Abstract
Cryptochromes are blue-light absorbing photoreceptors found in many organisms where they have been involved in numerous growth, developmental, and circadian responses. In Arabidopsis thaliana, two cryptochromes, CRY1 and CRY2, mediate several blue-light-dependent responses including hypocotyl growth inhibition. Our study shows that an increase in the intensity of the ambient magnetic field from 33–44 to 500 μT enhanced growth inhibition in A. thaliana under blue light, when cryptochromes are the mediating photoreceptor, but not under red light when the mediating receptors are phytochromes, or in total darkness. Hypocotyl growth of Arabidopsis mutants lacking cryptochromes was unaffected by the increase in magnetic intensity. Additional cryptochrome-dependent responses, such as blue-light-dependent anthocyanin accumulation and blue-light-dependent degradation of CRY2 protein, were also enhanced at the higher magnetic intensity. These findings show that higher plants are sensitive to the magnetic field in responses that are linked to cryptochrome-dependent signaling pathways. Because cryptochromes form radical pairs after photoexcitation, our results can best be explained by the radical-pair model. Recent evidence indicates that the magnetic compass of birds involves a radical pair mechanism, and cryptochrome is a likely candidate for the avian magnetoreception molecule. Our findings thus suggest intriguing parallels in magnetoreception of animals and plants that appear to be based on common physical properties of photoexcited cryptochromes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The geomagnetic field is an omnipresent source of information for magnetosensitive organisms (Wiltschko and Wiltschko 1995). Migratory birds are known to use a magnetic compass; their responses in orientation tests depend on the direction and intensity of the local magnetic field. Moreover, the magnetic compass responses of birds have been found to depend on the wavelength of the ambient light: under light from the blue to green part of the spectrum, they headed into the normal migratory direction, whereas they were disoriented under yellow and red light (Wiltschko and Wiltschko 2002). The sensitivity to magnetic fields as weak as the geomagnetic field of 25–60 μT is linked to the effect of magnetic fields on electron transfer reactions of photoreceptors through radical-pair processes (Schulten 1982; Ritz et al. 2000), with the blue-light receptor cryptochrome suggested as a promising candidate for the receptor molecule (Ritz et al. 2000). The effect of magnetic fields in this model would modulate photoreceptor signaling and could manifest itself by forming direction- and intensity-dependent activation patterns (Ritz et al. 2000). In birds, reception of magnetic compass information takes place in the eye (Semm and Demaine 1986; Wiltschko et al. 2002); the proposed function of cryptochromes in magnetoreception is supported by their occurrence in the retina (Möller et al. 2004; Mouritsen et al. 2004).
Cryptochrome photoreceptors were first identified in higher plants where they are ubiquitous and mediate a number of blue-light-dependent developmental and growth responses (Ahmad and Cashmore 1993; Briggs and Olney 2001; Ahmad 2003); they have since been identified in animals and prokaryotes (Lin and Shalitin 2003). In Arabidopsis, cryptochromes are encoded by two similar genes, cry1 and cry2, and show partial functional overlap in mediating responses such as blue-light-dependent inhibition of hypocotyl elongation, anthocyanin accumulation, vegetative growth, floral initiation, maintenance of circadian rhythms and expression of blue-light regulated genes. In addition, CRY2 protein levels in seedlings decrease rapidly upon illumination by blue light, presumably as a result of protein degradation of the light-activated form of the receptor (Ahmad et al. 1998; Lin et al. 1998). Like photolyases, plant cryptochromes have been shown to undergo a light-dependent electron transfer reaction, known as photoactivation, that leads to photoreduction of the flavin cofactor, FAD (Giovani et al. 2003). This photoreduction presumably plays a role in signaling, as mutants that inactivate this reaction in the purified protein in vitro also show impaired photoreceptor function in vivo (Zeugner et al. 2005). Given that light-dependent electron transfer may play a role in cryptochrome signaling, it appeared to be an intriguing possibility that cryptochrome-controlled responses in plants may also be affected by weak magnetic fields.
In the present study, we wanted to test (1) whether a change in ambient magnetic conditions affects physiological responses in plants, and (2) whether any observed magnetic effects are specific to cryptochrome-mediated pathways. For this purpose we choose hypocotyl growth, anthocyanin accumulation and CRY2 protein stability as reference responses, exposing seedlings to two distinct magnetic conditions: the local magnetic conditions of 33, 40 or 44 μT and a more than ten times increased field of 500 μT.
