, Aivars J (2004) Static magnetic field influence on rat brain function detected by heart rate monitoring. Bioelectromagnetics 25(3):211–215
Vidal-Gadea A, Ward K, Beron C, Ghorashian N, Gokce S, Russell J, Truong N, Parikh A, Gadea O, Ben-Yakar A, Pierce-Shimomura J (2015) Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. Elife 4:e07493
Wan G, Hayden A, Iiams S, Merlin C (2021) Cryptochrome 1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat Commun 12(1):771
Wang X, Ma Q, Jiang W, Lv J, Pan W, Song T, Wu LF (2008) Effects of hypomagnetic field on magnetosome formation of Magnetospirillum magneticum AMB-1. Geomicrobiol J 25:296–303
Wang X, Liang L, Song T, Wu L (2009) Magnetosome formation and expression of mamA, mms13, mms6 and magA in Magnetospirillum magneticum AMB-1 exposed to pulsed magnetic field. Curr Microbiol 59(3):221–226
Wang N, Wang X, Qin W, Yeung SH, Kwok D, Wong H, Xue Q, Chu P, Leung CW, Ruotolo A (2011) Multiple-mode excitation in spin-transfer nanocontacts with dynamic polarizer. Appl Phys Lett 98(24):242506
Wang L, Du H, Guo X, Wang X, Wang M, Wang Y, Wang M, Chen S, Wu L, Xu A (2015) Developmental abnormality induced by strong static magnetic field in Caenorhabditis elegans. Bioelectromagnetics 36(3):178–189
Ward BK, Tan GX, Roberts DC, Della Santina CC, Zee DS, Carey JP (2014) Strong static magnetic fields elicit swimming behaviors consistent with direct vestibular stimulation in adult zebrafish. PLoS One 9(3):e92109
Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ (2000) Curvature in Arabidopsis inflorescence stems is limited to the region of amyloplast displacement. Plant Cell Physiol 41(6):702–709
Weiss J, Herrick RC, Taber KH, Contant C, Plishker GA (1992) Bio-effects of high magnetic fields: a study using a simple animal model. Magn Reson Imaging 10(4):689–694
Xu S, Tomita N, Ohata R, Yan Q, Ikada Y (2001) Static magnetic field effects on bone formation of rats with an ischemic bone model. Biomed Mater Eng 11(3):257–263
Xu S, Okano H, Tomita N, Ikada Y (2010) Recovery effects of a 180 mT static magnetic field on bone mineral density of osteoporotic lumbar vertebrae in ovariectomized rats. Evid Based Complement Alternat Med 2011:620984
Xu C, Lv Y, Chen C, Zhang Y, Wei S (2014) Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis. Adv Space Res 53(7):1118–1124
Xu J, Jarocha LE, Zollitsch T, Konowalczyk M, Henbest KB, Richert S, Golesworthy MJ, Schmidt J, Déjean V, Sowood DJC, Bassetto M, Luo J, Walton JR, Fleming J, Wei Y, Pitcher TL, Moise G, Herrmann M, Yin H, Wu H, Bartölke R, Käsehagen SJ, Horst S, Dautaj G, Murton PDF, Gehrckens AS, Chelliah Y, Takahashi JS, Koch KW, Weber S, Solov'yov IA, Xie C, Mackenzie SR, Timmel CR, Mouritsen H, Hore PJ (2021) Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 594(7864):535–540
Yakir-Blumkin MB, Loboda Y, Schächter L, Finberg JPJN (2020) Static magnetic field exposure in vivo enhances the generation of new doublecortin-expressing cells in the sub-ventricular zone and neocortex of adult rats. Neuroscience 425:217–234
Yan Q, Tomita N, Ikada Y (1998) Effects of static magnetic field on bone formation of rat femurs. Med Eng Phys 20(6):397–402
Yang P, Hu L, Wang Z, Ding C, Zhang W, Qian A, Shang P (2009) Inhibitory effects of moderate static magnetic field on leukemia. IEEE Trans Magn 45(5):2136–2139
Yang Y, Yan Y, Zou X, Zhang C, Zhang H, Xu Y, Wang X, Janos P, Yang Z, Gu H (2011) Static magnetic field modulates rhythmic activities of a cluster of large local interneurons in drosophila antennal lobe. J Neurophysiol 106(5):2127–2135
Yang J, Wang S, Zhang G, Fang Y, Fang Z, Shang P, Zhang H (2021) Static magnetic field (2–4 T) improves bone microstructure and mechanical properties by coordinating osteoblast/osteoclast differentiation in mice. Bioelectromagnetics 42(3):200–211
