Abstract
Man-made static magnetic fields (SMFs) widely exist in human life as a physical environmental factor. However, the biological responses to moderate SMFs exposure and their underlying mechanisms are largely unknown. The present study was focused on exploring the nervous responses to moderate-intensity SMFs at 0.5 T and 1 T in Caenorhabditis elegans (C. elegans). We found that SMFs at either 0.5 T or 1 T had no statistically significant effects on the locomotor behaviors, while the 1 T magnetic field increased pharyngeal pumping. The avoidance behavior of the pathogenic Pseudomonas aeruginosa was greatly decreased in either 0.5 T or 1 T SMFs exposed nematodes, and the learning index was reducede from 0.52 ± 0.11 to 0.23 ± 0.17 and 0.16 ± 0.11, respectively. The total serotonin level was increased by 17.08% and 16.45% with the treatment of 0.5 T and 1 T SMF, compared to the control group; however, there were minimal effects of SMFs on other three neurotransmitters including choline, γ-aminobutyric acid (GABA), dopamine. RT-qPCR was used to further investigate the expression of serotonin-related genes, including rate-limiting enzymes, transcription factors and transport receptors. The expression levels of tph-1 and unc-86 genes were increased by SMF exposure, while those of ocr-2, osm-9, ser-1 and mod-1 genes were decreased. With the staining of lipid in either wild-type N2 or tph-1 mutants, we found that 0.5 T and 1 T SMFs decreased fat storage in C. elegans via serotonin pathway. Our study demonstrated that moderate-intensity SMFs induced neurobehavioral disorder and the reduction of fat storage by disturbing the secretion of serotonin in C. elegans, which provided new insights into elucidating nervous responses of C. elegans to moderate-intensity SMFs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since life on Earth originated and evolved in the presence of the geomagnetic field (25–65 μT) (Van Huizen et al. 2019), many animals perform orientation and migration rely on the geomagnetic field (Clites &Pierce 2017, Wu &Dickman 2012). With the development and application of superconducting magnetic technology, the intensity of static magnetic fields (SMFs) to which living organisms are exposed is constantly increasing. Long-term or short-term exposure to SMFs whose intensity is much higher than the Earth’s magnetic field may result in various disorders in neurobehavioral and homeostasis. SMFs were classified into weak (< 1 mT), moderate (1 mT to 1 T), strong (1–5 T) and ultra-strong (> 5 T) types according to the field intensities (Dini and Abbro 2005). The human-made SMF is usually presented in specific industrial processes, magnetic health products from commercially available and medical diagnosis. In particular, the intensity of SMFs provided by the core components of most MRI machines is between 0.5 T and 3 T. Considering that there are varying degrees of health risk for clinical magnetic therapy exposure, occupational exposure and daily magnetic field exposure, more extensive researches are needed on the health effects associated with occupational and non-occupational group exposure to moderate-intensity SMFs.
Accumulating evidence has indicated that moderate-intensity SMFs are capable of affecting several biological systems, especially the nervous system that has a pretty-close contact with the properties of membrane channels and internal electrophysiological activities (Rosen 2003; Shen et al. 2007; Todorovic et al. 2020). A survey of MRI (1.0 and 1.5 T) subjects found significant occurrences of vertigo, dizziness and nausea, while these occurrences gradually restored after a period of exposure (de Vocht et al. 2006), which was in consistence with the observations in various model organisms. Short-term (30 min) exposure to SMFs above 7 T elicited head tilt, locomotor circling, conditioned taste aversion and suppression of hind leg upright behavior in rats and mice (Houpt et al. 2011, 2012; Lockwood et al. 2003). Intermittent exposure to the 1.7 T SMF induced a pronounced but transient derangement in locomotor behavior of Caenorhabditis elegans (C. elegans) (BESSHO et al. 1995). The associated mechanisms of these effects are still lacking. The electric currents induced in the body by movement through the magnetic field are also one of the factors that should be considered. In addition to the perspective of potential hazards, moderate SMFs also exhibit a possible positive influence on the nervous system in vivo. Electroencephalogram analysis revealed that 0.5 T SMF reduced epileptiform activity in anesthetized rats and monkeys (Rivadulla et al. 2018). Exposure to the SMF of 0.15 T for 30 min significantly increased response latency of snail Helix pomatia and produced analgesia by affecting the serotonergic and the opioid system (Hernadi and Laszlo 2014). In general, the neurobehavioral effects of moderate-intensity SMFs are milder, but controversial compared to strong magnetic fields.
Although the effects of moderate-intensity SMFs on the physiological and biochemical basis of the nervous system have been extensively investigated for decades, the inconsistency of biological system or magnetic field parameters between different studies significantly compromised risk assessment of SMFs exposure. Osuga reported that the neuroconduction in damaged nerves is not affected by the 1.5 T SMF used in MRI (Osuga and Tatsuoka 1999). In contrast, exposure to 0.7 T SMF significantly increased the conduction velocity of action potentials in the C fibers of the frog sciatic nerve (Okano et al. 2012), while the compound action potential of guinea pigs was decreased after 0.5 T SMF treatment (Coots et al. 2004). Disturbances in the level of the neurotransmitters and their receptors are also the potential reasons for abnormal neurobehavior induced by SMFs. A previous study has reported that exposure to SMF enhanced the blockage of N-Methyl D-aspartate (NMDA) receptor blockers (Goraghani et al. 2019). Previous studies have also shown that moderate-intensity SMFs in different orientations increased the activities of triphosphatases and acetylcholinesterase in rat synaptosomes (Dincic et al. 2018), which revealed the regulatory of moderate SMFs on neurotransmitter levels. However, systematic research on the integrity and function of the neurotransmitter system is relatively lacking, which is insufficient to fully illustrate the mechanisms of moderate-intensity SMFs exposure on the nervous system.