Materials and methods
Plant materials and growth conditions
As wild-type strain of Arabidopsis thaliana (L.) Heynh, we used the ecotype Landsberg erecta (Ler) (Redei 1962), originally obtained from Lehle Seeds, Tucson, AZ, USA. The double mutant hy4-3 fha1 used in the hypocotyl growth tests is defective in both cry1 and cry2 (Ahmad et al. 2002). Arabidopsis seeds were sterilized by rinsing them in 70% (v/v) ethanol and in 5% (v/v) sodium hypochloride solution. Seeds were sown on half-strength Murashige and Skoog salts medium (Sigma) on Petri plates containing 2% (w/v) sucrose and 0.9% (w/v) agar. Plates were maintained for 2 days at 5°C, and then placed at room temperature for 2 days, during which time the seedlings for studying hypocotyl growth and anthocyanin accumulation were irradiated under white fluorescent light for typically 24 h (100 μmol m−2 s−1) to induce germination, and later transferred to the relevant light condition at the respective magnetic field intensity or, in the case of the seedlings used for the CRY2 stability experiments, returned to darkness at the local magnetic field for an additional 48 h.
For all experiments, the seedlings received identical growth and germination treatment and were subsequently placed simultaneously in the two different magnetic fields. The initial series of tests on hypocotyl growth (tests F1–F6) was carried out in Frankfurt am, Germany, where the technique to generate the 500 μT magnetic field was available. This required shipping plates of seedlings from the Paris laboratory to Frankfurt and often storing them at 5°C before the tests, resulting in some variation in the state of germination of seedlings (between radicle emergence and early cotyledon emergence) from one trial to the next. Therefore, to avoid this source of variation between trials, the equipment for the magnetic field and the light sources (see below) were moved to Paris, to achieve greater uniformity in germination state between independent trials (P1–P6). However, all plates of seedlings used within a trial and compared had undergone identical germination conditions, with the only variable within a trial being the difference in magnetic intensity to which the test samples were exposed (see below).
Magnetic fields and blue-light irradiation
All tests were performed in indoor rooms where because of the construction materials, the local magnetic field was slightly lower than the geomagnetic field outside. It was 44 μT for the hypocotyl growth trials F1–F6, and and 33 μT for trials P1–P6. Anthocyanin accumulation and cry2 stability was studied in a laboratory with 40 μT.
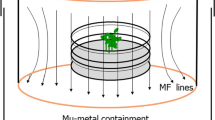
The 500 μT field was produced by Helmholtz coils (diameter 44 cm; 22 cm clearance) aligned in a way that the axis of the coils coincided with inclination; as a result, magnetic intensity was the only parameter altered (Fig. 1). The plates carrying the seedlings were placed in the center of each coil system. To control against any non-magnetic effects from the coils, the control plates in the local field were placed in a similar system of double-wrapped coils with the current in each coils running in opposite directions, thus producing a net zero magnetic field. The field intensity was measured with the magnetometer 428B from Hewlett-Packard using the probe 3529A. The two coil systems always stood in the same room; it was checked that the experimental 500 μT field had no detectable influence on the local control field.
Experimental setup for irradiating seedlings of Arabidopsis in a magnetic field generated by a Helmholtz coil. The coil was tilted in order to assure that the magnetic field lines (thin black arrows) were parallel to those of the local field (large grey arrow). Seedlings grew on two standard Petri dishes that were placed horizontally in the center of the coil where the magnetic field is homogeneous. The lower plate was wrapped in aluminum foil (not shown) and served as a dark control. Blue (465 nm) or red (633 nm) overhead light was provided by an array of LEDs. Seedlings: 1–8 mm; Petri dish: diameter 94 mm, height 16 mm; Helmholtz coil: diameter 44 cm, 22 cm clearance. For further details see Materials and methods
Overhead blue light with a peak wavelength of 465 nm and with λ/2 at 454 and 476 nm was generated by arrays of 19 LEDs (light-emitting diodes, Conrad)) each mounted on a 12 × 12 cm plastic board 7 cm above the plates. Red-light emitting LEDs had a peak wavelength of 633 nm. Fluence rates were adapted to the physiological response studied (see below) and were measured with Optometer P9710-1 (Gigahertz-Optik, Pucheim, Germany) using the radiometric probe RW-3703-2 (a silicium photoelement for the wavelength range 400–800 nm) and kept constant during the respective experiments and was identical in the two magnetic conditions.
