Abstract
As the basic unit of living organisms, the cell is where the macroscopic phenomenon meets the microscopic mechanisms. The focus of this chapter is on current evidence of SMFs on human cells and some animal cells, with a special focus on the factors that contributed to the seemingly inconsistent experimental results in the literature. We summarize cellular effects of static magnetic fields (SMFs), including cell orientation, proliferation, microtubule and cell division, actin, viability, attachment/adhesion, morphology, migration, membrane, cell cycle, DNA, reactive oxygen species (ROS), adenosine triphosphate (ATP) as well as calcium. Although it is obvious that for each aspect, the experimental results are highly variable, there are some effects that have clear physical explanations and confirmed phenomenon. For example, magnetic properties of the cells and their subcellular structures are determined by their compositions and structures, which will directly affect their orientation in high SMFs. However, there are still many unanswered questions. For example, the effects of SMFs on cellular ROS have been reported by numerous studies, but the effects are highly variable in different magnetic settings and sample types and there are still not clear physical explanations. Although the upscaling of the mechanisms from cells to tissues and living organisms is still a huge challenge, given the essential roles of cells in various living organisms, they are no doubt the central hub for researchers in this field to unravel the underlying mechanism and explore the future application of various SMFs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Magnetic field (MF)
- Static magnetic field (SMF)
- Cell type
- Cell density
- Red blood cell (RBC)
- Orientation
- Microtubule
- Calcium
6.1 Introduction
Just like temperature and pressure, magnetic field is an important physical tool that could impact multiple objects and processes. Although there are numerous reports about the SMF bioeffects, their results are highly variable. However, as we have discussed in Chaps. 1 and 2, the seemingly inconsistent observations are mostly due to the different SMF parameters, such as different types of magnetic fields (static or time-varying, pulsed or noise), magnetic fields with various flux densities (weak, moderate, or strong) or frequencies (extremely low frequency, low frequency, or radiofrequency), as well as biological sample types, which can all lead to diverse and sometimes even completely opposite results.
In addition, as we have discussed in Chaps. 3–5, cells are filled with various cellular contents and biomolecules of different magnetic properties that will respond to the MF differently. For example, it has been shown that the peptide bonds united into organized structures, such as α-helix, which confers proteins diamagnetic anisotropy (Pauling 1979). Organized polymers, such as microtubules that are composed of well-organized tubulin, are also demonstrated to have strong diamagnetic anisotropy and could be aligned in the presence of magnetic fields (Vassilev et al. 1982; Bras et al. 1998, 2014). In fact, it has been found that even the dissolved oxygen in water could be modulated by high SMFs (Ueno and Harada 1982; Ueno et al. 1994, 1995). The effects of SMFs on cells have been reviewed and discussed previously (Adair 2000; Dini and Abbro 2005; Miyakoshi 2005, 2006; Ueno 2012; Albuquerque et al. 2016). Recently, Torbati et al. published a very comprehensive review about the coupling of mechanical deformation and electromagnetic fields in biological cells (Torbati et al. 2022). In this review, besides electric field, they also summarized and discussed the major mechanisms governing the interaction for MFs with cellular functions (Fig. 6.1), which proposed that deformation mediated interaction is likely to be one of the primary mechanisms governing the impact of magnetic fields on cellular function. This provides a very important point of view that once the MFs are first translated into mechanical deformation in the cell and cell membrane, which in turn may trigger an electrical response via mechanisms such as tension-activated ion channels. Consequently, cellular mechanical signal can affect multiple aspects of cellular behaviors, including cell proliferation, endocytosis, etc. Chap. 5 of this book also provided a detailed in-depth analysis for SMF-induced membrane changes, with special focus on ion channels, membrane potential, and gradient SMFs.
Major mechanisms governing the interaction for magnetic fields with cellular functions. (a) In the presence of magnetic particles, their interaction with an applied magnetic field could conceivably activate sensory mechanisms. The cell’s magnetic susceptibility may become different than the ambient medium, leading to a noticeable magnetic Maxwell stress. (b) Anisotropic diamagnetism of cell membrane, which means that the magnetic susceptibility of a biological cell membrane is anisotropic, and its in-plane component differs from its out-of-plane value, which causes deformation. Physically, the deformation proceeds due to the attempt by the lipid molecules to reorient under the action of an applied magnetic field such that the vesicle then stretches parallel to the field. (c) For nonhomogeneous magnetic fields, a force proportional to the magnetic field gradient is developed, i.e., B × ∇B. (d) In the phenomenon of magnetic induction, an electric current is generated due to the temporal variation of the magnetic field. Alternatively, this also occurs when a charged object moves in a magnetic field. (e) Magnetic fields can in principle alter chemical reactions and have been proposed to impact free-radical recombination rates. [Reprinted with permission from (Torbati et al. 2022)]
Here in this chapter, our goal is to provide an overview for the current evidence of SMF effects on cells, with a special focus on the differential cellular effects reported in previous studies, which seems contradictory in many aspects. We try to analyze the reasons that have caused these inconsistencies. Here we mainly discuss about human cells and some animal cells, while cells of plants, bacteria, and other organisms will be discussed in Chap. 7.
6.2 Cellular Effects of Static Magnetic Fields
SMFs could induce multiple cellular effects depending on the magnetic field itself as well as the cells examined. Here we will mainly discuss some cellular effects that have been reported by multiple independent studies, such as SMF-induced changes in cell orientation, proliferation, microtubule and cell division, actin, viability, attachment/adhesion, morphology, migration, cell membrane, cell cycle, DNA, intracellular reactive oxygen species (ROS), and calcium. Our focus here is mainly on human cells.
6.2.1 Cell Orientation
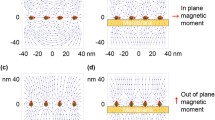
The orientation changes of biomolecules and cells are one of the most well studied aspects of SMF bioeffects. The magnetic properties of biological samples have been discussed in Chap. 3. It has been proved that when objects with high magnetic susceptibility anisotropy are exposed to strong SMFs, they will change their orientations. There are multiple examples for cells align themselves in parallel to the SMF direction. Among them, the best studied example was erythrocytes (red blood cells, RBCs). The first reported RBC orientation change induced by SMF was in 1965 by Murayama, who found that sickled RBCs were oriented perpendicular to a 0.35 T SMF (Murayama 1965). It is interesting that in 1993, a work carried out by Higashi et al. showed that normal RBCs were also aligned by an 8 T SMF but the orientation direction was different from what Murayama has observed (Higashi et al. 1993). Their results showed that normal RBCs oriented with their disk planes parallel to the field direction (Fig. 6.2). In 1995, they reported that the cell membrane components, including the transmembrane proteins and lipid bilayers, were the major reasons for RBC alignment in 8 T SMF (Higashi et al. 1995). In addition, they found that the paramagnetism of membrane-bound hemoglobin contributes significantly to this orientation (Takeuchi et al. 1995; Higashi et al. 1996). These results clearly demonstrate that cells can be oriented by strong SMFs and the effects depend on the molecular components of the cell. Besides RBCs, more components in the blood stream have also been studied, such as platelets (Yamagishi et al. 1992; Higashi et al. 1997) and fibrinogen (Torbet et al. 1981; Yamagishi et al. 1990; Iwasaka et al. 1994).
Red blood cells were aligned by an 8 T static magnetic field. Left: red blood cells in control condition, with no SMF. Right: red blood cells in an 8 T SMF. The field direction was normal to the paper. [Illustration courtesy of Shu-tong Maggie Wang and Ding Joe Wang, based on experimental results from reference (Higashi et al. 1993)]
Moreover, some other cells like osteoblast cells, smooth muscle cells, and Schwann cells could also be aligned in parallel to the direction of the strong magnetic fields when they are exposed for a prolonged period. In 2000 and 2002, Kotani et al. found that osteoblast cells were oriented in parallel to the field direction by an 8 T SMF and the bone formation was significantly stimulated to grow along the direction of the magnetic field (Kotani et al. 2000, 2002). In 2001, Umeno et al. found that smooth muscle cell was aligned along the magnetic field direction after they were exposed to an 8 T SMF for 3 days (Umeno et al. 2001). In 2003, Iwasaka et al. found that the 14 T SMF aligned smooth muscle cell assemblies and the cell colonies were extended along the field direction (Iwasaka et al. 2003). Eguchi et al. found that Schwann cells were also oriented in parallel to the 8 T SMF after 60 h exposure (Eguchi et al. 2003). They used linearly polarized light and observed changes in the intracellular macromolecule behavior in 8 T and 14 T SMFs (Iwasaka and Ueno 2003a, b). In 2005, they also examined the actin cytoskeleton in Schwann cells and found that actin fibers were oriented in the direction of 8 T SMF (Eguchi and Ueno 2005). More interestingly, the Schwann cells did not orient in the 8 T SMF when an inhibitor of small GTPase (guanosine triphosphatase) Rho-associated kinase was added, which indicated that the SMF-induced Schwann cell orientation was dependent on Rho-regulated actin fibers (Eguchi and Ueno 2005). In 2007, Coletti et al. found that 80 mT SMF-induced myogenic L6 cells to align in parallel bundles, an orientation conserved throughout differentiation. They proposed that SMF-enhanced parallel orientation of myotubes was relevant to tissue engineering of a highly organized tissue such as skeletal muscle (Coletti et al. 2007).
In the meantime, there are also multiple examples showing that cells could align in perpendicular to the direction of the magnetic fields, such as the bull sperm. The orientation of bull sperm was examined by a few studies, which actually showed stronger alignment effects than RBCs and platelets. The bull sperm cell has a head that mainly contains diamagnetic cell membrane and DNA. It also has a long tail with microtubules inside. In 2001, Emura et al. found that the orientation of bull sperm cells could be affected by SMFs in an MF strength-dependent manner (Emura et al. 2001). They found that the bull sperm could reach 100% alignment perpendicular to the direction of the MF at just below 1 T (Emura et al. 2001). In 2003, Emura et al. showed that the whole bull sperm and the sperm heads were orientated perpendicular to 1.7 T SMF while the paramecium cilia were aligned in parallel to 8 T SMF (Emura et al. 2003). It was interesting that the sperm tail is theoretically predicted to be in parallel with the field direction due to the diamagnetic anisotropy of microtubules, which will be discussed later. But why the whole sperm is aligned in perpendicular to the field direction is still unclear. It is possible that the sperm head has a stronger diamagnetic anisotropy, which dominates the whole sperm.
Another example of cell orientation in perpendicular to the direction of the magnetic field is neurite outgrowth. In 2008, Kim et al. showed that the application of 0.12 T SMF for 3–5 days could be used to modulate the orientation and direction of neurite formation in cultured human neuronal SH-SY5Y cells and PC12 cells (Kim et al. 2008). It is interesting that they found the neurites perpendicular to the SMF had long, thin, and straight appearance while the neurites in parallel to the SMF direction had “thickened or beaded” dystrophic appearance. More importantly, they not only found the neurites tended to orient perpendicular to the direction of SMF, the direction can also be changed after the SMF direction has changed (Kim et al. 2008).
From evidences mentioned above, it is clear that SMF-induced cell orientation is cell type-dependent. Actually, Ogiue-Ikeda and Ueno compared three different cell lines, including the smooth muscle A7r5 cells, human glioma GI-1 cells, and human kidney HFK293 cells for their orientation changes under 8 T for 60-h exposure. They found that while the smooth muscle A7r5 cells and the human glioma GI-1 cells aligned along the field direction, the human kidney HEK293 cells were not aligned (Ogiue-Ikeda and Ueno 2004). They proposed that this was probably due to their different cell shapes because both A7r5 and GI-1 cells were spindle shaped while HEK293 cells were polygonal shaped. In addition, the orientation of adherent cells such as osteoblasts, smooth muscle cells, and Schwann cells in strong SMFs usually took a few days while floating/suspended cells such as RBCs exhibited a diamagnetic torque rotation in only a few seconds under SMFs of the same flux density. This also implies that when our human bodies are exposed to externally applied SMFs, the orientation of free circulating blood cells would be affected more readily compared to other types of cells.
Table 6.1 summarizes some reported studies about the orientation of cells in SMFs (Table 6.1). It is apparent that other than cell types, the SMF-induced cell orientation change is largely dependent on the MF intensity. The reported that cell orientation changes were all achieved in SMFs of at least 80 mT, and actually most of them were done in ultra-strong magnets, such as in 8 T SMF. Therefore, it is not surprising when Gioia et al. investigated the effect of chronic exposure to a 2 mT SMF on in vitro cultured swine granulosa cells (GCs) and did not observe cell orientation changes (Gioia et al. 2013). In addition, the cell type is an important factor because most cells do not have strong structure characteristics like sperm cell, nor RBCs.
Besides the orientation change of cells themselves in magnetic fields, cells can also be oriented by moderate and strong SMFs when they are embedded in collagen, a macromolecule that has strong diamagnetic anisotropy (Torbet and Ronziere 1984). In 1993, it was found that human foreskin fibroblasts embedded in collagen gel were oriented by 4.0 and 4.7 T SMFs (Guido and Tranquillo 1993). Human glioblastoma A172 cells embedded in collagen gels, but not A172 cells alone, oriented perpendicular to the field direction of 10 T SMF (Hirose et al. 2003). Therefore, the orientation for cells embedded in collagen is largely due to the diamagnetic anisotropy of collagen fibers, which orient in perpendicular direction of SMF. Another example was provided in 2000 by Kotani et al., who found that osteoblast cells themselves were oriented in parallel to the field direction by an 8 T SMF, but the mixture of osteoblast cells and collagen oriented perpendicular to the magnetic fields (Kotani et al. 2000). This is interesting and promising because the stimulation of bone formation to an intended direction using a combination of strong SMF and potent osteogenic agents could possibly lead to a clinically viable treatment of bone fractures and defects. In addition, in 2003, Eguchi et al. found that Schwann cells themselves oriented in parallel to the 8 T SMF after 60-h exposure but when they were embedded in collagen, they were aligned in perpendicular to the field direction (Eguchi et al. 2003). These data all showed that the collagen has a strong alignment effect on cells embedded in SMFs.
The shapes of most mammalian somatic cells are symmetric and surrounded by and attached to their extracellular matrix and neighboring cells. Therefore, they are less likely to have strong alignment effects in SMFs like sperm cells or RBCs in weak to moderate SMFs. However, the SMF-induced orientation effects can potentially affect their cell division and subsequently tissue development. In addition, it was very promising that Kotani et al. found that an 8 T SMF could cause osteoblasts to orient in parallel to the magnetic field and stimulate bone formation along the field direction. This implies that people may be able to apply SMFs in clinical treatment such as bone disorders. In fact, the orientation effects of RBCs might also provide some insights to help understanding the working mechanism of some magnetic therapy products. Continued efforts are encouraged to investigate more on blood cells, muscles, neurons, bones and sperms, as well as their potential medical applications in the future.
6.2.2 Cell Proliferation/Growth
Not surprisingly, the effect of SMFs on cell proliferation is also cell type-dependent. We summarize some reported studies about the SMF-induced cell proliferation/growth changes (Table 6.2).
