Abstract
Aquaporins (AQPs) are a family of membrane water channels that basically function as regulators of intracellular and intercellular water flow. To date, 13 AQPs, distributed widely in specific cell types in various organs and tissues, have been characterized in humans. A pair of NPA boxes forming a pore is highly conserved among all aquaporins and is also key residues for the classification of AQP superfamily into four groups according to primary sequences. AQPs may also be classified based on their transport properties. So far, chromosome localization and gene structure of 13 human AQPs have been identified, which is definitely helpful for studying phenotypes and potential targets in naturally occurring and synthetic mutations in human or cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Classification of Aquaporins
A large number of evidences have shown an unexpected diversity of aquaporins (AQPs) in both prokaryotic and eukaryotic organisms [1, 2] since the discovery of AQP1. More than 300 different aquaporins have been discovered so far in which 13 isoforms have been identified (AQP0–AQP12) in human. AQPs are integral, hydrophobic, transmembrane proteins that primarily facilitate the passive transport of water depending on the osmotic pressure on both sides of membrane. Subsequent studies show that AQPs can transport not only water molecules but also other small, uncharged molecules, i.e., glycerol, urea, down their concentration gradients.
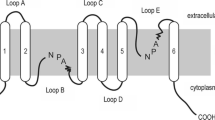
Structural analysis of several AQPs has established that these protein channels share a common structural feature. The functional aquaporin unit is a homotetramer, which comprises six α-helix transmembrane domains with two conserved asparagine–proline–alanine (NPA) motifs embedding into the plasma membrane, a signature sequence of water channels (see Chap. 3). Conformational changes of AQP protein permit other molecules passing through plasma membrane, i.e., urea, glycerol, H2O2, NH3, CO2, etc.
According to their structural and functional similarities, AQPs are initially subdivided into two subfamilies, classical AQPs (water-selective) and aquaglyceroporins (glycerol channel, Glps) aquaporins. However, further studies revealed that both the subfamilies overlap functionally, for examples, some classical AQPs transport water and other small solutes, e.g., glycerol. In addition, a new group of AQPs discovered showed that their structure is highly deviated from the previous AQPs especially around the AQP NPA box [3,4,5]. This subfamily was later named superaquaporin (also called unorthodox aquaporin) as it has very low homology with the previous two subfamilies [4]. This classification was usually accepted in physiology.
Later, it was found out that several members, e.g., AQP8 and AQP6 in classical AQP family have unique characteristics. Aquaporins are therefore organized into four categories, classical aquaporins, unorthodoxaquaporins, Aqp8-type aquaammoniaporins, and aquaglyceroporns, according to the phylogenetic tree or phylogenetic topology inferred from Bayesian inference (Fig. 1.1) [2, 4, 6]. This classification is identified based on the transport functions and properties of aquaporins.
The phylogenetic tree of 13 human AQPs. The tree shows the classical AQPs (AQP0, AQP1, AQP2, AQP4, AQP5, and AQP6) (light pink oval); the aquaammoniaporin AQP8 (light blue oval); the aquaglyceroporins (AQP3, AQP7, AQP9, AQP10, light green oval); and the superaquaporins (AQP11, AQP12, light yellow oval). (Modified from [4])
The first subfamily is that of aquaporins, the water selective or specific water channels, also named as “orthodox,” “classical” aquaporins, including AQP0, AQP1, AQP2, AQP4, AQP5, and AQP6. This subfamily of AQPs has been extensively studied, which help us define regulation of AQP expression in the body and their potential roles in physiological and pathophysiological states. Evidence, however, appears to suggest that AQP6 be classified as unorthodox aquaporins, due to low water permeability of AQP6 [7, 8].
The second subfamily of related proteins has low conserved amino acid sequences around the NPA boxes unclassifiable to the first two subfamilies [4]. Mammalian AQP11 and AQP12 are the only two members in this subfamily, which have been called “superaquaporins” or “unorthodox aquaporins.” The NPA boxes of these two AQPs are highly deviated from those of other classical AQPs with homology less than 20%, indicating that they belong to a supergene family of AQPs. The signature sequence for these AQPs is the cysteine residue at the nine residues downstream of the C-terminal of the second NPA, which is exposed on the surface of the protein at the periplasmic side of the membrane [9, 10]. The structure and function of AQP11 and AQP12 are currently poorly understood. As this subfamily focuses on deviated NPA itself and unconventional functions, AQP6 and AQP8 are also included previously [11].
The third subfamily is AQP8-type aquaammoniaporins. The structure and function of AQP8 indicate that AQP8 should not be regarded as either a conventional water channel or an aquaglyceroporin. In AQP8, both NPA motifs are conserved (although the first motif is followed by VS, instead of VT). AQP8 has the highest homology to the plant AQP, γTIP, than any mammalian AQPs [11]. AQP8 is characterized as a Hg2+-inhibitable water channel when expressed in Xenopus oocytes [12,13,14]. AQP8 is unique due to its permeability of NH3/NH4+ [15, 16] in Xenopus oocytes and in AQP8-containing proteoliposomes [17]. While more evidence suggests that AQP8 is not the only aquaporin transporting ammonia, some other classical aquaporins (AQP1, −6) and aquaglyceroporins are also capable of facilitating ammonia transport.
The fourth subfamily is represented by aquaglyceroporins that are permeable to water and other small uncharged molecules (ammonia, urea, in particular glycerol). They also facilitate the diffusion of arsenite and antimonite and play a crucial role in metalloid homeostasis [18]. The aquaglyceroporins, including AQP3, AQP7, AQP9, and AQP10, can be distinguished from aquaporins based on amino acid sequence alignments [19]. The aspartic acid residue in the second NPA box is the signature key for AQP members of this subfamily. This residue is located just the downstream of the arginine forming the aromatic residues/arginine (Ar/R) narrowest filter for the selective water permeation [20]. The aspartic acid residue enlarges this pore constriction and makes more hydrophobic, permeating small molecules larger than water [10]. AQP3 is the first mammalian aquaglyceroporin to be cloned, and it is permeable to glycerol and water [21, 22]. AQP7, AQP9, and AQP10 transport water, glycerol, and urea when expressed in Xenopus oocytes [23,24,25]. AQP9 is also permeable to a wide range of other solutes in oocytes [25]. Most aquaglyceroporins that transport glycerol and urea are less understood yet.
