Abstract
Conventional energy sources are limited and expected to deplete soon, so natural alternative sustainable and safer sources for the environment have to be explored. Researchers have studied various biomass sources to produce solid, liquid, and gaseous fuels. Different treatment processes like physical, chemical, and enzymatic can produce valuable alternative fuels like biodiesel. The quality and yield of the products depend on the type of reactor, reaction conditions (temperature, pressure, holding time), and particle size of feed. Homogenous base catalysts (like sodium hydroxide), homogenous acid catalysts (like hydrochloric acid), heterogeneous base catalysts (like rice husk), heterogeneous acid catalysts (like zirconia), and biocatalyst like lipase can help to improve the selectivity and energy efficiency of the biofuel production process. Recent trends show the potential of nanocatalysts to enhance the biofuel production capacity. The present chapter discusses various feed sources, treatment processes, and catalysts to produce biodiesel fuel with properties comparable to conventional diesel fuel. Emphasis has been given to the different classifications of catalysts and a comparison between their ability to improve biofuel production. Furthermore, the chapter explores prospects and challenges in developing biodiesel as an alternative energy source.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biodiesel
- Oil extraction
- Transesterification
- Homogenous catalyst
- Heterogeneous catalyst
- Nanocatalyst
- Utilization potential
1 Introduction
Biomass availability sources have emerged as a potential alternative to ever-shrinking fossil fuel reserves. In present times, from the overall global energy demand, approximately 81% of the energy is being obtained from fossil fuels, which will negatively impact the environment and human health (Aderibigbe et al. 2021). According to the oil market report (2021) from the Organization of the Petroleum Exporting Countries, the global oil demand could rise from 90.5 to 96.5 million barrels per day (mb/day) from 2020 to 2021, which could be due to the inevitable industrial and anthropogenic overcome of the globally imposed COVID-19 lockdown courses of action taken in 2020. Likewise, utilization of diesel and gasoline for the first quarter of the year 2021 was around 24.0 and 26.3 mb/day, which can increase further. Also, by the year 2030, it is predicted that the oil consumption around the world will rise to 118 mb/day and could ultimately lead to depletion in the global crude oil reserve by 2060 (OPEC 2021; Bharti et al. 2021). In this sequence, India’s utility for automobile fuel is more than 0.5. On average, India’s annual consumption is about 450 kg/vehicle. Our dependency on fossil fuel-based energy in powering transportation, agriculture, and accommodation indicates an alarming situation and calls for immediate actions to preserve the ever-diminishing fossil fuel and search for a better and more efficient alternative to rendering the need without fail (Mohiddin et al. 2021). Fossil-based fuels produce an ample number of harmful products such as CO, NOx, CO2, SOx, and carbon specifically into the environment (Perera 2018; Kumar et al. 2020). Various global initiatives like the Paris Agreement signed by members (191) of the United Nations Framework Convention on Climate Change (UNFCCC) aim at reducing the global risks of climate change through constant attempts (UN 2015).
Thus, researchers are looking for a non-toxic, high-energy capacity, clean, and green alternative to fossil fuels. Biodiesel, on this note, is the most preferred biofuel to be utilized for its inexhaustibility; innocuous, high lubricating qualities; and cleaner-burning properties (Atabani et al. 2012; Kim et al. 2018; Bharti et al. 2021). Biodiesel is clean, cheap, and environment-friendly and emits fewer hydrocarbons, smoke, and toxic gases. Being produced from local resources, biodiesel is considered renewable. A high amount of excess oxygen allows complete combustion and lower emissions. First-generation raw materials like starch and potato through second-generation non-edible oil cakes, waste oils to third-generation algae, and energy crops can generate high-quality biodiesel through a low-cost, simple transesterification process at moderate reaction conditions. The process involves a reaction between triglyceride and alcohol in the presence of an acid or basic catalyst to produce fatty acid alkyl esters and alcohol (Kanwar Gaur and Goyal 2022; Nagappan et al. 2022). High cetane number, longer ignition delay, low ash value, better engine ignition, and emission performance have motivated researchers to explore different blend ratios of biodiesel in petrol (Gad and Ismail 2021; Suzihaque et al. 2022). Another pathway to produce biodiesel is through thermo-chemical route. Pyrolysis of biomass in specially designed reactors can produce biochar, syngas, and bio-oil of suitable properties. Feedstock type, reaction conditions, and reactor types can affect the relative proportions of products. To produce commercial-scale biodiesel to replace fossil fuel, interest in biomass, including agricultural waste algae-derived biofuel, has risen considerably (Ghesti et al. 2022).
To promote biofuel as a commercial fuel, policies are designed globally. The major bio-ethanol-producing countries are the United States and Brazil. The United States had created around 1557 petajoules with 38% of global biofuel in 2019 (Kohler 2019). India has also proposed a 20% ethanol blending in petrol and 5% biodiesel in the diesel by 2030 as per its National Policy on Biofuels, 2018 (Devi et al. 2021). American standard ASTM D7467 mentions the biodiesel to commercial diesel from 6% to 20%. Blends with 13% are allowed in Brazil, with the target of increasing to 15% by 2023 (Bukkarapu and Krishnasamy 2022).
The present chapter explores the various types of raw materials to be used for biodiesel production, their merits, and their limitations. Different pathways for converting feedstock to biodiesel generation, yield, and multiple factors affecting the quality of biodiesel produced are discussed here. The chapter also compares the performance of catalytic and non-catalytic transesterification processes. The chapter offers insight into technical, social, and financial barriers hindering the acceptance of biodiesel and the future scope of biodiesel as a commercial fuel.
