Abstract
Antimicrobial peptides (AMPs) are emerging as promising alternative to antibiotics, especially due to their activity against drug-resistant pathogenic strains. A vast number of AMPs have been characterized and purified from various natural sources, ranging from bacteria, fungus, plants, to higher vertebrates including mammals. Many of them have also been produced using either chemical synthesis or the recombinant technology. Both synthesized and expressed AMPs showed significant inhibitory activity against a number of economically significant pathogens. Majority of AMPs kill pathogens by disruption of cell membranes. Hence, chances of developing resistance by the microbes are less. Fish are reservoirs of these peptides, and they express majority of AMPs. However, application of AMPs in field condition is not at advanced stage, which may be due to issues like cost of production, stability, and toxicity to host cells. This can be addressed through artificial designing of short peptides to reduce the manufacturing cost and to enhance stability. AMPs not only kill microbes directly but also help in immunomodulation and may be highly useful for fish, which mainly depends on its innate immune system to fight against pathogens.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Natural defense systems are vital for survival of organisms in varying conditions and environments. In vertebrates, this system is composed of two subdivisions, the innate immune system and the adaptive immune system. The innate immune system acts quickly and non-specifically providing the first line of defense against invading pathogens. It is not specific to a particular antigen and has no immunologic memory. On the other hand, adaptive immunity is antigen specific and has memory, enabling the host to produce effective immune response on later exposure to the antigen (Marshall et al. 2018). In fish, innate immunity is the central mechanism of defense as their adaptive immune system has some constraints such as limited repertoire of antibodies, affinity maturation and memory, and slow proliferation of lymphocytes (Magnadóttir 2006).
Innate immunity acts through defense mechanisms, physical barriers such as the skin which prevents the entry of pathogens, cellular components (e.g., macrophages), and humoral responses (e.g., antimicrobial peptides), which may produce direct bactericidal effects (Magnadóttir 2006). Antimicrobial peptides (AMPs) are gene-encoded, ribosomally synthesized, host defense peptides carrying a net positive (cationic) and has molecular weight less than 10 kDa (de Zoysa et al. 2015; Raju et al. 2020). AMPs are mainly encountered at the portal of pathogen entry that encompass circulating myeloid cells and skin and mucosal and epithelial surfaces where they are usually stored in secretory granules, destined for extracellular secretion (Adem Bahar and Ren 2013). The main tissues or organs of fish that are at the high-risk targets of pathogens are epithelial cells of the skin, gills, gastrointestinal tract, gut, and respiratory organs. Hence, AMPs are typically localized in these tissues (Scocchi et al. 2016). AMPs are synthesized as pre-proproteins that include a signal sequence which undergoes hydrolytic degradation to form the active molecules (Raju et al. 2020). They are conserved effector molecules that play a key role in the first line of defense against invading pathogens prior to the activation of adaptive immunity. Although these peptides are enormously diverse in terms of their biosynthesis, sequences, and structures, they all share certain structural characteristic features like small size (<100 amino acids), cationic nature at physiological pH, amphipathicity, broad-spectrum antimicrobial activity, and similar mode of action (Sathyan et al. 2012; Chaithanya et al. 2013). The cationic nature of AMPs helps them in initial binding with the anionic bacterial surface, and their amphipathic nature enables them to ravage the bacterial membrane structure, ultimately killing the bacteria (Brogden 2005). In most organisms, AMPs are multifunctional molecules that are involved not only in attacking the pathogen but also in other important biological functions like immune modulation, wound healing, and cancer cell growth inhibition and/or as signaling molecules, for example, hepcidins, besides acting as antimicrobial, are also involved in iron regulations (Shi and Camus 2006; Tincu and Taylor 2004). The common functional characteristic of most AMPs is their induction, following the exposure of host to pathogen-associated molecular patterns (PAMPs) (Rončević et al. 2020). Their expression is either constitutive (within secretory cells) or upregulated during an infection, highlighting their role as antimicrobial. The induction pathways for AMP synthesis have been conserved evolutionarily in almost all organisms (Hancock and Diamond 2000; Acosta et al. 2019).
Increase in antibiotic-resistant bacteria and re-emergence of various infectious diseases have stimulated the exploration of these evolutionarily ancient components of the defense system as new therapeutic candidates to replace the conventional antibiotics and as cancer therapeutics. AMPs show exceptional specificity against prokaryotes, with low toxicity toward eukaryotic cells. Further, their rapid mode of killing mechanism gives very narrow escape for microbes to develop resistance (Chen and Lu 2020; Deslouches et al. 2015). They are highly effective even at micromolar concentrations and often exhibit synergistic effects with conventional antibiotics (Hancock and Diamond 2000; Yan and Hancock 2001). When many antibiotic treatments result in sepsis due to release of endotoxins from dead bacterial cells, AMPs bind to those endotoxins, reducing the septic shock (Bao et al. 2006). They have broad-range activity against Gram-positive and Gram-negative bacteria, fungi, some parasites, and enveloped viruses and on cancer cells. AMPs are one of the most promising alternatives to antibiotics because they are effective even against multidrug-resistant pathogens (Rima et al. 2021). Many researchers are also with the opinion that AMPs could offer the best promising solution to fight against microbial infection in aquaculture with no harmful effect on the environment (Chaturvedi et al. 2020; Valero et al. 2020). Interestingly, many AMPs, including those originated from fish, are being discovered and studied. Fish are considered as potential sources of AMPs, owing to their innate immunity being the major defense system. Here, we discuss the naturally occurring AMPs derived from fish, artificial designing of AMPs, and mechanism of action and highlight the possibility of their large-scale production for therapeutic applications.