Hypocotyl growth
The experiments were carried out in temperature-controlled rooms with temperature varying between 20.5 and 22.7°C (F1–F6) and 19 and 21°C (P1–P6). In each coil, two plates were placed one above the other. The overhead blue light passed two diffusers (white Plexiglass) before reaching the top plate where it had a fluence rate of 20 W m−2 (Fig. 1); it was further dimmed by the agar of the upper plate to 12 W m−2 at the lower plate, or, in trials F1–F6, a neutral density filter (40% transmittance; cellulose acetate LEE filters, Panavision France, Paris) placed between the Petri dishes such that the lower plate was exposed to 3.3 W m−2. After an exposure time of about 66 h (F1–F6) and 72 h (P1–P6, see Tables), the length of ideally 30 hypocotyls was measured. These data obtained in the two magnetic fields were compared using the z-test.
In the same manner, we also tested in the two magnetic conditions (1) the double mutant hy4-3fha1 that is defective in the genes cry1 and cry2 under blue light, (2) wild-type Arabidopsis and the cry-deficient mutant in total darkness, with the plates of seedlings densely wrapped in aluminum foil, and (3) wild-type Arabidopsis under red light produced by LEDs (Börsig, Neckarsulm, Germany) with a peak wavelength of 633 nm with λ/2 at 623 and 644 nm; the fluence rates were 42.5 W m−2 and 8.5 W m−2, respectively, at the top and the lower plate.
Anthocyanin accumulation
Anthocyanin assays were performed following the protocol described previously (Kubasek et al. 1992; Ahmad et al. 1995). Seedlings were grown under blue light of 12 W m−2 for 48–55 h at 25–26°C. Twenty seedlings from plates at the two magnetic intensities were harvested and transferred into 1 ml of acidified methanol (1% HCl/methanol) and the pigments extracted over 3–4 h at room temperature. The absorbance of the solution with extracted pigments was taken at 530 nm and also at 657 nm to correct for presence of chlorophyll. Anthocyanin contents were determined by the empirical formula A530–0.25*A657 where A is the absorbance at 530 and 657 nm, respectively (Kubasek et al. 1992). All determinations were performed in triplicate from the same experimental sample plate, and the absorbance data in the two magnetic fields were compared using the t test.
Stability of the CRY2 protein
After germination, the seedlings were kept in darkness for 2 days at the local magnetic field to generate etiolated plants. Then, at a hypocotyl length of between 0.6 and 0.8 mm, duplicate plates were transferred to each of the respective magnetic field conditions under blue light of the low intensity of 2.5 W m−2 so as not to saturate the CRY2 degradation response, with seedlings kept in the local field in the dark serving as controls. After an exposure of 30 min, seedlings were harvested and assayed for CRY2 protein levels essentially as previously described (Ahmad et al. 1998). Thirty seedlings from experimental sample plates were homogenized into 0.1 ml SDS gel electrophoresis sample buffer (0.1 M Tris/HCl pH 6.8, 10% glycerol, 2% SDS, 100 mM β-mercaptoethanol, 0.01% bromophenol blue dye) and boiled immediately for 10 min. Proteins were subsequently loaded onto polyacrylamide gels, electrophoresed, and subjected to Western transfer, followed by immunodetection of the CRY2 protein with anti-CRY2 antibodies on the blots. Duplicate gels were run for staining with Coomassie blue dye to verify equivalent protein load. Visualization of the proteins was by a dye-based detection assay (TMB stabilized substrate for HRP from Promega, Madison, WI, USA).
Results
We found a significant effect of the increase in magnetic intensity in all three responses studied.
Hypocotyl growth
We first looked for a possible effect of magnetic intensity on the blue-light-dependent hypocotyl growth that is largely under the control of cryptochome photoreceptors, in particular CRY1. A clear effect of the increased magnetic intensity on hypocotyl growth was already observed in the initial series of tests F1–F3 performed in Frankfurt am. In this series, there was a considerable amount of variance in hypocotyl growth due to differences in germination states (see Materials and methods). In series P1–P3, performed in Paris, the variance of the controls is smaller and the magnetic effect is more pronounced. Table 1, upper section, gives the mean lengths of the hypocotyls grown under the two magnetic conditions and the various light regimes, together with the standard deviations and the test statistic of the z-test comparing those grown in the local magnetic field and those grown under increased intensity.