Additionally, a few isoforms, for example, AQP1, AQP3, AQP8, also facilitate hydrogen peroxide membrane permeation and are called peroxiporins.
As AQPs are present in three domains of life including bacteria, eukaryotes, and archaea, a generally accepted classification will be useful to obtain an overview of widely distributed AQP family in every kingdom of lives. AQP superfamily may therefore be classified based on the primary sequence around highly conserved a pair of NPA boxes, which is critical for the function of AQPs. Four AQP subfamilies are identified: AQP1-like, AQP3-like, AQP8-like, and AQP11-like. Compared to the above, consistency of primary sequence is emphasized in this classification. For example, the presence of Asp (D) in the second NPA box is the key for AQP3-like, while Cys (C) at nine residues downstream of the second NPA box is the key for AQP11-like.
1.2 Isoforms of AQPs
To date, at least 13 isoforms of AQPs have been discovered in humans (Table 1.1). The biological roles of these proteins have been thoroughly investigated in the past 30 years after the discovery of the first water channel AQP1. We have learned substantial base of knowledge on the structure, cellular localization, biological function, and potential pathophysiological significance of these mammalian AQPs, although there are some questions still need to answer.
1.2.1 Classical Aquaporins
1.2.1.1 AQP0
AQP0 is the protein in the fiber cells of the eye lens where it is required for homeostasis and transparency of the lens [26]. AQP0 showed lower water permeability than AQP1, about to 1/40 that of AQP1 [27]. AQP0 in lens also functions as peroxiporins to facilitate membrane transport of hydrogen peroxide [28]. The water transport via AQP0 is regulated by C-terminal cleavage [29]. Deletion of amino acids at the C-terminal end of AQP0 impairs lens fiber organization, integrity, mechanical properties, and lens development [30,31,32]. AQP0 is also regulated by pH and Ca2+/calmodulin (CaM) [33]. Lowering internal Ca2+ concentration or inhibiting calmodulin increased AQP0 water permeability. The molecular dynamics and functional mutation studies reveal that binding to calmodulin inhibits AQP0 water permeability by allosterically closing the cytoplasmic gate of AQP0 [34]. Emerging evidence showed that AQP0 could be a marker of erythroid differentiation and play a critical role of AQP0 in erythropoiesis [35].
1.2.1.2 AQP1
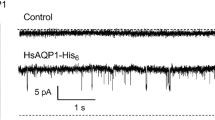
AQP1 is the first water channel discovered [36,37,38] and the first AQP that was found to function as a gas channel [39, 40]. AQP1 is a widely distributed water channel in the body [41], where it plays a central role in the regulation of water transport through those tissues. Aside of facilitating water movement, studies have revealed that AQP1 could enhance CO2 and NH3 permeability [7, 42] and function as a nonselective monovalent cation channel when activated by intracellular cGMP [43]. Phosphorylation of tyrosine Y253 in the C-terminus is involved in the regulation of AQP1 as a cGMP-gated cation channel [44]. Early evidence showed that threonine and serine protein kinase also regulate AQP1 ion channel activity [45]. Recent studies revealed a role of human AQP1 in the facilitated transport of H2O2 in smooth muscle [46] and cardio myocytes cell [47] hypertrophy.
1.2.1.3 AQP2
AQP2 is an arginine vasopressin (AVP)-regulated aquaporin which is probably the most thoroughly studied to date. AQP2 displays permeability only to H2O but not any other small molecules. AQP2 is expressed in principal cells of the collecting ducts and is abundant both in the apical plasma membrane and subapical vesicles [48,49,50] in the kidney where it deeply involved in urine concentration. Translocation of AQP2 from intracellular compartment to the apical membrane is dependent on the binding of vasopressin to its V2 receptor [49, 50] located in the basolateral plasma membrane, by which vasopressin increases the water permeability.
1.2.1.4 AQP4
AQP4 is a predominant AQP located in central nervous system and is permeable to water [51, 52] and CO2 [7]. Phosphorylation of AQP4 at cytosolic serine residues (Ser111 and Ser180) is indicated mediating water permeability by gating [53]. AQP4 possesses Ca2+-dependent calmodulin-binding domains at both its cytosolic N- and C-termini. The S276 residue of AQP4 was able to be phosphorylated in vivo and was linked to Ca2+-CaM-dependent, reversible translocation of AQP4 to the cell surface during extracellular hypotonic challenge of astrocytes [54, 55]. Phosphorylation at AQP4 C-terminus by protein kinase C (PKC) is required for Golgi transition [56].
1.2.1.5 AQP5
AQP5 expression was described in the digestive, renal, respiratory, integumentary, and reproductive systems as well as in sense organs [57]. AQP5 is permeable to water and CO2 [7, 58]. AQP5 can be directly phosphorylated at Ser156 and Thr259 by protein kinase A (PKA) in the cytoplasmic loop and the C-terminus [59, 60]. However, it increases intracellular Ca2+, but not PKA-induced phosphorylation, that induces AQP5 trafficking to plasma membrane [61, 62].
1.2.1.6 AQP6
AQP6 colocalizes with the H+-ATPase in intracellular vesicles in the renal collecting duct type-A intercalated cells [8], indicating that AQP6 may functionally interact with H+-ATPase in the vesicles to regulate intravesicle pH. In response to acid–base changes H+-ATPase in the intercalated cells is observed translocating from the cytoplasmic vesicles to the apical plasma membrane [63], where no AQP6 is found, indicating that AQP6 lacks intracellular trafficking and functions exclusively at the intracellular sites. The lack in intracellular trafficking of AQP6 is likely due to its intracellular retention [64]. A region within loop C of AQP6 that is responsible for severely hampering plasma membrane expression was recently identified. Serine substitution corroborated that amino acids present within AQP6 194–213 of AQP6 loop C contribute to its intracellular endoplasmic reticulum (ER) retention [64]. This signal may preclude proper plasma membrane trafficking and severely curtail expression of AQP6 in heterologous expression systems [64]. AQP6 appears impermeable to H2O [7, 65], but in the presence of HgCl2 or at acidic pH (<5.5), the water and anion permeability of AQP6 in oocytes was rapidly increased [8]. Moreover, AQP6 also enables transport of urea, glycerol, and nitrate [66, 67]. The N-terminus of AQP6 seems critical for the trafficking of the protein to the intracellular sites and intracellular vesicles localization [68]. Calcium signals may be involved in internalization of AQP6 as calmodulin can bind AQP6 in a calcium-dependent manner at the N-terminus [69].
