Abstract
Aquaporins, a highly conserved group of membrane proteins, are involved in the bidirectional transfer of water and small solutes across cell membranes taking part in many biological functions all over the human body. In view of the wide range of cancer malignancies in which aquaporin-5 (AQP5) has been detected, an increasing interest in its implication in carcinogenesis has emerged. Recent publications suggest that this isoform may enhance cancer cell proliferation, migration and survival in a variety of malignancies, with strong evidences pointing to AQP5 as a promising drug target and as a novel biomarker for cancer aggressiveness with high translational potential for therapeutics and diagnostics. This review addresses the structural and functional features of AQP5, detailing its tissue distribution and functions in human body, its expression pattern in a variety of tumors, and highlighting the underlying mechanisms involved in carcinogenesis. Finally, the actual progress of AQP5 research, implications in cancer biology and potential for cancer detection and prognosis are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aquaporins
Water homeostasis is central to the physiology of all living cells. Channels that facilitate water permeation through cell membranes, nowadays known as aquaporins (AQPs), were first described in red blood cells in the late 1950s [1] and later in renal epithelia [2]. The first recognized water channel, initially designated as Channel-like integral protein of 28 kDa (CHIP28) [3], was accidently isolated and co-purified with the Rh blood group antigen from erythrocytes membranes by Agre and co-workers in 1987 [4]. The deduced protein sequence [5] revealed a shared homology with the major intrinsic protein (MIP) from bovine lens cells suggesting that this protein belongs to the MIP family of transmembrane channel proteins [3]. Further experiments with CHIP28 inserted in liposomes [6] and heterologously expressed in Xenopus oocytes [7], revealed its water channel activity and finally unveiled the protein entity predicted 30 years before. In 2003 Agre and co-workers were awarded the Nobel Prize in Chemistry for the identification and characterization of CHIP28 that was later renamed aquaporin-1 (AQP1). Ever since the discovery of AQP1, many other members of the MIP family have been found, today comprising more than 1700 integral membrane proteins present in virtually all-living organisms [8]. In mammals 13 AQPs were identified with specific organ, tissue, and cellular localizations. Importantly, these proteins are involved in many biological functions including transepithelial fluid transport [9], brain edema, neuroexcitation [10], cell migration [11], adhesion [12], proliferation [13, 14], differentiation [15] and metabolism [16].
The most remarkable feature of AQP channels is their high selectivity and efficiency regarding water or glycerol permeation. AQPs allow water/glycerol to move freely and bidirectionally across the cell membrane in response to osmotic and/or hydrostatic gradients, but exclude ions, such as hydroxide or hydronium ions and protons [17], the latter being essential to preserve the electrochemical potential across the membrane. Apart from water and glycerol, many other permeants such as urea [18], hydrogen peroxide [19], ammonia [20], nitrate [21], arsenite/antimonite [22, 23], nitric oxide [24], silicon [25], carbon dioxide [26] and even anions [27] may permeate specific AQPs.
Based on their primary sequences, mammalian AQPs are divided in three subfamilies: classical or orthodox AQPs, aquaglyceroporins and unorthodox AQPs as illustrated in Fig. 1 [28]. This subdivision also reflects AQPs permeation specificities: (1) orthodox or classical AQPs (AQP0, 1, 2, 4, 5, 6, 8) are primarily selective to water, (2) aquaglyceroporins (AQP3, 7, 9, 10) are permeable to water and small neutral solutes such as glycerol, and (3) unorthodox AQPs (AQP11, 12), also named “S-aquaporins”, “superaquaporins”, “subcellular aquaporins” or “sip-like aquaporins” are located intracellularly with selectivity not clearly established [29, 30], although recent reports revealed AQP11 ability for water [31] and glycerol [32] permeation.
Dendrogram depicting the phylogenetic relationship of mammalian aquaporins (AQP), showing the three main subfamilies. Dendogram was generated by neighbor-joining method (applied to 1000 bootstrap data sets) using the MEGA6 software [145]. The accession numbers are as follows: MIP (XP_011536656), AQP1 (NP_932766), AQP2 (NP_000477), AQP3 (NP_004916), AQP4 (NP_001641), AQP5 (NP_001642), AQP6 (NP_001643), AQP7 (NP_001161), AQP8 (NP_001160), AQP9 (NP_066190), AQP10 (NP_536354), AQP11 (NP_766627), AQP12 (NP_945349)
Recently, a crescent interest has been paid to AQPs and their roles in cancer development. It is well established that tumor growth, development, invasion and metastasis depend on tumor microenvironment and metabolism [33] and it is also known that AQPs play an important role in tissue water balance in response to osmotic gradients, essential to maintain cell function, including in malignant cells [34, 35]. AQPs may also facilitate tumor growth, local infiltration and metastasis by enhancing cell migration and angiogenesis towards a chemotactic stimulus. The exact mechanism is not clear yet, but may involve rapid changes in cell shape and volume induced by AQPs polarization at the front edge of migrating cells, facilitating transmembrane water fluxes driven by changes in osmolality (produced by transmembrane ion fluxes) that promote lamellipodium formation and stabilization by actin polymerization [34, 35] (Fig. 2a). In addition, AQPs may also promote cell–matrix adhesion (Fig. 2b), important for tumor cell spread and migration, although the underlying mechanism remains unclear. AQPs have also been associated with tumor proliferation by facilitating glycerol uptake, which is essential for cellular biosynthesis and, consequently for cell division [34, 35]. Therefore, AQP expression can be advantageous for high metabolic turnover or tumor-specific metabolic pathways needed for survival of malignant cells. Additionally, the possible interaction between AQPs and oncogenes/oncoproteins can ultimately activate the transcription of genes involved in cell growth, transformation and survival [34, 35]. The involvement of AQPs in cancer may be also due to the effects triggered by their permeants (Fig. 2c). In fact, it was recently reported that some AQP isoforms mediate the uptake of hydrogen peroxide [36, 37], a reactive oxygen species that is involved in intracellular signaling and may promote many aspects of tumor progression [38].
Aquaporins (AQPs) roles in cancer. a At the leading edge of the migrating cell, AQPs facilitate water fluxes driven by an increase in local osmolarity due to transmembrane ion fluxes, promoting lamellipodium formation and stabilization by actin polymerization. b AQPs may increase cell–matrix adhesion, important for tumor spread. c AQPs facilitate permeants (glycerol, hydrogen peroxide) uptake or may interact with oncoproteins, which activate intracellular signaling cascades promoting the transcription of genes involved in tumor cell proliferation
In the last decade, an increasing number of reports showed that AQP5 is abundantly expressed in different tumors and could serve as biomarker with prognostic value of cancer aggressiveness [39–42]. In this review, we discuss the present knowledge on AQP5 function and regulation as a membrane channel, focusing on AQP5 expression in human cancers, as well as on its implication in cancer biology and involvement in tumor progression.
Aquaporin-5
Aquaporin-5 (AQP5) was first cloned from rat submandibular gland [43] and is widely distributed among the human body, as depicted in Fig. 3. In fact, its expression has been described in the digestive [44, 45], renal [46], respiratory [47–49], integumentary [50–53] and reproductive systems [54, 55] as well as in sense organs [56–60], as summarized in Table 1. Being primarily selective for water, AQP5 thus plays important water flux control throughout the several body systems.
Tissue distribution of human aquaporin-5 (AQP5). In the eye AQP5 is expressed in the cytoplasm and/or plasma membrane of lens fibber cells depending on its differentiation, in the cytoplasm and plasma membrane of corneal epithelium and in the apical membrane of acinar cells in lacrimal glands. In the inner ear this isoform is expressed in the cochlea, in the apical membrane of outer sulcus cells of the apical turn. In the respiratory system AQP5 is expressed in airways at the apical membrane of columnar epithelial cells and at the apical membrane of serous acinar cells of sub-mucosal glands. In lungs, AQP5 is expressed at the apical membrane of type I pneumocytes. It is also expressed in the apical membrane of ductal cells in mammary glands. In the digestive system this isoform is expressed in the pancreas, at the apical membrane of intercalated and intralobular ductal cells and at the apical membrane of acinar cells in salivary glands, as well as at the apical and basolateral membranes of secretory cells of Brunner’s glands in the duodenum. In integumentary system AQP5 is expressed in the plasma membrane of keratinocytes in the skin granular layer and in apical and basolateral membranes of secretory cells in sweat glands. AQP5 is also expressed in renal cortex, in the apical membrane of type B intercalated cells in the collecting duct. In the female reproductive system AQP5 is expressed in the cytoplasm of vaginal epithelial cells, in the basolateral membrane of endometrial glandular epithelial cells and in the plasma membrane of uterus smooth muscle cells
AQP5 structure and selectivity
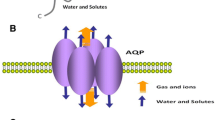
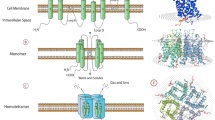
The 3D structure of AQP5 (Fig. 4) shows that this isoform shares the core structural features found in other AQP family members [69]. These channels are inserted in cell membranes as tetramers (Fig. 4a, b) composed by four identical monomers, each behaving as a water pore (Fig. 4c). Each monomer interacts with two of its neighbors, forming a central pore (Fig. 4a) that is not involved in water conductance [17], but was suggested to permeate gases [48, 70, 71] and ions [72, 73]. Interestingly, AQP5 structure was solved with a lipid molecule (phosphatidylserine) occluding this central pore [69], whose physiological relevance is still unclear but may inhibit the permeation of oxygen [74].