Under blue light, we observed the expected growth inhibition, i.e., seedlings exposed to elevated fluence rates were substantially more inhibited than those grown at lower ones. However, in the higher magnetic field of 500 μT, this growth inhibition was enhanced when compared to that of seedlings grown in the local field of 44 or 33 μT. Figure 2 gives the lengths of the seedlings at 500 μT as percentage of the control seedlings grown simultaneously in the local field under identical conditions. The reduction in length due to the increased magnetic intensity was comparable for all fluence rates of blue light and statistically highly significant (Table 1, last column). Dark-grown seedlings, on the other hand, were unaffected by the change in magnetic intensity (Fig. 3; Table 1, central section).
Effect of magnetic intensity on hypocotyl growth inhibition in Arabidopsis thaliana: length of seedlings grown in 500 μT as percentage of those grown in parallel in the local field. Round symbols indicate data of the initial tests in Frankfurt, diamonds those of the tests in Paris. Symbols indicate difference to those in the local field by the z-test: solid, P < 0.001; semi-open, P < 0.05; open, no significant difference (for numerical data, see Tables 1 and 2)
Effect of magnetic intensity on anthocyanin accumulation (A530-0.25A657) in seedlings grown in parallel in the local magnetic field of 40 μT and in a higher magnetic field of 500 μT. Three independent trials are shown, with error bars representing the standard deviation and asterisks indicating significance level of the differences: *P < 0.05; **P < 0.01
To establish a crucial role of cryptochrome for the observed magnetic sensitivity, we repeated the growth inhibition experiments in the presence of monochromatic 633 nm red light. Under these conditions, hypocotyl growth inhibition in Arabidopsis is mediated by phytochromes (Ahmad and Cashmore 1993; Quail et al. 1995), and not by cryptochromes (Ahmad et al. 2002). Red light also led to substantial hypocotyl growth inhibition (Table 1, lower section), but in contrast to that observed under blue light, the growth inhibition remained unaffected by the increased magnetic field intensity (Fig. 2). The cryptochrome-deficient double mutant, cry1/cry2, of A. thaliana exposed to blue light showed neither growth inhibition nor did it respond to the increased magnetic field intensity of 500 μT (Fig. 2; Table 2).
In summary, we observed a significant magnetic effect on hypocotyl growth inhibition, but this effect is manifested only when growth is controlled by the blue-light pathway, and it requires functional cryptochrome. The increased magnetic intensity seemed to enhance the effect of the blue light.
Anthocyanin accumulation
We next studied the effect of magnetic intensity on anthocyanin accumulation. In each of three independent trials, the levels of anthocyanin accumulation were significantly higher in seedlings that had been grown in the higher magnetic 500 μT field compared to seedlings grown in the local magnetic field of 40 μT under identical light intensity, with the differences of 28, 43 and 45%, respectively, being significant all three independent trials (Fig. 3). This magnetic field effect on anthocyanin accumulation is consistent with the effect on hypocotyl inhibition, as here, too, the response to blue light is enhanced.
Stability of CRY2
In contrast to CRY1, the CRY2 photoreceptor undergoes light-dependent degradation in response to blue light activation (Ahmad et al. 1998; Lin et al. 1998). Because the blue-light-dependent degradation of CRY2 represents the most rapid and direct cryptochrome response that has as yet been identified in plants, CRY2 stability is a very suitable system to address the question whether or not the magnetic field exerts its effect at the level of this photoreceptor or close to it. To establish whether this cryptochrome-dependent response may also be sensitive to the magnetic field, we performed a rapid assay for CRY2 protein stability in etiolated (dark-grown) Arabidopsis seedlings that had been exposed for 30 min to blue light in the local 40-μT field and in the 500-μT field. The results are given in Fig. 4: CRY2 protein levels were markedly decreased relative to those in dark-control seedlings. However, as seen in three independent trials, the rate of decrease is clearly greater under the higher magnetic field intensity. Here, too, the increased magnetic intensity enhances the effect of the blue light, which is in agreement with the results of two experiments previously described.