17.2 History of Antimicrobial Peptides
AMP was discovered in 1939, when an antimicrobial agent named gramicidin, isolated from a soil bacterium, Bacillus brevis, protected mice from pneumococcal infection (Dubos 1939). Hirsch (1956) reported the first AMP of animal origin, defensin, isolated from leukocytes of rabbit. AMPs have also been isolated from plants, for example, purothionin, obtained from Triticum aestivum, is effective against fungi and some phytopathogenic bacteria (Balls et al. 1942; De Caleya et al. 1972). The field of AMP research expanded further with the contribution of Hans Boman, Michael Zasloff, and Robert Lehrer, who autonomously identified and purified insect cecropins, amphibian magainins, and mammalian defensins, respectively (Steiner et al. 1981; Zasloff 1987; Ganz and Lehrer 1994). Over the last few years, a number of unconventional AMPs have also been discovered that are proteolytically processed from larger and functionally different proteins usually following a microbial infection (Bulet et al. 2004; Reverter et al. 2018). Although a lot of vertebrate antimicrobial peptides were discovered by the mid-1980s, it took yet another decade to discover the antimicrobial activity of fish peptides. In 1980, a toxic peptide named pardaxin from Moses sole flatfish was characterized, but its antimicrobial activity was not observed until 1996 (Primor and Tu 1980; Oren and Shai 1996). Since then, a number of AMPs have been identified in fishes. AMP family characterized in fish includes piscidins which are homologous to cecropins, while other AMPs such as defensin, hepcidin, and cathelicidin have equivalent counterpart in vertebrates (Masso-Silva and Diamond 2014).
17.3 Fish Antimicrobial Peptide
As aquatic animals are rich source of AMPs, a myriad of them have been identified from these organisms. Many of these peptide families express in more than single species and cell type with different gene copy number in different fish species. AMPs have been isolated from mucus, skin surfaces, and mast cells of different aquatic organisms. The AMPs identified from fishes are reported to possess antibacterial, antiviral, antifungal, antiparasitic, and in some cases antitumor properties as well (Campagna et al. 2007; Falco et al. 2008; Chang et al. 2011). Most of the AMPs in fish are detected in early developmental stages with the highest expression level at post-fertilization for some and post-hatching for others, indicating the critical role of AMPs in protection against infections during embryonic development. Majority of the AMP mRNA transcripts expression are upregulated after bacterial infection, confirming their role in fish defense (Magnadóttir 2006; Milne et al. 2019). There are five major families of AMPs in fish and some peptides with no clear homology with any known bioactive peptides (Su 2011).
17.3.1 Piscidins
They are linear, amphipathic, α-helical antimicrobial peptides, widely distributed in various species of teleost fishes (Milne et al. 2019). Piscidin was first purified from the mast cells of hybrid striped bass (Morone chrysops (white bass) × Morone saxatilis (striped bass)) (Silphaduang et al. 2006). Their amino acid sequence length ranges from 18 to 46 residues, comprising a high proportion of basic amino acids (mainly histidine), phenylalanine, and isoleucine (Qiao et al. 2021). A broad-spectrum antibiotic activity of piscidins includes antibacterial, antifungal, antiviral, and antiparasitic properties (Colorni et al. 2008; Zahran and Noga 2010; Hu et al. 2019; Zheng et al. 2021). Members/paralogues, comprising piscidin family, are enlisted in Table 17.1.
In addition, there are also multiple structurally similar but functionally different piscidin isoforms identified in different fish species as well as within the same species. Among other piscidin isomers (i.e., Piscidin 1–7), Piscidin-1 shows the highest antibacterial activity, even against MRSA and has potential to permeabilize cancer cell membranes as well (Noga and Silphaduang 2003; Lin et al. 2012; Raju et al. 2020). LcP5L4, a piscidin-5 isoform isolated from Larimichthys crocea, showed antiparasitic activity against the parasite Cryptocaryon irritans (Zheng et al. 2021). Five piscidin isoforms have been identified from Nile tilapia Oreochromis niloticus (named TP1–5) (Peng et al. 2012) and six different piscidins from Dicentrarchus labrax that possess broad-spectrum antimicrobial activity (Barroso et al. 2020). A lot of studies have reported that piscidins exhibit strong activity against both fish and human bacterial pathogens, including some MDR-bacteria such as MRSA, vancomycin resistant Enterococci (VRE), etc. It interacts and disrupts the target cell membrane using toroidal pore mechanism (Falco et al. 2008).
17.3.2 Hepcidins
Hepcidin was first isolated from bacterially challenged hybrid striped bass, and since then, it has been screened in more than 40 teleost fish species (Shike et al. 2004). Mammalian, fish, and other predicted hepcidins share four to eight cysteine residues at conserved positions in N-terminal, which is crucial for both its optimal conformation and its antimicrobial activity (Hocquellet et al. 2012). The peptide has been detected in several tissues, and a high amount of hepcidin transcripts was rather found in acidophilic granulocytes of the spleen, heart, and stomach instead of the liver (Cuesta et al. 2008; Wang et al. 2009). Accumulating evidences have indicated that all fish hepcidin antimicrobial peptide (HAMP) isoforms can be categorized into two classes: HAMP1 and HAMP2. HAMP1 is more involved in iron regulation while HAMP2 in antimicrobial activity (Hilton and Lambert 2008; Mu et al. 2018; Neves et al. 2017). Hepcidin isolated from Salmo caspius (Caspian trout), having antimicrobial activity against Streptococcus iniae and Aeromonas hydrophila, belongs to HAMP2 class (Shirdel et al. 2019). Hepcidin from Epinephelus coioides shows rapid and potent inhibitory activity against S. aureus and P. stutzeri (Mohapatra et al. 2019). The teleost hepcidin is also found to be effective against protozoan parasitic infections caused by Trypanosoma carassii (Xie et al. 2019). Some hepcidins like PsHepcidin, from starry flounder Platichthys stellatus, show synergistic interaction with antibiotics and hence are used in combination with antibiotic therapy for the treatment of bacterial infections (Liu et al. 2018).