Yang B, Yang Z, Cheng L, Li Y, Zhou T, Han Y, Du H, Xu A (2022) Effects of 10 T static magnetic field on the function of sperms and their offspring in Caenorhabditis elegans. Ecotoxicol Environ Saf 240:113671
Yano A, Hidaka E, Fujiwara K, Iimoto M (2001) Induction of primary root curvature in radish seedlings in a static magnetic field. Bioelectromagnetics 22(3):194–199
Ye S, Yang J, Chen C (2004) Effect of static magnetic fields on the amplitude of action potential in the lateral giant neuron of crayfish. Int J Radiat Biol 80(10):699–708
Yeh S, Yang J, Lee Y, Tsai L (2008) Static magnetic field expose enhances neurotransmission in crayfish nervous system. Int J Radiat Biol 84(7):561–567
Yoshie S, Ikehata M, Hirota N, Takemura T, Minowa T, Hanagata N, Hayakawa T (2012) Evaluation of mutagenicity and co-mutagenicity of strong static magnetic fields up to 13 Tesla in Escherichia coli deficient in superoxide dismutase. J Magn Reson Imaging 35(3):731–736
Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C (2009) The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. J Neurosci 29(8):2597–2610
Yoshino T, Matsunaga T (2006) Efficient and stable display of functional proteins on bacterial magnetic particles using Mms13 as a novel anchor molecule. Appl Environ Microbiol 72(1):465–471
Yu X, Liu H, Klejnot J, Lin C (2010) The cryptochrome blue light receptors. Arabidopsis Book 8:e0135
Yu B, Liu J, Cheng J, Zhang L, Song C, Tian X, Fan Y, Lv Y, Zhang X (2021) A static magnetic field improves iron metabolism and prevents high-fat-diet/streptozocin-induced diabetes. Innovation (N Y) 2(1):100077
Zahedi Y, Zaun G, Maderwald S, Orzada S, Pütter C, Scherag A, Winterhager E, Ladd ME, Grümmer R (2014) Impact of repetitive exposure to strong static magnetic fields on pregnancy and embryonic development of mice. J Magn Reson Imaging 39(3):691–699
Zaun G, Zahedi Y, Maderwald S, Orzada S, Pütter C, Scherag A, Winterhager E, Ladd ME, Grümmer R (2014) Repetitive exposure of mice to strong static magnetic fields in utero does not impair fertility in adulthood but may affect placental weight of offspring. J Magn Reson Imaging 39(3):683–690
Zhang H, Gan L, Zhu X, Wang J, Han L, Cheng P, Jing D, Zhang X, Shan Q (2018a) Moderate-intensity 4 mT static magnetic fields prevent bone architectural deterioration and strength reduction by stimulating bone formation in streptozotocin-treated diabetic rats. Bone 107:36–44
Zhang J, Meng X, Ding C, Shang P (2018b) Effects of static magnetic fields on bone microstructure and mechanical properties in mice. Electromagn Biol Med 37(2):76–83
Zhang QM, Tokiwa M, Doi T, Nakahara T, Chang PW, Nakamura N, Hori M, Miyakoshi J, Yonei S (2003) Strong static magnetic field and the induction of mutations through elevated production of reactive oxygen species in Escherichia coli soxR. Int J Radiat Biol 79:281–286
Zhu Y, Wang S, Long H, Zhu J, Jian F, Ye N, Lai W (2017) Effect of static magnetic field on pain level and expression of P2X3 receptors in the trigeminal ganglion in mice following experimental tooth movement. Bioelectromagnetics 38(1):22–30
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yang, B., Cheng, L., Liu, Z., Zhao, Y., Xu, A. (2023). Impact of SMFs on Microorganisms, Plants, and Animals. In: Zhang, X. (eds) Biological Effects of Static Magnetic Fields. Springer, Singapore. https://doi.org/10.1007/978-981-19-8869-1_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-8869-1_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8868-4
Online ISBN: 978-981-19-8869-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)