Multiple evidence showed that SMFs could inhibit cell proliferation. For example, Malinin et al. exposed mouse fibroblast L-929 cells and human fetal lung fibroblast WI-38 cells to 0.5 T SMF for 4–8 h after they were frozen in liquid nitrogen and found that the subsequent cell growth was significantly inhibited (Malinin et al. 1976). In 1999, Pacini et al. examined the effects of 0.2 T SMF in human breast cancer cells and found that 0.2 T not only reduced cell proliferation but also enhanced the vitamin D anti-proliferative effect (Pacini et al. 1999b). In 2003, Pacini et al. examined human skin fibroblasts for their effects in 0.2 T SMF generated by a magnetic resonance tomography and found that the cell proliferation was reduced (Pacini et al. 2003). In 2008, Hsieh et al. found that 3 T SMF inhibited human chondrocytes growth in vitro and affected recovery of damaged knee cartilage in vivo in the pig model. They also mentioned that these results may be specific to the parameters used in this study and may not apply to other situations, field strengths, forms of cartilage injury, or animal species (Hsieh et al. 2008). In 2012, Li et al. found that the proliferation of human umbilical artery smooth muscle cells (hUASMCs) was significantly decreased after 5 mT SMF exposure for 48 h compared with the non-treated group (Li et al. 2012). In 2013, Mo et al. showed that magnetic shielding increased human neuroblastoma SH-SY5Y cell proliferation (Mo et al. 2013), which indicated that the geomagnetic field may have an inhibitory effect on SH-SY5Y neuroblastoma cell proliferation. In 2013, Gioia et al. investigated the effect of a 2 mT SMF on GCs and found that the doubling time was significantly reduced (p < 0.05) in exposed samples after 72 h of culture (Gioia et al. 2013). In 2016, Wang et al. exposed adipose-derived stem cells (ASCs) to 0.5 T SMF for 7 days and found that the cell proliferation was inhibited (Wang et al. 2016). We found that 1 T and 9 T SMFs could inhibit the proliferation of human nasopharyngeal carcinoma CNE-2Z and colon cancer HCT116 cells (Zhang et al. 2015, 2016).
There are also some studies showing that SMFs could promote proliferation of some cell types, such as bone marrow cells, stem cells as well as endothelia cells. For example, Martino et al. found that 60 and 120 μT SMFs increased the cell proliferation of human umbilical vein endothelial cell (Martino et al. 2010). In 2013, Chuo et al. found that a 0.2 T SMF increased the proliferation of bone marrow stem cells (Chuo et al. 2013). In 2007, Stolfa et al. used MTT assay to study the effect of 0.6 T SMF on human chondrocytes and found that the MTT reading was increased by 0.6 T SMF (Stolfa et al. 2007), which was probably due to the increased cell proliferation and/or cell viability or metabolic activity. Maredziak et al. found that 0.5 T SMF increased the proliferation rate of human adipose-derived mesenchymal stromal stem cells (hASCs) via activation of the phosphoinositide 3-kinase/Akt (PI3K/Akt) signaling pathway (Maredziak et al. 2017). Recently, Wu et al. reported that exposure to SMFs of 140 mT (Max) causes membrane depolarization transduced by T-type voltage-gated calcium channels into second-messenger cascades that regulate downstream gene expression, which increase human mesenchymal stem cells (MSCs) proliferation (Wu et al. 2022).
However, there are also some studies shown that cell proliferation was not affected by SMFs. For example, in 1992 Short et al. found that 4.7 T SMF treatment did not affect cell number of either human malignant melanoma cells or the normal human cells (Short et al. 1992). In 2005, using a nuclear magnetic resonance (NMR) spectrometer, Gao et al. found that even 14.1 T SMF exposure for 12 h did not affect cell growth of bacterial strain Shewanella oneidensis MR-1 (Gao et al. 2005). In 2007, Coletti et al. found that 80 mT SMF did not affect myotube cell proliferation (Coletti et al. 2007). In 2010, Hsu and Chang found that 0.29 T SMF did not affect the cell proliferation of dental pulp cells (Hsu and Chang 2010). In 2015, Reddig et al. found that exposure of unstimulated mononuclear blood cells to 7 T SMF alone or in combination with varying gradient magnetic fields and pulsed radiofrequency fields did not affect cell proliferation (Reddig et al. 2015). Iachininoto et al. investigated the effects of 1.5 T and 3 T gradient SMFs for their effects on hematopoietic stem cells and found that the cell proliferation was not affected (Iachininoto et al. 2016).
Moreover, there are some studies that have compared different cell types. For example, in 2003 Aldinucci et al. tested the effects of combining a 4.75 T SMF and a pulsed electromagnetic field (EMF) of 0.7 mT generated by an NMR apparatus. They found that the 4.75 T SMF did not affect cell proliferation in both normal and PHA-activated peripheral blood mononuclear cells (PBMC), but significantly reduced proliferation in Jurkat leukemia cells (Aldinucci et al. 2003b). We found that 1–9 T SMFs inhibited CNE-2Z and HCT116 cancer cells but not the Chinese hamster ovary (CHO) cells (Zhang et al. 2016). In addition, we found that the EGFR/Akt/mTOR signaling pathway, which was upregulated in many cancers, was involved in SMF-induced cancer cell proliferation inhibition (Zhang et al. 2015, 2016). In addition, as we have mentioned before, SMF-induced effects on cell proliferation were not only cell type-dependent, but also dependent on SMF flux density as well as cell density. More investigations are needed to unravel additional mechanisms and specific effects of a given SMF on a specific cell type.
6.2.3 Microtubule and Cell Division
Purified microtubules have been known for a long time to be a target of SMFs as well as electric fields, which align along the magnetic field and electric field direction due to diamagnetic anisotropy of tubulin dimers (Vassilev et al. 1982; Bras et al. 1998, 2014; Minoura and Muto 2006; Wang et al. 2008). It was also shown that tubulin assembly in vitro was disordered by a 10–100 nT hypogeomagnetic field (HGMF; magnetic fields <200 nT) (Wang et al. 2008). These studies demonstrated that microtubules could be affected by SMFs in vitro, but the effects of SMFs on microtubules in cells were less reported. In 2005, Valiron et al. showed that the microtubule and actin cytoskeleton could be affected by 7–17 T ultra-high SMFs in some cell types during interphase (Valiron et al. 2005). In 2013, Gioia observed actin and alpha-tubulin cytoskeleton modifications in swine granulosa cells after 3 days exposure to a 2 mT SMF (Gioia et al. 2013). However, this effect seems to be cell type- and/or exposure time-dependent because our group did not observe obvious microtubule abnormalities in CNE-2Z or RPE1 interphase cells when we exposed them to 1 T SMF for 3 days or 27 T ultra-strong SMF for 4 h (Zhang et al. 2017b).
Microtubule is a key component for mitotic spindle, which is mainly composed of microtubules and chromosomes and is the fundamental machinery for cell division. However, information about the mitotic spindles in SMFs was not provided in the above-mentioned studies. In contrast, time-varying magnetic fields and electric fields have been shown to be able to affect mitotic spindle and cell division. For example, in 1999, Zhao et al. found that a small physiological electric field could orient cultured human corneal epithelial cells through affecting cell division (Zhao et al. 1999). In 2011, Schrader et al. observed spindle disturbances in human-hamster hybrid (A(L)) cells induced by the electrical component of the mobile communication frequency range signal (Schrader et al. 2011). However, for time-varying magnetic fields, people need to distinguish the effects caused by the magnetic fields per se or the thermal effect. In 2011, Ballardin et al. found that 2.45 GHz microwaves could disrupt spindle assembly (inducing multipolar spindles) in Chinese hamster V-79 cells, which was not due to the thermal effects (Ballardin et al. 2011). In contrast, in 2013, Samsonov and Popov found that exposure to 94 GHz radiation increased the rate of microtubule assembly and that effect was actually caused by the thermal effect (Samsonov and Popov 2013). The thermal effect in Samsonov and Popov’s study is likely due to the high frequency compared to Ballardin et al.’s study. Moreover, there is a well-known electromagnetic approach called tumor treating fields (TTF, TTFields) that use low-intensity (1–3 V/cm) and intermediate-frequency (100–300 kHz) alternating electric fields to treat cancers such as glioblastoma. The mechanism has been proved to be mainly through disturbing mitotic spindle formation (Kirson et al. 2004; Pless and Weinberg 2011; Davies et al. 2013). TTFields destroy cells within the process of mitosis via apoptosis and have no effect on non-dividing cells (Pless and Weinberg 2011). In fact, the U.S. Food and Drug Administration has approved this technology for use in glioblastoma (Davis 2013).
We previously found that mitotic spindles could be affected by SMFs (Luo et al. 2016). Our results show that 1 T SMF treatment for 7 days could increase the abnormal mitotic spindles and mitotic index (% of cells in mitosis) in HeLa cells, which is likely due to the effect of SMF on microtubules. In addition, this phenotype is also time-dependent because when cells were treated for shorter time, the effects were not obvious. Although 1 T SMF did not affect the overall cell cycle distribution, it could delay the mitotic exit using synchronization experiment (Luo et al. 2016), which will be discussed in the cell cycle section later in this chapter.
Since purified microtubules can be aligned by SMFs, we predict that the spindle orientation could also be affected, which is a critical determining factor for cell division orientation. In fact, back in 1998, Denegre et al. found that 16.7 T large gradient ultra-high SMF could affect the division orientation of Xenopus eggs (Fig.6.3) (Denegre et al. 1998). In 2006, Eguchi et al. showed that 8 T SMF could also change the cleavage plan formation in frog embryo division (Eguchi et al. 2006). It was proposed that SMFs may affect the orientation of astral microtubules and/or spindles, which was theoretically proven later by Valles (2002), but no experimental evidence has been reported. In 2012, Mo et al. found that hypogeomagnetic field (HGMF; magnetic fields <200 nT) could cause a decrease in horizontal third cleavage furrows and abnormal morphogenesis in Xenopus embryos (Mo et al. 2012). In addition, they used immunofluorescence staining of tubulin to show the reorientation of the spindle of four-cell stage blastomeres. Their results indicated that a brief (2-h) exposure to HGMF was sufficient to interfere with the development of Xenopus embryos at cleavage stages. Also, the mitotic spindle could be an early sensor to the deprivation of the geomagnetic field, which provided a clue to the molecular mechanism underlying the morphological and other changes observed in the developing and/or developed embryos (Mo et al. 2012).
Third cleavage in an animal-vegetal (AV)-parallel static magnetic field. Top (a, c, e, g and i) and side (b, d, f, h and j) views of eight-cell embryos from an AV-parallel field, showing the classes of third cleavage reorientation. For the side view, the embryo in the top view was rotated with the animal pole away from the viewer. The numbers of horizontal cleavages depicted are four (normal; a, b), three (c, d), two (e, f), one (g, h), and zero (i, j). (k) The average number of horizontal third cleavages per embryo as a function of field strength. [Reprinted with permission from (Denegre et al. 1998). Copyright © 1998, National Academy of Sciences, USA]
In the meantime, although it was shown that the microtubule and actin cytoskeleton in interphase cells could be affected by 7–17 T ultra-high SMFs in some cell types (Valiron et al. 2005), information about the mitotic spindle in ultra-high SMFs was not provided. Using human nasopharyngeal cancer CNE-2Z cells and human retinal pigment epithelial RPE1 cells, we found that the spindle orientation could be altered by a 27 T ultra-high SMF. More interestingly, we found that the spindle orientation was determined by both microtubules and chromosomes (Zhang et al. 2017b) (Fig. 6.4). High SMF-induced spindle orientation and morphology changes are recoverable for the non-cancer RPE1 cells, but not for the CNE-2Z cancer cells, which caused cancer cell growth arrest.
Mitotic spindle orientation changes in high static magnetic fields are determined by the balance between microtubules and chromosomes. A water-cooled magnet (WM4 in the High Magnetic Field Facility of Chinese Academy of Sciences) and a specialized cell incubation system were used to provide a homogeneous 27 T SMF on cells. [Figures are adapted from (Zhang et al. 2017b), open access]
6.2.4 Actin
Besides microtubules, the actin cytoskeleton has also been reported to be affected by SMFs in some cell types. For example, Mo et al. showed that in the absence of the geomagnetic field (GMF), the so-called hypomagnetic field (HMF) environment, the adhesion and migration of human neuroblastoma SH-SY5Y cells were inhibited, which were accompanied with a reduction in cellular F-actin amount and disordered kinetics of actin assembly in vitro (Mo et al. 2016). These results indicated that elimination of the GMF affected assembly of the motility-related actin cytoskeleton and suggested that F-actin was a target of HMF exposure and probably a mediator of GMF sensation (Mo et al. 2016).
Although whether actin could serve as a mediator of GMF sensation still needs to be further confirmed, there are multiple other studies have shown that actin could be affected in cells by SMFs. The most striking and convincing data was provided in 2005 by Eguchi and Ueno (2005), which was briefly mentioned in the cell orientation section above. They examined the actin cytoskeleton in 8 T ultra-high SMF-treated Schwann cells and found that actin fibers were oriented in the direction of the magnetic field. However, when the Schwann cells were treated with an inhibitor of small GTPase Rho-associated kinase, which disrupted actin fibers, the orientation phenotype induced by 8 T SMF no longer existed. This indicated that the SMF-induced Schwann cell orientation was dependent on Rho-regulated actin fibers (Eguchi and Ueno 2005). Therefore, their data directly showed that the Rho-regulated actin fibers were involved in SMF-induced cell orientation, at least in Schwann cell. Another example for SMF-induced actin alteration was in 2007 by Coletti et al. who used myogenic cell line L6 and found that 80 mT SMF promoted myogenic cell alignment and differentiation (Coletti et al. 2007), which was also introduced in the previous cell orientation section (Table 6.1). More specifically, they observed increased accumulation of actin and myosin as well as formation of large multinucleated myotubes, which was derived from increased cell fusion efficiency, but not cell proliferation (Coletti et al. 2007). In addition, a few other studies also showed SMF-induced actin alterations. For example, in 2009, Dini et al. found that 72-h of 6 mT SMF exposure caused human leukemia U937 cell F-actin modification (Dini et al. 2009). In 2013, Gioia found actin cytoskeleton modifications in swine granulosa cells after 3 days exposure to a 2 mT SMF (Gioia et al. 2013). Lew et al. found that 0.4 T SMF could increase the fluorescence intensity of the F-actin (Lew et al. 2018).
There are also some studies that reported the unchanged actin in SMF-treated cells. For example, in 2005, Bodega et al. examined primary cultures of astroglial cells for their responses to 1 mT sinusoidal, static, or combined magnetic field for various timepoints and did not observe any significant changes on actin (Bodega et al. 2005). In my opinion, their magnetic flux density in their study might be too low to induce actin alteration. We examined multiple human cancer cells, such as human nasopharyngeal cancer CNE-2Z and colon cancer HCT116 cells, for their responses to 1 T SMF for 2–3 days and did not observe any significant changes on actin (data not shown). However, the cells we examined are different from above-mentioned cell types that have actin alterations upon SMF exposure, such as neuroblastoma cells, Schwann cells, and myogenic cell. These cells may have different actin regulation network than the cancer cell lines we examined. From the above-mentioned studies, it is likely that actin cytoskeleton in cells respond to SMFs in a cell type- and magnetic field flux density-dependent way, which will need more systematic investigations.