Discussion
There are numerous reports describing a multitude of magnetic effects in plants and fungi, yet most studies remain largely on a phenomenological level and are in general characterized by a lack of physiological and mechanistic insights (for review: Galland and Pazur 2005). Our results on hypocotyl growth inhibition are the first example of magnetic effects in plants that are clearly shown to be blue-light-dependent. They indicate a link between phototransduction and magnetic sensitivity observable on the level of in vivo growth responses of plants. Our results further demonstrate that this magnetic sensitivity to blue light occurs in responses that are under the control of the cryptochrome blue-light photoreceptors of Arabidopsis.
Not only in hypocotyl growth, but also in anthocyanin accumulation and CRY2 stability, the responses in the stronger magnetic field are consistent with an increased sensitivity to blue light. It should be noted that the differences in responses between the two magnetic fields are mostly between 12 and 37% in the hypocotyl growth inhibition assay, and between 28 and 45% in the anthocyanin accumulation assay. The absolute size of these differences may appear small compared to differences that can be caused by other factors. We observed considerable variability in absolute growth and in absolute anthocyanin accumulation from experiment to experiment, which is most likely due to small variations in the state of germination, time of growth etc. Anthocyanin accumulation in particular is known to be under tight developmental control and reaches a peak at a relatively defined point in seedling development (Kubasek et al. 1992). Thus, magnetic field effects on plant responses can easily be missed or attributed to general scatter when different sets of seedlings are compared. However, for sets of seedlings prepared, grown, and treated under identical conditions, the effect of the ambient magnetic field is obvious: we consistently found effects of the increased magnetic intensities on blue light responses. Magnetic field effects were statistically significant for the vast majority of individual experiments, thereby providing conclusive evidence that the ambient magnetic field affects the cryptochrome-dependent response pathways. This effect was consistently observed despite some differences in growth between experiments as in the initial series, which suggests that it is a robust effect not bound to a particular developmental state.
Radical-pair mechanism
The question arises by which mechanism magnetic fields as weak as those used in our experiments can influence responses involving photoreceptors like cryptochrome. Schulten et al. (1976) and others (Brocklehurst 1976) pointed out that certain chemical reactions, so-called radical-pair reactions, can be influenced by weak magnetic fields. In the radical-pair mechanism, a light-induced electron-transfer reaction results in the generation of radical-pair intermediates in either a singlet or triplet electronic state. The reaction rates and products from a radical pair depend on the spin state. Singlets and triplet states have chemically different fates and the transient radical pair thus decays into two different types of products, i.e., singlet or triplet products.
In the specific case of cryptochrome, the primary events could occur as follows (Fig. 5). In darkness the flavin chromophore of cryptochrome exists in the oxidized state (FAD); after near-UV or blue-light absorption, cryptochrome reaches the singlet excited state (FAD*) from where it undergoes a photoreduction, i.e., it receives via intraprotein electron transfer an electron from a tryptophan residue. The electron transfer results in a radical pair consisting of a flavosemiquinone radical (FADH•) and a tryptophanyl radical (Giovani et al. 2003). The radical pair is initially formed in either a singlet or a triplet state, defined by the relative orientation of the electron spins in the two radicals. For simplicity, we assume in the following that the radical pair is generated in a singlet state; however, the arguments can be applied completely analogously if the radical pair is generated in a triplet state.
Radical-pair mechanism as exemplified by the blue-light activated photoreceptor cryptochrome. FAD chromophore in darkness, FAD* singlet excited state after absorption of near-UV or blue light, FADH• flavosemiquinone radical, Trp• neutral tryptophanyl radical. FADH• and Trp• form radical pairs that can exsist either in the singlet S[FADH• Trp•] or in the triplet T[FADH• Trp•] state. External magnetic fields modulate the singlet-triplet ratio of the radical pairs and thus subsequent biological reactions that depend on the radical yield (modified after Ritz et al. 2000). The proton donor required for the formation of the FADH• radical has been omitted. For further explanations see text
Singlet radical pairs can convert to triplet radical pairs, i.e., T[FADH• Trp•], which in turn can convert back to singlet radical pairs (Fig. 5, double arrow inside the box). Both triplet and singlet states of radical pairs are transient and react within at most 100 μs to form singlet or triplet products. Since singlet and triplet products are chemically different, they will react with distinct reaction partners and/or form different reaction products before they revert back to the ground state. For example, if one of the radical products can diffuse, interaction of radical products with (as of yet unknown) downstream signaling partners can become spin-selective, providing a mechanism by which magnetic field effects on radical pair spin states can result in neurophysiological signals (Weaver et al. 2000).
