Abstract
Scum from grease traps and sludge from wastewater are an oil-rich waste from the wastewater treatment plants. To mitigate possible negative impacts on the environment, the production of biodiesel from grease traps and sludge from sumps and septic tanks was investigated. These wastes are characterized by a high content of free fatty acids (FFA) and processed towards biodiesel by acidic esterification. The reaction of FFAs by an acid catalyst was optimized through a response surface methodology. The best conversion (95.3%) was obtained with acid catalyser (1.5% w/w oil/4 h) and raw material from the grease trap at the university restaurant at 70 °C and a molar ratio of 1:9 (oil/alcohol). In conclusion, there is viability of biofuels production through the use of sanitation waste as raw material.
Graphical Abstract
Scum and sludge are an oil-rich waste from the sewage treatment plants. To mitigate possible negative impacts on the environment, these raw materials were used as feedstock for biodiesel production.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of alternative technologies has led to the production of biodiesel. This biofuel is used as a substitute for petroleum, which traditionally has been considered as the best candidate compared to all other energy sources (Leung et al. 2010). Biodiesel has been used directly or blended with diesel oil at various levels in many countries. The increasing demand for biodiesel is also due to awareness of the environmental impact of emissions from conventional fossil fuels combustion. Compared to petroleum-based diesel, biodiesel has a more favourable combustion emission profile such as low emission of carbon monoxide, particulate matter and unburned hydrocarbon (Chen et al. 2008).
Biodiesel can be produced by the transesterification reaction, in which animal fat or vegetable oil is reacted with alcohol in the presence of a catalyst (Adewale et al. 2015). Price and availability are important factors that determine different types of feedstocks used for biodiesel production from one region of the world to another (Ramadhas et al. 2005; Balat 2011; Kumar et al. 2011; Badday et al. 2013; Sanli et al. 2013). Despite the current growth, sustainability of the biodiesel industries may be limited due to the industry’s inability to secure cheap feedstock (Rhee et al. 1989).
The use of waste oil and grease (O&G), such as waste frying oil, fatty acids, scum from grease traps and sewage, as raw materials for the generation of biodiesel can significantly contribute to the reduction of production costs and characterizes the process as sewage treatment (Oliveira et al. 2014), since O&G affects the operation of traditional wastewater treatment plants, inhibiting biological activity in activated sludge reactors and causing clogging and fouling of pumps and piping (Rhee et al. 1989). In a study conducted by the National Renewable Energy Laboratory in the USA, 30 metropolitan areas generated an average of 6 kg of fats, oil and grease per year per person. This figure is even larger than the estimated volume of restaurant waste cooking oil generated in the USA, which is just around 4 kg/person/year (Wiltsee 1998).
Previous studies have focused on fats, oil and grease samples obtained directly from food establishments (Ramadhas et al. 2005; Kulkarni and Dalai 2006; Badday et al. 2013; Ezebor et al. 2014). Moreover, there are no published articles on the esterification of sanitation waste. In the present study, biodiesel production of scum from grease traps and sludge from sumps and septic tanks using the acid-catalysed reaction was studied. The reactions were optimized using response surface methodology. The produced biodiesel was analysed and compared to the standard specifications by the Brazilian National Agency of Petroleum, Natural Gas and Biofuels. With the completion of this work, it will be possible to determine whether the oily from sanitation waste can be an alternative for the generation of biodiesel.
Experimental
Raw material for esterification
The raw materials used in this study for the production of biodiesel were the following: scum from the grease trap of a food processing plant (SGT-FPP) from the municipality Grande Vitoria; scum from the grease trap of the university restaurant (SGT-UR) at the Federal University of Espírito Santo (UFES); scum from the grease trap at the UFES wastewater treatment station (SGT-WTS); and sludge from sumps and septic tanks (SSST) at the campus of UFES. The grease trap samples were collected according to the Brazilian Regulatory Standard 10007 (ABNT 2004).
Extraction and characterization of sanitation waste oil
The extraction of oil and grease and the physical–chemical characterization of the oil extracted as well as the chromatography analysis (fatty acid composition) can be observed in Oliveira et al. (2016), previously published by this research group.
Experimental design/statistical analysis
To check the effect of the variables on the reaction conversion, as well as to determine the conditions that maximize the synthesis of esters, a factorial design (32) with 3 levels and 2 variables was adopted. Table 1 shows the ranges of the study variables. These ranges are defined to cover most of the studies described in the literature (Freedman et al. 1984; Bondioli 2004; Mondala et al. 2009; Oliveira et al. 2010; Muhammad and Rohani 2011; Aitao et al. 2012).
