Abstract
This investigation is performed to evaluate the impact of static magnetic field on the Cell growth alignment, and differentiation potential in Human Mesenchymal Stem cells derived from human newborn cords. In vitro-cultured mesenchymal stem cells derived from human newborn cords were exposed to SMF up to 24 mT and compared with the control (unexposed) cultures. Viability was assessed via Trypan Blue staining and MTT assay. Cell cycle progression was studied after flow cytometry data analysis. Sox-2, Nanong, and Oct-4 Primers used for RT-PCR experiment. Morphological studies showed that the exposed cells were significantly aligned in parallel bundles in a correlation with the magnetic field lines. Viability measurements showed a significant reduction in cell viability which was noted after exposure to static magnetic field and initiated 36 h after the end of exposure time. Flow cytometric data analysis confirmed a decrease in G1 phase cell population within the treated and cultured groups compared with the corresponding control samples. However, the induced changes were recovered in the cell cultures after the post-exposure culture recovery time which may be attributed to the cellular repair mechanisms. Furthermore, the proliferation rate and Oct-4 gene expression were reduced due to the 18 mT static magnetic field exposure. The significant proliferation rate decrease accompanied by the Sox-2, Nanong, and Oct-4 gene expression decline, suggested the differentiation inducing effects of SMF exposure. Exposure to Static Magnetic fields up to 24 mT affects mesenchymal stem cell alignment and proliferation rate as well as mRNA expression of Sox-2, Nanong, and Oct-4 genes, therefore can be considered as a new differentiation inducer in addition to the other stimulators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is presently known that life is an electromagnetic event in an aqueous medium due to existence of moving ions and charged groups within the living cells. Therefore, an appropriate choice of magnetic field can be expected to initiate systemic changes that result in efficacious effects at the cellular level.12–14 Curative application of magnetic fields (MFs) is well-established and the magnet therapy is used for the both acute and chronic pain reliefs.9,57 Therapeutic advantages of MFs have been shown for the treatment of wounds,28 bone fractures9,30 and killing of cancer cells.19,27
The interaction of (MFs) with living organisms has been a rapidly growing field of investigation in the past decades.18,35 Studying these effects has become a considerable public concern due to mankind’s confrontation with these magnetic fields from different sources such as imaging with MRI, power lines and in-home devices. Exposure to high intensity magnetic fields is currently on the rise because of the widespread use of magnetic resonance tomography devices for diagnostic purposes.18 Prolonged and systematic exposure (for months and even years) to low and moderate intensity of magnetic fields on human beings under industrial conditions is reported to cause fatigue, dizziness, sleeplessness, headache, and heart pain.32 However, few environmental health issues are as contentious as the question of whether exposure to magnetic fields increases the risk for diseases.54 Among matters of controversy, exposure to static magnetic field has yielded conflicting results.
Early epidemiological studies found a positive correlation between MF exposure and some cancer types, especially childhood leukemia.54 Nevertheless, several other studies have failed to confirm these findings and basically excluded the potential genotoxic and oncogenic effects of MFs.36,56
Despite the increasing number of in vivo and in vitro studies on the effects of the MFs interaction with living organisms, major gaps in our knowledge still remain. At the current state of knowledge, the biological effects of SMFs have yet to be unequivocally interpreted. SMF time of exposure and intensity are critical variables in the study of the mentioned effects on a particular cell type. As regards cell type, there are reported effects of SMF as well as a lack of any effects. Absence of SMF effect on cell growth include human fetal lung fibroblasts.56 In contrast other studies detected SMF influence on apoptosis1,49 and neuron response.38 Moderate-intensity SMF induced modifications of cell shape, cell surface, and cytoskeleton progressively inflicted during the entire period of exposure.12,31,53
It has been investigated that exposure to strong static magnetic field (up to 10 T) had no effect on changes in cell growth rate but in the presence of trace amounts of ferrous ions in the culture medium micronucleus formation increased as a consequence of cellular DNA damage in the cancer cells.40 On the other hand, many effects have been investigated to alter cell growth in the moderate intensity (up to 0.1 T) static magnetic field exposure,1,10 but it doesn’t cause significant growth changes in high intensities underline the fact that SMF affects living cells in a magnetic intensity and cell type manner.
Stem cells are primitive cells, present in all human organisms, which are capable of dividing and reproducing themselves, or switching to become more specialized cells in human body such as cells in brain, heart, muscles, and kidney and can be used for therapeutic purposes.33,39 Mesenchymal stem cells (MSCs) are a heterogeneous subset of stromal stem cells that can be isolated from many adult tissues.17 They can interact with cells of both the innate and adaptive immune systems. After in vivo administration they induce peripheral tolerance and migrate to injured tissues, where they can inhibit the release of pro-inflammatory cytokines, be differentiated into other cell types and promote the survival of damaged tissues.34
MSCs exhibit immune-suppressive properties and a pattern of multilineage differentiation potential.47 These cells can grow and differentiate toward different phenotypes throughout life.17 These cells in blood or tissues can be differentiated into adipocytes, chondrocytes, osteocytes, cardio myocytes, and neurons. Bone marrow (BM) has been recognized as one major source and the first one reported to contain these cells for both experimental and clinical studies46 and human MSCs are precious tools for regenerative medicine and cell based therapy.51
However, BM may be detrimental for clinical use due to the highly invasive donation procedure, decline in MSC number and reduced differentiation potential with increasing donor age.29 As this method is considered to be painful and invasive, many scientists prefer to obtain MCSs from other resources in adult human body, fetus, amniotic fluid, and umbilical cord. Umbilical cord derived Mesenchymal stem cells (UCMSCs) have drawn new interest as useful materials for cell based therapy.25 Multipotent mesenchymal stem cells derived from human umbilical cord blood (UCB) are a promising candidate for the development of future strategies in regenerative medicine. Another advantage of umbilical cord derived MSCs is the fact that it does not entangle the researcher in ethical issues associated with harvesting stem cells from live donors.23
The aim of this study is to determine probable effects of SMF intensities up to 24 milliTesla on probable cell cycle progression alterations, cell viability, growth, and orientation of in vitro cultured human mesenchymal stem cells. We used human mesenchymal stem cell as a target for SMF due to its high sensitivity to physical stimulus and therapeutic applications in medical assessments. Samples were studied after a 24-h exposure to SMF and cultured for various times.
