Abstract
Abnormal changes in glycosylation have been positively correlated with tumorigenesis and progression in cancer. In particular, an elevation in the level of core fucosylation has been observed. Core fucosylation is driven by fucosyltransferase 8 (FUT8), which catalyzes transfer of the fucosyl moiety from GDP-fucose to the innermost GlcNAc residue of N-glycans via an α1,6-linkage. Core fucosylation is known to be critical for various biological processes including inflammation and immune response, tumor initiation, and metastasis as well as in central nervous system diseases. FUT8 and/or core-fucosylated glycoproteins serve as important diagnosis biomarkers that provide a set of specific targets for therapeutic intervention. In this review, we summarize the existing evidence for the influence of core fucosylation in regulating cell surface protein stability and functional expression, as well as cancer cell signal transduction pathways in pancreatic cancer (PC) and in other cancers. In addition, we also discuss the relevance between FUT8 and cancer stemness, which is another aspect of FUT8 that could be applied to tumor therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Glycosylation is a commonly occurring posttranslational modification that occurs under the catalysis of a variety of glycosyltransferases to attach glycans to proteins or other organic molecules (Fuster and Esko 2005; Ohtsubo and Marth 2006). In eukaryotic cells, according to sugar-amino acid linkages, glycosylation is classified into one of two major forms: N-glycosylation (sequon: Asn-X-Ser/Thr, X can be any amino acid except proline) and O-glycosylation (sequon: Ser/Thr) (Eichler 2019; Christiansen et al. 2014). Protein glycosylation is involved in various pathophysiological processes of tumor development and progression such as regulating tumor cell proliferation, invasion, metastasis, and angiogenesis (Ohtsubo and Marth 2006; Hakomori 2002; Chandler et al. 2019). Aberrant cancer-associated changes in protein glycosylation have been observed in cancers, including truncated O-glycans (Moreira et al. 2020), altered N-glycan branching (Kizuka and Taniguchi 2016), increased fucosylation (Miyoshi et al. 2008), and terminal sialylation (Vajaria et al. 2016), which serve as important tumor diagnostic biomarkers and/or provide a range of possible targets for therapeutic intervention.
Fucosylation is one of the most common modifications that take place when deoxyhexose fucose is transferred from GDP-fucose to glycoproteins or glycolipids under the catalysis of fucosyltransferases (FUTs) (Becker and Lowe 2003; Miyoshi et al. 2008). GDP-fucose is manufactured via de novo synthesis and salvage pathways (Fig. 1). In de novo synthesis, GDP-mannose is converted to GDP-fucose through enzymatic reactions catalyzed by GDP-mannose 4,6-dehydratase (GMD) and GDP-4-keto-6-deoxymannaose 3,5-epimerase-4-reductase (FX) (Tonetti et al. 1996; Niittymäki et al. 2006). In the salvage pathway, GDP-fucose is generated by the catalysis of fucose kinase and GDP-fucose pyrophosphorylase (Becker and Lowe 2003; Niittymäki et al. 2004). Subsequently, the GDP-fucose is transported into the Golgi complex via the membrane-anchored GDP-L-fucose transporter (Puglielli and Hirschberg 1999) where GDP-fucose serves as a donor substrate for 11 fucosyltransferases (FUT1-11) to transfer the fucose to proper N-glycan substrates (Hirschberg 2001) (Fig. 1). Alterations in the expression of FUTs are closely related to malignant transformations of cancer such as invasion and metastasis (Lu et al. 2020; Park et al. 2020; Tu et al. 2013). In particular, FUT8 is considered to be directly related to tumors; it is upregulated in various cancers and has shown involvement in tumor characteristics (Liu et al. 2011; Zhao et al. 2008; Hutchinson et al. 1991).
2 Biological Functions and Implications of FUT8
Fucosyltransferase 8 (FUT8) is the only enzyme responsible for catalyzing core fucosylation, which is responsible for the transfer of fucose from GDP-L-fucose to the innermost GlcNAc residue of N-glycans via α-1,6 linkage (Norton and Mehta 2019; Bastian et al. 2021). Many pivotal glycoproteins in mammalian tissues are core fucosylated, and core fucosylation is essential to the functions of these proteins: E-cadherin, integrin, epidermal growth factor (EGF) receptor, transforming growth factor (TGF)-β receptor, c-mesenchymal-epithelial transition factor (c-Met), and Fms-like tyrosine kinase 3 (FLT3) (Zhao et al. 2006; Liu et al. 2019; Wang et al. 2005, 2015b; Duan et al. 2020). A deficiency in FUT8 can lead to disorders in normal organisms, causing cancers and a series of other diseases. Disruption of the FUT8 gene in mice (FUT8−/− mice) is known to cause severe growth retardation, death during postnatal development, emphysema-like changes (Wang et al. 2005), and neuronal disorders (Fukuda et al. 2011).
Core fucosylation is widely observed in mammalian tissues and is particularly abundant in brain tissue (Uozumi et al. 1996; Shimizu et al. 1993; Miyoshi et al. 1997). FUT8−/− mice exhibit increased locomotion that is particularly obvious as strenuous hopping behavior in a novel environment, which is consistent with a schizophrenia-like phenotype that is significantly reduced by treatment with haloperidol, an antipsychotic drug (Fukuda et al. 2011). Research has shown that FUT8−/− mice exhibit decreased performance in working memory and impaired prepulse inhibition through dysregulation of AMPA receptor, which is an ionotropic glutamate receptor (Gu et al. 2015). In addition, a deficiency of FUT8 promotes neuroinflammation by increasing the sensitivity of glial cells to inflammatory mediators, suggesting that the disorders of FUT8−/− mice are caused not only by neurons but also by glial cell dysfunction (Lu et al. 2019).
FUT8 also participates in immune response. Systemic lupus erythematosus (SLE) is an autoimmune multisystem disease characterized by loss of tolerance towards nuclear antigens, and the activation of CD4+ T cells promotes the pathogenic process of SLE (Suárez-Fueyo et al. 2016; Abdirama et al. 2021). The recognition by T cell receptors (TCRs) of peptide-loaded major histocompatibility complex II (pMHC-II) on the B cell surface is important for the determination of CD4+ T cell activation (Krogsgaard et al. 2005). Recent research has shown that SLE patients exhibit hyper core fucosylation in CD4+ T cells, which is crucial for regulating TCR-pMHC-II interaction in CD4+ T cell activation (Liang et al. 2018; Sun et al. 2020). In addition, a lack of core fucosylation attenuates T-B cell communication via TCR-pMHC and suppresses IgG class switching by impairing CD4+ T cell activation (Liang et al. 2018).
The importance of core fucosylation has also been established in individuals with FUT8-congenital disorder of glycosylation (FUT8-CDG), because these individuals carry pathogenic variants of FUT8. Individuals with FUT8-CDG typically experience intrauterine growth retardation, severe delays in growth and development, significantly shortened limbs, neurological impairments, and respiratory complications (Ng et al. 2020, 2018). These characteristics are consistent with some aspects observed in FUT8−/− mice. Taken together, these data clearly show that FUT8 is significantly involved in many important biological functions.
3 High Expression of FUT8 in Cancer
Altered FUT8 expression has been linked to clinical features of tumors and to the outcomes in extensive studies. Increased expression and enzymatic activity of FUT8 has been observed in cancers such as breast, lung, liver, colorectal, prostate, melanoma, and pancreatic (Table 1). Core fucosylation affects receptor activation, modulates cancer cell signal transduction pathways, and promotes tumor cell growth and metastasis (Liao et al. 2021; Bastian et al. 2021).