2 Process for Production of Biodiesel
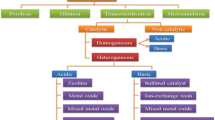
The waste biomass-derived biodiesel process can involve any of the two routes. The first pathway is the production of lipids through hydrolysis of cellulose and hemicellulose of biomass, which can be further processed to produce biodiesel. The second pathway involves the production of cheap and eco-friendly catalysts from waste biomass components, like stems, leaves, stalks, etc., to enhance the speed of the conventional transesterification process of multiple raw materials (Fig. 10.1).
2.1 Enzyme-Catalyzed Biodiesel Production
Biomass consists of lignin, cellulose, and hemicellulose, which are difficult to be converted into simple sugar using conventional methods. Lignocellulosic materials can undergo pretreatment (physical, chemical, or biological) to produce biomass of suitable properties. Pretreatment can involve physical, chemical, or biological methods. Mechanical treatments like milling, grinding, and steaming increase surface area and improve raw material conversion (Wang et al. 2021). Chemical treatment, including acid treatment, enhances cellulose decomposition efficiency. Alkali treatment is done to remove lignin altogether. Biological treatment methods like microbial treatment can be done in a low-cost, environment-friendly manner to degrade hemicellulose and lignin (Mahmood et al. 2019). Cellulase consists of endo-1,4-β-glucanase, cellobiohydrolase, and β-glucosidase, which can be converted into sugar to utilize biodiesel further. Oleaginous microorganisms including yeast, mold, and bacteria, under suitable conditions, can convert carbohydrates into microbial lipids, also known as single-cell oils (SCOs).
Yeast species such as Meyerozyma guilliermondii and Rhodosporidium fluviale have shown significant lipid production potential. Further, biodiesel can be produced using both catalytic and non-catalytic processes. Non-catalytic processes include treatments like emulsification, pyrolysis, and transesterification, among which transesterification is the most popular.
2.2 Transesterification Process
The general procedure for oil extraction involves various common steps. The feedstock is pretreated to enhance oil yield during the pressing stage. Kernel removal and drying are carried out using stompers and mallets, followed by oil extraction. Figure 10.2 shows the general biodiesel production process.
2.3 Oil Extraction
Mechanical extraction can be done using a manual ram or an automated screw press. An oil yield as high as 91% on filtering and degumming can be achieved. The solvent extraction process involves using a solvent to extract oil from the seed. The most common solvent, n-hexane, can generate oil of 41% and 95–99% yield using J. curcas and P. pinnata seeds. There are associated environmental concerns related to wastewater treatment after the solvent extraction process. The enzymatic process uses an enzyme to extract oil from sources. Though the process is slow, it is still an attractive technique due to its eco-friendly nature and no volatile component emission. Recently, researchers have used a combination of solvent extraction and microwave-based hybrid processes to enhance the oil yield efficiency at low cost, time, and energy.
2.4 Transesterification
As shown in Fig. 10.2, the oil extracted from the seed is sent for the transesterification process, which is the heart of the biodiesel production process. Since reactants, oils, and alcohols are immiscible with each other, there is a need to mechanically agitate the vessel or increase the reaction time and temperature with additional costs. Reaction temperature, time, mixing intensity, and reactor geometry are the most significant parameters. The production can be done using both catalytic and non-catalytic methods. For the non-catalytic process, the transesterification of vegetable oil takes place above the critical temperature and pressure (temperature, 513 K methanol and 514 K ethanol and 10–45 MPa) (Da Silva and Oliveira 2014). However, high temperatures lead to increased energy consumption and are not economically feasible. The catalytic process utilizes biocatalysts or chemical catalysts to transform the triglyceride molecules into fatty acid alkyl esters (FAAEs) (Nasreen et al. 2018; Gupta and Rathod 2021).
Catalytic processes can be further classified into various categories based on the types of material of construction, i.e., homogeneous or heterogeneous types. If a catalyst is soluble in the reactant, it becomes a simple, low-energy-consuming homogeneous catalyst. Certain limitations are associated with a low recovery rate and less reusability (Mohiddin et al. 2021), which prompted researchers to look for heterogeneous catalysts. Being produced from insoluble materials, they are easily detachable using simple centrifugation or filtration processes and are reusable many times. Recently biomass-derived heterogeneous catalysts have gained wide popularity among researchers worldwide (Cheng and Li 2018). Nanocatalysts, including nanoferrites synthesized from ceramic material, showcase tremendous advantages over traditional heterogeneous catalysts regarding their chemical, physical, structural, electrical, and mechanical characteristics (Thakur et al. 2020).
2.5 Homogeneous Catalysts
Homogeneous catalysis involves the application of catalysts present in its liquid form, mainly basic and acidic. Homogeneous catalysts such as acids (H2SO4, HCl, H3PO4, AlCl3, ZnCl2, etc.) and bases (NaOH, KOH, CH3NaO, CH3KO, etc.) are among the widely used catalysts. While homogeneous acid catalysts require high temperature and pressure (Marchetti et al. 2011), homogeneous base catalysts need milder conditions (Vyas et al. 2010). The drawbacks mentioned above notably showcase the inevitable difficulty in separation and reusability of the catalyst and restrict its significance and use in biodiesel’s industrial-scale synthesis by using homogeneous catalysts (Bharti et al. 2021).