Materials and Methods
Static Magnetic Field Exposure
Exposure to MF was performed using a locally designed SMF generator. The magnetic field generator consisted of two coils and a DC switching power supply. The coils were built from a 3.0 mm diameter wire, and resistant to heat up to 200 °C. Wire length in each coil was about 1 km and each coil weighs approximately 40 kg. Coils had a total resistance of 3 Ω and the inductance of 2 Henry. These two coils guided the magnetic field through two iron blades with the height of 1 m and the cross section of 10 cm.
The electrical power was provided using a 220 V/AC power supply equipped with a variable transformer as well as a single-phase full-wave rectifier. The switching power supply could apply a DC voltage up to 50 V and a current up to 16 A to the coil, for moderate intensity static magnetic field generation as needed. In order to cool off the system, a gas chiller with optimum control on temperature was used. This cooling system consisted of an evaporator, an engine, a condenser, and refrigerant gas. The evaporator covered the outer surface of the coils. This enables the system to effectively cool the system down. The gas introduced to this motor was of R12 kind (Fig. 1).
The field between the iron blades was measured by a 13610.93 PHYWE (Gottingen, Germany) Teslameter. Presence of any pulsation in the current from rectifier into the SMF generating apparatus, was tested by an oscilloscope (8040, Leader Electronics Co., Yokohama, Japan). However, the ripple voltage had not been zero, it still was enough small to be neglected and consider the generated magnetic field homogenous.
The field produced by the system, was simulated using the CST STUDIO 2011 software. The uniformity of the field is represented with surfaces of the same color. The profile of field emission in coils is illustrated in Fig. 2. Calibration of the system as well as tests for the accuracy and uniformity of the SMF were performed by a Teslameter (13610.93, PHYWE, Gottingen, Germany), and also was calculated with Complete Technology for 3D Simulation CST STUDIO 2011 software (http://www.CST.com) for the best site selection of experimental samples within the exposure chamber of SMF generator (Fig. 2).
Isolation and Purification of Umbilical-Cord-Derived Mesenchymal Stem Cells (UCHMSCs)
Cord samples, collected at delivery from newborn infants with their mothers’ consent, were donated to Royan Cord Blood Bank, Research and Development section (Tehran, Iran). This study has been conducted according to Declaration of Helsinki principles. Blood vessels were initially detached from the cords in order to extract Wharton’s Jelly (WJ) which subsequently was treated with Collagenase Type IV 1% powder in PBS (Phosphate Buffered Saline).
WJ pieces were discarded and the suspension was centrifuged (1500 rpm, 5 min, 20 °C). After 45 min of incubation the pellet was cultured in Low Glucose DMEM medium (Gibco—life technologies TM—Cat # 21885) supplemented with 15% FBS (Fetal Bovine Serum), 1% penicillin/streptomycin, and 1% l-Glutamin. HMSs were purificated and adhered to the bottom of the plate after 2 weeks. Each experimental sample which were harvested and counted to provide 70% confluent monolayers contained about 1 million cells in each cell culture flask with an area equal to 25 cm2.34
All experiments were performed according to the established protocols provided by the Ethics Committees of Tarbiat Modares University and Royan Stem Cell Technology Company (Cord Blood Bank) Research Center, and the new born parents’ written informed consent letters were collected prior to participation.
Identification of HMSCs
Immunofluorescence experiments showed the presence of surface antigens of mesenchymal stem cells (CD44, CD73, CD90, and CD105) and the absence of hematopoietic ones (CD133, CD45, and CD34). The lake of HLA-DR surface antigen was also examined in the cord derived MSCs. Multilineage differentiation potential of UCHMSCs into bone, cartilage, and fat has been described as one of their main characteristics by several laboratories.3,20 The potential of HMSCs for differentiation was investigated by providing the proper differentiation conditions in our laboratory in order to ensure that the samples are truly mesenchymal stem cells.
HMSCs were cultured in DMEM medium supplemented with 10 μM l-ascorbic acid-2-phosphate, 1 nm (1 μL mL−1) dexamethasone for 21 days for adipogenic differentiation then stained with Oil Red dye. The samples were similarly cultured for three weeks in order to induce osteogenic differentiation. The medium contained 10−2 M β-glycerol phosphate and 50 µg mL−1 ascorbic acid in high glucose DMEM, 1 nm dexamethasone, 5 µg mL−1 insulin, 60 µM indomethacin. The cultured cells were then stained using Alizarin Red to illustrate differentiated cells. Chondrogenic differentiation occurred when MSCs were grown under a three-dimensional culture format namely, the volume at the bottom of testing tube. The medium contained DMAM: F12 (1:1 mix with 1× ITS, 10 ng mL−1 TGF-Beta; 5 μg mL−1 ITS premix; 100 μg mL−1 sodium pyruvate, 100 µM ascorbic acid, 1 nM dexamethasone, 40 μg mL−1 proline (Sigma). The cells differentiated into chondrocytes after 21 days of incubation and chondrocytes were observed in blue by staining the microtome samples with Toluidine Blue.
SMF Treatment of Cells
HMSC cultures at the passage number 3 were harvested and counted to provide cultures at the 60% confluency for the experiments. Before each exposure, the MF intensity was set using the Teslameter to make sure that no other magnetic appliance was working. The SMF generator also provided standard cell culture conditions using a locally designed incubator placed between the arms of a U shape iron core (Fig. 1). Samples were exposed to SMF (12, 18, and 24 mT) for 24 h, and then cultured for different time periods of 24, 36, 48, 60, and 72 h. The control samples at the time were placed inside another incubator in the isolation under standard cell culture conditions. They were only exposed to geomagnetic field of earth which was 0.047 mT in our lab. It would be impressive if we could but the measurement was performed by Institute of Geophysics, University of Tehran.