Structure of AQP5. a Extracellular and b side views of AQP5 homotetramer showing the central pore with lipid (phosphatidylserine). c Diagram illustrating how water molecules permeate through AQP pore. d Topology map of the basic monomeric AQP5 fold, showing the six transmembrane alpha-helices (1–6) connected by loops (A–E), the conserved asparagine–proline–alanine (NPA) motifs embed in the membrane, the histidine residues involved in gating (His67 and His173) and the serine residue involved in intracellular signaling (Ser156). e Detailed view of AQP5 pore and schematic representation of the proposed AQP5 gating mechanism. The two half-helices are depicted in white and the positioning of ar/R and NPA selectivity filters is indicated. The grey mesh represents the residues lining AQP5 pore. Key histidine residues involved in AQP5 gating are highlighted: His67, which controls the transition between closed and open conformations, and His173, controlling the transition between wide and narrow states. Structures were generated with Chimera (http://www.cgl.ucsf.edu/chimera) and are based on AQP5 X-ray structure (protein data bank code: 3D9S)
The different structural domains of one AQP5 monomer are represented in Fig. 4d. Each monomer is composed of 265 amino acids distributed along six transmembrane alpha-helices (1–6) connected by five loops, with both amino and carboxyl termini located in the cytoplasm [5, 75]. Loops B and E fold back into the membrane forming two half-helices containing two short hydrophobic stretches of amino acid residues asparagine–proline–alanine (NPA), highly conserved and considered the signature sequence for AQPs [76]. The NPA repeats are embedded in the membrane and are critical to water and solute permeation (Fig. 4d). AQP5 pore (grey mesh in Fig. 4e) contains two conserved filters that prevent permeation of too large-sized molecules and positive charges including protons, which is crucial to avoid dissipation of proton gradients. From the exoplasmic side and running along the pore, the narrowest region of the pore is found to be the aromatic/R constriction selectivity filter (ar/R) composed by an arginine residue in an aromatic environment that excludes solutes larger than 2.8 Å. Further down in the center of the pore, the second selective filter is formed by the NPA-capped ends of the two half-helixes which, together with the backbone α-carbonyl groups, act as hydrogen-bond donors and acceptors that coordinate the transport of water through the pore. Both filters are responsible for proton exclusion by mechanisms still in debate in the literature. The first studies pointed to proton exclusion due to water molecule dipoles reorientation near the NPA-motifs causing interruption of hydrogen bonds and preventing proton transfer through a Grotthuss-type ‘proton-wire’ mechanism [17, 77]. In addition, a strong electrostatic field spanning the aquaporin pore, peaking at the NPA and ar/R regions, is responsible for blocking proton conduction through the pore [70, 78, 79]. A recent study has shed new lights on this mechanism highlighting that in addition to the NPA-motifs the ar/R selectivity filter contributes to prevent proton conduction via a Grotthuss mechanism [80].
AQP5 regulation
The molecular mechanisms for the regulation of AQP5 protein expression are not yet fully understood. However, it is known that promoter region of AQP5 gene encompasses two nuclear factor-κB (NF-κB) responsive elements, two activator protein 1 (AP-1) binding sequences [81], a number of putative GATA-binding sites, multiple putative specificity protein 1 (Sp1) consensus sites [82], and an estrogen response element [83], suggesting that these elements may directly regulate AQP5 expression. In addition, GATA-6 mediates transcriptional activation of AQP5 indirectly through synergic interactions with the transcription factor Sp1 [84]. Regulation through osmosensitive transcription nuclear factor of activated T-cells 5 (NFAT5) is implicated in the hyperosmotic expression of AQP5 [85, 86] and hypoxia is implicated in AQP5 downregulation in lung epithelial cells through both hypoxia inducible factor (HIF-1α) and proteasome-mediated pathways [87].
Similar to other eukaryotic AQPs, post-transcriptional regulation of AQP5 function has been reported. AQPs are frequently regulated by trafficking, whereby shuttling from intracellular sites to the plasma membrane occurs in response to various stimuli. The translocation of AQP5 in response to neurotransmitters, hormones [88] and cyclic adenosine monophosphate (cAMP) [89, 90] has been described. cAMP regulates AQP5 expression at both transcriptional and post-transcriptional levels through a protein kinase A (PKA) pathway [89]. AQP5 has two cAMP-dependent protein kinase target motifs at loop D and C-terminal tail (Ser156 and Thr259, respectively). AQP5 translocation is affected by cAMP in a PKA-mediated manner with dual effects: short term exposure to cAMP results in AQP5 internalization from the plasma membrane while long term (8 h) exposure increases AQP5 abundance and labeling on the apical membrane [90]. Woo et al. reported that phosphorylation of AQP5 at its Ser156 is not responsible for AQP5 translocation to the plasma membrane [91] but rather promotes cell proliferation through the RAS signaling pathway [92]. Interestingly, Ser156 is preferentially phosphorylated in tumor cells [93] thus supporting PKA-dependent phosphorylation of Ser156 involvement in cell proliferation.
Recently, Kitchen et al. [94] proposed that AQP5 membrane abundance which results from the balance between translocation to and internalization from the membrane, is regulated by three independent mechanisms: phosphorylation of AQP5 in Ser156 resulting in either increased targeting or decreased internalization or both; involvement of PKA in basal recycling of AQP5 between the plasma membrane and intracellular sites independently of Ser156 phosphorylation; and environmental tonicity-regulated changes in AQP5 localization that are not mediated by phosphorylation of Ser156 nor by PKA. Acting together, these pathways dictate the number of AQP molecules present in the target membrane and regulate cellular water flow.
AQPs are also regulated by “gating”, which are mechanisms that produce a change in the 3D structure, ultimately affecting the rate of water/solute permeation across the channel [95]. An interesting gating mechanism regulating human AQP5 activity has been recently proposed by computational studies [96] that will benefit from further experimental evidence. By means of molecular dynamics simulations Janosi et al. [96] found that AQP5 channel can switch between different conformations characterized by distinct rates of water flux, thus changing between open and closed, and between wide and narrow conformations, respectively (Fig. 4e). The transition between open and closed states occurs through a tap-like mechanism and involves the displacement of the His67 residue located at the cytoplasmic part of the channel. In addition, being in the open conformation, the channel may transition between wide and narrow states, thus conducting maximum and lower water fluxes, respectively. This latter mechanism is governed by the orientation of His173 residue, which is located near the selectivity ar/R filter. The trigger for AQP5 gating is still unclear. It is well established that eukaryotic AQPs can be gated by phosphorylation [95]. Several consensus phosphorylation sites are present in AQP5 [69], however, none of these residues clearly qualifies as the regulatory site [96].
Despite the wide distribution of AQP5 in human body and the detailed information available of its structure and regulatory mechanisms, not much is known about the correlation between AQP5 dysfunction and disease. Recent studies have demonstrated that AQP5 mutations were associated with the development of palmoplantar keratoderma, a disease that is characterized by an abnormal thickening of the skin on the palms of the hands and soles of feet [52, 53]. An AQP5 transport defect was also associated with Sjögren’s syndrome, a chronic autoimmune disease that destroys the salivary and lacrimal glands [66, 97]. AQP5 decreased expression in secretory cells of lacrimal and salivary glands probably contribute to the reduced lacrimation and salivation in humans. In addition, Aqp5-null mice have reduced saliva and airway submucosal secretions [98, 99], reduced water permeability across the alveolar epithelium [100] and thicker corneas [101]. Therefore, further functional and mechanistic studies are required to disclose AQP5 implication in these pathologies.
AQP5 in cancer
Due to AQP5 particular structural characteristics and up-regulation in different tumors, an increasing interest in its involvement in cancer has emerged from 2003 onwards. Recent publications suggest that this isoform enhances cancer cell proliferation, migration and survival through multiple pathways that are not yet fully understood [92, 93, 102–113]. In general tumor cells overexpress this protein that is primarily located in plasma membranes although also found intracellularly for several cancer types. AQP5 expression profile in human cancers is summarized in Table 2.
Figure 5 depicts the proposed molecular events accounting for AQP5 involvement in tumorigenesis. In tumors, the cAMP dependent phosphorylation of AQP5 on Ser156 by PKA activates the RAS/Mitogen-activated protein kinases (MAPK) pathway involved in cell proliferation and survival [92, 102]. AQP5 also binds to the SH3 domain of adaptor molecules, such as c-Src [102] that can in turn activate intracellular pathways, such as RAS/MAPK [92, 103]. AQP5 seems only to interact with the activated form of c-Src [102], which is associated with epithelial mesenchymal transition (EMT), a common process in invasive tumors by which cells lose their epithelial characteristics and acquire migratory mesenchymal properties, starting to express mesenchymal markers [33] and presenting a marked spindle cell-like/fibroblastic phenotype [102]. Activated c-Src promotes EMT by enhancing integrin mediated cell–matrix adhesion and disrupting E-cadherin dependent cell–cell contacts [131]. The activation of integrins and focal adhesion kinase (FAK)–MAPK signaling pathway may also play a prominent role in tumor spread and proliferation [132, 133] related with AQP5 up-regulation [104]. AQP5 may additionally facilitate cancer cell motility due to its preferential polarization in the leading edge of migrating cells [106, 108, 111–113], as described for other oncogenic AQPs [35] (represented in Fig. 2a).