Homogeneous base catalysts accompanied by milder reaction condition like low temperature and pressure conditions, easy availability, and shorter reaction time (4000 times faster than acid-catalyzed transesterification) make homogenous base catalysts highly acceptable (Kawashima et al. 2009; Williams 2015). Generally, the base catalytic transesterification process undergoes at a minimum pressure (1.4–4.2 bar), temperatures (333–338 K), with a minimum concentration of catalyst (0.5–2 wt%), atmospheric pressure, average stoichiometric alcohol/oil molar ratio, and reaction times (typically 1 h) (Bhuiya et al. 2016).
The mechanism for the action of homogeneous base catalyst is given in Fig. 10.3. Verma et al. (2017) used both methanol and ethanol for the synthesis of Karanja biodiesel through KOH as a catalyst and reported the highest yield of 88.7% and 77% for methanolysis and ethanolysis, respectively, at a reaction temperature of 333 K with a molar ratio of 1:9 and 1.25 wt% of catalyst loading for 2 h. In another study, the waste soybean oil was transesterified with 7 wt% NaOH and gave 95% biodiesel yield with an oil-to-methanol molar ratio of 1:9, reaction temperature of 40 °C, and reaction time of 1 h 33 min (Tan et al. 2019). Another study reported that canola oil is transesterified using NaOH catalyst (3 wt%), yielding 85% biodiesel at the oil-to-methanol molar ratio of 1:6.5 at a slightly higher temperature of 70 °C for 2 h (Hariprasath et al. 2019).
Mechanism for base-catalyzed transesterification process. (Source: Kumar et al. 2021)
The most commonly used acid catalysts are sulfuric acid, ferric sulfate, hydrochloric acid, and sulfonic acid (Atadashi et al. 2013). The acid-catalyzed reaction proceeds between triglycerides, alcohols, and an acid catalyst, producing glycerol and biodiesel. Figure 10.4 shows the homogeneous acid-catalyzed reaction mechanism. Unlike base catalysts, acid catalysts are more economically viable and insensitive to the oil’s free fatty acid content. Sulfuric acid and hydrochloric acid are favored catalysts in this process (Lam et al. 2010). Oliveira et al. (2017) reported the yield of biodiesel as 93.5% using H2SO4 as a catalyst with oil to methanol ratio of 1:9 at a temperature of 70 °C. The alcohol-to-oil molar ratio is crucial in influencing the reaction.
Mechanism for acid-catalyzed transesterification process. (Source: Kumar et al. 2021)
2.6 Heterogeneous Catalysts
Heterogeneous catalysts are anti-corrosive and can be recovered quickly. This ease of recovery attributes to its different reactant phase systems, which assist an easy pathway for separating the catalyst from the reaction mixture through conventional separation techniques like filtration, sedimentation, and centrifugation (Diamantopoulos 2015). Examples of acidic heterogeneous catalysts are ZnO/I2, ZrO2/SO2–4, TiO2/SO2–4, niobic acid, sulfated zirconia, amberlyst-15, and Nafion NR50. In contrast, CaO, CaTiO3, CaZrO3, CaO-CeO2, CaMnO3, Ca2Fe2O5, KOH/Al2O3, KOH/NaY, Al2O3/KI, ETS-10 zeolite, and alumina/silica-supported K2CO3 are some examples of alkali heterogeneous catalysts (Leung et al. 2010).
A suitable heterogeneous catalyst must be mesoporous, highly stable, benign, and inexpensive (Changmai et al. 2020). It must also possess multi-functionality and strong, active sites, making it highly versatile and competitive (Chouhan and Sarma 2011). Presently, researchers are trying to make bifunctional heterogeneous catalysts that can be employed for both esterification and transesterification simultaneously, which could save energy, time, material, and production cost (Farooq et al. 2013; Mohiddin et al. 2021).
The commonly used solid catalysts are functionalized by sulfonated zirconia (SZ), Nafion resins, sulfonated saccharides, tungsten oxides, and organosulfur mesoporous silicas (Chopade et al. 2012). For instance, around 95% yield of biodiesel was obtained from neem oil at a temperature condition of 65 °C for a reaction time of 2 h with a catalyst loading of 1 and molar ratio of 9:1 (alcohol to oil) using sulfated zirconia as a catalyst (Muthu et al. 2010). Another study by Shu et al. (2010) reported the use of carbon-based solid acid catalyst at a temperature of 220 °C with a catalyst loading of 0.2 with the molar ratio of 16.8:1 (alcohol to oil) for 4.5 h using waste vegetable oil as a feedstock for the conversion of biodiesel which yielded to be 94.8%.
The heterogeneous alkali catalyst shows enhanced catalytic activity during transesterification under milder reaction conditions (Calero et al. 2014). Table 10.1 displays several types of base heterogeneous catalysts sued for the transesterification of lipids. Researchers have reported the complete conversion (100% conversion) to biodiesel of soybean oil using snail shell as a heterogeneous base catalyst at room temperature with the reaction time of 7 h at the oil-to-methanol molar ratio of 1:6 and a catalyst loading of 3 wt% (Laskar et al. 2018).
Several researchers experimented with kitchen waste as a heterogeneous base catalyst and found encouraging results. Likewise, researchers have obtained a biodiesel yield of 93% by transesterification of refined soybean oil at a temperature of 57.5 °C and reaction time of 3 h with 1:10 to be the molar ratio of oil to methanol at a catalyst loading of 7 wt% (Goli and Sahu 2018). Using chicken eggshell as a heterogeneous catalyst at 57.5 °C for 5 h and the oil-to-methanol ratio of 1:13 with 8.5 wt% of catalyst loading reported 90.41% biodiesel yield (Kirubakaran and Arul Mozhi Selvan 2018).