Cell Orientation
Cell orientation was analyzed using circular statistics.5 Such a technique is recently gaining much interest for analyzing periodic variables including angle distribution. Rayleigh test is particularly suited for detecting a unimodal deviation from uniformity and uses the mean vector length (resultant vector) as a statistical index which indicates moderate concentration about the mean direction. The mean vector length is used in a range of 0 (in case of multiple random cell directions) to 1 (indicating a perfect anisotropy i.e., the existence of a preferred-aligned-cell direction).55 The higher is the value of resultant vector length quantity the more probable is the existence of a preferred cell alignment rather than chaotic state in cell direction. The cut-off between an aligned and a random cell growth direction distribution has been fixed below Rayleigh test p value (p = 0.05) for aligned cell direction distribution.4
In this study, cell direction angles from light microscopic photographs were calculated and analyzed using Microstructure Measurement software and Rayleigh test. The results then were used to verify the hypothesis of uniform distribution of cell directions in the SMF exposed cultures, or the existence of null hypothesis and multiple random cell directions. Three independent experiments for 18 mT exposures were performed and the number of resultant vectors inserted was at least 50 for each image. Then the p value was calculated for each sample and compared for the treated and control non-exposed cell culture photographs.6,55
Viability Measurements
The samples were exposed to Static Magnetic Field and went through various cultivation periods of time. Afterwards, the cell viability and the counts were measured instantly by Trypan Blue staining methods.48 An aliquot of cell suspension was diluted to 1:1 with 0.4% trypan blue and the cells were studied with a light microscope (Motic AE31) equipped with Micro-scan (DCM510, 5 M pixcel CMOS Chip) to measure the count and viability percentage. The provided photographs were then compared to evaluate the morphological and cell orientation changes.
Cell Cycle Progression Analysis
Flow cytometric assay was performed by Flow Cytometer (Facscalibur) to investigate cell cycle progression in HMSCs. Control samples which were not exposed to magnetic field, and the treated ones after having spent various culture times, were harvested and centrifuged at 1300 rpm for 5 min at the room temperature. The samples were washed with the culture medium and centrifuged again and the supernatant was decanted to provide pellet containing HMSCs. Samples were permeabilized by overnight incubation in 4.5 mL cold 70% ethanol at 4 °C. Then they were stained with PI (propidium iodide) 5 mg mL−1 and studied using Flow Cytometer (Facscalibur) in FL3 channel for PI detection to determine the cell-cycle progression rate.37 Flow cytometry data were analyzed using Win MDI 2.9 software to determine the distribution of cell population in each cell growth phase.41 After data analysis the cell count percentages of G0/G1, S, G2, and M phases were determined.
G0 is a resting phase where the cell has left the cycle and stopped dividing. In G1 phase (or Gap1) Cells increase in size and the G1 checkpoint control mechanism provides that everything is ready for DNA synthesis. DNA replication occurs in the S phase and the gap between DNA synthesis and mitosis is named G2 (Gap2) where the cells continue to grow. The G2 checkpoint control mechanism ensures that everything is ready to enter the M (mitosis) phase and divide.15 Most normal and healthy cells were observed in the G0/G1 phase. Most of apoptotic cells can be detected as a population of pre-G0/G1 at the left of the G0/G1 peak. Development through the cell-cycle for treated samples and control ones was assessed after every culture time by determining the cell population distributed in cell cycle phases (preG1, G0/G1, S, and G2/M).
Population Doubling Time (PDT) Measurement
Population doubling time (PDT) is a scale which represents cell proliferation speed.7 Long doubling time period shows low proliferation ability and the best passage number in which HMSCs were sharp and viable has been selected by calculating this value. PDT was measured for the first to fifth passages using the 2.1 Formula to select are more sensitive.
C t is the culture time, N 0 the primary cell count, and N the final cell count.
RT-PCR
In the next step the control and SMF treated samples which were cultured for three weeks, were analyzed for gene expression using reverse transcriptase polymerase chain reaction (RT-PCR) procedure. RNA extraction and cDNA synthesis were performed using (Qiagene Rneasy mini kit #7104) and (Fermentas Revert Aid First Strand cDNA Synthesis Kit #1622).
Gene expression analysis for Sox-2, Nanong, and Oct4 genes was performed and the PCR products were assessed after agarose Gel Electrophoresis. The primer sequences are shown in Table 1.
Statistical Analysis
Data analysis was performed using GraphPad Prism5 Software for Analysis of variance (ANOVA)11 which determines the significant changes. Flow cytometric data was analyzed using WinMDI 2.9 software which provides a functional tool for this purpose. It is noteworthy that the coefficient of variation (CV) represented in the statistic table of each analysis was less than 8 for the phase regions. Circular statistics (Rayleigh test) was performed to evaluate cell direction uniformity and the p value (p < 0.05) was fixed for aligned cell direction distribution.55
Results
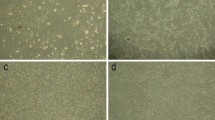
Human Mesenchymal Stem Cells Purification
Cultivation in low glucose medium gradually favored mesenchymal stem cells over hematopoietic and other cell types. In the third passage other cells namely macrophages and hematopoietic ones were mostly excluded and HMSCs remained which are illustrated in Fig. 3.
Characterization of Mesenchymal Stem Cells
Mesenchymal stem cells are reported to have immune-suppressive effects and fail to induce proliferation of allogeneic lymphocytes.16 They express surface antigens which have been considered as their characteristics.52 Data analysis was performed using WinMDI2.9 software. Results showed a negligible contamination of hematopoietic cells in the umbilical cord-derived cells at the selected passage. The cells represented mesenchymal stem cell markers including CD44, CD73, CD90, and CD105 versus the rest of markers (CD34, CD45, CD133, and HLA-DR) indicating the absence of hematopoietic pollution as illustrated in (Figs. 4a and 4b). The lack expression of HLA-DR surface molecules was also evident in the flow cytometry data.