AQP5 intracellular signaling pathways involved in cancer. Phosphorylation of AQP5, mediated by cAMP-dependent protein kinase A (PKA), promotes the binding of adaptor molecules with SH3 domain, such as c-Src, triggering intracellular signaling cascades. Downstream, there is signal transduction through RAS/Raf-1/mitogen activated protein kinase 1 and 2 (MEK1/2)/extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathway to the nucleus, where cyclin (Cy)/cyclin-dependent kinases (CDK) complexes phosphorylate retinoblastoma protein (Rb) that releases the transcription factor E2F, leading to expression of genes that are involved in cell transformation, proliferation, cycle progression and survival. Cell–matrix adhesion mediated by integrins is required for the continuous activation of focal adhesion kinase–mitogen-activated protein kinases (FAK–MAPK) pathway that is essential for cytoskeleton integrity. This pathway also promotes epithelial mesenchymal transition (EMT) by increasing the expression of mesenchymal markers and decreasing the expression of epithelial markers, essential for cancer cell migration and spread. Epidermal growth factor receptor (EGFR) activation by growth factors can activate RAS/MAPK signal transduction pathway and phosphoinositide 3-kinase (PI3K), which in turn activate protein kinase B (AKT) that is able to block caspase-9 activation. In the cytoplasm, nuclear factor-κB (NF-κB) binds to its inhibitor IκBα and stays in an inactive state. After receptor activation, IκBα is phosphorylated and undergoes proteossomal degradation. NF-κB is released and translocates into the nucleus where it activates the transcription of target genes, namely anti-apoptotic genes, resulting in increased AQP5 expression, cell proliferation and survival. AQP5 expression was also recently implicated in colon cancer multidrug resistance mechanisms due to activation of p38 MAPK pathway in response to stress signals
Microarray analysis after AQP5 silencing revealed its influence on the expression of genes related with cell growth, development, cycle progression and apoptosis [124]. This agrees with higher susceptibility to apoptosis after down-regulating AQP5 in cancer cells [107]. The link between AQP5 and tumor resistance to apoptosis is unclear, but may involve the activation of the epidermal growth factor receptor (EGFR). Up-regulating AQP5 in lung cancer cells activated EGFR [111], which is known to trigger the RAS/MAPK as well as phosphatidylinositol-3-kinase (PI3K)/AKT signal pathways [134]. PI3K activates AKT that in turn blocks caspase-9, ultimately blocking apoptosis in AQP5 expressing cancer cells [107]. It is also known that AQP5 promoter contains sequences for nuclear factor-κB (NF-κB) [81], suggesting that NF-κB may regulate AQP5 expression [109, 110]. In the cytoplasm NF-κB binds to its inhibitor IκBα and stays inactive. After receptor activation, IκBα is degraded and NF-κB translocates into the nucleus where it activates the transcription of AQP5 and anti-apoptotic genes, promoting cell proliferation and survival [109, 110].
In colon cancer cells, AQP5 is also associated with the p38 MAPK pathway [105], which is activated in response to stress signals, like chemotherapy-damaged DNA. Activation of p38 MAPK pathway leads to the expression of multidrug resistance proteins responsible for tumor drug resistance [33, 105, 114].
In the next sections we will further review and discuss the current knowledge on AQP5 in cancer, describing its expression profile and highlighting the underlying mechanisms involved in carcinogenesis in a variety of malignancies occurring in different body systems.
Digestive system
Oral and esophageal cancer
Aquaporin-5 is down-regulated in salivary gland adenoid cystic carcinoma, with weak or no expression comparing with normal glands [104]. On the other hand, AQP5 is up-regulated in tongue squamous cell carcinoma (SCC) [104], esophageal SCC [124, 125] and in intramural esophageal SCC [124], suggesting a possible role of this isoform in SCC tumorigenesis.
AQP5 silencing inhibited cell growth in a tongue SCC cell line probably by disrupting the actin alignment and by suppressing the expression of integrins α5 and β1 in an initial phase, and by inhibiting the MAPK signaling pathway in a second phase [104]. In addition, AQP5 knockdown partially reduced cell cycle progression from G1 to S phase and induced apoptosis [104]. These results suggest that AQP5 plays an important role in cell growth, survival and adhesion promoting cytoskeleton integrity and that its suppression could ultimately lead to cell death by anoikis resulting from loss of cell–matrix interaction and cytoskeleton disruption [104, 135].
AQP5 overexpression relates with clinicopathological variables, such as tumor size and histological type as well as with tumor malignancy and the risk of recurrence after curative surgery, pointing that it could constitute a basis for selecting an appropriate postoperative treatment [124]. Interestingly, co-expression of AQP5 and AQP3 is related with invasion depth, lymph node involvement, metastasis and a poorer survival rate [125], which suggest that the combined detection of these two isoforms may be an useful prognostic biomarker for esophageal SCC. Although the correlation between AQP5 overexpression and oral cancer requires validation, the available data indicate that AQP5 could be a drug target and/or a useful biomarker for this disease.
Gastric cancer
Aquaporin-5 is overexpressed in gastric cancer cell lines and tissue samples, especially in intestinal histological type, in parallel with a decrease in AQP4 that is extensively expressed in normal gastric epithelia [112, 128, 129]. However, AQP5 expression in intestinal metaplasia, a precursor event of gastric cancer, is still controversial [112, 129]. Confirming AQP5 expression in metaplastic and then in cancer cells will establish its importance in gastric cancer tumorigenesis and progression. AQP5 overexpression is also correlated with lymph node metastasis and lymphovascular invasion, which are related with gastric cancer aggressiveness [112, 128]. In addition, induced AQP5 overexpression in a poorly differentiated human gastric adenocarcinoma cell line (MKN45) decreased cell proliferation and the number of cells with spindle-like shape and increased alkaline phosphatase activity and laminin β3 expression, which are known markers of differentiated gastric cells, suggesting an involvement of this isoform in cell differentiation [129]. In contrast, another report demonstrated that AQP5 stimulates cell proliferation and migration rather than differentiation [112]. Although these contradictory results may be explained by cell genetic background differences, further studies are needed to disclose the possible involvement of AQP5 in gastric cell differentiation and its clinical implications for cancer patients.
Colon and colorectal cancer
Aquaporin-5 expression is up-regulated in colorectal cancer (CRC) compared with normal colonic tissue where its expression is rarely detected [42, 92, 103, 105, 114, 115]. Some studies also detected AQP5 expression in pre-neoplastic lesions, such as mild dysplasia, as well as early and late adenomas [92, 103, 114]. On the other hand, AQP8 expression seems to be down-regulated [115], suggesting that a loss of this isoform in association with a gain of AQP5 may be a critical event in colorectal carcinogenesis by still unclear molecular mechanisms.
It is well established that activation of RAS/MAPK pathway can lead to EMT and, consequently, to metastatic progression of colorectal cancer [136]. Accordingly, overexpression of AQP5 increased ERK1/2 phosphorylation in HCT116 colon cancer cells while overexpression of AQP1 and AQP3 had no effect [103]. Based on these findings it was proposed that the oncogenic property of AQP5 is mediated by the activation of RAS/MAPK signaling pathway [92], which is critical for cell cycle regulation (Fig. 5).
AQP5 silencing in HT-29 cells demonstrated that AQP5 expression increases cell proliferation, even with chemotherapeutic treatment [105]. Actually, a positive correlation was found between AQP5 expression and the expression of multidrug resistance (MDR) proteins, such as P-glycoprotein (P-gp) [105]. It was also found that treatment of HT-29 cells with AQP5-siRNA or with a p38 MAPK inhibitor decreased p38 phosphorylation, suggesting that this pathway is involved in colon cancer MDR [105] (Fig. 5). Although further investigation is needed to reveal the exact contribution of p38 MAPK pathway in mediating MDR in AQP5 overexpressing cells, these interesting findings propose that AQP5 could be a promissory therapeutic target for CRC.
Independent statistical studies found a positive correlation between AQP5 overexpression and tumor-nodes-metastasis (TNM) stage [115], distant lymph node metastasis [42, 115] and liver metastasis [93]. However, the relationship between the overexpression of this isoform and the degree of tumor differentiation is still controversial and requires additional studies. In fact, AQP5 overexpression was related with decreased tumor differentiation, with higher tumor aggressiveness and, consequently, with a poorer prognosis [105]. On the contrary, another study related AQP5 overexpression with high/medium tumor differentiation and thus less aggressive tumors [115]. Finally, in a more recent study AQP5 was not associated with overall patient survival and disease-free survival, implying that it cannot be considered an independent prognostic marker for CRC [42].
Hepatic cancer
A recent study suggested for the first time that AQP5 overexpression in hepatocellular carcinoma (HCC) cells may be strongly related with more aggressive clinicopathological features, such as higher tumor stage, lymph node involvement and a poorer survival rates [39]. In addition, combined AQP5 and AQP3 overexpression was associated with the poorest prognostic for HCC patients, suggesting that these two proteins could be used as helpful prognosis markers in this type of cancer [39]. Since only one report associates AQP5 overexpression with a poorer survival for HCC patients, additional studies are needed to understand the potential involvement of this isoform in cell proliferation, migration and metastasis, as well as its potential use as a drug target.