C. elegans as a well-established in vivo model organism has a simple structure of its nervous system The neuron location and connection are known, and almost every neuron with precise specificity has a fluorescent reporter gene. Since the main neurotransmitter types and their metabolism, vesicle circulation and synaptic transmission are highly homologous with higher animals, C. elegans is widely used in neurological research (Corsi et al. 2015). More importantly, previous studies have reported that C. elegans senses the magnetic field through AFD neurons and performs geomagnetic orientation and vertical migration (Vidal-Gadea et al. 2015). However, there is a lack of evidence from the effects of SMFs on the development and functions of nervous system. This study focused on the effects of moderate-intensity SMFs on neurobehavior, neurotransmitter levels and functions in C. elegans. First, we evaluated locomotor and learning behavior to elucidate behavioral changes mediated by moderate-intensity SMFs. Then, we assessed the neuronal integrity and neurotransmitter secretion levels of the four neurotransmitter systems including choline, γ-aminobutyric acid (GABA), dopamine, and serotonin (5-hydroxytryptamine, 5-HT) by using transgenic strains. A statistically significant increase in serotonin level was found in worms exposed to 0.5 T and 1 T SMF, while there are no statistically significant effects on the other three neurotransmitter systems. Furthermore, we detected serotonin-related gene expression by using RT-qPCR, as well as the effect of serotonin disorder mediated by moderate-intensity SMFs on fat metabolism. This study sought to reveal the impact of moderate-intensity SMFs on the nervous system and provided a new insight for exploring the potential therapeutic value.
Materials and methods
Worm and bacterial strains and cultivation
C. elegans were cultured on nematode growth medium (NGM) plates seeded with Escherichia coli (E. coli) OP50 at 20 °C, according to standard protocols (BRENNER 1974). The strains used in this study, such as GR1366 (mgIs42 [tph-1::GFP + rol-6(su1006)]), BZ555 (egIs1 [dat-1p::GFP]), LX929 (vsIs48 [unc-17::GFP]), EG1285 (oxIs12 [unc-47p::GFP + lin-15( +)]), LIU1 (ldrIs1 [dhs-3p::dhs-3::GFP + unc-76( +)]), MT15434 tph-1(mg280), wild-type strain N2 and E. coli OP50, were obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota, USA). Pathogenic Pseudomonas aeruginosa (P. aeruginosa) PA14 was obtained from the Institute of Microbiology, Chinese Academy of Sciences. The worms were maintained in the same stage via the synchronization procedure for further experiments.
Magnetic field intensity detection and exposure
The 0.5 T and 1 T permanent magnets were bought from Jiangsu Zhongxin magnetoelectricity, and the intensity and polarity of permanent magnets were detected by FE-2100RD magnet analyzer (HuNan Forever Elegance Technology Co., Ltd., CN). The magnet analyzer scans a plane three millimeters high from the surface of the permanent magnet, so the magnetic field intensity of the permanent magnets of 0.5 T and 1 T was, respectively, displayed as 4000 Gs and 8000 Gs, N pole showed red, and S pole showed blue (Fig. 1b and c). The permanent magnets have a homogeneous distribution of magnetic field in a wide range of 15 mm (0.5 T permanent magnets) and 20 mm (1 T permanent magnets) of the central area on the top. After synchronization, nematodes in L1 stage were transferred into 18 mm NGM plates and E. coli OP50 was added as food source, which was placed right on top of the magnets and exposed for either 48 h or 72 h. To ensure the proper nematodes culturing conditions, the permanent magnets were placed in the 20 °C incubator. The control groups were in the same incubator, around 30–40 cm away from the magnets. The geomagnetic field in our laboratory was 50.101 ± 0.145 μT by using the link: https://www.ngdc.noaa.gov/geomag/magfield.shtml.
Locomotor behavior assay
The endpoints of head thrash, body bend and pharynx pump were used to evaluate locomotion behaviors of C. elegans. After exposure, nematodes were washed with fresh M9 buffer and placed on individual non-seeded NGM plates to roam freely for one minute. The frequency of body bend and head thrash assay was followed the protocol outlined in previous studies (Tsalik and Hobert 2003, Wu et al. 2015). Pharyngeal pumping was scored as the number of contractions and relaxations of the pharynx that occur in 30 s. Each endpoint was recorded for at least 30 nematodes per group, and four replicates were performed.