3.1 Core-Fucosylated Proteins as Tumor Diagnostic Biomarkers
Pancreatic cancer (PC) is one of the worlds’ deadliest malignant diseases, and the overall 5-year survival rate among patients with PC is <5% (Jemal et al. 2008; Li et al. 2004). The poor survival rate is mainly due to the lack of validated, specific screening tests that reliably detect early-stage PC in people who have no symptoms. In most cases, patients with PC come to the hospital in an advanced stage when the cancer can no longer be removed with surgery or has spread from the pancreas to other parts of the body (Kamisawa et al. 2016). Therefore, there is an urgent clinical need to develop novel diagnostic methods for PC.
The detection of serum tumor markers is an effective diagnostic tool for pancreatic cancer, as well as for other cancers. FUT8 participates in α-fetoprotein (AFP) core fucosylation and can be used as a clinical marker for the early detection of hepatocellular carcinoma (HCC) (Nakagawa et al. 2006). Core-fucosylated prostate-specific antigen (PSA) is a diagnostic biomarker for prostate cancer (Lang et al. 2018). Chronic pancreatitis (CP) is a chronic inflammatory and fibrotic disease of the pancreas and a strong risk factor for the occurrence of pancreatic ductal adenocarcinoma (PDAC) (Malka et al. 2002; Talamini et al. 1999). In at least one study, serum core-fucosylated haptoglobin levels were significantly increased in CP patients compared with healthy volunteers, and these levels have since been used as an independent determinant for CP diagnosis (Ueda et al. 2016). Intraductal papillary mucinous neoplasm (IPMN) is a type of tumor that grows within the pancreatic ducts and has a high risk for the development of PDAC, which accounts for 90% of all pancreatic cancers (Muraki et al. 2021; Kleeff et al. 2016). FUT8 expression is upregulated in IPMN and correlates with the size of tumors. FUT8 levels in serum should be considered for evaluation in a future prospective study of IPMN (Watanabe et al. 2016). A report of remarkable increases (40%) in core-fucosylated biantennary glycans on ribonuclease 1 (RNase 1) in PC serum compared with healthy controls suggests that RNase 1 could be of clinical value for the detection of PC (Barrabés et al. 2007). In addition, aberrantly expressed mucin glycoproteins (MUC) are known to play important roles in PC. FUT8 could regulate the expression of MUC4 and MUC1 at protein levels. Thus, core-fucosylated MUC1/4 could also serve as a diagnosis marker for PC (Kumar et al. 2015).
3.2 Roles of Core Fucosylation in Cell Adhesion and Cell Migration
Dysregulation of glycosylation is not only a consequence of cancer, it also is involved in fundamental molecular and cell biology processes, such as regulating cell signaling and cell adhesion, and eventually controlling tumor cell growth and metastatic and invasive behaviors (Pinho and Reis 2015). Several studies have shown that core fucosylation plays a critical role in cellular events through a variety of mechanisms. Abnormal expression of core fucosylation has been identified as a feature of metastatic cancer (Magalhães et al. 2017; Taniguchi et al. 2021; Shao et al. 2016). For example, core fucosylation of the L1 cell adhesion molecule (L1CAM) hinders its cleavage by protease plasmin, which facilitates L1CAM-mediated cell invasion and tumor dissemination in melanoma (Agrawal et al. 2017). FUT8 deficiency suppresses cell migration by interfering with the integrin/FAK pathway in breast cancer (Liu et al. 2019). In fact, FUT8-KO has been used to block α3β1 integrin-mediated cell migration and cell signaling, which was reinstated via reintroduction of the FUT8 gene (Zhao et al. 2006). FUT8 is upregulated in pancreatic cancer patients and correlates with cancer invasiveness, lymph node metastases, and a worsened state of relapse-free survival, whereas inhibition of FUT8 expression reduces tumor cell invasion and peritoneal metastasis (Tada et al. 2020). These results suggest that FUT8 plays an important role in both cell adhesion and cell migration.
3.3 Roles of FUT8 in Cellular Signaling
Altering the expression of core-fucosylated proteins interferes with cell signaling molecules that regulate the activation of tyrosine kinase protein receptors such as epidermal growth factor receptor (EGFR), IGF-1R, and FLT3 (Fig. 2). The EGFR signaling cascade is a key regulator in essential cellular functions that include proliferation, differentiation, survival, and migration (Sabbah et al. 2020). Core fucosylation plays an important role in the EGFR mediation of signaling pathways. A lack of core fucosylation in EGFR could change its conformation, which would block EGF binding, and subsequently reduce the phosphorylation levels of EGFR and EGFR-mediated ERK activation for cell proliferation, which may partially explain the growth retardation in FUT8-KO mice (Wang et al. 2006). In a similar manner, the functions of c-Met and HGF receptor were also downregulated by a lack of core fucosylation. In a recent study, FUT8-KO blocked DEN/PB-induced hepatocellular carcinoma formation (Wang et al. 2015b) and liver regeneration (Wang et al. 2015a). This particular inhibitory function was also confirmed using 2-fluoro-L-fucose (2FF), an analog of L-fucose, which inhibits core fucosylation by interfering with the normal synthesis of GDP-fucose (Fig. 1) in hepatoma cell lines (Zhou et al. 2017) and in pancreatic cancer cell lines (Liang et al. 2021). Interestingly, increasing the sialylation and fucosylation (catalyzed by FUT4 or FUT6) of EGFR suppresses its activation and function, but FUT8 is known to promote EGFR dimerization and phosphorylation in lung cancer cells (Liu et al. 2011). Further studies have indicated that the overexpression of FUT8 on EGFR can enhance EGF-mediated cell growth and increase the sensitivity to gefitinib (Matsumoto et al. 2008). Thus, the impact of FUT8-mediated core glycosylation on EGFR signaling may provide a therapeutic target for cancer.
Insulin-like growth factor receptor (IGF-1R) is a heterotetrameric receptor that consists of two α subunits located extracellularly and two β subunits that span the membrane. The α subunit is responsible for binding to the ligand IGF-1, and the β subunits exhibit tyrosine kinase activity and activate a variety of intracellular signaling pathways (MAPK, PI3K/AKT), which promotes cell survival and motility (Díez 1999; Vincent and Feldman 2002). IGF-1R requires core fucosylation for its cellular signaling, and the knockdown of FUT8 reduces IGF-1 signaling (Vanhooren et al. 2011). This phenomenon has also been observed in human trophoblast and choriocarcinoma cells (Yu et al. 2019). Recent studies have shown that impairment of the self-repair function of alveolar epithelial cells (AECs) is an important cause of idiopathic pulmonary fibrosis (IPF). IGF-1 is a primary factor of increases in cell senescence (Kritschil et al. 2020). Suppressing core fucosylation in IGF-1R prevents its binding to IGF-1 and inhibits IGF-1/PI3K/AKT signaling, thus blocking the IPF process induced by AEC senescence (Sun et al. 2021).