Biliary tract cancer
In biliary tract cancer, which comprises both gallbladder and bile duct carcinomas, AQP5 is overexpressed and, in some cases, associated with loss of subcellular polarization [130]. Surprisingly, a positive correlation was found between AQP5 overexpression and small tumor size, favorable response to postoperative chemotherapy and disease-free survival of patients [130], indicating that this isoform acts as a tumor suppressor rather than an oncogenic protein thus playing a different role in this type of cancer. AQP5 may facilitate bile transport and reabsorption and may be important for drug sensitivity in biliary tract cancer.
Respiratory system
Lung cancer
The subcellular localization of AQP5 in lung cancer cells seems to be determined by cellular differentiation. Basolateral membrane expression in nonmucinous bronchioloalveolar carcinoma cells and in atypical adenomatous hyperplasia is unique and might represent an early event in lung adenocarcinoma development [118].
Many reports demonstrated that AQP5 is up-regulated in non-small cell lung cancer (NSCLC), especially in well to moderately differentiated adenocarcinomas [85, 93, 111, 117, 118]. However, an association between AQP5 expression and clinicopathological variables for lung cancer patients is still controversial. AQP5 overexpression was found to be associated with the histological tumor type, TNM staging and lymph node metastasis [137]. A positive correlation was also reported between AQP5 overexpression and worst clinical outcomes, with higher rates of tumor recurrence, early disease progression [102] and decreased survival rates [137] suggesting that AQP5 could be a novel prognostic marker in NSCLC. However, these observations were not confirmed in a posterior study [118] thus rendering necessary additional investigation.
Site-directed mutagenesis demonstrated that both AQP5 phosphorylation by PKA and AQP5 plasma membrane localization are important for migration and proliferation in NSCLC cell lines [102]. Consistent with these findings AQP5 silencing reduced both tumor cell migration and proliferation [85, 111, 119] at least partly through osmosensitive transcription factor NFAT5 regulation [85].
AQP5-overexpressing tumor cells have increased tumor growth rates, produce more and bigger metastasis and are preferably located at the leading edge of the lesions with more alveolar wall invasion. These cells also express higher levels of proliferating cell nuclear antigen and c-Myc, which are mitotic markers [111], and increased amounts of Mucin 5AC [111, 117] and Mucin 5B, in part through EGFR signaling pathway [117]. These findings are important because mucins can affect the formation of tumoral tissues, facilitating tumor progression through an increase in cell proliferation and metastatic potential [138]. Chae et al. [102] found that AQP5-overexpressing bronchial epithelial cells lost their cell–cell contacts, their characteristic polarity and epithelial cell markers, such as E-cadherin, α-catenin and γ-catenin, expressing instead mesenchymal markers, such as vimentin and fibronectin. Contradictory to these findings, Chen et al. [119] observed that the acquisition of a fibroblast-like morphology occurs in parallel with a decrease of AQP5 expression, as well as zonula occludens-1 and E-cadherin. Thus, the influence of AQP5 overexpression in EMT remains to be elucidated for lung cancer.
Integumentary system
Breast cancer
In invasive breast cancer cells AQP5 is up-regulated [67, 120] and its polarity in the apical plasma membrane domain of benign ductal epithelial cells is lost during the progression of breast tumors. In fact, in cancer cells, AQP5 is diffused intracellularly and this diffusion is more prominent in invasive tumor cells, especially in cases with lymph node metastasis [67]. The prominent intracellular location observed in poorly differentiated high-grade tumor cells may result from the lost membrane polarity and subsequent impairment of AQP5 targeting to the membrane; however AQP5 subcellular location may contribute for activation of intracellular pathways involved in proliferation and drug-resistance mechanisms. In fact, knockdown of AQP5 in MCF7 breast cancer cell line reduced cell migration and proliferation, indicating that this isoform plays an important role in tumor spread [67], although the specific pathways involved remain to be elucidated.
AQP5 overexpression is associated with metastasis [67, 120], poor prognosis [40, 67, 120], higher tumor grade and tumor recurrence [120]. Interestingly, in early breast cancer cases AQP5 overexpression was found to be correlated with patient survival in hormone positive (that express progesterone and/or estrogen receptors) and/or human epidermal growth factor receptor 2 (HER2) positive tumors [40]. It is possible that in these early cases, AQP5 may facilitate the entry of chemotherapeutic agents into the cells. It is known that triple negative tumors, that do not express progesterone, estrogen nor HER2 receptors, have an unfavorable prognosis when compared with HER2 and hormone positive tumors due to the lack of therapies other than chemotherapy [139]. A decrease in cell proliferation of a triple negative cell line (MDA-MB-231) was observed after AQP5 silencing [67], which suggests that this protein could be used as an efficient drug target for these cases. AQP5 seems to actively participate in breast cancer tumorigenesis, progression and spread. However, the relationship between the expression of this isoform and survival rates of patients with triple negative tumors needs further investigation.
Kasimir and co-workers [140] found a relationship between functional promoter AQP5-1364C>A polymorphism (AC and CC genotypes) and progesterone receptor positivity. It is known that binding of a transcription factor to the C allele diminishes promoter activity and, consequently decreases AQP5 expression [141]. However, no association was found with increased risk of breast cancer development or with clinicopathological variables such as tumor size and grade, lymph node involvement, metastasis and expression of estrogen or HER2 receptors. Since no differences were found in patients’ survival rates, functional regulation of progesterone receptors by AQP5 and implications for adjuvant therapy remain to be elucidated.
Reproductive system
Ovarian cancer
Aquaporin-5 is overexpressed in malignant and borderline ovarian tumors when compared with benign tumors and normal ovarian tissue, where its expression is almost absent [108, 109, 121, 122]. In addition, the subcellular localization of AQP5 differs between benign and malignant tumors: it is expressed at the basolateral membrane of benign tumor cells, at the apical and basolateral membranes of borderline tumor cells and scattered at the plasma membrane of malignant tumor cells [109, 110].
AQP5 silencing decreased both proliferation and migration of 3AO ovarian cancer cells and tumor growth rates [108], which supports AQP5 implication in tumor growth and spread. AQP5 overexpression was positively correlated with lymph node metastasis and ascites formation; however, no relationship was found with other clinicopathological variables, such as tumor histological type and grade [121].
Treatment of ovarian cancer cell lines SKOV3 and CAOV3 with epigallocatechin gallate (EGCG) [109], a green tea polyphenol, or cisplatin [110], a first-line chemotherapy drug against ovarian cancer, inhibited cell growth and proliferation in a dose and time-dependent manner. These treatments also reduced the expression of AQP5 and NF-κB and increased the expression of IκBα [109, 110]. Experiments with ammonium pyrrolidine dithiocarbamate, a NF-κB specific inhibitor, suggested that blocking NF-κB activation and consequently its nuclear translocation could directly inhibit AQP5 gene transcription and expression, leading to decreased cell proliferation [109, 110]. Treatment with EGCG also activated the apoptosis-signaling pathway by enhancing endonuclease activity leading to DNA fragmentation [109].
Gynecological cancer
Aquaporin-5 expression was found to be up-regulated in cervical [123] and endometrial cancers [113]. In cervical cancer AQP5 overexpression was positively correlated with lymph node metastasis, Ki-67 (proliferative cell marker) expression and poor outcome [123]. In addition, the cumulative expression of AQP5 and Ki-67 is related with the worst survival rates [123]. Since the expression of these proteins is not observed in normal cervical tissues, this may be considered a cancer-specific event. However, further characterization of the pathways by which AQP5 and Ki-67 are involved in the pathogenesis of cervical cancer is still needed, as well as their role as prognostic indicators.
AQP5 silencing in an endometrial adenocarcinoma cell line decreased cell migration suggesting that this isoform may be involved in this process [113]. It is known from previous studies that the expression of endometrial AQP5 is menstrual cycle-dependent and correlates with serum levels of estradiol, which is indicative of an estrogen regulation mechanism [113]. It is also documented that AQP5 promoter region has an estrogen response element that is directly activated by estrogen [83], similarly to AQP2, which expression mediated by estrogen increases cell migration, invasion and adhesion [142]. Since endometrial cancer is an estrogen dependent disease, a similar regulation mechanism may be involved in AQP5 expression.
Prostate cancer
A recent study has revealed for the first time the involvement of AQP5 in prostate cancer. AQP5 can be both overexpressed and lost in subgroups of prostate cancers [143] and both alterations are linked to unfavorable outcome. AQP5 expression was related with AQP5 gene amplification, tumor grade, circulating tumor cells, lymph node metastasis, Ki-67 expression, gene deletions, high Gleason scale and post-operative PSA levels [106, 143]. It is hypothesized that this dichotomous role of AQP5 in cancer cells may be due to different mechanisms: AQP5 may influence migration and may activate intracellular pathways leading to cancer cell proliferation.
Nervous system and hematologic tumors
Chronic myelogenous leukemia
Up to now only one study reported alterations of AQP5 expression in chronic myelogenous leukemia (CML) [107]. In fact, Chae and co-workers showed that AQP5 is overexpressed in CML cell lines and bone marrow samples when compared with peripheral blood lymphocytes and normal bone marrow cells. In addition, they found a positive correlation between higher AQP5 expression and accelerated or blast crisis phase. There is evidence that AQP5 overexpression may be related with increased cell proliferation, which seems to be due to an augment in BCR-ABL1 and Akt phosphorylation at Tyr177 and Thr308 positions, respectively. These two critical cell-signaling molecules for CML cell proliferation can be deactivated by AQP5 ablation, as shown by siRNA assays. It was also found that AQP5 silencing increases caspase 9 activity, resulting in an increasing number of cells undergoing apoptosis [107]. Resistance to imatinib mesylate, a tyrosine-kinase inhibitor used in CML treatment at chronic phase, was also associated with a higher AQP5 expression. However, this drug resistance seems to be due to an independent secondary molecular event rather than to amplification or mutation in BCR-ABL fusion genes, common in CML cells. AQP5 ablation studies with imatinib mesylate therapy are needed to examine if AQP5 down-regulation increases the drug susceptibility of CML cells in patients at chronic phase. If AQP5 is involved in CML cell proliferation and aggressiveness as suggested, it will be important to identify the underlying BCR-ABL1 and apoptosis pathways affected by AQP5 expression. Expression profile studies in other hematologic malignancies would also be of great interest to look for similarities with solid tumors.