Learning behavior Assay
The behavioral avoidance was examined as a learning and memory behavior model and performed as previously described (Lee and Mylonakis 2017, Meisel and Kim 2014). E. coil OP50 and P. aeruginosa PA14 were grown in Luria broth until optical density 600 (OD600) reached 1.0. Then, 10 μL of each bacteria was spotted on assay plates at the same distance from the center. We spread 200 μL bacterial OP50 for naive growth plate, and 200 μL bacterial PA14 for training growth plate to train nematodes to avoid the pathogenic PA14. All plates were used after incubation for 24 h at 37 °C. After exposure, nematodes were washed with fresh M9 buffer and incubated on naive and training growth plates for 4 h. Then, nematodes were washed 3 times and placed in the center of the assay plates. After moving freely for 1 h, the number of nematodes on bacteria spots after paralyzing with 1 mM NaN3 was counted. Six assay plates were used in each naive or training group, with at least 100 nematodes per assay plate. The learning index was calculated as follows:
The choice index greater than 0 indicates the preference for OP50 of C. elegans, and vice versa for PA14, which means that the lower the learning index, the lower the avoidance behavior of C. elegans. In naive conditions, it learns to avoid PA14 after exposure to training growth plate compared with nematodes in naive growth plates without PA14 training.
Imaging and neuronal morphology analysis
The most common neurotransmitters in C. elegans include choline, GABA, dopamine and serotonin (Hobert 2013). To investigate the effects of SMF on neurotransmitter systems, we used GFP-labeled transgenic nematodes to examine the changes in the above four neurotransmitter systems in C. elegans after 0.5 T and 1 T SMFs exposure, including the level of neurotransmitter secretion, neuron cell body and nerve wire integrity. Nematodes were exposed to 0.5 and 1 T for 48 h from hatching. For each group at least 30 nematodes were performed neuron structure modeling under Leica DM4B fluorescence microscope (Leica, Germany) state evaluation. In cholinergic neurons and GABAergic neurons, nerve damage was examined according to whether the overall structures of the neurons were broken or missing (Ijomone et al. 2020; Liang et al. 2020). In dopaminergic neurons, damage was examined according to whether the two pairs of CEP neurons and a pair of ADE neurons in the head were broken or notched (Nass et al. 2002). In serotoninergic neurons, nerve changes were examined based on whether the NSM and ADF neurons were damaged, missing and weakened (Donohoe et al. 2008; Tatum et al. 2015). Mean fluorescence intensity in neuron cell bodies that secrete the corresponding neurotransmitter was quantified with Image J software to indicate the level of neurotransmitter.
Quantitative Real-Time PCR
Total RNA from nematodes were isolated using TRIzol, and cDNA was synthesized by a reverse transcriptase kit (transgen, China). The qRT-PCR was performed on a Step One real-time cycler (Roche, Shanghai, China), using a SYBR Green qPCR Mix (transgen, China). The final results were expressed as the relative expression ratio between the targeted gene and the reference (act-1) gene. The primer sequence is presented in Table 1. Three independent replicates were performed.
Determination of serotonin levels
After exposure to SMF, all nematodes of each group were washed three times and fully suspended in 1 mL M9 buffer. Then, 10 µl of suspension was pipetted on slides and counted the exact number of nematodes, repeating 3 times independently to get the average value to reduce the error. According to the density method above, 3000 nematodes were obtained. The nematodes were homogenized in pre-chilled 1 mL PBS liquid and centrifuged at 3000 rpm for 20 min to obtain the supernatant for detection. The concentration of serotonin was performed using a 5-hydroxytryptamine enzyme-linked immunosorbent assay kit (J&L Biological Company, China) as described (Cao et al. 2019).
Quantitative analysis of lipid droplets using DHS-3::GFP worms
All nematodes were anesthetized in a droplet of 20 mM NaN3 and mounted on slides before imaging. For each group at least 30 nematodes were scored. The fluorescence intensity of DHS-3::GFP was measured by Image-Pro Plus Version 6.0, and three independent experiments were repeated.
Oil Red O staining and quantitation
The Oil Red O staining was performed based on a previously described with minor modification. 0.5% Oil Red O solution was dissolved in 1,2-propa-nediol and filtered through a 0.22 μm syringe filter before staining. The exposed nematodes were washed 3 times with M9, then added 1000μL M9 buffer and 50μL 10% paraformaldehyde, frozen and thawed three cycles repeatedly at -80 °C. After thawing completely, nematodes were washed with pre-chilled M9 and dehydrated in 1,2-propanediol for 5 min and stained with Oil Red O for 5 h at room temperature. After staining, nematodes were washed with 85% 1,2-propanediol and PBS for image. 2000 nematodes were obtained according to the above density method, and the stained lipids of the nematodes were extracted with 200μL of ethanol and quantified at OD 510 nm.
Statistical analysis
All presented values were expressed as mean ± standard deviation (SD) based on data from at least three replicates. Statistical analysis was performed by the Mann–Whitney test using software Origin 2020. P < 0.05 was considered statistically significant. The relative fluorescence intensity from the fluorescence images was determined metrically using Image-Pro Plus, version 6.0.
Results and discussion
Effects of moderate SMFs on locomotor behavior
Locomotion behavior is a sensitive endpoint of neural responses in C. elegans. In our study, the body bends, head thrashes and pharynx pumps were tested to elucidate the effects of moderate SMF on locomotor behaviors of C. elegans. As shown in Fig. 2a and b, moderate intensities of SMF had no statistically significant difference of body bends and head thrashes in C elegans exposed to either 0.5 T and 1 T SMFs compared to controls. In contrast, a statistically significant increase in pharynx pumps was observed in worms exposed to 1 T SMF at either 48 h or 72 h. These results indicated that moderate SMFs did not induce the abnormal movement of C. elegans, while feeding behavior was disturbed by 1 T SMF.