17.3.3 Defensins
Defensins are another cysteine-rich CAMP that are extensively distributed in nearly all life forms. Based upon the bonding pattern of conserved 6 cysteine residues to form intramolecular disulfide bonds, defensin family can be sub-categorized into α, β, and θ. Whilst α- and θ-defensins have only been found in mammals to date, the β-subfamily has a widespread distribution in all major vertebrate lineages from fish, amphibians, birds, reptiles, to mammals (Zou et al. 2007). In contrary to mammals, fish β-defensins are found to be encoded by three exons, producing a pro-peptide and a mature peptide with a signature motif of 6 conserved cysteine residues (Casadei et al. 2009). The peptide acts through interaction with bacterial membrane followed by insertion and permeabilization by generating multiple pores in the membrane (Chaturvedi et al. 2015). The peptide is reported to be an effective antimicrobial with activity against G+ and G- bacteria, fungi, protozoa, and enveloped viruses (Chang et al. 2011; Contreras et al. 2020; Cuesta et al. 2011). ScBD, a beta-defensin type 2, isolated from mandarin fish Siniperca chuatsi, effectively inhibited E. coli, S. aureus, and A. hydrophila (Wang et al. 2012).
17.3.4 Cathelicidins
Cathelicidins are another class of antimicrobials, produced as pre-propeptides, which include a signal sequence and a highly conserved cathelin-like domain and a variable C-terminus antimicrobial domain (Tomasinsig and Zanetti 2005). Cathelicidin was first isolated from Atlantic hagfish Myxine glutinosa, and first jawed-fish cathelicidin, rtCATH_1, was identified from rainbow trout (Sun et al. 2007; Chang et al. 2005). It has been reported that CATH possess antibacterial activity against fish pathogen V. anguillarum with no hemolytic activity and also has a role in immunomodulation of fishes (Bridle et al. 2011). Cathelicidin also acts against a number of Gram-positive and Gram-negative bacteria by permeabilizing their lipid membranes (Uzzell et al. 2003). Fish cathelicidins are enriched with Arg, Gly, and Ser, forming β-sheet and/or random coil and exhibit no cytotoxic activities (Jiang et al. 2018; Maier et al. 2008). Based on the secondary structure, fish cathelicidins are roughly divided into two groups: peptides forming a disulfide bond and peptides forming an extended structure (Chen et al. 2019). Like other AMPs, gene copy number of cathelicidins also varies species to species (Maier et al. 2008; Zhang et al. 2015a, b).
17.4 Mechanism of Action
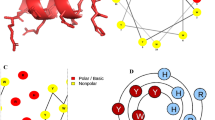
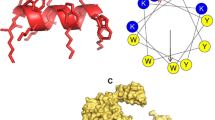
The mode of action of AMPs is one of the reasons for being popularly considered as a replacement over antibiotics. The secondary structure of AMPs, which is influenced by their microenvironment, is crucial for their antimicrobial activity. It has been reported that the α-helical structure is responsible for the salt-insensitivity of the peptide, as in the case of piscidins and some α-helical cathelicidins (Broekman et al. 2011). AMPs typically target the microbial cell membrane rather than a specific receptor. The cytoplasmic membrane of both Gram-positive and Gram-negative bacteria is rich in phospholipids with negatively charged head groups like phosphatidylglycerol, cardiolipin and phosphatidylserine. An additional electronegative charge is conferred on the outer leaflet due to the presence of teichoic acid in Gram-positive and LPS in Gram-negative bacterium (Gong et al. 2020). In contrast to bacteria, the outer leaflet phospholipid composition in higher vertebrates is predominantly of zwitterion molecules like phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin, while the negatively charged head groups, if present, are found mostly in the inner leaflet of the membrane facing the cytoplasm (Mahlapuu et al. 2016; Kumar et al. 2018). Thus, the positively charged AMP interacts with bacterial membrane selectively. After the initial interactions, the AMPs accumulate and then self-assemble on the bacterial membrane after reaching a certain concentration (Epand et al. 2016). AMPs thus embed themselves into the hydrophobic regions of the lipid membrane, thereby causing disintegration and permeabilization of the bacterial membrane, leading to leakage of cell contents and dissipation of transmembrane potential and/or pose multiple stress on the membrane proteins, ultimately leading to cell death (Bessin et al. 2004). There are three models explaining the action of AMPs on the target membranes: (1) carpet model, (2) barrel-stave model, and (3) toroidal-pore model.
The carpet model is a non-pore-forming type also known as the self-promoted uptake model. According to this technique, AMPs act by adsorbing parallel to the lipid bilayer of the target’s membrane till it reaches a threshold concentration to cover the entire membrane surface, thus, forming a “carpet.” Consequently, this unfavorable interaction leads to loss of membrane integrity eventually resulting in thinning of membrane bilayer, followed by disintegration of the membrane in the form of micelles, producing a detergent-like effect. The barrel-stave model is a trans-membrane pore type where the antimicrobials initially align parallel to the membrane and then insert in the bilayer in perpendicular manner, which facilitates lateral peptide-peptide interaction. The amphipathic structure of peptide is crucial for pore formation, where the hydrophobic region interacts with the membrane lipids and hydrophilic residues form the channel lumen. The toroidal-pore model is another trans-membrane pore formation type. In this, AMP insertion is similar to barrel-stave model; however, the specific peptide-bilayer interaction is absent. The aggregation of AMPs bends the membrane bilayer, and a pore is formed partly by the peptide and partly by phospholipid head groups. Some peptides are able to translocate to the cytoplasmic leaflet of the membrane and enter cytoplasm, thereby, targeting intracellular components (Epand et al. 2016; Dawood and Koshio 2016; Kumar et al. 2018).