2.7 Nanocatalysts
Along with the development of heterogeneous catalysts, which are recyclable, stable, highly selective, and efficient, demand has been growing simultaneously concerning its improvement or modification as a catalyst that can be used for biodiesel production. Nanocatalysis has emerged as a suitable option. According to the reports, the market value of nanocatalysts had reached up to USD 9.58 billion in 2020, which is further expected to reach USD 22.9 billion by 2027. Nanoscale heterogeneous catalysts with larger surface areas allow them to be an attractive candidate for transesterification reactions (Bharti et al. 2020, 2021). Their catalytic properties can be modified accordingly by simply making acceptable changes to the shape and size of the active phase of the nanomaterials (Somorjai and Materer 1994). Nanoferrites exhibit strong ferrimagnetic properties containing iron oxide as the prime component. They are classified as (a) spinel (MFe2O4), (b) garnet (M3Fe5O12), and (c) hexagonal (MFe12O19), where M denotes transition metals like Mn, Fe, Co, Ni, Cu, and Zn (Bharti et al. 2020; Punia et al. 2020; Rana et al. 2015). The introduction of magnetic property into nanocatalyst helps with easier separation. It shows good recovery on applying an external magnetic field which facilitates the minor catalyst weight loss and high catalyst reusability compared to traditional separation techniques (Gardy et al. 2019). Amidst all the base nanocatalysts available to prepare biodiesel, CaO, zeolites, and hydrotalcite have received humongous attention. Among them, CaO has been extensively studied for its high activity, basicity, and milder reaction condition. Despite its advantages, it also shows some limitations in the recovery step of the catalyst from the reaction mixture, as during the process of a transesterification hydrogen bond is formed between the lattice oxygen species with methanol and glycerine, which increases the viscosity of glycerine and forms solid in suspension with CaO which hence inhibit the process of recovery (Kouzu and Haidaka 2012). Magnetic functionalized CaO helps overcome these limitations. Thus, a magnetic material is combined with the CaO and SrO samples to prepare a magnetic catalyst. This prepared catalyst was utilized to transesterify soybean oil into biodiesel by Zhang et al. (2016) and obtained a yield of 94.9% at 70 °C for 2 h with an oil-to-methanol molar ratio of 1:12 and 0.5 wt% of catalyst loading (Bashir et al. 2022). As the magnetic property of the catalyst allowed it to be recovered quickly after every cycle, it showed stability upon five reusable runs.
The biodiesel obtained using the oil extraction process possesses high viscosity and low volatility, contains polyunsaturated hydrocarbons, and is not suitable for use as a fuel. It needs further purification and modification to be used in vehicles (Table 10.2).
3 Scope and Challenges for Biodiesel Usage
The properties of biodiesel are a vital function of the type of feedstock used, with non-edible oils and waste materials as the most promising for its preparation. As a sustainable option, biodiesel is producible in more significant amounts from vegetable oils and fats of animal origin. The choice of raw material for biodiesel production depends on the availability of bio-resources in that specific region. As shown in Table 10.3, based on research trends, it was found that while countries like Cuba, India, Malaysia, Mali, Mozambique, Pakistan, Peru, Tanzania, Thailand, and Zimbabwe have explored Jatropha curcas, similarly, oilseed palm was researched in Brazil, Iran, Malaysia, Mexico, Peru, and Thailand; rapeseed in Canada, Chile, China, Greece, Italy, and Turkey; sunflower in Argentina, Canada, Greece, Italy, and Turkey; Pongamia pinnata in Australia, Bangladesh, and India; Callophyllum inophyllum in Australia and Malaysia; soybean in Argentina, Canada, Italy, and the United States; fish oil in Iran; used cooking oil in Ireland; animal fat and fish residues in Norway and Bangladesh; and microalgae in China as feedstock materials (Alagumalai et al. 2021; Jayakumar et al. 2021). Computing biodiesel from biomass is an acceptable strategy for the future bioenergy-based economic development path.
Other factors like reaction temperature, reaction and holding time, types of catalyst used, and reactor configuration also affect the quality and yield of biodiesel. Different researchers have examined various sources and response conditions to maximize oil output. Optimizing reaction parameters is essential to obtain biodiesel at a low cost. Further experiments are underway to alleviate the properties of biodiesel. Using convention transesterification methods, biodiesel with properties compared to conventional diesel oil can be obtained. As shown in Table 10.4, the flashpoint of biodiesel is 171 °C, much higher than mineral diesel. Thus, they are highly safe to use. In addition to this, the cetane number of biodiesel is much higher than conventional diesel, indicating its high combustion efficiency (Mishra and Goswami 2018). Researcher investigations have shown that using the ultrasonic method in the traditional process of transesterification can lead to the production of biodiesel with better lower heating values, lower viscosity, and density at less reaction time (4 min) (Ponnappan et al. 2021). Similar experiments are underway globally to improve the properties of biodiesel to improve its utilization potential further. Table 10.4 shows the benefits and drawbacks of various catalysts.
The cetane number of biodiesel indicates its compatibility with engines, including smooth start and running, i.e., engine knock and nitrous oxide emissions (Abomohra et al. 2022). While high heating value (HHV) defines its calorific value, fuel’s viscosity and blending ratio of biodiesel to diesel are the fundamental properties affecting the engine’s performance. Table 10.5 shows the properties of biodiesel, including its kinematic viscosity, cloud point, and cetane number obtained from different sources, which are in the range mentioned by the American Society for Testing and Materials (ASTM) and European Standards.