Effects of 0.5 T and 1 T SMFs exposure for 48 h and 72 h on locomotion behaviors in C. elegans. (a) Body bends (n ≥ 30 nematodes/group). (b) Head thrashes (n ≥ 30 nematodes/group). (c) Pharynx pump s(n ≥ 30 nematodes/group). Data are means ± SD of four independent experiments (n = 4). *P < 0.05, ns., not significant, compared with the control groups
However, LEE et.al reported that body movement of C. elegans was decreased by chronic-term treatment (exposure for 2 to 8 days) of SMF with magnetic field intensity at 0.2 T (LEE et al. 2012). This inconsistency was partially attributed to the shorter exposure time of magnetic fields. Considering that the C. elegans laid eggs after being exposed for 3 days from L1 stage, we did not expose them for more than 3 days as reported. Interestingly, we found pharynx pumping increased statistically significantly by 1 T SMF-treatment, unveiling that SMF may affect the normal diet of C. elegans. At the cellular level, it has been reported that 0.5 T SMF did not affect ATP levels, while 1 T SMF could increase ATP levels of multiple cell types (Wang et al. 2018). Consistent changes in pharynx pumping and ATP levels revealed the potential impact of moderate-intensity SMFs on energy metabolism. In general, our data showed that moderate-intensity SMFs treatment for 48 or 72 h had no statistically significant effects on locomotor behavior of C. elegans, except for increases in pharynx pumping mediated by 1 T SMF.
Effects of moderate SMF on learning behavior
E. coli OP50 is the main food source for C. elegans under laboratory condition. However, worms often exhibit an initial preference for pathogenic P. aeruginosa PA14, whereas avoidance behavior was observed after hours of exposure to the pathogen (Meisel &Kim 2014). Since behavioral avoidance is controlled by the nervous system, we examined this avoidance behavior to evaluate the effect of moderate SMFs on the learning behavior of worms (Fig. 3a). As shown in Fig. 3b, the moderate-intensity SMFs did not affect initial preference for P. aeruginosa PA14 of naive nematodes, while the behavioral avoidance of P. aeruginosa PA14 in the trained nematodes treated with 0.5 T and 1 T SMF was reduced. The learning index was decreased from 0.52 ± 0.11 to 0.23 ± 0.17 and 0.16 ± 0.11 in wild-type worms exposed to 0.5 T and 1 T SMFs for 48 h, respectively (Fig. 3c). These results indicated that exposure to 0.5 T and 1 T SMFs for 48 h attenuated the learning ability of C. elegans to avoid pathogenic bacteria.
There is evidence that short-term SMF exposure can alter the vestibular system of individual organisms including zebrafish, rats and mice, which may further affect their development and learning behavior (Houpt et al. 2012; Pais-Roldan et al. 2016; Ward et al. 2014). In this study, we found that exposure to 0.5 T and 1 T SMFs for 48 h reduced the aversive olfactory learning behavior of C. elegans. This may be the initial manifestation of nervous system damage (Tkac et al. 2021). However, it has also been reported that the decrease in learning behavior may be caused by the increased tolerance of organisms to pathogenic bacteria (Meisel and Kim 2014). The stability of the structure and number of synapses is the key to balance the excitation and inhibition of the nervous system, as well as the basis of neurotransmitter transmissions (Barbagallo et al. 2017). In light of the observation that SMF had minimal effects on locomotor behaviors of worms, we postulated that the decline of learning behavior of worms was probably derived from the neurotransmitter systems abnormalities induced by SMF.
Effects of moderate SMF on neurons and neurotransmitters
Neurobehavioral alteration is usually associated with the change of neuronal integrity and neurotransmitter secretion level (Jha et al. 2017). Therefore, we analyzed whether 0.5 and 1 T SMFs exposure for 48 h had any influence on various neurotransmitter systems including choline, GABA, dopamine and serotonin by using transgenic strains. As shown in Fig. 4a, the overall structure of the four neurotransmitter systems was intact after SMFs exposure, without neuronal loss and nerve line breakage; however, a slight elevation of serotonin localized outside the NSM neuron cell body was observed in C. elegans exposed to SMFs for 48 h. Then, we set out to determine the levels of neurotransmitter by quantifying the fluorescence intensity of four neurotransmitter systems. As shown in Fig. 4b, 0.5 T and 1 T SMF had no statistically significant effects on fluorescence intensity of cholinergic neurons, GABAergic neurons or dopaminergic neurons. On the other hand, a statistically significant increase on fluorescence intensity of serotonergic neurons was found in worms exposed to 0.5 T and 1 T SMFs. Moreover, using Elisa Kit to examine the concentrations of serotonin on wild-type N2 worms, serotonin concentrations of nematodes treated with 0.5 T and 1 T SMFs were 7.23 ± 0.63 ng mL−1 and 7.19 ± 0.29 ng mL−1, which were 17.08% and 16.45% higher than 6.18 ± 0.26 ng mL−1 of the control group, respectively (Fig. 4c).