17.5 Artificial Designing of AMPs
Many studies have reported the antimicrobial activity of natural AMPs. However, they are associated with several disadvantages such as cytotoxicity, low stability, and high cost of production (Hancock and Scott 2000). It is also reported that antimicrobial activity decreases with chain length and longer peptides are more cytotoxic (Dong et al. 2018). To overcome the problems associated with natural AMPs, many studies have been carried out to develop compositionally simple and short peptides through artificial designing (Hu et al. 2011; Kim et al. 2014; Qi et al. 2010). We have designed a short peptide of 12 residues using only 3 types of amino acids (RRWYRRWYRRWY). It was synthesized by solid-phase peptide synthesis using Fmoc chemistry. The peptide showed antimicrobial activity different fish bacteria such as Edwardsiella tarda, Aeromonas sobria, and Vibrio parahaemolyticus and is also potent against important oomycete, Saprolegnia parasitica. The peptide was effective even against gentamicin and methicillin-resistant Staphylococcus aureus. The peptide showed stability at higher temperature and even in the presence of serum (Hussain Bhat et al. 2020). The main advantage of artificial designing and chemical synthesis of AMPs is the possibility to modify to make the peptide more stable, potent, less toxic to host cells, and less costly in terms of production due to shorter length (Bagheri et al. 2016; Carotenuto et al. 2008; Grieco et al. 2013). In the similar line, another peptide of 16 residues was designed and synthesized (Bhat et al. 2022). The peptide also produced antimicrobial effect against various pathogens and could inhibit the growth of S. parasitica in embryonated fish eggs. The peptide has less cytotoxic and hemolytic activity and retained its activity even in the presence of serum and salt. Findings in our studies indicate that artificially designed and chemically synthesized AMPs may serve as an alternative to antibiotic for combating bacterial and fungal pathogen encountered in aquaculture.
17.6 Production of AMPs
Isolation of AMPs from natural sources is not feasible enough to meet the rising demands of basic research and clinical trials. Therefore, exploring alternative strategies for large-scale production of AMPs is important for downstream application. For production of AMPs, two approaches, viz., chemical synthesis and recombinant technology, have been reported. Though chemical synthesis might prove to be economical, it can be done for peptide having a length up to 40 residues. Hence, heterologous expression is preferred for synthesis of longer peptides (Meng et al. 2021). The recombinant technology for AMP production offers less complicated, environment-friendly, and cost-effective method. Among various reported microbial systems, E. coli is one of the most widely used host systems. However, it poses some limitations such as contamination of final product with bacterial LPS. Other popular expression hosts are yeast Pichia pastoris, and now insect cells have also come up as an attractive option for AMP expression (Karbalaei et al. 2020; Käßer et al. 2022). Insect cells are used for insect AMP expression since they might pose toxicity to bacterial or fungal host. P. pastoris has been used for the low-cost production of Tilapia piscidin-4, Ch-penaeidins, and Mytichitin-CB (Meng et al. 2021; Li et al. 2005; Neshani and Eidgahi 2018). E. coli was used for large-scale production of myticusin-beta, Vpdef, and was studied for their potential as antibiotics and immunomodulatory molecule (Oh et al. 2020; Zhang et al. 2015a, b). Sathyan et al. (2012) reported that some of the peptides, obtained through recombinant approach, showed reduced potency compared to their chemically synthesized counterparts.
Many of the naturally occurring AMPs have been chemically synthesized for use in research to elucidate their biological activity. These synthetic AMPs were found to be as effective as naturally occurring ones, and in some cases, they gave better performance. Synthetic Fi-His1–21 showed significant inhibition of V. vulnificus, P. aeruginosa, V. parahaemolyticus, V. cholerae, and S. aureus. It also showed DNA-binding activity and anti-cancerous activity (Sruthy et al. 2019). Synthetic BsHep elevated the expressions of immune-relevant genes in liver of Bostrychus sinensis and also improved its survival against V. parahaemolyticus infection (Shen et al. 2021).
17.7 Conclusion
A number of researches on AMPs are going on, leading to discovery of different peptides with antimicrobial property. With increase in comprehensive studies of AMPs at structural, functional, and genetic level, it is expected that soon AMPs will be commercialized as therapeutic replacement of antibiotics. In order to facilitate their commercial development, more attempts should be made on developing strategies to reduce the cost of production, to enhance stability, and to reduce toxicity to host cells. As isolation of AMPs from the natural source for application will be an expensive venture, heterologous expression may be considered. For development of short and effective AMPs, artificial designing and chemical synthesis may be advantageous.