Non-edible oils like palm oil and cottonseed oil are attractive options to prepare biodiesel of international standards. In addition to this, locally available and low-cost biomass feedstocks are also investigated as a potential source of biodiesel. However, the significant challenges with biodiesel are its high viscosity, leading to the formation of larger droplets, poor fuel combustion, and deposition of black smoke in the combustion chamber. The polymerization of unsaturated fatty acids at higher temperatures results in gumming and injection choking. Transesterification of oil is the most common method to reduce viscosity and improve engine efficiency. A few investigations have shown concerns like low stability against oxidation, lack of long-duration shelf life, high cetane number, and high flash point compared to fossil fuels. The studies have shown that lowering NOx emission is still a challenge by using non-edible oils as substitutes in diesel engines.
Thus, biodiesel blending with petroleum fossil fuels has already been implemented worldwide. Various terms are used to define the proportion of biodiesel in petroleum. Bzz indicates the relative balance of biodiesel, where zz is the quantity of biodiesel in the blend. For instance, B20 and B80 terms are used to define 20% and 80% biodiesel.
4 Global Biodiesel Policies
In an attempt to foster the growth of biofuel production to improve the share of renewable energy, different countries have initiated various policy-level interventions like mandatory blending targets, tax exemptions, etc. The ethical issues with first-generation edible products have given impetus to research on biofuel production from non-edible oil cakes and waste biomass material. National biofuel policies of Brazil, India, and Indonesia are generally framed by leveraging the use of local resources like sugarcane, agro-waste, and palm oil, respectively (Saravanan et al. 2018; Lima et al. 2020; Halimatussadiah et al. 2021). Studies have shown that shells of groundnut, rubber seed, castor seed, sunflower, linseed, groundnut, and sesame seed can be used to produce biodiesel in Bangladesh annually. In addition, the country is reported to have a great potential to use non-edible waste animal skin to produce biodiesel (Mahmud et al. 2022).
Initially, the European Commission 2003 Biofuel Directive to increase the use of biofuel and other renewable fuels to 5.75% has impacted both production and consumption of biofuel in European nations. While biofuel policies in Brazil were driven by the petroleum fuel shortage, the US policies were framed to reduce greenhouse gas emissions in the framework. The Canadian Environmental Protection Act Bill C-33 (Canada), Renewable Fuel Standard (RFS2) (the United States), National Alcohol Program Protocol (Brazil), EU Directive 2009/28/EC (European Union), National Policy on Biofuels 2018 (India), and National Policy on Biofuels (Malaysia) are some of the critical global policies targeted at supporting research and development activities and commercialization of the same. In Germany, the Biofuel Quota Act, 2007, had set a target of 4.4% biodiesel in diesel, which was supported by many other policy initiatives like targeting a share of 18% for renewables to total energy share (Saravanan et al. 2018).
Key instruments adopted in the United States to promote biofuel production are supporting the feed crop supply, which include incentivizing farmers to grow biomass feedstock through the Biomass Crop Assistance Program (BCAP). Likewise, the Biorefinery Assistance Program assists biorefineries by providing easy loans (Saravanan et al. 2018). Import tariffs also play a significant role in protecting national industries from the external competition (Sorda et al. 2010). Technology, policies, environmental sustainability, and economics are the pillar of success for biofuel implementation programs.
Overall, the success of these policies depends on competition from other fuels and faster implementation of these policies. Issues related to efficient land use, soil health, and biodiversity need to be addressed through multifaceted, efficient policies encompassing these dimensions.
5 Conclusion and Future Direction
Biofuels are fuels obtained from biological sources and take much lesser time to form than fossil fuels. It can play a critical role in mitigating climate change, improving global energy security, and strengthening local economies. Four generations of fuels have been tested under lab and industrial-scale conditions. There are ethical issues associated with the first and second generations of raw materials where edible feedstock is used for biodiesel generation. Thus, more focus should be given to the application of third and fourth generations of fuels, which can serve the dual purpose of waste utilization and energy generation. With more advanced technology like synthetic biology-based raw materials, supercritical oil extraction, and ultrasonic treatment, plasma reactors are tested on a laboratory scale to improve the final product yield and quality.
The potential of biodiesel to replace conventional fuel is very high. Already many nations have adopted biodiesel blends with diesel oil. For many years, international and national policy-makers are also making efforts to reduce fossil fuel usage. Their focus is to encourage low-carbon technologies, and develop energy-efficient technologie. The reduction in environmental CO2 was observed due to biodiesel in the place of fossil fuels. It may be so because the ratio between CO2 emitted from biodiesel combustion is lesser than the CO2 utilized by the plants for photosynthesis (Lapuerta et al. 2008). SO2 emission was also lower using biodiesel (Basha et al. 2009). Newer techniques like particulate traps, catalysts, and recirculation of exhaust gas can also further improve the efficiency of biodiesel-based vehicles. Though sulfate and aromatic compound emissions are very low, research efforts are still underway to improve engine performance and reduce nitrous oxide emissions from biodiesel-based fuels.
With the collective efforts from researchers across the globe in advanced reactor configuration, along with the use of third and fourth generations of biofuel and optimization of reaction parameters, the scope of biodiesel as a low-cost, eco-friendly, and sustainable fuel is very high.