The effects of each of the selected variables were analysed with regard to the conversion using the computer program STATISTICA version 10 as a statistical tool. The statistical analysis allowed us to express the process conversion as a polynomial model; that is, the response can be written as a function of the variables. An analysis of variance (ANOVA) of the data was also performed, and the values were considered significant when p < 0.05. The optimal values of the independent variables were determined by performing a three-dimensional analysis of the response surface of the independent and dependent variables.
Acid esterification
The esterification procedure with acid catalysis is based on the reaction between the waste oil and the ethanol in an acid medium to obtain ethyl esters from fatty acids. The catalyser used was sulphuric acid (H2SO4, Sigma-Aldrich) at a fixed concentration of 1.5% (w/w oil) based on the data in the literature for this type of waste (Freedman et al. 1984, Oliveira et al. 2010). The reactions were conducted using 125-mL Erlenmeyer flasks, in which 10 g of oil, ethanol in the molar ratio determined by the experimental design and sulphuric acid at 1.5% w/w relative to the oil were held at temperatures of 50, 60 and 70 °C and continuously stirred for 4 h. After the 4 h, an aliquot was extracted from the medium for quantification of the biodiesel, and subsequently, the mixture was washed twice with water to neutralize the pH, followed by centrifugation to separate the glycerol and impurities.
Calculation of the conversion to ethyl esters
The conversion into biodiesel was calculated according to measured reductions in the values of acidity index. As the esters form, the amount of free fatty acids decreases, and the acidity index therefore decreases. According to Oliveira et al. (2010), this methodology can provide a good prediction of the conversion to esters, with very similar values compared to data obtained with gas chromatography. Other authors have already demonstrated the efficiency of this methodology (Mello et al. 2008; Lucena et al. 2011; Ma et al. 2015) by using Eq. 1, used to determine conversion:
where IA0 is the oil acidity and IAF is the final acidity of the biodiesel.
Biodiesel analysis
To verify that the product meets the Brazilian specifications, we used several analytical methodologies described by the American Oil Chemists Society (AOCS 1997) and American Society for Testing and Materials (ASTM 2001), such as the acidity index (%), water content (%), density, flash point and total glycerine (%).
Results and discussion
The extraction of oil and grease and the physical–chemical characterization of the oil extracted can be observed in Oliveira et al. (2016), previously published by this research group. In Fig. 1 are shown pictures of stages of oils extraction of raw grease trap waste for biodiesel production.
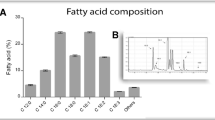
The amounts of oils from the grease traps and from sludge from sumps and septic tank are shown in Fig. 2a. The acid index from the extracted oils is shown in Fig. 2b. According to Kulkarni and Dalai (2006), if the FFA content exceeds 1–3%, acid transesterification is considered the best route to convert the FFA into esters. The composition of free fatty acids is shown in Fig. 2c. The heterogeneity of FFAs in oils and greases from sanitation waste samples is explained by the high use of animal fats and vegetable oils from the kitchens that are discharged in the effluent.
Mean values with SD of oils and grease from sanitation wastes (a); mean values with SD of acid index (mg KOH g−1) from extracted oils (b) and composition of free fatty acids in this raw materials (c). SGT-FPP scum from the grease trap of a food processing plant; SGT-UR scum from the grease trap of the university restaurant; SGT-WTS scum from the grease trap at the UFES wastewater treatment station; SSST sludge from sumps and septic tanks
Acid esterification
The conversion percentages of the esterification reactions via acid catalysis with the 1.5% concentration of H2SO4 and the reaction time of 4 h were obtained at different molar ratios and temperatures and are shown in Table 2.
Table 2 shows the result of the conversions of waste oils and grease to esters by acid catalysis with sulphuric acid. It is noted that the highest conversion with this type of catalysis was 95.3% at a temperature of 70 °C and a molar ratio of 1:9 (oil/alcohol) using oils and grease from the SGT-UR. For the SGT-FPP, the highest conversion was 93.4% at 60 °C and a molar ratio of 1:6 (oil/alcohol). For the SGT-WTS, the highest conversion obtained was 92.1% at 70 °C and a molar ratio of 1:9 (oil/alcohol), whereas the SSST oils and grease exhibited the lowest biodiesel conversions among the evaluated waste types, with a maximum conversion of 74.9% at 60 °C and a molar ratio of 1:9. The ANOVA is described in Table 3.
