Abstract
The current research work focuses on evaluation of workability, compressive strength and microstructure of geopolymer concrete (GPc) made with fly ash (FA) and ground granulated blast furnace slag (GGBFS) and that made with fly ash and OPC. The FA-GGBFS, and FA-OPC based GPc were made by replacing FA with GGBFS and OPC, respectively, at 10% and 20% by the mass of total binder. For the preparation of GPc mixes, sodium silicate (SS) solution and sodium hydroxide (SH) solution were used with a mass ratio of 1.2, and NaOH solution was prepared at 6 M and 8 M concentrations. In this study, tests for workability, compressive strength at 7 days and X-ray diffraction (XRD) analysis were conducted on geopolymer composites. The results showed reduction in slump value with increase in the concentration of SH solution and with GGBFS and OPC content in the GPc mixes. Further, the FA-OPC based GPc showed lower slump value than FA-GGBFS geopolymer composites. The FA-OPC geopolymer composites mostly showed lower strength as compared to FA-GGBFS based GPc. From XRD analysis, anorthoclase and albite peak intensities were higher in the geopolymer composites made with FA-GGBFS than GPc with FA-OPC.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Geopolymer composites
- Fly ash
- Ground granulated blast furnace slag
- OPC
- Compressive strength
- X-Ray diffraction analysis
1 Introduction
Around the world, the most widely utilized construction material for infrastructure development is concrete [1]. From the last few decades, the utilization of concrete has been increased enormously due to the rapid growth in the urbanization. Therefore, the utilization of ordinary Portland cement (OPC) as a basic binding material in concrete has been increased. However, the manufacturing of OPC emits 5–6% of carbon dioxide into the environment [2]. So, sustainable binding materials are required to replace OPC either partially or completely in the preparation of concrete. In this context, waste materials generated from industries like fly ash (FA), rice husk ash, copper slag and GGBFS can be utilized as alternative binding materials in combination with alkaline activators to replace the OPC partially or fully while producing the concrete.
The combined reaction of industrial wastes rich in aluminosilicates along with alkaline activators is known as a geopolymerization [3]. The fly ash-based geopolymers reaction is very slow at ambient temperature, so an elevated curing temperature is required for better geopolymerization reaction [4]. Few studies examined ways to improve the reactivity of FA, such as reducing the particle size of fly ash or adding calcium-based compounds [5]. Besides the aluminosilicate-based geopolymer network, the addition of calcium oxide (CaO) leads in the evolution of calcium silicate hydrates [6]. The materials such as GGBFS, OPC, metakaolin, rice hush ash (RHA) and silica fume can be utilized as source materials along with fly ash for the preparation of GPc at ambient environment. There have been a few studies that compare GPc prepared with a mixture of FA and GGBFS, and FA and OPC. The current study examines the workability (slump value), early age compressive strength and XRD studies of geopolymer composites made with various proportions of FA-GGBFS and FA-OPC, as well as varied molarities of NaOH solution.
2 Experimental Program
2.1 Materials Utilized in Making of Geopolymer Composites
In the present research investigation, GGBFS or OPC was added with FA in the preparation of GPc mixes. OPC 43 grade cement confirming to ASTM C150/C150M-20 (Type I) [7] was used in the GPc mixes. The relative density of FA, GGBFS and OPC were 2.20, 2.67 and 3.10, respectively. The coarse aggregates utilized in preparation of GPc mixes were a combination of maximum size 25 mm and 12.5 mm having relative density of 2.64 and 2.66 respectively. Further, relative density of fine aggregate (river sand) was 2.70 and fineness modulus was 2.62. Alkaline solution utilized was a blend of sodium silicate (Na2SiO3) solution and sodium hydroxide (NaOH) solution.
2.2 Geopolymer Composite Proportions and Preparation
The proportions of geopolymer composites prepared with various replacement levels of GGBFS and OPC, and NaOH (SH) solution molarity are interpreted in Table 1. FA-GGBFS and FA-OPC based GPc were prepared by replacing fly ash with GGBFS and OPC, respectively, at 10% and 20% by mass of binder. The binder content and alkaline solution to binder content ratio were taken as 400 kg/m3 and 0.6, respectively. The coarse aggregate was utilized at the proportion of 58% (25 mm aggregate) and 42% (12.5 mm aggregate) by mass of coarse aggregate. The SH solution was made at 6 M and 8 M concentrations. The SS and SH solutions were mixed at a mass ratio (SS/SH) of 1.2 for preparing the GPc mixes.