Core fucosylation may also negatively regulate receptor functions. Fms-like tyrosine kinase 3 (FLT3) is a member of the tyrosine kinase receptor type III family, which is known to exert a significant effect on the expansion of hematopoietic progenitors in the pathogenesis of acute myeloid leukemia (AML) (Spiekermann et al. 2003). Oddly enough, in one study we found that a lack of core fucosylation in FLT3 induced ligand-independent dimerization on the cell surface, which then led to the aberrant activation of downstream signaling pathways such as p-STAT5, p-ERK, and p-AKT. This activation is known to induce IL-3-independent cell proliferation in Ba/F3 cells (Duan et al. 2020). Activation by deficient FUT8 was also observed in the signaling pathway for activin/P-Smad2, which is a member of the transforming growth factor (TGF)-β superfamily (Gu et al. 2013). Decreased core fucosylation of activin receptors, ACVR2A and ACVR1B, via knockdown of the FUT8 gene, enhanced activin binding and activin receptor-mediated signaling, which were cancelled by the restoration of FUT8 expression.
3.4 Roles of FUT8 in TGF-β-Induced EMT
Epithelial-mesenchymal transition (EMT) is a biological process that allows polarized epithelial cells to undergo multiple biochemical changes that enable them to acquire a mesenchymal phenotype and migrate to secondary sites (Thiery and Sleeman 2006). EMT is characterized by a loss of cell-cell adhesion and by the acquisition of cell motility. This process features a decreased expression of cell-cell adhesion molecules and epithelial markers such as E-cadherin and an increased expression of intermediate filament proteins and mesenchymal cell markers such as N-cadherin and integrins (Bhowmick et al. 2001; Maeda et al. 2005).
FUT8 is upregulated during TGF-β-induced EMT in breast cancer cells (Tu et al. 2017). The increase in FUT8 modifies TGF-β receptors I and II on a cell surface, which facilitates TGF-β binding and enhances downstream signaling. These developments stimulate breast cancer cell invasion and metastasis (Tu et al. 2017). The molecular mechanism for the induction of FUT8 during TGF-β-induced EMT has been explored in lung cancer cells (Chen et al. 2013). In that study, the expression of E-cadherin was suppressed during EMT, leading to the nuclear accumulation of β-catenin, which then formed a complex with lymphoid enhancer-binding factor-1 (LEF-1) to activate FUT8 expression (Chen et al. 2013). In addition, activation of the Wnt/β-catenin signaling pathway is also known to induce the expression of FUT8, which improves both cell invasion in hepatocellular carcinoma (HCC) (Zhang et al. 2020a) and EMT in breast cancer cells (Yang et al. 2017). Lin et al. found that blocking core fucosylation of TGF-β1 receptors by siRNA inhibits the phosphorylation of Smad2/3, which leads to the interruption of TGF-β/Smad2/3 signaling activation and subsequently suppresses EMT development (Lin et al. 2011). These results strongly suggest that TGF-β signaling partially regulates EMT via FUT8.
4 Core Fucosylation Regulates the Stability of Glycoproteins Expressed on the Cell Surface
Many pivotal glycoproteins of tumors are highly core-fucosylated, and their functions are known to be regulated by core fucosylation (Okada et al. 2017; Matsumoto et al. 2008; Zhao et al. 2006; Geng et al. 2004). The latest research reports indicate that core fucosylation plays an important role in regulating the stability of cell surface glycoproteins. Programmed cell death protein-1 (PD-1) is an immune checkpoint, and binding with its ligand (PD-L1) plays a crucial role in T lymphocyte activation and tumor immune escape (Keir et al. 2008; Han et al. 2020). Blocking core fucosylation has reduced the PD-1 expression on the cell surface (Zhang et al. 2020b), which downregulated PD-1/PD-L1 interaction and enhanced T cell activation and led to more efficient tumor eradication (Okada et al. 2017). The underlying mechanism showed that suppression of core fucosylation in PD-1 promoted PD-1 ubiquitination and then increased the degradation of PD-1 by proteasomes (Zhang et al. 2020b; Okada et al. 2017). A similar result has been observed in the molecules of triple negative breast cancer (TNBC) patients who are not responsive to anti-PD1/PDL1 immunotherapy. B7 homolog 3 protein (B7H3) is also known as CD276 and is regarded as an immune checkpoint molecule for immunosuppressive activity. B7H3 is a highly N-glycosylated protein with core fucosylation, which makes it less prone to ubiquitin-proteasome degradation and allows it to be stably expressed on a cell surface, which inhibits the occurrence of a tumor immune response (Huang et al. 2021).
The lack of FUT8 is also known to reduce the expressions of E-cadherin and impaired E-cadherin-dependent cell-cell adhesion in colon carcinoma cells (Osumi et al. 2009). Our recent research has shown that FUT8-KO significantly suppresses the expression of EGFR on the surface of PC cells. Furthermore, treatment with cycloheximide, a protein synthesis inhibitor, showed that defective core fucosylation accelerates EGFR degradation, which suggests that core fucosylation plays an important role in maintaining the stability of the protein (Liang et al. 2021). However, the precise molecular mechanisms for how core fucosylation affects protein structure to regulate its stability remains to be elucidated in future study.
5 Glycosylation in Cancer Stem Cells
Cancer is a major cause of death worldwide. Despite significant progress in the diagnosis and treatment of cancer, the survival rate of progressive cancers remains insufficient. One of the most important reasons has been attributed to a distinct subpopulation of tumor cells that are referred to as cancer stem cells (CSCs). These cells have the capacity to self-renew and a facility for differentiation that drives tumor initiation, metastasis, relapse, and chemoresistance (Jordan et al. 2006; Clarke and Fuller 2006). Many well-known CSC markers such as CD133, CD44, EpCAM, and CD24 are heavily glycosylated, and alterations in glycosylation are associated with tumor development and tumor cell stemness (Barkeer et al. 2018). Bellis et al. revealed a significant upregulation of ST6Gal-I in human colon tumors, and high ST6Gal-I expression has been associated with the expression of the cancer stem cell markers CD133 and ALDH1, while the shRNA-mediated downregulation of ST6Gal-I reduces the percentage of CSCs in cancer cell populations (Swindall et al. 2013). Orospheres are CSC-rich oral squamous cell populations that are obtained via a spheroid culture technique. The expression levels of FUT3 and FUT6 (involved in SLex epitope synthesis) were more highly upregulated in orospheres compared with their adherent counterparts; also, orospheres expressed higher CSC markers and were more resistant to cisplatin/radiation in oral squamous cell carcinoma (Desiderio et al. 2015). FUT9 expression boosts the acquisition of stemness both in murine and human colon cancer cells towards a cancer stem-like transcriptional profile, phenotype, and function; other functional implications of CSCs also are promoted such as tumor growth and resistance to chemotherapy (Blanas et al. 2020). Breast cancer cells expressing high levels of CD44 and low levels of CD24 are resistant to chemotherapy and often lead to cancer relapse. In some studies FUT8 knockdown has reduced the CD44+/CD24− population and increased the susceptibility to chemotherapy, indicating that FUT8 is essential for maintaining the stemness phenotype (Ma et al. 2021).