6.2.5 Cell Viability
So far, most studies showed that SMFs had minimum effects on cell viability. For example, in 1992, Short et al. found that 4.7 T SMF treatment did not affect cell viability in both human malignant melanoma cells and normal human fibroblast cells (Short et al. 1992). In 2003, Pacini et al. found that 0.2 T SMF could affect the cell morphology and proliferation but not the cell viability of human skin fibroblasts (Pacini et al. 2003). In 2009, Dini et al. reported that 72-h exposure of 6 mT SMF did not affect cell viability in human leukemia U937 cells (Dini et al. 2009). In 2013, Gioia et al. investigated the effect of chronic exposure to a 2 mT SMF on in vitro cultured swine granulosa cells (GCs) and found that the SMF exposure did not affect the cell viability (Gioia et al. 2013). In 2016, Romeo et al. examined human fetal lung fibroblasts MRC-5 exposed to 370 mT SMF and found that the cell viability was not affected (Romeo et al. 2016). We examined 1 T SMF-induced effects on cell viability in 15 different cell lines, including human cancer cell lines CNE-2Z, A431 and A549, non-cancer cell line 293 T as well as CHO cells, etc. In fact, we checked four different cell densities and found that the cell viability was not obviously changed by 1 T SMF in any of these cell types (Zhang et al. 2017c). These studies, including more than 20 different cell types, showed that SMFs do not have obviously effect on cell viability.
However, there are a few studies indicate that SMFs could increase apoptosis in some cell types. In 2005, Chionna et al. reported that 6 mT SMF-induced apoptosis in Hep G2 cells in a time-dependent manner. The apoptosis was almost negligible at the beginning of experiment but increased to about 20% after 24-h of continuous exposure (Chionna et al. 2005). In 2006, Tenuzzo et al. found that 6 mT SMF could promote apoptosis in T hybridoma 3DO cells, human liver cancer Hep G2 cells, and rat thyroid FRTL cells, but not human lymphocytes, mice thymocytes, human histiocytic lymphoma or human cervical cancer HeLa cells (Tenuzzo et al. 2006). In 2008, Hsieh et al. found that 3 T SMF-induced human chondrocytes apoptosis through p53, p21, p27, and Bax protein expression (Hsieh et al. 2008). In 2016, Wang et al. exposed adipose-derived stem cells (ASCs) to 0.5 T SMF for 7 days and found that the cell viability was inhibited (Wang et al. 2016).
It is interesting and puzzling that when SMFs are combined with some other treatments, they have been shown to have totally opposite effects. For example, in 2001, Tofani et al. found that when 3 mT SMF was combined with 3 mT 50 Hz time-varying magnetic fields, the apoptosis of human colon carcinoma WiDr and breast cancer MCF-7 cells were increased, while the MRC-5 cells were not affected (Tofani et al. 2001). In 2006, Ghibelli et al. found that exposure to SMFs of NMR (1 T) could increase damage-induced apoptosis in tumor cells of hematopoietic origin, but not mononuclear white blood cells, showing that NMR may increase the differential cytotoxicity of antitumor drugs on tumor vs. normal cells (Ghibelli et al. 2006). These studies show that SMF could promote the apoptosis effects of time-varying magnetic fields or antitumor drugs. However, there are also evidences showing that SMF could protect some cells from apoptosis. For example, in 1999, Fanelli et al. showed that 0.3–60 mT SMFs could reduce cell apoptosis induced by damaging agents such as etoposide (VP16) and puromycin (PMC) (Fanelli et al. 1999). It was also interesting that although Tenuzzo et al. found that 6 mT SMF could promote apoptosis in T hybridoma 3DO cells, human liver cancer Hep G2 cells, and rat thyroid FRTL cells, when the SMF was combined with apoptotic inducing drugs, such as cycloheximide and puromycin, it had a protective effect because the majority of cells could be rescued from apoptosis, except for 3DO (Tenuzzo et al. 2006).
Therefore, the effect of SMFs on cell apoptosis is magnetic field intensity, treatment time, and most importantly, cell type-dependent. In most reported cases, the cell viability was not affected by SMFs. However, there were also a few reports indicating that some cells could be affected. In addition, SMFs could have combinational or antagonistic effects when they are combined with other treatments, such as time-varying magnetic fields or different cell damaging agents. Further investigations are strongly needed to unravel the underlying mechanisms.
6.2.6 Cell Attachment/Adhesion
There are several studies showing that the cell attachment could be affected by SMFs. For example, in 2011, Sullivan et al. exposed the cells directly to SMFs right after seeding with an exposure time of 18 h and found that WI-38 (human fetal lung fibroblast cells) attachment was significantly reduced by 35–120 mT SMFs (Sullivan et al. 2011). In 2012, Li et al. exposed human umbilical artery smooth muscle cells (hUASMCs) to 5 mT SMF for 48-h and found that the cell adhesion was obviously decreased (Li et al. 2012). In 2014, Wang et al. found that moderate SMFs of 0.26–0.33 T could reduce human breast cancer MCF-7 cell attachment (Wang et al. 2014).
Although these results indicate that cell attachment/adhesion may be affected by SMFs, the consensus result is still lacking. In most cases, SMFs seem to inhibit the cell attachment/adhesion, there are also opposite evidences. For example, Mo et al. found that shielding of the geomagnetic field also inhibited cell adhesion and migration accompanied with a reduction in cellular F-actin amount in human neuroblastoma SH-SY5Y cells (Mo et al. 2016). This indicates that in the absence of SMF, the cell attachment could also be reduced. Moreover, in our own experience, the cell attachment/adhesion of most cells was not affected by moderate SMFs.
Not surprisingly, the SMF-induced changes in cell attachment also seemed to be cell type-dependent. In 1992, Short et al. tested both human malignant melanoma cells and the normal human cells and found that the malignant melanoma cells had reduced attachment to the tissue culture surface while the normal fibroblasts were not affected by the 4.7 T SMF (Short et al. 1992). Wang et al. found that although human breast cancer MCF-7 cell attachment was reduced by moderate SMFs of 0.26–0.33 T, the HeLa cell attachment was not affected (Wang et al. 2014). In addition to the different cell types, the experimental procedure, such as the timing of SMF exposure before or after the cells have been attached to the cell culture plates, is also likely to be a key factor that influences the experimental outcomes. Moreover, we found that the supporting substrate, such as the cell culture plate and the coverslip, can also influence the experimental results about cell attachment/adhesion. Therefore, more researches are certainly needed to examine the exact effects of SMFs on cell attachment/adhesion, as well as their consequences in vivo.
6.2.7 Cell Morphology
Multiple studies have shown that the cell shape can be altered by SMFs. In 2003, Pacini et al. found that the morphology of human skin fibroblast cells was modified by 0.2 T SMF (Pacini et al. 2003). In the same year, Iwasaka et al. found that 14 T SMF affected the morphology of smooth muscle cell assemblies, and the shapes of the cell colonies extended along the direction of the magnetic flux (Iwasaka et al. 2003). Chinonna et al. also reported time-dependent cell shape and membrane microvilli changes in human histiocytic lymphoma U937 cells and human lymphocytes by a 6 mT SMF (Chionna et al. 2003). In 2005, Chionna et al. found that Hep G2 cells exposed to 6 mT SMF for 24 h were elongated with many irregular microvilli randomly distributed on the cell surface, as well as a less flat shape due to partial detachment from the culture dishes. In addition, cytoskeleton was also modified in a time-dependent manner (Chionna et al. 2005). In 2009, Dini et al. found that 72 h of 6 mT SMF caused human leukemia U937 cell shape change and F-actin modification, appearance of membrane roughness and large blebs and impaired expression of specific macrophagic markers on the cell surface (Dini et al. 2009). It was also interesting that although the cell growth was inhibited, the average cell size of rat pituitary adenoma GH3 cells was increased by prolonged exposure to 0.5 T SMF (Rosen and Chastney 2009). In 2013, Gioia found cell length and thickness changes, as well as actin and alpha-tubulin cytoskeleton modifications in swine granulosa cells after 3 days exposure to a 2 mT SMF (Gioia et al. 2013). Mo et al. found that magnetic shielding made the human neuroblastoma SH-SY5Y cells smaller in size and more round in shape, which was likely due to the disordered kinetics of actin assembly (Mo et al. 2016).
Not surprisingly, there are also many studies that did not observe cell morphology changes after SMF exposure. For example, in 1992, Sato et al. found that there were no cell shape changes in HeLa cells after 1.5 T SMF exposure for 96 h (Sato et al. 1992). In 2003, Iwasaka et al. found that no distinct changes in cell morphology in smooth muscle cells including cell membrane components occurred during the 3-h exposure to 8 T magnetic field (Iwasaka and Ueno 2003b). In 2005, Bodega et al. examined primary cultures of astroglial cells for their responses to 1 mT sinusoidal, static, or combined magnetic fields for various timepoints and did not observe any significant changes on actin (Bodega et al. 2005). Again, the cell type may play a very important role in the SMF-induced cell morphology changes. For example, in 1999, Pacini et al. found that a 0.2 T magnetic field-induced obvious morphology change in human neuronal FNC-B4 cell but did not affect mouse leukemia or human breast carcinoma cells (Pacini et al. 1999a).
In addition, multiple other factors could also determine whether people can observe cell morphology changes after SMF exposure, such as magnetic flux density and exposure time, as well as detection techniques and experimental setup. There are two studies that both used freezing and SMF but the experimental results are totally different. The first one was in 1976, Malinin et al. exposed mouse fibroblast L-929 cells and human fetal lung fibroblast WI-38 cells to 0.5 T SMF for 4–8 h after they were frozen and found that the cell morphology was significantly changed after they were thawed and cultured for 1–5 weeks (Malinin et al. 1976). In contrast, in 2013, Lin et al. found that when 0.4 or 0.8 T SMFs were used during the slow cooling procedures of RBCs, the survival rates of frozen-thawed RBCs were increased and there was no morphological changes (Lin et al. 2013). The mechanisms of the SMF + freezing-induced cell growth and/or morphological changes between these two studies are still unknown, which could be due to the SMF + freezing procedure differences, or cell type differences. More studies are needed to test more cells in both procedures to reveal the underlying mechanisms.
6.2.8 Cell Migration
There are some studies showing that SMFs could affect cell migration. On the one hand, studies show that cell migration can be inhibited by SMFs. For example, back in 1990, Papatheofanis found that 0.1 T SMF could inhibit cell migration of human polymorphonuclear leukocytes (PMNs) (Papatheofanis 1990). In 2012, Li et al. found that 5 mT SMF treatment for 48 h inhibited human umbilical artery smooth muscle cells (hUASMCs) migration (Li et al. 2012). In 2021, our group found that an upward direction gradient SMF provided by a NdFeB permanent magnet (Fig. 6.5a, b) can increase the cellular ROS level of ovarian cancer HO8910 and SKOV3 cells and inhibit their migration. In contrast, the normal human ovarian cells (IOSE386) migration was not affected (Fig. 6.5c–e) (Song et al. 2021). These results show that the SMF effects on cell migration are also cell type-dependent. We further performed RNA sequencing and found that these moderate SMFs increased the oxidative stress level and reduced the stemness of ovarian cancer cells. Consistently, the expressions of stemness-related genes were significantly decreased, including hyaluronan receptor (CD44), SRY-box transcription factor 2 (Sox2), and cell myc proto-oncogene protein (C-myc). The ovarian cancer metastasis in mice was also inhibited (Song et al. 2021).
Moderate static magnetic fields increase ovarian cancer cell ROS levels and inhibit cell migration. (a) Illustration of cells exposed to a moderate SMF provided by a permanent magnet. (b) Magnetic field distribution on the magnet surface was measured by a magnet analyzer. The SMF range in the cell culture dish area is 0.1–0.5 T. (c) ROS levels of HO8910 and SKOV3 cells exposed to the moderate SMF at different time points and one-way analysis of variance (ANOVA) with Bonferroni correction for comparison between three groups. (d) Wound healing assays of IOSE386, HO8910, and SKOV3 cells exposed to moderate SMF. Quantification of the relative healing area is shown on the right. Comparisons were made between two groups by Student’s t test. (e) Transwell invasion assays of IOSE386, HO8910 and SKOV3 cells treated with or without 20 μM H2O2. Quantification of the invasive cells is shown on the right. Comparisons were made between two groups by Student’s t test. (f) Relative cell numbers of IOSE386, HO8910, and SKOV3 cells exposed to moderate SMF for 24 h. Comparisons were made between the experimental group and the sham control group by Student’s t test. *p < 0.05, **p < 0.01, and ***p < 0.001. [Reprinted from (Song et al. 2021), open access]
On the other hand, there are also studies indicate that SMFs can increase cell migration. For example, in 2016, Mo et al. found that in the absence of the geomagnetic field, the human neuroblastoma cell migration was inhibited accompanied with a reduction in cellular F-actin amount (Mo et al. 2016). This indicates that geomagnetic field may be important for cell migration. Recently, by NIH3T3 cellular experiments in vitro and diabetic wound healing experiments in vivo, we show that high glucose-induced impairments in cell migration can be improved by moderate SMF treatment, which makes them a potential tool to improved diabetic wound healing (Feng et al. 2022). However, the relevant studies about SMFs and cell migration are too few to find some clues about the magnetic parameter and cell types.
It should be mentioned that there are many studies using gradient SMFs to separate different cell populations based on their different migration ability, which is called magnetophoresis. Based on the measured magnetic moments of hemoglobin and the relatively high hemoglobin concentration of human RBCs, the differential migration of RBCs was possible if exposed to a high gradient SMF. For example, in 2003, Zborowski et al. used a mean magnetic field of 1.40 T and a mean gradient of 0.131 T/mm to separate deoxygenated and methemoglobin (metHb)-containing RBCs (Zborowski et al. 2003). The existence of unpaired electrons in the four heme groups of deoxy and metHb gives them paramagnetic properties, which is very different from the diamagnetic property of oxyhemoglobin. Zborowski et al. showed that the magnetophoretic mobility for erythrocytes with 100% deoxygenated hemoglobin and for erythrocytes containing 100% metHb were similar, while oxygenated erythrocytes were diamagnetic (Zborowski et al. 2003). Magnetophoresis could provide a way to characterize and separate cells based on magnetic properties of biological macromolecules in cells (Zborowski et al. 2003). In fact, this technique has been used in both malaria detection and infected erythrocyte separation. Although many other techniques are also available, magnetophoretic is very promising because of their high specificity for malaria parasite-infected RBCs (Kasetsirikul et al. 2016).
There are also some studies using gradient SMFs to “guide” cell migration. For example, in 2013, Zablotskii et al. showed that SMF gradient could assist cell migration to those areas with the strongest magnetic field gradient, thereby allowing the buildup of tunable interconnected stem cell networks, which is an elegant route for tissue engineering and regenerative medicine (Zablotskii et al. 2013).
6.2.9 Stem Cell Differentiation
Stem cell is probably one of the most susceptible cell types that are responsive to MFs. In fact, there have been multiple studies that have investigated the effect of SMFs on stem cells, such as dental pulp stem cells (DPSCs), bone marrow stromal cells (BMSCs), human adipose-derived stem cells (hASCs), etc., which have been previously discussed in some reviews (Sadri et al. 2017; Marycz et al. 2018; Ho et al. 2019).
In recent few years, there are more studies that have reported the promotion effects of SMFs on stem cells. For example, it was shown that a 0.4 T SMF can enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism (Lew et al. 2018). In 2019, using the planarian regeneration model, Van Huizen et al. found that weak magnetic fields (WMFs) of <1 mT altered stem cell proliferation and subsequent differentiation via changes in ROS accumulation and downstream heat shock protein 70 (Hsp70) expression, indicating that by adjusting SMF strength, SMFs can increase or decrease new tissue formation in vivo (Van Huizen et al. 2019). In 2021, Zhang et al. investigated the effect of moderate SMF on the chondrogenesis and proliferation of mandibular bone marrow mesenchymal stem cells (MBMSCs) in the MBMSC/mandibular condylar chondrocyte (MCC) coculture system. They found that the proliferation of MBMSCs was significantly enhanced in the experimental group with MBMSCs cocultured with MCCs under SMF stimulation relative to controls. Glycosaminoglycan (GAG) content was increased, and SOX9, collagen type II alpha 1 (COL2A1), and aggrecan (ACAN) were also increased at the mRNA and protein levels. This indicates the potential of moderate SMF in repairing condylar cartilage defects in medicine (Zhang et al. 2021). Recently, Wu et al. found that ~100 mT SMF regulates T-type calcium ion channels and mediates mesenchymal stem cells proliferation (Wu et al. 2022). There are also two studies that have investigated the SMF effects on cancer cell stemness (Zhao et al. 2021; Song et al. 2022), which will be discussed in Chap. 9 of this book.