References
Acosta J, Roa F, González-Chavarría I, Astuya A, Maura R, Montesino R, Muñoz C, Camacho F, Saavedra P, Valenzuela A, Sánchez O, Toledo JR (2019) In vitro immunomodulatory activities of peptides derived from Salmo salar NK-lysin and cathelicidin in fish cells. Fish Shellfish Immunol 88:587–594
Adem Bahar A, Ren D (2013) Antimicrobial peptides. Pharmaceuticals (Basel) 6:1543–1575
Bagheri M, Arasteh S, Haney EF, Hancock REW (2016) Tryptic stability of synthetic Bactenecin derivatives is determined by the side chain length of cationic residues and the peptide conformation. J Med Chem 59(7):3079–3086
Balls AK, Hale WS, Harris TH (1942) A crystalline protein obtained from a lipoprotein of wheat flour. Cereal Chem 19(19):279–288
Bao B, Peatman E, Xu P, Li P, Zeng H, He C, Liu Z (2006) The catfish liver-expressed antimicrobial peptide 2 (LEAP-2) gene is expressed in a wide range of tissues and developmentally regulated. Mol Immunol 43(4):367–377
Barroso C, Carvalho P, Carvalho C, Santarém N, Gonçalves JFM, Rodrigues PNS, Neves JV (2020) The diverse Piscidin repertoire of the European Sea bass (Dicentrarchus labrax): molecular characterization and antimicrobial activities. Int J Mol Sci 21(13):4613
Bessin Y, Saint N, Marri L, Marchini D, Molle G (2004) Antibacterial activity and pore-forming properties of ceratotoxins: a mechanism of action based on the barrel stave model. Biochim Biophys Acta 1667(2):148–156
Bhat RAH, Khangembam VC, Thakuria D, Pant V, Tandel RS, Tripathi G, S arma, D. (2022) Antimicrobial activity of an artificially designed peptide against fish pathogens. Microbiol Res 260:127039
Bridle A, Nosworthy E, Polinski M, Nowak B (2011) Evidence of an antimicrobial-immunomodulatory role of Atlantic Salmon Cathelicidins during infection with Yersinia ruckeri. PLoS One 6(8):e23417
Broekman DC, Zenz A, Gudmundsdottir BK, Lohner K, Maier VH, Gudmundsson GH (2011) Functional characterization of codCath, the mature cathelicidin antimicrobial peptide from Atlantic cod (Gadus morhua). Peptides 32(10):2044–2051
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Browne MJ, Feng CY, Booth V, Rise ML (2011) Characterization and expression studies of Gaduscidin-1 and Gaduscidin-2; paralogous antimicrobial peptide-like transcripts from Atlantic cod (Gadusmorhua). Dev Comp Immunol 35(3):399–408
Bulet P, Stöcklin R, Menin L (2004) Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev 198(1):169–184
Campagna S, Saint N, Molle G, Aumelas A (2007) Structure and mechanism of action of the antimicrobial peptide piscidin. Biochemistry 46(7):1771–1778
Carotenuto A, Malfi S, Saviello MR, Campiglia P, Gomez-Monterrey I, Mangoni ML, Gaddi LMH, Novellino E, Grieco P (2008) A different molecular mechanism underlying antimicrobial and hemolytic actions of temporins a and L. J Med Chem 51(8):2354–2362
Casadei E, Wang T, Zou J, González Vecino JL, Wadsworth S, Secombes CJ (2009) Characterization of three novel β-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss). Mol Immunol 46(16):3358–3366
Chaithanya ER, Philip R, Sathyan N, Kumar PRA, Greenwood M, Molenaar AJ, Waltzer L (2013) Molecular characterization and phylogenetic analysis of a histone-derived antimicrobial peptide Teleostin from the marine teleost fishes, Tachysurus jella and Cynoglossus semifasciatus. ISRN Mol Biol 2013:185807
Chang CI, Pleguezuelos O, Zhang YA, Zou J, Secombes CJ (2005) Identification of a novel cathelicidin gene in the rainbow trout, Oncorhynchus mykiss. Infect Immun 73(8):5053–5064
Chang WT, Pan CY, Rajanbabu V, Cheng CW, Chen JY (2011) Tilapia (Oreochromis mossambicus) antimicrobial peptide, hepcidin 1–5, shows antitumor activity in cancer cells. Peptides 32(2):342–352
Chaturvedi P, Dhanik M, Pande A (2015) Molecular characterization and in silico analysis of Defensin from Tor putitora (Hamilton). Probiotics Antimicrob Proteins 7(3):207–215
Chaturvedi P, Bhat RAH, Pande A (2020) Antimicrobial peptides of fish: innocuous alternatives to antibiotics. Rev Aquac 12(1):85–106
Chen CH, Lu TK (2020) Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 9(1):24
Chen C, Wang A, Zhang F, Zhang M, Yang H, Li J, Su P, Chen Y, Yu H, Wang Y (2019) The protective effect of fish-derived cathelicidins on bacterial infections in zebrafish, Danio rerio. Fish Shellfish Immunol 92:519–527
Cole AM, Weis P, Diamond G (1997) Isolation and characterization of Pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder *. J Biol Chem 272(18):12008–12013
Colorni A, Ullal A, Heinisch G, Noga EJ (2008) Activity of the antimicrobial polypeptide piscidin 2 against fish ectoparasites. J Fish Dis 31(6):423–432
Contreras G, Shirdel I, Braun MS, Wink M (2020) Defensins: transcriptional regulation and function beyond antimicrobial activity. Dev Comp Immunol 104:103556
Cuesta A, Meseguer J, Esteban MÁ (2008) The antimicrobial peptide hepcidin exerts an important role in the innate immunity against bacteria in the bony fish gilthead seabream. Mol Immunol 45(8):2333–2342
Cuesta A, Meseguer J, Esteban MÁ (2011) Molecular and functional characterization of the gilthead seabream β-defensin demonstrate its chemotactic and antimicrobial activity. Mol Immunol 48(12–13):1432–1438
Dawood MAO, Koshio S (2016) Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture 454:243–251
de Caleya RF, Gonzalez-Pascual B, García-Olmedo F, Carbonero P (1972) Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Microbiol 23(5):998–1000