Brain cancer
Aquaporin-5 was found to be up-regulated in primary glioblastomas and astrocytomas [127]. However, due to the low number of samples tested, further investigation is needed to corroborate these preliminary findings.
In meningiomas, whose majority are benign tumors, AQP5 overexpression associates with A(−1364)C polymorphism in the promoter region [126]. The C(−1364)allele, known to be involved in decreased promoter activity [141] was associated with low AQP5 expression and with less severe brain edema [126]. In fact, AA genotype seems to result in an AQP5 up-regulation and is related with severe brain edema and poorer outcome, however this correlation appears relatively week and needs to be confirmed [126]. Since AQP5 appears to increase water movement into the surrounding brain parenchyma, preventing AQP5 induced brain edema might have a positive clinical impact. It is also known that RAS pathway may play an important role in meningioma cell proliferation [144] and that AQP5 is involved in RAS signaling transduction [103]. Thus, it would be interesting to investigate if this pathway is involved in the tumorigenesis of meningiomas.
Conclusions and perspectives
Aquaporin-5 overexpression in cancer cells and tumor tissues has been extensively reported. Consistent observations demonstrate that AQP5 is up-regulated in cancer, strongly suggesting its implication in carcinogenesis in different organs and systems. Due to AQP5 involvement in cell migration, proliferation and adhesion in human cancer, this protein emerges as a promising drug target and its modulators as useful anti-tumor agents. Although the mechanisms by which AQP5 interferes with cell differentiation and participates in tumorigenesis are not completely clear, the information available supports its interaction with oncoproteins, such as RAS and c-Src and, thus, its interplay with intracellular signaling transduction pathways. In addition, AQP5-facilitated transmembrane water transport was also proposed to be involved in tumor cell migration, where AQP5 polarization at the front edge of the migrating cell facilitating rapid changes in cell volume and subsequent changes in cell shape is crucial for the suggested mechanism [35]. Despite the precise molecular events responsible for AQP polarization in cells are not clear, the recently described mechanism whereby AQP5 membrane abundance is regulated by phosphorylation [94] may help explain the preferential AQP5 phosphorylated state found in tumors, the common pattern of AQP5 overexpression and the enhance of cell migration and proliferation.
On the contrary, other lines of evidence indicate that AQP5 may instead act as tumor suppressor in some malignancies as indicated by preliminary findings in biliary tract cancer. The complexity of AQP5 therapeutic use is further illustrated by its involvement in both chemosensitivity and drug-resistance mechanisms in tumors. In addition, AQP5 differential expression among human tissues and tumors within different body systems, suggests the need of a tissue-targeted approach for anticancer treatment.
The development of therapeutic strategies using AQP5 as drug target, however promising, needs a stronger basis on the molecular mechanisms responsible for its participation in tumor biology. In addition to AQP5 interaction with oncogenes, it is also possible that its function as a channel (transporting water or any signaling molecule such as hydrogen peroxide) is crucial for tumorigenesis. In this context, the modulation of AQP5 gating (open and closed channel) possibly through phosphorylation might be a powerful tool to prevent tumor development. In fact, the contrasting phosphorylation status between cancer and normal tissues suggests that the key role of this isoform in carcinogenesis is related with its phosphorylation rather than with its expression in cancer cells. Blocking the particular phosphorylation site of AQP5 in loop D may provide a unique opportunity in designing tumor-specific molecular inhibitors.
In conclusion, there is a great translational potential in AQP5-based therapeutics and diagnostics. In view of the wide range of cancer malignancies in which AQP5 is implicated, the potential of AQP5 as a biomarker for cancer detection and prognosis should be explored. Further pathophysiological investigation is required to establish AQP5 as cancer drug target and as a biomarker for cancer detection and follow up.
Abbreviations
- AQP:
-
Aquaporin
- cAMP:
-
Cyclic adenosine monophosphate
- CDK:
-
Cyclin-dependent kinases
- CHIP28:
-
Channel-like integral protein of 28 kDa
- CML:
-
Chronic myelogenous leukemia
- CRC:
-
Colorectal cancer
- EGCG:
-
Epigallocatechin gallate
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial mesenchymal transition
- ERK1/2:
-
Extracellular signal-regulated kinases 1 and 2
- FAK:
-
Focal adhesion kinase
- HCC:
-
Hepatocellular carcinoma
- HIF-1α:
-
Hypoxia inducible factor
- HER2:
-
Epidermal growth factor receptor 2
- IκBα:
-
Nuclear factor-κB inhibitor alpha
- MAPK:
-
Mitogen-activated protein kinases
- MIP:
-
Major intrinsic protein
- MDR:
-
Multidrug resistance
- NAFTA5:
-
Nuclear factor of activated T-cells 5
- NF-κB:
-
Nuclear factor-κB
- NPA:
-
Asparagine–proline–alanine
- NSCLC:
-
Non-small cell lung cancer
- PI3K:
-
Phosphatidylinositol-3-kinase
- PKA:
-
Protein kinase A
- SCC:
-
Squamous cell carcinoma
- Sp1:
-
Specificity protein 1
- TNM:
-
Tumor-nodes-metastasis
References
Paganelli CV, Solomon AK (1957) The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol 41(2):259–277
Whittembury G (1960) Ion and water transport in the proximal tubules of the kidney of Necturus maculosus. J Gen Physiol 43:43–56
Smith BL, Agre P (1991) Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem 266(10):6407–6415
Agre P, Saboori AM, Asimos A, Smith BL (1987) Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem 262(36):17497–17503
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88(24):11110–11114
Zeidel ML, Ambudkar SV, Smith BL, Agre P (1992) Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry 31(33):7436–7440
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256(5055):385–387
Abascal F, Irisarri I, Zardoya R (2014) Diversity and evolution of membrane intrinsic proteins. Biochim Biophys Acta 1840(5):1468–1481. doi:10.1016/j.bbagen.2013.12.001
Noda Y, Sohara E, Ohta E, Sasaki S (2010) Aquaporins in kidney pathophysiology. Nat Rev Nephrol 6(3):168–178. doi:10.1038/nrneph.2009.231
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14(4):265–277. doi:10.1038/nrn3468
Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS (2005) Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434(7034):786–792. doi:10.1038/nature03460
Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi Y (2006) Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol 355(4):628–639. doi:10.1016/j.jmb.2005.10.081
Hara-Chikuma M, Verkman AS (2008) Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med (Berl) 86(2):221–231. doi:10.1007/s00109-007-0272-4
Serna A, Galan-Cobo A, Rodrigues C, Sanchez-Gomar I, Toledo-Aral JJ, Moura TF, Casini A, Soveral G, Echevarria M (2014) Functional inhibition of aquaporin-3 with a gold-based compound induces blockage of cell proliferation. J Cell Physiol 229(11):1787–1801. doi:10.1002/jcp.24632
Madeira A, Mosca AF, Moura TF, Soveral G (2015) Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life 67(1):54–60. doi:10.1002/iub.1345
Verkman AS, Anderson MO, Papadopoulos MC (2014) Aquaporins: important but elusive drug targets. Nat Rev Drug Discov 13(4):259–277. doi:10.1038/nrd4226
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407(6804):599–605. doi:10.1038/35036519
Ma T, Yang B, Verkman AS (1997) Cloning of a novel water and urea-permeable aquaporin from mouse expressed strongly in colon, placenta, liver, and heart. Biochem Biophys Res Commun 240(2):324–328. doi:10.1006/bbrc.1997.7664
Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282(2):1183–1192. doi:10.1074/jbc.M603761200
Holm LM, Jahn TP, Moller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T (2005) NH3 and NH4 + permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch 450(6):415–428. doi:10.1007/s00424-005-1399-1