For the raw material from the SGT-FPP, the ANOVA showed that the linear and quadratic molar ratio, the quadratic temperature and the linear interaction between the variables are significantly related to the conversion percentage of the acid catalysis at a significance level of 0.05. For the evaluated reactions with oils and grease from the SGT-UR, it was found that only the linear and quadratic molar ratios were significant. For the catalyses involving raw materials from other waste (SGT-WTS and SSST), only the linear molar ratio was significant.
Considering the regression coefficients obtained at a 95% confidence interval, it was possible to write mathematical model corresponding to the response variable (conversion) according to the equations below:
where C (%) is the conversion to biodiesel given as a percentage, R is the molar ratio (oil/alcohol), and T is the temperature in °C.
The analyses of the biodiesel conversions with the four types of raw materials are given in the surface response plots as shown in Fig. 3.
For acid-catalysed reactions with all four types of waste can be no increase in conversion when the molar ratio (oil/alcohol) is increased. The temperature had little or no significance for the conversion of esters under the conditions evaluated, and the transesterification using SSST oils exhibited very low conversions. It is worth noting that the conversions were almost entirely above or near 90%, with the exception of biodiesel made with oils and grease from the sludge from sumps and septic tanks. This low conversion can be explained by high moisture content, which may have negatively influenced esterification or the presence of non-esterifiable lipid material.
Overall, the oily waste from grease traps showed promising results with this catalyser. The acid catalysis results for biodiesel production from sanitation oily waste studied herein were superior to those reported in the literature by several authors. Barros et al. (2008), using demulsified waste fat from grease traps in a mall in Blumenau (Brazil), obtained a maximum biodiesel conversion of 80% using sulphuric acid (H2SO4) as a catalyser. In Canada, Muhammad and Rohani (2011) studied acid catalysis for the production of biodiesel from oils and grease derived from primary and secondary sludge and obtained conversion of 41.25 and 38.94% (w/w dry sludge), respectively, with the use of natural zeolites for the removal of water formed during the esterification process. Other authors have also studied the production of biodiesel from sewage sludge using sulphuric acid as a catalyst, including Mondala et al. (2009) in Tuscaloosa, USA., who obtained a 10% methyl ester conversion, and Revellame et al. (2010), who found values close to 4% of biodiesel per dry weight of sludge.
Considering the high yield results obtained here validates the use of oils and grease from sanitation waste as raw material for acid esterification reaction for further production of commercial biodiesel.
Quality analysis of biodiesel synthesized
Table 4 presents the fuel properties of biodiesel compared with standards ANP—Brazilian National Agency of Petroleum, Natural Gas and Biofuels (Resolution 7) (ANP 2010) for pure biodiesel (B100). The characterization was done using the conditions of the best yield of biodiesel: SGT-UR under the highest temperature (70 °C) and highest oil/alcohol molar ratio (1:9) conditions. The only parameter that was not within the specifications was the acidity index. It is expected that a larger number of washes during the purification of the biodiesel should decrease the acidity index because the FFA are drawn into the aqueous phase due to the polarity.
Conclusions
The sanitation waste is available in large amounts in urban centres and represents an environmental issue. Based on results of this work, it is suggested that oils and grease from grease traps and sludge from sumps and septic tanks are a potential source of lipids for the production of the biodiesel. The results showed high biodiesel conversion rates via the optimization modelling for studied catalytic route. The best conversion rate found was 95.3%, and the optimum set of operational conditions was obtained using the lipid material from the grease trap of the university restaurant catalysed with the sulphuric acid at an optimal temperature of 70 °C and a molar ratio of 1:9 (oil/alcohol).