Effects of 0.5 T and 1 T SMFs exposure for 48 h on neurons and neurotransmitters of C. elegans. (a) Fluorescence imaging of each neurotransmitter neurons; from top to bottom are cholinergic neurons, GABAergic neurons, dopaminergic neurons and serotonergic neurons. (b) Analysis of fluorescence intensity of four neurotransmitter systems (n ≥ 30 nematodes/group). (c) Serotonin concentrations after long-term exposure to SMF. The data in (b) and (c) are presented as mean ± SD of three independent experiments (n = 3). *p < 0.05, **p < 0.01, compared with the control groups
The present study performed a comprehensive analysis of choline, GABA, dopamine and serotonin synthesis in worms exposed to moderate SMFs. Among the above neurotransmitters, serotonin could be considered as an indicator that was affected by either 0.5 T or 1 T SMFs. Serotonin has shown to modulate foraging, mating, egg laying, metabolism, aversive olfactory learning and feeding behaviors in C. elegans (Flavell et al. 2013; Ishita et al. 2020; Srinivasan et al. 2008). There are five classes of the neurons that accumulate serotonin, including ADF, NSM, AIM and RIH neurons shortly after hatching, and in the HSN neurons at the end of the fourth larval stage (Sze et al. 2000). A previous study reported that pregnant rats exposed to extremely low-frequency magnetic fields led to a significant increase in serotonin level on the brain cortex of offspring rats at birth, 15 days and 21 days (Cañedo et al. 2003). However, it has also been reported that the level of pontine medullary serotonin did not significantly change in rats exposed to 0.08 T SMF for 12 h to 8 days (Kroeker et al. 1996). Our data showed that compared with the control group, the serotonin levels of nematodes exposed to 0.5 T and 1 T SMFs for 48 h from the L1 stage were statistically significantly increased, which might be associated with the deficit in learning behavior caused by chronic-term SMF exposure. The change of serotonin level could be due to an alteration of gene expression, properties of membrane channels, ATPases and neurotransmitter enzyme activities (Cañedo et al. 2003; Dincic et al. 2018; Rosen 2003). As the genome of C. elegans is highly homologous to mammals, and the metabolic pathway is similar as well (Corsi et al. 2015), it is essential to explore the expression of serotonin-related gene and associated mechanisms of serotonin changes after SMF exposure, including gene transcription of rate-limiting enzyme and receptors.
Effects of moderate SMF on serotonin-related gene expressions
Serotonin-related genes included tryptophan hydroxylase tph-1, which is the rate-limiting enzyme in the first step of serotonin biosynthesis. Gene unc-86 regulates serotonin biosynthesis and neurodevelopment in four classes of serotonergic neurons (NSM, AIM, AIH, HSN) (Sze et al. 2002), while the biosynthesis of serotonin in ADF neurons is regulated by complex encoded by osm-9 and ocr-2 that is independent of unc-86 (Zhang et al. 2004). Metabotropic G protein-coupled receptors (GPCRs), SER-1 and serotonin-gated chloride channel, MOD-1, also play crucial roles in the functions of serotonin (Anderson et al. 2013; Ranganathan et al. 2000). By using qRT-PCR, we found that the mRNA levels of unc-86 and tph-1 were increased in nematodes exposed to 0.5 T and 1 T SMFs for 48 h, while ocr-2, osm-9, ser-1 and mod-1 were significantly decreased (Fig. 5).
The low expression of ocr-2 and osm-9 mRNA levels indicated that the secretion of serotonin in ADF neurons was reduced, whereas the high expression of unc-86 increased the secretion of serotonin in other types of serotonergic neurons, resulting in up-regulation of total serotonin level. Among the serotonergic neurons of C. elegans, only ADF neurons are involved in aversive olfactory learning behavior (Shao et al. 2019). The statistically significant decrease in the aversion of olfactory learning behavior of C. elegans may be caused by the decline of ADF neuron function. Furthermore, the reduced expression of receptor genes ser-1 and mod-1 also leads to impaired serotonin function. On the other hand, downregulation of receptor gene expression may provided a negative feedback to the total serotonin level. Based on the critical role of serotonin in energy homeostasis and fat metabolism (Zheng and Greenway 2012), we hypothesized that chronic-term exposure to moderate-intensity SMFs altered fat metabolism through central serotonin pathway.
Effects of moderate SMF on fat storage
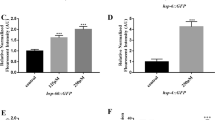
Given the modulating effect of moderate intensities of SMF at 0.5 T and 1 T on serotonin level, we asked whether the increased serotonin influenced fat storage of worms. DHS-3 localized on lipid droplets of C. elegans, which is used as a lipid droplets marker protein (Zhang et al. 2012). Utilizing DHS-3::GFP nematodes, the fat storage in 0.5 T and 1 T magnetic field exposure groups decreased by 13.08% and 10.20% compared with the control group, respectively, as shown in Fig. 6a and c, which was confirmed by Oil Red O staining for lipid droplets. Furthermore, tph-1 mutants showed increased fat storage than wild-type N2 nematodes, whereas both 0.5 T and 1 T SMF did not elicit any statistically significant change on the fat storage in this strain (Fig. 6b and d). The results indicated that long-term treatment of 0.5 T and 1 T SMF induced fat loss on wild-type N2 worms by increasing serotonin levels.