de Zoysa GH, Cameron AJ, Hegde VV, Raghothama S, Sarojini V (2015) Antimicrobial peptides with potential for biofilm eradication: synthesis and structure activity relationship studies of battacin peptides. J Med Chem 58(2):625–639
Della Pelle G, Perà G, Belardinelli MC, Gerdol M, Felli M, Crognale S, Scapigliati G, Ceccacci F, Buonocore F, Porcelli F (2020) Trematocine, a novel antimicrobial peptide from the Antarctic fish Trematomus bernacchii: identification and biological activity. Antibiotics 9(2):66
Deslouches B, Steckbeck JD, Craigo JK, Doi Y, Burns JL, Montelaro RC (2015) Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob Agents Chemother 59(2):1329–1333
Dong N, Chou S, Li J, Xue C, Li X, Cheng B, Shan A, Xu L (2018) Short symmetric-end antimicrobial peptides centered on β-turn amino acids unit improve selectivity and stability. Front Microbiol 9:2832
Dubos RJ (1939) Studies on a bactericidal agent extracted from a soil bacillus: II. Protective effect of the bactericidal agent against experimental pneumococcus infections in mice. J Exp Med 70(1):11
Epand RM, Walker C, Epand RF, Magarvey NA (2016) Molecular mechanisms of membrane targeting antibiotics. Biochim Biophys Acta 1858(5):980–987
Falco A, Chico V, Marroquí L, Perez L, Coll JM, Estepa A (2008) Expression and antiviral activity of a β-defensin-like peptide identified in the rainbow trout (Oncorhynchus mykiss) EST sequences. Mol Immunol 45(3):757–765
Ganz T, Lehrer RI (1994) Defensins. Curr Opin Immunol 6(4):584–589
Gong H, Sani MA, Hu X, Fa K, Hart JW, Liao M, Hollowell P, Carter J, Clifton LA, Campana M, Li P, King SM, Webster JRP, Maestro A, Zhu S, Separovic F, Waigh TA, Xu H, McBain AJ, Lu JR (2020) How do self-assembling antimicrobial lipopeptides kill bacteria? ACS Appl Mater Interfaces 12(50):55675–55687
Grieco P, Carotenuto A, Auriemma L, Limatola A, di Maro S, Merlino F, Mangoni ML, Luca V, di Grazia A, Gatti S, Campiglia P, Gomez-Monterrey I, Novellino E, Catania A (2013) Novel α-MSH peptide analogues with broad spectrum antimicrobial activity. PLoS One 8(4):e61614
Hancock REW, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defenses. Trends Microbiol 8(9):402–410
Hancock REW, Scott MG (2000) The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A 97(16):8856–8861
Hilton KB, Lambert LA (2008) Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene 415(1–2):40–48. https://doi.org/10.1016/J.GENE.2008.02.016
Hirsch JG (1956) Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med 103(5):589
Hocquellet A, le Senechal C, Garbay B (2012) Importance of the disulfide bridges in the antibacterial activity of human hepcidin. Peptides 36(2):303–307
Hu J, Chen C, Zhang S, Zhao X, Xu H, Zhao X, Lu JR (2011) Designed antimicrobial and antitumor peptides with high selectivity. Biomacromolecules 12(11):3839–3843
Hu H, Guo N, Chen S, Guo X, Liu X, Ye S, Chai Q, Wang Y, Liu B, He Q (2019) Antiviral activity of Piscidin 1 against pseudorabies virus both in vitro and in vivo. Virol J 16(1):1–10
Hussain Bhat RA, Thakuria D, Pant V, Khangembam VC, Tandel RS, Shahi N, Sarma D, Tripathi G, Krishnani KK, Krishna G (2020) Antibacterial and antioomycete activities of a novel designed RY12WY peptide against fish pathogens. Microb Pathog 149:104591
Jiang H, Hu Y, Wei X, Xiao X, Jakovlić I, Liu X, Su J, Yuan G (2018) Chemotactic effect of β-defensin 1 on macrophages in Megalobrama amblycephala. Fish Shellfish Immunol 74:35–42
Karbalaei M, Rezaee SA, Farsiani H (2020) Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol 235(9):5867–5881
Käßer L, Rotter M, Coletta L, Salzig D, Czermak P (2022) Process intensification for the continuous production of an antimicrobial peptide in stably-transformed Sf-9 insect cells. Sci Rep 12(1):1–10
Kim H, Jang JH, Kim SC, Cho JH (2014) De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J Antimicrob Chemother 69(1):121–132
Ko SJ, Kang NH, Kim MK, Park J, Park E, Park GH, Kang TW, Na DE, Park JB, Yi YE, Jeon SH, Park Y (2019) Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus. Microb Pathog 127:70–78
Kumar P, Kizhakkedathu JN, Straus SK (2018) Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 8(1):4
Lauth X, Shike H, Burns JC, Westerman ME, Ostland VE, Carlberg JM, van Olst JC, Nizet V, Taylor SW, Shimizu C, Bulet P (2002) Discovery and characterization of two isoforms of Moronecidin, a novel antimicrobial peptide from hybrid striped bass *. J Biol Chem 277(7):5030–5039
Li L, Wang JX, Zhao XF, Kang CJ, Liu N, Xiang JH, Li FH, Sueda S, Kondo H (2005) High level expression, purification, and characterization of the shrimp antimicrobial peptide, Ch-penaeidin, in Pichia pastoris. Protein Expr Purif 39(2):144–151
Lin HJ, Huang TC, Muthusamy S, Lee JF, Duann YF, Lin CH (2012) Piscidin-1, an antimicrobial peptide from fish (hybrid striped bass Morone saxatilis x M. chrysops), induces apoptotic and necrotic activity in HT1080 cells. Zoolog Sci 29(5):327–332. https://doi.org/10.2108/Zsj.29.327
Liu ZM, Chen J, Lv YP, Hu ZH, Dai QM, Fan XL (2018) Molecular characterization of a hepcidin homologue in starry flounder (Platichthys stellatus) and its synergistic interaction with antibiotics. Fish Shellfish Immunol 83:45–51
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20(2):137–151