1.2.2 Superaquaporins
1.2.2.1 AQP11
AQP11 has a conventional N-terminal Asn-Pro-Ala (NPA) signature motif and an unique amino acid sequence pattern that includes an Asn-Pro-Cys (NPC) motif, which appears essential for full expression of molecular function [3]. Recent evidence strongly suggests that Cys227 of AQP11 plays an important role in the formation of its quaternary structure and molecular function [70]. One reconstruction vesicle study has clearly shown that AQP11 is indeed a water channel that transports water as efficient as AQP1 [71, 72]. Although detailed subcellular localization of AQP11 remains clarified, it has been observed that AQP11 colocalizes with markers of the endoplasmic reticulum [73] and HA-tagged AQP11-transgenic mice [74]. Recent studies showed that AQP11 colocalized to the mitochondrial-associated membrane (MAM) which regulates essential signal transduction [75]. AQP11 facilitates specifically H2O2 transport to ER [75] and thus AQP11 constitutes an important regulator of renal and hepatic ER redox homeostasis and signaling. Deficiency or downregulation of AQP11 is associated with endoplasmic reticulum stress and apoptosis in the kidney proximal tubules [73] and in adipocytes [76].
1.2.2.2 AQP12
AQP12 is more closely related to AQP11 than to other aquaporins. With regard to the signature motifs, the first NPA motif of AQP12 is substituted by an Asn-Pro-Thr (NPT) motif and the C-terminal NPA motif is conserved [5, 9]. AQP12 seems to be expressed specifically in pancreatic acinar cells and retained in intracellular structures [5]. The osmotic water permeability measured by using vesicles from the AQP12 knockout and wild-type mouse pancreas showed only a small nonsignificant difference [77]. One study suggests that AQP12 may function as controlling the proper secretion of pancreatic fluid following rapid and intense stimulation [77].
1.2.3 AQP8-Type Aquaammoniaporins
1.2.3.1 AQP8
So far, AQP8 is the only member in this family. It is a water channel first found in intracellular domains of the proximal tubule and the collecting duct cells [78]. Several studies showed that AQP8 transports water [7, 79] and ammonia [7, 17]. Although AQP8 was shown ultrastructurally localized at inner mitochondrial membrane (IMM) in the liver and functionally permeable to water [79], this was not supported by water permeability study in AQP8-deleted mouse liver cell IMM [80]. In the kidney, AQP8 facilitates transport of NH3 released from glutamine and glutamate out of the IMM [81] for secretion into the tubule lumen, where the NH3 buffers acid excreted by epithelial cells, particularly during metabolic acidosis [82]. AQP8 may also facilitate the diffusion of hydrogen peroxide across membranes of mitochondrial in situations when reactive oxygen species is generated, e.g., electron transport chain is highly reduced [75, 83, 84].
1.2.4 Aquaglyceroporins
1.2.4.1 AQP3
AQP3 has a wide tissue distribution. It is permeable to water, glycerol, and urea. Recent studies revealed the pH gating of human AQP3 on both water and glycerol permeabilities using a human red blood cell model and in silico [85]. AQPs also differ in their capacity to transport various substances, such as urea, glycerol, H2O2, ions, and gas. Emerging evidence showed that AQP3 is regulated on short-term basis likely via cAMP-PKA pathway [86,87,88]. In the kidney, the increased basolateral diffusion of AQP3 induced by elevated intracellular cAMP likely altered AQP3 interactions with other proteins or lipids in the plasma membrane, which may be a physiological adaptation to the increased water flow mediated by apical AQP2 [86]. AQP3 was shown to transport H2O2 through the plasma membrane [84, 89, 90], which likely plays an important role in initiating intracellular signaling in cell migration [91], inflammation [92], and cancer progression [93, 94].
1.2.4.2 AQP7
AQP7 facilitates transport of water, glycerol, urea, ammonia, arsenite, and NH3 [7, 23, 95]. Hydropathy analysis predicts six putative transmembrane domains with the N- and C-terminal localized in the cytosol. Six prospective sites of AQP7 for PKA phosphorylation have been identified based on database analysis [96], but the direct regulation by PKA remains to be elucidated, whereas a potential PKC phosphorylation site is found at residue Thr-174 [23]. AQP7 is abundantly expressed in adipose tissue [97] and pancreatic β-cells [98, 99].
1.2.4.3 AQP9
AQP9 is expressed at the sinusoidal plasma membrane of hepatocytes [100], where it serves as a conduit for the uptake of NH3 and mediates the efflux of newly synthesized urea. AQP9 may also function as a glycerol channel to facilitate glycerol uptake in the liver. AQP9 is also permeable to water, carbamides, CO2, and NH3; moreover, AQP9 is suggested playing a crucial role in metalloid homeostasis by transporting antimonite and arsenite [2, 11]. Interestingly, it also transports much larger substrates such as lactate, purine, pyrimidine [2, 25], probably due to a larger pore size disclosed by a 3D structure analysis [101]. AQP9 facilitates the membrane transport of H2O2 in mammalian cells and regulates redox-regulated downstream cell signaling [102]. Human AQP9 has a potential N-glycosylation site at Asn142, a potential PKC phosphorylation sites at Ser11 and Ser222, a potential casein kinase II phosphorylation site at Ser28 [25, 103]. However, little is known about short-term regulation of AQP9.