Before 48 h of GPc mix preparation, the SH pellets were mixed in tap water for the making of SH solution. Before 24 h of GPc mix preparation, the SS solution (commercially available) was added with SH solution to make the alkaline solution. The process followed for preparation of GPc mixes is as follows: Firstly, all the aggregates were introduced into the drum mixer and mixed thoroughly. Then, the binders, i.e. FA and GGBFS, or FA and OPC were mixed along with the aggregates. Once the binders and aggregates were thoroughly mixed, the alkaline solution was added, and further mixed to obtain a fresh geopolymer composite. Slump test was carried out on the freshly prepared concrete mix. Thereafter, the fresh GPc was filled in cube moulds (size: 150 mm) in three layers with proper compaction of each layer on the vibration table. The cube moulds with the specimens were then stored for 24 h after casting in the laboratory. The cube mould samples were then demolded and let to ambient condition until the testing date.
2.3 Tests on Geopolymer Concrete (GPc)
2.3.1 Workability and Compressive Strength Test on GPc
The workability of fresh GPc mix was determined by conducting the slump test on fresh GPc mix. The 7 days strength test was done on concrete cube (size: 150 mm) specimens as per IS 516-2021 [8]. The mean value of 3 replicate 150 mm GPc cube samples was noted as the compressive strength value of a given geopolymer composite.
2.3.2 Microstructure Analysis
Following the completion of strength test, the broken cube pieces were grounded in a jaw crusher and the pulverized material was sieved through a sieve (with 75 micron meter square mesh). The passed geopolymer powder samples were collected in plastic zipper bags and stored in a desiccator. These powder samples were used for the microstructure study, i.e. through XRD analysis. The XRD analysis was performed on the GPc powder samples in Rigaku SmartLab X-ray diffractometer with a radiation of CuKα having a wavelength (λ) of 1.5405 Å. The GPc powder was scanned with in a range from 5° to 60° 2θ, with 0.03° 2θ as step size.
3 Results and Discussion
3.1 Workability of Geopolymer Composites
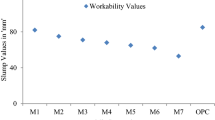
The slump test values of FA-GGBFS based and FA-OPC based geopolymer composites are depicted in Fig. 1. As noted from Fig. 1, among all the geopolymer composites, the highest slump value, i.e. 210 mm, was found in FA-GGBFS based GPc prepared with 10% GGBFS and 6 M NaOH solution. Similarly, FA-OPC (20%) based GPc with 8 M NaOH solution showed the lowest slump value of 145 mm. From Fig. 1, as the NaOH solution molarity was increased, the slump value was reduced. The viscosity of alkaline solution increased as NaOH solution molarity was increased [9], thereby reducing the slump of FA-GGBFS and FA-OPC based geopolymer composites.
Further, the slump value was reduced as the GGBFS or OPC content increased in geopolymer composites. This could be due to the presence of more angular-shaped particles of GGBFS, and OPC in the geopolymer composites [10, 11] that decreased the slump value of geopolymer composites. Further, irrespective of NaOH solution molarity and fly ash content, the FA-OPC based GPc showed a lower slump than FA-GGBFS based GPc as evident from Fig. 1. This is due to the influence of faster reactivity of OPC in the alkaline solution, which resulted in faster setting [12] of FA-OPC based GPc.
3.2 Compressive Strength of Geopolymer Composites
The 7 days strength of FA-GGBFS and FA-OPC geopolymer composites is presented in Fig. 2.