5.1 FUT8 Regulates Cancer Stemness
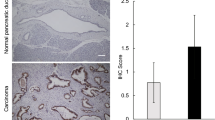
Pancreatic cancer has a high mortality rate, and one of the reasons could be the presence of pancreatic cancer stem cells, which were first identified by Simeone et al. in 2007 (Li et al. 2007). Terao et al. reported that increased cellular fucosylation is a common glycan change in pancreatic cancer stem cells (Terao et al. 2015), which is consistent with our recent study using PANC-1 and MIA PaCa-2 cell lines. Compared with MIA PaCa-2 cells, PANC-1 cells exhibit a greater degree of staining with Aleuria aurantia lectin (AAL), which specifically recognizes fucosylation. RT-PCR has shown that much higher expression levels of CSC biomarkers such as EpCAM, CD24, CD44, and CD133 are present in PANC-1 cells, which indicates that fucosylation modification could be related to cell stemness. Interestingly, a deficiency of FUT8 (FUT8-KO) greatly decreases the expression levels of these CSC biomarkers as well as sphere formation (Fig. 3). Furthermore, re-expression of FUT8 in FUT8-KO cells rescues the cell stemness features of these PC cells (Liang et al. 2021). In fact, this phenomenon is not restricted to PC; decreased or blocked core fucosylation has reduced stemness and EMT, which has also been observed in breast cancer cells (Yang et al. 2017). In addition, upregulated FUT8 has also been found in esophageal cancer stem-like cells, which were enriched using the sphere-formation method (Sadeghzadeh et al. 2020). These results strongly suggest that FUT8 could regulate cancer stemness.
Schematic diagram of core fucosylation and CSCs in PC. Tumor cells are heterogeneous and include CSC populations. CSCs are resistant to chemotherapy, which can lead to tumor recurrence and metastasis. PC cells express high levels of core fucosylation; blocking core fucosylation can inhibit cell proliferation, migration, and cancer stemness, which could be beneficial to prevent or inhibit tumor regrowth or recurrence
5.2 FUT8 Increases Multidrug Resistance
Drug resistance is also a characteristic of cancer stemness and remains a major challenge to the efficiency of curative treatments (Gottesman 2002). Recent studies have revealed that changes in glycosylation regulate cancer stemness and interfere with tumor response to anticancer drugs, leading to the emergence of cancer cells resistant to chemotherapy treatment (Mallard and Tiralongo 2017; Zhang et al. 2012; Ferreira et al. 2016). Aberrant glycosylation has proven to be related to chemotherapy resistance in malignant cells (Very et al. 2018). Fucosylated glycans are common modifications on the cell surface, and these participate in various biological processes such as differentiation, development, and tumor multidrug resistance (MDR). For example, compared with wild-type RMG-1 cells, the transfection of FUT1 and FUT2 genes into human ovarian carcinoma-derived RMG-I cells greatly promotes cell viability in the presence of 5-fluorouracil (5-FU) (Iwamori et al. 2005). The expression levels of FUT4 and its product, Lewis X antigen, are dramatically increased in patients with metastatic colorectal cancer (Blanas et al. 2019). Overexpression of FUT4 induces the RAF-MEK-ERK signaling pathway and exhibits significant resistance to anti-EGFR (cetuximab) and anti-VEGF (bevacizumab) chemotherapeutical agents (Giordano et al. 2015).
A growing body of evidence indicates that FUT8 is one of the main causes of cancer drug resistance (Cheng et al. 2013; Lv et al. 2019). Altered levels of FUT8 are responsible for resistance to 5-FU in human hepatocellular carcinoma via modulation of the PI3K/AKT signaling pathway with the involvement of MDR-related protein 1 (MRP1) expression (Cheng et al. 2013). HCV infection induces FUT8 expression in human hepatoma cells, which promotes expression of the drug-resistant proteins P-glycoprotein and MRP1 via activation of the PI3K/AKT/NF-κB signaling pathways; HCV also enhances 5-FU drug resistance (Li et al. 2019). On the other hand, FUT8-KO in a pro-B cell line has induced autodimerization of FLT3 and activated intracellular signaling such as the phosphorylations of STAT5, AKT, and ERK. An important development is that FUT8-KO increases the sensitivities for PKC412, a tyrosine kinase inhibitor, which suppresses cell proliferation (Duan et al. 2020). In a similar manner, an increase in core fucosylation in ovarian cancer cells has led to cisplatin resistance by suppressing copper transporter 1 (CTR1)-cisplatin interactions, while FUT8-KO weakens cisplatin resistance by promoting cellular cisplatin uptake (Lv et al. 2019). The effect of FUT8 on drug resistance has also been observed in pancreatic cancer cells. FUT8-KO has significantly increased the chemosensitivity for gemcitabine, which is a first-line therapy for advanced pancreatic cancer (Liang et al. 2021).
6 Conclusion and Perspectives
The prevalence of aberrant FUT8 and core-fucosylated glycoproteins, EGFR, TGFβR, PD-1, E-cadherin, and α3β1 integrin, have been reported in different types of cancers, as described above. The upregulation of core fucosylation is involved in regulating tumor malignance that results in promoting cell proliferation, metastasis, cancer stemness, and multidrug resistance (Fig. 3). Thus, targeting core fucosylation could be a feasible approach for the treatment of cancers.
Blocking core fucosylation via the use of an analog of L-fucose could be a useful method for the downregulation of cancer stemness and drug resistance. A lack of specificity, however, could mean that other forms of fucosylation could be affected, as well as other forms of glycosylation via sugar metabolism. Therefore, an increase in specificity for inhibiting core fucosylation or directly targeting FUT8 genes or proteins could open new opportunities to develop novel therapeutic strategies to eradicate CSCs, which will help lower the rates of cancer recurrence and enhance the survival rates for cancer patients.
References
Abdirama D, Tesch S, Grießbach AS, von Spee-Mayer C, Humrich JY, Stervbo U, Babel N, Meisel C, Alexander T, Biesen R, Bacher P, Scheffold A, Eckardt KU, Hiepe F, Radbruch A, Burmester GR, Riemekasten G, Enghard P (2021) Nuclear antigen-reactive CD4(+) T cells expand in active systemic lupus erythematosus, produce effector cytokines, and invade the kidneys. Kidney Int 99(1):238–246. https://doi.org/10.1016/j.kint.2020.05.051
Agrawal P, Fontanals-Cirera B, Sokolova E, Jacob S, Vaiana CA, Argibay D, Davalos V, McDermott M, Nayak S, Darvishian F, Castillo M, Ueberheide B, Osman I, Fenyo D, Mahal LK, Hernando E (2017) A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell 31(6):804–819.e807. https://doi.org/10.1016/j.ccell.2017.05.007