1.2.4.4 AQP10
AQP10 is an aquaglyceroporin expressed only in the human gastrointestinal tract, but not in the mouse small intestine where it has been demonstrated to be a pseudogene [24, 104]. AQP10 is able to transport water, glycerol, and urea when expressed in Xenopus oocytes [24]. AQP10 is also a glycerol channel expressed in the plasma membrane of human adipocytes [105]. Silence of AQP10 in human differentiated adipocytes resulted in a 50% decrease of glycerol and osmotic water permeability, suggesting that AQP10, together with AQP7, is particularly important for the maintenance of normal or low glycerol contents inside the adipocyte, thus protecting humans from obesity [105]. Three potential glycosylated sites for AQP10 were predicted, at least one of them Asn133 in the extracellular loop of AQP10 was confirmed. Glycosylation at Asn133 may increase thermostability of AQP10 when challenged with low temperature, indicating a stabilizing effect of the N-linked glycan [106]. AQP10 mediated increased glycerol flux activated by acidification in human adipocytes [107], likely by a unique gating mechanism combining complex interaction networks between water molecules and protein residues at the loop interface [108].
1.3 Gene Structures of AQPs
Table 1.1 shows chromosome localization and numbers of exons of 13 human AQPs. The gene of AQP0 spans 3.6 kb, contains four exons, and is present in single copy in the haploid human genome. Transcription is initiated from a single site 26 nucleotides downstream from the TATA box [109].
Genomic Southern analysis indicated the existence of a single AQP1 gene that was localized to human 7p14 by in situ hybridization [110,111,112]. AQP2 cDNA was cloned as the water channel of the apical membrane of the kidney collecting tubule in the rat [48], which shows 42% identity in amino acid sequence to AQP1. Human AQP2 encodes a deduced protein with 89.7–91% amino acid identity to the rat protein [112,113,114,115]. By in situ hybridization, AQP2 gene was mapped to chromosome 12q13 [113, 115], very close to the site of major intrinsic protein (MIP).
Using a rat AQP3 probe, Ishibashi [116] screened a human kidney cDNA library and isolated a cDNA coding for human AQP3 protein. AQP3 gene is located at 9p13 and appeared to exist as a single copy with six exons. The initiation site of transcription was identified to be located 64-bp upstream of the first ATG codon. The 5-prime flanking region contained a TATA box, 2 Sp1 sequences, and some consensus sequences including AP-2 sites [117].
Human AQP4 (initially called mercurial-insensitive water channel, MIWC) cDNA cloned from a fatal brain cDNA library showed that the longest open reading frame encoded 301 amino acids with 94% identity to rat AQP4. Analysis of MIWC genomic indicated two distinct but overlapping transcription units from which multiple MIWC mRNAs are transcribed. Later reports revealed that the AQP4 gene is composed of four exons encoding 127, 55, 27, and 92 amino acids separated by introns of 0.8, 0.3, and 5.2 kb. Genomic Southern blot analysis indicated the presence of a single MIWC gene, localized on chromosome 18q [51, 118].
Human AQP5 cDNA and gene was isolated and characterized from a human submaxillary gland library, which contained a 795-bp open reading frame encoding a 265-amino acid polypeptide with a transcription initiation site 518 bp upstream of the initiating methionine. AQP5 gene was mapped to chromosome 12q13 [119].
Ma et al. isolated the cDNA by using degenerate PCR from a human kidney cDNA library that was related to AQP2, having four exons and was organized similarly to AQP0 and AQP2 and later was referred to this gene as AQP6, assigned to chromosome 12q13 [120, 121].
Human AQP7 gene contains 10 exons. An Alu repetitive sequence and binding sites for several different transcription factors within the AQP7 promoter was determined, including a putative peroxisome proliferator response element and a putative insulin response element, indicating potential involvement of AQP7 in energy metabolism [23, 122, 123].
Like the genes of non-water-selective aquaporins, the AQP8 gene contains six exons; however, its exon–intron boundaries are different from the boundaries of those other aquaporin genes. AQP8 gene was mapped to chromosome 16p12 [14, 124].
A partial AQP9 cDNA was isolated by using RT-PCR of leukocyte RNA with primers based on conserved regions of aquaporins [125]. AQP9 shares greater sequence identity with AQP3 and AQP7 than with other members of the family, suggesting that these three proteins belong to a subfamily.
The cDNA encoding AQP10 was isolated from jejunum cDNA library. Sequence analysis predicted that AQP10 is approximately 53% identical to AQP3 and AQP9, Northern blot analysis revealed expression of a 2.3-kb AQP10 transcript in jejunum but not liver [126].
Human AQP11 gene contains three exons and spans 8 kb and was mapped to chromosome 11q14. Human AQP12A gene contains four exons and encodes a 1.5-kb transcript only in pancreas [73, 127].
Genetic variants of AQPs may result in disturbance of molecule selection and transport by AQPs; disruption of the formation of tetramers or arrays; and misfolding, faulty sorting of AQPs, or other dysfunction [81].