Ikeda M, Beitz E, Kozono D, Guggino WB, Agre P, Yasui M (2002) Characterization of aquaporin-6 as a nitrate channel in mammalian cells. Requirement of pore-lining residue threonine 63. J Biol Chem 277(42):39873–39879. doi:10.1074/jbc.M207008200
Sanders OI, Rensing C, Kuroda M, Mitra B, Rosen BP (1997) Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J Bacteriol 179(10):3365–3367
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP (2002) Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA 99(9):6053–6058. doi:10.1073/pnas.092131899
Herrera M, Hong NJ, Garvin JL (2006) Aquaporin-1 transports NO across cell membranes. Hypertension 48(1):157–164. doi:10.1161/01.HYP.0000223652.29338.77
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440(7084):688–691. doi:10.1038/nature04590
Nakhoul NL, Davis BA, Romero MF, Boron WF (1998) Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol 274(2 Pt 1):C543–C548
Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P (1999) Rapid gating and anion permeability of an intracellular aquaporin. Nature 402(6758):184–187. doi:10.1038/46045
Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA (2008) A current view of the mammalian aquaglyceroporins. Annu Rev Physiol 70:301–327. doi:10.1146/annurev.physiol.70.113006.100452
Benga G (2012) On the definition, nomenclature and classification of water channel proteins (aquaporins and relatives). Mol Aspects Med 33(5–6):514–517. doi:10.1016/j.mam.2012.04.003
Ishibashi K, Tanaka Y, Morishita Y (2014) The role of mammalian superaquaporins inside the cell. Biochim Biophys Acta 1840(5):1507–1512. doi:10.1016/j.bbagen.2013.10.039
Yakata K, Tani K, Fujiyoshi Y (2011) Water permeability and characterization of aquaporin-11. J Struct Biol 174(2):315–320. doi:10.1016/j.jsb.2011.01.003
Madeira A, Fernandez-Veledo S, Camps M, Zorzano A, Moura TF, Ceperuelo-Mallafre V, Vendrell J, Soveral G (2014) Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity (Silver Spring) 22(9):2010–2017. doi:10.1002/oby.20792
Videira M, Reis RL, Brito MA (2014) Deconstructing breast cancer cell biology and the mechanisms of multidrug resistance. Biochim Biophys Acta 1846(2):312–325. doi:10.1016/j.bbcan.2014.07.011
Verkman AS, Hara-Chikuma M, Papadopoulos MC (2008) Aquaporins–new players in cancer biology. J Mol Med (Berl) 86(5):523–529. doi:10.1007/s00109-008-0303-9
Papadopoulos MC, Saadoun S (2015) Key roles of aquaporins in tumor biology. Biochim Biophys Acta 1848(10 Pt B):2576–2583. doi:10.1016/j.bbamem.2014.09.001
Miller EW, Dickinson BC, Chang CJ (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA 107(36):15681–15686. doi:10.1073/pnas.1005776107
Cordeiro RM (2015) Molecular dynamics simulations of the transport of reactive oxygen species by mammalian and plant aquaporins. Biochim Biophys Acta 1850(9):1786–1794. doi:10.1016/j.bbagen.2015.05.007
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5):479–496. doi:10.3109/10715761003667554
Guo X, Sun T, Yang M, Li Z, Li Z, Gao Y (2013) Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. Biomed Res Int 2013:206525. doi:10.1155/2013/206525
Lee SJ, Chae YS, Kim JG, Kim WW, Jung JH, Park HY, Jeong JY, Park JY, Jung HJ, Kwon TH (2014) AQP5 expression predicts survival in patients with early breast cancer. Ann Surg Oncol 21(2):375–383. doi:10.1245/s10434-013-3317-7
Shan T, Cui X, Li W, Lin W, Li Y (2014) AQP5: a novel biomarker that predicts poor clinical outcome in colorectal cancer. Oncol Rep 32(4):1564–1570. doi:10.3892/or.2014.3377
Kang BW, Kim JG, Lee SJ, Chae YS, Jeong JY, Yoon GS, Park SY, Kim HJ, Park JS, Choi GS, Jeong JY (2015) Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncology 88(6):369–376. doi:10.1159/000369073
Raina S, Preston GM, Guggino WB, Agre P (1995) Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem 270(4):1908–1912
Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K (2004) Aquaporins in the digestive system. Med Electron Microsc 37(2):71–80. doi:10.1007/s00795-004-0246-3
Delporte C (2014) Aquaporins in salivary glands and pancreas. Biochim Biophys Acta 1840(5):1524–1532. doi:10.1016/j.bbagen.2013.08.007
Procino G, Mastrofrancesco L, Sallustio F, Costantino V, Barbieri C, Pisani F, Schena FP, Svelto M, Valenti G (2011) AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney. Cell Physiol Biochem 28(4):683–692. doi:10.1159/000335762
Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE (2001) Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol 24(3):224–234. doi:10.1165/ajrcmb.24.3.4367
Wang K, Feng YL, Wen FQ, Chen XR, Ou XM, Xu D, Yang J, Deng ZP (2007) Decreased expression of human aquaporin-5 correlated with mucus overproduction in airways of chronic obstructive pulmonary disease. Acta Pharmacol Sin 28(8):1166–1174. doi:10.1111/j.1745-7254.2007.00608.x
Seno S, Ogawa T, Shibayama M, Kouzaki H, Shimizu T (2012) Expression and localization of aquaporin 1, 2, 3, 4, and 5 in human nasal mucosa. Am J Rhinol Allergy 26(3):167–171. doi:10.2500/ajra.2012.26.3742
Iizuka T, Suzuki T, Nakano K, Sueki H (2012) Immunolocalization of aquaporin-5 in normal human skin and hypohidrotic skin diseases. J Dermatol 39(4):344–349. doi:10.1111/j.1346-8138.2011.01327.x
Inoue R, Sohara E, Rai T, Satoh T, Yokozeki H, Sasaki S, Uchida S (2013) Immunolocalization and translocation of aquaporin-5 water channel in sweat glands. J Dermatol Sci 70(1):26–33. doi:10.1016/j.jdermsci.2013.01.013
Cao X, Yin J, Wang H, Zhao J, Zhang J, Dai L, Zhang J, Jiang H, Lin Z, Yang Y (2014) Mutation in AQP5, encoding aquaporin 5, causes palmoplantar keratoderma Bothnia type. J Invest Dermatol 134(1):284–287. doi:10.1038/jid.2013.302
Blaydon DC, Lind LK, Plagnol V, Linton KJ, Smith FJ, Wilson NJ, McLean WH, Munro CS, South AP, Leigh IM, O’Toole EA, Lundstrom A, Kelsell DP (2013) Mutations in AQP5, encoding a water-channel protein, cause autosomal-dominant diffuse nonepidermolytic palmoplantar keratoderma. Am J Hum Genet 93(2):330–335. doi:10.1016/j.ajhg.2013.06.008
Kim SO, Oh KJ, Lee HS, Ahn K, Kim SW, Park K (2011) Expression of aquaporin water channels in the vagina in premenopausal women. J Sex Med 8(7):1925–1930. doi:10.1111/j.1743-6109.2011.02284.x
Skowronski MT, Frackowiak L, Skowronska A (2012) The expression of aquaporin 1 and 5 in uterine leiomyomata in premenopausal women: a preliminary study. Reprod Biol 12(1):81–89
Kenney MC, Atilano SR, Zorapapel N, Holguin B, Gaster RN, Ljubimov AV (2004) Altered expression of aquaporins in bullous keratopathy and Fuchs’ dystrophy corneas. J Histochem Cytochem 52(10):1341–1350. doi:10.1369/jhc.4A6385.2004
Garfias Y, Navas A, Perez-Cano HJ, Quevedo J, Villalvazo L, Zenteno JC (2008) Comparative expression analysis of aquaporin-5 (AQP5) in keratoconic and healthy corneas. Mol Vis 14:756–761
Hirt B, Penkova ZH, Eckhard A, Liu W, Rask-Andersen H, Muller M, Lowenheim H (2010) The subcellular distribution of aquaporin 5 in the cochlea reveals a water shunt at the perilymph-endolymph barrier. Neuroscience 168(4):957–970. doi:10.1016/j.neuroscience.2009.09.002
Shankardas J, Patil RV, Vishwanatha JK (2010) Effect of down-regulation of aquaporins in human corneal endothelial and epithelial cell lines. Mol Vis 16:1538–1548
Grey AC, Walker KL, Petrova RS, Han J, Wilmarth PA, David LL, Donaldson PJ, Schey KL (2013) Verification and spatial localization of aquaporin-5 in the ocular lens. Exp Eye Res 108:94–102. doi:10.1016/j.exer.2012.12.004
Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S (2003) Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut 52(7):1008–1016
Kouznetsova I, Gerlach KL, Zahl C, Hoffmann W (2010) Expression analysis of human salivary glands by laser microdissection: differences between submandibular and labial glands. Cell Physiol Biochem 26(3):375–382. doi:10.1159/000320561
Gresz V, Kwon TH, Hurley PT, Varga G, Zelles T, Nielsen S, Case RM, Steward MC (2001) Identification and localization of aquaporin water channels in human salivary glands. Am J Physiol Gastrointest Liver Physiol 281(1):G247–G254
Collaco AM, Jakab RL, Hoekstra NE, Mitchell KA, Brooks A, Ameen NA (2013) Regulated traffic of anion transporters in mammalian Brunner’s glands: a role for water and fluid transport. Am J Physiol Gastrointest Liver Physiol 305(3):G258–G275. doi:10.1152/ajpgi.00485.2012