Effects of 0.5 T and 1 T SMFs exposure for 48 h on fat storage in N2 and tph-1(mg280) worms. (a) The representative images of green fluorescence in DHS-3::GFP worms. (b) The representative images of Oil Red O staining in N2 and tph-1(mg280) worms. (c) Quantification of DHS-3::GFP fluorescence intensity (n ≥ 30 nematodes/group). (d) Quantification of Oil Red O staining N2 and tph-1(mg280) worms. The data in (c) and (d) are presented as mean ± SD of three independent experiments (n = 3). *p < 0.05
The fat storage of C. elegans is regulated by hundreds of genes, which functions in food sensation, neuroendocrine signaling, lipolysis and synthesis (Lin et al. 2019). Considering the effect of SMFs on lipid peroxidation (Vergallo et al. 2020; WATANABE et al. 1997), the underlying mechanisms of fat reduction by SMFs are yet to be further studied in C. elegans. In addition, lipid metabolism disorders and diabetes are highly correlated (Thongnak et al. 2020). Many previous studies reported the therapeutic effect of moderate SMF on type 2 diabetes, diabetic wounds healing and anti-thrombosis (Carter et al. 2020; Jing et al. 2010; Li et al. 2020), which provided theoretical evidence into safe application of moderate SMF to cure related diseases in the presence or absence of drugs. Given that the changes of serotonergic system alone cannot account for serotonin-mediated alterations in the fat storage in mammals, the mechanism merits direct exploring in higher animals. Our results provided clear evidence that the moderate SMF reduced the fat storage of wild-type worms by regulating the level of serotonin. However, to determine whether the moderate SMF is effective for fat accumulation caused by diet or disease requires further systematic investigations.
Conclusion
Our results showed that exposure to 0.5 and 1 T SMFs had no effect on the movement behavior, but significantly reduced the aversion learning behavior in C. elegans. 1 T magnetic field increased pharyngeal pumping. Further, long-term exposure to 0.5 and 1 T SMFs had differential effects on the expressions of tph-1 in ADF and NSM neurons. Specifically, the moderate-intensity SMFs decreased the secretion of serotonin in ADF neurons, while increasing the secretion of serotonin in NSM neurons, resulting in an increased in the total level of serotonin in C. elegans. Moreover, wild-type nematodes exposed to moderate-intensity SMFs exhibited the phenotype of decreased fat storage caused by increased serotonin level. Our study provided new insights into elucidating nervous responses of C. elegans to moderate-intensity SMFs.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anderson A, Laurenson-Schafer H, Partridge FA, Hodgkin J, McMullan R (2013): Serotonergic Chemosensory Neurons Modify the C. elegans Immune Response by Regulating G-Protein Signaling in Epithelial Cells. PLOS Pathog 9
Barbagallo B, Philbrook A, Touroutine D, Banerjee N, Oliver D, Lambert CM, Francis MM (2017) Excitatory neurons sculpt GABAergic neuronal connectivity in the C-elegans motor circuit. Development 144:1807–1819
Bessho K, Yamada S, Kunitani T, Nakamura T, Hashiguchi T, Tanimoto Y, Harada S, Yamamoto H, Hosono R (1995) Biological responses in caenorhabditis-elegans to high magnetic-fields. Experientia 51:284–288
Brenner S (1974) Genetics of caenorhabditis-elegans. Genetics 77:71–94
Cañedo L, Cantú RGX, Hernández-R J (2003) Magnetic field exposure during gestation: pineal and cerebral cortex serotonin in the rat. Int J Dev Neurosci 21:263–266
Cao X, Wang X, Chen H, Li H, Tariq M, Wang C, Zhou Y, Liu Y (2019) Neurotoxicity of nonylphenol exposure on Caenorhabditis elegans induced by reactive oxidative species and disturbance synthesis of serotonin. Environ Pollut 244:947–957
Carter CS et al (2020) Exposure to Static Magnetic and Electric Fields Treats Type 2 Diabetes (vol 32, pg 561, 2020). Cell Metab 32:1076–1076
Clites BL, Pierce JT (2017): Identifying Cellular and Molecular Mechanisms for Magnetosensation. In: Zoghbi HY (Zoghbi HY)^(Zoghbi HYs)|,*Annual Review of Neuroscience, pp. 231–250
Coots A, Shi RY, Rosen AD (2004) Effect of a 0.5-T static magnetic field on conduction in guinea pig spinal cord. J Neurol Sci 222:55–57
Corsi AK, Wightman B, Chalfie M (2015): A Transparent window into biology: A primer on Caenorhabditis elegans. WormBook : the online review of C. Elegans Biol 1–31
de Vocht F, van Drooge H, Engels H, Kromhout H (2006) Exposure, health complaints and cognitive performance among employees of an MRI scanners manufacturing department. J Magn Reson Imaging 23:197–204
Dincic M, Krstic DZ, Colovic MB, Ostojic JN, Kovacevic S, De Luka SR, Djordjevic DM, Cirkovic S, Brkic P, Todorovic J (2018) Modulation of rat synaptosomal ATPases and acetylcholinesterase activities induced by chronic exposure to the static magnetic field. Int J Radiat Biol 94:1062–1071