References
Abascal F, Irisarri I, Zardoya R (2014) Diversity and evolution of membrane intrinsic proteins. Biochim Biophys Acta 1840(5):1468–1481
Finn RN, Cerda J (2015) Evolution and functional diversity of aquaporins. Biol Bull 229(1):6–23
Ikeda M, Andoo A, Shimono M, Takamatsu N, Taki A, Muta K, Matsushita W, Uechi T, Matsuzaki T, Kenmochi N, Takata K, Sasaki S, Ito K, Ishibashi K (2011) The NPC motif of aquaporin-11, unlike the NPA motif of known aquaporins, is essential for full expression of molecular function. J Biol Chem 286(5):3342–3350
Ishibashi K, Tanaka Y, Morishita Y (2014) The role of mammalian superaquaporins inside the cell. Biochim Biophys Acta 1840(5):1507–1512
Itoh T, Rai T, Kuwahara M, Ko SB, Uchida S, Sasaki S, Ishibashi K (2005) Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res Commun 330(3):832–838
Ishibashi K, Tanaka Y, Morishita Y (2021) The role of mammalian superaquaporins inside the cell: an update. Biochim Biophys Acta Biomembr 1863(7):183617
Geyer RR, Musa-Aziz R, Qin X, Boron WF (2013) Relative CO(2)/NH(3) selectivities of mammalian aquaporins 0-9. Am J Physiol Cell Physiol 304(10):C985–C994
Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P (1999) Rapid gating and anion permeability of an intracellular aquaporin. Nature 402(6758):184–187
Calvanese L, Pellegrini-Calace M, Oliva R (2013) In silico study of human aquaporin AQP11 and AQP12 channels. Protein Sci 22(4):455–466
Ishibashi K, Tanaka Y, Morishita Y (2020) Perspectives on the evolution of aquaporin superfamily. Vitam Horm 112:1–27
Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA (2008) A current view of the mammalian aquaglyceroporins. Annu Rev Physiol 70:301–327
Koyama Y, Yamamoto T, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K, Kihara I (1997) Molecular cloning of a new aquaporin from rat pancreas and liver. J Biol Chem 272(48):30329–30333
Ma T, Yang B, Verkman AS (1997) Cloning of a novel water and urea-permeable aquaporin from mouse expressed strongly in colon, placenta, liver, and heart. Biochem Biophys Res Commun 240(2):324–328
Koyama N, Ishibashi K, Kuwahara M, Inase N, Ichioka M, Sasaki S, Marumo F (1998) Cloning and functional expression of human aquaporin8 cDNA and analysis of its gene. Genomics 54(1):169–172
Jahn TP, Moller AL, Zeuthen T, Holm LM, Klaerke DA, Mohsin B, Kuhlbrandt W, Schjoerring JK (2004) Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett 574(1–3):31–36
Holm LM, Jahn TP, Moller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T (2005) NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch 450(6):415–428
Saparov SM, Liu K, Agre P, Pohl P (2007) Fast and selective ammonia transport by aquaporin-8. J Biol Chem 282(8):5296–5301
Bienert GP, Thorsen M, Schussler MD, Nilsson HR, Wagner A, Tamas MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of as(OH)3 and Sb(OH)3 across membranes. BMC Biol 6:26
Borgnia M, Nielsen S, Engel A, Agre P (1999) Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68:425–458
Verma RK, Gupta AB, Sankararamakrishnan R (2015) Major intrinsic protein superfamily: channels with unique structural features and diverse selectivity filters. Methods Enzymol 557:485–520
Yang B, Verkman AS (1997) Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem 272(26):16140–16146
Echevarria M, Windhager EE, Tate SS, Frindt G (1994) Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci U S A 91(23):10997–11001
Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, Sasaki S (1997) Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem 272(33):20782–20786
Ishibashi K, Morinaga T, Kuwahara M, Sasaki S, Imai M (2002) Cloning and identification of a new member of water channel (AQP10) as an aquaglyceroporin. Biochim Biophys Acta 1576(3):335–340
Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA (1999) Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am J Physiol 277(5):F685–F696
Clemens DM, Nemeth-Cahalan KL, Trinh L, Zhang T, Schilling TF, Hall JE (2013) In vivo analysis of aquaporin 0 function in zebrafish: permeability regulation is required for lens transparency. Invest Ophthalmol Vis Sci 54(7):5136–5143
Chandy G, Zampighi GA, Kreman M, Hall JE (1997) Comparison of the water transporting properties of MIP and AQP1. J Membr Biol 159(1):29–39
Varadaraj K, Kumari SS (2020) Lens aquaporins function as peroxiporins to facilitate membrane transport of hydrogen peroxide. Biochem Biophys Res Commun 524(4):1025–1029
Gonen T, Cheng Y, Kistler J, Walz T (2004) Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol 342(4):1337–1345
Varadaraj K, Kumari S (2019) Deletion of seventeen amino acids at the C-terminal end of aquaporin 0 causes distortion aberration and cataract in the lenses of AQP0DeltaC/DeltaC mice. Invest Ophthalmol Vis Sci 60(4):858–867
Varadaraj K, FitzGerald PG, Kumari SS (2021) Deletion of beaded filament proteins or the C-terminal end of aquaporin 0 causes analogous abnormal distortion aberrations in mouse lens. Exp Eye Res 209:108645
Varadaraj K, Gao J, Mathias RT, Kumari S (2019) C-terminal end of aquaporin 0 regulates lens gap Junction Channel function. Invest Ophthalmol Vis Sci 60(7):2525–2531
Nemeth-Cahalan KL, Kalman K, Hall JE (2004) Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol 123(5):573–580
Reichow SL, Clemens DM, Freites JA, Nemeth-Cahalan KL, Heyden M, Tobias DJ, Hall JE, Gonen T (2013) Allosteric mechanism of water-channel gating by Ca2+−calmodulin. Nat Struct Mol Biol 20(9):1085–1092
Hu C, Peng K, Wu Q, Wang Y, Fan X, Zhang DM, Passerini AG, Sun C (2021) HDAC1 and 2 regulate endothelial VCAM-1 expression and atherogenesis by suppressing methylation of the GATA6 promoter. Theranostics 11(11):5605–5619
Denker BM, Smith BL, Kuhajda FP, Agre P (1988) Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem 263(30):15634–15642