References
ABNT (2004) Associação Brasileira de Normas Técnicas, Amostragem de Resíduos–procedimento–NBR 10007. São Paulo, Brazil. https://www.abntcatalogo.com.br. Accessed 25 Sept 2015
Adewale P, Dumont MJ, Ngadi M (2015) Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew Sustain Energy Rev 45:574–588
Aitao LI, Thao PN, Jinyong Y, Kaiyuan T, Zhi L (2012) Whole-cell based solvent-free system for one-pot production of biodiesel from waste grease. Bioresour Technol 114:725–729
ANP (2010) Brazilian National Agency of Petroleum Natural Gas and Biofuels, Rio de Janeiro, Brazil. www.anp.gov.br/petro/legis_biodiesel.asp. Accessed 08 May 2014
AOCS—American Oil Chemists Society (1997) Official method Ca-5a-40- free fatty acids. Boulder, Urbana
ASTM—American Society for Testing and Materials (2001) Annual book of ASTM, section 5 petroleum products. Lubricants and Fossil Fuels, West Conshohocken
Badday AS, Abdullah AZ, Lee KT (2013) Optimization of biodiesel production process from Jatropha oil using supported heteropolyacid catalyst and assisted by ultrasonic energy. Renew Energy 50:427–432
Balat M (2011) Potential alternatives to edible oils for biodiesel production–a review of current work. Energy Convers Manage 52:1479–1492
Barros AAC, Wust E, Meier HF (2008) Evaluate the waste fatty acid by scientific and technical study to obtain biodiesel. Eng Sanit Ambient 13:255–262
Bondioli P (2004) The preparation of fatty acids esters by means of catalytic reactions. Top Catal 27:77–82
Chen X, Du W, Liu D (2008) Response surface optimization of biocatalytic biodiesel production with acid oil. Biochem Eng J 40:423–429
Ezebor F, Khairuddean M, Abdullah AZ, Boey PL (2014) Esterification of oily-FFA and transesterification of high FFA waste oils using novel palm trunk and bagasse-derived catalysts. Energy Convers Manag 88:1143–1150
Freedman B, Pryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. JAOCS 61:1638–1643
Kulkarni MG, Dalai AK (2006) Waste cooking oil—an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913
Kumar G, Kumar D, Johari RP, Singh CP (2011) Enzymatic transesterification of Jatropha curcas oil assisted by ultrasonication. Ultrason Sonochem 18:923–927
Leung DYC, Xuan W, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87:1083–1095
Lucena IL, Saboya RMA, Oliveira JFG, Rodrigues ML, Torres JAEB, Cavalcante CL (2011) Oleic acid esterification with ethanol under continuous water removal conditions. Fuel 90:902–904
Ma LL, Han Y, Sun KA, Lu J, Ding JC (2015) Kinetic and thermodynamic studies of the esterification of acidified oil catalyzed by sulfonated cation exchange resin. J Energy Chem 24:456–462
Mello VM, Oliveira FCC, Fraga WG, Nascimento CJ, Suarez PAZ (2008) Determination of the content of fatty acid methyl esters (FAME) in biodiesel samples obtained by esterification using 1H-NMR spectroscopy. Magn Reson Chem 46:1051–1054
Mondala A, Liang K, Toghiani H, Hernandez R, French T (2009) Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour Technol 100(1203):1210
Muhammad NS, Rohani S (2011) Experimental analysis of lipid extraction and biodiesel production from wastewater sludge. Fuel Process Technol 92:2241–2251
Oliveira JFG, Lucena IL, Saboya RMA, Rodrigues ML, Torres AEB, Fernandes FAN, Cavalcante CL, Parente EJ (2010) Biodiesel production from waste coconut oil by esterification with ethanol: the effect of water removal by adsorptionRenewable. Energy 35:2581–2584
Oliveira JP, Antunes PWP, Pinotti LM, Cassini STA (2014) Physico-chemical characterization of oily sanitary waste and of oils and greases extracted for conversion into biofuels. Quim Nova 37:597–602
Oliveira JP, Antunes PWP, Santos AR, Mordente TZ, Pinotti LM, Cassini STA (2016) Transesterification of Sanitation Waste for Biodiesel production. Waste Biomass Valorization. doi:10.1007/s12649-016-9581-6
Ramadhas AS, Jayaraj S, Muraleedharan C (2005) Biodiesel production from high FFA rubber seed oil. Fuel 84:335–340
Revellame E, Hernandez R, French W, Holmes W, Alley E (2010) Biodiesel from activated sludge via in situ transesterification. J Chem Technol Biotechnol 85:614–620
Rhee CH, Martyn PC, Kremer JG (1989) Removal of oil and grease in oil processing wastewater sanitation district of Los Angeles County, U.S.A
Sanli H, Canakci M, Alptekin E (2013) Predicting the higher heating values of waste frying oils as potential biodiesel feedstock. Fuel 115:850–854
Wiltsee G (1998) Urban waste grease resource assessment. National Renewable Energy Laboratory, Colorado: NREL/SR-570-26141
Funding
This study was funded by FINEP PROSAB—BRAZIL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Oliveira, J.P., Antunes, P.W.P., Mordente, T.Z. et al. Biodiesel production from scum of grease traps and sludge from septic tanks. Clean Techn Environ Policy 19, 1231–1237 (2017). https://doi.org/10.1007/s10098-016-1308-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1308-7