Barkeer S, Chugh S, Batra SK, Ponnusamy MP (2018) Glycosylation of cancer stem cells: function in stemness, tumorigenesis, and metastasis. Neoplasia (New York, NY) 20(8):813–825. https://doi.org/10.1016/j.neo.2018.06.001
Barrabés S, Pagès-Pons L, Radcliffe CM, Tabarés G, Fort E, Royle L, Harvey DJ, Moenner M, Dwek RA, Rudd PM, De Llorens R, Peracaula R (2007) Glycosylation of serum ribonuclease 1 indicates a major endothelial origin and reveals an increase in core fucosylation in pancreatic cancer. Glycobiology 17(4):388–400. https://doi.org/10.1093/glycob/cwm002
Bastian K, Scott E, Elliott DJ, Munkley J (2021) FUT8 alpha-(1,6)-fucosyltransferase in cancer. Int J Mol Sci 22(1):455. https://doi.org/10.3390/ijms22010455
Becker DJ, Lowe JB (2003) Fucose: biosynthesis and biological function in mammals. Glycobiology 13(7):41r–53r. https://doi.org/10.1093/glycob/cwg054
Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL (2001) Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem 276(50):46707–46713. https://doi.org/10.1074/jbc.M106176200
Blanas A, Cornelissen LAM, Kotsias M, van der Horst JC, van de Vrugt HJ, Kalay H, Spencer DIR, Kozak RP, van Vliet SJ (2019) Transcriptional activation of fucosyltransferase (FUT) genes using the CRISPR-dCas9-VPR technology reveals potent N-glycome alterations in colorectal cancer cells. Glycobiology 29(2):137–150. https://doi.org/10.1093/glycob/cwy096
Blanas A, Zaal A, van der Haar ÀI, Kempers M, Kruijssen L, de Kok M, Popovic MA, van der Horst JC, JvV S (2020) FUT9-driven programming of colon cancer cells towards a stem cell-like state. Cancers (Basel) 12(9):2580. https://doi.org/10.3390/cancers12092580
Chandler KB, Costello CE, Rahimi N (2019) Glycosylation in the tumor microenvironment: implications for tumor angiogenesis and metastasis. Cell 8(6):544. https://doi.org/10.3390/cells8060544
Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Yang PC, Hsiao M, Hsu TL, Wong CH (2013) Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci U S A 110(2):630–635. https://doi.org/10.1073/pnas.1220425110
Cheng L, Luo S, Jin C, Ma H, Zhou H, Jia L (2013) FUT family mediates the multidrug resistance of human hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell Death Dis 4(11):e923. https://doi.org/10.1038/cddis.2013.450
Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH (2014) Cell surface protein glycosylation in cancer. Proteomics 14(4-5):525–546. https://doi.org/10.1002/pmic.201300387
Clark DJ, Schnaubelt M, Hoti N, Hu Y, Zhou Y, Gooya M, Zhang H (2020) Impact of increased FUT8 expression on the extracellular vesicle proteome in prostate cancer cells. J Proteome Res 19(6):2195–2205. https://doi.org/10.1021/acs.jproteome.9b00578
Clarke MF, Fuller M (2006) Stem cells and cancer: two faces of eve. Cell 124(6):1111–1115. https://doi.org/10.1016/j.cell.2006.03.011
Desiderio V, Papagerakis P, Tirino V, Zheng L, Matossian M, Prince ME, Paino F, Mele L, Papaccio F, Montella R, Papaccio G, Papagerakis S (2015) Increased fucosylation has a pivotal role in invasive and metastatic properties of head and neck cancer stem cells. Oncotarget 6(1):71–84. https://doi.org/10.18632/oncotarget.2698
Díez J (1999) Insulin-like growth factor I in essential hypertension. Kidney Int 55(2):744–759. https://doi.org/10.1046/j.1523-1755.1999.00300.x
Duan C, Fukuda T, Isaji T, Qi F, Yang J, Wang Y, Takahashi S, Gu J (2020) Deficiency of core fucosylation activates cellular signaling dependent on FLT3 expression in a Ba/F3 cell system. FASEB J 34(2):3239–3252. https://doi.org/10.1096/fj.201902313RR
Eichler J (2019) Protein glycosylation. Curr Biol 29(7):R229–r231. https://doi.org/10.1016/j.cub.2019.01.003
Ferreira JA, Peixoto A, Neves M, Gaiteiro C, Reis CA, Assaraf YG, Santos LL (2016) Mechanisms of cisplatin resistance and targeting of cancer stem cells: adding glycosylation to the equation. Drug Resist Updat 24:34–54. https://doi.org/10.1016/j.drup.2015.11.003
Fukuda T, Hashimoto H, Okayasu N, Kameyama A, Onogi H, Nakagawasai O, Nakazawa T, Kurosawa T, Hao Y, Isaji T, Tadano T, Narimatsu H, Taniguchi N, Gu J (2011) Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: importance of the balance between the dopamine and serotonin systems. J Biol Chem 286(21):18434–18443. https://doi.org/10.1074/jbc.M110.172536
Fuster MM, Esko JD (2005) The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5(7):526–542. https://doi.org/10.1038/nrc1649
Geng F, Shi BZ, Yuan YF, Wu XZ (2004) The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: prognostic implications. Cell Res 14(5):423–433. https://doi.org/10.1038/sj.cr.7290243
Giordano G, Febbraro A, Tomaselli E, Sarnicola ML, Parcesepe P, Parente D, Forte N, Fabozzi A, Remo A, Bonetti A, Manfrin E, Ghasemi S, Ceccarelli M, Cerulo L, Bazzoni F, Pancione M (2015) Cancer-related CD15/FUT4 overexpression decreases benefit to agents targeting EGFR or VEGF acting as a novel RAF-MEK-ERK kinase downstream regulator in metastatic colorectal cancer. J Exp Clin Cancer Res 34:108. https://doi.org/10.1186/s13046-015-0225-7
Gottesman MM (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627. https://doi.org/10.1146/annurev.med.53.082901.103929
Gu W, Fukuda T, Isaji T, Hashimoto H, Wang Y, Gu J (2013) α1,6-fucosylation regulates neurite formation via the activin/phospho-Smad2 pathway in PC12 cells: the implicated dual effects of Fut8 for TGF-β/activin-mediated signaling. FASEB J 27(10):3947–3958. https://doi.org/10.1096/fj.12-225805
Gu W, Fukuda T, Isaji T, Hang Q, Lee HH, Sakai S, Morise J, Mitoma J, Higashi H, Taniguchi N, Yawo H, Oka S, Gu J (2015) Loss of α1,6-fucosyltransferase decreases hippocampal long term potentiation: implications for core fucosylation in the regulation of AMPA receptor heteromerization and cellular signaling. J Biol Chem 290(28):17566–17575. https://doi.org/10.1074/jbc.M114.579938
Hakomori S (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A 99(16):10231–10233. https://doi.org/10.1073/pnas.172380699
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10(3):727–742
Herrera H, Dilday T, Uber A, Scott D, Zambrano JN, Wang M, Angel PM, Mehta AS, Drake RR, Hill EG, Yeh ES (2019) Core-fucosylated tetra-antennary N-glycan containing a single N-acetyllactosamine branch is associated with poor survival outcome in breast cancer. Int J Mol Sci 20(10):2528. https://doi.org/10.3390/ijms20102528
Hirschberg CB (2001) Golgi nucleotide sugar transport and leukocyte adhesion deficiency II. J Clin Invest 108(1):3–6. https://doi.org/10.1172/jci13480