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A 88(24):11110–11114
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256(5055):385–387
Prasad GV, Coury LA, Finn F, Zeidel ML (1998) Reconstituted aquaporin 1 water channels transport CO2 across membranes. J Biol Chem 273(50):33123–33126
Nakhoul NL, Davis BA, Romero MF, Boron WF (1998) Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol 274(2):C543–C548
Day RE, Kitchen P, Owen DS, Bland C, Marshall L, Conner AC, Bill RM Conner MT (2014) human aquaporins: regulators of transcellular water flow. Biochim Biophys Acta 1840(5):1492–1506
Ripoche P, Goossens D, Devuyst O, Gane P, Colin Y, Verkman AS, Cartron JP (2006) Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: a stopped-flow analysis. Transfus Clin Biol 13(1–2):117–122
Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ (2000) Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol Pharmacol 57(3):576–588
Campbell EM, Birdsell DN, Yool AJ (2012) The activity of human aquaporin 1 as a cGMP-gated cation channel is regulated by tyrosine phosphorylation in the carboxyl-terminal domain. Mol Pharmacol 81(1):97–105
Zhang W, Zitron E, Homme M, Kihm L, Morath C, Scherer D, Hegge S, Thomas D, Schmitt CP, Zeier M, Katus H, Karle C, Schwenger V (2007) Aquaporin-1 channel function is positively regulated by protein kinase C. J Biol Chem 282(29):20933–20940
Kramolowsky EV, Tucker RD (1990) Use of 5F bipolar electrosurgical probe in endoscopic urological procedures. J Urol 143(2):275–277
Montiel V, Bella R, Michel LYM, Esfahani H, De Mulder D, Robinson EL, Deglasse JP, Tiburcy M, Chow PH, Jonas JC, Gilon P, Steinhorn B, Michel T, Beauloye C, Bertrand L, Farah C, Dei Zotti F, Debaix H, Bouzin C, Brusa D, Horman S, Vanoverschelde JL, Bergmann O, Gilis D, Rooman M, Ghigo A, Geninatti-Crich S, Yool A, Zimmermann WH, Roderick HL, Devuyst O, Balligand JL (2020) Inhibition of aquaporin-1 prevents myocardial remodeling by blocking the transmembrane transport of hydrogen peroxide. Sci Transl Med 12(564):eaay2176
Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S (1993) Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361(6412):549–552
Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW (1993) Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A 90(24):11663–11667
Marples D, Knepper MA, Christensen EI, Nielsen S (1995) Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol 269(3 Pt 1):C655–C664
Yang B, Ma T, Verkman AS (1995) cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel. Evidence for distinct transcriptional units. J Biol Chem 270(39):22907–22913
Yang B, Brown D, Verkman AS (1996) The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem 271(9):4577–4580
Gunnarson E, Zelenina M, Aperia A (2004) Regulation of brain aquaporins. Neuroscience 129(4):947–955
Kitchen P, Salman MM, Halsey AM, Clarke-Bland C, MacDonald JA, Ishida H, Vogel HJ, Almutiri S, Logan A, Kreida S, Al-Jubair T, Winkel Missel J, Gourdon P, Tornroth-Horsefield S, Conner MT, Ahmed Z, Conner AC, Bill RM (2020) Targeting Aquaporin-4 subcellular localization to treat central nervous system edema. Cell 181(4):784–799e719
Ishida H, Vogel HJ, Conner AC, Kitchen P, Bill RM, MacDonald JA (2022) Simultaneous binding of the N- and C-terminal cytoplasmic domains of aquaporin 4 to calmodulin. Biochim Biophys Acta Biomembr 1864(2):183837
Assentoft M, Kaptan S, Fenton RA, Hua SZ, de Groot BL, MacAulay N (2013) Phosphorylation of rat aquaporin-4 at Ser(111) is not required for channel gating. Glia 61(7):1101–1112
Direito I, Madeira A, Brito MA, Soveral G (2016) Aquaporin-5: from structure to function and dysfunction in cancer. Cell Mol Life Sci 73(8):1623–1640
Musa-Aziz R, Chen LM, Pelletier MF, Boron WF (2009) Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci U S A 106(13):5406–5411
Hasegawa T, Azlina A, Javkhlan P, Yao C, Akamatsu T, Hosoi K (2011) Novel phosphorylation of aquaporin-5 at its threonine 259 through cAMP signaling in salivary gland cells. Am J Physiol Cell Physiol 301(3):C667–C678
Woo J, Lee J, Kim MS, Jang SJ, Sidransky D, Moon C (2008) The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem Biophys Res Commun 367(2):291–298
Tada J, Sawa T, Yamanaka N, Shono M, Akamatsu T, Tsumura K, Parvin MN, Kanamori N, Hosoi K (1999) Involvement of vesicle-cytoskeleton interaction in AQP5 trafficking in AQP5-gene-transfected HSG cells. Biochem Biophys Res Commun 266(2):443–447
Ishikawa Y, Skowronski MT, Inoue N, Ishida H (1999) Alpha(1)-adrenoceptor-induced trafficking of aquaporin-5 to the apical plasma membrane of rat parotid cells. Biochem Biophys Res Commun 265(1):94–100
Verlander JW, Madsen KM, Tisher CC (1987) Effect of acute respiratory acidosis on two populations of intercalated cells in rat cortical collecting duct. Am J Physiol 253(6 Pt 2):F1142–F1156
Soler DC, Kowatz T, Sloan AE, McCormick TS, Cooper KD, Stepanyan R, Engel A, Vahedi-Faridi A (2021) A region within the third extracellular loop of rat aquaporin 6 precludes trafficking to plasma membrane in a heterologous cell line. Sci Rep 11(1):13673
Liu K, Kozono D, Kato Y, Agre P, Hazama A, Yasui M (2005) Conversion of aquaporin 6 from an anion channel to a water-selective channel by a single amino acid substitution. Proc Natl Acad Sci U S A 102(6):2192–2197
Holm LM, Klaerke DA, Zeuthen T (2004) Aquaporin 6 is permeable to glycerol and urea. Pflugers Arch 448(2):181–186
Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M (2002) Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem 277(42):39873–39879
Beitz E, Liu K, Ikeda M, Guggino WB, Agre P, Yasui M (2006) Determinants of AQP6 trafficking to intracellular sites versus the plasma membrane in transfected mammalian cells. Biol Cell 98(2):101–109