Dini L, Abbro L (2005) Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 36:195–217
Donohoe DR, Phan T, Weeks K, Aamodt EJ, Dwyer DS (2008) Antipsychotic drugs up-regulate tryptophan hydroxylase in ADF neurons of Caenorhabditis elegans: Role of calcium-calmodulin-dependent protein kinase II and transient receptor potential vanilloid channel. J Neurosci Res 86:2553–2563
Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI (2013) Serotonin and the Neuropeptide PDF Initiate and Extend Opposing Behavioral States in C. elegans. Cell 154:1023–1035
Goraghani MS, Ahmadi-Zeidabadi M, Bakhshaei S, Shabani M, Ravandi SG, Rezaei-Zarchi S, Nozari M (2019) Behavioral consequences of simultaneous postnatal exposure to MK-801 and static magnetic field in male Wistar rats. Neurosci Lett 701:77–83
Hernadi L, Laszlo JF (2014) Pharmacological analysis of response latency in the hot plate test following whole-body static magnetic field-exposure in the snail Helix pomatia. Int J Radiat Biol 90:547–553
Hobert O (2013): The neuronal genome of Caenorhabditis elegans. WormBook : the online review of C. Elegans Biol 1–106
Houpt TA, Carella L, Gonzalez D, Janowitz I, Mueller A, Mueller K, Neth B, Smith JC (2011) Behavioral effects on rats of motion within a high static magnetic field. Physiol Behav 102:338–346
Houpt TA, Cassell J, Carella L, Neth B, Smith JC (2012) Head tilt in rats during exposure to a high magnetic field. Physiol Behav 105:388–393
Ijomone OM, Miah MR, Akingbade GT, Bucinca H, Aschner M (2020) Nickel-Induced Developmental Neurotoxicity in C. elegans Includes Cholinergic, Dopaminergic and GABAergic Degeneration, Altered Behaviour, and Increased SKN-1 Activity. Neurotox Res 37:1018–1028
Ishita Y, Chihara T, Okumura M (2020) Serotonergic modulation of feeding behavior in Caenorhabditis elegans and other related nematodes. Neurosci Res 154:9–19
Jha SK, Jha NK, Kumar D, Sharma R, Shrivastava A, Ambasta RK, Kumar P (2017) Stress-Induced Synaptic Dysfunction and Neurotransmitter Release in Alzheimer’s Disease: Can Neurotransmitters and Neuromodulators be Potential Therapeutic Targets? J Alzheimers Dis 57:1017–1039
Jing D, Shen G, Cai J, Li F, Huang J, Wang Y, Xu Q, Tang C, Luo E (2010) Effects of 180 mT static magnetic fields on diabetic wound healing in rats. Bioelectromagnetics 31:640–648
Kroeker G, Parkinson D, Vriend J, Peeling J (1996) Neurochemical effects of static magnetic field exposure. Surg Neurol 45:62–66
Lee C, Chen H, Yeh L, Hong M, Huang GS (2012) Dosage-dependent Induction of Behavioral Decline in Caenorhabditis elegans by Long-term Treatment of Static Magnetic Fields. J Radiat Res 53:24–32
Lee K, Mylonakis E (2017) An Intestine-Derived Neuropeptide Controls Avoidance Behavior in Caenorhabditis elegans. Cell Rep 20:2501–2512
Li Q, Liao Z, Gu L, Zhang L, Zhang L, Tian X, Li J, Fang Z, Zhang X (2020): Moderate Intensity Static Magnetic Fields Prevent Thrombus Formation in Rats and Mice. Bioelectromagnetics 41:52–62
Liang X, Wang Y, Cheng J, Ji Q, Wang Y, Wu T, Tang M (2020) Mesoporous Silica Nanoparticles at Predicted Environmentally Relevant Concentrations Cause Impairments in GABAergic Motor Neurons of Nematode Caenorhabditis elegans. Chem Res Toxicol 33:1665–1676
Lin Y, Bao B, Yin H, Wang X, Feng A, Zhao L, Nie X, Yang N, Shi G, Liu J (2019): Peripheral cathepsin L inhibition induces fat loss in C. elegans and mice through promoting central serotonin synthesis. BMC BIOL 17
Lockwood DR, Kwon B, Smith JC, Houpt TA (2003) Behavioral effects of static high magnetic fields on unrestrained and restrained mice. Physiol Behav 78:635–640
Meisel JD, Kim DH (2014) Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol 35:465–470
Nass R, Hall DH, Miller DM, Blakely RD (2002) Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. P Natl Acad Sci USA 99:3264–3269
Okano H, Ino H, Osawa Y, Osuga T, Tatsuoka H (2012) The effects of moderate-intensity gradient static magnetic fields on nerve conduction. Bioelectromagnetics 33:518–526
Osuga T, Tatsuoka H (1999) Effect of 1.5 T steady magnetic field on neuroconduction of a bullfrog sciatic nerve in a partially active state within several hours after extraction. Magn Reson Imaging 17:791–794
Pais-Roldan P, Singh AP, Schulz H, Yu X (2016): High magnetic field induced otolith fusion in the zebrafish larvae. SCI REP-UK 6
Ranganathan R, Cannon SC, Horvitz HR (2000) MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408:470–475
Rivadulla C, Aguilar J, Coletti M, Aguila J, Prieto S, Cudeiro J (2018): Static magnetic fields reduce epileptiform activity in anesthetized rat and monkey. SCI REP-UK 8
Rosen AD (2003) Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys 39:163–173
Shao J, Zhang X, Cheng H, Yue X, Zou W, Kang L (2019) Serotonergic neuron ADF modulates avoidance behaviors by inhibiting sensory neurons in C-elegans. Pflug Arch Eur J Phy 471:357–363