References
Abomohra AEF, Eladel H, Mohammed S (2022) Dual use of a local Protosiphon isolate BENHA2020 for biodiesel production and antioxidant activity of lipid-free biomass: a novel biorefinery approach for biomass valorization. Renew Energy 184:1104–1111
Aderibigbe FA, Shiru S, Amosa MK et al (2021) Heterogeneous catalysis of second generation oil for biodiesel production: a review. Chem Bio Eng Rev 8(2):78–89
Aigba P, Anyadiegwu F, Ogoke J (2021) Characterization of jatropha oil and its biodiesel. Adv Environ Stud 5:376–381
Alagumalai A, Mahian O, Hollmann F, Zhang W (2021) Environmentally benign solid catalysts for sustainable biodiesel production: a critical review. Sci Total Environ 768:144856
Aleman-Ramirez JL, Moreira J, Torres-Arellano S et al (2021) Preparation of a heterogeneous catalyst from moringa leaves as a sustainable precursor for biodiesel production. Fuel 284:118983
Ashraful AM, Masjuki HH, Kalam MA, Rizwanul Fattah IM, Imtenan S, Shahir SA, Mobarak HM (2014) Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: a review. Energy Convers Manag 80:202–228
Atabani AE, Silitonga AS, Badruddin IA et al (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev 16:2070–2093
Atadashi IM, Aroua MK, Aziz ARA, Sulaiman NMN (2013) The effects of catalysts in biodiesel production: a review. J Ind Eng Chem 19(1):14–26
Basha SA, Gopal KR, Jebaraj S (2009) A review on biodiesel production, combustion, emission and performance. Renew Sustain Energy Rev 13:1628–1634
Bashir MA, Wu S, Zhu J et al (2022) Recent development of advanced processing technologies for biodiesel production: a critical review. Fuel Process Technol 227:107120
Bharti MK, Gupta S, Chalia S et al (2020) Potential of magnetic nanoferrites in removal of heavy metals from contaminated water: mini review. J Supercond Nov Magn 33:3651. https://doi.org/10.1007/s10948-020-05657-1
Bharti MK, Chalia S, Thakur P et al (2021) Nanoferrites heterogeneous catalysts for biodiesel production from soybean and canola oil: a review. Environ Chem Lett 19:1–20
Bhuiya MMK, Rasul MG, Khan MMK et al (2016) Prospects of 2nd generation biodiesel as a sustainable fuel – Part: 1 Selection of feedstocks, oil extraction techniques and conversion technologies. Renew Sustain Energy Rev 55:1109–1128
Boz N, Degirmenbasi N, Kalyon DM (2009) Conversion of biomass to fuel: transesterification of vegetable oil to biodiesel using KF loaded nano-γ-Al2O3 as catalyst. Appl Catal B 89:590–596
Bukkarapu KR, Krishnasamy A (2022) Predicting engine fuel properties of biodiesel and biodiesel-diesel blends using spectroscopy based approach. Fuel Process Technol 230:107227
Calero J, Luna D, Sancho ED et al (2014) Development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 122:94–102
Celante D, Schenkel JVD, de Castilhos F (2018) Biodiesel production from soybean oil and dimethyl carbonate catalyzed by potassium methoxide. Fuel 212:101–107
Chang AC, Louh RF, Wong D et al (2011) Hydrogen production by aqueous-phase biomass reforming over carbon textile supported Pt-Ru bimetallic catalysts. Int J Hydrogen Energy 36:8794–8799
Changmai B, Sudarsanam P, Rokhum L (2020) Biodiesel production using a renewable mesoporous solid catalyst. Ind Crop Prod 145:111911
Cheng F, Li X (2018) Preparation and application of biochar-based catalysts for biofuel production. Catalysts 8(9):346
Chopade SG, Kulkarni KS, Kulkarni AD et al (2012) Solid heterogeneous catalysts for production of biodiesel from trans-esterification of triglycerides with methanol: a review. Acta Chim Pharm Indica 2(1):8–14
Chouhan APS, Sarma AK (2011) Modern heterogeneous catalysts for biodiesel production: a comprehensive review. Renew Sustain Energy Rev 15:4378–4399
Da Silva C, Oliveira JV (2014) Biodiesel production through non-catalytic supercritical transesterification: current state and perspectives. Braz J Chem Eng 31(2):271
Devi A, Singh A, Bajar S et al (2021) Nanomaterial in liquid biofuel production: applications and current status. Environ Sustain 4:343–353
Diamantopoulos N (2015) Comprehensive review on the biodiesel production using solid acid heterogeneous catalysts. J Thermodyn Catal 06:1–8
el Sherbiny SA, Refaat AA, el Sheltawy ST (2010) Production of biodiesel using the microwave technique. J Adv Res 1:309–314
Farooq M, Ramli A, Subbarao D (2013) Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J Clean Prod 59:131–140
Feyzi M, Hassankhani A, Rafiee H (2013) Preparation and characterization of CsAlFe3O4 nanocatalysts for biodiesel production. Energy Convers Manage 71:62–68
Gad MS, Ismail MA (2021) Effect of waste cooking oil biodiesel blending with gasoline and kerosene on diesel engine performance, emissions and combustion characteristics. Process Saf Environ Prot 149:1–10
Gardy J, Nourafkan E, Osatiashtiani A et al (2019) A core-shell SO4/Mg-Al-Fe3O4 catalyst for biodiesel production. Appl Catal B Environ 259:118093
Ghesti GF, Silveira EA, Guimarães MG, Evaristo RBW, Costa M (2022) Towards a sustainable waste-to-energy pathway to pequi biomass residues: biochar, syngas, and biodiesel analysis. Waste Manag 143:144–156