Höti N, Lih TS, Pan J, Zhou Y, Yang G, Deng A, Chen L, Dong M, Yang RB, Tu CF, Haffner MC, Kay Li Q, Zhang H (2020) A comprehensive analysis of FUT8 overexpressing prostate cancer cells reveals the role of EGFR in castration resistance. Cancers (Basel) 12(2):468. https://doi.org/10.3390/cancers12020468
Huang Y, Zhang HL, Li ZL, Du T, Chen YH, Wang Y, Ni HH, Zhang KM, Mai J, Hu BX, Huang JH, Zhou LH, Yang D, Peng XD, Feng GK, Tang J, Zhu XF, Deng R (2021) FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun 12(1):2672. https://doi.org/10.1038/s41467-021-22618-x
Hutchinson WL, Du MQ, Johnson PJ, Williams R (1991) Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology 13(4):683–688
Iwamori M, Tanaka K, Kubushiro K, Lin B, Kiguchi K, Ishiwata I, Tsukazaki K, Nozawa S (2005) Alterations in the glycolipid composition and cellular properties of ovarian carcinoma-derived RMG-1 cells on transfection of the alpha1,2-fucosyltransferase gene. Cancer Sci 96(1):26–30. https://doi.org/10.1111/j.1349-7006.2005.00005.x
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics. CA Cancer J Clin 58(2):71–96. https://doi.org/10.3322/ca.2007.0010
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355(12):1253–1261. https://doi.org/10.1056/NEJMra061808
Kamisawa T, Wood LD, Itoi T, Takaori K (2016) Pancreatic cancer. Lancet 388(10039):73–85. https://doi.org/10.1016/s0140-6736(16)00141-0
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331
Kizuka Y, Taniguchi N (2016) Enzymes for N-glycan branching and their genetic and nongenetic regulation in cancer. Biomol Ther 6(2):25. https://doi.org/10.3390/biom6020025
Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP (2016) Pancreatic cancer. Nat Rev Dis Primers 2:16022. https://doi.org/10.1038/nrdp.2016.22
Kritschil R, Zhang Z, Lei C, Zhong J, Dong Q, Lee J, Conover CA, Sowa G, Vallejo AN, Vo N (2020) Effects of suppressing bioavailability of insulin-like growth factor on age-associated intervertebral disc degeneration. JOR Spine 3(4):e1112. https://doi.org/10.1002/jsp2.1112
Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM (2005) Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature 434(7030):238–243. https://doi.org/10.1038/nature03391
Kumar S, Das S, Rachagani S, Kaur S, Joshi S, Johansson SL, Ponnusamy MP, Jain M, Batra SK (2015) NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene 34(37):4879–4889. https://doi.org/10.1038/onc.2014.409
Lang R, Leinenbach A, Karl J, Swiatek-de Lange M, Kobold U, Vogeser M (2018) An endoglycosidase-assisted LC-MS/MS-based strategy for the analysis of site-specific core-fucosylation of low-concentrated glycoproteins in human serum using prostate-specific antigen (PSA) as example. Clin Chim Acta 480:1–8. https://doi.org/10.1016/j.cca.2018.01.040
Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363(9414):1049–1057. https://doi.org/10.1016/s0140-6736(04)15841-8
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM (2007) Identification of pancreatic cancer stem cells. Cancer Res 67(3):1030–1037. https://doi.org/10.1158/0008-5472.can-06-2030
Li S, Liu XY, Pan Q, Wu J, Liu ZH, Wang Y, Liu M, Zhang XL (2019) Hepatitis C virus-induced FUT8 causes 5-FU drug resistance in human hepatoma Huh7.5.1 cells. Viruses 11(4):378. https://doi.org/10.3390/v11040378
Li F, Zhao S, Cui Y, Guo T, Qiang J, Xie Q, Yu W, Guo W, Deng W, Gu C, Wu T (2020) α1,6-fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC). Am J Cancer Res 10(3):816–837
Liang W, Mao S, Sun S, Li M, Li Z, Yu R, Ma T, Gu J, Zhang J, Taniguchi N, Li W (2018) Core fucosylation of the T cell receptor is required for T cell activation. Front Immunol 9:78. https://doi.org/10.3389/fimmu.2018.00078
Liang C, Fukuda T, Isaji T, Duan C, Song W, Wang Y, Gu J (2021) α1,6-Fucosyltransferase contributes to cell migration and proliferation as well as to cancer stemness features in pancreatic carcinoma. Biochim Biophys Acta Gen Sub 1865(6):129870. https://doi.org/10.1016/j.bbagen.2021.129870
Liao C, An J, Yi S, Tan Z, Wang H, Li H, Guan X, Liu J, Wang Q (2021) FUT8 and protein core fucosylation in tumours: from diagnosis to treatment. J Cancer 12(13):4109–4120. https://doi.org/10.7150/jca.58268
Lin H, Wang D, Wu T, Dong C, Shen N, Sun Y, Sun Y, Xie H, Wang N, Shan L (2011) Blocking core fucosylation of TGF-β1 receptors downregulates their functions and attenuates the epithelial-mesenchymal transition of renal tubular cells. Am J Physiol Renal Physiol 300(4):F1017–F1025. https://doi.org/10.1152/ajprenal.00426.2010
Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, Juan YH, Chen CH, Khoo KH, Yu CJ, Yang PC, Hsu TL, Wong CH (2011) Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci U S A 108(28):11332–11337. https://doi.org/10.1073/pnas.1107385108
Liu D, Gao Z, Yue L (2019) Fucosyltransferase 8 deficiency suppresses breast cancer cell migration by interference of the FAK/integrin pathway. Cancer Biomark 25(4):303–311. https://doi.org/10.3233/cbm-190209
Lu X, Zhang D, Shoji H, Duan C, Zhang G, Isaji T, Wang Y, Fukuda T, Gu J (2019) Deficiency of α1,6-fucosyltransferase promotes neuroinflammation by increasing the sensitivity of glial cells to inflammatory mediators. Biochim Biophys Acta Gen Subj 1863(3):598–608. https://doi.org/10.1016/j.bbagen.2018.12.008
Lu HH, Lin SY, Weng RR, Juan YH, Chen YW, Hou HH, Hung ZC, Oswita GA, Huang YJ, Guu SY, Khoo KH, Shih JY, Yu CJ, Tsai HC (2020) Fucosyltransferase 4 shapes oncogenic glycoproteome to drive metastasis of lung adenocarcinoma. EBioMedicine 57:102846. https://doi.org/10.1016/j.ebiom.2020.102846
Lv X, Song J, Xue K, Li Z, Li M, Zahid D, Cao H, Wang L, Song W, Ma T, Gu J, Li W (2019) Core fucosylation of copper transporter 1 plays a crucial role in cisplatin-resistance of epithelial ovarian cancer by regulating drug uptake. Mol Carcinog 58(5):794–807. https://doi.org/10.1002/mc.22971
Ma M, Guo D, Tan Z, Du J, Guan F, Li X (2021) Fucosyltransferase 8 regulation and breast cancer suppression by transcription factor activator protein 2γ. Cancer Sci 112(8):3190–3204. https://doi.org/10.1111/cas.14987
Maeda M, Johnson KR, Wheelock MJ (2005) Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci 118(Pt 5):873–887. https://doi.org/10.1242/jcs.01634
Magalhães A, Duarte HO, Reis CA (2017) Aberrant glycosylation in cancer: a novel molecular mechanism controlling metastasis. Cancer Cell 31(6):733–735. https://doi.org/10.1016/j.ccell.2017.05.012
Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P (2002) Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 51(6):849–852. https://doi.org/10.1136/gut.51.6.849
Mallard BW, Tiralongo J (2017) Cancer stem cell marker glycosylation: nature, function and significance. Glycoconj J 34(4):441–452. https://doi.org/10.1007/s10719-017-9780-9