In the next step the differentiation ability of umbilical cord-derived cells into the osteogenic, adipogenic, and chondrogenic tissues was assessed and the results showed that the cells could be differentiated into all of the mentioned cell types after three weeks in the favorable culture medium conditions (Fig. 5).
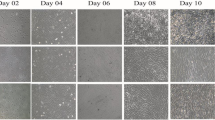
The SMF Effect on the Cell Orientation
Previous investigations have shown that after 60 h of exposure to the SMF, the cultured mouse osteoblasts were transformed to rod-like shapes and were orientated in the direction parallel to the magnetic field.30
The morphological examination of HMSCs revealed that exposure to magnetic field did not cause any significant changes in cell morphology, however it was observed that the exposed cells’ orientation was different from the control ones.
The treated samples were more oriented compared to the control samples and the cell growth direction was also correlated with the magnetic field in the treated samples (Fig. 6).
Analysis of directions is a branch of mathematical statistics which provides methods for inferential statistical analysis of circular data.5 Circular statistics has been used to compute the average heading direction, assert the prevalence of a common heading direction for circular data in many investigations.
There are multiple tests for this problem namely Rayleigh test which asks how large the resultant vector length (R) must be to indicate a non-uniform distribution that share a common null hypothesis as described in “Materials and Methods” section.6 We used this approach to compare the average heading directions of the treated and control groups and reveal whether significant changes have occurred. Light microscopic images were analyzed and the quantity of resultant vector of each group was compared using Rayleigh test.55
The p value computed for the control non-exposed samples was 0.322 which indicates that the orientation was arbitrary in the control samples, whereas the value for the SMF treated samples was measured 0.02 (less than 0.05) which proves that cell growth had already been oriented and according to data analysis a preferred cell alignment in the SMF treated samples was evident.
This is in support of previous studies which indicate that moderate intensity static magnetic fields affect cell shape and culture direction.50
SMF Effect on the Proliferation Rate
Proliferation rate estimation showed the optimum passage number equal to 3 for the experiments because of the minimum proliferation doubling time (PDT) value (Fig. 7a). This is due to the improved proliferation ability of these cells rather than the other passage numbers. Afterwards the samples were treated and compared with the control ones and the results showed a significant reduced proliferation rate, so exposure to 18 mT SMF caused a longer PDT compared to the control samples (Fig. 7b). The cell population in G1 phase significantly (p < 0.01) dropped after 36 h after the SMF exposure in the treated samples. The recovery of G1 phase population in the 72-h post exposure cultured samples was significant (p < 0.01) in comparison to the 36-h cultured samples according to one-way ANOVA Tukey analysis11 as illustrated in (Fig. 7b).
SMF Effect on the Viability Percentage
The cell cultures were initially exposed to 12, 18, and 24 mT SMF and then viability percentage of each sample was measured according to the well-established procedure.48 All of the exposed and control samples were then incubated for 24, 36, 48, 60, and 72 h for recovery. Although the decrease in viability percentage wasn’t significant right after 24 h of exposure but the viable cell rates were dramatically decreased after 36 h from the termination of the exposure time. However, 72 h after the exposure the viability was significantly recovered.
The modulation of viability percentages was confirmed using one-way ANOVA Tukey’s multiple comparison tests (Fig. 8). It has been suggested that various exposure system conditions always complicate the evaluation of studies. In this study we found that there was no significant relation between the magnetic field moderate intensity (12, 18, and 24 mT) exposure of mesenchymal stem cell and viability percentage according to our data and 2-way ANOVA statistical tests.
In all of the mentioned intensities significant viability drops occurred 36 h after the exposure which was leveled off in the 72 h cultured samples after exposure to moderate static magnetic field. The change was dramatic for the 18 mT exposure intensity (p < 0.01) so the 18 mT static magnetic field was used for the next steps of experience.
SMF Effect on the Cell Cycle Progression
Cell cycle progression was assessed to investigate the DNA fragmentation in the exposed and control samples.16 When the cells were permeabilized, by 70% ethanol, DNA molecules leaked out of them and the result was a profile of DNA molecules which were stained by PI and analyzed by Flow Cytometer. Data analysis which was performed using Win MDI 2.9 software revealed the DNA length profile which was proportional to each cell population of cell cycle phases.41 Data analysis showed the presence of the following populations in the following cell growth phases: pre-G1, G0/G1, S, and G2/M (Fig. 9).
RT-PCR
RT-PCR product agarose gel-electrophoresis was performed to determine the expression of Oct-4, Nanong, and Sox-2 genes which are stemness markers and mainly expressed in undifferentiated cells. According to RT-PCR results, the treated samples exhibited low expression of these genes due to the 18 mT static magnetic field exposure. Lower Oct-4 expression in the SMF exposed HMSCs compared with the control ones indicated more differentiation in the treated samples59 (Fig. 10).
Oct-4 PCR products agarose gel-electrophoresis photograph. 1: Ladder100, 2: GAPDH (control+), 3: HMSCs after 3 weeks, 4: the SMF treated samples after 3 weeks, 5: the SMF treated samples after 2 weeks, 6: control non-exposed sample, 7: SMF treated sample after the first week, 8: control− (without oct-4 primer).
Longer PDT and significant proliferation rate decrease in our experiments accompanied by the Oct-4, Sox-2, and Nanong genes expression decline suggest improved differentiation of 18 mT SMF exposed HMSC cultures (Fig. 11).
Sox-2 and Nanong PCR products agarose gel-electrophoresis photograph. Sox-2: (1: treated sample after 1 week, 2: treated sample after 2 weeks, 3: treated sample after 3 weeks, 4: control unexposed sample after 2 weeks, 5: control sample after 3 weeks, 6: treated sample after 4 weeks, 7: control−) 8: Ladder, Nanong: (9: treated sample after 1 week, 10: control unexposed sample after 1 week, 11: control sample after 2 weeks, 12: GAPDH (control+), 13: control sample after 3 weeks, 14: treated sample after 2 weeks, 15: treated sample after 3 weeks, 16: control−).