Goli J, Sahu O (2018) Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew Energy 128:142–154
Gupta AR, Rathod VK (2021) Application of catalysts in biodiesel production. Chapter 3. In: Inamuddin, Ahamed MI, Boddula R, Rezakazemi M (eds) Biodiesel technology and applications. https://doi.org/10.1002/9781119724957.ch3
Halimatussadiah A, Nainggolan D, Yui S, Moeis FR, Siregar AA (2021) Progressive biodiesel policy in Indonesia: does the Government’s economic proposition hold. Renew Sustain Energy Rev 150:111431
Hariprasath P, Selvamani ST, Vigneshwar M et al (2019) Comparative analysis of cashew and canola oil biodiesel with homogeneous catalyst by transesterification method. Mater Today Proc 16:1357
Hossain ABMS, Mazen MA (2010) Effects of catalyst types and concentrations on biodiesel production from waste soybean oil biomass as renewable energy and environmental recycling process. Aust J Crop Sci 4(7):550–555
Hu S, Guan Y, Wang Y, Han H (2011) Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl Energy 88:2685–2690
Jayakumar M, Karmegam N, Gundupalli MP et al (2021) Heterogeneous base catalysts: synthesis and application for biodiesel production – a review. Bioresour Technol 331:125054
Kanwar Gaur R, Goyal R (2022) A review: Effect on performance and emission characteristics of waste cooking oil biodiesel-diesel blends on IC engine. Mater Today Proc 63:643–646
Kawashima A, Matsubara K, Honda K (2009) Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresour Technol 100(2):696–700
Khan K, Kumar G, Sharma AK, Kumar PS, Mandal C, Chintala V (2018) Performance and emission characteristics of a diesel engine using complementary blending of castor and karanja biodiesel. Biofuels 9:53–60
Kim DS, Hanifzadeh M, Kumar A (2018) Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ Prog Sustain Energy 37(1):7–19
Kirubakaran K, Arul Mozhi Selvan V (2018) Eggshell as heterogeneous catalyst for synthesis of biodiesel from high free fatty acid chicken fat and its working characteristics on a CI engine. J Environ Chem Eng 6:4490–4503
Kohler M (2019) Economic assessment of ethanol production. In: Basile A, Iulianelli A, Dalena F, Veziroğlu TN (eds) Ethanol. Elsevier, Oxford, pp 505–521
Kongprawes G, Wongsawaeng D, Ngaosuwan K et al (2021) Low-temperature and atmospheric pressure plasma for palm biodiesel hydrogenation. Sci Rep 11:14224. https://doi.org/10.21203/rs.3.rs-282528/v1
Kouzu M, Haidaka J (2012) Transesterification of vegetable oil into biodiesel catalyzed by CaO: a review. Fuel 93:1–12
Kumar N, Sonthalia A, Tomar M et al (2020) An experimental investigation on spray, performance and emission of hydrotreated waste cooking oil blends in an agricultural engine. Int J Engine Res 22(7):1468087420928734
Kumar P, Sharma PK, Tripathi S et al (2021) Realization of second generation of biofuel and extraction techniques. In: Prasad L, Pradahan S, Naik SN (eds) Biofuel extraction techniques. Wiley, Hoboken, NJ
Lam MK, Lee KT, Mohamed AR (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28(4):500–518
Lapuerta M, Armas O, Fernandez JR (2008) Effect of biodiesel fuels on diesel engine emissions. Prog Energy Combust Sci 34:198–223
Laskar LB, Rajkumari K, Gupta R et al (2018) Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv 8:20131–20142
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87(4):1083
Lima M, da Silva Junior CA, Pelissari TD, Lourençoni T, Luz IMS, Lopes FJA (2020) Sugarcane: Brazilian public policies threaten the Amazon and Pantanal biomes. Perspect Ecol Conserv 18:210–212
Mahmood H, Moniruzzaman M, Iqbal T, Khan MJ (2019) Recent advances in the pretreatment of lignocellulosic biomass for biofuels and value-added products. Curr Opin Green Sustain Chem 20:18–24
Mahmud S, Haider ASMR, Shahriar ST, Salehin S, Hasan ASMM, Johansson MT (2022) Bioethanol and biodiesel blended fuels—feasibility analysis of biofuel feedstocks in Bangladesh. Energy Rep 8:1741–1756
Marchetti JM, Pedernera MN, Schbib NS (2011) Production of biodiesel from acid oil using sulfuric acid as catalyst: kinetics study. Int J Low Carbon Technol 6:38–43
Mishra VK, Goswami R (2018) A review of production, properties and advantages of biodiesel. Biofuels 9(2):273–289
Mohiddin MNB, Tan YH, Seow YX et al (2021) Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: a review. J Ind Eng Chem 98:60–81
Muthu H, Selvabala VS, Varathachary TK et al (2010) Synthesis of biodiesel from neem oil using sulfated zirconia via transesterification. Braz J Chem Eng 27(4):601–608
Nagappan M, Devaraj AM, Babu J, Vibhav Saxena N, Prakash O, Kumar P, Sharma A (2022) Impact of additives on combustion, performance and exhaust emission of biodiesel fueled direct injection diesel engine. Mater Today Proc 62:2326
Nakatani N, Takamori H, Takeda K et al (2009) Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour Technol 100(3):1510–1513
Nasreen S, Nafees ML, Qureshi LA et al (2018) Review of catalytic transesterification methods for biodiesel production. In: Krzysztof B (ed) Biofuels - state of development. IntechOpen, London, pp 93–119
Nath B, Das B, Kalita P, Basumatary S (2019) Waste to value addition: utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J Clean Prod 239:118112