Rabaud NE, Song L, Wang Y, Agre P, Yasui M, Carbrey JM (2009) Aquaporin 6 binds calmodulin in a calcium-dependent manner. Biochem Biophys Res Commun 383(1):54–57
Takahashi S, Muta K, Sonoda H, Kato A, Abdeen A, Ikeda M (2014) The role of cysteine 227 in subcellular localization, water permeability, and multimerization of aquaporin-11. FEBS Open Bio 4:315–320
Yakata K, Hiroaki Y, Ishibashi K, Sohara E, Sasaki S, Mitsuoka K, Fujiyoshi Y (2007) Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim Biophys Acta 1768(3):688–693
Yakata K, Tani K, Fujiyoshi Y (2011) Water permeability and characterization of aquaporin-11. J Struct Biol 174(2):315–320
Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, Ikeda M, Yamamoto T, Verkman A, Kusano E, Ookawara S, Takata K, Sasaki S, Ishibashi K (2005) Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol 25(17):7770–7779
Inoue Y, Sohara E, Kobayashi K, Chiga M, Rai T, Ishibashi K, Horie S, Su X, Zhou J, Sasaki S, Uchida S (2014) Aberrant glycosylation and localization of polycystin-1 cause polycystic kidney in an AQP11 knockout model. J Am Soc Nephrol 25(12):2789–2799
Bestetti S, Galli M, Sorrentino I, Pinton P, Rimessi A, Sitia R, Medrano-Fernandez I (2020) Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol 28:101326
Fruhbeck G, Balaguer I, Mendez-Gimenez L, Valenti V, Becerril S, Catalan V, Gomez-Ambrosi J, Silva C, Salvador J, Calamita G, Malagon MM, Rodriguez A (2020) Aquaporin-11 contributes to TGF-beta1-induced endoplasmic reticulum stress in human visceral adipocytes: role in obesity-associated inflammation. Cell 9(6):1403
Ohta E, Itoh T, Nemoto T, Kumagai J, Ko SB, Ishibashi K, Ohno M, Uchida K, Ohta A, Sohara E, Uchida S, Sasaki S, Rai T (2009) Pancreas-specific aquaporin 12 null mice showed increased susceptibility to caerulein-induced acute pancreatitis. Am J Physiol Cell Physiol 297(6):C1368–C1378
Elkjaer ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, Frokiaer J, Nielsen S (2001) Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am J Physiol Renal Physiol 281(6):F1047–F1057
Calamita G, Ferri D, Gena P, Liquori GE, Cavalier A, Thomas D, Svelto M (2005) The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem 280(17):17149–17153
Yang B, Zhao D, Verkman AS (2006) Evidence against functionally significant aquaporin expression in mitochondria. J Biol Chem 281(24):16202–16206
Soria LR, Fanelli E, Altamura N, Svelto M, Marinelli RA, Calamita G (2010) Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem Biophys Res Commun 393(2):217–221
Molinas SM, Trumper L, Marinelli RA (2012) Mitochondrial aquaporin-8 in renal proximal tubule cells: evidence for a role in the response to metabolic acidosis. Am J Physiol Renal Physiol 303(3):F458–F466
Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282(2):1183–1192
Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840(5):1596–1604
de Almeida A, Martins AP, Mosca AF, Wijma HJ, Prista C, Soveral G, Casini A (2016) Exploring the gating mechanisms of aquaporin-3: new clues for the design of inhibitors? Mol Biosyst 12(5):1564–1573
Marlar S, Arnspang EC, Koffman JS, Locke EM, Christensen BM, Nejsum LN (2014) Elevated cAMP increases aquaporin-3 plasma membrane diffusion. Am J Physiol Cell Physiol 306(6):C598–C606
Jourdain P, Becq F, Lengacher S, Boinot C, Magistretti PJ, Marquet P (2014) The human CFTR protein expressed in CHO cells activates aquaporin-3 in a cAMP-dependent pathway: study by digital holographic microscopy. J Cell Sci 127(Pt 3):546–556
Hua Y, Ding S, Zhang W, Zhou Q, Ye W, Chen M, Zhu X (2015) Expression of AQP3 protein in hAECs is regulated by camp-PKA-CREB signalling pathway. Front Biosci 20:1047–1055
Almasalmeh A, Krenc D, Wu B, Beitz E (2014) Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J 281(3):647–656
Miller EW, Dickinson BC, Chang CJ (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A 107(36):15681–15686
Hara-Chikuma M, Chikuma S, Sugiyama Y, Kabashima K, Verkman AS, Inoue S, Miyachi Y (2012) Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J Exp Med 209(10):1743–1752
Hara-Chikuma M, Satooka H, Watanabe S, Honda T, Miyachi Y, Watanabe T, Verkman AS (2015) Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nat Commun 6:7454
Hara-Chikuma M, Watanabe S, Satooka H (2016) Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem Biophys Res Commun 471(4):603–609
Satooka H, Hara-Chikuma M (2016) Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol Cell Biol 36(7):1206–1218
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP (2002) Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A 99(9):6053–6058
Maeda N (2012) Implications of aquaglyceroporins 7 and 9 in glycerol metabolism and metabolic syndrome. Mol Aspects Med 33(5–6):665–675
Miranda M, Escote X, Ceperuelo-Mallafre V, Alcaide MJ, Simon I, Vilarrasa N, Wabitsch M, Vendrell J (2010) Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: differences and similarities between depots. J Clin Endocrinol Metab 95(7):3470–3479
Mendez-Gimenez L, Becerril S, Camoes SP, da Silva IV, Rodrigues C, Moncada R, Valenti V, Catalan V, Gomez-Ambrosi J, Miranda JP, Soveral G, Fruhbeck G, Rodriguez A (2017) Role of aquaporin-7 in ghrelin- and GLP-1-induced improvement of pancreatic beta-cell function after sleeve gastrectomy in obese rats. Int J Obes (Lond) 41(9):1394–1402
Matsumura K, Chang BH, Fujimiya M, Chen W, Kulkarni RN, Eguchi Y, Kimura H, Kojima H, Chan L (2007) Aquaporin 7 is a beta-cell protein and regulator of intraislet glycerol content and glycerol kinase activity, beta-cell mass, and insulin production and secretion. Mol Cell Biol 27(17):6026–6037
Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frokiaer J, Nielsen S (2000) Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 276(3):1118–1128