Matsumoto K, Yokote H, Arao T, Maegawa M, Tanaka K, Fujita Y, Shimizu C, Hanafusa T, Fujiwara Y, Nishio K (2008) N-glycan fucosylation of epidermal growth factor receptor modulates receptor activity and sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Sci 99(8):1611–1617. https://doi.org/10.1111/j.1349-7006.2008.00847.x
Miyoshi E, Uozumi N, Noda K, Hayashi N, Hori M, Taniguchi N (1997) Expression of alpha1-6 fucosyltransferase in rat tissues and human cancer cell lines. Int J Cancer 72(6):1117–1121. https://doi.org/10.1002/(sici)1097-0215(19970917)72:6<1117::aid-ijc29>3.0.co;2-#
Miyoshi E, Moriwaki K, Nakagawa T (2008) Biological function of fucosylation in cancer biology. J Biochem 143(6):725–729. https://doi.org/10.1093/jb/mvn011
Moreira IB, Pinto F, Gomes C, Campos D, Reis CA (2020) Impact of truncated O-glycans in gastric-cancer-associated CD44v9 detection. Cell 9(2):264. https://doi.org/10.3390/cells9020264
Muraki T, Jang K-T, Reid MD, Pehlivanoglu B, Memis B, Basturk O, Mittal P, Kooby D, Maithel SK, Sarmiento JM, Christians K, Tsai S, Evans D, Adsay V (2021) Pancreatic ductal adenocarcinomas associated with intraductal papillary mucinous neoplasms (IPMNs) versus pseudo-IPMNs: relative frequency, clinicopathologic characteristics and differential diagnosis. Mod Pathol 35(1):96–105. https://doi.org/10.1038/s41379-021-00902-x
Nakagawa T, Uozumi N, Nakano M, Mizuno-Horikawa Y, Okuyama N, Taguchi T, Gu J, Kondo A, Taniguchi N, Miyoshi E (2006) Fucosylation of N-glycans regulates the secretion of hepatic glycoproteins into bile ducts. J Biol Chem 281(40):29797–29806. https://doi.org/10.1074/jbc.M605697200
Ng BG, Xu G, Chandy N, Steyermark J, Shinde DN, Radtke K, Raymond K, Lebrilla CB, AlAsmari A, Suchy SF, Powis Z, Faqeih EA, Berry SA, Kronn DF, Freeze HH (2018) Biallelic mutations in FUT8 cause a congenital disorder of glycosylation with defective fucosylation. Am J Hum Genet 102(1):188–195. https://doi.org/10.1016/j.ajhg.2017.12.009
Ng BG, Dastsooz H, Silawi M, Habibzadeh P, Jahan SB, Fard MAF, Halliday BJ, Raymond K, Ruzhnikov MRZ, Tabatabaei Z, Taghipour-Sheshdeh A, Brimble E, Robertson SP, Faghihi MA, Freeze HH (2020) Expanding the molecular and clinical phenotypes of FUT8-CDG. J Inherit Metab Dis 43(4):871–879. https://doi.org/10.1002/jimd.12221
Niittymäki J, Mattila P, Roos C, Huopaniemi L, Sjöblom S, Renkonen R (2004) Cloning and expression of murine enzymes involved in the salvage pathway of GDP-L-fucose. Eur J Biochem 271(1):78–86. https://doi.org/10.1046/j.1432-1033.2003.03904.x
Niittymäki J, Mattila P, Renkonen R (2006) Differential gene expression of GDP-L-fucose-synthesizing enzymes, GDP-fucose transporter and fucosyltransferase VII. APMIS 114(7-8):539–548. https://doi.org/10.1111/j.1600-0463.2006.apm_461.x
Norton PA, Mehta AS (2019) Expression of genes that control core fucosylation in hepatocellular carcinoma: systematic review. World J Gastroenterol 25(23):2947–2960. https://doi.org/10.3748/wjg.v25.i23.2947
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126(5):855–867. https://doi.org/10.1016/j.cell.2006.08.019
Okada M, Chikuma S, Kondo T, Hibino S, Machiyama H, Yokosuka T, Nakano M, Yoshimura A (2017) Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells. Cell Rep 20(5):1017–1028. https://doi.org/10.1016/j.celrep.2017.07.027
Osumi D, Takahashi M, Miyoshi E, Yokoe S, Lee SH, Noda K, Nakamori S, Gu J, Ikeda Y, Kuroki Y, Sengoku K, Ishikawa M, Taniguchi N (2009) Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci 100(5):888–895. https://doi.org/10.1111/j.1349-7006.2009.01125.x
Park S, Lim JM, Chun JN, Lee S, Kim TM, Kim DW, Kim SY, Bae DJ, Bae SM, So I, Kim HG, Choi JY, Jeon JH (2020) Altered expression of fucosylation pathway genes is associated with poor prognosis and tumor metastasis in non-small cell lung cancer. Int J Oncol 56(2):559–567. https://doi.org/10.3892/ijo.2019.4953
Pinho SS, Reis CA (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 15(9):540–555. https://doi.org/10.1038/nrc3982
Puglielli L, Hirschberg CB (1999) Reconstitution, identification, and purification of the rat liver golgi membrane GDP-fucose transporter. J Biol Chem 274(50):35596–35600. https://doi.org/10.1074/jbc.274.50.35596
Sabbah DA, Hajjo R, Sweidan K (2020) Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr Top Med Chem 20(10):815–834. https://doi.org/10.2174/1568026620666200303123102
Sadeghzadeh Z, Khosravi A, Jazi MS, Asadi J (2020) Upregulation of fucosyltransferase 3, 8 and protein O-fucosyltransferase 1, 2 genes in esophageal cancer stem-like cells (CSLCs). Glycoconj J 37(3):319–327. https://doi.org/10.1007/s10719-020-09917-z
Shao K, Chen ZY, Gautam S, Deng NH, Zhou Y, Wu XZ (2016) Posttranslational modification of E-cadherin by core fucosylation regulates Src activation and induces epithelial-mesenchymal transition-like process in lung cancer cells. Glycobiology 26(2):142–154. https://doi.org/10.1093/glycob/cwv089
Shimizu H, Ochiai K, Ikenaka K, Mikoshiba K, Hase S (1993) Structures of N-linked sugar chains expressed mainly in mouse brain. J Biochem 114(3):334–338. https://doi.org/10.1093/oxfordjournals.jbchem.a124177
Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W (2003) Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res 9(6):2140–2150
Suárez-Fueyo A, Bradley SJ, Tsokos GC (2016) T cells in systemic lupus erythematosus. Curr Opin Immunol 43:32–38. https://doi.org/10.1016/j.coi.2016.09.001
Sun Y, Li Z, Liang W, Zhang Y, Song W, Song J, Xue K, Wang M, Sun W, Gu J, Li M, Li W (2020) A novel immunochromatographic strips assay for rapid and simple detection of systemic lupus erythematosus. Sci Rep 10(1):14178. https://doi.org/10.1038/s41598-020-71137-0
Sun W, Jing X, Yang X, Huang H, Luo Q, Xia S, Wang P, Wang N, Zhang Q, Guo J, Xu Z (2021) Regulation of the IGF1 signaling pathway is involved in idiopathic pulmonary fibrosis induced by alveolar epithelial cell senescence and core fucosylation. Aging (Albany NY) 13(14):18852–18869. https://doi.org/10.18632/aging.203335
Swindall AF, Londoño-Joshi AI, Schultz MJ, Fineberg N, Buchsbaum DJ, Bellis SL (2013) ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res 73(7):2368–2378. https://doi.org/10.1158/0008-5472.can-12-3424
Tada K, Ohta M, Hidano S, Watanabe K, Hirashita T, Oshima Y, Fujnaga A, Nakanuma H, Masuda T, Endo Y, Takeuchi Y, Iwashita Y, Kobayashi T, Inomata M (2020) Fucosyltransferase 8 plays a crucial role in the invasion and metastasis of pancreatic ductal adenocarcinoma. Surg Today 50(7):767–777. https://doi.org/10.1007/s00595-019-01953-z
Talamini G, Falconi M, Bassi C, Sartori N, Salvia R, Caldiron E, Frulloni L, Di Francesco V, Vaona B, Bovo P, Vantini I, Pederzoli P, Cavallini G (1999) Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol 94(5):1253–1260. https://doi.org/10.1111/j.1572-0241.1999.01075.x