6.2.10 Cell Membrane
The cell membrane itself is dielectric and plays important roles in cellular responses to external stimuli, especially for electromagnetic fields. As we have mentioned in the introduction, SMF could affect cellular function via membrane, which has been reviewed from a physical point of view, focusing on deformation (Torbati et al. 2022), and gradient SMF-induced membrane changes involving ion channels and membrane potential (Chap. 5 of this book).
In fact, high SMF-induced membrane alignment change is one of the best studied effects on biomolecules. The cell membrane mainly consists of phospholipids and embedded proteins, and the phospholipids of a cell membrane are orderly arranged in a double layer, called lipid bilayers. Due to the diamagnetic anisotropy of phospholipid molecules in the lipid bilayer (Braganza et al. 1984; Helfrich 1973), the phospholipid molecules would align or reorient in the high SMFs, which consequently affect the bulk biophysical properties of the cell membrane. In fact, the RBC orientation changes mentioned earlier are one of the best examples illustrating the action of SMF on cell membrane to affect cellular behaviors (Fig. 6.2). Moreover, in a more simplified model, the lipid vesicles made from egg lecithin are shown to be able to completely align parallelly to an external 1.5 T SMF in seconds (Fig. 6.6) (Boroske and Helfrich 1978). These studies demonstrate that the origin of magnetic alignment of nonspherical vesicles is the interaction of the magnetically anisotropic bilayer with the externally applied SMFs.
Alignments of a cylindrical lipid vesicle in a static magnetic field. To measure the field-induced alignment of cylindrical vesicles made from egg lecithin, a homogeneous field of 1.5 T was applied parallel to the sample slides. Simultaneously, the sample was observed under a phase contrast microscope, with the optical axis being normal to the slides. The vesicle movements, translational and rotational were recorded. The vesicles could be moved parallel and perpendicular to the magnetic field and rotated around the microscope axis so that the initial angle of orientation made with the field was variable. [Reprinted with permission from (Boroske and Helfrich 1978)]
Later, multiple studies have shown that the cell membrane permeability can be increased by SMFs. For example, in 2011, Liu et al. used Atomic Force Microscope (AFM) to reveal that a 9 mT SMF could increase the number and size of the holes on the cell membrane of K562 cells, which may increase the membrane permeability and the flow of the anticancer drugs (Liu et al. 2011). In 2012, Bajpai et al. found that 0.1 T SMF could suppress both gram positive (S. epidermidis) and gram negative bacteria (E. coli) growth, which was likely due to SMF-induced cell membrane damages (Bajpai et al. 2012). There are also multiple studies indicated that SMFs could increase the membrane rigidity in cells. For example, in 2013, Lin et al. found that a 0.8 T SMF decreased membrane fluidity and enhanced erythrocyte membrane stability to resist dehydration damage caused by slow cooling procedures (Lin et al. 2013). They found that the SMF coupled with the slow cooling procedure increased the survival rates of frozen-thawed erythrocytes without obvious cellular damage. Therefore they proposed that the SMFs increased the biophysical stability of the cell membrane, which reduced dehydration damage to the erythrocyte membrane during the slow cooling procedure (Lin et al. 2013). In 2015, Hsieh et al. showed that dental pulp cells (DPCs) treated with a 0.4 T SMF had a higher tolerance to lipopolysaccharide (LPS)-induced inflammatory response when compared to untreated controls. They suggested that 0.4 T SMF attenuates LPS-induced inflammatory response to DPCs by changing cell membrane stability/rigidity (Hsieh et al. 2015). Lew et al. used 0.4 T SMF to treat dental pulp stem cells (DPSCs) and suggested that the cell membranes of the DPSCs were affected to influence intracellular calcium (Lew et al. 2018).
The effects of SMFs on cell membrane are also cell type-dependent. In 2006, Nuccitelli et al. showed that 6 mT SMF exposure for 5 min affected cell membrane potential differently in various cell types. Specifically, the 6 mT SMF caused depolarization in Jurkat cells but hyperpolarization in U937 cells (Nuccitelli et al. 2006). In addition, high resolution imaging techniques like AFM or electron microscopy are also important to reveal the SMF-induced cell membrane changes, which have been used in multiple studies to reveal the membrane changes or membrane associated protein changes caused by SMFs (Jia et al. 2007; Liu et al. 2011; Wang et al. 2014). In contrast, low resolution imaging techniques are less likely to unravel the membrane changes. In 2010, Wang et al. used an illustration to show the potential mechanism of SMFs on cell membrane, some of the associated receptor and channel proteins, as well as the downstream effectors (Wang et al. 2010). In addition, since membrane dynamics changes can affect the activity of membrane embedded proteins, SMFs may also affect some of the membrane associated proteins, such as mechanosensitive ion channels or other embedded proteins (Petrov and Martinac 2007; Wang et al. 2010).
Since SMF-induced membrane bending not only affects ion channels, but also leads to the generation of electrical fields via flexoelectricity, the effects of SMFs on cell membrane could potentially affect a large number of cellular processes, which are still underexplored. It is possible that some of the SMF-induced effects on nervous system (Chap. 13), ROS and calcium changes that will be discussed later in this chapter, are all related to the SMF-induced membrane deformation, which should be investigated in more details in the future.
6.2.11 Cell Cycle
There are a few studies indicating that SMFs may be able to affect cell cycle in some types of cells or at specific conditions. For example, in 2010, Chen et al. found that 8.8 mT SMF increased the G2/M phase and decreased G1 and S phases in K562 cells (Chen et al. 2010). In 2013, Mo et al. showed that magnetic shielding promoted cell cycle progression in the G1 phase of SH-SY5Y cells (Mo et al. 2013). We found that 1 T SMF could cause a mitotic arrest to reduce cell number in synchronized HeLa cells (Luo et al. 2016).
On the other hand, most other studies found that the cell cycle was not affected by SMFs. For example, in 2010, Hsu and Chang found that 0.29 T SMF did not affect the cell cycle of dental pulp cells (Hsu and Chang 2010). Also in 2010, Sarvestani et al. investigated the effects of a 15 mT SMF on cell cycle progression in rat bone marrow stem cells (BMSCs) and did not find any cell cycle changes (Sarvestani et al. 2010). We analyzed multiple cell types seeded at different cell densities for the effects of 1 T SMF (Zhang et al. 2017c). For all the cell lines we tested, 1 T SMF exposure for 2 days did not significantly affect the cell cycle. In addition, we exposed human colon cancer HCT116 cells and human nasopharyngeal cancer CNE-2Z cells to 9 T SMF for 3 days (Zhang et al. 2016), or exposed CNE-2Z cells to an ultra-high 27 T SMF for 4 h and did not observe obvious cell cycle changes (Zhang et al. 2017b).
However, the effect of SMFs on cell cycle is likely to be cell type-dependent, just like most other SMF-induced cellular effects. In 2010, Zhao et al. found that 13 T SMF had no obvious effect on the cell cycle distribution in both CHO cells and DNA double-strand break repair-deficient mutant XRS-5 cells, but decreased the G0/G1 phase and increased S phase cell percentage in human primary skin AG1522 cells (Zhao et al. 2010). This indicates that maybe SMFs have more effects on cell cycles in primary cells than immortalized cells. In addition, the specific cell cycle changes in SMF-induced are different in reported studies (Chen et al. 2010; Zhao et al. 2010). Therefore, further investigations are needed to examine more cell types and/or experimental conditions for the exact effect of SMFs on cell cycle.
Although most results so far showed that SMFs did not change the overall cell cycle distribution of a given cell population, we found that prolonged exposure (7 days) to 1 T SMF could increase the abnormal spindle percentage and the mitotic index in HeLa cells (Luo et al. 2016). Moreover, we found that the duration of mitosis was increased by 1 T SMF. Using cell synchronization experiment, we found that 1 T SMF could delay cells exiting from mitosis. In the absence of 1 T SMF, most of the double thymidine synchronized cells exit from mitosis 12 h after thymidine release. However, there were a significantly increased number of HeLa cells staying in mitosis in the presence of 1 T SMF.
6.2.12 DNA
Due to the public health concerns about the power lines, mobile phones, and cancer, DNA integrity is frequently studied in pulsed MFs (McCann et al. 1993; Cridland et al. 1996; Olsson et al. 2001; Zhou et al. 2002; Williams et al. 2006; Ruiz-Gomez et al. 2010). As early as 1984, Liboff et al. show that DNA synthesis in cells could be increased by time-varying MFs (Liboff et al. 1984). Although so far there are still not enough evidences to confirm the harmful mutagenesis effects of these time-varying MFs on human bodies, more researches are still needed since people have increased exposure to various time-varying magnetic fields nowadays.
In contrast, SMF-induced DNA damage and mutation are relatively less revealed. In 2004, Takashima et al. used somatic mutation and recombination test system in DNA repair-proficient and -deficient strains of Drosophila melanogaster to test strong SMFs for their possible effects on DNA damage and mutation in flies. They found that 2, 5, or 14 T fields exposure for 24 h caused a statistically significant enhancement in somatic recombination frequency in the postreplication repair-deficient flies, whereas the frequency remained unchanged in the nucleotide excision repair-deficient flies and in the DNA repair-proficient flies after exposure. In addition, they found that exposure to high magnetic fields induces somatic recombination in Drosophila and that the dose-response relationship is not linear (Takashima et al. 2004). Other than this work in flies, most other studies revealed that SMFs do not cause DNA damage or mutation. For example, in 2015, Reddig et al. found that exposure of unstimulated human mononuclear blood cells to 7 T SMF alone or combined with varying gradient magnetic fields and pulsed radiofrequency fields did not induce DNA double-strand breaks (Reddig et al. 2015). In 2016, Romeo et al. examined human fetal lung fibroblasts MRC-5 exposed to 370 mT SMF and found that the DNA integrity was not affected (Romeo et al. 2016). Wang et al. exposed adipose-derived stem cells (ASCs) to 0.5 T SMF for 7 days and did not observe DNA integrity changes (Wang et al. 2016). Therefore, these studies did not reveal the direct DNA damage. Interestingly, in 2014, Teodori et al. found that the DNA damage in primary glioblastoma cells caused by X-ray irradiation could be prevented by an 80 mT SMF exposure, which is likely due to the SMF-induced protection effect on mitochondria membrane potential (Teodori et al. 2014). So 80 mT SMF might have a protective role in X-ray-induced DNA damage. However, it was also shown that combining 10 T SMF with X-ray-irradiation could promote the micronucleus formation, although the 10 T SMF itself does not have any effects on micronucleus formation (Nakahara et al. 2002). The available evidences so far about SMF-induced DNA damage and mutation are still not sufficient to a solid conclusion. Most studies revealed that SMFs do not cause DNA damage or mutation in human cells. However, more investigations are encouraged to examine different cell types and magnetic field intensities to help us to achieve a more complete understanding on this issue.
Besides DNA damage that we discussed above, the alignment of DNA in the presence of magnetic field was also studied. It was reported that the DNA chain can be aligned by strong SMFs because of its relative large diamagnetic anisotropy (Maret et al. 1975), which is mainly due to their stacked aromatic bases. In addition, it has been theoretically predicted that the highly compacted mitotic chromosome arms can generate electromagnetic fields along the chromosome arm direction (Zhao and Zhan 2012) and chromosomes should be able to be fully aligned by SMFs of around 1.4 T (Maret 1990). In addition, Andrews et al. showed that the isolated mitotic chromosomes can be aligned by an electric field (Andrews et al. 1980). We found that a 27 T ultra-high SMF could affect the mitotic spindle orientation in human cells, in which chromosomes played important roles (Zhang et al. 2017b).
Moreover, the nature of intertwined double-strand DNA determines that the DNA has to rotate in cells (Keszthelyi et al. 2016). Since DNA is negatively charged and undergoes fast rotation during replication in living cells, we predict that its movement will be affected by Lorentz force, especially in high SMFs. Combined theoretical calculation and cellular experiments, we show that moderate to high SMFs can directly inhibit DNA synthesis/replication (Yang et al. 2020). We used two colon cancer and two lung cancer cell lines to detect the SMF effect on DNA synthesis, which was determined by BrdU incorporation. We observed that the DNA replication was decreased by about 5–15% by upward direction SMF in four different cell lines, while downward direction SMF did not generate such effect (Fig. 6.7). The differential effects of SMFs of different directions on DNA synthesis have been discussed in Chap. 2 of this book.
DNA synthesis is decreased by 1 T upward but not downward magnetic field. Inhomogeneous SMFs were generated by permanent magnets. *p < 0.05, **p < 0.01. [Reprinted from (Yang et al. 2020), open access]
6.2.13 Intracellular Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) are highly active radicals, ions, and molecules that have a single unpaired electron in their outer shell of electrons. ROS include free oxygen radicals (·O2−, ·OH, NO·, etc.) and non-radical ROS (H2O2, N2O2, ROOH, HOCl, etc.). It is well known that low levels of ROS can act as intracellular signaling messengers that oxidize protein thiol groups, modify protein structure and functions while higher levels of ROS could nonspecifically attack proteins, lipids, and DNA to disrupt normal cellular processes (Liou and Storz 2010; Shi et al. 2014). There are also multiple studies showing that the elevated ROS levels in cancer cells compared to normal cells could contribute to the cancer progression (Gao et al. 2007). However, there are also some studies indicating that excessive oxidant stress slows cancer cell proliferation, threatens their survival and therapeutic interventions to further increase the oxidant stress level in newly formed tumor cells, which is likely to make them prone to death (Schumacker 2006, 2015; Trachootham et al. 2006).
ROS level change after SMF treatment is probably the most frequently reported phenomenon in the field of magnetobiology. We have previously reviewed the literature about ROS changes by various magnetic fields in 2017 (Wang and Zhang 2017). However, in the past few years, there are a large number of new studies that have reported the effects of SMFs on ROS levels. We categorize the reported studies according to their effects, including ROS level elevation (Tables 6.3 and 6.6), reduction (Table 6.4), or no change (Tables 6.5).
However, there is no rules we can find in these studies yet. For example, for hypomagnetic fields, some studies showed ROS elevation (Fu et al. 2016), some showed reduction (Politanski et al. 2013), and some showed no difference (Politanski et al. 2013; Van Huizen et al. 2019). These variations could be due to the cell type, magnetic flux density, or even timepoint differences. For example, Sullivan et al. showed that the oxidant production increased 37% in WI-38 cells exposed to SMF (230–250 mT) during the first 18 h after seeding, but no change was observed after a prolonged 5-day exposure (Sullivan et al. 2011), which indicates that the SMF-induced ROS elevation is time-dependent. Moreover, ROS was known to be different in different cell types, as well as different cell densities (Limoli et al. 2004; Wang and Zhang 2019). Furthermore, we recently found that gradient moderate SMFs can regulate the oxidative stress and inhibit metastasis in the ovarian cancer cells (Song et al. 2021).