Discussion
In this study we have successfully isolated HMSCs through a non-invasive procedure and made sure that they didn’t contain contaminating hematopoietic stem cells and their progenitors using flow cytometric assessment. This was confirmed by our Immunofluorescence experimental data which represented the presence of mesenchymal markers (CD105, CD90, CD73, and CD44) against lack expression of CD45, CD34, CD133 hematopoietic surface antigens in the cultures52 (Fig. 4). Our experiments have also shown that the cord-derived HMSCs were devoid of Human leukocyte antigen (HLA)-DR which originally is defined as antigen responsible for rejection of tissue transplants in HLA-mismatched donors. Poor immunogenicity and broad differentiation ability of MSCs favor the use of this type of stem cells for successful grafting and therapeutic uses since differentiated pluripotent stem cells provide new tools for tissue engineering and regenerative medicine.17,24
Expression of mesenchymal markers and differentiation ability of the cells confirmed that they are human mesenchymal stem cells, so this cell type was chosen as a target for investigating SMF possible effects in the present study. Furthermore, differentiation potential of human cord-derived mesenchymal stem cells which has also been experienced (Fig. 5) favored them for stem cell therapy23,39 hence they were chosen in this study.
Previous studies suggested that SMF affects living cells in a magnetic intensity, time of exposure and cell type-dependent manner, and cell viability can be influenced by some of the magnetic exposures. Buemi et al. examined the effects of a 0.5 mT SMF on the cell proliferation/cell death balance in renal cells and cortical astrocyte cultures from rats. They observed a gradual decrease in proliferation rate due to a magnetic field exposure.10
A further area of interest is to evaluate whether exposure to static magnetic field affects cell-growth cycle distribution and cause micronucleus formation in the exposed cells. The presence or absence of such micronuclei represents whether a particular treatment damages cellular DNA.19 Most but not all of studies have shown that a static magnetic field alone does not have a detrimental effect on the basic properties of cell growth and survival under normal culture conditions.35
However, the frequency of micronucleus formation leading to cell death significantly increases when certain treatments (e.g., X-irradiation45 or nanoparticles19) are given prior to the exposure. Ahmadianpour et al. demonstrated apoptosis induction initiated 36 h after the end of exposure to 6 mT SMF and was accompanied with an oxidative stress in Jurcat cell line. A temporary G2 arrest and the increase in cellular population of S and G2 phases were also evident after this treatment according to flow cytometry data analysis.1
Our results confirmed the finding and demonstrated some effects on the cell viability percentage (Fig. 8) and cell-cycle progression in the HMSCs (Fig. 9). Furthermore, we have shown that exposure to SMF leads to late consequences including a viability percentage drop which could be reversed after the recovery time. In the next step cell-cycle progression data analysis confirmed this finding and a temporary decline in G1 phase population was noticed. Viability and cell cycle G1 phase population percentages dropped after completion of 36 h SMF exposure time. The viability percentage decrease accompanied with DNA fragmentation (where this occurred) has been known as one of the apoptosis characteristics.1
We speculate that apoptosis might have been induced in a fraction of the cell sample population and led to a reduction in viability percentage; in contrast the remainder which were subject to more trivial changes would restore and regenerate, therefore after 3 days of cultivation it resulted in an increase in the cell viability percentage and the G1 population recovery (Figs. 8 and 9). This phenomenon which was neglected in the previous studies may be attributed to the cellular repair system and the powerful regenerative ability of human mesenchymal stem cells.
An explanation for the obtained results of variation in viability percentage and cell cycle progression in the SMF treated cells might be related to the variation in reactive oxygen spices (ROS) concentration after the exposure. According to the previous studies SMF exposure up to 30 mT can cause increased activity of superoxide dismutase (SOD) and decreased activity of catalase and ascorbate peroxidase which consequently result in free radical accumulation.43
Aerobic metabolism induces ROS which are diverse and abundant in biological systems, and are capable of inducing oxidative stress and apoptosis. While excessive ROS production clearly damages DNA, low levels of ROS affect cell signaling particularly at the level of redox modulation and result in calcium ion concentration changes. Therefore, ROS dosage is a critical parameter in determining the ultimate cellular response.58
The mechanisms of ROS-induced modifications in ion transport pathways involve:
-
1.
oxidation of sulfhydryl groups located in the ion transport proteins which can alter cytosolic calcium concentration, which in turn, proceeds cellular differentiation,
-
2.
peroxidation of membrane phospholipids,
-
3.
inhibition of membrane-bound regulatory enzymes, and 4-modification of oxidative phosphorylation.58
Essential decisions that every cell is constantly challenged to make in multi-cellular organisms to ensure proper development are; Maintain stemness, proliferate, differentiate, and die. Many internal and external factors can affect cell fate such as chemicals or environmental conditions.
In particular, assessment of ROS generation provides an accurate picture of cell fate. A sequential dysregulation of mitochondrial function that precedes cell shrinkage and nuclear fragmentation, leads to programmed cell death (PCD) after ROS concentration modulation due to some physical or chemical treatments. However, some of the low energy SMF effects on cell growth which seem negligible might be compensated by cellular repair system.58
Furthermore, one important event during the MFs exposure is the modification in Ca2+ influx. There are many ions and charged groups in the trance-membrane proteins which are crucial in cell fate and susceptible to the external magnetic field. This seems to be a crucial event in the exposed cells, since Ca2+ can contribute to many cell functions. For example, modified Ca2+ homeostasis affects cell growth and differentiation.44 while some of the available studies suggest that the mechanism of organization and breakdown of different cytoskeleton elements depends on altered phosphorylation/dephosphorylation state of proteins in the exposed cells.13,22
The specific ion conduction properties of microfilaments combined with their proposed role in Ca (2+) signaling make microvilli the most likely cellular site for the interaction with external electric and magnetic fields.31 The mechanism suggested to explain these effects is based on the diamagnetic anisotropic properties of membrane phospholipids.