Taniguchi N, Ohkawa Y, Maeda K, Harada Y, Nagae M, Kizuka Y, Ihara H, Ikeda Y (2021) True significance of N-acetylglucosaminyltransferases GnT-III, V and α1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer. Mol Asp Med 79:100905. https://doi.org/10.1016/j.mam.2020.100905
Terao N, Takamatsu S, Minehira T, Sobajima T, Nakayama K, Kamada Y, Miyoshi E (2015) Fucosylation is a common glycosylation type in pancreatic cancer stem cell-like phenotypes. World J Gastroenterol 21(13):3876–3887. https://doi.org/10.3748/wjg.v21.i13.3876
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7(2):131–142. https://doi.org/10.1038/nrm1835
Tonetti M, Sturla L, Bisso A, Benatti U, De Flora A (1996) Synthesis of GDP-L-fucose by the human FX protein. J Biol Chem 271(44):27274–27279. https://doi.org/10.1074/jbc.271.44.27274
Tu Z, Lin YN, Lin CH (2013) Development of fucosyltransferase and fucosidase inhibitors. Chem Soc Rev 42(10):4459–4475. https://doi.org/10.1039/c3cs60056d
Tu CF, Wu MY, Lin YC, Kannagi R, Yang RB (2017) FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res 19(1):111. https://doi.org/10.1186/s13058-017-0904-8
Ueda M, Kamada Y, Takamatsu S, Shimomura M, Maekawa T, Sobajima T, Fujii H, Nakayama K, Nishino K, Yamada M, Kobayashi Y, Kumada T, Ito T, Eguchi H, Nagano H, Miyoshi E (2016) Specific increase in serum core-fucosylated haptoglobin in patients with chronic pancreatitis. Pancreatology 16(2):238–243. https://doi.org/10.1016/j.pan.2016.01.004
Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N (1996) Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-beta-D-glucosaminide alpha1—>6fucosyltransferase. J Biol Chem 271(44):27810–27817. https://doi.org/10.1074/jbc.271.44.27810
Vajaria BN, Patel KR, Begum R, Patel PS (2016) Sialylation: an avenue to target cancer cells. Pathol Oncol Res 22(3):443–447. https://doi.org/10.1007/s12253-015-0033-6
Vanhooren V, Dewaele S, Kuro OM, Taniguchi N, Dollé L, van Grunsven LA, Makrantonaki E, Zouboulis CC, Chen CC, Libert C (2011) Alteration in N-glycomics during mouse aging: a role for FUT8. Aging Cell 10(6):1056–1066. https://doi.org/10.1111/j.1474-9726.2011.00749.x
Very N, Lefebvre T, El Yazidi-Belkoura I (2018) Drug resistance related to aberrant glycosylation in colorectal cancer. Oncotarget 9(1):1380–1402. https://doi.org/10.18632/oncotarget.22377
Vincent AM, Feldman EL (2002) Control of cell survival by IGF signaling pathways. Growth Hormon IGF Res 12(4):193–197. https://doi.org/10.1016/s1096-6374(02)00017-5
Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, Uozumi N, Ihara S, Lee SH, Ikeda Y, Yamaguchi Y, Aze Y, Tomiyama Y, Fujii J, Suzuki K, Kondo A, Shapiro SD, Lopez-Otin C, Kuwaki T, Okabe M, Honke K, Taniguchi N (2005) Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci U S A 102(44):15791–15796. https://doi.org/10.1073/pnas.0507375102
Wang X, Gu J, Miyoshi E, Honke K, Taniguchi N (2006) Phenotype changes of Fut8 knockout mouse: core fucosylation is crucial for the function of growth factor receptor(s). Methods Enzymol 417:11–22. https://doi.org/10.1016/s0076-6879(06)17002-0
Wang Y, Fukuda T, Isaji T, Lu J, Gu W, Lee HH, Ohkubo Y, Kamada Y, Taniguchi N, Miyoshi E, Gu J (2015a) Loss of α1,6-fucosyltransferase suppressed liver regeneration: implication of core fucose in the regulation of growth factor receptor-mediated cellular signaling. Sci Rep 5:8264. https://doi.org/10.1038/srep08264
Wang Y, Fukuda T, Isaji T, Lu J, Im S, Hang Q, Gu W, Hou S, Ohtsubo K, Gu J (2015b) Loss of α1,6-fucosyltransferase inhibits chemical-induced hepatocellular carcinoma and tumorigenesis by down-regulating several cell signaling pathways. FASEB J 29(8):3217–3227. https://doi.org/10.1096/fj.15-270710
Watanabe K, Ohta M, Yada K, Komori Y, Iwashita Y, Kashima K, Inomata M (2016) Fucosylation is associated with the malignant transformation of intraductal papillary mucinous neoplasms: a lectin microarray-based study. Surg Today 46(10):1217–1223. https://doi.org/10.1007/s00595-015-1299-8
Yang HF, Yu M, Jin HD, Yao JQ, Lu ZL, Yabasin IB, Yan Q, Wen QP (2017) Fentanyl promotes breast cancer cell stemness and epithelial-mesenchymal transition by upregulating α1, 6-fucosylation via Wnt/β-catenin signaling pathway. Front Physiol 8:510. https://doi.org/10.3389/fphys.2017.00510
Yu M, Cui X, Wang H, Liu J, Qin H, Liu S, Yan Q (2019) FUT8 drives the proliferation and invasion of trophoblastic cells via IGF-1/IGF-1R signaling pathway. Placenta 75:45–53. https://doi.org/10.1016/j.placenta.2018.11.005
Zhang Z, Zhao Y, Jiang L, Miao X, Zhou H, Jia L (2012) Glycomic alterations are associated with multidrug resistance in human leukemia. Int J Biochem Cell Biol 44(8):1244–1253. https://doi.org/10.1016/j.biocel.2012.04.026
Zhang C, Wu Q, Huang H, Chen X, Huang T, Li W, Zhang J, Liu Y (2020a) Caveolin-1 upregulates Fut8 expression by activating the Wnt/β-catenin pathway to enhance HCC cell proliferative and invasive ability. Cell Biol Int 44(11):2202–2212. https://doi.org/10.1002/cbin.11426
Zhang N, Li M, Xu X, Zhang Y, Liu Y, Zhao M, Li P, Chen J, Fukuda T, Gu J, Jin X, Li W (2020b) Loss of core fucosylation enhances the anticancer activity of cytotoxic T lymphocytes by increasing PD-1 degradation. Eur J Immunol 50(11):1820–1833. https://doi.org/10.1002/eji.202048543
Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J (2006) Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J Biol Chem 281(50):38343–38350. https://doi.org/10.1074/jbc.M608764200
Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, Kitazume S, Taniguchi N (2008) Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci 99(7):1304–1310. https://doi.org/10.1111/j.1349-7006.2008.00839.x
Zhou Y, Fukuda T, Hang Q, Hou S, Isaji T, Kameyama A, Gu J (2017) Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci Rep 7(1):11563. https://doi.org/10.1038/s41598-017-11911-9
Acknowledgments
This work was supported in part by a Grants-in-Aid for Scientific Research (19H03184 to J.G.) from the Japan Society for the Promotion of Science and by a Grants-in-Aid for Scientific Research on Innovative Areas (20H04909 to J.G.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Liang, C., Song, W., Gu, J. (2023). Significance of FUT8 in Pancreatic Cancer and Others. In: Furukawa, K., Fukuda, M. (eds) Glycosignals in Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-7732-9_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-7732-9_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7731-2
Online ISBN: 978-981-19-7732-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)