If we assume that the singlet but not triplet products act as biological effector to initiate signaling, the well-understood theory of magnetic field effects on radical pairs (e.g., Timmel et al. 1998) would predict increased signaling for stronger magnetic fields. The interconversion between the singlet and triplet states of radical pairs is driven by the so-called hyperfine interaction of magnetic nuclei (H1, N14) with the unpaired electron spins. An external magnetic field changes the dynamics of singlet-triplet interconversion and, hence, the yield of reaction products. One needs to distinguish two types of magnetic field effects, typically referred to as ‘Zeeman’ or ‘high’-field effect and as ‘low’-field effect, respectively (Brocklehurst 1976; Timmel et al. 1998). The strength of the field is measured in comparison with the strength of the internal magnetic field created by the hyperfine interactions. Typically, internal magnetic fields are on the order 100 μT to several mT. For an external magnetic field of comparable size or stronger (‘high’ field), two of the three triplet states, namely T+1 and T−1, become progressively decoupled, restricting singlet-triplet conversion to the S and T0 states. If the radical pair is created in a singlet state, the reduced efficiency of singlet-triplet mixing amounts to a reduction of triplet states generated and, hence, an increase of singlet over triplet product yields, and an enhanced response to light in stronger magnetic fields. In external magnetic fields weaker than the hyperfine interactions (‘low’ field), a different mechanism leads to a decrease of singlet product yields (Brocklehurst 1976; Timmel et al. 1998). However, this low-field effect is only expected to occur for relatively simple radical pairs with a high degree of symmetry and it is not clear whether it would occur for the Arabidopsis FADH• Trp• radical pair.
Effects of external magnetic fields are more pronounced the longer the lifetime of the radical pair (Brocklehurst 1976; Timmel et al. 1998). Lifetimes of >100 ns are common in many radical pairs and effects of 100 μT fields have been demonstrated in radical pairs with such lifetimes (Batchelor et al. 1993; Brocklehurst and McLauchlan 1996; Galland and Pazur 2005). The lifetime of FADH• Trp• radical pairs in Escherichia coli CPD photolyase is exceptionally long, on the order of 10 μs (Weber 2005; see also Cintolesi et al. 2003 and references therein). If the lifetime of the closely related Arabidopsis cryptochrome FADH• Trp• radical pair was similarly long, one would expect effects of even weaker magnetic fields.
It should be noted that the dose-response curve of radical pair reaction yields to magnetic fields is highly non-linear. This is a consequence of the above discussed fact that singlet-triplet interconversion is driven mostly by the internal magnetic field created by the hyperfine interactions on the order of mT. The effect of an external magnetic field will become difficult to notice if the external magnetic field is much smaller than the hyperfine interactions. In experiments with radical pairs in solution, the effect of a 10–100 μT magnetic field (compared to zero magnetic field) is a change of yield on the order of 1%. For external magnetic fields of the same order as or larger than hyperfine couplings, one expects larger changes of reaction yields. Magnetic field effects saturate quickly for higher fields. We chose an intensity of 500 μT for this proof-of-principle study, because for this intensity a noticeable increase of singlet yield compared to 33, 40 or 44 μT is expected, avoiding the complications of ‘low’-field effects that can potentially occur at lower intensities.
In the preceding discussion we have assumed that the FADH• Trp• radical pair is generated in the singlet state. The discussion can be adapted to yield the same conclusions for a radical pair generated in the triplet state: one only has to assume that the biological effector is a triplet product in this case.
Cryptochrome—a wide-spread magnetophotoreceptor?
Our study provides strong support for the suggested link between magnetic field sensitivity through radical-pair reactions and a crucial role of cryptochromes. The absence of magnetic field effects on hypocotyl growth under red light and in the cry1/cry2 double mutant, restricts the magnetosensitive reaction to the blue-light, i.e., the cryptochrome-mediated pathway. As our experiments involve in vivo assays of phenomena that occur in planta, we cannot rule out the possibility that the observed magnetic sensitivity occurs at a point in the respective signaling pathways downstream (and therefore not directly linked) to the primary photochemistry of the photoreceptor. However, it is significant that several independent responses known to be under the control of the cryptochromes are affected by magnetic fields in a similar manner, including a very rapid response of blue-light induced CRY2 protein degradation that occurs within only a half hour of light activation. This suggests that the magnetic field may, in fact, act at the point of cryptochrome photoreception, involving the cryptochrome molecule itself.