SMF interacting with the protein domains may induce a movement of condensed counter-ion clouds along the filaments axis. It is proposed that reorientation of the molecules during moderate SMF exposure results in the deformation of membrane embedded proteins including ion channels, thereby altering their activation kinetics.42
SMF exposure promotes movements of Ca2+, cell proliferation, and the eventual production of proinflammatory cytokines in human peripheral blood mononuclear cells (PBMC) as well as in Jurcat cells2 since modified Ca2+ homeostasis affects cell growth.42,44
Other signaling pathways are also investigated to respond SMF exposure of these, was detailed biochemical validation performed for the network linked to the inflammatory cytokine IL-6. Wang et al. showed that SMF affects membrane gangliosides as well as proteins which modulates calcium ion concenration in cytosol and leads to pre-oligodendrocyte differentiation.53
The question is how the applied magnetic fields affect cellular elements. One explanation for SMF effects on living cells might be the interaction between their charged components and the applied external field. The Lorentz force is the combination of electric and magnetic force on a point charge due to the magnetic fields exposure.
The force F exerted on a charged particle q moving with velocity v through an electric E and magnetic B fields can be calculated using Lorentz low.21 At the cellular level current of electrons or ions cause a locally weak electric field which is influenced by the magnetic field according to Lorentz’s low. Therefore, the interaction between external magnetic and weak charged groups of biomolecules produces a new force. The movement of membrane proteins (such as ion channels) determines a rearrangement of their position on the cell surface and alters their physical and chemical properties including ion transfer.50 Such a physical stimulus which changes cellular Ca2+ concentration, consequently can improve cell differentiation ability of some chemical inducers therefore can be considered as a tool for cell therapy by extracting differentiated tissues from stem cells.
SMF exposures have been reported to promote the rearrangement of F-actin filaments which, in turn could be responsible for the cell shape and orientation modifications. Displacement of the counter-ion clouds produces ion transfer from one site of actin filaments to the other and, thereby, facilitates cation conductance along the whole filament.13,50 Cytoskeleton modification affects cell shape and this is supported by the observation that F-actin in the differentiated cells is highly expressed in the cytoplasmic protrusions12,31 According to the report of Bras, changes in the cell surface micro-morphology in the SMF exposed cells were closely related to the reorganization of tubulin as a cytoskeleton element.8 Hirose et al. suggested that the orientation of glioblastoma cells is due to rearrangement of microtubules under the influence of magnetically oriented collagen fiber.26 It has also been investigated that SMF promotes myogenic differentiation and cell alignment in the parallel bundles.14
The effect of SMF on the morphology of certain cell types has been the subject of some other research projects. Pacini et al. examined 0.2 T static magnetic field effect on human neuronal cell cultures. They demonstrated that magnetic fields up to 0.5 T can cause significant changes in human neuronal cells. The data was consistent with induction of cell remodeling and differentiation without genome damage.38
Our results also showed that the treated HMSc monolayers would be aligned in a correlation with the magnetic field power lines which was surprising because of their adherence to the bottom of culture dishes. It was observed that the cells were detached and suspended after a few hours of exposure time and then significantly aligned in a directed pattern.
This has been in support of the previous findings and suggested that exposure to SMF affects not only the cell size, shape, and orientation but also the cell membrane surface components and actin filaments and interacts with adhesion molecules responsible for cell geometry and focal contacts.50 Understanding of these interactions may also represent a step forward the comprehension of the micro domains action mechanism and of ion channel/cytoskeleton interactions due to the exposure.
It is clear that magnetic field can affect living cells by exerting forces on cellular components and moving charged groups such as ions according to their magnetic properties based on Lorentz’s law,21 so an appropriate choice of magnetic field can be expected to initiate changes that result in efficacious effects on stem cell fate.
According to our results, exposure to SMF in the moderate range of intensities up to 24 mT made cell cultures more oriented in a correlation with the lines representing the direction of applied magnetic field (Fig. 6) which confirms the previous findings. The significant cell proliferation rate (Fig. 7) decline, accompanied by the Oct-4, Nanong, and Sox-2 genes expression decrease suggest more-differentiated cell sub-population forming in the treated samples (Figs. 10 and 11). These results are in support of the previous studies and provide justification for the fact that SMFs can affect cell fate through some mechanisms which must be subject of future investigations. However, more experiments which include exposures to different times and various SMF flux densities are required to determine the mechanism and threshold values for the magnetic field biological effects.
Conclusion
Mesenchymal stem cells have the potential to differentiate into multiple connective tissues. They can be easily isolated from human umbilical cord without ethical issues and successfully expanded in vitro for the needed therapeutic applications in regenerative medicine. This study demonstrates that SMF exposure affects the cell-growth cycle progression, proliferation rate, alignment, and differentiation. Viability percentage and G1 cell-cycle phase population drop in the treated samples have been leveled off after the post-exposure culture recovery time. The reduced proliferation rate and Oct-4, Nanong, and Sox-2 genes’ expression suggest the differentiation inducing effects of SMF exposure.
Abbreviations
- MF:
-
Magnetic field
- SMF:
-
Static magnetic field
- mT:
-
MilliTesla
- MSCs:
-
Mesenchymal stem cells
- HMCs:
-
Human mesenchymal stem cells
- UCHMSCs:
-
Umbilical Cord derived Human Mesenchymal Stem Cells
References
Ahmadianpour, M. R., P. Abdolmaleki, S. J. Mowla, and S. Hosseinkhani. Static magnetic field of 6 mT induces apoptosis and alters cell cycle in p53 mutant Jurkat cells. Electromagn. Biol. Med. 32:9–19, 2013.
Aldinucci, C., J. B. Garcia, M. Palmi, G. Sgaragli, A. Benocci, A. Meini, F. Pessina, C. Rossi, C. Bonechi, and G. P. Pessina. The effect of strong static magnetic field on lymphocytes. Bioelectromagnetics 24:109–117, 2003.
Barry, F. P., and J. M. Murphy. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36:568–584, 2004.
Batschelet, E. Circular Statistics in Biology. London: Academic Press, 1981.