6.2.14 Adenosine Triphosphate (ATP)
Whether SMFs could affect the enzymatic ATP synthesis in vitro has been debated. In 2008, Buchachenko and Kuznetsov reported magnetic interactions on the rate of enzymatic synthesis of ATP in vitro (Buchachenko and Kuznetsov 2008). They found that the ATP synthesis can be significantly increased by 55 and 80 mT SMFs in the presence of 25Mg2+. However, later studies by Crotty et al. failed to reproduce their results (Crotty et al. 2012) and the reason was still unclear (Hore 2012). Although the magnetic flux densities in these two studies were almost identical, the experimental details about the magnetic field setup were provided by Crotty et al. but not by Buchachenko and Kuznetsov. In addition, it is also possible that the difference was due to the fact that these two groups have used different sources of proteins. Buchachenko and Kuznetsov used a monomeric creatine kinase isozyme from snake venom, whereas Crotty et al. used dimeric creatine kinase. To our point of view, the above-mentioned factors about both the magnetic fields and the protein itself could potentially produce seemingly inconsistent results. Therefore, more investigations are encouraged to address this question.
Besides the in vitro catalytic studies, there are also some cellular works showing that the ATP level in cells could be affected by SMFs. However, the exact effects also seem to be case dependent. Back in 1995, Itegin et al. found that chronically applied SMF of 0.02 T had differential effects on various ATPase. The mean activities of Na+–K+ ATPase and Ca2+ ATPase were significantly increased by SMF but that of Mg2+ ATPase was non-significantly reduced (Itegin et al. 1995). It is possible that different cells have different ATPase network so that their responses to SMFs could be dissimilar. In 2010, Wang et al. tested moderate SMF (~0.25 T) on PC12 cells (derived from a pheochromocytoma of the rat adrenal medulla) and found that the ATP level was moderately, but statistically significantly increased (Wang et al. 2010). There was another study by Kurzeja et al. that also reported ATP level increase induced by SMF, although it was done in the presence of fluoride. In 2013, Kurzeja et al. found that moderate SMFs (0.4, 0.6, and 0.7 T) could rescue fluoride-induced ATP decrease in fibroblasts. In addition, the effect was magnetic flux density-dependent, in which 0.7 T SMF produced more significant effects than 0.4 and 0.6 T SMFs (Kurzeja et al. 2013).
There were also some studies showing that the cellular ATP level could be reduced by SMFs in a magnetic flux density- and cell type-dependent manner. For example, in 2011, Zhao et al. used 8.5 T strong homogeneous SMF to test its effects in three cell lines, including human-hamster hybrid A(L) cells, mitochondria-deficient [ρ(0) A(L)] cells, and double-strand break (DSB) repair-deficient (XRS-5) cells. They found that SMF-induced ATP content change was magnetic flux density, time, as well as cell type-dependent (Zhao et al. 2011). Moreover, their results indicated that the 8.5 T SMF-induced cellular ATP decrease was partially mediated by mitochondria and the DNA DSB repair process because the ATP level in wild type A(L) cells could recover 12–24 h after SMF exposure but the mitochondria-deficient or double-strand break repair-deficient (XRS-5) cells could not (Zhao et al. 2011). In 2018, our group used rat adrenal PC12 cells to compare SMFs of different flux densities for their effects on ATP. Our results show that although 0.26 or 0.50 T SMFs did not affect ATP, 1 T and 9 T SMFs affected ATP level differently and time-dependently. Moreover, SMF-induced ATP level fluctuations are correlated with mitochondrial membrane potential changes (Wang et al. 2018).
6.2.15 Calcium
Calcium plays important roles in a number of biological systems, especially in signal transduction cascades. The magnetic field-induced calcium changes in cells are mostly studied in time-varying magnetic fields (Walleczek and Budinger 1992; Barbier et al. 1996; Tonini et al. 2001; Zhou et al. 2002; Fassina et al. 2006; Yan et al. 2010) and was found to be dependent on cell status and magnetic flux density (Walleczek and Budinger 1992) as well as other magnetic field parameters (Carson et al. 1990). There are multiple studies showing that the calcium level was increased by 50–60 Hz magnetic fields (Barbier et al. 1996; Tonini et al. 2001; Fassina et al. 2006).
Similar to time-varying magnetic fields, there are also many studies show that the calcium level was increased by SMFs. For example, in 1998, Flipo et al. examined the in vitro effects of 0.025–0.15 T SMFs on the cellular immune parameters of the C57BL/6 murine macrophages, spleen lymphocytes, and thymic cells (Flipo et al. 1998). Exposure to the SMF for 24 h resulted in increased intracellular Ca2+ level in macrophages and increased Ca2+ influx in concanavalin A-stimulated lymphocytes (Flipo et al. 1998). In 2006, Tenuzzo et al. showed that 6 mT SMF could increase the calcium level in multiple cell lines (Tenuzzo et al. 2006). Prina-Mello et al. exposed rat cortical neurons to SMF of 0.75 T for 1 h and observed increased calcium level (Prina-Mello et al. 2006). In 2009, Dini et al. found that 6 mT SMF could cause significant increase in calcium level in human leukemia U937 cells (Dini et al. 2009). In 2010, Wang et al. found that 0.23–0.28 T SMF could increase extracellular calcium level in rat adrenal pheochromocytoma PC12 cells (Wang et al. 2010). In addition, they found that SMFs could antagonize CGS21680-induced calcium reduction, which was similar to the effect of a selective A(2A)R antagonist ZM241385 (Wang et al. 2010). In the same year, Hsu and Chang also found that 0.29 T SMF in combination with Dex/β-GP significantly increased the extracellular calcium concentration at the early stage, followed by obvious calcium deposits later, which may contribute to the accelerated osteogenic differentiation and mineralization of dental pulp cells (DPCs) (Hsu and Chang 2010). In 2014, Surma et al. found that weak SMFs increased the intracellular calcium and accelerated the development of skeletal muscle cells from newborn Wistar rats in primary culture (Surma et al. 2014). In the same year, Bernabo et al. showed that a 2 mT SMF could cause a reversible cell membrane depolarization wave (of about 1 min), which induced intracellular calcium increase and mitochondrial activity decrease in vital granulosa cells (Bernabo et al. 2014).
In the meantime, there are also some studies showing that the intracellular calcium was not affected by SMFs. For example, in 1986, Bellossi exposed neonatal isolated chick brains to SMFs of 0.2–0.9 T and did not observe calcium efflux changes (Bellossi 1986). Papatheofanis et al. exposed mice to 1 T SMF for 30 min/day for 10 days and did not observe calcium alteration (Papatheofanis and Papatheofanis 1989). In 1990, Calson et al. found that 0.15 T SMF did not affect the cytosolic calcium level in HL-60 cells (Carson et al. 1990). In 1992, Yost and Liburdy combined extremely low frequency (ELF) time-varying magnetic fields with SMFs and examined their effects on calcium signaling in the lymphocyte (Yost and Liburdy 1992). Their results showed that a 1 h exposure of thymic lymphocytes to a 16 Hz, 42.1 μT magnetic field combined with a colinear SMF of 23.4 μT inhibited calcium influx in mitogen-activated cells but not resting lymphocytes. However, it was interesting that either the time-varying magnetic fields or the SMF alone did not have such effects (Yost and Liburdy 1992). In 2008, Belton et al. found that application of 1, 10, or 100 mT SMF did not affect the calcium response to ATP in HL-60 cells (Belton et al. 2008). In 2009, Belton et al. and Rozanski et al. depleted GSH in HL-60 cells and then examined their responses to 0.1 T SMF and did not observe obvious calcium changes (Belton et al. 2009; Rozanski et al. 2009).
So far as we know, there are only a few studies that have reported the inhibition effect of SMFs on calcium. In 1992, Yost and Liburdy found that a combination of 16 Hz, 42.1 μT time-varying magnetic fields with 23.4 μT SMF could decrease calcium level in thymic lymphocytes (Yost and Liburdy 1992). In 1996, Rosen et al. found that a 120 mT SMF caused a minor reduction in the peak calcium current amplitude and shift in the current–voltage relationship in cultured GH3 cells (Rosen 1996). In 2012, Li et al. found that 5 mT SMF could decrease cytosolic free calcium concentration in human vascular smooth muscle cells (VSMCs) (Li et al. 2012).
There are also many indirect evidences showing that calcium is involved in SMF-induced cellular effects. For example, in 1990, a study using human polymorphonuclear leukocytes (PMNs) showed that 0.1 T SMF could induce degranulation and cell migration inhibition, which could be prevented by pretreatment of calcium channel antagonists diltiazem, nifedipine, and verapamil in dose-dependent manner (Papatheofanis 1990). In 2005, Okano and Ohkuno found that neck exposure to 180 mT (B(max)) SMF alone for 5–8 weeks significantly suppressed or retarded the development of hypertension together with increased baroreflex sensitivity (BRS) in SMF group. Their results indicated that SMF may increase the L-type voltage-gated calcium channel blocker nicardipine-induced hypotension by more effectively antagonizing the Ca2+ influx through the calcium channels compared with the nicardipine injection (NIC) treatment alone (Okano and Ohkubo 2005). In 2006, Ghibelli et al. found that 1 T SMF could potentiate the cytotoxic effects of puromycin and VP16, which could be prevented by calcium chelating agents EGTA and BAPTA-AM as well as the calcium channel blocker nifedipine (Ghibelli et al. 2006). In 2008, Yeh et al. found that 8 mT SMF increased the efficacy of synaptic-transmission in crayfish tail-flip escape circuit in a calcium-dependent way (Yeh et al. 2008). Also in 2008, Morris et al. used pharmacological agents for L-type calcium channel to show that SMF-induced anti-edema effect may work through the L-type calcium channels in vascular smooth muscle cells (Morris and Skalak 2008).
The differential effects of SMF-induced calcium changes are likely due to multiple reasons, such as cell types, magnetic flux density as well as incubation time. There are multiple studies indicating that different cell types have differential calcium changes when exposed to SMFs. In 1999, Fanelli et al. found that the calcium level in different cell types responded to 6 mT SMF differently, which seemed to be correlated to the SMF-induced anti-apoptotic effect (Fanelli et al. 1999). They further found that both the protective and potentiating effects of 6 mT and 1 T SMFs in drug-treated cells were mediated by the Ca2+ influx from the extracellular medium, which only happened in some cell types (Fanelli et al. 1999; Ghibelli et al. 2006). In 2003, Aldinucci et al. tested the effects of combining a 4.75 T SMF and a pulsed EMF of 0.7 mT generated by an NMR apparatus for 1 h. They found that in Jurkat leukemia cells the calcium level was reduced significantly after exposure (Aldinucci et al. 2003b) but in normal or in phytohemagglutinin (PHA) challenged lymphocytes the calcium level was increased (Aldinucci et al. 2003a). In addition, the SMF-induced calcium changes are also magnetic flux density-dependent. In 2006, Ghibelli et al. proposed that both the anti-apoptotic effect of a 6 mT SMF and the potentiating effect of a 1 T SMF were mediated by calcium influx (Ghibelli et al. 2006). In 2014, Zhang et al. examined multiple mineral elements for MC3T3-E1 cells during osteoblast mineralization when they were exposed to 500 nT, control geomagnetic field, 0.2 T, and 16 T SMFs. They found that the calcium level was decreased by 500 nT and 0.2 T SMFs but increased by the 16 T SMF (Zhang et al. 2014). This magnetic flux density-induced difference may have contributed to some of the inconsistencies in the literature, in addition to the cell type-induced variations. Moreover, the SMF-induced calcium changes are also likely to be time-dependent. In 2005, Chionna et al. found that Hep G2 cells exposed to 6 mT SMF had increased calcium level in a time-dependent manner and it reached the highest level at 4 h (Chionna et al. 2005). Table 6.7 summarizes the calcium changes induced by SMFs in the literature (Table 6.7).
Since calcium plays crucial roles in cellular processes such as cell proliferation as well as apoptosis, it is not surprising that different magnetic flux densities could cause differential effects on calcium levels in various cell types, which lead to totally diverse cellular effects. In addition, there are also several studies that reported some signal transduction pathway changes, which are probably due to, or at least partially due to, the SMF-induced calcium modulation. For example, in 2012, Li et al. found that 5 mT SMF could influence the proliferation, migration, and adhesion of human umbilical artery smooth muscle cells (hUASMCs) by inhibiting the clustering of integrin β1, decreasing cytosolic free calcium concentration, and inactivating FAK (Li et al. 2012). We previously found that 1 T SMF could inhibit human CNE-2Z cancer cell proliferation, which was related to the EGFR-Akt-mTOR pathways (Zhang et al. 2015, 2016). As mentioned earlier in this chapter, we found that EGFR and its downstream pathways likely contribute to the cell type- and cell plating density-induced variations in SMF-induced cell proliferation changes (Zhang et al. 2017c). In fact, the kinase activity of EGFR protein itself could be directly inhibited by SMFs (Zhang et al. 2016), which will be further discussed in Chap. 9. Lew et al. used 0.4 T SMF to treat dental pulp stem cells and found that the cell proliferation rate was increased. Their results indicated that 0.4 T SMF affected the cellular membranes of the DPSCs and activated intracellular calcium ions, which may activate p38 MAPK signaling to reorganize the cytoskeleton and increase cell proliferation of the DPSCs (Lew et al. 2018). Moreover, Maredziak et al. showed that 0.5 T SMF increased the proliferation rate of human adipose-derived mesenchymal stromal stem cells via activation of the phosphoinositide 3-kinase/Akt (PI3K/Akt) signaling pathway (Maredziak et al. 2017).
6.3 Conclusion
Since the human body is composed of various cells, which are filled with various components that can respond to the magnetic fields, most studies in the bioeffects of magnetic fields are carried out at cellular level. The parameters of the magnetic fields as well as the cells examined both have enormous impact on the experimental outcomes. So far, most cellular effects of SMFs are largely dependent on magnetic field types, flux density, cell types, as well as other factors mentioned in Chap. 1. The cellular effects not only include the above-mentioned aspects such as cell orientation, proliferation, calcium level changes, but also some other aspects that are relatively less studied and not included in this chapter, such as gene expression, mitochondria, and immune system. It is obvious that further investigations are needed to get a more complete understanding of the cellular effects of SMFs. Overall, most cellular effects of SMFs are relative mild, except for the orientation changes in strong SMFs. In our own lab, to get unbiased and reproducible results throughout our studies, we always have at least two researchers to conduct the same sets of experiments independently and gathered their results together for data analysis. More importantly, people should know that the cellular effects of SMFs are influenced by various factors and parameters of magnetic field and the cells, as well as the experimental procedure, such as incubation time and magnetic field direction. In addition, the absence of magnetic field effects in some experiments contrasted with the positive findings reported by other investigators. These discrepancies may be attributable to an inadequate detection capacity of instrument or techniques. Therefore, people should not only carefully record and analyze all experimental factors, but also try to take advantages of the advanced modern technologies to get a more comprehensive understanding of the cellular effects of SMFs.