Mahlapuu M, Håkansson J, Ringstad L, Björn C (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Maier VH, Dorn KV, Gudmundsdottir BK, Gudmundsson GH (2008) Characterisation of cathelicidin gene family members in divergent fish species. Mol Immunol 45(14):3723–3730
Marshall JS, Warrington R, Watson W, Kim HL (2018) An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol 14(2):1–10
Mason AJ, Bertani P, Moulay G, Marquette A, Perrone B, Drake AF, Kichler A, Bechinger B (2007) Membrane interaction of chrysophsin-1, a histidine-rich antimicrobial peptide from red sea bream. Biochemistry 46(51):15175–15187
Masso-Silva JA, Diamond G (2014) Antimicrobial peptides from fish. Pharmaceuticals 7(3):265–310
Meng DM, Yang XM, Sun XQ, Cheng L, Fan ZC (2021) Application of antimicrobial peptide Mytichitin-A in pork preservation during refrigerated storage. J Food Process Preserv 45(5):e15404
Milne DJ, Fernández-Montero Á, Gundappa MK, Wang T, Acosta F, Torrecillas S, Montero D, Zou J, Sweetman J, Secombes CJ (2019) An insight into piscidins: the discovery, modulation and bioactivity of greater amberjack, Seriola dumerili, piscidin. Mol Immunol 114:378–388
Mohapatra A, Dixit A, Garg LC, Sahoo PK (2019) Hepcidin gene of Indian major carp, Labeo rohita: molecular, structural and functional characterization, and antibacterial activity of recombinant hepcidin. Aquaculture 511:734218
Mu Y, Huo J, Guan Y, Fan D, Xiao X, Wei J, Li Q, Mu P, Ao J, Chen X (2018) An improved genome assembly for Larimichthys crocea reveals hepcidin gene expansion with diversified regulation and function. Commun Biol 1(1):1–12
Neshani A, Eidgahi MA (2018) Extended-Spectrum antimicrobial activity of the low cost produced tilapia Piscidin 4 (TP4) marine antimicrobial peptide identification of pathogenic bacteria in blood cultures and susceptibility testing of isolates with various antibiotics view project making fc-fusion protein for immunisation and therapy view project. J Res Med Dent Sci 6(5):327–334
Neves JV, Ramos MF, Moreira AC, Silva T, Gomes MS, Rodrigues PNS (2017) Hamp1 but not Hamp2 regulates ferroportin in fish with two functionally distinct hepcidin types. Sci Rep 7(1):14793
Noga EJ, Silphaduang U (2003) Piscidins: a novel family of peptide antibiotics from fish. Drug News Perspect 16(2):87–92
Oh R, Lee MJ, Kim YO, Nam BH, Kong HJ, Kim JW, Park JY, Seo JK, Kim DG (2020) Myticusin-beta, antimicrobial peptide from the marine bivalve, Mytilus coruscus. Fish Shellfish Immunol 99:342–352
Olivieri C, Buonocore F, Picchietti S, Taddei AR, Bernini C, Scapigliati G, Dicke AA, Vostrikov VV, Veglia G, Porcelli F (2015) Structure and membrane interactions of chionodracine, a piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus. Biochim Biophys Acta 1848(6):1285–1293
Oren Z, Shai Y (1996) A class of highly potent antibacterial peptides derived from Pardaxin, a pore-forming peptide isolated from moses sole fish Pardachirus marmoratus. Eur J Biochem 237(1):303–310
Park CB, Lee JH, Park IY, Kim MS, Kim SC (1997) A novel antimicrobial peptide from the loach, Misgurnus anguillicaudatus. FEBS Lett 411(2–3):173–178
Peng K-C, Lee S-H, Hour A-L, Pan C-Y, Lee L-H, Chen J-Y (2012) Analysis of their expressions and biological functions. PLoS One 7(11):50263
Primor N, Tu AT (1980) Conformation of pardaxin, the toxin of the flatfish Pardachirus marmoratus. Biochim Biophys Acta 626(2):299–306
Qi X, Zhou C, Li P, Xu W, Cao Y, Ling H, Ning Chen W, Ming Li C, Xu R, Lamrani M, Mu Y, Leong SSJ, Wook Chang M, Chan-Park MB (2010) Novel short antibacterial and antifungal peptides with low cytotoxicity: efficacy and action mechanisms. Biochem Biophys Res Commun 398(3):594–600
Qiao Y, Ma X, Zhang M, Zhong S (2021) Cerocin, a novel piscidin-like antimicrobial peptide from black seabass, Centropristis striata. Fish Shellfish Immunol 110:86–90
Raju S v, Sarkar P, Kumar P, Arockiaraj J (2020) Piscidin, fish antimicrobial peptide: structure, classification, properties, mechanism, gene regulation and therapeutical importance. Int J Pept Res Ther 27(1):91–107
Reverter M, Tapissier-Bontemps N, Lecchini D, Banaigs B, Sasal P (2018) Biological and ecological roles of external fish mucus: a review. Fishes 3(4):41
Rima M, Rima M, Fajloun Z, Sabatier JM, Bechinger B, Naas T (2021) Antimicrobial peptides: a potent alternative to antibiotics. Antibiotics 10(9):1095
Rončević T, Čikeš-Čulić V, Maravić A, Capanni F, Gerdol M, Pacor S, Tossi A, Giulianini PG, Pallavicini A, Manfrin C (2020) Identification and functional characterization of the astacidin family of proline-rich host defence peptides (PcAst) from the red swamp crayfish (Procambarus clarkii, Girard 1852). Dev Comp Immunol 105:103574
Saitoh T, Seto Y, Fujikawa Y, Iijima N (2019) Distribution of three isoforms of antimicrobial peptide, chrysophsin-1, −2 and −3, in the red sea bream, Pagrus (Chrysophrys) major. Anal Biochem 566:13–15
Salerno G, Parrinello N, Roch P, Cammarata M (2007) cDNA sequence and tissue expression of an antimicrobial peptide, dicentracin; a new component of the moronecidin family isolated from head kidney leukocytes of sea bass, Dicentrarchus labrax. Comp Biochem Physiol B Biochem Mol Biol 146(4):521–529
Sathyan N, Philip R, Chaithanya ER, Anil Kumar PR, Antony SP (2012) Identification of a histone derived, putative antimicrobial peptide Himanturin from round whip ray Himantura pastinacoides and its phylogenetic significance. Res Immunol 2:120–124