In migratory birds, recent evidence strongly supports a radical pair mechanism underlying the detection of the geomagnetic field (Ritz et al. 2004; Thalau et al. 2005; Wiltschko et al. 2005); this finding, combined with the above mentioned evidence implicating a role of cryptochromes (Möller et al. 2004; Mouritsen et al. 2004) makes it an appealing hypothesis that radical pairs of cryptochrome represent the physical basis of the avian magnetic compass, even if a direct link between cryptochromes and magnetic sensitivity could not yet be established for birds. Together with our present findings, this would mean that magnetic sensitivity is based on similar mechanisms in very different organisms.
In animals, magnetic fields have an important biological function: they provide navigational information for spatial orientation (Wiltschko and Wiltschko 1995, 2005). In plants, the biological significance of the observed magnetic sensitivity is not obvious. Our demonstration of magnetic field effects in Arabidopsis calls for further, more exhaustive studies of possible magnetic field effects on cryptochrome-mediated responses in plants. For example, the role of cryptochromes in entraining circadian rhythms suggests the possibility of magnetic effects on the internal clock (Devlin and Kay 2000; Harmer et al. 2000). For plants, further such studies are necessary to establish what biological significance the observed magnetic sensitivity might have. Yet it is also possible that the magnetic effects observed in plants represent side-effects of their flavin-containing cryptochromes. Responding to changes in magnetic fields might be an intrinsic property of the photochemistry of cryptochromes—animals, using the same pigments, may have developed this property in the course of evolution to serve an important function, namely to provide magnetic information for compass orientation.
Conclusions
Beyond its relevance to plants, our study on the role of cryptochrome in the context of magnetic sensitivity will likely have a broader impact on sensory biology at large. As Arabidopsis is well amenable to mutagenic and molecular methods, this may facilitate the analysis of molecular and physical details about cryptochrome-based magnetic receptors that are technically inaccessible, e.g., in the birds’ eyes. Although the radical-pair mechanism is an established mechanism by which weak magnetic fields can alter chemical reaction rates and yields, experimental studies of magnetic effects on radical pairs have so far been confined to in-vitro systems, e.g., re-engineered photosynthetic reaction centers (Liu et al. 2005). Our study is the first to demonstrate magnetic effects on a functional in vivo system. It thus opens the avenue to a direct verification of a radical-pair mechanism mediating magnetic sensitivity through identification of magnetically sensitive radical-pair reaction steps within the blue-light pathway, with optical and electron spin resonance studies identifying spin-sensitive reaction steps and radical-pair intermediates in Arabidopsis. In this sense, Arabidopsis may well emerge in the future as a useful minimal model for magnetoreception in other organisms, even animals like birds.
Abbreviations
- cry:
-
Cryptochrome
- FAD:
-
Flavin adenindinucleotide
- phy:
-
Phytochrome
- Trp:
-
Tryptophan
References
Ahmad M (2003) Cryptochromes and flavoprotein blue-light photoreceptors In: Nalwa HS (ed) Handbook of photochemistry and photobiology, vol 4. Academic, New York, pp 149–182
Ahmad M, Cashmore AR (1993) HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162–166
Ahmad M, Lin C, Cashmore AR (1995) Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyls elongation. Plant J 8:653–658
Ahmad M, Jarillo J, Cashmore AR (1998) Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell 10:197–208
Ahmad M, Grancher N, Heil M, Black RC, Giovani B, Galland P, Lardemer D (2002) Action spectrum for hypocotyl growth inhibition suggests dosage-dependent synergism among cryptochrome photoreceptors of Arabidopsis thaliana. Plant Physiol 129:774–785
Batchelor S, Kay C, McLauchlan K, Shkrob (1993) Time-resolved and modulation methods in the study of the effects of magnetic fields on the yields of free radical reactions. J Phys Chem 97:13250–13258
Briggs W R, Olney M (2001) Photoreceptors in plant photomorphogenesis to date: Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol 125:85–88
Brocklehurst B (1976) Spin correlation in the geminate recombination of radical ions in hydrocarbons. 1. Theory of the magnetic field effect. J Chem Soc Faraday Trans 2:1869–1884