References
Adair RK (2000) Static and low-frequency magnetic field effects: health risks and therapies. Rep Prog Phys 63(3):415–454
Albuquerque WW, Costa RM, e Fernandes TDS, Porto AL (2016) Evidences of the static magnetic field influence on cellular systems. Prog Biophys Mol Biol 121(1):16–28
Aldinucci C, Garcia JB, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP (2003a) The effect of exposure to high flux density static and pulsed magnetic fields on lymphocyte function. Bioelectromagnetics 24(6):373–379
Aldinucci C, Garcia JB, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP (2003b) The effect of strong static magnetic field on lymphocytes. Bioelectromagnetics 24(2):109–117
Alipour M, Hajipour-Verdom B, Javan M, Abdolmaleki P (2022) Static and electromagnetic fields differently affect proliferation and cell death through acid enhancement of ROS generation in mesenchymal stem cells. Radiat Res 198(4):384–395. https://doi.org/10.1667/RADE-21-00037.1
Anand A, Nagarajan S, Verma AP, Joshi DK, Pathak PC, Bhardwaj J (2012) Pre-treatment of seeds with static magnetic field ameliorates soil water stress in seedlings of maize (Zea mays L.). Indian J Biochem Biophys 49(1):63–70
Andrews MJ, McClure JA, Malinin GI (1980) Induction of chromosomal alignment by high frequency electric fields. FEBS Lett 118(2):233–236
Ashta A, Motalleb G, Ahmadi-Zeidabadi M (2020) Evaluation of frequency magnetic field, static field, and temozolomide on viability, free radical production and gene expression (p53) in the human glioblastoma cell line (A172). Electromagn Biol Med 39(4):298–309
Baby SM, Narayanaswamy GK, Anand A (2011) Superoxide radical production and performance index of photosystem II in leaves from magnetoprimed soybean seeds. Plant Signal Behav 6(11):1635–1637
Bae JE, Huh MI, Ryu BK, Do JY, Jin SU, Moon MJ, Jung JC, Chang Y, Kim E, Chi SG, Lee GH, Chae KS (2011) The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 32(35):9401–9414
Bajpai I, Saha N, Basu B (2012) Moderate intensity static magnetic field has bactericidal effect on E. coli and S. epidermidis on sintered hydroxyapatite. J Biomed Mater Res B Appl Biomater 100(5):1206–1217
Bajpai I, Balani K, Basu B (2014) Synergistic effect of static magnetic field and HA-Fe3O4 magnetic composites on viability of S. aureus and E. coli bacteria. J Biomed Mater Res B Appl Biomater 102(3):524–532
Ballardin M, Tusa I, Fontana N, Monorchio A, Pelletti C, Rogovich A, Barale R, Scarpato R (2011) Non-thermal effects of 2.45 GHz microwaves on spindle assembly, mitotic cells and viability of Chinese hamster V-79 cells. Mutat Res 716(1–2):1–9
Barbier E, Dufy B, Veyret B (1996) Stimulation of Ca2+ influx in rat pituitary cells under exposure to a 50 Hz magnetic field. Bioelectromagnetics 17(4):303–311
Bekhite MM, Finkensieper A, Abou-Zaid FA, El-Shourbagy IK, Omar KM, Figulla HR, Sauer H, Wartenberg M (2010) Static electromagnetic fields induce vasculogenesis and chondro-osteogenesis of mouse embryonic stem cells by reactive oxygen species-mediated up-regulation of vascular endothelial growth factor. Stem Cells Dev 19(5):731–743
Bekhite MM, Figulla HR, Sauer H, Wartenberg M (2013) Static magnetic fields increase cardiomyocyte differentiation of Flk-1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int J Cardiol 167(3):798–808
Bellossi A (1986) Lack of an effect of static magnetic-field on calcium efflux from isolated chick brains. Bioelectromagnetics 7(4):381–386
Belton M, Commerford K, Hall J, Prato FS, Carson JJ (2008) Real-time measurement of cytosolic free calcium concentration in HL-60 cells during static magnetic field exposure and activation by ATP. Bioelectromagnetics 29(6):439–446
Belton M, Prato FS, Rozanski C, Carson JJ (2009) Effect of 100 mT homogeneous static magnetic field on Ca2+ response to ATP in HL-60 cells following GSH depletion. Bioelectromagnetics 30(4):322–329
Bernabo N, Saponaro I, Tettamanti E, Mattioli M, Barboni B (2014) Acute exposure to a 2 mT static magnetic field affects ionic homeostasis of in vitro grown porcine granulosa cells. Bioelectromagnetics 35(3):231–234
Bertagna F, Lewis R, Silva SRP, McFadden J, Jeevaratnam K (2022) Thapsigargin blocks electromagnetic field-elicited intracellular Ca2+ increase in HEK 293 cells. Physiol Rep 10(9):e15189
Bhardwaj J, Anand A, Nagarajan S (2012) Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol Biochem 57:67–73
Bodega G, Forcada I, Suarez I, Fernandez B (2005) Acute and chronic effects of exposure to a 1-mT magnetic field on the cytoskeleton, stress proteins, and proliferation of astroglial cells in culture. Environ Res 98(3):355–362
Boroske E, Helfrich W (1978) Magnetic anisotropy of egg lecithin membranes. Biophys J 24(3):863–868
Braganza LF, Blott BH, Coe TJ, Melville D (1984) The superdiamagnetic effect of magnetic fields on one and two component multilamellar liposomes. Biochim Biophys Acta 801(1):66–75
Bras W, Diakun GP, Diaz JF, Maret G, Kramer H, Bordas J, Medrano FJ (1998) The susceptibility of pure tubulin to high magnetic fields: a magnetic birefringence and X-ray fiber diffraction study. Biophys J 74(3):1509–1521
Bras W, Torbet J, Diakun GP, Rikken GL, Diaz JF (2014) The diamagnetic susceptibility of the tubulin dimer. J Biophys 2014:985082
Buchachenko AL, Kuznetsov DA (2008) Magnetic field affects enzymatic ATP synthesis. J Am Chem Soc 130(39):12868–12869
Cakmak T, Cakmak ZE, Dumlupinar R, Tekinay T (2012) Analysis of apoplastic and symplastic antioxidant system in shallot leaves: impacts of weak static electric and magnetic field. J Plant Physiol 169(11):1066–1073
Calabro E, Condello S, Curro M, Ferlazzo N, Caccamo D, Magazu S, Ientile R (2013) Effects of low intensity static magnetic field on FTIR spectra and ROS production in SH-SY5Y neuronal-like cells. Bioelectromagnetics 34(8):618–629
Carson JJL, Prato FS, Drost DJ, Diesbourg LD, Dixon SJ (1990) Time-varying magnetic-fields increase cytosolic free Ca2+ in HL-60 cells. Am J Physiol 259(4):C687–C692
Chen WF, Qi H, Sun RG, Liu Y, Zhang K, Liu JQ (2010) Static magnetic fields enhanced the potency of cisplatin on K562 cells. Cancer Biother Radiopharm 25(4):401–408
Chen YP, Li R, He JM (2011) Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 20(4):760–769
Chen YP, Chen D, Liu Q (2017) Exposure to a magnetic field or laser radiation ameliorates effects of PB and CD on physiology and growth of young wheat seedlings. J Photochem Photobiol B 169:171–177
Chen WT, Lin GB, Lin SH, Lu CH, Hsieh CH, Ma BL, Chao CY (2018) Static magnetic field enhances the anticancer efficacy of capsaicin on HepG2 cells via capsaicin receptor TRPV1. PLoS One 13(1):e0191078
Chionna A, Dwikat M, Panzarini E, Tenuzzo B, Carla EC, Verri T, Pagliara P, Abbro L, Dini L (2003) Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur J Histochem 47(4):299–308
Chionna A, Tenuzzo B, Panzarini E, Dwikat MB, Abbro L, Dini L (2005) Time dependent modifications of HepG2 cells during exposure to static magnetic fields. Bioelectromagnetics 26(4):275–286
Chuo W, Ma T, Saito T, Sugita Y, Maeda H, Zhang G, Li J, Liu J, Lu L (2013) A preliminary study of the effect of static magnetic field acting on rat bone marrow mesenchymal stem cells during osteogenic differentiation in vitro. J Hard Tissue Biol 22(2):227–232
Coletti D, Teodori L, Albertini MC, Rocchi M, Pristera A, Fini M, Molinaro M, Adamo S (2007) Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A 71(10):846–856
Cridland NA, Cragg TA, Haylock RG, Saunders RD (1996) Effects of 50 Hz magnetic field exposure on the rate of DNA synthesis by normal human fibroblasts. Int J Radiat Biol 69(4):503–511
Crotty D, Silkstone G, Poddar S, Ranson R, Prina-Mello A, Wilson MT, Coey JM (2012) Reexamination of magnetic isotope and field effects on adenosine triphosphate production by creatine kinase. Proc Natl Acad Sci U S A 109(5):1437–1442
Csillag A, Kumar BV, Szabo K, Szilasi M, Papp Z, Szilasi ME, Pazmandi K, Boldogh I, Rajnavolgyi E, Bacsi A, Laszlo JF (2014) Exposure to inhomogeneous static magnetic field beneficially affects allergic inflammation in a murine model. J R Soc Interface 11(95):20140097
Davies AM, Weinberg U, Palti Y (2013) Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci 1291:86–95
Davis ME (2013) Tumor treating fields—an emerging cancer treatment modality. Clin J Oncol Nurs 17(4):441–443
De Nicola M, Cordisco S, Cerella C, Albertini MC, D'Alessio M, Accorsi A, Bergamaschi A, Magrini A, Ghibelli L (2006) Magnetic fields protect from apoptosis via redox alteration. Ann N Y Acad Sci 1090:59–68
Denegre JM, Valles JM Jr, Lin K, Jordan WB, Mowry KL (1998) Cleavage planes in frog eggs are altered by strong magnetic fields. Proc Natl Acad Sci U S A 95(25):14729–14732
Dini L, Abbro L (2005) Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 36(3):195–217
Dini L, Dwikat M, Panzarini E, Vergallo C, Tenuzzo B (2009) Morphofunctional study of 12-O-tetradecanoyl-13-phorbol acetate (TPA)-induced differentiation of U937 cells under exposure to a 6 mT static magnetic field. Bioelectromagnetics 30(5):352–364
Eguchi Y, Ueno S (2005) Stress fiber contributes to rat Schwann cell orientation under magnetic field. IEEE Trans Magn 41(10):4146–4148
Eguchi Y, Ogiue-Ikeda M, Ueno S (2003) Control of orientation of rat Schwann cells using an 8-T static magnetic field. Neurosci Lett 351(2):130–132
Eguchi Y, Ueno S, Kaito C, Sekimizu K, Shiokawa K (2006) Cleavage and survival of Xenopus embryos exposed to 8 T static magnetic fields in a rotating clinostat. Bioelectromagnetics 27(4):307–313
Elyasigorji Z, Mobasheri H, Dini L (2022) Static magnetic field modulates olfactory ensheathing cell’s morphology, division, and migration activities, a biophysical approach to regeneration. J Tissue Eng Regen Med 16(7):665–679
Emura R, Ashida N, Higashi T, Takeuchi T (2001) Orientation of bull sperms in static magnetic fields. Bioelectromagnetics 22(1):60–65
Emura R, Takeuchi T, Nakaoka Y, Higashi T (2003) Analysis of anisotropic diamagnetic susceptibility of a bull sperm. Bioelectromagnetics 24(5):347–355
Escobar JF, Vaca-Gonzalez JJ, Guevara JM, Vega JF, Hata YA, Garzon-Alvarado DA (2020) In vitro evaluation of the effect of stimulation with magnetic fields on chondrocytes. Bioelectromagnetics 41(1):41–51
Fan Z, Hu P, Xiang L, Liu Y, He R, Lu T (2020) A static magnetic field inhibits the migration and telomerase function of mouse breast cancer cells. Biomed Res Int 2020:7472618
Fanelli C, Coppola S, Barone R, Colussi C, Gualandi G, Volpe P, Ghibelli L (1999) Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J 13(1):95–102
Fassina L, Visai L, Benazzo F, Benedetti L, Calligaro A, De Angelis MG, Farina A, Maliardi V, Magenes G (2006) Effects of electromagnetic stimulation on calcified matrix production by SAOS-2 cells over a polyurethane porous scaffold. Tissue Eng 12(7):1985–1999
Feng C, Yu B, Song C, Wang J, Zhang L, Ji X, Wang Y, Fang Y, Liao Z, Wei M, Zhang X (2022) Static magnetic fields reduce oxidative stress to improve wound healing and alleviate diabetic complications. Cell 11(3):443
Filippi M, Dasen B, Guerrero J, Garello F, Isu G, Born G, Ehrbar M, Martin I, Scherberich A (2019) Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 223:119468
Flipo D, Fournier M, Benquet C, Roux P, Le Boulaire C, Pinsky C, LaBella FS, Krzystyniak K (1998) Increased apoptosis, changes in intracellular Ca2+, and functional alterations in lymphocytes and macrophages after in vitro exposure to static magnetic field. J Toxicol Environ Health A 54(1):63–76
Fu JP, Mo WC, Liu Y, He RQ (2016) Decline of cell viability and mitochondrial activity in mouse skeletal muscle cell in a hypomagnetic field. Bioelectromagnetics 37(4):212–222
Gao W, Liu Y, Zhou J, Pan H (2005) Effects of a strong static magnetic field on bacterium Shewanella oneidensis: an assessment by using whole genome microarray. Bioelectromagnetics 26(7):558–563
Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV (2007) HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 12(3):230–238
Ghibelli L, Cerella C, Cordisco S, Clavarino G, Marazzi S, De Nicola M, Nuccitelli S, D'Alessio M, Magrini A, Bergamaschi A, Guerrisi V, Porfiri LM (2006) NMR exposure sensitizes tumor cells to apoptosis. Apoptosis 11(3):359–365
Gioia L, Saponaro I, Bernabo N, Tettamanti E, Mattioli M, Barboni B (2013) Chronic exposure to a 2 mT static magnetic field affects the morphology, the metabolism and the function of in vitro cultured swine granulosa cells. Electromagn Biol Med 32(4):536–550
Guido S, Tranquillo RT (1993) A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels—correlation of fibroblast orientation and gel birefringence. J Cell Sci 105:317–331
Gurhan H, Bruzon R, Kandala S, Greenebaum B, Barnes F (2021) Effects induced by a weak static magnetic field of different intensities on HT-1080 fibrosarcoma cells. Bioelectromagnetics 42(3):212–223
Haghighat N, Abdolmaleki P, Ghanati F, Behmanesh M, Payez A (2014) Modification of catalase and MAPK in Vicia faba cultivated in soil with high natural radioactivity and treated with a static magnetic field. J Plant Physiol 171(5):99–103
Hajipour Verdom B, Abdolmaleki P, Behmanesh M (2018) The static magnetic field remotely boosts the efficiency of doxorubicin through modulating ROS behaviors. Sci Rep 8(1):990
Hamid HA, Ramasamy R, Mustafa MK, Hosseinpour Sarmadi V, Miskon A (2022) Magnetic exposure using samarium cobalt (SmCo5) increased proliferation and stemness of human umbilical cord mesenchymal stem cells (hUC-MSCs). Sci Rep 12(1):8904
He Y, Chen G, Li Y, Li Y, Yi C, Zhang X, Li H, Zeng B, Wang C, Xie W, Zhao W, Yu D (2021) Effect of magnetic graphene oxide on cellular behaviors and osteogenesis under a moderate static magnetic field. Nanomedicine 37:102435
Helfrich W (1973) Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C 28(11):693–703