Scocchi M, Furlan M, Venier P, Pallavicini A (2016) Cathelicidins: an ancient family of fish antimicrobial peptides. In: Lessons in immunity: from single-cell organisms to mammals. Academic Press, Cambridge, MA, pp 225–237
Shen B, Wei K, Yang J, Jing F, Zhang J (2021) Molecular characterization and functional analyses of a hepcidin gene from Bostrychus sinensis. Aquaculture 544:737114
Shi J, Camus AC (2006) Hepcidins in amphibians and fishes: antimicrobial peptides or iron-regulatory hormones? Dev Comp Immunol 30(9):746–755
Shike H, Shimizu C, Lauth X, Burns JC (2004) Organization and expression analysis of the zebrafish hepcidin gene, an antimicrobial peptide gene conserved among vertebrates. Dev Comp Immunol 28(7–8):747–754
Shirdel I, Kalbassi MR, Hosseinkhani S, Paknejad H, Wink M (2019) Cloning, characterization and tissue-specific expression of the antimicrobial peptide hepcidin from Caspian trout (Salmo caspius) and the antibacterial activity of the synthetic peptide. Fish Shellfish Immunol 90:288–296
Silphaduang U, Colorni A, Noga EJ (2006) Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis Aquat Organ 72(3):241–252
Sruthy KS, Nair A, Antony SP, Puthumana J, Singh ISB, Philip R (2019) A histone H2A derived antimicrobial peptide, fi-Histin from the Indian white shrimp, Fenneropenaeus indicus: molecular and functional characterization. Fish Shellfish Immunol 92:667–679
Steiner H, Hultmark D, Engström Å, Bennich H, Boman HG (1981) Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292(5820):246–248
Su Y (2011) Isolation and identification of pelteobagrin, a novel antimicrobial peptide from the skin mucus of yellow catfish (Pelteobagrus fulvidraco). Comp Biochem Physiol B Biochem Mol Biol 158(2):149–154
Sun BJ, Xie HX, Song Y, Nie P (2007) Gene structure of an antimicrobial peptide from mandarin fish, Siniperca chuatsi (Basilewsky), suggests that moronecidins and pleurocidins belong in one family: the piscidins. J Fish Dis 30:335–343
Subramanian S, Ross NW, MacKinnon SL (2009) Myxinidin, a novel antimicrobial peptide from the epidermal mucus of hagfish, Myxine glutinosa L. Marine Biotechnol 11(6):748–757
Tincu JA, Taylor SW (2004) Antimicrobial peptides from marine invertebrates. Antimicrob Agents Chemother 48(10):3645–3654
Tomasinsig L, Zanetti M (2005) The cathelicidins-structure, function and evolution. Curr Protein Pept Sci 6(1):23–34
Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M (2003) Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides 24(11):1655–1667
Valero Y, Saraiva-Fraga M, Costas B, Guardiola FA (2020) Antimicrobial peptides from fish: beyond the fight against pathogens. Rev Aquac 12(1):224–253
Villalobos-Delgado LH, Nevárez-Moorillon GV, Caro I, Quinto EJ, Mateo J (2019) Natural antimicrobial agents to improve foods shelf life. In: Galanakis CM (ed) Food quality and shelf life. Academic Press, pp 125–157
Wang KJ, Cai JJ, Cai L, Qu HD, Yang M, Zhang M (2009) Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides 30(4):638–646
Wang G, Li J, Zou P, Xie H, Huang B, Nie P, Chang M (2012) Expression pattern, promoter activity and bactericidal property of β-defensin from the mandarin fish Siniperca chuatsi. Fish Shellfish Immunol 33(3):522–531
Xie J, Obiefuna V, Hodgkinson JW, McAllister M, Belosevic M (2019) Teleost antimicrobial peptide hepcidin contributes to host defense of goldfish (Carassius auratus L.) against Trypanosoma carassii. Dev Comp Immunol 94:11–15
Yan H, Hancock REW (2001) Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob Agents Chemother 45(5):1558–1560
Yin ZX, He W, Chen WJ, Yan JH, Yang JN, Chan SM, He JG (2006) Cloning, expression and antimicrobial activity of an antimicrobial peptide, epinecidin-1, from the orange-spotted grouper, Epinephelus coioides. Aquaculture 253(1–4):204–211
Zahran E, Noga EJ (2010) Evidence for synergism of the antimicrobial peptide piscidin 2 with antiparasitic and antioomycete drugs. J Fish Dis 33(12):995–1003. https://doi.org/10.1111/J.1365-2761.2010.01205.X
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A 84(15):5449–5453
Zhang L, Yang D, Wang Q, Yuan Z, Wu H, Pei D, Cong M, Li F, Ji C, Zhao J (2015a) A defensin from clam Venerupis philippinarum: molecular characterization, localization, antibacterial activity, and mechanism of action. Dev Comp Immunol 51(1):29–38
Zhang X-J, Zhang X-Y, Zhang N, Guo X, Peng K-S, Wu H, Lu L-F, Wu N, Chen D-D, Li S, Nie P, Zhang Y-A (2015b) Distinctive structural hallmarks and biological activities of the multiple cathelicidin antimicrobial peptides in a primitive teleost fish. J Immunol 194(10):4974–4987
Zheng L, Qiu J, Liu H, Shi H, Chi C, Pan Y (2021) Molecular characterization and antiparasitic activity analysis of a novel piscidin 5-like type 4 from Larimichthys crocea. Mol Immunol 129:12–20
Zou J, Mercier C, Koussounadis A, Secombes C (2007) Discovery of multiple beta-defensin like homologues in teleost fish. Mol Immunol 44(4):638–647
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pant, V., Chanu, K.V., Thakuria, D. (2023). Antimicrobial Peptides: An Alternative to Antibiotics for Environment-Friendly Hill Aquaculture. In: Pandey, P.K., Pandey, N., Akhtar, M.S. (eds) Fisheries and Aquaculture of the Temperate Himalayas. Springer, Singapore. https://doi.org/10.1007/978-981-19-8303-0_17
Download citation
DOI: https://doi.org/10.1007/978-981-19-8303-0_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8302-3
Online ISBN: 978-981-19-8303-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)