Shen J, Chao Y, Du L (2007) Effects of static magnetic fields on the voltage-gated potassium channel currents in trigeminal root ganglion neurons. Neurosci Lett 415:164–168
Srinivasan S, Sadegh L, Elle IC, Christensen AGL, Faergeman NJ, Ashrafi K (2008) Serotonin regulates C-elegans fat and feeding through independent molecular mechanisms. Cell Metab 7:533–544
Sze JY, Victor M, Loer C, Shi Y, Ruvkun G (2000) Food and metabolic signaling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403:560–564
Sze JY, Zhang SY, Li J, Ruvkun G (2002) The C-elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development 129:3901–3911
Tatum MC, Ooi FK, Chikka MR, Chauve L, Martinez-Velazquez LA, Steinbusch HWM, Morimoto RI, Prahlad V (2015) Neuronal Serotonin Release Triggers the Heat Shock Response in C. elegans in the Absence of Temperature Increase. Curr Biol 25:163–174
Thongnak L, Pongchaidecha A, Lungkaphin A (2020) Renal Lipid Metabolism and Lipotoxicity in Diabetes. Am J Med Sci 359:84–99
Tkac I, Benneyworth MA, Nichols-Meade T, Steuer EL, Larson SN, Metzger GJ, Ugurbil K (2021): Long-term behavioral effects observed in mice chronically exposed to static ultra-high magnetic fields. Magn Reson Med 81:1544–1559
Todorovic D, Ilijin L, Mrdakovic M, Vlahovic M, Grcic A, Petkovic B, Peric-Mataruga V (2020) The impact of chronic exposure to a magnetic field on energy metabolism and locomotion ofBlaptica dubia. Int J Radiat Biol 96:1076–1083
Tsalik EL, Hobert O (2003) Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol 56:178–197
Van Huizen AV, Morton JM, Kinsey LJ, Von Kannon DG, Saad MA, Birkholz TR, Czajka JM, Cyrus J, Barnes FS, Beane WS (2019): Weak magnetic fields alter stem cell-mediated growth. SCI ADV 5, eaau7201-eaau7201
Vergallo C, Panzarini E, Tenuzzo BA, Mariano S, Tata AM, Dini L (2020) Moderate Static Magnetic Field (6 mT)-Induced Lipid Rafts Rearrangement Increases Silver NPs Uptake in Human Lymphocytes. Molecules 25:1398
Vidal-Gadea A, Ward K, Beron C, Ghorashian N, Gokce S, Russell J, Truong N, Parikh A, Gadea O, Ben-Yakar A, Pierce-Shimomura J (2015): Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. ELIFE 4
Wang D, Wang Z, Zhang L, Li Z, Tian X, Fang J, Lu Q, Zhang X (2018) Cellular ATP levels are affected by moderate and strong static magnetic fields. Bioelectromagnetics 39:352–360
Ward BK, Tan GX, Roberts DC, Della Santina CC, Zee DS, Carey JP (2014): Strong Static Magnetic Fields Elicit Swimming Behaviors Consistent with Direct Vestibular Stimulation in Adult Zebrafish. PLOS ONE 9
Watanabe Y, Nakagawa M, Miyakoshi Y (1997) Enhancement of Lipid Peroxidation in the Liver of Mice Exposed to Magnetic Fields. Ind Health 35:285–290
Wu L, Dickman JD (2012) Neural Correlates of a Magnetic Sense. Science 336:1054–1057
Wu T, He K, Zhan Q, Ang S, Ying J, Zhang S, Zhang T, Xue Y, Tang M (2015) MPA-capped CdTe quantum dots exposure causes neurotoxic effects in nematode Caenorhabditis elegans by affecting the transporters and receptors of glutamate, serotonin and dopamine at the genetic level, or by increasing ROS, or both. Nanoscale 7:20460–20473
Zhang P, Na H, Liu Z, Zhang S, Xue P, Chen Y, Pu J, Peng G, Huang X, Yang F, Xie Z, Xu T, Xu P, Ou G, Zhang SO, Liu P (2012) Proteomic Study and Marker Protein Identification of Caenorhabditis elegans Lipid Droplets. Mol Cell Proteomics 11:317–328
Zhang SY, Sokolchik I, Blanco G, Sze JY (2004) Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons. Development 131:1629–1638
Zheng J, Greenway FL (2012) Caenorhabditis elegans as a model for obesity research. Int J Obes (lond) 36:186–194
Funding
This work was supported by Dean’s Fund of Hefei Institute of Physical Science (YZJJZX202014), the National Natural Science Foundation of China (52177227, 22006149) and Hundred Talents Program (E09BR22). A portion of this work was supported by the High Magnetic Field Laboratory of Anhui Province.
Author information
Authors and Affiliations
Contributions
LC contributed to methodology, formal analysis, investigation, data curation, writing-original draft, validation and writing—review and editing.
BY was involved in validation and methodology.
HD contributed to resources and funding acquisition.
TZ was involved in investigation.
YL contributed to methodology and formal analysis.
JW and ZC were involved in investigation and data curation.
AX contributed to resources, writing—review and editing, funding acquisition and supervision.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, L., Yang, B., Du, H. et al. Moderate intensity of static magnetic fields can alter the avoidance behavior and fat storage of Caenorhabditis elegans via serotonin. Environ Sci Pollut Res 29, 43102–43113 (2022). https://doi.org/10.1007/s11356-022-18898-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18898-5