Jiang XX, Wu RJ, Xu KH, Zhou CY, Guo XY, Sun YL, Lin J (2010) Immunohistochemical detection of aquaporin expression in eutopic and ectopic endometria from women with endometriomas. Fertil Steril 94(4):1229–1234. doi:10.1016/j.fertnstert.2009.08.053
Tsubota K, Hirai S, King LS, Agre P, Ishida N (2001) Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet 357(9257):688–689. doi:10.1016/S0140-6736(00)04140-4
Jung HJ, Park JY, Jeon HS, Kwon TH (2011) Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLoS One 6(12):e28492. doi:10.1371/journal.pone.0028492
McManaman JL, Reyland ME, Thrower EC (2006) Secretion and fluid transport mechanisms in the mammary gland: comparisons with the exocrine pancreas and the salivary gland. J Mammary Gland Biol Neoplasia 11(3–4):249–268. doi:10.1007/s10911-006-9031-3
Horsefield R, Norden K, Fellert M, Backmark A, Tornroth-Horsefield S, Terwisscha van Scheltinga AC, Kvassman J, Kjellbom P, Johanson U, Neutze R (2008) High-resolution X-ray structure of human aquaporin 5. Proc Natl Acad Sci USA 105(36):13327–13332. doi:10.1073/pnas.0801466105
Hub JS, Grubmuller H, de Groot BL (2009) Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations? Handb Exp Pharmacol 190:57–76. doi:10.1007/978-3-540-79885-9_3
Wang Y, Tajkhorshid E (2010) Nitric oxide conduction by the brain aquaporin AQP4. Proteins 78(3):661–670. doi:10.1002/prot.22595
Yool AJ, Weinstein AM (2002) New roles for old holes: ion channel function in aquaporin-1. News Physiol Sci 17:68–72
Boassa D, Stamer WD, Yool AJ (2006) Ion channel function of aquaporin-1 natively expressed in choroid plexus. J Neurosci 26(30):7811–7819. doi:10.1523/JNEUROSCI.0525-06.2006
Zhang YB, Chen LY (2013) In silico study of aquaporin V: effects and affinity of the central pore-occluding lipid. Biophys Chem 171:24–30. doi:10.1016/j.bpc.2012.09.004
Tani K, Fujiyoshi Y (2014) Water channel structures analysed by electron crystallography. Biochim Biophys Acta 1840(5):1605–1613. doi:10.1016/j.bbagen.2013.10.007
Preston GM, Jung JS, Guggino WB, Agre P (1994) Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis. J Biol Chem 269(3):1668–1673
Kozono D, Yasui M, King LS, Agre P (2002) Aquaporin water channels: atomic structure molecular dynamics meet clinical medicine. J Clin Investig 109(11):1395–1399. doi:10.1172/JCI15851
de Groot BL, Grubmuller H (2001) Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science 294(5550):2353–2357. doi:10.1126/science.1062459
Soveral G, Prista C, Moura TF, Loureiro-Dias MC (2010) Yeast water channels: an overview of orthodox aquaporins. Biol Cell 103(1):35–54. doi:10.1042/BC20100102
Kosinska Eriksson U, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R (2013) Subangstrom resolution X-ray structure details aquaporin-water interactions. Science 340(6138):1346–1349. doi:10.1126/science.1234306
Yao C, Purwanti N, Karabasil MR, Azlina A, Javkhlan P, Hasegawa T, Akamatsu T, Hosoi T, Ozawa K, Hosoi K (2010) Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-kappaB and p-c-Jun/c-Fos. Am J Pathol 177(2):724–734. doi:10.2353/ajpath.2010.090282
Borok Z, Li X, Fernandes VF, Zhou B, Ann DK, Crandall ED (2000) Differential regulation of rat aquaporin-5 promoter/enhancer activities in lung and salivary epithelial cells. J Biol Chem 275(34):26507–26514. doi:10.1074/jbc.M910007199
Kobayashi M, Takahashi E, Miyagawa S, Watanabe H, Iguchi T (2006) Chromatin immunoprecipitation-mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes Cells 11(10):1133–1143. doi:10.1111/j.1365-2443.2006.01009.x
Zhou B, Francis TA, Yang H, Tseng W, Zhong Q, Frenkel B, Morrisey EE, Ann DK, Minoo P, Crandall ED, Borok Z (2008) GATA-6 mediates transcriptional activation of aquaporin-5 through interactions with Sp1. Am J Physiol Cell Physiol 295(5):C1141–C1150. doi:10.1152/ajpcell.00120.2008
Guo K, Jin F (2015) NFAT5 promotes proliferation and migration of lung adenocarcinoma cells in part through regulating AQP5 expression. Biochem Biophys Res Commun 465(3):644–649. doi:10.1016/j.bbrc.2015.08.078
Hollborn M, Vogler S, Reichenbach A, Wiedemann P, Bringmann A, Kohen L (2015) Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: involvement of NFAT5. Mol Vis 21:360–377
Kawedia JD, Yang F, Sartor MA, Gozal D, Czyzyk-Krzeska M, Menon AG (2013) Hypoxia and hypoxia mimetics decrease aquaporin 5 (AQP5) expression through both hypoxia inducible factor-1alpha and proteasome-mediated pathways. PLoS One 8(3):e57541. doi:10.1371/journal.pone.0057541
Ishikawa Y, Cho G, Yuan Z, Inoue N, Nakae Y (2006) Aquaporin-5 water channel in lipid rafts of rat parotid glands. Biochim Biophys Acta 1758(8):1053–1060. doi:10.1016/j.bbamem.2006.03.026
Yang F, Kawedia JD, Menon AG (2003) Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem 278(34):32173–32180. doi:10.1074/jbc.M305149200
Sidhaye V, Hoffert JD, King LS (2005) cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 280(5):3590–3596. doi:10.1074/jbc.M411038200
Woo J, Chae YK, Jang SJ, Kim MS, Baek JH, Park JC, Trink B, Ratovitski E, Lee T, Park B, Park M, Kang JH, Soria JC, Lee J, Califano J, Sidransky D, Moon C (2008) Membrane trafficking of AQP5 and cAMP dependent phosphorylation in bronchial epithelium. Biochem Biophys Res Commun 366(2):321–327. doi:10.1016/j.bbrc.2007.11.078
Woo J, Lee J, Kim MS, Jang SJ, Sidransky D, Moon C (2008) The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem Biophys Res Commun 367(2):291–298. doi:10.1016/j.bbrc.2007.12.073
Woo J, Lee J, Chae YK, Kim MS, Baek JH, Park JC, Park MJ, Smith IM, Trink B, Ratovitski E, Lee T, Park B, Jang SJ, Soria JC, Califano JA, Sidransky D, Moon C (2008) Overexpression of AQP5, a putative oncogene, promotes cell growth and transformation. Cancer Lett 264(1):54–62. doi:10.1016/j.canlet.2008.01.029
Kitchen P, Oberg F, Sjohamn J, Hedfalk K, Bill RM, Conner AC, Conner MT, Tornroth-Horsefield S (2015) Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS One 10(11):e0143027. doi:10.1371/journal.pone.0143027
Tornroth-Horsefield S, Hedfalk K, Fischer G, Lindkvist-Petersson K, Neutze R (2010) Structural insights into eukaryotic aquaporin regulation. FEBS Lett 584(12):2580–2588. doi:10.1016/j.febslet.2010.04.037
Janosi L, Ceccarelli M (2013) The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS One 8(4):e59897. doi:10.1371/journal.pone.0059897
Delporte C (2009) Aquaporins in secretory glands and their role in Sjogren’s syndrome. Handb Exp Pharmacol 190:185–201. doi:10.1007/978-3-540-79885-9_9
Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS (1999) Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem 274(29):20071–20074
Song Y, Verkman AS (2001) Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem 276(44):41288–41292. doi:10.1074/jbc.M107257200
Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS (2000) Lung fluid transport in aquaporin-5 knockout mice. J Clin Investig 105(1):93–100. doi:10.1172/JCI8258
Thiagarajah JR, Verkman AS (2002) Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J Biol Chem 277(21):19139–19144. doi:10.1074/jbc.M202071200
Chae YK, Woo J, Kim MJ, Kang SK, Kim MS, Lee J, Lee SK, Gong G, Kim YH, Soria JC, Jang SJ, Sidransky D, Moon C (2008) Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS One 3(5):e2162. doi:10.1371/journal.pone.0002162
Kang SK, Chae YK, Woo J, Kim MS, Park JC, Lee J, Soria JC, Jang SJ, Sidransky D, Moon C (2008) Role of human aquaporin 5 in colorectal carcinogenesis. Am J Pathol 173(2):518–525. doi:10.2353/ajpath.2008.071198
Ishimoto S, Wada K, Usami Y, Tanaka N, Aikawa T, Okura M, Nakajima A, Kogo M, Kamisaki Y (2012) Differential expression of aquaporin 5 and aquaporin 3 in squamous cell carcinoma and adenoid cystic carcinoma. Int J Oncol 41(1):67–75. doi:10.3892/ijo.2012.1445
Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong J, Lu B, Jiang G, Zhao W (2014) AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumour Biol 35(7):7035–7045. doi:10.1007/s13277-014-1956-3
Li J, Wang Z, Chong T, Chen H, Li H, Li G, Zhai X, Li Y (2014) Over-expression of a poor prognostic marker in prostate cancer: AQP5 promotes cells growth and local invasion. World J Surg Oncol 12:284. doi:10.1186/1477-7819-12-284
Chae YK, Kang SK, Kim MS, Woo J, Lee J, Chang S, Kim DW, Kim M, Park S, Kim I, Keam B, Rhee J, Koo NH, Park G, Kim SH, Jang SE, Kweon IY, Sidransky D, Moon C (2008) Human AQP5 plays a role in the progression of chronic myelogenous leukemia (CML). PLoS One 3(7):e2594. doi:10.1371/journal.pone.0002594