Brocklehurst B, McLauchlan K (1996) Free radical mechanism for the effects of environmental electromagnetic fields on biological systems. Int J Rad Biol 69:3–24
Cintolesi F, Ritz T, Kay C, Timmel C, Hore P (2003) Anisotropic recombination of an immobilized photoinduced radical pair in a 50-μT magnetic field: a model avian photomagnetoreceptor. Chem Phys 294:385–399
Devlin PF, Kay SA (2000) Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 12:2499–2510
Galland P, Pazur A (2005) Magnetoreception in plants. J Plant Res 118:371–389
Giovani B, Byrdin M, Ahmad M, Brettel K (2003) Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nature Struct Biol 6:489–490
Harmer SL, Hogenesch JB, Staume M, Chang HS, Han B, Zhu T, Wang X, Kreps J A, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circdian clock. Science 290:2110–2113
Kubasek WL, Shirley BW, McKillop A, Goodman H, Briggs W, Ausubel FM (1992) Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell 4:1229–1236
Lin C, Shalitin D (2003) Cryptochrome structure and signal transduction. Annu Rev Plant Biol 54:469–496
Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR (1998) Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc Natl Acad Sci USA 95:2686–2690
Liu Y, Edge R, Henbest K, Timmel CR, Hore PJ, Gast P (2005) Magnetic field effect on singlet oxygen production in a biochemical system. Chem Communicat 2:174–176
Möller A, Sagasser S, Wiltschko W, Schierwater B (2004) Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91:585–588
Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, Dirks P, Weiler R (2004) Cryptochromes and neuronal-activity markers colocalize in the retina of migra-tory birds durign migratory orienttaion. Proc Natl Acad Sci USA 101:14294–14299
Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D (1995) Phytochromes: photosensory perception and signal transduction. Science 268:675–680
Redei GP (1962) Single locus heterosis. Z Vererbungsl 93:164–170
Ritz T, Adem S, Schulten K (2000) A model for photoreceptor-based magnetoreception in birds. Biophys J 78:707–718
Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W (2004) Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429:177–180
Schulten K (1982) Magnetic field effects in chemistry and biology. Festkörperprobleme 22:61–83
Schulten K, Staerk H, Weller A, Werner HJ, Nickel B (1976) Magnetic field dependence of the geminate recombination of radical ion pairs in polar solvents. Z Phys Chem NF 101:371–390
Semm P, Demaine C (1986) Neurophysiological properties of magnetic cells in the pigeon’s visual system. J Comp Physiol A 159:619–625
Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W (2005) Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 92:86–90
Timmel C, Till U, Brocklehurst K, McLauchlan K, Hore P (1998) Effects of weak magnetic fields on free radical recombination reactions. Mol Phys 95:71–89
Weaver J, Vaughan T, Astumian D (2000) Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature 405:707–709
Weber S (2005) Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. Biochem Biophys Acta 1707:1–23
Wiltschko R, Wiltschko W (1995) Magnetic orientation in animals. Springer, Berlin Heidelberg New York
Wiltschko W, Wiltschko R (2002) Magnetic compass orientation in birds and its physiolo-gical basis. Naturwissenschaften 89:445–452
Wiltschko W, Wiltschko R (2005) Magnetic orientation and magnetoreception. J Comp Physiol 191:675–693
Wiltschko W, Traudt J, Güntürkün O, Prior H, Wiltschko R (2002) Lateralisation of magnetic compass orientation in a migratory birds. Nature 419:467–470
Wiltschko R, Ritz T, Stapput K, Thalau P, Wiltschko W (2005) Two different types of light-dependent responses to magnetic fields in birds. Curr Biol 15:1518–1523
Zeugner A, Byrdin M, Bouly J-P, Bakrim N, Giovani B, Brettel K, Ahmad M (2005) Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J Biol Chem 280:19437–19440
Acknowledgments
This work was supported by the National Science Foundation (grant 0343737 to M.A.), the Human Frontier Science Foundation (grant to R.W. and T.R.) and the Deutsche Forschungsgemeinschaft (grant to W.W.). T.R. thanks the Sloan foundation for support. We thank K. Stapput and B. Siegmund for their help.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ahmad, M., Galland, P., Ritz, T. et al. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana . Planta 225, 615–624 (2007). https://doi.org/10.1007/s00425-006-0383-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0383-0