Batschelet, E. Animal migration, navigation, and homing. In: Second-Order Statistical Analysis of Directions: Proceedings in Life Sciences, edited by K. Schmidt-Koenig, and W. T. Keeton. Berlin: Springer, 1978, pp. 3–24.
Berens, P. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31:1–21, 2009.
Bernice, M. Martin Tissue Culture Techniques: An Introduction. Boston: Birkhauser, 1994.
Bras, W., G. P. Diakun, J. Diaz, G. Maret, H. Kramer, J. Bordas, and F. Medrano. The susceptibility of pure tubulin to high magnetic fields: a magnetic birefringence and X-ray fiber diffraction study. Biophys. J . 74:1509–1521, 1998.
Bruce, G., C. Howlett, and R. Huckstep. Effect of a static magnetic field on fracture healing in a rabbit radius: preliminary results. Clin. Orthop. Relat. Res. 222:300–306, 1987.
Buemi, M., D. Marino, G. Di Pasquale, F. Floccari, M. Senatore, C. Aloisi, F. Grasso, G. Mondio, P. Perillo, and N. Frisina. Cell proliferation/cell death balance in renal cell cultures after exposure to a static magnetic field. Nephron 87:269–273, 2001.
Casella, G. Statistical Design. New York: Springer, 2008.
Chionna, A., M. Dwikat, E. Panzarini, B. Tenuzzo, E. Carla, T. Verri, P. Pagliara, L. Abbro, and L. Dini. Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur. J. Histochem. 47:299–308, 2009.
Chionna, A., B. Tenuzzo, E. Panzarini, M. B. Dwikat, L. Abbro, and L. Dini. Time dependent modifications of Hep G2 cells during exposure to static magnetic fields. Bioelectromagnetics 26:275–286, 2005.
Coletti, D., L. Teodori, M. C. Albertini, M. Rocchi, A. Pristera, M. Fini, M. Molinaro, and S. Adamo. Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A 71:846–856, 2007.
Cooper, G. The eukaryotic cell cycle. In: The Cell: A Molecular Approach, 2nd ed. Sunderland, MA: Sinauer Associates, 2000.
Dazzi, F., and F. M. Marelli-Berg. Mesenchymal stem cells for graft-versus-host disease: close encounters with T cells. Eur. J. Immunol. 38:1479–1482, 2008.
Deans, R. J., and A. B. Moseley. Mesenchymal stem cells: biology and potential clinical uses. Exp. Hematol. 28:875–884, 2000.
Dini, L., and L. Abbro. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 36(3):195–217, 2005.
Dobson, J. Cancer therapy: death by magnetism. Nat. Mater. 11:1006–1008, 2012.
Donzelli, E., A. Salvadè, P. Mimo, M. Viganò, M. Morrone, R. Papagna, F. Carini, A. Zaopo, M. Miloso, and M. Baldoni. Mesenchymal stem cells cultured on a collagen scaffold: in vitro osteogenic differentiation. Arch. Oral Biol. 52:64–73, 2007.
Durrant, C., M. Hertzberg, and P. Kuchel. Magnetic susceptibility: further insights into macroscopic and microscopic fields and the sphere of Lorentz. Concepts Magn. Reson. Part A 18:72–95, 2003.
D’Ascenzo, M., R. Piacentini, P. Casalbore, M. Budoni, R. Pallini, G. B. Azzena, and C. Grassi. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur. J. Neurosci. 23(4):935–944, 2006.
Feldmann, R. E., K. Bieback, M. H. Maurer, A. Kalenka, H. F. Bürgers, B. Gross, C. Hunzinger, H. Klüter, W. Kuschinsky, and H. Eichler. Stem cell proteomes: a profile of human mesenchymal stem cells derived from umbilical cord blood. Electrophoresis 26:2749–2758, 2005.
Ghannam, S., C. Bouffi, F. Djouad, C. Jorgensen, and D. Noël. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res. Ther. 1:2, 2010.
Goodwin, H., A. Bicknese, S. Chien, B. Bogucki, D. Oliver, C. Quinn, and D. Wall. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol. Blood Marrow Transplant. 7:581–588, 2001.
Hirose, H., T. Nakahara, and J. Miyakoshi. Orientation of human glioblastoma cells embedded in type I collagen, caused by exposure to a 10 T static magnetic field. Neurosci. Lett. 338:88–90, 2003.
Hu-sheng, Z., Y. Xuan-dong, L. Zong-shan, Z. Fan-qing, L. Hong-qing, D. Ren-qing, and D. Bi. Inducing apoptosis of cancer cell and inhibiting mice’s malignant tumour growth by magnetic fields. Wuhan Univ. J. Nat. Sci. 4:363–366, 1999.
Jing, D., G. Shen, J. Cai, F. Li, J. Huang, Y. Wang, Q. Xu, C. Tang, and E. Luo. Effects of 180 mT static magnetic fields on diabetic wound healing in rats. Bioelectromagnetics 31:640–648, 2010.
Kern, S., H. Eichler, J. Stoeve, H. Klüter, and K. Bieback. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301, 2006.
Kotani, H., H. Kawaguchi, T. Shimoaka, M. Iwasaka, S. Ueno, H. Ozawa, K. Nakamura, and K. Hoshi. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 17:1814–1821, 2002.
Lange, K. Microvillar ion channels: cytoskeletal modulation of ion fluxes. J. Theor. Biol. 206(4):561–584, 2000.
Manzetti, S., and O. Johansson. Global electromagnetic toxicity and frequency-induced diseases: theory and short overview. Pathophysiology 19:185–191, 2012.
Metallo, C. M., S. M. Azarin, L. Ji, J. J. De Pablo, and S. P. Palecek. Engineering tissue from human embryonic stem cells. J. Cell Mol. Med. 12:709–729, 2008.
Minguell, J. J., A. Erices, and P. Conget. Mesenchymal stem cells. Exp. Biol. Med. 226:507–520, 2001.
Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 87:213–223, 2005.