Yan C, Zhu Y, Zhang X, Chen X, Zheng W, Yang J (2014) Down-regulated aquaporin 5 inhibits proliferation and migration of human epithelial ovarian cancer 3AO cells. J Ovarian Res 7:78. doi:10.1186/s13048-014-0078-2
Yan C, Yang J, Shen L, Chen X (2012) Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch Gynecol Obstet 285(2):459–467. doi:10.1007/s00404-011-1942-6
Yang J, Yan C, Zheng W, Chen X (2012) Proliferation inhibition of cisplatin and aquaporin 5 expression in human ovarian cancer cell CAOV3. Arch Gynecol Obstet 285(1):239–245. doi:10.1007/s00404-011-1908-8
Zhang Z, Chen Z, Song Y, Zhang P, Hu J, Bai C (2010) Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J Pathol 221(2):210–220. doi:10.1002/path.2702
Huang YH, Zhou XY, Wang HM, Xu H, Chen J, Lv NH (2013) Aquaporin 5 promotes the proliferation and migration of human gastric carcinoma cells. Tumour Biol 34(3):1743–1751. doi:10.1007/s13277-013-0712-4
Jiang XX, Xu KH, Ma JY, Tian YH, Guo XY, Lin J, Wu RJ (2012) Reduced migration of Ishikawa cells associated with downregulation of aquaporin-5. Oncol Lett 4(2):257–261. doi:10.3892/ol.2012.738
Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, Trink B, Chang YS, Sidransky D, Mao L (2003) Involvement of aquaporins in colorectal carcinogenesis. Oncogene 22(43):6699–6703. doi:10.1038/sj.onc.1206762
Wang W, Li Q, Yang T, Bai G, Li D, Li Q, Sun H (2012) Expression of AQP5 and AQP8 in human colorectal carcinoma and their clinical significance. World J Surg Oncol 10:242. doi:10.1186/1477-7819-10-242
Zhang ZQ, Zhu ZX, Bai CX (2012) Lung fluorescence imaging to evaluate tumor metastasis induced by AQP5 expression in murine model. Med Oncol 29(1):205–211. doi:10.1007/s12032-010-9788-5
Zhang ZQ, Zhu ZX, Bai CX, Chen ZH (2011) Aquaporin 5 expression increases mucin production in lung adenocarcinoma. Oncol Rep 25(6):1645–1650. doi:10.3892/or.2011.1241
Machida Y, Ueda Y, Shimasaki M, Sato K, Sagawa M, Katsuda S, Sakuma T (2011) Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential. Hum Pathol 42(5):669–678. doi:10.1016/j.humpath.2010.07.022
Chen Z, Zhang Z, Gu Y, Bai C (2011) Impaired migration and cell volume regulation in aquaporin 5-deficient SPC-A1 cells. Respir Physiol Neurobiol 176(3):110–117. doi:10.1016/j.resp.2011.02.001
Shi Z, Zhang T, Luo L, Zhao H, Cheng J, Xiang J, Zhao C (2012) Aquaporins in human breast cancer: identification and involvement in carcinogenesis of breast cancer. J Surg Oncol 106(3):267–272. doi:10.1002/jso.22155
Yang JH, Shi YF, Cheng Q, Deng L (2006) Expression and localization of aquaporin-5 in the epithelial ovarian tumors. Gynecol Oncol 100(2):294–299. doi:10.1016/j.ygyno.2005.08.054
Yang JH, Yu YQ, Yan CX (2011) Localisation and expression of aquaporin subtypes in epithelial ovarian tumours. Histol Histopathol 26(9):1197–1205
Zhang T, Zhao C, Chen D, Zhou Z (2012) Overexpression of AQP5 in cervical cancer: correlation with clinicopathological features and prognosis. Med Oncol 29(3):1998–2004. doi:10.1007/s12032-011-0095-6
Shimizu H, Shiozaki A, Ichikawa D, Fujiwara H, Konishi H, Ishii H, Komatsu S, Kubota T, Okamoto K, Kishimoto M, Otsuji E (2014) The expression and role of aquaporin 5 in esophageal squamous cell carcinoma. J Gastroenterol 49(4):655–666. doi:10.1007/s00535-013-0827-9
Liu S, Zhang S, Jiang H, Yang Y, Jiang Y (2013) Co-expression of AQP3 and AQP5 in esophageal squamous cell carcinoma correlates with aggressive tumor progression and poor prognosis. Med Oncol 30(3):636. doi:10.1007/s12032-013-0636-2
Lambertz N, Hindy NE, Adler C, Rump K, Adamzik M, Keyvani K, Bankfalvi A, Siffert W, Erol Sandalcioglu I, Bachmann HS (2013) Expression of aquaporin 5 and the AQP5 polymorphism A(−1364)C in association with peritumoral brain edema in meningioma patients. J Neurooncol 112(2):297–305. doi:10.1007/s11060-013-1064-z
McCoy E, Sontheimer H (2007) Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia 55(10):1034–1043. doi:10.1002/glia.20524
Shen L, Zhu Z, Huang Y, Shu Y, Sun M, Xu H, Zhang G, Guo R, Wei W, Wu W (2010) Expression profile of multiple aquaporins in human gastric carcinoma and its clinical significance. Biomed Pharmacother 64(5):313–318. doi:10.1016/j.biopha.2009.12.003
Watanabe T, Fujii T, Oya T, Horikawa N, Tabuchi Y, Takahashi Y, Morii M, Takeguchi N, Tsukada K, Sakai H (2009) Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci 59(2):113–122. doi:10.1007/s12576-008-0017-3
Sekine S, Shimada Y, Nagata T, Moriyama M, Omura T, Watanabe T, Hori R, Yoshioka I, Okumura T, Sawada S, Fukuoka J, Tsukada K (2012) Prognostic significance of aquaporins in human biliary tract carcinoma. Oncol Rep 27(6):1741–1747. doi:10.3892/or.2012.1747
Avizienyte E, Brunton VG, Fincham VJ, Frame MC (2005) The SRC-induced mesenchymal state in late-stage colon cancer cells. Cells Tissues Organs 179(1–2):73–80. doi:10.1159/000084511
Zhao JH, Reiske H, Guan JL (1998) Regulation of the cell cycle by focal adhesion kinase. J Cell Biol 143(7):1997–2008
Schlaepfer DD, Hunter T (1997) Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem 272(20):13189–13195
McCubrey JA, Abrams SL, Fitzgerald TL, Cocco L, Martelli AM, Montalto G, Cervello M, Scalisi A, Candido S, Libra M, Steelman LS (2015) Roles of signaling pathways in drug resistance, cancer initiating cells and cancer progression and metastasis. Adv Biol Regul 57:75–101. doi:10.1016/j.jbior.2014.09.016
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2(5):249–256. doi:10.1038/35010517
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196. doi:10.1038/nrm3758
Song T, Yang H, Ho JC, Tang SC, Sze SC, Lao L, Wang Y, Zhang KY (2015) Expression of aquaporin 5 in primary carcinoma and lymph node metastatic carcinoma of non-small cell lung cancer. Oncol Lett 9(6):2799–2804. doi:10.3892/ol.2015.3108
Sugihara I, Yoshida M, Shigenobu T, Takagi H, Maruyama K, Takeuchi N, Toda M, Inoue M, Nakada H (2006) Different progression of tumor xenografts between mucin-producing and mucin-non-producing mammary adenocarcinoma-bearing mice. Cancer Res 66(12):6175–6182. doi:10.1158/0008-5472.CAN-05-3663
Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N (2009) Triple-negative breast cancer–current status and future directions. Ann Oncol 20(12):1913–1927. doi:10.1093/annonc/mdp492
Kasimir-Bauer S, Heubner M, Otterbach F, Kimmig R, Siffert W, Adamzik M (2009) Prognostic relevance of the AQP5 -1364C>A polymorphism in primary breast cancer. Mol Med Rep 2(4):645–650. doi:10.3892/mmr_00000151
Adamzik M, Frey UH, Bitzer K, Jakob H, Baba HA, Schmieder RE, Schneider MP, Heusch G, Peters J, Siffert W (2008) A novel-1364A/C aquaporin 5 gene promoter polymorphism influences the responses to salt loading of the renin-angiotensin-aldosterone system and of blood pressure in young healthy men. Basic Res Cardiol 103(6):598–610. doi:10.1007/s00395-008-0750-z
Zou LB, Zhang RJ, Tan YJ, Ding GL, Shi S, Zhang D, He RH, Liu AX, Wang TT, Leung PC, Sheng JZ, Huang HF (2011) Identification of estrogen response element in the aquaporin-2 gene that mediates estrogen-induced cell migration and invasion in human endometrial carcinoma. J Clin Endocrinol Metab 96(9):E1399–E1408. doi:10.1210/jc.2011-0426
Pust A, Kylies D, Hube-Magg C, Kluth M, Minner S, Koop C, Grob T, Graefen M, Salomon G, Tsourlakis MC, Izbicki J, Wittmer C, Huland H, Simon R, Wilczak W, Sauter G, Steurer S, Krech T, Schlomm T, Melling N (2015) Aquaporin 5 expression is frequent in prostate cancer and shows a dichotomous correlation with tumor phenotype and PSA recurrence. Hum Pathol. doi:10.1016/j.humpath.2015.09.026
Shu J, Lee JH, Harwalkar JA, Oh-Siskovic S, Stacey DW, Golubic M (1999) Adenovirus-mediated gene transfer of dominant negative Ha-Ras inhibits proliferation of primary meningioma cells. Neurosurgery 44(3):579–587 (discussion 587–578)
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (PEst-OE/SAU/UI4013/2011–2014 to iMed.ULisboa).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Direito, I., Madeira, A., Brito, M.A. et al. Aquaporin-5: from structure to function and dysfunction in cancer. Cell. Mol. Life Sci. 73, 1623–1640 (2016). https://doi.org/10.1007/s00018-016-2142-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2142-0