From Fig. 2, the strength (at 7 days age) of FA-GGBFS and FA-OPC based GPc mixes enhanced with increase in GGBFS and OPC content, respectively. The increase in GGBFS content led to more calcium content that resulted in the development of more amount of geopolymer gels leading to compact microstructure of FA-GGBFS based GPc mixes [10]. The higher OPC content led to more amount of calcium oxide (CaO) content in the GPc mixes, which reacted faster than other oxides in the presences of alkaline solution [12] and resulted in the development of higher amount of calcium-rich gels. From Fig. 2, as the concentration of NaOH (SH) solution increased, the strength of FA-GGBFS based and FA-OPC based GPc was increased. The higher SH solution molarity in FA-GGBFS and FA-OPC geopolymer composites resulted in dissolution of Si and Al from source materials to a comparatively larger extent, thereby resulting in improved polycondensation process in the mixes made with higher molarity of NaOH (SH) solution [13, 14]. From Fig. 2, it is inferred that the geopolymer composites made with FA and GGBFS mostly exhibited greater strength than GPc mixes made with FA and OPC, which could be due to the influence of comparatively greater extent of polymerization in FA-GGBFS geopolymer composites.
3.3 X-Ray Diffraction Analysis of GPc
Figures 3 and 4 represent the X-ray diffraction (XRD) plots of GPc mixes made with 6 M and 8 M SH solution, respectively. The obtained XRD plots of GPc showed the phases of muscovite (8.8° 2θ), albite (22.01° 2θ and 28.02° 2θ), anorthoclase (27.5° 2θ) and aragonite (CaCO3) (45.8° 2θ) in all the GPc mixes. Similarly, the crystalline phases of quartz (SiO2) and mullite (Al4.75Si1.25O9.63) were also identified in all mixes. Nepheline (NaAlSiO4) and C–S–H (calcium silicate hydrate) gel peaks were found at 27.1° 2θ and 29.5° 2θ, respectively, in XRD plots. The peaks corresponding to nepheline (NaAlSiO4) were observed in all 8 M NaOH solution-GPc mixes and in fly ash-OPC (20%) based GPc mix with NaOH solution of 6 M. Further, the peak related to calcium silicate hydrate gel was observed in OPC (10% and 20%) based GPc mixes, and GPc mix with 20% GGBFS, activated with 8 M SH solution.
From Figs. 3 and 4, with increase in GGBFS content and NaOH solution concentration, albite and nepheline peaks were increased in FA-GGBFS based GPc. This indicates that more GGBFS content led to more amount of calcium that resulted in greater extent of geopolymerization reaction, thereby showing higher peak intensity of albite. In addition, greater dissolution of Al and Si from source materials with increase in concentration of NaOH (SH) solution resulted in higher peak intensity of albite and nepheline in FA-GGBFS based GPc mixes. From Figs. 3 and 4, as OPC replacement and concentration of NaOH (SH) solution increased, the albite peak intensity was increased in FA-OPC geopolymer composites. This indicates that increase in calcium oxide content at higher OPC content enhanced the polymerization process thus showing higher peaks related to albite. In addition, in fly ash-OPC GPc mixes, the larger peak intensity of albite with increase in SH concentration led to larger dissolution of Si and Al from the precursor materials.
From Figs. 3 and 4, albite and anorthoclase peak intensities were higher in FA-GGBFS geopolymer composites than FA-OPC geopolymer composites. This is consistent with the variation in strength where FA-GGBFS mixes mostly showed higher strength than FA-OPC based GPc mixes (Fig. 2). Further, C–S–H gel peak was not identified in FA-GGBFS geopolymer composites with 10% GGBFS and 8 M SH solution. This might be the influencing factor for lower strength of FA-GGBFS (90%: 10%) based GPc mix than FA-OPC (90%: 10%) based GPc mix made with 8 M SH solution although the albite and anorthoclase peaks were higher in FA-GGBFS based GPc mix (90%: 10%) than FA-OPC based GPc mix (90%: 10%).
4 Conclusions
The outcomes of the current investigations were as follows:
-
The slump values of FA-GGBFS and FA-OPC based GPc mixes reduced as the GGBFS or OPC content, and NaOH solution concentration increased.
-
The slump values were greater in the geopolymer composites with GGBFS as compared to OPC, irrespective of GGBFS or OPC content, and NaOH solution concentration.
-
The compressive strength was increased in FA-GGBFS and FA-OPC based geopolymer composites with increase in the GGBFS and OPC replacement, respectively. Similarly, the strength was also increased with molarity of NaOH solution.