Nakahara, T., H. Yaguchi, M. Yoshida, and J. Miyakoshi. Effects of exposure of CHO-K1 cells to a 10-T static magnetic field. Radiology 224:817–822, 2002.
Nicoletti, I., G. Migliorati, M. Pagliacci, F. Grignani, and C. Riccardi. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271–279, 1991.
Pacini, S., G. B. Vannelli, T. Barni, M. Ruggiero, I. Sardi, P. Pacini, and M. Gulisano. Effect of 0.2 T static magnetic field on human neurons: remodeling and inhibition of signal transduction without genome instability. Neurosci. Lett. 267:185–188, 1999.
Passier, R., and C. Mummery. Origin and use of embryonic and adult stem cells in differentiation and tissue repair. Cardiovasc. Res. 58:324–335, 2003.
Raylman, R. R., A. C. Clavo, and R. L. Wahl. Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics 17:358–363, 1996.
Riccardi, C., and I. Nicoletti. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 1:1458–1461, 2006.
Rosen, A. D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 39:163–173, 2003.
Sahebjamei, H., P. Abdolmaleki, and F. Ghanati. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 28:42–47, 2007.
Salido, G. Oxidative stress, intracellular calcium signals and apoptotic processes. In: Apoptosis: Involvement of Oxidative Stress and Intracellular Ca2+ Homeostasis, edited by G. M. Salido, and J. A. Rosado. Netherlands: Springer, 2009, pp. 1–16.
Sarvestani, A. S., P. Abdolmaleki, S. J. Mowla, F. Ghanati, E. Heshmati, Z. Tavasoli, and A. M. Jahromi. Static magnetic fields aggravate the effects of ionizing radiation on cell cycle progression in bone marrow stem cells. Micron 41:101–104, 2010.
Satija, N. K., V. K. Singh, Y. K. Verma, P. Gupta, S. Sharma, F. Afrin, M. Sharma, P. Sharma, R. Tripathi, and G. Gurudutta. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J. Cell Mol. Med. 13:4385–4402, 2009.
Siegel, G., R. Schäfer, and F. Dazzi. The immunosuppressive properties of mesenchymal stem cells. Transplantation 87:S45–S49, 2009.
Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 111:A3.B.1–A3.B.3, 2001.
Tavasoli, Z., P. Abdolmaleki, S. J. Mowla, F. Ghanati, and A. S. Sarvestani. Investigation of the effects of static magnetic field on apoptosis in bone marrow stem cells of rat. Environmentalist 29:220–224, 2009.
Teodori, L., M. C. Albertini, F. Uguccioni, E. Falcieri, M. B. Rocchi, M. Battistelli, C. Coluzza, G. Piantanida, A. Bergamaschi, and A. Magrini. Static magnetic fields affect cell size, shape, orientation, and membrane surface of human glioblastoma cells, as demonstrated by electron, optic, and atomic force microscopy. Cytometry Part A 69:75–85, 2006.
Uccelli, A., L. Moretta, and V. Pistoia. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8:726–736, 2008.
De Ugarte, D. A., Z. Alfonso, P. A. Zuk, A. Elbarbary, M. Zhu, P. Ashjian, P. Benhaim, M. H. Hedrick, and J. K. Fraser. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 89:267–270, 2003.
Wang, Z., A. Sarje, P.-L. Che, and K. J. Yarema. Moderate strength (0.23–0.28 T) static magnetic fields (SMF) modulate signaling and differentiation in human embryonic cells. BMC Genom. 10:356, 2009.
Wertheimer, N., and E. Leeper. Electrical wiring configurations and childhood cancer. Am. J. Epidemiol. 109:273–284, 1979.
Wilkie, D. Rayleigh test for randomness of circular data. Appl. Stat. 32(3):311, 1983.
Wiskirchen, J., E. F. Grönewäller, F. Heinzelmann, R. Kehlbach, E. Rodegerdts, M. Wittau, H. P. Rodemann, C. D. Claussen, and S. H. Duda. Human fetal lung fibroblasts. In vitro study of repetitive magnetic field exposure at 0.2, 1.0, and 1.5 T. Radiology 215:858–862, 2000.
Wolsko, P., D. Eisenberg, L. Simon, R. Davis, J. Walleczek, M. Mayo-Smith, T. Kaptchuk, and R. Phillips. Double-blind placebo-controlled trial of static magnets for the treatment of osteoarthritis of the knee: results of a pilot study. Altern. Ther. Health Med. 10:36–43, 2004.
Zamzami, N., P. Marchetti, M. Castedo, D. Decaudin, A. Macho, T. Hirsch, S. A. Susin, P. X. Petit, B. Mignotte, and G. Kroemer. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 182:367–377, 1995.
Zeineddine, D., A. A. Hammoud, M. Mortada, and H. Boeuf. The Oct4 protein: more than a magic stemness marker. Am. J. Stem Cells 3(2):74–82, 2014.
Acknowledgments
This study was supported by grants from the Tarbiat Modares University (TMU), Tehran, Iran and Iranian council of stem cell Technology Tehran, Iran. The experiments with the HMSCs extraction were performed at Royan Stem Cell Technology Company (Cord Blood Bank), Tehran, Iran since the author Dr. Abrun is a member of cord blood bank.
Conflict of interest
Author Parviz Abdolmaleki declares that he has no conflict of interest. Author Maryam Sadri declares that she has no conflict of interest. Author Saied Abrun declares that he has no conflict of interest. Author Baharah Beiki declares that she has no conflict of interest. Author Fazel Sahraneshin Samani declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors and does not contain any studies with human participants or animals performed by any of the authors. Human umbilical cord which is characterized as medical waste was used in this study with the parents’ consent letters were collected prior to participation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Roger D. Kamm oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Sadri, M., Abdolmaleki, P., Abrun, S. et al. Static Magnetic Field Effect on Cell Alignment, Growth, and Differentiation in Human Cord-Derived Mesenchymal Stem Cells. Cel. Mol. Bioeng. 10, 249–262 (2017). https://doi.org/10.1007/s12195-017-0482-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-017-0482-y