Viadiu H, Gonen T, Walz T (2007) Projection map of aquaporin-9 at 7 a resolution. J Mol Biol 367(1):80–88
Watanabe S, Moniaga CS, Nielsen S, Hara-Chikuma M (2016) Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem Biophys Res Commun 471(1):191–197
Loitto VM, Huang C, Sigal YJ, Jacobson K (2007) Filopodia are induced by aquaporin-9 expression. Exp Cell Res 313(7):1295–1306
Morinaga T, Nakakoshi M, Hirao A, Imai M, Ishibashi K (2002) Mouse aquaporin 10 gene (AQP10) is a pseudogene. Biochem Biophys Res Commun 294(3):630–634
Laforenza U, Scaffino MF, Gastaldi G (2013) Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS One 8(1):e54474
Oberg F, Sjohamn J, Fischer G, Moberg A, Pedersen A, Neutze R, Hedfalk K (2011) Glycosylation increases the thermostability of human aquaporin 10 protein. J Biol Chem 286(36):31915–31923
Gotfryd K, Mosca AF, Missel JW, Truelsen SF, Wang K, Spulber M, Krabbe S, Helix-Nielsen C, Laforenza U, Soveral G, Pedersen PA, Gourdon P (2018) Human adipose glycerol flux is regulated by a pH gate in AQP10. Nat Commun 9(1):4749
Truelsen SF, Missel JW, Gotfryd K, Pedersen PA, Gourdon P, Lindorff-Larsen K (1864) Helix-Nielsen C (2022) the role of water coordination in the pH-dependent gating of hAQP10. Biochim Biophys Acta Biomembr 1:183809
Pisano MM, Chepelinsky AB (1991) Genomic cloning, complete nucleotide sequence, and structure of the human gene encoding the major intrinsic protein (MIP) of the lens. Genomics 11(4):981–990
Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S (1993) Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol 265(4 Pt 2):F463–F476
van Lieburg AF, Verdijk MA, Knoers VV, van Essen AJ, Proesmans W, Mallmann R, Monnens LA, van Oost BA, van Os CH, Deen PM (1994) Patients with autosomal nephrogenic diabetes insipidus homozygous for mutations in the aquaporin 2 water-channel gene. Am J Hum Genet 55(4):648–652
Deen PM, Verdijk MA, Knoers NV, Wieringa B, Monnens LA, van Os CH, van Oost BA (1994) Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science 264(5155):92–95
Deen PM, Weghuis DO, Sinke RJ, Geurts van Kessel A, Wieringa B, van Os CH (1994) Assignment of the human gene for the water channel of renal collecting duct Aquaporin 2 (AQP2) to chromosome 12 region q12-->q13. Cytogenet Cell Genet 66(4):260–262
Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F (1997) Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8(6):861–867
Sasaki S, Fushimi K, Saito H, Saito F, Uchida S, Ishibashi K, Kuwahara M, Ikeuchi T, Inui K, Nakajima K et al (1994) Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J Clin Invest 93(3):1250–1256
Ishibashi K, Sasaki S, Saito F, Ikeuchi T, Marumo F (1995) Structure and chromosomal localization of a human water channel (AQP3) gene. Genomics 27(2):352–354
Inase N, Fushimi K, Ishibashi K, Uchida S, Ichioka M, Sasaki S, Marumo F (1995) Isolation of human aquaporin 3 gene. J Biol Chem 270(30):17913–17916
Lu M, Lee MD, Smith BL, Jung JS, Agre P, Verdijk MA, Merkx G, Rijss JP, Deen PM (1996) The human AQP4 gene: definition of the locus encoding two water channel polypeptides in brain. Proc Natl Acad Sci U S A 93(20):10908–10912
Lee MD, Bhakta KY, Raina S, Yonescu R, Griffin CA, Copeland NG, Gilbert DJ, Jenkins NA, Preston GM, Agre P (1996) The human Aquaporin-5 gene. Molecular characterization and chromosomal localization. J Biol Chem 271(15):8599–8604
Ma T, Yang B, Kuo WL, Verkman AS (1996) cDNA cloning and gene structure of a novel water channel expressed exclusively in human kidney: evidence for a gene cluster of aquaporins at chromosome locus 12q13. Genomics 35(3):543–550
Ma T, Yang B, Umenishi F, Verkman AS (1997) Closely spaced tandem arrangement of AQP2, AQP5, and AQP6 genes in a 27-kilobase segment at chromosome locus 12q13. Genomics 43(3):387–389
Kondo H, Shimomura I, Kishida K, Kuriyama H, Makino Y, Nishizawa H, Matsuda M, Maeda N, Nagaretani H, Kihara S, Kurachi Y, Nakamura T, Funahashi T, Matsuzawa Y (2002) Human aquaporin adipose (AQPap) gene. Genomic structure, promoter analysis and functional mutation. Eur J Biochem 269(7):1814–1826
Ishibashi K, Yamauchi K, Kageyama Y, Saito-Ohara F, Ikeuchi T, Marumo F, Sasaki S (1998) Molecular characterization of human Aquaporin-7 gene and its chromosomal mapping. Biochim Biophys Acta 1399(1):62–66
Viggiano L, Rocchi M, Svelto M, Calamita G (1999) Assignment of the aquaporin-8 water channel gene (AQP8) to human chromosome 16p11. Cytogenet Cell Genet 84(3–4):208–210
Ishibashi K, Kuwahara M, Gu Y, Tanaka Y, Marumo F, Sasaki S (1998) Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem Biophys Res Commun 244(1):268–274
Hatakeyama S, Yoshida Y, Tani T, Koyama Y, Nihei K, Ohshiro K, Kamiie JI, Yaoita E, Suda T, Hatakeyama K, Yamamoto T (2001) Cloning of a new aquaporin (AQP10) abundantly expressed in duodenum and jejunum. Biochem Biophys Res Commun 287(4):814–819
Ishibashi K, Kuwahara M, Kageyama Y, Sasaki S, Suzuki M, Imai M (2000) Molecular cloning of a new aquaporin superfamily in mammals: AQPX1 and AQPX2. In: Hohmann SN (ed) Molecular biology and physiology of water and solute transport, vol 1, 1st edn. Kluwer Academic/Plenum, New York, pp 123–126
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82170693, 81870465, 81970623, 81670646).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Xu, L., Guo, X., Wang, W., Li, C. (2023). Classification and Gene Structure of Aquaporins. In: Yang, B. (eds) Aquaporins. Advances in Experimental Medicine and Biology, vol 1398. Springer, Singapore. https://doi.org/10.1007/978-981-19-7415-1_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-7415-1_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7414-4
Online ISBN: 978-981-19-7415-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)