Higashi T, Yamagishi A, Takeuchi T, Kawaguchi N, Sagawa S, Onishi S, Date M (1993) Orientation of erythrocytes in a strong static magnetic field. Blood 82(4):1328–1334
Higashi T, Yamagishi A, Takeuchi T, Date M (1995) Effects of static magnetic-fields on erythrocyte rheology. Bioelectrochem Bioenerg 36(2):101–108
Higashi T, Sagawa S, Ashida N, Takeuchi T (1996) Orientation of glutaraldehyde-fixed erythrocytes in strong static magnetic fields. Bioelectromagnetics 17(4):335–338
Higashi T, Ashida N, Takeuchi T (1997) Orientation of blood cells in static magnetic field. Physica B 237:616–620
Hirose H, Nakahara T, Miyakoshi J (2003) Orientation of human glioblastoma cells embedded in type I collagen, caused by exposure to a 10 T static magnetic field. Neurosci Lett 338(1):88–90
Ho SY, Chen IC, Chen YJ, Lee CH, Fu CM, Liu FC, Liou HH (2019) Static magnetic field induced neural stem/progenitor cell early differentiation and promotes maturation. Stem Cells Int 2019:8790176
Hore PJ (2012) Are biochemical reactions affected by weak magnetic fields? Proc Natl Acad Sci U S A 109(5):1357–1358
Hsieh CH, Lee MC, Tsai-Wu JJ, Chen MH, Lee HS, Chiang HO, Wu CH, Jiang CC (2008) Deleterious effects of MRI on chondrocytes. Osteoarthr Cartil 16(3):343–351
Hsieh SC, Tsao JT, Lew WZ, Chan YH, Lee LW, Lin CT, Huang YK, Huang HM (2015) Static magnetic field attenuates lipopolysaccharide-induced inflammation in pulp cells by affecting cell membrane stability. Sci World J 2015:492683
Hsu SH, Chang JC (2010) The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology 62(2):143–155
Iachininoto MG, Camisa V, Leone L, Pinto R, Lopresto V, Merla C, Giorda E, Carsetti R, Zaffina S, Podda MV, Teofili L, Grassi C (2016) Effects of exposure to gradient magnetic fields emitted by nuclear magnetic resonance devices on clonogenic potential and proliferation of human hematopoietic stem cells. Bioelectromagnetics 37(4):201–211
Itegin M, Gunay I, Logoglu G, Isbir T (1995) Effects of static magnetic field on specific adenosine-5′-triphosphatase activities and bioelectrical and biomechanical properties in the rat diaphragm muscle. Bioelectromagnetics 16(3):147–151
Iwasaka M, Ueno S (2003a) Detection of intracellular macromolecule behavior under strong magnetic fields by linearly polarized light. Bioelectromagnetics 24(8):564–570
Iwasaka M, Ueno S (2003b) Polarized light transmission of smooth muscle cells during magnetic field exposures. J Appl Phys 93(10):6701–6703
Iwasaka M, Ueno S, Tsuda H (1994) Diamagnetic properties of fibrin and fibrinogen. IEEE Trans Magn 30(6):4695–4697
Iwasaka M, Miyakoshi J, Ueno S (2003) Magnetic field effects on assembly pattern of smooth muscle cells. In Vitro Cell Dev Biol Anim 39(3–4):120–123
Jia C, Zhou Z, Liu R, Chen S, Xia R (2007) EGF receptor clustering is induced by a 0.4 mT power frequency magnetic field and blocked by the EGF receptor tyrosine kinase inhibitor PD153035. Bioelectromagnetics 28(3):197–207
Kamalipooya S, Abdolmaleki P, Salemi Z, Javani Jouni F, Zafari J, Soleimani H (2017) Simultaneous application of cisplatin and static magnetic field enhances oxidative stress in HeLa cell line. In Vitro Cell Dev Biol Anim 53(9):783–790
Kasetsirikul S, Buranapong J, Srituravanich W, Kaewthamasorn M, Pimpin A (2016) The development of malaria diagnostic techniques: a review of the approaches with focus on dielectrophoretic and magnetophoretic methods. Malar J 15(1):358
Keszthelyi A, Minchell NE, Baxter J (2016) The causes and consequences of topological stress during DNA replication. Genes (Basel) 7(12):134
Kim S, Im WS, Kang L, Lee ST, Chu K, Kim BI (2008) The application of magnets directs the orientation of neurite outgrowth in cultured human neuronal cells. J Neurosci Methods 174(1):91–96
Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y (2004) Disruption of cancer cell replication by alternating electric fields. Cancer Res 64(9):3288–3295
Kotani H, Iwasaka M, Ueno S, Curtis A (2000) Magnetic orientation of collagen and bone mixture. J Appl Phys 87(9):6191–6193
Kotani H, Kawaguchi H, Shimoaka T, Iwasaka M, Ueno S, Ozawa H, Nakamura K, Hoshi K (2002) Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J Bone Miner Res 17(10):1814–1821
Kurzeja E, Synowiec-Wojtarowicz A, Stec M, Glinka M, Gawron S, Pawlowska-Goral K (2013) Effect of a static magnetic fields and fluoride ions on the antioxidant defense system of mice fibroblasts. Int J Mol Sci 14(7):15017–15028
Lew WZ, Huang YC, Huang KY, Lin CT, Tsai MT, Huang HM (2018) Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. J Tissue Eng Regen Med 12(1):19–29
Li Y, Song LQ, Chen MQ, Zhang YM, Li J, Feng XY, Li W, Guo W, Jia G, Wang H, Yu J (2012) Low strength static magnetic field inhibits the proliferation, migration, and adhesion of human vascular smooth muscle cells in a restenosis model through mediating integrins β1-FAK, Ca2+ signaling pathway. Ann Biomed Eng 40(12):2611–2618
Liboff AR, Williams T Jr, Strong DM, Wistar R Jr (1984) Time-varying magnetic fields: effect on DNA synthesis. Science 223(4638):818–820
Limoli CL, Rola R, Giedzinski E, Mantha S, Huang TT, Fike JR (2004) Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci USA 101(45):16052–16057
Lin CY, Wei PL, Chang WJ, Huang YK, Feng SW, Lin CT, Lee SY, Huang HM (2013) Slow freezing coupled static magnetic field exposure enhances cryopreservative efficiency—a study on human erythrocytes. PLoS One 8(3):e58988
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5):479–496
Liu Y, Qi H, Sun RG, Chen WF (2011) An investigation into the combined effect of static magnetic fields and different anticancer drugs on K562 cell membranes. Tumori 97(3):386–392
Luo Y, Ji XM, Liu JJ, Li ZY, Wang WC, Chen W, Wang JF, Liu QS, Zhang X (2016) Moderate intensity static magnetic fields affect mitotic spindles and increase the antitumor efficacy of 5-Fu and Taxol. Bioelectrochemistry 109:31–40
Malinin GI, Gregory WD, Morelli L, Sharma VK, Houck JC (1976) Evidence of morphological and physiological transformation of mammalian cells by strong magnetic fields. Science 194(4267):844–846
Maredziak M, Tomaszewski K, Polinceusz P, Lewandowski D, Marycz K (2017) Static magnetic field enhances the viability and proliferation rate of adipose tissue-derived mesenchymal stem cells potentially through activation of the phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway. Electromagn Biol Med 36(1):45–54
Maret G (1990) Recent biophysical studies in high magnetic-fields. Physica B 164:205–212
Maret GS, Schickfus MV, Mayer A, Dransfeld K (1975) Orientation of nucleic acids in high magnetic fields. Phys Rev Lett 35(6):397–400
Martino CF, Castello PR (2011) Modulation of hydrogen peroxide production in cellular systems by low level magnetic fields. PLoS One 6(8):e22753
Martino CF, Perea H, Hopfner U, Ferguson VL, Wintermantel E (2010) Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 31(4):296–301
Marycz K, Maredziak M, Lewandowski D, Zachanowicz E, Ziecina A, Wiglusz RJ, Pazik R (2017) The effect of Co0.2Mn0.8Fe2O4 ferrite nanoparticles on the C2 canine mastocytoma cell line and adipose-derived mesenchymal stromal stem cells (ASCs) cultured under a static magnetic field: possible implications in the treatment of dog mastocytoma. Cell Mol Bioeng 10(3):209–222
Marycz K, Kornicka K, Rocken M (2018) Static magnetic field (SMF) as a regulator of stem cell fate-new perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev Rep 14(6):785–792
McCann J, Dietrich F, Rafferty C, Martin AO (1993) A critical review of the genotoxic potential of electric and magnetic fields. Mutat Res 297(1):61–95
Minoura I, Muto E (2006) Dielectric measurement of individual microtubules using the electroorientation method. Biophys J 90(10):3739–3748
Miyakoshi J (2005) Effects of static magnetic fields at the cellular level. Prog Biophys Mol Biol 87(2–3):213–223
Miyakoshi J (2006) The review of cellular effects of a static magnetic field. Sci Technol Adv Mater 7(4):305–307
Mo WC, Liu Y, Cooper HM, He RQ (2012) Altered development of Xenopus embryos in a hypogeomagnetic field. Bioelectromagnetics 33(3):238–246
Mo WC, Zhang ZJ, Liu Y, Bartlett PF, He RQ (2013) Magnetic shielding accelerates the proliferation of human neuroblastoma cell by promoting G1-phase progression. PLoS One 8(1):e54775
Mo WC, Zhang ZJ, Wang DL, Liu Y, Bartlett PF, He RQ (2016) Shielding of the geomagnetic field alters actin assembly and inhibits cell motility in human neuroblastoma cells. Sci Rep 6:22624
Morris CE, Skalak TC (2008) Acute exposure to a moderate strength static magnetic field reduces edema formation in rats. Am J Physiol Heart Circ Physiol 294(1):H50–H57
Murayama M (1965) Orientation of sickled erythrocytes in a magnetic field. Nature 206(982):420–422
Naarala J, Kesari KK, McClure I, Chavarriaga C, Juutilainen J, Martino CF (2017) Direction-dependent effects of combined static and ELF magnetic fields on cell proliferation and superoxide radical production. Biomed Res Int 2017:5675086
Nakahara T, Yaguchi H, Yoshida M, Miyakoshi J (2002) Effects of exposure of CHO-K1 cells to a 10-T static magnetic field. Radiology 224(3):817–822
Nuccitelli S, Cerella C, Cordisco S, Albertini MC, Accorsi A, De Nicola M, D'Alessio M, Radogna F, Magrini A, Bergamaschi A, Ghibelli L (2006) Hyperpolarization of plasma membrane of tumor cells sensitive to antiapoptotic effects of magnetic fields. Ann N Y Acad Sci 1090:217–225
Ogiue-Ikeda M, Ueno S (2004) Magnetic cell orientation depending on cell type and cell density. IEEE Trans Magn 40(4):3024–3026
Okada R, Yamato K, Kawakami M, Kodama J, Kushioka J, Tateiwa D, Ukon Y, Zeynep B, Ishimoto T, Nakano T, Yoshikawa H, Kaito T (2021) Low magnetic field promotes recombinant human BMP-2-induced bone formation and influences orientation of trabeculae and bone marrow-derived stromal cells. Bone Rep 14:100757
Okano H, Ohkubo C (2005) Exposure to a moderate intensity static magnetic field enhances the hypotensive effect of a calcium channel blocker in spontaneously hypertensive rats. Bioelectromagnetics 26(8):611–623
Olsson G, Belyaev IY, Helleday T, Harms-Ringdahl M (2001) ELF magnetic field affects proliferation of SPD8/V79 Chinese hamster cells but does not interact with intrachromosomal recombination. Mutat Res 493(1–2):55–66
Pacini S, Vannelli GB, Barni T, Ruggiero M, Sardi I, Pacini P, Gulisano M (1999a) Effect of 0.2 T static magnetic field on human neurons: remodeling and inhibition of signal transduction without genome instability. Neurosci Lett 267(3):185–188
Pacini S, Aterini S, Pacini P, Ruggiero C, Gulisano M, Ruggiero M (1999b) Influence of static magnetic field on the antiproliferative effects of vitamin D on human breast cancer cells. Oncol Res 11(6):265–271
Pacini S, Gulisano M, Peruzzi B, Sgambati E, Gheri G, Gheri Bryk S, Vannucchi S, Polli G, Ruggiero M (2003) Effects of 0.2 T static magnetic field on human skin fibroblasts. Cancer Detect Prev 27(5):327–332
Papatheofanis FJ (1990) Use of calcium-channel antagonists as magnetoprotective agents. Radiat Res 122(1):24–28
Papatheofanis FJ, Papatheofanis BJ (1989) Short-term effect of exposure to intense magnetic fields on hematologic indices of bone metabolism. Investig Radiol 24(3):221–223
Pauling L (1979) Diamagnetic anisotropy of the peptide group. Proc Natl Acad Sci U S A 76(5):2293–2294
Petrov E, Martinac B (2007) Modulation of channel activity and gadolinium block of MscL by static magnetic fields. Eur Biophys J 36(2):95–105
Pless M, Weinberg U (2011) Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs 20(8):1099–1106
Politanski P, Rajkowska E, Brodecki M, Bednarek A, Zmyslony M (2013) Combined effect of X-ray radiation and static magnetic fields on reactive oxygen species in rat lymphocytes in vitro. Bioelectromagnetics 34(4):333–336
Poniedzialek B, Rzymski P, Karczewski J, Jaroszyk F, Wiktorowicz K (2013) Reactive oxygen species (ROS) production in human peripheral blood neutrophils exposed in vitro to static magnetic field. Electromagn Biol Med 32(4):560–568
Prasad A, Teh DBL, Blasiak A, Chai C, Wu Y, Gharibani PM, Yang IH, Phan TT, Lim KL, Yang H, Liu X, All AH (2017) Static magnetic field stimulation enhances oligodendrocyte differentiation and secretion of neurotrophic factors. Sci Rep 7(1):6743
Prina-Mello A, Farrell E, Prendergast PJ, Campbell V, Coey JM (2006) Influence of strong static magnetic fields on primary cortical neurons. Bioelectromagnetics 27(1):35–42
Reddig A, Fatahi M, Friebe B, Guttek K, Hartig R, Godenschweger F, Roggenbuck D, Ricke J, Reinhold D, Speck O (2015) Analysis of DNA double-strand breaks and cytotoxicity after 7 Tesla magnetic resonance imaging of isolated human lymphocytes. PLoS One 10(7):e0132702
Roman A, Tombarkiewicz B (2009) Prolonged weakening of the geomagnetic field (GMF) affects the immune system of rats. Bioelectromagnetics 30(1):21–28
Romeo S, Sannino A, Scarfi MR, Massa R, d'Angelo R, Zeni O (2016) Lack of effects on key cellular parameters of MRC-5 human lung fibroblasts exposed to 370 mT static magnetic field. Sci Rep 6:19398
Rosen AD (1996) Inhibition of calcium channel activation in GH3 cells by static magnetic fields. Biochim Biophys Acta 1282(1):149–155
Rosen AD, Chastney EE (2009) Effect of long term exposure to 0.5 T static magnetic fields on growth and size of GH3 cells. Bioelectromagnetics 30(2):114–119
Rozanski C, Belton M, Prato FS, Carson JJ (2009) Real-time measurement of cytosolic free calcium concentration in DEM-treated HL-60 cells during static magnetic field exposure and activation by ATP. Bioelectromagnetics 30(3):213–221
Ruiz-Gomez MJ, Sendra-Portero F, Martinez-Morillo M (2010) Effect of 2.45 mT sinusoidal 50 Hz magnetic field on saccharomyces cerevisiae strains deficient in DNA strand breaks repair. Int J Radiat Biol 86(7):602–611
Sadri M, Abdolmaleki P, Abrun S, Beiki B, Samani FS (2017) Static magnetic field effect on cell alignment, growth, and differentiation in human cord-derived mesenchymal stem cells. Cell Mol Bioeng 10(3):249–262