-
The FA-GGBFS geopolymer composites mostly exhibited greater strength as compared to FA-OPC geopolymer composites.
-
The XRD analysis of GPc mixes with GGBFS showed higher peak intensity of albite and nepheline with increase in GGBFS content and NaOH (SH) solution concentration. Similarly, peaks related to albite increased with OPC content and concentration of NaOH (SH) solution in FA-OPC geopolymer composites. The increase in intensity of peak related to albite with concentration of NaOH (SH) solution is consistent with the variation in strength of all geopolymer composites.
-
In comparison between FA-GGBFS based and FA-OPC based geopolymer composites, the intensity of peak related to albite and anorthoclase were greater in geopolymer composites with FA and GGBFS than geopolymer composites with FA and OPC. This corroborates the variation in strength where geopolymer composites with GGBFS mostly attained greater strength than geopolymer composites with OPC.
References
Luga, E., Atis, C.D.: Optimization of heat cured fly ash/slag blend geopolymer mortars designed by “Combined Design” method: Part 1. Constr. Build. Mater. 178, 393–404 (2018)
Arıoğlu Akan, M.O., Dhavale, D.G., Sarkis, J.: Greenhouse gas emissions in the construction industry: an analysis and evaluation of a concrete supply chain. J. Clean. Prod. 167, 1195–1207 (2017)
Almalkawi, A.T., Balchandra, A., Soroushian, P.: Potential of using industrial wastes for production of geopolymer binder as green construction materials. Constr. Build. Mater. 220, 516–524 (2019)
Somna, K., Jaturapitakkul, C., Kajitvichyanukul, P., Chindaprasirt, P.: NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 90(6), 2118–2124 (2011)
Changmai, M., Das, P. P., Mondal, P., Pasawan, M., Sinha, A., Biswas, P., ... & Purkait, M. K.: Hybrid electrocoagulation–microfiltration technique for treatment of nanofiltration rejected steel industry effluent. Int. J. Environ. Anal. Chem. 102, 62–83 (2022)
Samanta, N. S., Das, P. P., Mondal, P., Bora, U., & Purkait, M. K.: Physico-chemical and adsorption study of hydrothermally treated zeolite A and FAU-type zeolite X prepared from LD (Linz–Donawitz) slag of the steel industry. Int. J. Environ. Anal. Chem. 13, 1–23 (2022)
ASTM C150/C150M-20 (Type I), Standard specification for Portland cement. ASTM International, West Conshohocken, PA (2020)
IS 516: 2021 (Part 1/Sec 1), Method of tests for strength of concrete. Bureau of Indian Standard, Manak Bhavan, New Delhi (2021)
Chareerat, T., Chindaprasirt, P., Sirivivatnanon, V.: Workability and strength of coarse high calcium fly ash geopolymers. Cement Concr. Compos. 29, 224–229 (2007)
Deb, P.S., Nath, P., Sarker, P.K.: The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 62, 32–39 (2014)
Jindal, B.B.: Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: a review. Constr. Build. Mater. 227, 116644 (2019)
Amer, I., Kohail, M., El-Feky, M.S., Rashad, A., Khalaf, M.A.: Characterization of alkali-activated hybrid slag/cement concrete. Ain Shams Eng. J. 12(1), 135–144 (2021)
Chindaprasirt, P., Chalee, W.: Effect of sodium hydroxide concentration on chloride penetration and steel corrosion of fly ash-based geopolymer concrete under marine site. Constr. Build. Mater. 63, 303–310 (2014)
Mohapatra, A.K., Pradhan, B.: A study on early age properties of hybrid alkali activated mortars cured under ambient condition. Mater. Today: Proc. 65, 954–960 (2022)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Leela Sai Rangarao, M., Mohapatra, A.K., Pradhan, B. (2023). A Study on Workability, Strength and Microstructure of Geopolymer Composites Made with Sustainable Materials. In: Deka, D., Majumder, S.K., Purkait, M.K. (eds) Sustainable Environment. NERC 2022. Springer, Singapore. https://doi.org/10.1007/978-981-19-8464-8_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-8464-8_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8463-1
Online ISBN: 978-981-19-8464-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)