Nath B, Kalita P, Das B, Basumatary S (2020) Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew Energy 151:295–310
Navas MB, Lick ID, Bolla PA et al (2018) Transesterification of soybean and castor oil with methanol and butanol using heterogeneous basic catalysts to obtain biodiesel. Chem Eng Sci 187:444–454
Oliveira JP, Antunes PWP, Mordente TZ et al (2017) Biodiesel production from scum of grease traps and sludge from septic tanks. Clean Technol Environ Policy 19(4):1231
OPEC (2021) OPEC monthly oil market report April 2021. OPEC, Vienna, Austria. https://www.opec.org/opec_web/en/publications/338.htm
Pali HS, Kumar N (2016) Comparative assessment of sal and kusum biodiesel properties. Energy Sour A Recovery Util Environ Effects 38:3391–3396
Perera F (2018) Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: solutions exist. Int J Environ Res Public Health 15(1):16
Ponnappan VS, Babu MD, Nagappan B et al (2021) Investigation on the effect of ultrasound irradiation on biodiesel properties and transesterification parameters. Environ Sci Pollut Res 28:64769–64777
Punia P, Bharti MK, Chalia S et al (2020) Recent advances in synthesis, characterization, and applications of nanoparticles for contaminated water treatment - a review. Ceram Int 47(2):1526–1550
Quesada J, Olivares P (2010) Supercritical biodiesel production from raw soybean oil. J Biofuels 1:115
Rana K, Thakur P, Sharma P et al (2015) Improved structural and magnetic properties of cobalt nanoferrites: influence of sintering temperature. Ceram Int 41:4492–4497
Saravanan AP, Mathimani T, Deviram G, Rajendran K, Pugazhendhi A (2018) Biofuel policy in India: a review of policy barriers in sustainable marketing of biofuel. J Cleaner Product 193:734–747. https://doi.org/10.1016/j.jclepro.2018.05.033
Shu Q, Gao J, Nawaz Z et al (2010) Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl Energy 87(8):2589–2596
Silva GF, Camargo FL, Ferreira ALO (2011) Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol. Fuel Process Technol 92(3):407–413
Singh D, Sharma D, Soni SL et al (2021) A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 285:1–19
Somorjai GA, Materer N (1994) Surface structures in ammonia synthesis. Top Catal 1:215–231
Sorda G, Banse M, Kemfert C (2010) An overview of biofuel policies across the world. Energy Policy 38(11):6977–6988. https://doi.org/10.1016/j.enpol.2010.06.066
Suzihaque MUH, Alwi H, Kalthum Ibrahim U, Abdullah S, Haron N (2022) Biodiesel production from waste cooking oil: a brief review. Mater Today Proc 63:S490–S495
Tamjidi S, Esmaeili H, Moghadas BK (2021) Performance of functionalized magnetic nanocatalysts and feedstocks on biodiesel production: a review study. J Clean Prod 305:127200
Tan CH, Nagarajan D, Show PL et al (2019) Biodiesel from microalgae. In: Pandey A, Larroche C, Dussap CG, Gnansounou E, Khanal SK, Ricke S (eds) Biofuels: alternative feedstocks and conversion processes for the production of liquid and gaseous biofuels. Elsevier, Amsterdam, pp 601–628
Thakur P, Chahar D, Taneja S et al (2020) A review on MnZn ferrites: synthesis, characterization and applications. Ceram Int 46:15740–15763
Topare NS, Paitl KD, Naik P et al (2015) Application of ultrasound for synthesis of biodiesel. Emerg Trends Chem Eng 2(1):1–8
UN (2015) Paris Agreement. https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement
Velusamy K, Devanand J, Senthil Kumar P, Soundarajan K et al (2021) A review on nano-catalysts and biochar-based catalysts for biofuel production. Fuel 306:121632
Verma P, Dwivedi G, Sharma MP (2017) Comprehensive analysis on potential factors of ethanol in Karanja biodiesel production and its kinetic studies. Fuel 188:586
Vyas AP, Verma JL, Subrahmanyam N (2010) A review on FAME production processes. Fuel 89:1–9
Wan L, Liu H, Nasreen S et al (2018) High temperature transesterification of soybean oil with methanol using manganese carbonate as catalyst. Chem Ind Chem Eng Q 24(1):9–22
Wang H, Peng X, Zhang H, Yang S, Li H (2021) Microorganisms-promoted biodiesel production from biomass: a review. Energy Convers Manag 12:100137
Williams L (2015) Synthesis and characterization of biodiesel fuels by clay catalyzed transesterifications. Stephen F. Austin State University
Zahan K, Kano M (2018) Biodiesel production from palm oil its by-products and mill effluent: a review. Energies 11(8):2132. https://doi.org/10.3390/en11082132
Zhang PB, Shi M, Liu YL et al (2016) Influence of crystal of Fe2O3 in magnetism and activity of nanoparticle CaO@Fe2O3 for biodiesel production. Fuel 186:787–791
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kaur, P.J., Sharma, P.K., Chaturvedi, S. (2023). Biodiesel from Biomass: Production of Sustainable Biodiesel Fuel. In: Pathak, P.D., Mandavgane, S.A. (eds) Biorefinery: A Sustainable Approach for the Production of Biomaterials, Biochemicals and Biofuels. Springer, Singapore. https://doi.org/10.1007/978-981-19-7481-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-7481-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7480-9
Online ISBN: 978-981-19-7481-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)