Samsonov A, Popov SV (2013) The effect of a 94 GHz electromagnetic field on neuronal microtubules. Bioelectromagnetics 34(2):133–144
Sarvestani AS, Abdolmaleki P, Mowla SJ, Ghanati F, Heshmati E, Tavasoli Z, Jahromi AM (2010) Static magnetic fields aggravate the effects of ionizing radiation on cell cycle progression in bone marrow stem cells. Micron 41(2):101–104
Sato K, Yamaguchi H, Miyamoto H, Kinouchi Y (1992) Growth of human cultured cells exposed to a non-homogeneous static magnetic field generated by Sm–Co magnets. Biochim Biophys Acta 1136(3):231–238
Schrader T, Kleine-Ostmann T, Munter K, Jastrow C, Schmid E (2011) Spindle disturbances in human-hamster hybrid (AL) cells induced by the electrical component of the mobile communication frequency range signal. Bioelectromagnetics 32(4):291–301
Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10(3):175–176
Schumacker PT (2015) Reactive oxygen species in cancer: a dance with the devil. Cancer Cell 27(2):156–157
Shi Y, Nikulenkov F, Zawacka-Pankau J, Li H, Gabdoulline R, Xu J, Eriksson S, Hedstrom E, Issaeva N, Kel A, Arner ES, Selivanova G (2014) ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ 21(4):612–623
Shine MB, Guruprasad KN (2012) Impact of pre-sowing magnetic field exposure of seeds to stationary magnetic field on growth, reactive oxygen species and photosynthesis of maize under field conditions. Acta Physiol Plant 34(1):255–265
Shine MB, Guruprasad KN, Anand A (2012) Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 33(5):428–437
Short WO, Goodwill L, Taylor CW, Job C, Arthur ME, Cress AE (1992) Alteration of human tumor cell adhesion by high-strength static magnetic fields. Investig Radiol 27(10):836–840
Song C, Yu B, Wang J, Ji X, Zhang L, Tian X, Yu X, Feng C, Wang X, Zhang X (2021) Moderate static magnet fields suppress ovarian cancer metastasis via ROS-mediated oxidative stress. Oxid Med Cell Longev 2021:7103345
Song C, Yu B, Wang J, Zhu Y, Zhang X (2022) Effects of moderate to high static magnetic fields on reproduction. Bioelectromagnetics 43(4):278–291
Stolfa S, Skorvanek M, Stolfa P, Rosocha J, Vasko G, Sabo J (2007) Effects of static magnetic field and pulsed electromagnetic field on viability of human chondrocytes in vitro. Physiol Res 56(Suppl 1):S45–S49
Sullivan K, Balin AK, Allen RG (2011) Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 32(2):140–147
Surma SV, Belostotskaya GB, Shchegolev BF, Stefanov VE (2014) Effect of weak static magnetic fields on the development of cultured skeletal muscle cells. Bioelectromagnetics 35(8):537–546
Takashima Y, Miyakoshi J, Ikehata M, Iwasaka M, Ueno S, Koana T (2004) Genotoxic effects of strong static magnetic fields in DNA-repair defective mutants of Drosophila melanogaster. J Radiat Res 45(3):393–397
Takeuchi T, Mizuno T, Higashi T, Yamagishi A, Date M (1995) Orientation of red-blood-cells in high magnetic-field. J Magn Magn Mater 140:1462–1463
Tenuzzo B, Chionna A, Panzarini E, Lanubile R, Tarantino P, Di Jeso B, Dwikat M, Dini L (2006) Biological effects of 6 mT static magnetic fields: a comparative study in different cell types. Bioelectromagnetics 27(7):560–577
Teodori L, Giovanetti A, Albertini MC, Rocchi M, Perniconi B, Valente MG, Coletti D (2014) Static magnetic fields modulate X-ray-induced DNA damage in human glioblastoma primary cells. J Radiat Res 55(2):218–227
Tofani S, Barone D, Cintorino M, de Santi MM, Ferrara A, Orlassino R, Ossola P, Peroglio F, Rolfo K, Ronchetto F (2001) Static and ELF magnetic fields induce tumor growth inhibition and apoptosis. Bioelectromagnetics 22(6):419–428
Tonini R, Baroni MD, Masala E, Micheletti M, Ferroni A, Mazzanti M (2001) Calcium protects differentiating neuroblastoma cells during 50 Hz electromagnetic radiation. Biophys J 81(5):2580–2589
Torbati M, Mozaffari K, Liu L, Sharma P (2022) Coupling of mechanical deformation and electromagnetic fields in biological cells. Rev Mod Phys 94:025003
Torbet J, Ronziere MC (1984) Magnetic alignment of collagen during self-assembly. Biochem J 219(3):1057–1059
Torbet J, Freyssinet JM, Hudryclergeon G (1981) Oriented fibrin gels formed by polymerization in strong magnetic-fields. Nature 289(5793):91–93
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10(3):241–252
Ueno S (2012) Studies on magnetism and bioelectromagnetics for 45 years: from magnetic analog memory to human brain stimulation and imaging. Bioelectromagnetics 33(1):3–22
Ueno S, Harada K (1982) Redistribution of dissolved-oxygen concentration under strong dc magnetic-fields. IEEE Trans Magn 18(6):1704–1706
Ueno S, Iwasaka M, Kitajima T (1994) Redistribution of dissolved-oxygen concentration under magnetic-fields up to 8 T. J Appl Phys 75(10):7174–7176
Ueno S, Iwasaka M, Furukawa G (1995) Dynamic behavior of dissolved-oxygen under magnetic-fields. IEEE Trans Magn 31(6):4259–4261
Umeno A, Ueno S (2003) Quantitative analysis of adherent cell orientation influenced by strong magnetic fields. IEEE Trans Nanobiosci 2(1):26–28
Umeno A, Kotani H, Iwasaka M, Ueno S (2001) Quantification of adherent cell orientation and morphology under strong magnetic fields. IEEE Trans Magn 37(4):2909–2911
Valiron O, Peris L, Rikken G, Schweitzer A, Saoudi Y, Remy C, Job D (2005) Cellular disorders induced by high magnetic fields. J Magn Reson Imaging 22(3):334–340
Valles JM (2002) Model of magnetic field-induced mitotic apparatus reorientation in frog eggs. Biophys J 82(3):1260–1265
Van Huizen AV, Morton JM, Kinsey LJ, Von Kannon DG, Saad MA, Birkholz TR, Czajka JM, Cyrus J, Barnes FS, Beane WS (2019) Weak magnetic fields alter stem cell-mediated growth. Sci Adv 5(1):eaau7201
Vassilev PM, Dronzine RT, Vassileva MP, Georgiev GA (1982) Parallel arrays of microtubules formed in electric and magnetic fields. Biosci Rep 2(12):1025–1029
Vergallo C, Ahmadi M, Mobasheri H, Dini L (2014) Impact of inhomogeneous static magnetic field (31.7–232.0 mT) exposure on human neuroblastoma SH-SY5Y cells during cisplatin administration. PLoS One 9(11):e113530
Walleczek J, Budinger TF (1992) Pulsed magnetic-field effects on calcium signaling in lymphocytes-dependence on cell status and field intensity. FEBS Lett 314(3):351–355
Wang H, Zhang X (2017) Magnetic fields and reactive oxygen species. Int J Mol Sci 18(10):2175
Wang H, Zhang X (2019) ROS reduction does not decrease the anticancer efficacy of X-ray in two breast cancer cell lines. Oxid Med Cell Longev 2019:3782074
Wang DL, Wang XS, Xiao R, Liu Y, He RQ (2008) Tubulin assembly is disordered in a hypogeomagnetic field. Biochem Biophys Res Commun 376(2):363–368
Wang Z, Che PL, Du J, Ha B, Yarema KJ (2010) Static magnetic field exposure reproduces cellular effects of the Parkinson’s disease drug candidate ZM241385. PLoS One 5(11):e13883
Wang Z, Hao F, Ding C, Yang Z, Shang P (2014) Effects of static magnetic field on cell biomechanical property and membrane ultrastructure. Bioelectromagnetics 35(4):251–261
Wang J, Xiang B, Deng J, Freed DH, Arora RC, Tian G (2016) Inhibition of viability, proliferation, cytokines secretion, surface antigen expression, and adipogenic and osteogenic differentiation of adipose-derived stem cells by 7-day exposure to 0.5 T static magnetic fields. Stem Cells Int 2016:7168175
Wang D, Zhang L, Shao G, Yang S, Tao S, Fang K, Zhang X (2018) 6 mT 0–120 Hz magnetic fields differentially affect cellular ATP levels. Environ Sci Pollut Res Int 25(28):28237–28247
Wang S, Huyan T, Lou C, Shang P, Zhang H (2022) 12 T high static magnetic field suppresses osteosarcoma cells proliferation by regulating intracellular ROS and iron status. Exp Cell Res 417(2):113223
Williams PA, Ingebretsen RJ, Dawson RJ (2006) 14.6 mT ELF magnetic field exposure yields no DNA breaks in model system salmonella, but provides evidence of heat stress protection. Bioelectromagnetics 27(6):445–450
Wosik J, Chen W, Qin K, Ghobrial RM, Kubiak JZ, Kloc M (2018) Magnetic field changes macrophage phenotype. Biophys J 114(8):2001–2013
Wu H, Li C, Masood M, Zhang Z, Gonzalez-Almela E, Castells-Garcia A, Zou G, Xu X, Wang L, Zhao G, Yu S, Zhu P, Wang B, Qin D, Liu J (2022) Static magnetic fields regulate T-type calcium ion channels and mediate mesenchymal stem cells proliferation. Cell 11(15):2460
Yamagishi A, Takeuchi T, Higashi T, Date M (1990) Magnetic-field effect on the polymerization of fibrin fibers. Physica B 164(1–2):222–228
Yamagishi A, Takeuchi T, Higashi T, Date M (1992) Diamagnetic orientation of blood-cells in high magnetic-field. Physica B 177(1–4):523–526
Yan J, Dong L, Zhang B, Qi N (2010) Effects of extremely low-frequency magnetic field on growth and differentiation of human mesenchymal stem cells. Electromagn Biol Med 29(4):165–176
Yang J, Zhang J, Ding C, Dong D, Shang P (2018) Regulation of osteoblast differentiation and iron content in MC3T3-E1 cells by static magnetic field with different intensities. Biol Trace Elem Res 184(1):214–225
Yang X, Li Z, Polyakova T, Dejneka A, Zablotskii V, Zhang X (2020) Effect of static magnetic field on DNA synthesis: the interplay between DNA chirality and magnetic field left-right asymmetry. FASEB Bioadv 2(4):254–263
Yeh SR, Yang JW, Lee YT, Tsai LY (2008) Static magnetic field expose enhances neurotransmission in crayfish nervous system. Int J Radiat Biol 84(7):561–567
Yost MG, Liburdy RP (1992) Time-varying and static magnetic fields act in combination to alter calcium signal transduction in the lymphocyte. FEBS Lett 296(2):117–122
Yuan Z, Memarzadeh K, Stephen AS, Allaker RP, Brown RA, Huang J (2018a) Development of a 3D collagen model for the in vitro evaluation of magnetic-assisted osteogenesis. Sci Rep 8(1):16270
Yuan LQ, Wang C, Zhu K, Li HM, Gu WZ, Zhou DM, Lai JQ, Zhou D, Lv Y, Tofani S, Chen X (2018b) The antitumor effect of static and extremely low frequency magnetic fields against nephroblastoma and neuroblastoma. Bioelectromagnetics 39(5):375–385
Zablotskii V, Dejneka A, Kubinova S, Le-Roy D, Dumas-Bouchiat F, Givord D, Dempsey NM, Sykova E (2013) Life on magnets: stem cell networking on micro-magnet arrays. PLoS One 8(8):e70416
Zablotskii V, Syrovets T, Schmidt ZW, Dejneka A, Simmet T (2014) Modulation of monocytic leukemia cell function and survival by high gradient magnetic fields and mathematical modeling studies. Biomaterials 35(10):3164–3171
Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN (2003) Red blood cell magnetophoresis. Biophys J 84(4):2638–2645
Zhang J, Ding C, Shang P (2014) Alterations of mineral elements in osteoblast during differentiation under hypo, moderate and high static magnetic fields. Biol Trace Elem Res 162(1–3):153–157
Zhang L, Yang XX, Liu JJ, Luo Y, Li ZY, Ji XM, Wang WC, Zhang X (2015) 1 T moderate intensity static magnetic field affects Akt/mTOR pathway and increases the antitumor efficacy of mTOR inhibitors in CNE-2Z cells. Sci Bull 60(24):2120–2128
Zhang L, Wang J, Wang H, Wang W, Li Z, Liu J, Yang X, Ji X, Luo Y, Hu C, Hou Y, He Q, Fang J, Wang J, Liu Q, Li G, Lu Q, Zhang X (2016) Moderate and strong static magnetic fields directly affect EGFR kinase domain orientation to inhibit cancer cell proliferation. Oncotarget 7(27):41527–41539
Zhang HT, Zhang ZJ, Mo WC, Hu PD, Ding HM, Liu Y, Hua Q, He RQ (2017a) Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase. Protein Cell 8(7):527–537
Zhang L, Hou Y, Li Z, Ji X, Wang Z, Wang H, Tian X, Yu F, Yang Z, Pi L, Mitchison TJ, Lu Q, Zhang X (2017b) 27 T ultra-high static magnetic field changes orientation and morphology of mitotic spindles in human cells. elife 6:e22911
Zhang L, Ji X, Yang X, Zhang X (2017c) Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 8(8):13126–13141
Zhang M, Li W, He W, Xu Y (2021) Static magnetic fields enhance the chondrogenesis of mandibular bone marrow mesenchymal stem cells in coculture systems. Biomed Res Int 2021:9962861
Zhao Y, Zhan Q (2012) Electric fields generated by synchronized oscillations of microtubules, centrosomes and chromosomes regulate the dynamics of mitosis and meiosis. Theor Biol Med Model 9:26. https://doi.org/10.1186/1742-4682-9-26
Zhao M, Forrester JV, McCaig CD (1999) A small, physiological electric field orients cell division. Proc Natl Acad Sci U S A 96(9):4942–4946
Zhao G, Chen S, Zhao Y, Zhu L (2010) Effects of 13 T static magnetic fields (SMF) in the cell cycle distribution and cell viability in immortalized hamster cells and human primary fibroblasts cells. Plasma Sci Technol 12(1):123–128
Zhao G, Chen S, Wang L, Zhao Y, Wang J, Wang X, Zhang W, Wu R, Wu L, Wu Y, Xu A (2011) Cellular ATP content was decreased by a homogeneous 8.5 T static magnetic field exposure: role of reactive oxygen species. Bioelectromagnetics 32(2):94–101
Zhao B, Yu T, Wang S, Che J, Zhou L, Shang P (2021) Static magnetic field (0.2–0.4 T) stimulates the self-renewal ability of osteosarcoma stem cells through autophagic degradation of ferritin. Bioelectromagnetics 42(5):371–383
Zheng L, Zhang L, Chen L, Jiang J, Zhou X, Wang M, Fan Y (2018) Static magnetic field regulates proliferation, migration, differentiation, and YAP/TAZ activation of human dental pulp stem cells. J Tissue Eng Regen Med 12(10):2029–2040
Zhou J, Yao G, Zhang J, Chang Z (2002) CREB DNA binding activation by a 50-Hz magnetic field in HL60 cells is dependent on extra- and intracellular Ca2+ but not PKA, PKC, ERK, or p38 MAPK. Biochem Biophys Res Commun 296(4):1013–1018
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ji, X., Zhang, X. (2023). Impact of Static Magnetic Fields on Cells. In: Zhang, X. (eds) Biological Effects of Static Magnetic Fields. Springer, Singapore. https://doi.org/10.1007/978-981-19-8869-1_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-8869-1_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8868-4
Online ISBN: 978-981-19-8869-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)